 ,, 邱海阳, MuhammadUZAIR, 房静静, 赵金凤, 李学勇
,, 邱海阳, MuhammadUZAIR, 房静静, 赵金凤, 李学勇 ,*中国农业科学院作物科学研究所 / 农作物基因资源与基因改良国家重大科学工程, 北京100081
,*中国农业科学院作物科学研究所 / 农作物基因资源与基因改良国家重大科学工程, 北京100081Phenotypic Analysis and Gene Mapping of the Rice Narrow-leaf Mutant nal20
LONG Hai-Xin ,, QIU Hai-Yang, Muhammad UZAIR, FANG Jing-Jing, ZHAO Jin-Feng, LI Xue-Yong
,, QIU Hai-Yang, Muhammad UZAIR, FANG Jing-Jing, ZHAO Jin-Feng, LI Xue-Yong ,*National Key Facility for Crop Gene Resource and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China
,*National Key Facility for Crop Gene Resource and Genetic Improvement, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China通讯作者:
第一联系人:
收稿日期:2018-02-7接受日期:2018-06-12网络出版日期:2018-07-02
| 基金资助: |
Received:2018-02-7Accepted:2018-06-12Online:2018-07-02
| Fund supported: |

摘要
关键词:
Abstract
Keywords:
PDF (3900KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
龙海馨, 邱海阳, MuhammadUZAIR, 房静静, 赵金凤, 李学勇. 水稻窄叶突变体nal20的表型分析与基因定位[J]. 作物学报, 2018, 44(9): 1301-1310. doi:10.3724/SP.J.1006.2018.01301
LONG Hai-Xin, QIU Hai-Yang, Muhammad UZAIR, FANG Jing-Jing, ZHAO Jin-Feng, LI Xue-Yong.
水稻(Oryza sativa L.)作为全世界最重要的粮食作物之一, 其产量关系着粮食生产安全。随着人口数量的增加和耕地面积的减少, 进一步提高水稻单位面积的产量是育种家们长期以来的育种目标。在水稻中超过90%的物质积累来源于叶片光合作用, 只有少部分来自于根部吸收[1], 故叶片形态是影响水稻产量的重要因素之一。叶片形态的改变会直接影响光合作用、呼吸作用、蒸腾作用和最终生物学产量。因此研究调控水稻叶片形态的分子机制对提高粮食产量有很重要的理论意义和应用价值。
叶片是植物生长中的重要器官, 其发育是一个十分复杂的过程, 主要通过一系列的极性发育和细胞分化来完成[2,3]。叶片起始发育于顶端分生组织(shoot apical meristem, SAM)附近的细胞, 在一定浓度的生长素诱导下通过细胞分裂沿着近端和远端迅速地延伸生长。起始细胞在远端横向生长, 在近端叶柄处持续细胞分裂。早期叶片发育主要是指从叶片远端到叶片基部的发育, 主要以细胞分裂方式为主[3,4], 在此阶段产生了很多大小、形状一致的细胞, 细胞数目大量增加。在随后的叶片发育进程中细胞分裂逐渐减弱直至停止, 新细胞的生长主要以细胞增大为主, 直至形成成熟的叶片[5], 故叶片的最终形态是由细胞大小和细胞数目共同决定的。
近年来, 随着分子克隆技术的快速发展, 愈来愈多的水稻窄叶突变体被深入研究, 通过对突变基因的图位克隆和功能研究, 逐步揭示了水稻叶片发育的分子机制。通常认为, 水稻的窄叶性状受质量性状等位基因控制。根据Gramene网站和水稻数据库报道, 目前已报道11个窄叶突变体, 分别为nal1、nal2/3、nal4、nal5、nal6、nal7、nal8、nal9、nal10、nal11和nrl1, 以单基因控制为主, 主要分布在第1、第3、第4、第11和第12染色体上[6], 且以第3、第4染色体居多, 其中NAL1、NAL2/3、NAL7、NRL1已被克隆并进行了部分功能分析。比如NAL1基因位于水稻第4染色体上, 编码一个与生长素极性运输有关的植物特异性蛋白, 但该蛋白的生化功能仍未知。突变体nal1主要表现为叶片宽度变窄, 而叶片长度的变化比较小[7]。NAL1基因功能缺失导致突变表型的出现[8]。COW1/NAL7基因位于水稻第3染色体短臂上, 编码一个与生长素生物合成有关的YUCCA家族蛋白。突变体cow1/nal7的叶片主要表现为宽度变窄、卷曲, 但是在叶片长度上没有明显的改变[9,10]。与生长素缺乏突变体类似, 生长素响应因子ARF11 (auxin-response factor 11)的功能缺失突变体osarf11-1的叶片也适度变窄, 剑叶角度减小, 株高轻度降低[11]。NRL1/ND1基因位于水稻第12染色体上, 编码一个类纤维素合成酶蛋白D4 (OsCSLD4)。突变体nrl1/nd1主要表现为叶片宽度变窄和长度变短[12,13,14]。NAL2和NAL3是2个WUSCHEL-related homeobox (WOX)同源基因, 分别位于水稻第11和第12染色体上, 与玉米NS1/2和拟南芥PRS基因同源。特别是, 只在双突变体nal2/3中才会出现突变性状, 单一突变体性状正常。突变体nal2/3主要表现为叶片变窄、分蘖数目增多、侧根数目减少等。进一步细胞学研究发现突变体的窄叶性状主要是由于顶端分生组织异常, 导致叶片生成细胞缺陷[15,16,17]。RICE MINUTE-LIKE1 (RML1)基因位于水稻第11染色体短臂上, 编码核糖体大亚基L3B (RPL3B)。突变体rml1表现为延迟生长、维管束缺陷和叶片变窄。进一步实验表明, RPL3B蛋白的突变导致核糖体60S亚基和多核糖体减少, 证明RPL3B蛋白在调控水稻正常叶片形态和植物结构方面起重要作用[18]。
随着越来越多新技术的应用, 耗时的传统叶片性状测量方式已被高通量叶片评分技术(high- throughput leaf scoring, HLS)代替。该技术可对水稻叶片数目、面积、颜色、形态等性状进行评估, 比传统测量方式更省时、精准, 并可与全基因组关联研究(genome-wide association study, GWAS)相结合, 成为一种新型寻找控制水稻叶片性状遗传位点的实验方法[19]。
水稻不仅是世界上最重要的粮食作物之一, 还是单子叶植物的模式植物。尽管叶片形态对植物光合效率起着关键作用, 但是目前对其遗传机制的研究并不深入, 特别是单子叶植物。本研究利用60Co-γ射线诱变粳稻品种春江06, 获得了一个窄叶突变体, 命名为nal20, 对其进行了表型分析、细胞学观察、遗传分析和基因定位, 为进一步了解调控叶片宽度的分子机制提供了良好的遗传材料。
1 材料与方法
1.1 材料
粳稻品种春江06经60Co-γ射线诱变处理后, M1代按单株收种, M2代按株系种植, 每个株系插秧12株。从其中1个株系中筛选获得了窄叶突变体nal20, 经多代自交, 其窄叶性状能够稳定遗传。遗传分析和基因定位所用的父本材料为表型正常的广亲和籼稻品种Dular。1.2 突变体的表型鉴定
在水稻抽穗期分别统计突变体(nal20)和野生型(春江06)各20株的株高、分蘖数、叶片形态(主茎倒一叶、倒二叶和倒三叶)、抽穗期、主茎穗型、主茎穗粒型等形态指标。其中主要重点观察和测定倒一叶(剑叶)、倒二叶、倒三叶的长度和最宽处的宽度。剑叶面积可通过公式进行计算: 剑叶面积(cm2): FLA = FLL×FLW×0.75 (FLA: flag leaf area; FLL: flag leaf length; FLW: flag leaf width) [28]。1.3 叶片下表皮观察
在水稻抽穗期时, 挑选长势正常的野生型和突变体各10株, 分别取植株主茎的倒一叶(剑叶)、倒二叶和倒三叶, 共30片叶子, 截取每片叶子最宽处5 cm。根据Yoshikawa等[20]的方法, 将叶片标记好, 置FAA固定液(95 mL 70%酒精 + 5 mL 37%乙醛 + 5 mL冰醋酸), 固定24 h以上。用于以下2个叶片试验。1.3.1 大小叶脉分析 取出固定好的3个部位的叶片, 分别截取1 cm, 以清水简单冲洗后, 用吉列刀片切取适宜宽度(可将叶片夹在切好的土豆条中, 用腕力切取), 置载玻片上, 在体视镜(Olympus SZX16)下观察, 统计叶片大小叶脉数目, 照相并记录。
1.3.2 表皮细胞大小 将固定的叶片依次按照50%、70%、90%、100%乙醇逐级脱水(其中100%乙醇洗脱两次效果更佳), 每次脱水1 h; 将脱水后的材料转入3 mol L-1水合氯醛, 96°C加热1~2 h, 转入蒸馏水, 浸泡一会儿, 用刀片轻轻刮除叶片上部, 直至剩余单层的下表皮。制作临时装片, 用光学显微镜观察(Olympus BX53), 统计细胞数目, 测量细胞宽度并照相。由于单个细胞的宽度较小, 难以准确测量, 故实际操作中可以一次横向选取多个细胞, 测量其总宽度, 再根据选取细胞的数目, 计算每个细胞的平均宽度[21]。
1.4 F2定位群体的构建与遗传分析
将窄叶突变体nal20与籼稻品种Dular杂交, F1代单株自交获得F2代种子。F2群体按单株插秧, 在抽穗期统计正常和突变个体的株数, 用于遗传学分析, 并收取突变表型单株作为基因定位群体。1.5 群体DNA提取及检测
从F2突变表型单株和2个亲本(父本Dular; 母本nal20)每株取约0.1 g叶片, 采用CTAB (十六烷基三甲基溴化铵)法[4]提取水稻基因组DNA, 用于基因定位。参照分离群体分组分析法(bulked segregant analysis, BSA)[22], 分别构建亲本和突变个体混池。经1%琼脂糖凝胶电泳检测后, 调节两个亲本DNA浓度使其一致, 等量混合构成亲本混池; 选取亮度一致的10株F2代突变体DNA, 等量混合构成突变个体混池, 用于后续连锁标记的筛选。1.6 基因初步定位
根据籼稻、粳稻之间序列的差异, 设计均匀分布在水稻12条染色体上的170对InDel (insertion and deletion)标记, 对突变个体和亲本混池进行BSA分析, 初步筛选出与窄叶突变体表型可能连锁的标记, 并用20株F2代突变个体对可能的连锁标记进行验证。其中使用的PCR体系为10 μL, 包含2× Mix buffer 5 μL、两端引物(10 μmol L-1)各0.5 μL、模板DNA 1 μL和ddH2O 3 μL。PCR程序为94°C预变性5 min, 94°C变性30 s, 56°C退火30 s, 72°C延伸30 s, 共35个循环, 72°C延伸10 min。PCR产物经8%的非变性聚丙烯酰胺凝胶电泳及0.1% AgNO3银染后, 统计每个F2突变个体的基因型。1.7 基因精细定位
根据初步定位结果, 利用本实验室已测序的籼稻品种Dular全基因组序列(未发表)与NCBI (1.8 候选基因分析
通过水稻基因组注释数据库(Rice Genome Annotation Project)网站(http://rice.plantbiology.msu. edu/), 进行基因预测, 查阅位于基因精细定位的区间内所有开放阅读框, 初步了解每个基因功能, 并分析可能的候选基因。1.9 突变材料的二代测序
选取突变体抽穗期的叶片(2~3片), 采用CTAB法提取DNA, 由北京诺禾致源生物信息科技有限公司对突变体全基因组进行二代测序。根据反馈的测序结果和初步定位的区间深入分析, 寻找突变位点。2 结果与分析
2.1 窄叶突变体nal20的叶片表型分析
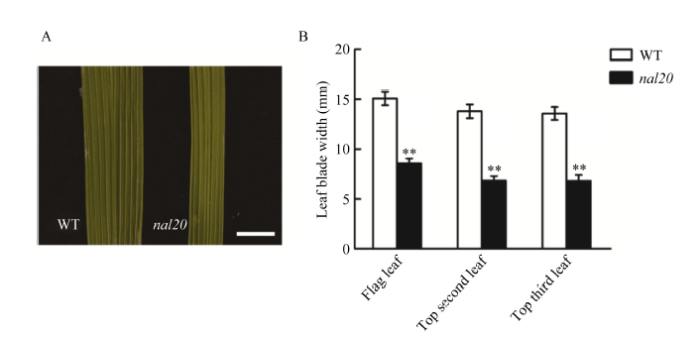
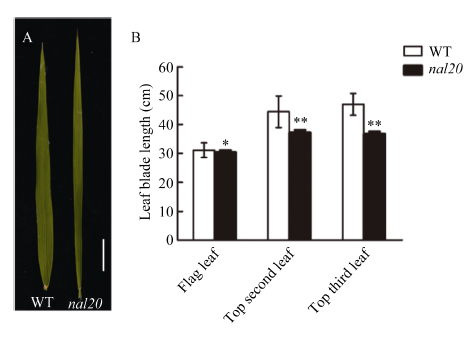
在抽穗期, 突变体nal20主要突变性状表现在叶片形态上(叶宽变窄和叶长缩短), 并且不同位置的突变程度有所差异。分别调查剑叶(倒一叶)、倒二叶和倒三叶, 发现突变体叶片宽度显著变窄(图1-A), 与野生型春江06相比分别减少47.04%、50.25%和49.56% (图1-B)。在叶片长度上也有一定程度上的变短, 比野生型分别减少2.38%、16.26%和21.92% (图2-B), 在倒二叶、倒三叶上的表型较为明显, 但在剑叶上表现不太明显(图2-A)。另外, 突变体nal20叶片宽度的变窄程度不受环境条件的影响。例如, 在北京昌平长日照和海南冬季短日照条件下, 与野生型相比, 突变体nal20剑叶宽度分别减少43.05%和47.06% (表1)。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1野生型和突变体nal20的叶片宽度
A: 野生型(左)和突变体nal20 (右)抽穗期的剑叶形态比较, 标尺为10 mm; B: 剑叶、倒二叶和倒三叶叶片宽度比较。以t测验计算P值, 柱形图中**表示突变体在P <0.01水平差异显著(n = 20)。
Fig. 1Leaf width of wild type (WT) and the nal20 mutant
A: flag leaf size of wild type and the nal20 mutant at heading stage (bar = 10 mm); B: leaf width comparison of flag, top second, and top third leaves between WT and nal20. P-values were analyzed using Student’s t-tests. ** significantly different at P < 0.01 (n = 20).
图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2野生型和突变体nal20的叶片长度
A: 野生型(左)和突变体nal20 (右)抽穗期的剑叶形态比较, 标尺为5 cm; B: 剑叶、倒二叶和倒三叶叶片长度比较。t测验计算P值, 柱形图中 *表示突变体在P <0.05水平差异显著; **表示突变体在P < 0.01水平差异显著(n = 20)。
Fig. 2Leaf length of wild type (WT) and the nal20 mutant
A: top leaf size of wild type and the nal20 mutant at heading stage (bar = 5 cm); B: length comparison of flag, second, and top third leaves between WT and nal20. P-values were analyzed using Student’s t-tests. * P < 0.05, ** P < 0.01 (n = 20).
Table 1
表1
表1叶片宽度和抽穗天数在不同日照条件下的比较
Table 1
| 性状 Trait | 日照条件 Daylength condition | 野生型春江06 Wild-type Chunjiang 06 | nal20 Mutant nal20 |
|---|---|---|---|
| 剑叶宽度 | 长日照(北京昌平) Long-day (Changping, Beijing) | 15.10±0.67 | 8.60±0.49** |
| Flag leaf width (mm) | 短日照(海南三亚) Short-day (Sanya, Hainan) | 15.62±0.85 | 8.27±0.46** |
| 抽穗天数 | 长日照(北京昌平) Long-day (Changping, Beijing) | 131.65±2.04 | 94.92±1.57** |
| Heading date (d) | 短日照(海南三亚) Short-day (Sanya, Hainan) | 89.96±1.82 | 92.31±1.79 |
新窗口打开|下载CSV
2.2 窄叶突变体nal20叶片的细胞学观察
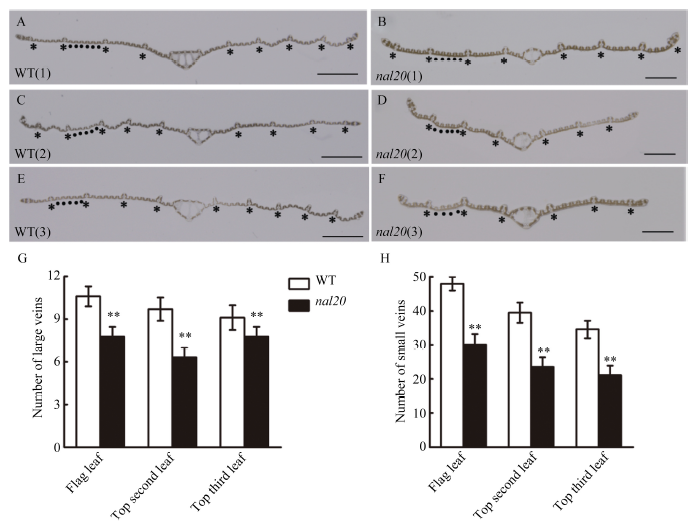
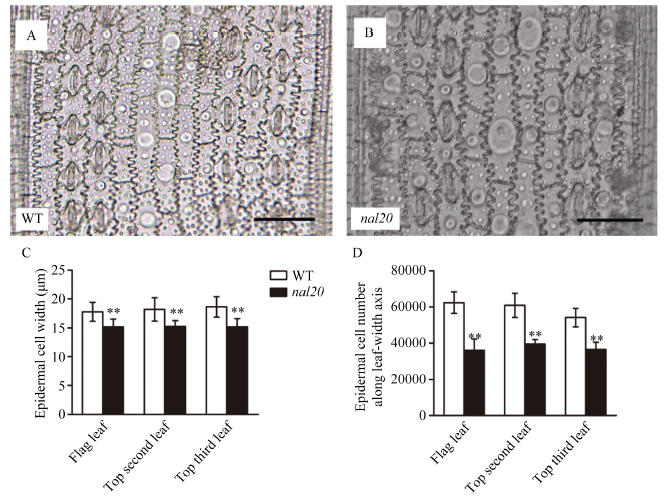
水稻的叶片主要由表皮(上表皮和下表皮)、叶肉和叶脉构成。为进一步研究突变体叶片变窄的机制, 我们对野生型春江06和突变体nal20的大小叶脉和下表皮细胞进行细胞学观察, 分别比较两者抽穗期时倒一叶、倒二叶和倒三叶的差异。突变体与野生型叶脉形态差异较一致(图3-A~F), 在大叶脉数目上, 突变体的倒一叶、倒二叶和倒三叶分别比野生型减少26.62%、35.57%和14.63% (图3-G); 在小叶脉数目上, 与野生型相比, 突变体3个位置的叶片分别减少37.27%、41.25%和38.91% (图3-H), 其中小叶脉数目的减少幅度比大叶脉更明显。在叶片下表皮, 突变体的倒一叶、倒二叶和倒三叶并未出现畸形细胞, 整体细胞形态与野生型没有明显变化(图4-A, B)。但是在细胞大小和数目上有比较明显的差异, 沿着叶宽方向, 突变体3个位置的细胞宽度分别比野生型减少14.56%、16.40%和18.41% (图4-C), 其细胞数目分别减少42.11%、35.24%和32.51% (图4-D)。因此, nal20叶片变窄主要是叶片横轴方向细胞数目减少造成的。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3野生型和突变体nal20叶脉数目比较
A, C, E: 野生型剑叶、倒二叶和倒三叶的叶脉, 标尺为1 mm; B, D, F: 突变体剑叶、倒二叶和倒三叶的叶脉, 标尺为1 mm; 图中*表示大叶脉, 2个大叶脉中的圆点表示小叶脉; G, H: 野生型和突变体大叶脉数目(G)和小叶脉数目(H)的比较。t测验计算P值, 柱形图中**表示突变体在P < 0.01水平差异显著(n = 20)。
Fig. 3Leaf vein number of wild type (WT) and the nal20 mutant
A, C, E: leaf vein shape of the flag leaf, top second leaf and top third leaf in the wild type (bars = 1 mm); B, D, F: leaf vein of the flag leaf, top second leaf and top third leaf in the mutant (bars = 1 mm); G: comparison of large vein number in the wild type and mutant; H: comparison of small vein number in the wild type and mutant. P-values were analyzed using Student’s t-tests. ** P < 0.01 (n = 20).
图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4野生型和突变体nal20叶片细胞大小和数目
A, B: 野生型(A)和突变体nal20 (B)成熟期剑叶下表皮细胞形态, 标尺为50 μm; C, D: 野生型和突变体nal20抽穗期的叶片下表皮细胞大小和细胞数目的比较。t测验计算P值, **表示突变体在P < 0.01水平差异显著(n = 20)。
Fig. 4Epidermal cell width and cell number of wild type (WT) and the nal20 mutant
A, B: Epidermal cell shape of flag leaf in the wild-type (A) and mutant (B) (bars = 50 μm); C, D: comparison of epidermal cell width and cell number along leaf-width axis. P-values were analyzed from Student’s t-tests. ** P < 0.01 (n = 20).
2.3 窄叶突变体nal20的其他表型
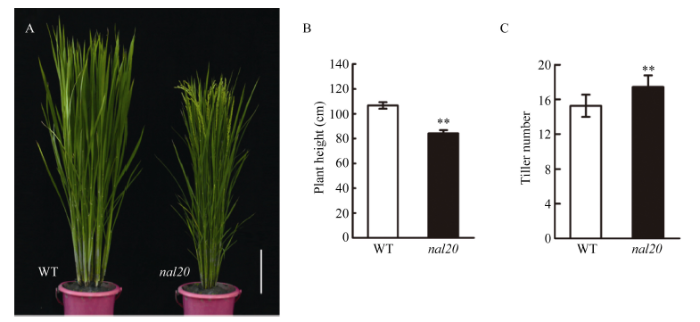
由图5和图6可见, 与野生型春江06相比, 突变体nal20还表现出株高变矮、分蘖数目增多、节间缩短、抽穗期提前等表型。图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5野生型和突变体nal20的整体株型
A: 野生型(左)和突变体nal20 (右)抽穗期的株型比较, 标尺为5 cm; B: 植株高度比较; C: 分蘖数目比较。t测验计算P值, 柱形图中**表示突变体在P < 0.01水平差异显著(n = 20)。
Fig. 5Gross morphology of wild type (WT) and the nal20 mutant
A: plant type of wild type and the nal20 mutant at heading stage (bar = 5 cm). Comparison of plant height (B) and tiller number (C) between WT and nal20. P-values were analyzed using Student’s tests. ** P < 0.01 (n = 20).
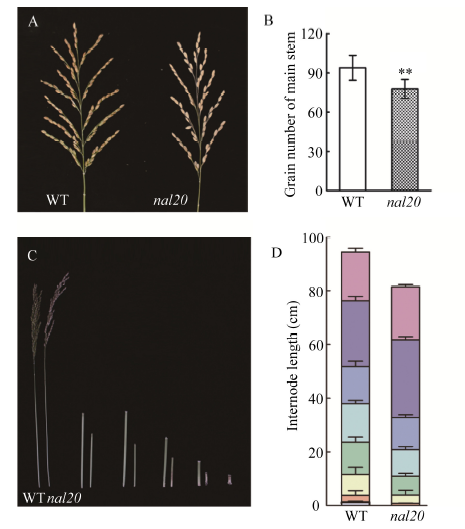
图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6野生型和突变体nal20穗型和茎节长度
A: 野生型(左)和突变体nal20 (右)抽穗期的主茎穗形态; B: 主茎穗粒数比较, t测验计算P值, 柱形图中**表示突变体在P < 0.01水平差异显著(n = 20); C: 野生型(左)和突变体nal20 (右)抽穗期的节间形态; D: 节间长度比较(由上而下分别是穗长、穗茎节、第1茎节、第2茎节、第3茎节、第4茎节、第5茎节、第6茎节)。
Fig. 6Panicle morphology and internode length of wild type (WT) and the nal20 mutant
A: panicle shape of wild type and the nal20 mutant at heading stage; B: comparison of grain number per panicle. The P-values were analyzed from student’s t-tests. ** P < 0.01 (n = 20); C: internodes of wild type and the nal20 mutant at heading stage; D: comparison of internode length (from the top to bottom is panicle, panicle internode, the first internode, the second internode, the third internode, the fourth internode, the fifth internode, and the sixth internode).
在整体形态上, 突变体的平均植株高度为84.35 cm, 为野生型的80% (图5-A, B), 分蘖数目比野生型增加了13.3% (图5-A, C)。进一步观察发现, 突变体株高的降低主要是由于突变体各个节间长度与野生型相比都有不同程度的缩短, 从第1茎节到第5茎节分别减少了14.03%、31.39%、40.62%、61.37%和66.14%; 茎节间数目上也有一定程度的减少, 突变体与野生型相比缺少一个节间(第6茎节)(图6-C, D), 主要是在第3茎节到第6茎节的缩短最为显著。
在主茎穗部形态和发育上, 从图6-A中可以看出, 突变体nal20的穗形与野生型没有太大差别, 但是与野生型相比, 突变体的主茎穗粒数显著减少17.25% (图6-B)。特别是在长日照条件下, 突变体nal20的抽穗期明显提前(图5-A和表1), 而在短日照条件下两者的抽穗时间并没有明显差异(表1)。
2.4 窄叶突变体nal20的遗传分析
用窄叶突变体nal20与叶片表型正常的籼稻品种Dular杂交, F1代植株叶片表型正常, 自交后得到的F2群体叶片性状分离, 分为正常叶型与窄叶型。在田间随机调查的200株中, 有46株表现出窄叶表型, 正常叶型和窄叶型的分离比例为3.4∶1.0, 经卡方检验得知(χ2 = 0.28 < χ2 0.05 = 3.84), 符合孟德尔遗传3∶1的分离比例, 推断窄叶突变体nal20受1对隐性基因控制。2.5 突变体nal20的基因定位
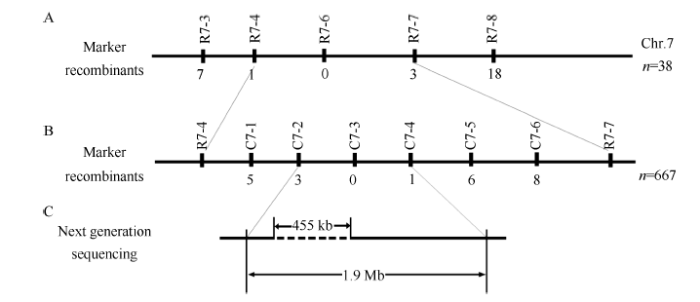
选取10株F2代窄叶表型明显的突变体, 构建突变体DNA混池, 采用BSA方法对覆盖水稻全基因组的170对具有多态性的InDel标记进行连锁分析, 发现目的基因可能与第7染色体的2个InDel标记R7-4和R7-7连锁(表2)。随后用38株F2突变个体DNA验证, 将突变体nal20目的基因初步定位在R7-4和R7-7标记之间(图7-A)。Table 2
表2
表2InDel分子标记
Table 2
| 分子标记 Marker | 正向引物序列 Forward primer sequence (5°-3°) | 反向引物序列 Reverse primer sequence (5°-3°) |
|---|---|---|
| R7-3 | GGCAAGTTAAAACCGAGCAG | CCATGGAAGGCTGTAACCAT |
| R7-4 | GATAGCTTGACAACGGTGGCAC | CCATACATTGTTGCACTTGTGAC |
| R7-6 | CCCCATGAGGCCTACACTT | AGCAGCATAATCAGATGAGACG |
| R7-7 | ATCGGTGCCGCTCCTAGAT | CACTCCACAGACATGCAATTT |
| R7-8 | TTCCAGGCTGCATCTTATTC | GCAGGACCCATGCTGAAAAG |
| C7-1 | ATCTAGCGGCTAGCACACTGG | ATCACCTCATGTCTCCGGACG |
| C7-2 | ACTGTGTGCTGCCTGACATAC | AGACGAATGGTCAAACATGTG |
| C7-3 | GATGGTAGGAGGCCGGACTGG | GCCTCCTTTACTACCGACCGC |
| C7-4 | GTGGTGACAATGTGGTACAAT | CCACTTATACGTGCGTAACAC |
| C7-5 | CGCCGTTCGCATAAAGTCCTG | TTGGACTTGTTTGGGCATACG |
| C7-6 | AAGGACTTGCCGTTTGATCTC | CATGATCGGTACTAGCAATTC |
新窗口打开|下载CSV
图7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7突变体nal20的基因定位图谱
A: 初步定位结果; B: 精细定位结果; C: 目的基因所在区域, 虚线表示455 kb片段缺失。A, B图中横线上方为定位所用标记, 下方数字为交换单株个数。
Fig. 7Gene mapping of the nal20 mutant
A: primary mapping result; B: fining mapping result; C: target gene region. The dashed line represents the 455 kb deletion. In panels A and B, the InDel markers and number of recombinants are marked above and below the horizontal line, respectively.
为了进一步精细定位目的基因, 将F2 群体扩大到667株突变个体, 在R7-4和R7-7标记之间新开发了6个新的InDel标记, 即C7-1、C7-2、C7-3、C7-4、C7-5和C7-6 (图7-B和表2)。利用这些标记进行连锁分析, 最终将目的基因定位在C7-2和C7-4之间, 物理距离约为1.9 Mb (图7-B)。
2.6 nal20突变体的重测序
通过对窄叶突变体nal20的精细定位, 最终将目的基因定位于C7-2和C7-4标记之间, 即Chr.7: 9020319-10974087 (Os-Nipponbare-Reference-IRGSP- 1.0 pseudomolecules)。由于该区域位于第7染色体的着丝粒区, 发生重组交换的频率很低, 而且多为重复序列, 很难开发特异性的分子标记来缩短定位区间。为了能够快速找到目的窄叶基因, 对nal20突变体全基因组进行了二代重测序, 发现在突变体第7染色体目标区间内有455 kb的缺失(g.9074601- g.9529600)(图7-B)。利用Rice Genome Annotation (水稻基因组注释系统)网站(http://rice.plantbiology.msu.edu/)查看此缺失区间内基因编码蛋白的详细功能信息, 发现在此缺失区间内共有60个基因, 其中12个具有已知的生化功能, 12个表达蛋白功能未知, 5个假定蛋白(表3), 以及31个转座子或逆转录转座子。其中一个功能基因LOC_Os07g15770, 是已报道的Ghd7基因。它是一个多效性基因, 能够延迟抽穗期、增加植株高度和每穗粒数, 显著提高水稻产量[23], 还对调控剑叶面积起重要作用[24]。推测窄叶突变体nal20的窄叶表型可能与Ghd7基因的缺失有关。Table 3
表3
表3455 kb缺失区间内的基因
Table 3
| 基因 Gene | 功能注释 Function annotation |
|---|---|
| LOC_Os07g15640 | CRR4, putative, expressed |
| LOC_Os07g15650 | Expressed protein |
| LOC_Os07g15670 | Peroxiredoxin, putative, expressed |
| LOC_Os07g15680 | Phospholipase D, putative, expressed |
| LOC_Os07g15720 | Hypothetical protein |
| LOC_Os07g15770 | CCT motif family protein, expressed |
| LOC_Os07g15820 | Expressed protein |
| LOC_Os07g15860 | Expressed protein |
| LOC_Os07g15870 | Expressed protein |
| LOC_Os07g15880 | Mitochondrial prohibitin complex protein 2, putative, expressed |
| LOC_Os07g15910 | Expressed protein |
| LOC_Os07g15920 | Expressed protein |
| LOC_Os07g15930 | Legume lectins beta domain containing protein, expressed |
| LOC_Os07g15940 | Legume lectins beta domain containing protein, expressed |
| LOC_Os07g15950 | Expressed protein |
| LOC_Os07g15959 | Expressed protein |
| LOC_Os07g15970 | Erythronate-4-phosphate dehydrogenase domain containing protein, expressed |
| LOC_Os07g15980 | Expressed protein |
| LOC_Os07g16030 | Expressed protein |
| LOC_Os07g16040 | Erythronate-4-phosphate dehydrogenase domain containing protein, expressed |
| LOC_Os07g16054 | Hypothetical protein |
| LOC_Os07g16130 | Acetyltransferase, GNAT family, putative, expressed |
| LOC_Os07g16140 | FAD binding protein, putative, expressed |
| LOC_Os07g16150 | Expressed protein |
| LOC_Os07g16180 | Hypothetical protein |
| LOC_Os07g16210 | Expressed protein |
| LOC_Os07g16224 | Piwi domain containing protein, putative, expressed |
| LOC_Os07g16260 | Expressed protein |
| LOC_Os07g16270 | Hypothetical protein |
新窗口打开|下载CSV
3 讨论
水稻是世界上最重要的粮食作物之一, 其叶片形态对最终产量有着至关重要的影响, 但是目前与叶片发育相关基因的研究大多集中在双子叶模式植物拟南芥中, 因此研究单子叶植物水稻的叶片发育分子机制具有重要的理论意义和应用价值。我们通过60Co-γ射线诱变粳稻品种春江06, 在M2代中得到了稳定遗传的窄叶突变体nal20, 其叶片宽度减少为野生型的一半左右。图位克隆结果显示nal20基因位于第7染色体着丝粒区, 进一步的二代测序结果表明在目标区域(1.9 Mb)内有455 kb缺失, 涵盖了60个基因, 其中1个基因(Ghd7)已报道与叶片发育相关。在本研究中, 由图位克隆结果可知, 目标突变区域位于着丝粒附近, 此处发生重组交换的频率极低, 需要使用数目非常庞大的F2代的突变个体来缩小目标区域, 是极其耗时的实验过程。目前, 二代测序技术(next-generation sequencing, NGS)日渐成熟, 具有高通量、快速、精准、费用低等特点, 应用该技术已经成为生物信息学研究及基因检测的发展趋势。在本研究中采用二代测序技术寻找目标突变位点无疑是一种快速有效的实验方法。Ghd7是一个多效基因, 对水稻的植株高度(plant height, PH)、抽穗期(heading date, HD)和每穗颖花数(spikelet number per panicle, SPP)都有很大的效应[23]。此外, 通过调查Ghd7近等基因系的FLL (剑叶长度)、FLW (剑叶宽度)和FLA (剑叶面积) 3个性状, 发现Ghd7对其有较大的遗传效应, 即Ghd7对调控剑叶面积起重要作用。相关性分析表明, 每穗颖花数与3个剑叶性状之间的相关性最高, 说明Ghd7通过产生较大的叶面积来保证充足的光合作用, 从而满足产生更多的颖花数的需求[24]。由于Ghd7能够提高产量, 目前在水稻生产上也得到了非常广泛的应用。Ghd7基因会延迟HD, 增加PH和SPP值[25]。Ghd7还可能在水稻开花途径中Ehd1和Hd3a基因的上游起作用, 在长日照条件下提高这2个控制花期基因的表达[26,27]。对Ghd7基因在水稻自然群体中的多态性分析(点突变或插入缺失)发现, Ghd7基因的不同等位变异(或称单倍型)具有不同的效应, 其中Ghd7蛋白多样性是调节表型变异的关键因素。比较Ghd7的3个性状发现, 只有在长日照条件下HD和SPP的变化极显著。在热带地区的Ghd7单倍型能够调控植物迅速适应当地生长季节的增长而增加产量, 而在温带地区的Ghd7单倍型会缩短水稻生命周期以保证结实率[23,25]。Ghd7的自然变异对水稻适应和遗传改良有很大的贡献, 使得这个多效基因能被灵活应用于现代分子育种中。同时还可为了满足不同生态类型的品种需求, 开发特定的分子标记来选择合适的单倍型。
本研究中的突变体nal20植株高度明显低于野生型(图5); 剑叶面积比野生型减少44.6%; 穗部发育上, 在长日照条件下, 抽穗时间比野生型提前37 d (图5-A和表1), 而在短日照下没有明显差异(表1)。野生型和突变体的穗粒数分别为93.95和77.75, 突变体的每穗粒数减少17.24%。综上所述, 突变体nal20表现为株高降低、开花期提前、每穗粒数减少、剑叶面积减少, 与已报道的Ghd7基因效应相反, 故我们推测nal20突变体表型很可能是缺失Ghd7基因功能所致。但是, 由于目标区域缺失范围内还有其他功能基因, 还需要通过转基因的方式来证实Ghd7基因缺失是导致叶片变窄的根本原因。
4 结论
利用60Co-γ射线诱变粳稻品种春江06获得了一个窄叶突变体nal20, 从剑叶到倒三叶均表现叶片宽度减少50%、植株高度降低、抽穗时间提前。叶片宽度变窄主要是细胞数目减少所致。该突变体基因定位于第7染色体着丝粒区, 目标区域内有455 kb大片段缺失, nal20的突变表型可能与Ghd7基因的缺失有关。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/S0168-9525(01)02601-4URLPMID:11858837 [本文引用: 1]

Interactions between members of three plant-specific gene femilies promote establishment of polarity in leaves of seed plants; polarity is required for subsequent lamina expansion.
DOI:10.1104/pp.015347URLPMID:12586863 [本文引用: 2]

Plant Physiol. 2003 Feb;131(2):389-94. Congresses
DOI:10.1093/nar/8.19.4321URLPMID:324241 [本文引用: 2]

Abstract A method is presented for the rapid isolation of high molecular weight plant DNA (50,000 base pairs or more in length) which is free of contaminants which interfere with complete digestion by restriction endonucleases. The procedure yields total cellular DNA (i.e. nuclear, chloroplast, and mitochondrial DNA). The technique is ideal for the rapid isolation of small amounts of DNA from many different species and is also useful for large scale isolations.
DOI:10.1105/tpc.109.069948URLPMID:2782284 [本文引用: 1]

Development of the flattened laminar structure in plant leaves requires highly regulated cell division and expansion patterns. Although tight regulation of these processes is essential during leaf development, leaf shape is highly diverse across the plant kingdom, implying that patterning of growth must be amenable to evolutionary change. Here, we describe the molecular identification of the classical tomato (Solarium lycopersicum) mutant lyrate, which is impaired in outgrowth of leaflet primodia and laminar tissues during compound leaf development. We found that the lyrate phenotype results from a loss-of-function mutation of the tomato JAGGED homolog, a well-described positive regulator of cell division in lateral organs. We demonstrate that LYRATE coordinates lateral outgrowth in the compound leaves of tomato by interacting with both the KNOX and auxin transcriptional networks and suggest that evolutionary changes in LYRATE expression may contribute to the fundamental difference between compound and simple leaves.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.4161/psb.5.2.10369URLPMID:19765235 [本文引用: 1]

Plant cell wall is a complex polysaccharide network and performs important developmental and physiological functions far beyond supplying the physical constrains. Plant cells have the ability to react to cell wall defects as exhibited by changes in gene expression, accumulation of ectopic lignin, stress responses, and growth arrest. It is a major challenge to understand how plants sense and response to wall integrity since very little is known about the signaling involved in the responses. Cellulose synthase-like D (CSLD) proteins mediating the biosynthesis of a wall polysaccharide polymer make up a common subfamily to all plants. Recently, we have reported the functional characterization of CSLD4 in rice. Mutation in OsCSLD4 shows morphological alterations and pleiotropic effects on wall compositions and structure. Our study demonstrates that OsCSLD4 plays critical roles in cell wall formation and plant growth. Here we show the subtle wall alterations through separating the culm residues into five fractions. Quantitative RT-PCR analysis further revealed that the expression of various genes involved in xylan synthesis and cell-cycle regulation was altered in mutant plants, as the responses to OsCSLD4 disruption. Therefore, plants may have a fine sensory machinery to react to wall defects and modulate growth for adapting to the changes.
DOI:10.1007/s00425-010-1180-3URLPMID:20443024 [本文引用: 1]

Appropriate leaf shape has proved to be useful in improving photosynthesis and increasing grain yield. To understand the molecular mechanism of leaf morphogenesis, we identified a rice mutant nrl1, which was characterized by a phenotype of narrow and rolled leaves. Microscopic observation showed that the mutation significantly decreased the number of vascular bundles of leaf and stem. Genetic analysis revealed that the mutation was controlled by a single nuclear-encoded recessive gene. To isolate the nrl1 gene, 756 F2 and F3 mutant individuals from a cross of the nrl1 mutant with Longtepu were used and a high-resolution physical map of the chromosomal region around the nrl1 gene was made. Finally, the gene was mapped in 16.5 kb region between marker RL21 and marker RL36 within the BAC clone OSJNBa0027H05. Cloning and sequencing of the target region from the mutant showed that there was a 58 bp deletion within the second exon of the cellulose synthase-like D4 gene (TIGR locus Os12g36890). The nrl1 mutation was rescued by transformation with the wild-type cellulose synthase-like D4 gene. Accordingly, the cellulose synthase-like D4 gene was identified as the NRL1 gene. NRL1 was transcribed in various tissues and was mainly expressed in panicles and internodes. NAL7 and SLL1 were found to be upregulated, whereas OsAGO7 were downregulated in the nrl1 mutant. These findings suggested that there might be a functional association between these genes in regulating leaf development.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1093/pcp/pct032URLPMID:23420902 [本文引用: 1]

Leaf shape is one of the key determinants of plant architecture. Leaf shape also affects the amount of sunlight captured and influences photosynthetic efficiency; thus, it is an important agronomic trait in crop plants. Understanding the molecular mechanisms governing leaf shape is a central issue of plant developmental biology and agrobiotechnology. Here, we characterized the narrow-leaf phenotype of FL90, a linkage tester line of rice (Oryza sativa). Light and scanning electron microscopic analyses of FL90 leaves revealed defects in the development of marginal regions and a reduction in the number of longitudinal veins. The narrow-leaf phenotype of FL90 shows a two-factor recessive inheritance and is caused by the loss of function of two WUSCHEL-related homeobox genes, NAL2 and NAL3 (NAL2/3), which are duplicate genes orthologous to maize NS1 and NS2 and to Arabidopsis PRS. The overexpression of NAL2/3 in transgenic rice plants results in wider leaves containing increased numbers of veins, suggesting that NAL2/3 expression regulates leaf width. Thus, NAL2/3 can be used to modulate leaf shape and improve agronomic yield in crop plants.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1038/ng.143URLPMID:18454147 [本文引用: 3]

Yield potential,plant height and heading date are three classes of traits that determine the productivity of many crop plants. Here we showed that the quantitative trait locus (QTL) Ghd7,isolated from an elite rice hybrid and encoding a CCT domain protein,had major effects on an array of traits in rice,including number of grains per panicle,plant height and heading date. Enhanced expression of Ghd7 under long-day conditions delays heading and increases plant height and panicle size. Natural mutants with reduced function enable rice to be cultivated in temperate and cooler regions. Thus,Ghd7 has played crucial roles for increasing productivity and adaptability of rice globally.
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
DOI:10.1038/nature01549URLPMID:12700762 [本文引用: 1]

Nature. 2003 Apr 17;422(6933):719-22. Comparative Study; Research Support, Non-U.S. Gov't
[本文引用: 1]
.
[本文引用: 1]
