 ,*, 彭昆鹏*, 贾妍*, 曾硕*, 王英枝*, 张静怡*信阳师范学院生命科学学院 / 大别山农业生物资源保护与利用研究院, 河南信阳 464000
,*, 彭昆鹏*, 贾妍*, 曾硕*, 王英枝*, 张静怡*信阳师范学院生命科学学院 / 大别山农业生物资源保护与利用研究院, 河南信阳 464000Functional Characterization of Soybean Cystatins Gene GmCYS2
KE Dan-Xia ,*, PENG Kun-Peng*, JIA Yan*, ZENG Shuo*, WANG Ying-Zhi*, ZHANG Jing-Yi*College of Life Sciences / Institute for Conservation and Utilization of Agro-bioresources in Dabie Mountains, Xinyang Normal University, Xinyang, Henan 464000, China
,*, PENG Kun-Peng*, JIA Yan*, ZENG Shuo*, WANG Ying-Zhi*, ZHANG Jing-Yi*College of Life Sciences / Institute for Conservation and Utilization of Agro-bioresources in Dabie Mountains, Xinyang Normal University, Xinyang, Henan 464000, China通讯作者:
收稿日期:2017-11-23接受日期:2018-03-26网络出版日期:2018-04-20
| 基金资助: |
Received:2017-11-23Accepted:2018-03-26Online:2018-04-20
| Fund supported: |

摘要
关键词:
Abstract
Keywords:
PDF (3819KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
柯丹霞, 彭昆鹏, 贾妍, 曾硕, 王英枝, 张静怡. 大豆半胱氨酸蛋白酶抑制剂基因GmCYS2的功能鉴定[J]. 作物学报, 2018, 44(8): 1159-1168. doi:10.3724/SP.J.1006.2018.01159
KE Dan-Xia, PENG Kun-Peng, JIA Yan, ZENG Shuo, WANG Ying-Zhi, ZHANG Jing-Yi.
半胱氨酸蛋白酶抑制剂CYS是一类小分子蛋白质, 能够可逆地抑制木瓜蛋白酶C1A和豆荚蛋白C13肽酶家族中的半胱氨酸蛋白酶活性[1]。它们的保守结构域包含一个甘氨酸残基和两个发夹环, 一个保守的QxVxG结构域和一个色氨酸残基[2]。此外, 在植物CYS中还普遍存在着另一个保守的LARFAV结构域([LVI]-[AGT]-[RKE]-[FY]-[AS]-[VI])[3]。CYS和它们的半胱氨酸蛋白酶之间的动态平衡在调控植物发育、植物响应生物胁迫以及非生物胁迫等过程中发挥重要作用, 如内源性蛋白质转换[4], 贮存蛋白的积累[5], 种子的萌发和成熟[6], 细胞程序性死亡[7], 非生物环境胁迫[8]以及植物防御螨[9]、真菌[10]的侵害等。
Tan等[11]利用已公布的苹果(Malus prunifolia)基因组数据库, 系统鉴定了苹果基因组中所有的CYS基因, 并详细分析了其全基因组特征, 检测了该类基因在干旱、高温、盐、脱落酸(ABA)等不同非生物胁迫下的表达模式。对单个基因的功能研究发现MpCYS2基因可能通过影响植株体内ROS的积累和根毛的发育从而增强转基因拟南芥的耐旱性[12]。MpCYS4基因参与ABA胁迫反应, 通过促进气孔关闭上调ABA和干旱相关基因的转录水平, 从而增强转基因拟南芥和苹果的抗旱能力, 能够与类受体蛋白激酶MpFER相互作用共同参与苹果干旱胁迫应答过程[13]。MpCYS4基因还能够延缓自然和应激条件下苹果叶片的衰老[14]。MpCYS5在拟南芥中的异位表达可以同时增强转基因拟南芥对盐胁迫和对衣霉素诱导的内质网胁迫的抗性[15]。最新的研究发现, 在拟南芥种子萌发过程中, AtCYS5基因的表达受热应激和外源ABA胁迫诱导, 过量表达该基因增强了拟南芥的耐高温能力和对外源ABA的不敏感性[16]。水稻OCXII基因沉默后, 转基因植株表现出较高的豆荚蛋白酶和木瓜蛋白酶活性, 幼苗生长受到促进, 碱胁迫下RNAi植株根的形成和茎的生长受到抑制, 说明OCXII基因具备双重功能, 同时参与植物发育过程和胁迫反应[17]。野生大豆GsCPI14通过与钙/钙调素结合的受体类激酶GsCBRLK相互作用增强植物的耐碱性[18]。此外, 拟南芥AtCYS1、AtCYS5 和AtCYS6基因受到甜菜胞囊线虫感染后表达量上升[19]。苎麻BnCPI的体外重组蛋白可以有效抑制木瓜蛋白酶和无花果蛋白酶的蛋白酶活性, 还能抑制一些重要植物病原真菌的菌丝生长[20]。
综上, CYS基因已经在许多植物如拟南芥、水稻、苹果、苎麻、大豆中得到了分离和鉴定, 其在胁迫反应中的作用研究得最细致和深入, 但关于该类基因在豆科植物结瘤固氮过程中的作用却知之甚少。随着大豆全基因组测序的完成和公布[21], 目前已经有20个大豆CYS家族基因被鉴定出来, RNA- seq数据显示, 这些基因在大豆的14个不同组织中均有表达[22], 其中7个基因在根瘤中具有转录活性[23]。Yuan等[24]对20个大豆CYS家族基因的序列结构特征、启动子调控元件、亲缘进化关系及组织表达特征等进行了系统详细的分析, 并检测了该类基因在大豆接种根瘤菌前后不同时期的根及根瘤中的表达情况, 以GmCYS16为候选基因, 利用发根农杆菌介导的百脉根毛根转化法, 深入研究了该基因在结瘤过程中的生物学功能。同时该研究指出, GmCYS2基因在接种后5 d和16 d根中表达水平明显升高, 在30 d根瘤中的表达量迅速上升, 为12 d根瘤的6倍, 在42 d和84 d根瘤中GmCYS2基因也能维持较高转录水平, 因此推测GmCYS2基因可能在根瘤的发生、发育和衰老过程中发挥功能。鉴于此, 本研究以GmCYS2为对象, 拟通过蛋白体外抑制活性的测定揭示其生化功能, 通过转GmCYS2基因的超表达百脉根植株共生结瘤表型的鉴定揭示其生物学功能, 为深入研究该类基因在共生信号转导途径中发挥的作用提供新的分子生物学证据, 为进一步寻找根瘤特异的半胱氨酸蛋白酶提供有益的线索。
1 材料与方法
1.1 试验材料
大豆Willimas 82 (W82)种子由中国科学院东北地理与农业生态研究所孔凡江研究员提供, 慢生型大豆根瘤菌菌株USDA110由复旦大学生命科学学院王应祥教授提供, 百脉根(Lotus japonicus) MG-20种子、百脉根中生根瘤菌菌株MAFF303099、植物表达载体pU1301由华中农业大学农业微生物国家重点实验室张忠明教授提供。1.2 植物材料处理
大豆W82种子经表面灭菌后, 置无菌水润湿的滤纸上萌发, 于16 h/8 h光照/黑暗, 相对湿度70%, 28℃培养2~3 d。再移入盛有无菌蛭石和沙(1:1)的花盆, 每天浇灌无菌Fahraeus无氮营养液, 以同样条件培养4~5 d后, 接种慢生型大豆根瘤菌菌株USDA110, 收集接种后10 d根、30 d和60 d根瘤, 液氮速冻, 于-80℃冰箱保存。1.3 RNA提取、RT-PCR及qPCR检测
参照RNA提取试剂盒(Invitrogen, USA)说明书, 提取大豆组织样品的总RNA。使用NanoDrop仪器检测RNA样品的浓度和纯度。按照TaKaRa公司反转录试剂盒操作说明得到cDNA第1链, RT-PCR扩增GmCYS2目的基因。按照TaKaRa公司PrimeScript RT reagent Kit操作说明进行荧光定量PCR检测, 根据相对定量法(2-ΔΔCt)公式计算结果。1.4 序列分析
利用NCBI网站的Blastp工具寻找GmCYS2蛋白的同源蛋白, 用DNAMAN软件进行序列同源性比对和进化树分析。1.5 重组蛋白GmCYS2的表达和纯化
根据NCBI网站公布的GmCYS2基因GenBank登录号为XP_003524913.1, 使用表1中F-GmCYS2- pro.和R-GmCYS2-pro.引物, 通过PCR扩增除去基因N端信号肽序列(1~78 bp)之外的cDNA片段, 并插入大肠杆菌表达载体pGS-21a。将测序正确的重组质粒pGS-21a-GmCYS2转化到大肠杆菌BL21 (DE3)中。将单个菌落接种到含有氨苄霉素的培养基中, 以37℃摇床培养。当细胞密度在600 nm达到OD = 0.6时, 加入IPTG诱导表达。诱导条件分别为15℃诱导16 h, 37℃诱导4 h。用SDS-PAGE和Western blot检测不同诱导条件下蛋白的表达情况。最终从37℃诱导4 h的细胞裂解液上清液中, 通过一步Ni柱纯化法得到GmCYS2蛋白。以BSA为标准品, 用Bradford法测定蛋白浓度, 通过考马斯亮蓝染色的SDS-PAGE凝胶扫描分析估计蛋白纯度。蛋白经0.22 μm滤膜过滤除菌, 为避免重复解冻, 同等分分装保存在-80℃冰箱备用, 存储缓冲液为50 mmol L-1 Tris-HCl, 150 mmol L-1 NaCl, 10% Glycerol, pH 8.0。Table 1
表1
表1本研究中所使用的引物
Table 1
| 引物名称 Primer name | 引物序列 Sequence of primer (5′-3′) |
|---|---|
| F-GmCYS2-pro. | TACGGGGGATTGGTC |
| R-GmCYS2-pro. | TCACTGCGTGGAAGGAG |
| F-OX | CGggatccATGGCGGCGTTGATAAG |
| R-OX | GGggtaccTCACTGCGTGGAAGGAG |
| F-NIN-rt | AACTCACTGGAAACAGGTGCTTTC |
| R-NIN-rt | CTATTGCGGAATGTATTAGCTAGA |
| F-ENOD40-1-rt | GGAGGTATGCTCAAACATTC |
| R-ENOD40-1-rt | GTAACTTCTCAAGAGAAGACC |
| F-ENOD40-2-rt | CAAAACTCGTTATGTTGCGG |
| R-ENOD40-2-rt | CACCTCAAAGGAAGAAGAACA |
| F-GmCYS2-rt | CAACAAGTGGTGTCTG |
| R-GmCYS2-rt | TCACTGCGTGGAAGGAG |
| F-Polyubiquitin | TTCACCTTGTGCTCCGTCTTC |
| R-Polyubiquitin | AACAACAGCACACACAGACAATC |
新窗口打开|下载CSV
1.6 GmCYS2蛋白的体外酶活性抑制实验
取出-80℃保存的30 d和60 d大豆根瘤样品, 在研钵中充分研磨, 加入0.15 mol L-1的NaCl重悬浮, 在50 mmol L-1 pH 6.0的磷酸钠与2 mmol L-1 EDTA缓冲液中, 4℃孵育1 h, 离心15 min, 收集上清液。采用Protein Assay试剂盒(Bio-Rad公司)定量分析蛋白质含量。通过根瘤提取物对特异性荧光底物的水解作用来测定重组蛋白GmCYS2的抑制活力。荧光标记的底物被酶解, AMC (Ex/Em = 365 nm/ 465 nm)荧光基团得以游离, 产生亮蓝色荧光, 因此很容易被DAPI滤光片所检测。反应过程如下: 在试管中加入1 μmol L-1 GmCYS2纯化蛋白和1 μg根瘤蛋白提取液, 25℃反应10 min, 随后分别加入蛋白酶特异底物Z-FR-AMC (组织蛋白酶L类)、Z-RR-AMC (组织蛋白酶B类)和Bz-FVR-AMC (组织蛋白酶H类), 终浓度为25 mmol L-1, 反应缓冲液为pH 8.0的HEPES, 反应体系为500 μL[4]。混合液在30℃孵育1 h后, 以同体积冰乙醇终止反应, 加去离子水3 mL。最后进行荧光测定, 以A值反映酶活性。所有的分析设置3组重复, 以不加抑制剂蛋白的试管作为空白对照。抑制率(%) = (A空白对照-A加抑制剂)/ A空白对照×100%。1.7 转GmCYS2基因的超表达百脉根植株的获得
根据NCBI网站公布的GmCYS2基因序列, 使用引物F-OX和R-OX (表1), 扩增GmCYS2基因全长序列并构建植物表达载体pU1301-GmCYS2, 重组质粒经冻融法转入发根农杆菌LBA1334菌株中备用。百脉根(Lotus japonicus)的毛根转化方法如前所述[25,26]。简言之, 收集剪去下胚轴的幼苗子叶部分, 放入准备好的携带有重组质粒的农杆菌LBA1334菌悬液中, 侵染30 min, 并转移到Murashige和Skoog (MS)平板上共培养5 d。随后移至含250 mg L-1羧卞的MS培养基上, 直至毛根长出。标记每根毛根并用GUS染液鉴定。每个幼苗只留下1~2个转基因阳性毛根。提取阳性毛根总RNA, 反转录后以表1中F-OX和R-OX为引物扩增GmCYS2基因, 百脉根多聚泛素(Polyubiquitin, Ubi)基因作为内参, 携带有空载体(pU1301)阳性毛根的复合体植株作为阴性对照。将RT-PCR检测为阳性的复合体植株移入无菌沙盆中炼苗1周, 接种根瘤菌MAFF303099, 每天浇灌无菌Fahraeus无氮营养液。1.8 复合体植株的共生表型鉴定及基因表达检测
接种30 d后, 对结瘤表型进行照相统计, 记录单株结瘤数和样本量(n)并计算每个植株(CK和GmCYS2-OX)的平均结瘤数, 重复2次。利用F-GmCYS2-rt和R-GmCYS2-rt引物, 荧光定量PCR检测超表达植株阳性毛根中GmCYS2的转录水平, 利用引物F-NIN-rt和R-NIN-rt, F-ENOD40-1-rt和R-ENOD40-1-rt, F-ENOD40-2-rt和R-ENOD40-2-rt分别检测结瘤标记基因NIN、ENOD40-1和ENOD40-2的转录水平, 百脉根Ubi基因作为内参。重复试验3次。采用Microsoft Excel 2007和SPSS 13.0软件分析数据并绘制图表。2 结果与分析
2.1 GmCYS2基因序列同源比对及进化树分析
序列分析表明, 完整的GmCYS2基因全长为393 bp, 克隆的基因序列与参考基因组序列完全一致。该基因编码130个氨基酸残基, N端包含26个氨基酸残基编码的信号肽。在NCBI网站上对GmCYS2基因的编码序列进行Blastp比对发现, 该序列与木豆(XP_020204069.1)、狭叶羽扇豆(XP_019445765.1)、绿豆(XP_014508215.1)、赤豆(XP_017435098.1)、鹰嘴豆(XP_004504436.1)、蒺藜苜蓿(XP_013446602.1)、落花生(XP_016196387.1)、蔓花生(XP_015958299.1)、水稻(XP_015635666.1)和烟草(XP_016482039.1)的CYS相似性分别为73%、67%、64%、60%、55%、54%、54%、54%、50%和47%。GmCYS2与它的同源蛋白都包含3个保守的结构域, 即N端的“G” 残基, 序列中央的“QxVxG”, C端的“W”残基, 另外还有1个可变的“LARFAV”序列(图1-A)。通过DNAMAN软件获得大豆GmCYS2蛋白与上述10种物种CYS蛋白的进化树(图 1-B), 大豆GmCYS2蛋白与木豆CYS蛋白处在同一进化分支上, 亲缘关系最近。图1
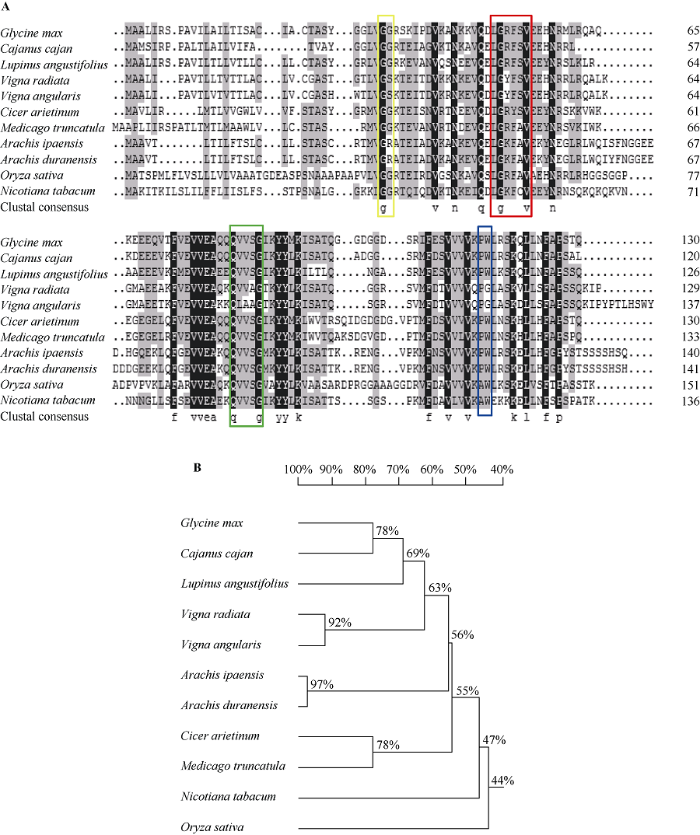 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1大豆GmCYS2与其他植物中同源蛋白的序列比对(A)及进化树分析(B)
图A中用黄色、红色、绿色和蓝色方框标出半胱氨酸蛋白酶抑制剂的主要保守结构域。图B中标尺代表遗传相似性, 指不同植物间同源蛋白进化关系的远近。
Fig. 1Amino acid sequence alignment (A) and phylogenetic tree (B) analysis of GmCYS2 with its homologs in some other plants
The main cystatin conserved motifs are in yellow, red, green, and blue boxes in panel A. The scale in panel B represents genetic similarity, indicating the proximity relationships among species.
2.2 重组蛋白GmCYS2的表达和纯化
由于蛋白酶抑制剂在E. coli中可溶性较差, 经包涵体复性纯化的蛋白酶活性很难保证, 需添加大标签GST用于蛋白表达。此外, GmCYS2序列的N端第1~26位氨基酸编码信号肽, 在构建体外表达载体时需将此信号肽去掉。因此我们构建的重组蛋白序列如图2所示, 包含双标签His-GST以及GmCYS2 C端的104个氨基酸, 蛋白大小为40 kDa左右(图2)。蛋白诱导条件分别为15℃诱导16 h和37℃诱导4 h。用SDS-PAGE和Western blot方法检测不同诱导条件下重组蛋白在E. coli中的表达情况。在2种诱导条件下的全细胞裂解液中都检测到了蛋白的表达(图3-A、B中1和2泳道)。全细胞裂解液经超声波破碎、离心, 分别取上清液和沉淀进行SDS-PAGE分析显示, 15℃诱导16 h条件下, 目的蛋白条带主要出现在沉淀中, 上清液中几乎检测不到(图3-A、B中3和4泳道)。37℃诱导4 h条件下, 目的蛋白条带在上清液和沉淀中都存在(图3-A, B中5和6泳道)。因此, 重组蛋白最终从37℃诱导4 h的细胞裂解液上清液中, 通过一步Ni 柱纯化法获得(图3-C)。以BSA为标准品, 用Bradford法测定, 纯化蛋白的浓度为1.80 mg mL-1, 通过考马斯亮蓝染色的SDS-PAGE凝胶扫描分析估计蛋白纯度约为85%。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2His-GST-GmCYS2融合蛋白的序列分析
氨基酸第5~10位: His标签; 第14~233位: GST标签; 第244~250位: TEV(烟草蚀纹病毒)切割位点; 251~355位: GmCYS2蛋白序列(去掉N端26个氨基酸编码的信号肽)。
Fig. 2Sequence analysis of His-GST-GmCYS2 fusion protein
5-10: His tag; 14-233: GST tag; 244-250: TEV cleavage site; 251-355: GmCYS2 protein sequence (Removing signal peptide of 26 amino acids at N end).
图3
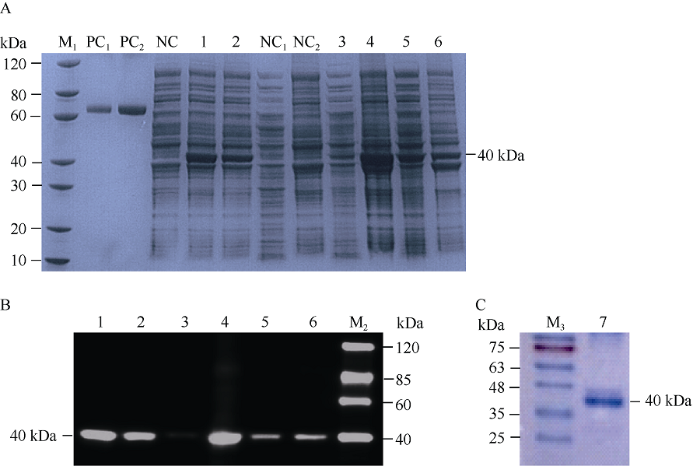 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3GmCYS2蛋白的体外表达、抗体检测和蛋白纯化
A: SDS-PAGE分析蛋白在BL21(DE3)菌株中的表达情况; B: Western blot分析, 抗-His抗体检测; C: 纯化蛋白的SDS-PAGE分析。M1: 蛋白marker; M2: Western marker; M3: 蛋白预染marker; PC1: BSA (1 μg); PC2: BSA (2 μg); NC: 未诱导的全细胞裂解液。1: 15℃诱导16 h的全细胞裂解液; 2: 37℃诱导4 h的全细胞裂解液; NC1: 未诱导的细胞裂解液上清液; NC2: 未诱导的细胞裂解液沉淀; 3: 15℃诱导16 h的细胞裂解液上清液; 4: 15℃诱导16 h的细胞裂解液沉淀; 5: 37℃诱导4 h的细胞裂解液上清液; 6: 37℃诱导4 h的细胞裂解液沉淀; 7: 从37℃诱导4 h的细胞裂解液上清液中纯化的蛋白。
Fig. 3Expression, antibody detection and purification of GmCYS2 protein in vitro
A: SDS-PAGE analysis of protein expression in BL21 (DE3) strain; B: Western blot analysis of protein with anti-His antibody; C: SDS-PAGE analysis of purified protein. M1: protein marker; M2: Western marker; M3: protein pre staining marker; PC1: BSA (1 μg); PC2: BSA (2 μg); NC: Non induced whole cell lysate. 1: Whole cell lysate induced under 15°C for 16 h; 2: Whole cell lysate induced under 37°C for 4 h; NC1: Supernatant of non-induced cell lysate; NC2: The precipitation of non-induced cell lysate; 3: Supernatant of cell lysate induced under 15°C for 16 h; 4: The precipitation of cell lysate induced under 15°C for 16 h; 5: Supernatant of cell lysate induced under 37°C for 4 h; 6: The precipitation of cell lysate induced under 37°C for 4 h; 7: Purified protein from supernatant of cell lysate induced under 37°C for 4 h.
2.3 GmCYS2蛋白的体外酶活检测
在30 d和60 d根瘤蛋白提取液中, GmCYS2重组蛋白主要抑制L类和B类组织蛋白酶的活性, 抑制率在26.7%~39.1%之间, 而对H类组织蛋白酶的抑制活性较低(< 15%)。此外, GmCYS2蛋白在30 d根瘤提取物中的抑制活性明显高于在60 d根瘤提取物中(P < 0.05)(图4)。说明GmCYS2蛋白在体外具有蛋白酶抑制活性, 且具有种类特异性和组织特异性。图4
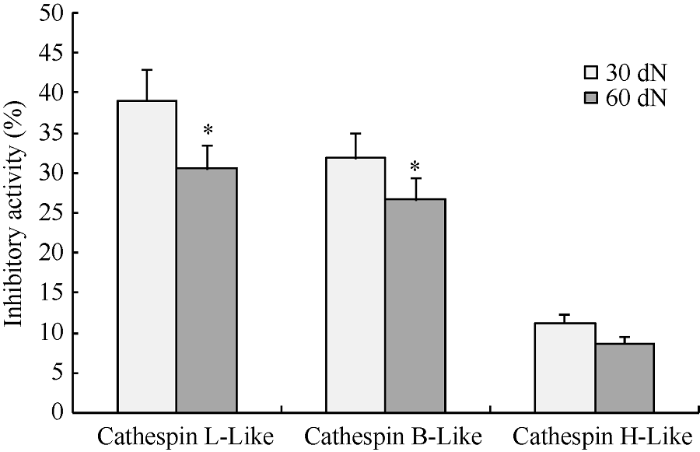 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4重组蛋白GmCYS2在不同时期根瘤提取物中的抑制活性分析
样品分别为30 d和60 d根瘤提取物。反应液中加入纯化的GmCYS2蛋白, 另外分别加入组织蛋白酶L类、B类和H类3种不同底物, 试验重复3次。* P < 0.05。
Fig. 4Inhibitory activity of the recombinant protein GmCYS2 in extracts from nodules in different periods in vitro
Samples were extracts of 30-day- and 60-day-old nodules. Reaction buffer added with purified GmCYS2 protein and the specific substrates cathepsin L-like, B-like, and H-like, respectively. Values are triplicate measurements. * P < 0.05.
2.4 过量表达GmCYS2对百脉根共生结瘤的影响
将GmCYS2基因在玉米(Zea mays)泛素启动子(ZmUb)的控制下表达于百脉根的转基因毛状根中, 分别获得pU1301-GmCYS2 (GmCYS2-OX)和空载体pU1301 (CK)复合体植株, 根瘤菌MAFF303099接种4周后记录结瘤表型。结果GmCYS2-OX毛状根长出更多的根瘤(图5-A), 每个植株的结瘤数从对照的5.8个增加到10.1个(图5-B和表2)。对单株结瘤数量分布的分析显示, 45%的GmCYS2-OX植株结瘤数目>10。与之相反, 对照中没有观察到结瘤数目>10的植株(图5-C)。转基因阳性毛根经GUS染色后呈现明显的蓝色, 阴性毛根仍为白色(图5-D), 进一步通过RT-PCR检测阳性毛根中GmCYS2基因的表达情况, 发现空载体对照中没有出现GmCYS2基因的转录信号, 而随机挑选的3个转GmCYS2基因复合体植株中均出现明显的转录信号(图5-E), 说明植物表达载体的构建是有效的。图5
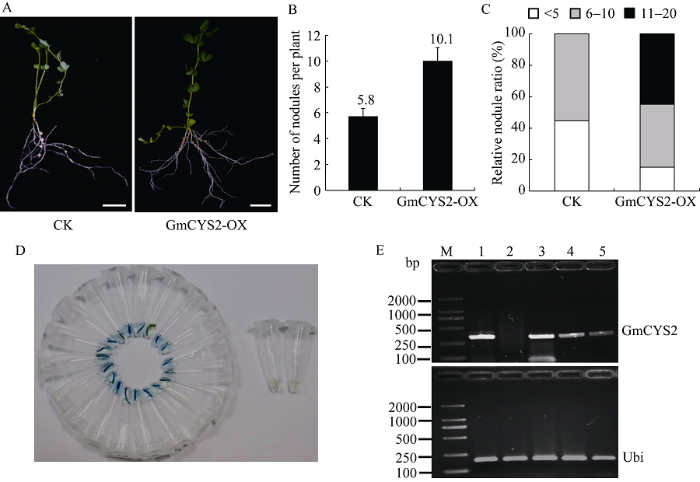 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5GmCYS2基因的过量表达对百脉根共生结瘤的影响
A: 复合体植株接种MAFF303099根瘤菌30 d后的结瘤表型。毛状根表达pMUb:GmCYS2 (GmCYS2-OX)或空载体pU1301 (CK), Bar = 10 mm; B: 单株复合体百脉根植株的平均结瘤数目; C: 根据单株根瘤数划分的不同结瘤种类所占的相对百分比; D: 阳性毛根的GUS鉴定; E: RT-PCR检测阳性毛根中GmCYS2基因的表达。M: DL2000 DNA marker; 1: 阳性质粒对照; 2: 空载体对照; 3~5: 复合体植株。
Fig. 5Effect of GmCYS2 overexpression on symbiosis in L. japonicus
A: Transgenic plants at 30 days after inoculation with MAFF303099 showing hairy roots expressing pMUb: GmCYS2 (GmCYS2-OX) or the empty vector pU1301 (CK). Bar = 10 mm; B: Mean numbers of nodules per plant of L. japonicus; C: The relative ratios of various groups divided by nodule number per plant; D: GUS identification of positive hairy roots; E: Detection of GmCYS2 gene expression in positive hairy roots by RT-PCR; M: DL2000 DNA marker; 1: plasmid as positive control; 2: empty vector control; 3-5: composite plants.
Table 2
表2
表2过表达GmCYS2复合体百脉根植株的结瘤数目统计
Table 2
| 试验 Experiment | 对照植株 CK | 超表达植株 GmCYS2-OX | P值 P-value |
|---|---|---|---|
| 1 | 5.42±1.53 (n=30) | 10.33±2.35 (n=25) | < 0.01 |
| 2 | 6.18±2.05 (n=23) | 9.87±2.48 (n=20) | < 0.01 |
新窗口打开|下载CSV
2.5 转基因毛根中GmCYS2以及共生基因的转录水平检测
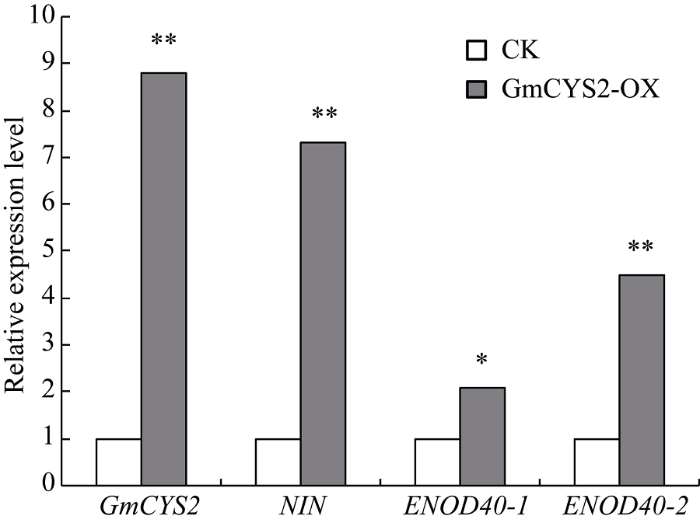
通过实时荧光定量PCR检测显示, 在超表达植株中GmCYS2 mRNA的表达水平为对照的8.8倍(图6), 表明GmCYS2基因的过量表达可以引起植株结瘤数目的增加。与此同时, 以qPCR分析NIN、ENOD40-1和ENOD40-2这3个与结瘤相关的基因在超表达植株中的表达水平, 进一步验证GmCYS2基因的过量表达对结瘤相关Marker基因的影响。结果表明这3种结瘤基因的表达水平, 特别是NIN和ENOD40-2基因, 与对照相比, 在GmCYS2-OX毛状根中显著增加(图6)。图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6转基因毛根中GmCYS2以及共生基因的转录水平检测
荧光定量PCR检测GmCYS2以及共生基因 NIN、ENOD40-1和ENOD40-2 在超表达植株(GmCYS2-OX)和对照植株(CK)阳性毛根中的转录水平。* P<0.05, ** P<0.01。
Fig. 6Detection of transcription levels of GmCYS2 and symbiotic marker genes in transgenic hairy roots
qPCR analysis of the transcript levels of GmCYS2 and symbiotic marker genes NIN, ENOD40-1, and ENOD40-2 in the GmCYS2-OX and control hairy roots. * P<0.05, ** P<0.01.
3 讨论
固氮效率与结瘤, 根瘤发育和衰老密切相关。已有证据表明, 半胱氨酸蛋白酶在几种豆科植物根瘤发育和衰老过程中扮演重要角色, 如基因沉默紫云英AsNODF32推迟根瘤发育和类菌体衰老, 延长了紫云英根瘤寿命[27]。抑制半胱氨酸蛋白酶CYP15A延缓蒺藜苜蓿根瘤的衰老[28]。豌豆PsCyp15A在不定型根瘤开始衰老时被激活[29]。在大豆中, 1983年最早有报道半胱氨酸蛋白酶在根瘤衰老时表达, 18个大豆半胱氨酸蛋白酶被发现在根瘤发育和衰老时有很强的转录活性[30]。然而, 这些半胱氨酸蛋白酶在根瘤发育和衰老过程中到底发挥怎样的功能?为什么如此多的半胱氨酸蛋白酶参与调控根瘤共生?这些问题目前并没有得到很好的阐明。先前的研究表明抑制半胱氨酸蛋白酶能够延迟根瘤衰老, 所以对它们的天然抑制剂CYS的研究将有助于解答这些疑问。大豆CYS家族基因的序列结构以及表达特征已经得到了详尽和细致的揭示, 但是对于单个基因具体功能的研究还十分有限, 特别是在共生结瘤信号途径中的功能及机制研究的报道很少。本研究克隆得到的GmCYS2基因与植物中大多数CYS基因一样, 氨基酸序列中包含3个典型基序即N端的“G”残基, 中部的“QVVSG”序列和C端的“W”残基, 还有一个保守的“LGRFSV”序列(图1), 这段序列在CYS家族基因的不同分支中是高度变化的。CYS通过抑制半胱氨酸蛋白酶的活性, 在植物种子萌发、宿主免疫、高敏感细胞死亡、病原体及昆虫的防御反应等过程中发挥关键作用。本研究在体外表达和纯化了GmCYS2重组蛋白(图3), 酶活性抑制实验显示, GmCYS2蛋白对L类组织蛋白酶的抑制活性最强, 其次是B类组织蛋白酶。此外, GmCYS2蛋白在成熟根瘤中的抑制活性明显高于衰老根瘤(图4)。该实验不仅证实GmCYS2蛋白在体外具有蛋白酶抑制活性, 还进一步揭示该蛋白对抑制的半胱氨酸蛋白酶种类具有选择性, 更有可能在成熟根瘤而不是衰老根瘤中发挥功能。
CYS基因的表达也受非生物胁迫和激素信号的影响[31]。启动子预测发现, GmCYS2启动子具有脱落酸、赤霉素、水杨酸、生长素和茉莉酸5种激素反应元件。有趣的是, GmCYS2启动子有一个结瘤特异因子结合位点元件, 该元件也存在于典型结瘤素基因大豆血红蛋白LBC3的启动子片段中。GmCYS2在接种根瘤菌的大豆根中表达量明显上升[24], 在根瘤衰老过程中表达量也是上升的[23]。这些研究结果表明, GmCYS2很可能参与大豆共生结瘤过程。水稻的1个CYS基因在大豆中异位表达, 正调控大豆的结瘤和缺氮反应[32]。本研究利用农杆菌介导的百脉根毛根转化法, 对转GmCYS2基因的超表达百脉根植株共生表型进行记录及统计分析表明, 大豆GmCYS2基因在百脉根中的异位表达能够显著增加百脉根的结瘤数目(图5), 并且上调共生Marker基因的表达(图6)。结瘤起始基因NIN是侵入线和根瘤原基形成必需的关键基因[33]。结瘤素基因ENOD40s也是根瘤起始和发育至关重要的Marker基因, 百脉根中有2个ENOD40基因, ENOD40-1和ENOD40-2 [34]。由此推测GmCYS2可能通过抑制特异的半胱氨酸蛋白酶活性, 进而在结瘤过程中发挥正调控作用。
4 结论
克隆了大豆半胱氨酸蛋白酶抑制剂基因GmCYS2, 该基因编码蛋白具有典型的CYS家族蛋白保守结构域。GmCYS2蛋白在体外具有蛋白酶抑制活性, 过量表达该基因能显著增加转基因百脉根的结瘤数目, 并且上调共生关键Marker基因的表达。推测GmCYS2可能通过抑制特异的半胱氨酸蛋白酶活性, 在结瘤过程中发挥正调控作用。该结果为揭示半胱氨酸蛋白酶抑制剂在共生结瘤过程中的功能及其调控机制提供了分子证据。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1016/j.febslet.2007.05.042URL [本文引用: 1]
[本文引用: 1]
DOI:10.1006/abbi.1998.0875URLPMID:9799556 [本文引用: 1]

Abstract The plant cystatins or phytocystatins (PhyCys) are cysteine proteinase inhibitors containing the QxVxG motif and have been placed in the cystatin superfamily of proteins. The primary sequences of PhyCys have a high degree of homology with the members of the cystatin family, but they resemble stefins by the absence of disulfide bonds and cysteine residues. A multialignment and a phylogenetic analysis of 63 cystatins, 32 of which are PhyCys, demonstrate that all PhyCys cluster in a major evolutionary tree branch and support the classification of PhyCys as a new cystatin family. The PhyCys also possess a specific consensus sequence [LVI]-[AGT]-[RKE]-[FY]-[AS]-[VI]-x-[EDQV]-[HYFQ] -N placed on the region corresponding to a predictable amino-terminal alpha-helix. This sequence can be used to specifically identify PhyCys on protein data banks and to differentiate them from the other members of the superfamily. Copyright 1998 Academic Press.
DOI:10.1104/pp.109.146019URL [本文引用: 2]
DOI:10.1016/j.biochi.2010.06.006URL [本文引用: 1]
DOI:10.1021/jf0201935URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/s00299-010-0948-zURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/s11103-016-0504-5URLPMID:27325119 [本文引用: 1]

Phytocystatins are well-known inhibitors of C1A cysteine proteinases. However, previous research has revealed legumain (C13) protease inhibition via a carboxy-extended phytocystatin. Among the 12 phytocystatins genes in rice, OcXII is the only gene possessing this carboxy-terminal extension. The specific legumain inhibition activity was confirmed, in our work, using a recombinant OcXII harboring only the carboxy-terminal domain and this part did not exhibit any effect on papain-like activities. Meanwhile, rice plants silenced at the whole OcXII gene presented higher legumain and papain-like proteolytic activities, resulting in a faster initial seedling growth. However, when germinated under stressful alkaline conditions, OcXII-silenced plants exhibited impaired root formation and delayed shoot growth. Interestingly, the activity of OcXII promoter gene was detected in the rice seed scutellum region, and decreases with seedling growth. Seeds from these plants also exhibited slower growth at germination under ABA or alkaline conditions, while maintaining very high levels of OcXII transcriptional activation. This likely reinforces the proteolytic control necessary for seed germination and growth. In addition, increased legumain activity was detected in OcXII RNAi plants subjected to a fungal elicitor. Overall, the results of this study highlight the association of OcXII with not only plant development processes, but also with stress response pathways. The results of this study reinforce the bifunctional ability of carboxy-extended phytocystatins in regulating legumain proteases via its carboxy-extended domain and papain-like proteases by its amino-terminal domain.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1038/nature08670URLPMID:20075913 [本文引用: 1]

Soybean (Glycine max) is one of the most important crop plants for seed protein and oil content, and for its capacity to fix atmospheric nitrogen through symbioses with soil-borne microorganisms. We sequenced the 1.1-gigabase genome by a whole-genome shotgun approach and integrated it with physical and high-density genetic maps to create a chromosome-scale draft sequence assembly. We predict 46,430 protein-coding genes, 70% more than Arabidopsis and similar to the poplar genome which, like soybean, is an ancient polyploid (palaeopolyploid). About 78% of the predicted genes occur in chromosome ends, which comprise less than one-half of the genome but account for nearly all of the genetic recombination. Genome duplications occurred at approximately 59 and 13 million years ago, resulting in a highly duplicated genome with nearly 75% of the genes present in multiple copies. The two duplication events were followed by gene diversification and loss, and numerous chromosome rearrangements. An accurate soybean genome sequence will facilitate the identification of the genetic basis of many soybean traits, and accelerate the creation of improved soybean varieties. 2010 Macmillan Publishers Limited. All rights reserved.
DOI:10.1186/1471-2229-10-160URLPMID:3017786 [本文引用: 1]

Abstract BACKGROUND: Next generation sequencing is transforming our understanding of transcriptomes. It can determine the expression level of transcripts with a dynamic range of over six orders of magnitude from multiple tissues, developmental stages or conditions. Patterns of gene expression provide insight into functions of genes with unknown annotation. RESULTS: The RNA Seq-Atlas presented here provides a record of high-resolution gene expression in a set of fourteen diverse tissues. Hierarchical clustering of transcriptional profiles for these tissues suggests three clades with similar profiles: aerial, underground and seed tissues. We also investigate the relationship between gene structure and gene expression and find a correlation between gene length and expression. Additionally, we find dramatic tissue-specific gene expression of both the most highly-expressed genes and the genes specific to legumes in seed development and nodule tissues. Analysis of the gene expression profiles of over 2,000 genes with preferential gene expression in seed suggests there are more than 177 genes with functional roles that are involved in the economically important seed filling process. Finally, the Seq-atlas also provides a means of evaluating existing gene model annotations for the Glycine max genome. CONCLUSIONS: This RNA-Seq atlas extends the analyses of previous gene expression atlases performed using Affymetrix GeneChip technology and provides an example of new methods to accommodate the increase in transcriptome data obtained from next generation sequencing. Data contained within this RNA-Seq atlas of Glycine max can be explored at http://www.soybase.org/soyseq.
DOI:10.1186/s12870-014-0294-3URL [本文引用: 2]
[本文引用: 2]
DOI:10.1016/j.plaphy.2016.08.015URLPMID:27592173 [本文引用: 1]

Roots of leguminous plants perceive Nod factor signals, and then root hair deformation responses such as swelling and curling are activated. However, very little is known about the molecular mechanisms of such root hair deformation. We have previously shown that LjROP6, a member of the Rho family of small GTPases, was identified as an NFR5 ( N od F actor R eceptor 5 )-interacting protein and participated in symbiotic nodulation in Lotus japonicus . In this study, we identified ten LjROP GTPases including LjROP6, and they were distributed into groups II, III, IV but not group I by phylogenetic analysis. The expression profiles of ten LjROP genes during nodulation were examined. LjROP6 belonged to group IV and interacted with NFR5 in a GTP-dependent manner. Overexpression of either wild-type ROP6 or a constitutively active mutant (ROP6-CA) generated root hair tip growth depolarization, while overexpression of a dominant negative mutant (ROP6-DN) exhibited normal root hair growth. After inoculating with Mesorhizobium loti or adding Nod factors to hairy roots, overexpression of ROP6 and ROP6-CA exhibited extensive root hair deformation, while overexpression of ROP6-DN inhibited root hair deformation. The infection event and nodule number were increased in ROP6 and ROP6-CA overexpressing transgenic plants; but decreased in ROP6-DN overexpressing transgenic plants. These studies provide strong evidence that ROP6 GTPase, which binds NFR5 in a GTP-dependent manner, is involved in root hair development as well as root hair deformation responses induced by NFs in the early stage of symbiotic interaction in L. japonicus .
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.plantsci.2005.07.003URL [本文引用: 1]
DOI:10.1104/pp.123.2.521URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.3969/j.issn.1003-0972.2015.04.038URL [本文引用: 1]

以模式豆科植物与根瘤菌的共生固氮为视角,介绍近年来在结瘤信号途径中筛选到的能够与已知关键调控蛋白相互作用的新蛋白,综述了相关新蛋白在共生结瘤过程中发挥的重要作用,进一步补充和完善了结瘤早期信号转导途径,为豆科植物与根瘤菌共生关系的研究提供参考.
DOI:10.3969/j.issn.1003-0972.2015.04.038URL [本文引用: 1]

以模式豆科植物与根瘤菌的共生固氮为视角,介绍近年来在结瘤信号途径中筛选到的能够与已知关键调控蛋白相互作用的新蛋白,综述了相关新蛋白在共生结瘤过程中发挥的重要作用,进一步补充和完善了结瘤早期信号转导途径,为豆科植物与根瘤菌共生关系的研究提供参考.
[本文引用: 1]
