 ,1, 刘峰1, 戴明剑1, 孙婷婷1, 苏炜华1, 王春风1, 张旭1, 毛花英1, 苏亚春
,1, 刘峰1, 戴明剑1, 孙婷婷1, 苏炜华1, 王春风1, 张旭1, 毛花英1, 苏亚春 ,1,2,*, 阙友雄
,1,2,*, 阙友雄 ,1,2,*
,1,2,*Cloning and Expression Characteristic Analysis of ScWRKY4 Gene in Sugarcane
WANG Ling ,1, LIU Feng1, DAI Ming-Jian1, SUN Ting-Ting1, SU Wei-Hua1, WANG Chun-Feng1, ZHANG Xu1, MAO Hua-Ying1, SU Ya-Chun
,1, LIU Feng1, DAI Ming-Jian1, SUN Ting-Ting1, SU Wei-Hua1, WANG Chun-Feng1, ZHANG Xu1, MAO Hua-Ying1, SU Ya-Chun ,1,2,*, QUE You-Xiong
,1,2,*, QUE You-Xiong ,1,2,*
,1,2,*通讯作者:
第一联系人:
收稿日期:2018-01-28接受日期:2018-06-12网络出版日期:2018-07-02
| 基金资助: |
Received:2018-01-28Accepted:2018-06-12Online:2018-07-02
| Fund supported: |

摘要
关键词:
Abstract
Keywords:
PDF (4186KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
王玲, 刘峰, 戴明剑, 孙婷婷, 苏炜华, 王春风, 张旭, 毛花英, 苏亚春, 阙友雄. 甘蔗ScWRKY4基因的克隆与表达特性分析[J]. 作物学报, 2018, 44(9): 1367-1379. doi:10.3724/SP.J.1006.2018.01367
WANG Ling, LIU Feng, DAI Ming-Jian, SUN Ting-Ting, SU Wei-Hua, WANG Chun-Feng, ZHANG Xu, MAO Hua-Ying, SU Ya-Chun, QUE You-Xiong.
植物在生长过程中, 依赖其体内各种功能基因的表达来适应环境, 特别是在生物和非生物逆境胁迫条件下, 这些相关功能基因在转录水平上的表达和修饰就显得尤其重要。转录因子又称反式作用因子, 可以结合在靶基因上游的顺式作用元件上, 对相关功能靶基因的转录效率进行正向或负向调控[1]。目前, 对植物逆境相关转录因子的研究较多的是WRKY、MYB (myeloblastosis)、NAC [NAM, ATAF1(2), CUC2]、bZIP (basic letcine-zipper)和AP2/ERF (APETALA 2/ethylene-responsive element binding factor)五大类[2]。自1994年首个甘薯WRKY转录因子家族基因SPF1 (SWEET POTATO FACTORS)[3]被克隆以来, 逐渐有大量的WRKY基因从多种植物中被分离鉴定。WRKY基因家族成员在模式植物拟南芥(Arabidopsis thaliana)中有72个[4]、水稻(Oryza sativa)中有105个[5]、高粱(Sorghum bicolor)中有68个[6]、大麦(Hordeum vulgare)中有45个[7]、玉米(Zea mays)中有119个[8]。
1996年Rushton等[9]发现欧芹(Petroselinum crispum)的WRKY转录因子WRKY1、WRKY2和WRKY3在PR1基因介导的免疫应答中具有调控作用。拟南芥72个WRKY基因中有49个受到丁香假单胞菌(Pseudomonas syringae)和水杨酸(salicylic acid, SA)的诱导表达[10]。利用全基因组分析大麦WRKY转录因子的表达图谱发现, HvWRKY1和HvWRKY2负调控大麦对白粉菌(Powdery mildew)的抗性[11]。水稻有15个WRKY转录因子可以被稻瘟病菌(Magnaporthe grisea)诱导表达, 其中12个能够同时被白叶枯病菌(Xanthomonas oryzae pv. oryzae)诱导表达[12]; 在水稻中过表达OsWRKY13基因能够显著增强其对白叶枯病病菌和稻瘟病菌的抗性[13]; OsWRKY62在水稻的先天免疫过程中起负调节作用[14]。拟南芥的WRKY转录因子中有30个响应盐胁迫, 其中23个上调表达, 7个下调表达[15]。菜豆(Phaseolus vulgaris)的88个WRKY转录因子中, 有19个受干旱胁迫诱导表达, 其中11个下调, 8个上调[16]。转录组分析发现, 杨树(Populus)的100个WRKY转录因子中, 有61个受到杨树黑斑病菌(Marssonina brunnea)、SA、茉莉酸甲酯(methyl jasmonate, MeJA)、损伤、冷胁迫、盐胁迫等诱导表达[17]。此外, 玉米中有58个WRKY基因受干旱胁迫诱导表达[18]。上述报道为研究植物WRKY转录因子在应答不同生物和非生物逆境胁迫下的耐受机制提供了基础。
甘蔗(Saccharum spp.)不仅是最重要的糖料作物, 也是最有潜力的生物能源作物, 具有重要的生物学和经济价值。研究甘蔗在生长发育及其适应外界环境变化过程中的信号转导, 特别是转录因子的功能, 对甘蔗的高产、高效分子育种理论具有重要的参考意义。目前, 在甘蔗上仅有1篇关于WRKY基因的研究报道[19]。Liu等[19]发现Sc-WRKY基因(GenBank登录号为GQ246458)在甘蔗品种福农22 (FN22)中的表达受甘蔗黑穗病菌(Sporisorium scitamineum)、SA、氯化钠(sodium chloride, NaCl)和聚乙二醇(polyethylene glycol, PEG)的诱导, 表明该基因可能在甘蔗对黑穗病菌、干旱和高盐胁迫的响应机制中发挥作用。本研究基于课题组前期构建的甘蔗转录组数据库, 从甘蔗品种新台糖22 (ROC22)中成功分离获得1个新的甘蔗WRKY转录因子基因ScWRKY4, 借助生物信息学软件预测了该基因的理化性质, 并验证了其亚细胞定位情况及转录激活活性, 同时利用实时荧光定量PCR (qRT-PCR)技术分析了ScWRKY4基因在黑穗病菌及不同植物激素和非生物胁迫下的表达水平变化, 目的是为甘蔗抗逆分子育种提供基因资源。
1 材料与方法
1.1 材料处理
试验所用的甘蔗材料由福建农林大学农业部福建甘蔗生物学与遗传育种重点实验室提供, 分别为感黑穗病品种ROC22和抗黑穗病品种崖城05-179。参照黄宁等[20]的方法, 从甘蔗田随机带土挖取9株健康并且长势一致的植株, 立即洗净根部泥土, 从乳白色幼嫩的蔗根取样; 从地面以上完整可见的第一节间算起, 选取第7~8节间, 用75%酒精擦净其表皮, 从侧芽、蔗皮和蔗肉取样; 选取+1叶的蔗叶, 用酒精擦拭干净。选择3株作为一个重复, 共3个生物学重复。用锡箔纸包好所有材料, 立即投入液氮冷冻, -80℃保存备用。为了分析目的基因在不同外源胁迫下的表达特性, 将ROC22健康植株的蔗茎砍成单芽茎段, 清洗干净并温水脱毒处理后, 在塑料托盆中沙培育苗3个月左右, 至其长出第4或第5叶, 挑选健壮且长势一致的蔗苗, 将根清洗干净后放入盛有清水的塑料盆中炼苗培养10 d, 开始试验处理。第一组分别在250 mmol L-1 NaCl和25.0% PEG-8000水溶液中培养, 于处理后0、0.5、3、6和24 h进行叶片取样; 第二组在叶面分别喷施100 μmol L-1脱落酸(abscisic acid, ABA)、5 mmol L-1 SA (含0.01%吐温-20, v/v)和25 μmol L-1 MeJA, ABA处理在0、0.5、3、6和24 h进行叶片取样, SA和MeJA处理在0、3、12和24 h进行叶片取样。黑穗病菌处理材料为32℃培养箱催芽至2 cm左右的蔗芽, 针刺接种5×106个 mL-1 (含0.01%吐温-20, v/v)浓度的黑穗病菌孢子悬浮液, 同时, 对照以无菌蒸馏水(含0.01%吐温-20, v/v)接种, 于28℃下16 h光照/8 h黑暗培养, 分别于处理后0、24、48和72 h进行蔗芽取样。每3株作为1次生物学重复, 每组设置3个生物学重复。用锡箔纸包好所有材料, 立即投入液氮冷冻, -80℃保存备用。
1.2 甘蔗ScWRKY4基因的克隆与生物信息学分析
利用TRIzol Reagent (Invitrogen, Carlsbad, CA, USA)提取ROC22叶片总RNA, 参照RevertAid First Strand cDNA Synthesis Kit (Fermentas, 中国上海)说明书将其逆转录为cDNA, 作为基因克隆的模板。从课题组前期构建的甘蔗受黑穗病菌侵染的转录组数据库[21]中, 挖掘到一条WRKY转录因子的Unigene序列(转录本ID: c128160.graph_c0), 命名为ScWRKY4, 利用NCBI在线软件设计该基因全长扩增引物对ScWRKY4-F/R (表1)。PCR反应总体系为25 μL, 包含cDNA模板1.0 μL、上下游引物(10 μmol L-1)各1.0 μL、Ex Taq DNA酶(5 U μL-1) 0.125 μL、10×Ex Taq缓冲液(Mg2+ plus) 2.5 μL、dNTPs混合物(2.5 mmol L-1) 2.0 μL和ddH2O 17.375 μL。PCR反应条件为94℃预变性4 min; 94℃变性30 s, 57℃退火30 s, 72℃延伸2 min, 35个循环; 最后72℃再延伸10 min。反应产物经1%琼脂糖凝胶电泳检测, 参照Gel Extraction Kit (天根, 中国北京)说明书纯化回收后, 再将其连接到pMD19-T (TaKaRa, 中国大连)克隆载体上并转化大肠杆菌DH5α感受态细胞(天根, 中国北京), 最后随机挑取单菌落进行PCR鉴定, 并将检测呈阳性的菌落送至尚亚有限公司测序分析。Table 1
表1
表1引物序列及用途
Table 1
| 引物名称 Primer name | 引物序列 Primer sequence (5°-3°) | 用途 Usage |
|---|---|---|
| ScWRKY4-F | TCCTCGGCATCTCCCATTCT | 扩增全长 Full length amplifcation |
| ScWRKY4-R | GTCTGGGAGCTCATGTTCGT | |
| ScWRKY4-QF | ATGAAGGTGAGGAGGAAGATG | 荧光定量 qRT-PCR |
| ScWRKY4-QR | CTTGTAGCCGTCGTCCAG | |
| GAPDH-QF | CACGGCCACTGGAAGCA | 内参基因 Reference gene |
| GAPDH-QR | TCCTCAGGGTTCCTGATGCC | |
| ScWRKY4-Subloc-F | CGGGATCCATGGAGGGGAGCA | 亚细胞定位 Subcellular localization |
| ScWRKY4-Subloc-R | TGCTCTAGAGAGCGACGTGAAAGC | |
| ScWRKY4-BD-F | GGAATTCCATATGATGGAGGGGAGCAGCCAGCT | 转录激活 Transactivation |
| ScWRKY4-BD-R | CGGGATCCGAGCGACGTGAAAGCGCAGC |
新窗口打开|下载CSV
通过NCBI的ORF finder (https://www.ncbi.nlm. nih.gov/orffinder/)在线程序和Conserved domains (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi)数据库, 分析测序获得的ScWRKY4 cDNA序列的开放阅读框(open reading frame, ORF)及保守功能结构域。运用ProtParam (https://web.expasy.org/protparam/)和GOR IV (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_ automat.pl?page=/NPSA/npsa_hnn.html/)程序, 预测ScWRKY4蛋白的一级和二级结构特征。利用在线程序SignalP 4.1 Server (http://www.cbs.dtu.dk/ services/SignalP/)、TMHMM Server v. 2.0 (http://www. cbs.dtu.dk/services/TMHMM/)和NetPhos 2.0 Server (http://www.cbs.dtu.dk/services/NetPhos/)分析ScWRKY4基因编码氨基酸的信号肽、跨膜结构域和磷酸化位点。借助Psort (http://www.psort.org/)工具预测ScWRKY4蛋白的亚细胞定位情况。采用NCBI中Blastp程序, 查找与甘蔗ScWRKY4蛋白同源的氨基酸序列, 而后利用DNAMAN 6.0软件进行序列比对, 并通过ClustalW对ScWRKY4蛋白与甘蔗Sc-WRKY[19]、大麦[7]、拟南芥[15]、玉米[22]的WRKY基因编码的氨基酸序列进行多重比对分析, 使用MEGA 6.0软件中的最大似然法(maximum- likelihood, ML) (1000 BootStrap)构建系统进化树。
1.3 甘蔗ScWRKY4基因的亚细胞定位
根据基因克隆测序正确的ScWRKY4序列的ORF (去除终止密码子)和亚细胞定位载体pCAMBIA 1300-GFP (Clontech, 中国北京)图谱设计引物对ScWRKY4-Subloc-F/R (表1), 以pMD19- T-ScWRKY4质粒为模板, 进行PCR扩增, 将其胶回收产物和亚细胞定位载体质粒分别用相同的限制性内切酶BamH I和Xba I (Fermentas, 中国上海)进行双酶切、胶回收及T4 DNA酶连接, 然后转化DH5α感受态细胞, 经菌液PCR检测、单酶切和双酶切验证及测序检测后, 得到pCAMBIA 1300-ScWRKY4- GFP亚细胞定位融合表达载体。将pCAMBIA 1300-ScWRKY4-GFP阳性质粒和pCAMBIA 1300-GFP空载分别转化农杆菌菌株GV3101 (天根, 中国北京), 添加含有50 μg mL-1卡那霉素和35 μg mL-1利福平的LB液体培养基, 于28℃下250 转 min-1振荡培养过夜。设置离心机温度4℃、转速4000×g、时间10 min, 收集菌体, 然后重悬于MS空白培养基并将浓度调至OD600=0.8, 加入200 μmol L-1乙酰丁香酮后黑暗静置30 min。选择5~8片叶龄且长势一致的本氏烟进行针刺注射, 在光照16 h/黑暗8 h条件下28℃培养2 d后, 将含有目的基因和空载的叶片剪下, 背光面朝下置1 μg mL-1 4’,6-二脒基-2-苯基吲哚(4’,6-diamidino-2-phenylindole, DAPI)染色剂中, 37℃暗培养30 min, 依次用NaCl水溶液脱色和清水洗净后, 背光面朝上置载玻片, 在Leica激光共聚焦显微镜(德国)下观察亚细胞定位情况[23]。1.4 甘蔗ScWRKY4的自激活活性分析
参照Matchmaker Gold酵母双杂交系统说明, 采用Y2HGold-GAL4酵母双杂交系统(含有4个报告基因: AUR1-C、HIS3、ADE2和MEL1)分析ScWRKY4的自激活活性。根据基因克隆测序正确的ScWRKY4序列的ORF (去除终止密码子)和BD (DNA-binding domain)载体pGBKT7的图谱设计引物对ScWRKY12-BD-F/R (表1), 以pMD19-T-ScWRKY4质粒为模板, 进行PCR扩增, 将其胶回收产物和pGBKT7载体质粒分别用相同的限制性内切酶Nde I和BamH I (Fermentas, 中国上海)进行双酶切、胶回收及T4 DNA酶连接, 转化DH5α感受态细胞, 经菌液PCR检测、单酶切和双酶切验证及测序比对后, 得到pGBKT7-ScWRKY4重组载体。按照Y2HGold Chemically Competent Cell (TaKaRa, 中国大连)说明书操作, 将200 μg pGBKT7-ScWRKY4重组质粒加入到100 μL酵母Y2HGold感受态细胞(全式金, 中国北京)中, 在SD/-Trp平板上29℃培养2~3 d, 至菌体长出。挑取3个单菌落同时于含有1 mL SD/-Trp液体培养基的2 mL无菌离心管中, 29℃、220 r min-1摇床震荡培养10 h左右, 稀释1×10-1、1×10-2和1×10-3三个浓度梯度, 取菌液8 μL分别于SD/-Trp、SD/-Trp (+X-α-Gal)和SD/-Trp (+X-α-Gal+AbA)平板上点样, 29℃培养箱中倒置培养, 对长出的菌落拍照记录。以杂交载体pGBKT7-53+pGADT7-T (已明确53蛋白和T蛋白在酵母细胞内能够发生结合, 该杂交载体在含有金担子素A (aureobasidin A, AbA)抗生素的平板上能激活报告基因AUR1-C)作为阳性对照, 空载pGBKT7 (含营养筛选标记TRP1)作为阴性对照, 分别转化Y2HGold, 并与pGBKT7- ScWRKY4在相同平板上培养。通过观察酵母生长情况来判断ScWRKY4是否具有自激活活性。1.5 甘蔗ScWRKY4基因表达模式分析
采用TRIzol法提取甘蔗不同组织(根、芽、叶、蔗肉和蔗皮)以及经ABA、SA、MeJA、PEG、NaCl和黑穗病菌胁迫处理样品的叶片总RNA, 按照PrimeScript RT Reagent Kit (Perfect Real Time) (TaKaRa, 中国大连)使用说明合成第1链cDNA, 以15倍稀释液作为qRT-PCR模板。在Primer Premier 5.0软件中设计ScWRKY4基因的qRT-PCR检测引物ScWRKY4-QF/R (表1), 以甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase, GAPDH) (GenBank Accession Number: CA254672)为内参基因[25], 引物GAPDH-Q/F如表1所示。采用ABI 7500 Real-time PCR System (美国)进行qRT-PCR检测, 并按照SYBR Green PCR Master Mix Kit (Roche, 中国上海)说明书配置定量反应体系。qRT-PCR扩增程序为50℃ 2 min; 95℃预变性10 min; 95℃变性15 s, 60℃退火延伸1 min, 40个循环; 增加熔解曲线, 95℃ 15 s, 60℃ 1 min, 95℃ 15 s, 60℃ 15 s。每个样品设置3次技术重复, 阴性对照以无菌水作模板。采用2-ΔΔCt [26]计算基因相对表达量, 利用DPS 9.50软件分析试验数据的显著性水平, 并用Origin 8.0软件作柱状图。在黑穗病菌接种试验中, 由于目标基因的表达量会受到机械损伤的影响, 参照苏亚春等[27]的方法, 用接种黑穗病病原菌材料中ScWRKY4基因的表达量减去对应时间接种无菌水材料中的表达量作为其相对表达水平。2 结果与分析
2.1 甘蔗ScWRKY4基因的克隆
以甘蔗ROC22的叶片cDNA为模板、ScWRKY4-F/R为PCR引物, 扩增到约1265 bp的单一条带(图1)。该扩增产物经纯化回收后与载体pMD19-T连接, 转化大肠杆菌DH5α感受态细胞, 经菌液PCR检测和测序鉴定, 得到图2序列。ORF finder分析显示, ScWRKY4基因的ORF (137~877 bp)全长为741 bp, 编码246个氨基酸。图1
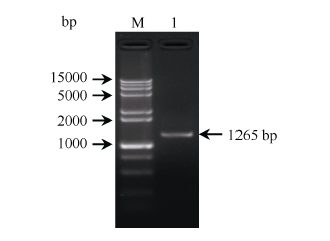 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1甘蔗ScWRKY4基因的PCR扩增
M: 15000+2000 bp DNA marker; 1: PCR产物。
Fig. 1PCR amplification of ScWRKY4 gene in sugarcane
M: 15000+2000 bp DNA marker; 1: PCR product.
图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2PCR扩增获得的ScWRKY4基因的核酸序列及其推导的氨基酸序列(*: 终止密码子)
红色框部分为WRKYGQK基序; 黑色框部分为C2H2基序(CX4CX23HXH)。
Fig. 2Nucleotide acid sequences and deduced amino acid sequences of sugarcane ScWRKY4 gene obtained by PCR amplification (*: stop codon)
The sequence of WRKYGQK motif is highlighted in red box, and that of C2H2 motif (CX4CX23HXH) in black box.
2.2 甘蔗ScWRKY4基因的生物信息学分析
利用生物学软件对ScWRKY4基因序列编码的蛋白进行理化性质预测, 发现该蛋白的分子式为C1166H1794N332O365S15, 理论分子量为26 767.4 Da, 平均疏水性(GRAVY)为-0.49, 不稳定系数(II)为42.69, 负电荷残基(Asp+Glu) 22个, 正电荷残基(Arg+Lys) 28个, 等电点为8.71, 推测ScWRKY4蛋白为一个亲水性的不稳定碱性蛋白。二级结构预测显示甘蔗ScWRKY4蛋白主要由无规则卷曲(70.73%)、α-螺旋(17.48%)和延伸链(11.79%)结构组成, 而缺少β-螺旋结构。另外, 预测发现ScWRKY4蛋白不具有信号肽和跨膜结构域。磷酸化位点分析结果显示ScWRKY4蛋白含有12个丝氨酸(serine)、8个苏氨酸(threonine)和2个酪氨酸(tyrosine), 推测这些位点上可能发生磷酸化反应, 从而对ScWRKY4蛋白的活性与功能产生调控作用。亚细胞定位预测发现, 甘蔗ScWRKY4蛋白位于细胞核的概率最大, 为73.9%。从图3可以看出, 甘蔗ScWRKY4基因编码蛋白包含一个从第166~223位的WRKY保守结构域, 确定该蛋白属于WRKY转录因子家族。采用NCBI中的BlastP在线程序, 查找ScWRKY4的同源氨基酸序列, 结果显示ScWRKY4蛋白与高粱SbWRKY57 (XP_002448267.2)、玉米ZmWRKY36 (NP_001151912.1)、II型少花古尔德草(Dichanthelium oligosanthes) DoWWRKY12 (OEL21317.1)、谷子(Setaria italic) SiWRKY12 (XP_ 004956420.1)和水稻OsWRKY12 (XP_015635033.1)的相似性分别为88%、85%、82%、79%和68%, 其WRKY结构域的氨基酸序列高度保守, 而在其他位置的多肽序列由于物种的差异而表现出较大的变化(图4)。参考前人报道的WRKY蛋白[7,15,22], 将ScWRKY4蛋白序列与甘蔗、大麦、拟南芥、玉米中不同家族类别的WRKY蛋白序列构建系统进化树(图5), 表明ScWRKY4与拟南芥AtWRKY12和AtWRKY13等聚在同一分支上, 属于WRKY家族中的IIc亚家族。
图3
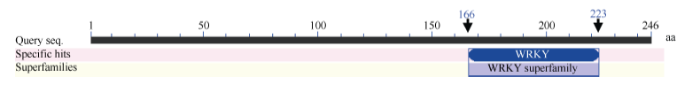 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3甘蔗ScWRKY4蛋白的保守结构域分析
Fig. 3Conserved domain prediction of ScWRKY4 protein
图4
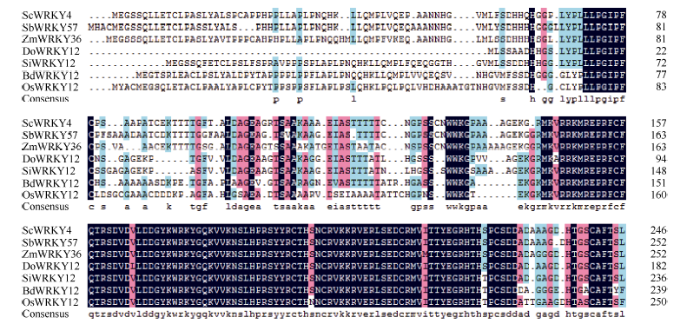 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4甘蔗ScWRKY4与其他植物WRKY蛋白的氨基酸序列比对
高粱: SbWRKY57 (XP_002448267.2); 玉米: ZmWRKY36 (NP_001151912.1); II型少花古尔德草: DoWWRKY12 (OEL21317.1); 谷子: SiWRKY12 (XP_004956420.1); 二穗短柄草: BdWRKY12 (XP_014751531.1); 水稻: OsWRKY12 (XP_015635033.1)。
Fig. 4Sequence alignment of ScWRKY4 and WRKYs in other species
Sorghum bicolor: SbWRKY57 (XP_002448267.2); Zea mays: ZmWRKY36 (NP_001151912.1); Dichanthelium oligosanthes: DoWWRKY12 (OEL21317.1); Setaria italic: SiWRKY12 (XP_004956420.1); Brachypodium distachyon: BdWRKY12 (XP_014751531.1); Oryza sativa OsWRKY12 (XP_015635033.1).
图5
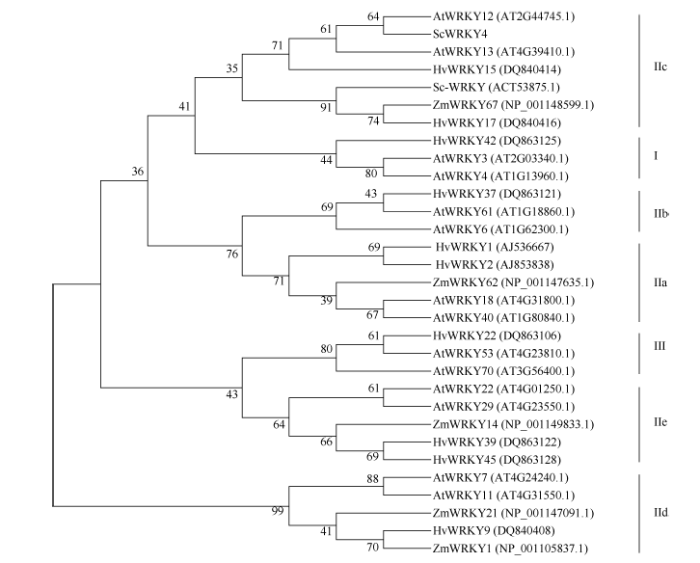 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5ScWRKY4蛋白与甘蔗及其他植物WRKY蛋白家族的系统进化树
Fig. 5Phylogenetic tree of ScWRKY4 protein and WRKYs in sugarcane and other species
2.3 甘蔗ScWRKY4基因的亚细胞定位分析
亚细胞定位表达载体35S::GFP和融合质粒35S::ScWRKY4::GFP分别用相同的内切酶BamH I和Xba I进行双酶切验证, 结果如图6-A所示。采用农杆菌转化及注射本氏烟叶片的方法进行亚细胞定位观察, 阳性对照35S::GFP在细胞核、细胞质和质膜上都有绿色荧光分布, 而35S::ScWRKY4::GFP融合蛋白只在细胞核中有很强的绿色荧光(图6-B), 表明35S::ScWRKY4::GFP融合蛋白定位于细胞核中。图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6甘蔗ScWRKY4亚细胞定位分析
(A) 35S::ScWRKY4::GFP亚细胞定位重组载体的酶切验证结果。1: 15000+2000 bp DNA marker; 2: 35S::GFP/Xba I; 3: ScWRKY4 ORF PCR产物; 4: 35S::ScWRKY4::GFP/Xba I; 5: 35S::ScWRKY4::GFP/Xba I+BamH I; 6: 100 bp ladder DNA marker。(B) 农杆菌介导转化的ScWRKY4及空载体注射本氏烟叶片48 h的亚细胞定位结果。本氏烟叶片表皮细胞被用于明场、绿色荧光、蓝色荧光、明场和绿色及蓝色荧光叠加后的图像分析; 白色箭头1、2和3分别表示质膜、细胞核和细胞质, 比例尺=25 μm; 35S::GFP: 携带空载pCAMBIA 1300-GFP的农杆菌菌株; 35S::ScWRKY4::GFP: 携带重组载体pCAMBIA 1300-ScWRKY4-GFP的农杆菌菌株; DAPI: 4’,6-二脒基-2-苯基吲哚。
Fig. 6Subcellular localization assay of sugarcane ScWRKY4
(A) The enzyme digestion to identify the insert-integrated subcellular localization expression vector 35S::ScWRKY4::GFP. 1: 15000+2000 bp DNA marker; 2: 35S::GFP/Xba I; 3: ScWRKY4 ORF PCR product; 4: 35S::ScWRKY4::GFP/Xba I; 5: 35S::ScWRKY4::GFP/Xba I+BamH I; 6: 100 bp ladder DNA marker. (B) Subcellular localizations of the Agrobacterium mediated transformation of ScWRKY4 and empty vector in Nicotiana benthamiana leaves 48 h after infiltration. The epidermal cells of N. benthamiana were used for taking images of visible light, green fluorescence, blue fluorescence, merged visible light and green fluorescence and blue fluorescence; white arrows 1, 2, and 3 indicate plasma membrane, nucleus and cytoplasm, respectively; scale bar = 25 μm; 35S::GFP: the Agrobacterium tumefaciens strain carrying the empty vector pCAMBIA 1300-GFP; 35S::ScWRKY4::GFP: the A. tumefaciens strain carrying the recombinant vector pCAMBIA 1300-ScWRKY4-GFP; DAPI: 4’,6-diamidino-2-phenylindole.
2.4 甘蔗ScWRKY4的自激活活性分析
重组质粒pGBKT7-ScWRKY4经酶切验证及测序分析, 初步显示酵母融合表达载体构建成功(图7-A)。利用Y2HGold-GAL4酵母双杂交系统对ScWRKY4的自激活活性分析表明, 在SD/-Trp平板上, pGBKT7-ScWRKY4与阳性对照pGADT7+ pGBKT7-p53和阴性对照pGBKT7一致, 均能长出菌落, 说明pGBKT7-ScWRKY4重组质粒已成功转入Y2HGold中, 且GAL4-BD与ScWRKY4蛋白结合, 能成功表达色氨酸。在添加X-α-Gal的SD/-Trp平板上发现, 由于阳性对照中MEL1基因被激活, X-α-Gal检测显蓝色, 而pGBKT7-ScWRKY4与阴性对照均不显蓝色, 说明ScWRKY4没有自激活活性。经过AbA抗性筛选, 阳性对照可正常生长, 且X-α-Gal检测显蓝色, 表明报告基因AUR1-C和MEL1基因都被激活, 而pGBKT7-ScWRKY4和阴性对照一样, 均不能正常生长, 进一步说明ScWRKY4蛋白不具有自激活活性(图7-b)。图7
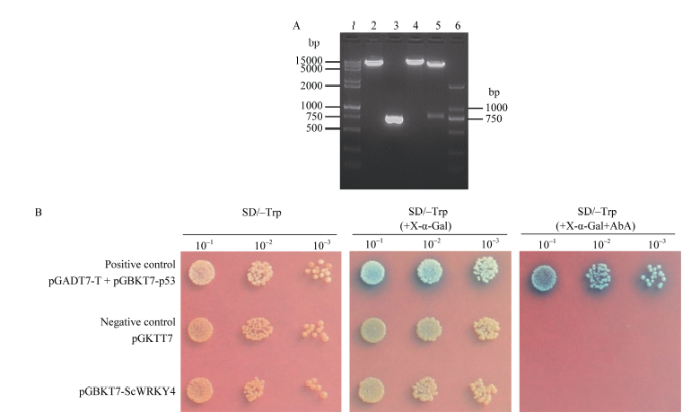 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7甘蔗ScWRKY4转录激活活性分析
(A) pGBKT7-ScWRKY4重组载体的酶切验证结果。1: 15000+2000 bp DNA marker; 2: pGBKT7/Nde I; 3: ScWRKY4 ORF PCR产物; 4: pGBKT7-ScWRKY4/Nde I; 5: pGBKT7-ScWRKY4/Nde I+BamH I; 6: 2000 bp ladder DNA marker。(B) ScWRKY4转录激活活性验证。SD/-Trp: 色氨酸营养缺陷型平板培养基; SD/-Trp (+ X-α-Gal): 色氨酸营养缺陷型平板培养基(添加5-溴-4-氯-3-吲哚-α-D-半乳糖苷); SD/-Trp (+ X-α-Gal+AbA): 色氨酸营养缺陷型平板培养基(添加5-溴-4-氯-3-吲哚-α-D-半乳糖苷和金担子素A)。
Fig. 7Transactivation activity assay of ScWRKY4 in sugarcane
(A) The enzyme digestion to identify the insert-integrated vector pGBKT7-ScWRKY4. 1: 15000+2000 bp DNA marker; 2: pGBKT7/Nde I; 3: ScWRKY4 ORF PCR product; 4: pGBKT7-ScWRKY4/Nde I; 5: pGBKT7-ScWRKY4/Nde I+BamH I; 6: 2000 bp ladder DNA marker. (B) The test of transactivation activity assay of ScWRKY4. SD/-Trp: synthetic dropout medium plate; SD/-Trp (+ X-α-Gal): synthetic dropout medium plate without tryptophan (plus 5-bromo-4-chloro-3-indoxyl-α-D-galactopyranoside); SD/-Trp (+ X-α-Gal+ AbA): synthetic dropout medium plate without tryptophan (plus 5-bromo-4-chloro-3-indoxyl-α-D-galactopyranoside and aureobasidin A).
2.5 ScWRKY4基因的组织特异性表达分析
ScWRKY4基因在甘蔗ROC22不同组织中的表达分析结果显示, 该基因在根、芽、叶和皮中的表达量差异不显著, 但在蔗肉中的表达量最高, 为对照蔗根中表达量的18.38倍(图8)。图8
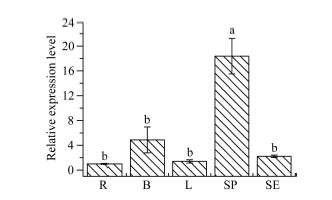 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8ScWRKY4基因在甘蔗ROC22组织中的表达情况
柱上不同的小写字母代表显著性的差异(P ≤ 0.05); n = 3; 内参基因为甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase, GAPDH); R: 蔗根; B: 蔗芽; L: 蔗叶; SP: 蔗肉; SE: 蔗皮。
Fig. 8Specificity analysis of ScWRKY4 gene in sugarcane ROC22 tissue
Bars superscripted by different lowercase letters are significantly different (P ≤ 0.05); n = 3; Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference gene; R: root; B: bud; L: leaf; SP: stem pith; SE: stem epidermis.
2.6 ScWRKY4基因在不同外源胁迫下的表达特性分析
2.6.1 ScWRKY4基因在黑穗病菌侵染下的表达特性 黑穗病菌侵染24 h时, ScWRKY4基因在崖城05-179中的表达量显著下调至对照组的0.20倍, 随后稳定在同一表达水平。而在ROC22中, 该基因在受黑穗病菌侵染72 h内的表达量与对照组相比, 并无显著差异(图9)。图9
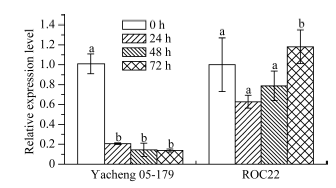 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图9甘蔗ScWRKY4基因在黑穗病侵染下的表达情况
Yacheng 05-179: 甘蔗抗黑穗病品种; ROC22: 甘蔗感黑穗病品种。柱上不同的小写字母代表显著性的差异(P ≤ 0.05); n = 3; 内参基因为甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphatedehydrogenase, GAPDH)。
Fig. 9Expression of sugarcane ScWRKY4 gene under the infection of smut pathogen
Yacheng 05-179: smut-resistant sugarcane variety; ROC22: smut-susceptible sugarcane variety. Bars superscripted by different lowercase letters are significantly different (P ≤ 0.05); n = 3; Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference gene.
2.6.2 ScWRKY4基因在不同植物激素和非生物胁迫下的表达特性 在SA和MeJA处理下, ScWRKY4基因的表达水平均高于对照组, 其中SA处理12 h时达到峰值, 为对照组的2.69倍, MeJA处理12 h时达到峰值, 为对照组的2.38倍。ABA胁迫下, ScWRKY4基因的表达量在各个时间点存在显著变化, 0.5 h时该基因显著上调至对照组的1.59倍, 3 h时恢复到对照组水平, 6 h时达到峰值且为对照组的2.87倍, 随后在24 h时表达量有所下调, 但仍高于对照(1.26倍)。经NaCl处理, ScWRKY4基因的表达量随胁迫时间的延长而呈现出逐渐上升的趋势, 在24 h时达到峰值, 其表达量为对照组的4.91倍。PEG处理下, ScWRKY4基因的表达量在3 h时达到高峰, 为对照组12.10倍, 而在该时间点前后, 该基因的表达水平与对照组相比没有明显差异(图10)。总体上看, ScWRKY4基因对SA、MeJA、ABA、NaCl和PEG胁迫都有一定程度的应答。
图10
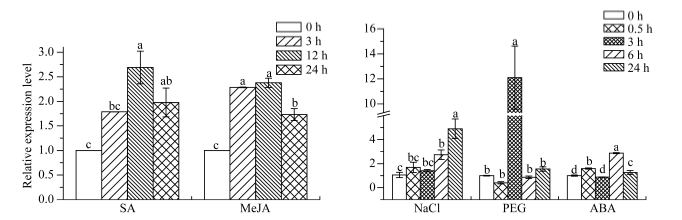 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图10甘蔗ScWRKY4基因在不同植物激素和非生物胁迫下的表达水平
柱上不同的小写字母代表显著性的差异(P ≤ 0.05); n = 3; 内参基因为甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase, GAPDH); SA: 水杨酸(5 mmol L-1); MeJA: 茉莉酸甲酯(25 μmol L-1); NaCl: 氯化钠(模拟盐胁迫) (250 mmol L-1); PEG: 聚乙二醇(模拟干旱胁迫) (25.0%); ABA: 脱落酸(100 μmol L-1)。
Fig. 10Relative expression level of the sugarcane ScWRKY4 gene under different plant hormones and abiotic stress treatments
Bars superscripted by different lowercase letters are significantly different (P ≤ 0.05); n = 3; Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference gene; SA: salicylic acid (5 mmol L-1); MeJA: methyl jasmonate (25 μmol L-1); NaCl: sodium chloride (simulating salt stress) (250 mmol L-1); PEG: polyethylene glycol (simulating drought treatment) (25.0%); ABA: abscisic acid (100 μmol L-1).
3 讨论
甘蔗是食糖总产占比最大的糖料作物, 同时也是可再生能源作物[28]。但是甘蔗在生产上常受到各种病害以及低温、干旱、高盐等非生物环境的影响, 导致其生物产量和质量的下降, 挖掘抗逆相关基因, 有助于利用转基因技术定向改良甘蔗品种的抗性[29]。当植物处于逆境环境时, 可以通过体内功能基因的表达变化来适应环境, 而转录因子对功能基因的表达和修饰起着重要的调控作用[4,6]。目前, 在甘蔗中, 与生物和非生物胁迫相关的转录因子及其相关基因已有报道[30]。其中WRKY转录因子的研究仅有一例Sc-WRKY报道[19]。WRKY转录因子包含WRKY蛋白结构域, 由60个左右的氨基酸残基组成, 可与DNA域结合, 且其N-末端有一个高度保守的核心序列WRKYGQK, C-末端则包含有锌指结构域[3]。根据WRKY蛋白所含有的WRKY结构域的个数和锌指结构的特点, 可将WRKY家族分为三类, 不同亚类可能反映其不同的功能, 其中第I类包含有2个WRKY结构域和锌指结构C2H2 (Cx4-5Cx22-23HxH); 第II类含1个WRKY结构域和锌指结构C2H2 (Cx4-5Cx22-23HxH), 根据其WRKY保守域外的保守结构序列特征, 又分IIa-IIe共5个亚类; 第III类含1个WRKY结构域, 锌指结构类型为C2HC (Cx7Cx23HxH)[30]。研究发现, 高度保守七肽序列WRKYGQK也并非完全保守, 如水稻中的高度保守的WRKY结构域, 共有9种变异类型, 其中较常见的为WRKYGEK和WRKYGKK[31]。已报道的甘蔗Sc-WRKY蛋白含有
一个WRKY结构域, 且存在变异, 为WRKYGKK类型, 锌指结构为Cx4Cx23HxH[19]。在本研究中, ScWRKY4基因编码蛋白与Sc-WRKY的氨基酸序列一致性仅为24.65%, 但其WRKY保守结构域七肽序列为普遍存在的WRKYGQK类型, 锌指结构与Sc-WRKY相同, 系统进化树分析显示两者都属于第IIc类WRKY转录因子, 推测Sc-WRKY和ScWRKY4可能具有某些相似的功能。
蛋白质的生物学功能与其在细胞中的定位紧密相关。向小华等[32]对普通烟草中的164个NtWRKY转录因子的亚细胞定位进行了预测分析, 发现其中有118个定位于细胞核, 主要为第I类和第II类WRKY家族成员, 而第III类家族成员中, 有77.4%定位于细胞质。亚细胞定位预测分析发现, ScWRKY4蛋白位于细胞核的概率最大, 为73.9%。本研究在本氏烟草叶片中对甘蔗ScWRKY4进行亚细胞定位分析, 发现其定位在细胞核, 与生物学软件及向小华等[32]的预测结果一致, 说明ScWRKY4可能作为核蛋白发挥作用。大部分WRKY转录因子的典型特征之一是具有转录激活活性, 但有些WRKY转录因子并不具有转录激活活性, 如大豆GmWRKY7、GmWRKY8、GmWRKY13和GmWRKY15转录因子在酵母中都无转录激活活性[33]。香蕉(Musa acuminata) MaWRKY11蛋白有2个WRKY保守结构域, N端具有转录自激活活性, 而C端没有[34]。没有自激活活性的转录因子序列可作为诱饵, 在cDNA文库中筛选与之互作的功能基因, 有助于进一步分析转录因子在响应外界信号过程中的作用。GmWRKY7能够与其下游功能基因GmALMT1的启动子相结合, 通过抑制GmALMT1基因的表达来影响大豆对铝毒害的响应[33]。除此之外, WRKY不仅作为转录因子起作用, 还可能具有信号传递功能, 参与到植物对外界胁迫信号的传递过程中[35]。本研究通过酵母体内转录激活试验, 发现ScWRKY4转录因子不具有自激活活性, 该研究结果有待在植物系统上作进一步鉴定, 关于ScWRKY4转录因子对下游基因的表达调控作用仍有待研究。
前人研究表明, 普通烟草中的WRKY基因, 大多数在根、茎和叶等不同组织中具有不同程度的表达[32]。本研究发现, ScWRKY4在甘蔗ROC22的根、芽、叶、肉、皮中均有表达, 且在蔗肉中的表达量最高, 说明ScWRKY4在甘蔗中的表达具有组成型表达特性。WRKY转录因子在植物对生物和非生物胁迫的应答反应中具有重要作用。有些WRKY转录因子在植物对病原菌等生物侵染的基础防御过程中起负调节作用, 如拟南芥中过表达AtWRKY38或AtWRKY62, 使得与抗病防御相关的PR1基因的表达量下调[36]。Liu等[19]发现Sc-WRKY基因在FN22中的表达受黑穗病菌的诱导, 说明Sc-WRKY基因在对黑穗病菌侵染的防御反应中具有积极作用。本研究中的ScWRKY4与Sc-WRKY同属于WRKY转录因子家族中的第IIc类, 然而, 研究发现, 受黑穗病菌侵染后, ScWRKY4基因在抗病甘蔗材料崖城05-179中表现出下调的趋势, 在感病品种ROC22中不受该病原菌诱导表达, 推测ScWRKY4可能不参与甘蔗对黑穗病的抗性反应或在该防御系统中起负调控作用, 暗示隶属于相同WRKY基因亚族的ScWRKY4与Sc-WRKY, 可能在响应黑穗病菌胁迫方面存在功能的分化。ScWRKY4基因在SA和MeJA处理下的表达量均高于对照水平, 且该基因均能被ABA、NaCl和PEG胁迫诱导表达。甘蔗Sc-WRKY基因也受SA、NaCl、PEG等诱导表达[19]。研究结果表明, ScWRKY4可能参与了甘蔗对盐胁迫和干旱胁迫的信号传导过程。同样, 水稻OsWRKY08的表达水平在PEG、NaCl和ABA胁迫处理后均可提高, 并通过ABA途径提高对渗透胁迫的耐受能力[37]。拟南芥AtWRKY63在ABA处理下诱导表达, 并参与到由ABA介导的信号途径中, 积极响应对干旱的耐受性[38]。
4 结论
从甘蔗ROC22中成功分离到1个ScWRKY4基因, 该基因长1265 bp, 包含1个741 bp的ORF, 编码246个氨基酸, 含1个WRKYGQK保守结构域和C2H2 (CX4CX23HXH)锌指结构域, 属于WRKY转录因子家族的IIc亚类。ScWRKY4蛋白为碱性的不稳定亲水性蛋白, 不存在信号肽和跨膜结构域; 其蛋白二级结构元件缺少β螺旋结构; 包含有22个磷酸化位点。ScWRKY4被定位于细胞核, 不具有自激活活性。ScWRKY4基因表达量在蔗肉中最高, 而在其他组织中差异不大; 该基因在甘蔗抗病品种崖城05-179与黑穗病菌互作过程中下调表达, 在感病品种ROC22中的表达水平较为稳定, 但在外源激素SA、MeJA和ABA以及非生物胁迫因子NaCl和PEG处理条件下均被诱导上调表达, 暗示ScWRKY4基因不参与甘蔗对黑穗病的抗性反应或在该防御系统起负调控作用, 并且有可能参与到甘蔗对盐胁迫及干旱胁迫的信号传导过程中。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1007/s12033-013-9713-1URLPMID:24122284 [本文引用: 1]

With current advances in genomics, several technological processes have been generated, resulting in improvement in different segments of molecular research involving prokaryotic and eukaryotic systems. A widely used contribution is the identification of new genes and their functions, which has led to the elucidation of several issues concerning cell regulation and interactions. For this, increase in the knowledge generated from the identification of promoters becomes considerably relevant, especially considering that to generate new technological processes, such as genetically modified organisms, the availability of promoters that regulate the expression of new genes is still limited. Considering that this issue is essential for biotechnologists, this paper presents an updated review of promoters, from their structure to expression, and focuses on the knowledge already available in eukaryotic systems. Information on current promoters and methodologies available for studying their expression are also reported.
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
DOI:10.1016/j.pbi.2007.04.020URLPMID:17644023 [本文引用: 2]

Members of the complex family of WRKY transcription factors have been implicated in the regulation of transcriptional reprogramming associated with plant immune responses. Recently genetic evidence directly proving their significance as positive and negative regulators of disease resistance has accumulated. WRKY genes were shown to be functionally connected forming a transcriptional network composed of positive and negative feedback loops and feed-forward modules. Within a web of partially redundant elements some WRKY factors hold central positions mediating fast and efficient activation of defense programs. A key mechanism triggering strong immune responses appears to be based on the inactivation of defense-suppressing WRKY proteins.
[本文引用: 1]
URL [本文引用: 2]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 3]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.5897/AJB11.3602URL [本文引用: 7]

The WRKY proteins comprise a superfamily of transcription factors which are unique in plants. In the present study, a WRKY gene of sugarcane was isolated. This gene is 1 003 bp in full length, which includes 93 bp 5rsquo; untranslated region (UTR) and 172 bp 3rsquo; UTR. The open reading frame (ORF) of this gene is 738 bp which encodes 245 amino acids, with a typical WRKY DNA-binding domain at the 127 ~ 186 aa of the deduced amino acid sequences. The cysteine and histidine are also rich at the 153 ~ 184 aa, which is a C2H2 zinc finger domain of Class II and located at the C-terminal end of this deduced amino acid sequence; thus, this gene belongs to WRKYII. Furthermore, the prokaryotic expression vector of Sc-WRKY gene was successfully constructed and expressed in Escherichia coli (BL21 strain), and the molecular weight of the targeted product was about 25.7 kD. Real-time quantitative PCR analysis demonstrated that the Sc-WRKY gene was strongly induced by Ustilago scitaminea, salicylic acid (SA), NaCl and PEG, which suggests that this gene might play an important role in smut-resistant, drought-tolerant and salt-tolerant mechanism. Key words: Sugarcane (Saccharum officinarum), WRKY transcription factors, prokaryotic expression, real-time quantitative PCR.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/BF02943642URL [本文引用: 1]

This paper discusses the economic importance and utilization of sugarcane byproducts (bagasse, molasses, filter cake, tops and trash, and fermentation waste or vinasse) in India. The composition of these byproducts and their use in the production of paper, boards/agglomerated products, moulded products, rayon grade pulp, electric power, biogas, alcohol/ethanol, furfural, food additives, animal ...
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/S1360-1385(00)01600-9URLPMID:10785665 [本文引用: 2]

The WRKY proteins are a superfamily of transcription factors with up to 100 representatives in Arabidopsis. Family members appear to be involved in the regulation of various physio-logical programs that are unique to plants, including pathogen defense, senescence and trichome development. In spite of the strong conservation of their DNA-binding domain, the overall structures of WRKY proteins are highly divergent and can be categorized into distinct groups, which might reflect their different functions.
DOI:10.1186/1471-2148-5-1URLPMID:544883 [本文引用: 1]

pAbstract/p pBackground/p pWRKY proteins are newly identified transcription factors involved in many plant processes including plant responses to biotic and abiotic stresses. To date, genes encoding WRKY proteins have been identified only from plants. Comprehensive search for WRKY genes in non-plant organisms and phylogenetic analysis would provide invaluable information about the origin and expansion of the WRKY family./p pResults/p pWe searched all publicly available sequence data for WRKY genes. A single copy of the WRKY gene encoding two WRKY domains was identified from itGiardia lamblia/it, a primitive eukaryote, itDictyostelium discoideum/it, a slime mold closely related to the lineage of animals and fungi, and the green alga itChlamydomonas reinhardtii/it, an early branching of plants. This ancestral WRKY gene seems to have duplicated many times during the evolution of plants, resulting in a large family in evolutionarily advanced flowering plants. In rice, the WRKY gene family consists of over 100 members. Analyses suggest that the C-terminal domain of the two-WRKY-domain encoding gene appears to be the ancestor of the single-WRKY-domain encoding genes, and that the WRKY domains may be phylogenetically classified into five groups. We propose a model to explain the WRKY familys origin in eukaryotes and expansion in plants./p pConclusions/p pWRKY genes seem to have originated in early eukaryotes and greatly expanded in plants. The elucidation of the evolution and duplicative expansion of the WRKY genes should provide valuable information on their functions./p
[本文引用: 3]
[本文引用: 3]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
DOI:10.1111/jipb.12513URLPMID:27995748 [本文引用: 1]

ABSTRACT WRKY gene family is among the largest families of transcription factors (TFs) in higher plants. By regulating the plant hormone signal transduction pathway, these TFs play critical roles in some plant processes in response to biotic and abiotic stress. Various research have demonstrated the important biological functions of WRKY TFs in plant response to different kinds of biotic and abiotic stresses and working mechanisms. However, very little summarization has been done to review their research progress. Not just important TFs function in plant response to biotic and abiotic stresses, WRKY are also participated in carbohydrate synthesis, senescence, development, and secondary metabolites synthesis. WRKY proteins can bind to W-box [TGACC (A/T)] in the promoter of its target genes and activate or repress the expression of downstream genes to regulate their stress response. Moreover, WRKY proteins can interact with other TFs to regulate plant defensive responses. In the present review, we focus on the structural characteristics of WRKY TFs and the research progress on their functions in plant responses to a variety of stresses.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
