 ,, 袁露, 徐文华, 郑英
,, 袁露, 徐文华, 郑英 ,扬州大学医学院组织学与胚胎学教研室,江苏省非编码RNA基础与临床转化重点实验室,扬州 225009
,扬州大学医学院组织学与胚胎学教研室,江苏省非编码RNA基础与临床转化重点实验室,扬州 225009Role and mechanism of intraflagellar transport in mammalian spermiogenesis
Tingting Ge ,, Lu Yuan, Wenhua Xu, Ying Zheng
,, Lu Yuan, Wenhua Xu, Ying Zheng ,Jiangsu Key Laboratory of Experimental & Translational Non-coding RNA Research, Department of Histology and Embryology, School of Medicine, Yangzhou University, Yangzhou 225009, China
,Jiangsu Key Laboratory of Experimental & Translational Non-coding RNA Research, Department of Histology and Embryology, School of Medicine, Yangzhou University, Yangzhou 225009, China通讯作者: 郑英,教授,博士生导师,研究方向:生殖医学。E-mail:yzzkl@163.com
编委: 刘默芳
收稿日期:2021-06-10修回日期:2021-08-29
| 基金资助: |
Received:2021-06-10Revised:2021-08-29
| Fund supported: |
作者简介 About authors
葛婷婷,在读硕士研究生,专业方向:生殖医学。E-mail:

摘要
纤毛/鞭毛是真核生物细胞表面伸出的进化保守的细胞器,独特的位置和特性使它们在细胞运动和信号传递等生命过程发挥重要作用。哺乳动物纤毛/鞭毛的组装和维持都依赖纤毛/鞭毛内运输(intraflagellar transport, IFT)。IFT是由IFT复合体A和复合体B在驱动蛋白或马达蛋白驱动下的双向运输系统。该过程可将货物蛋白在胞体的合成位点与纤毛/鞭毛尖端的装配位点之间进行运输。鞭毛是哺乳动物精子产生动力的特异性细胞器,其完整性对精子正常功能至关重要。近年来研究表明,IFT在哺乳动物精子鞭毛形成和雄性生殖能力方面必不可少。本文对参与IFT的蛋白在精子鞭毛形成中的作用和机制进行了综述,以探讨其在男性不育症中的发病机制,为不育症的诊断和治疗提供理论基础。
关键词:
Abstract
Eukaryotic cilia and flagella are evolutionarily conserved organelles that protrude from the cell surface. The unique location and properties of cilia allow them to function in vital processes such as motility and signaling. Ciliary assembly and maintenance rely on intraflagellar transport (IFT). Bidirectional movement of IFT particles composed of IFT-A and IFT-B complexes is powered by kinesin-2 and dynein-2 motors. IFT delivers building blocks between their site of synthesis in the cell body and the ciliary assembly site at the tip of the cilium. The integrity of the flagellum, a specialized organelle of mammalian sperm to generate the motility, is critical for normal sperm function. Recent findings suggest that IFT is indispensable for sperm flagellum formation and male fertility in mice and human. In this review, we summarize the role and mechanisms of IFT proteins during enflagellation in spermiogenesis, thereby discussing the pathological mechanisms of male infertility and providing theoretical basis for the diagnosis and treatment of male infertility.
Keywords:
PDF (842KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
葛婷婷, 袁露, 徐文华, 郑英. 哺乳动物纤毛/鞭毛内运输在精子形成中的作用及机制研究进展. 遗传[J], 2021, 43(11): 1038-1049 doi:10.16288/j.yczz.21-206
Tingting Ge.
真核生物的纤毛/鞭毛是进化上保守的、细胞表面伸出的细胞器,独特的位置和特性使其在运动和信号传导等过程中发挥作用。纤毛/鞭毛的组装和维持依赖于纤毛/鞭毛内运输(intraflagellar transport, IFT),即纤毛/鞭毛基部和顶端之间多组分运输的双向运输系统[1]。衣藻(Chlamydomonas reinhardtii)中IFT缺陷导致鞭毛组装异常,致使细胞出现鞭毛缩短、畸形或缺失;由于纤毛广泛分布于哺乳动物全身各组织器官,纤毛装配缺陷可出现多器官病变,统称为纤毛病(Ciliopathies),如呼吸道疾病、视网膜变性、肝肾多囊性改变、骨骼系统发育异常、神经系统异常、智力障碍、肥胖和男性不育等[2]。男性不育症大多与精子发生调控基因的异常表达有关,动物模型和基因筛选研究已经证实遗传因素是精子发生障碍的常见原因。尽管有许多研究强调了IFT与纤毛/鞭毛形成之间的关系,但IFT在哺乳动物精子鞭毛形成中的调控作用鲜有研究报道,IFT缺陷引起人类男性不育的临床案例更是报道极少。本文主要介绍了IFT复合体中的不同亚基,如IFT25、IFT27、IFT74、IFT81、IFT88、IFT20、IFT172、IFT140、IFT144和IFT139等在小鼠(Mus musculus)精子发生及人类男性生殖中的研究进展,它们作为影响男性生殖的潜在遗传因子通过IFT机制调控精子发生,对精子形成和其运动活性至关重要。
1 IFT参与精子鞭毛结构的形成
在哺乳动物精子发生过程中,精原细胞通过极其复杂的分化过程发育为成熟精子,涉及数千个基因,这些基因的正常表达是维持哺乳动物雄性生育能力的基础。精子发生始于生殖干细胞形成的精原细胞,精原细胞经有丝分裂后形成精母细胞,后者经过两次减数分裂后演变为单倍体圆形精子细胞。最后,圆形精子细胞经过复杂的形态变化形成蝌蚪状的精子。精子形成过程包括:细胞核浓缩和蛋白转型;高尔基复合体囊泡覆盖精子细胞核顶部,形成顶体;中心粒迁移到细胞核的尾侧,远端中心粒是鞭毛的成核结构,其微管延伸形成鞭毛[3];线粒体聚集在鞭毛起始处形成线粒体鞘;多余胞质形成残余体后丢失。精子形成过程中会出现一种独特的精子领结构,其出现在精子形成早期,消失于精子变长及核浓缩将近结束时,参与核浓缩、核延伸,并通过精子领内运输(intramanchette transport, IMT)输送精子尾部发育所需蛋白。轴丝是精子鞭毛的一个核心结构,由9组外周双联微管和2根中央微管(“9+2”结构)组成,每组外周双联微管伸出2个由动力蛋白组成的短臂,称为动力蛋白臂,分别为内动力蛋白臂(inner dynein arm, IDA)和外动力蛋白臂(out dynein arm, ODA)[4]。中央鞘向外周双联微管发出丝状结构,称放射辐,将外周微管和中央微管连接起来(图1)。图1
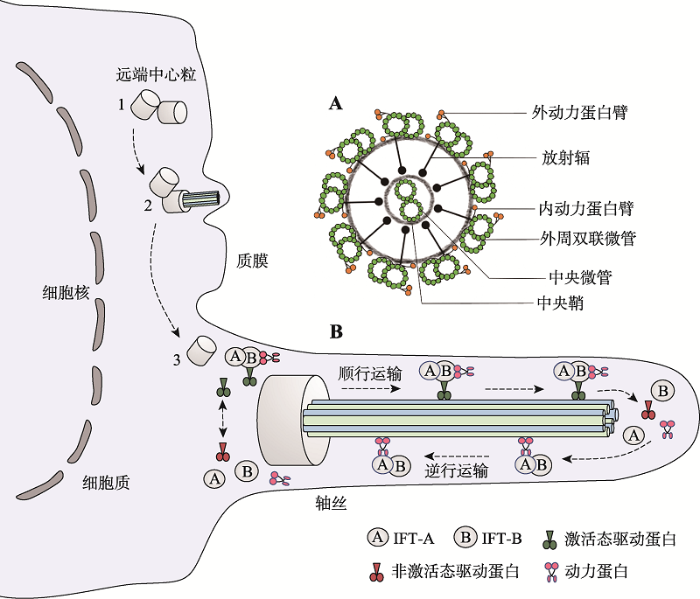 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1纤毛/鞭毛的形成过程及精子去细胞膜轴丝横断面模式图
A:精子去细胞膜轴丝横断面模式图;B:纤毛/鞭毛的形成及鞭毛内运输主要步骤示意图。
Fig. 1Diagram of the process of cilia/flagellum formation and the cross-sectional pattern of the axoneme in the acellular membranes of spermatozoa
精子尾部的发育涉及IMT和IFT两种转运机制,它们都通过微管轨道和运动蛋白来运输货物蛋白。精子尾部发育所需蛋白通过IMT储存和输送到基底体区域,后由IFT运输到发育中的精子尾部,参与轴丝组装。IFT是通过复合体A (IFT-A)和复合体B (IFT-B)在驱动蛋白或马达蛋白的作用下调控鞭毛组装的双向运输系统。IFT-A由IFT43、IFT121、IFT122、IFT139、IFT140和IFT144等6个亚单位组成;IFT-B由16个亚单位组成,其中核心复合体又分为核心复合体1 (IFT22、IFT25、IFT27、IFT74、IFT81)和核心复合体2 (IFT46、IFT52、IFT56、IFT70、IFT88),外周亚基包括IFT20、IFT38、IFT54、IFT57、IFT80和IFT172[1] (图2)。驱动蛋白承担复合体B的顺向运输,将鞭毛组装与维持所需的前体蛋白和信号分子由胞体运往鞭毛顶端的装配位点。鞭毛通过末端微管不断的拆卸来维持平衡,马达蛋白介导复合体A及相关周转产物从轴丝末端向胞体运送,称之为逆向运输,其运输的货物还包括复合体B上一些已经完成使命的蛋白质[5] (图1)。IFT复合体在精子发生过程中充当衔接子,介导货物蛋白和马达之间的结合,还在鞭毛和体细胞之间传导信号,IFT亚基的突变通常会导致鞭毛缩短或完全消失,顺向或逆向运输缺陷通常引起鞭毛末端肿胀。
图2
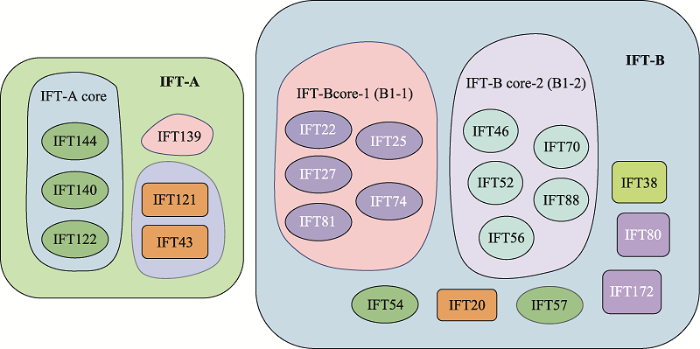 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2IFT复合体整体结构示意图
Fig. 2Schematic representation of the overall architecture of the IFT complex
2 IFT-B复合体调控精子鞭毛发生
IFT-B复合体由10个核心亚基和6个外周亚基组成。目前已有研究表明,Ift25、Ift27、Ift74、Ift81、Ift88、Ift20和Ift172突变会引起小鼠精子发生中鞭毛组装和维持出现不同程度的缺陷,进而影响雄性小鼠生育能力。2.1 IFT25通过多种途径调控精子发生
IFT25蛋白在小鼠睾丸中高表达,定位于精子细胞中心粒和鞭毛,是IFT蛋白的典型分布部位。小鼠出生后第12天,睾丸组织中IFT25蛋白开始表达,在第24天表达上调,即精子发生的最后阶段。IFT25缺失的生殖细胞在形成长形精子细胞时,由于细胞核形状改变而开始出现异常[6]。IFT25在体细胞和雄性生殖细胞的纤毛/鞭毛发育中起着不同的作用。Ift25基因敲除导致多种异常表型,这些表型与位于初级纤毛的Hedgehog信号通路被破坏有关,但纤毛生成不受影响[7]。在生殖细胞特异性缺失IFT25的小鼠模型中,所有小鼠都存活到成年,但精子鞭毛结构破坏,精子数量急剧减少,精子的运动性完全消失,且雄性不育。光镜和扫描电镜分析发现,一些精子鞭毛有分支、鞭毛厚度异常和尖端肿胀。透射电镜观察到鞭毛内存在空泡,尾部有囊泡聚集,“9+2”轴丝结构异常,外周致密纤维(outer dense fibers, ODF)和纤维鞘(fibrous sheath, FS)排列紊乱[6]。这可能是因为IFT25蛋白的缺乏破坏了精子细胞中的IFT,货物蛋白无法运输并整合到鞭毛中。虽然IFT25是IFT-B组分,IFT-B成分破坏通常会因为顺行运输受阻而导致鞭毛过短,但条件性敲除Ift25基因的雄性小鼠精子鞭毛尖端肿胀提示IFT25也可能参与逆行运输。
值得注意的是,Ift25敲除小鼠精子细胞中FS形成破坏[6]。FS是一种独特的细胞骨架结构,围绕着轴丝和ODF。它由两个纵列组成,通过紧密排列的圆周肋连接,在整个精子形成过程中从远端到近端组装。FS是信号通路中的蛋白质支架,可参于调节精子成熟、运动、获能、超活化和顶体反应,也是糖酵解通路中为精子超活化运动提供能量的酶的支架[8]。FS仅存在于精子细胞中,且IFT25在体细胞纤毛组装中非必需,精子细胞缺失IFT25会引起FS结构破坏,推测IFT25可能在FS的形成和维持中发挥独特作用。此外,一些精子中丢失“9+2”核心轴丝成分,这种成分只存在于活动鞭毛中,是调节运动的关键结构,其丢失可能是条件性敲除Ift25精子无运动活性的重要原因。
IFT25包含一个半乳糖结合样结构域,该结构域在其他蛋白质中可结合各种配体,如碳水化合物、磷脂和核酸等。研究表明,IFT25/Hsp16.2过表达可激活Hsp90,并通过PI3k-Akt信号通路稳定脂筏[9]。脂筏是专门的膜微域,在包括精子在内的许多细胞质膜上充当信号平台[10]。精子脂筏在cAMP合成的信号上游发挥作用,是精子激活钙依赖性途径所必需的[11],然而Ift25突变小鼠的精子脂筏被破坏,提示IFT25也可能负责将某些离子通道运输到精子鞭毛。
2.2 IFT27通过调控IFT-B蛋白运输参与鞭毛组装
IFT27与IFT88、IFT81、IFT74、IFT72、IFT52、IFT46构成IFT-B的核心复合体[12]。在小鼠出生后的第20天,IFT27蛋白在睾丸组织中首次检测到,其表达水平在第24天显著增加,直至第42天一直保持较高水平。IFT27分布于精母细胞和圆形精子细胞胞质及发育中的鞭毛。生殖细胞特异性Ift27基因敲除小鼠不育,仅2%的精子具有运动能力,附睾尾腔内精子呈现多种形态异常,如圆头、短且弯曲的尾部、精子鞭毛厚度不一、鞭毛末端肿胀,及头尾分离。超微结构观察显示,在IFT27缺失的小鼠附睾精子中发现断裂的“9+2”轴丝核心结构、断裂的轴丝微管、扭曲的膜、错位和缺失的微管、异常的FS和线粒体鞘等多种异常表型[13]。与IFT25相似,IFT27虽然不是体细胞纤毛形成所必须,但对小鼠精子发生和生殖能力都必不可少。IFT27与IFT25之间存在直接的相互作用,两者通过许多共同的机制调控精子形成。在Ift25敲除小鼠中,IFT27几乎不存在;相反,IFT25仍然存在于Ift27敲除小鼠中,这表明细胞和组织中的IFT27存在与否取决于IFT25。Ift25基因敲除小鼠不仅表现出与Ift27基因敲除小鼠相似的表型,而且具有IFT25缺失特有的表型,如精子脂筏被破坏、鞭毛出现分支[13]。因此推测,IFT25可能在IFT27之外还发挥其他的作用:其一,可能调节精子中的脂筏状态;其二,IFT25可能携带IFT25/IFT27未运输的其他货物蛋白。分别检测Ift25与Ift27两种基因敲除小鼠中其他IFT蛋白表达水平发现,Ift25敲除小鼠中IFT20和IFT81表达均下调。与Ift25相似,IFT81在条件性Ift27敲除小鼠内表达下调,但IFT20表达未受影响。Ift25和Ift27对睾丸内IFT20表达的不同影响,可能是Ift25和Ift27敲除小鼠两者表型存在差异的原因之一。
IFT27还参与其他多种蛋白质相互作用,CEP19- RABL2B-IFT通路是调控纤毛组装的分子机制之一。CEP19被中心粒CEP350/FOP复合体招募到纤毛基底部,RABL2B通过其固有的核苷酸交换活性和GTP结合,激活的RABL2B被CEP19特异性捕获引入纤毛。然后,RABL2B通过IFT74/81异二聚体与IFT-B结合,从而启动IFT-B的纤毛进入。RABL2在GTP结合状态下通过IFT74/81异二聚体与IFT-B复合体相互作用,这种相互作用在精子鞭毛运动缺陷的雄性不育小鼠中被破坏[14]。此外,IFT27被认为是一个功能连接体,协助IFT-B和BBSome这两个多蛋白复合体之间的纤毛蛋白转运[15]。
2.3 IFT74调控核心轴丝和纤维鞘的组装
IFT74分布于小鼠体内含有纤毛细胞的器官,如脑、肺和肾,在睾丸中高表达。小鼠出生后第12天首次在睾丸组织中检测到IFT74蛋白,第20天蛋白表达量显著上升。在雄性小鼠生殖细胞中,IFT74定位于精母细胞、圆形精子细胞囊泡以及长形精子细胞的顶体、中心体和发育中的尾部。Ift74敲除雄性小鼠不能生育,精子细胞数减少,活力降低,仅有少量发育成熟的精子,且形态异常,如圆头或其他畸形头、短尾、轴丝和微管呈多种异常形态;Ift74敲除小鼠精子顶体形成过程未观察到异常,可见IFT74虽定位在顶体,但不是调控顶体形成的关键因素[16]。IFT74是IFT-B复合体的核心组分,在精子形成过程中对其他IFT的稳定和功能有重要影响,IFT-B复合体组分如IFT27、IFT57、IFT81、IFT88和IFT140蛋白表达量在Ift74突变雄性小鼠体内都显著降低。而IFT-B复合体的另外两个组分IFT20和IFT25蛋白表达水平无明显变化,这两者位于IFT-B核心亚复合体之外,它们的功能可能不同于其他的IFT蛋白。
在IFT-B蛋白的核心亚群中,IFT25/27、IFT88/ 52/46和IFT81/74/72可以相互作用[12,17,18]。IFT74和IFT81通过中心和C端螺旋结构域的直接相互作用稳定IFT-B复合体,IFT74氨基末端和β-微管蛋白的高酸性羧基末端相互作用,使得IFT81氨基端与β-微管蛋白的亲和力增强了18倍[12]。结合Ift74敲除小鼠精子超微结构,推测IFT74的主要功能之一是在精子细胞轴丝形成过程中转运β-微管蛋白使其参与微管组装。IFT74也可以作为调节因子,通过控制IFT亚基进入鞭毛的频率和逆行速度,调节轴丝和微管装配来参与精子鞭毛的形成[19]。
IFT74在小鼠精子形成过程中的重要作用可能是通过调控核心轴丝和纤维鞘的组装来实现的。Ift74基因敲除后精子纤维鞘蛋白AKAP4的表达模式发生了变化。在野生型小鼠中,AKAP4 (110 kDa)是主要形式,且pro-AKAP4 (84 kDa)表达水平明显低于AKAP4,pro-AKAP4通过IFT由胞质运输到纤维鞘装配位点,经加工后,AKAP4进入纤维鞘。然而在Ift74敲除小鼠中,pro-AKAP4成为主要存在形式[16]。Ift74缺失导致pro-AKAP4不能被输送到纤维鞘的组装位置,也没有被加工为AKAP4。
2.4 IFT81与IFT74共同调节IFT的稳定性
IFT81作为IFT74的结合伴侣,在小鼠睾丸中的表达阶段性增加,在出生后第8天首次检测到,第16天时蛋白质表达显著增加,此后精子发生期间IFT81表达水平保持稳定。与IFT74的分布不同,IFT81均匀分布在粗线期精母细胞和圆形精子细胞胞质,以及长形精子尾部。生殖细胞特异性Ift81敲除小鼠精子数量减少、活力降低且精子鞭毛短小畸形,无生育能力。进一步研究发现IFT81的缺失扰乱了精子发生进程,出现精子头部异常、尾部微管丢失、ODF和线粒体鞘排列紊乱等异常,阻碍了鞭毛的装配和延长[20]。精子数量的减少表明IFT81不仅参与鞭毛形成,对生殖细胞的存活同样不可或缺。与条件性敲除Ift74基因相似,其他几种IFT组分如IFT20、IFT25、IFT27、IFT57、IFT74和IFT88,在IFT81突变小鼠中表达下调,此外作为纤维鞘主要加工形式的AKAP4水平也显著降低[16,20]。小鼠Ift74和Ift81两种条件性敲除后表现出相似表型,表明这两种相互作用的蛋白彼此间不能代偿,IFT81与IFT74一起充当IFT-B复合体的核心成分,共同运输精子尾部发育所需的微管蛋白和纤维鞘前体参与精子发生,并调节其稳定性。
2.5 IFT88调控纤维鞘的组装
与其他组织相比,IFT88在小鼠睾丸组织中呈现高水平表达。组织学分析发现,其首先出现在II-III期粗线期精母细胞的头部和尾部,并在VIII期精母细胞中高表达,此后蛋白表达量随着精子细胞发育成熟逐渐降低。与IFT20相同,IFT88也是存在于高尔基体中的IFT-B顺行蛋白[21],协助顶体和中心体正确及时地靶向运输货物,调控精子尾部发育。Ift88突变小鼠产生的精子较野生型小鼠少350倍,几乎无运动活性,精子短或无鞭毛。短鞭毛极少有正常排列的轴丝,异位微管和ODF聚集在一起,并积聚组装失败的纤维鞘蛋白[22]。Ift88缺失导致鞭毛轴丝及附属成分装配出现异常,FS尤为明显,精子尾部FS由两个连接到轴丝双联微管的纵向柱状蛋白和围绕轴丝的横向蛋白组成,在Ift88缺陷的突变体中,纵向柱状蛋白形成失败,或者不能与轴丝上的双联微管连接。部分精子细胞中参与运输但未被组装的鞭毛前体聚集,引起鞭毛末端异常肿胀[23]。此外,鞭毛肿胀的末端含有大量堆积的FS前体蛋白,提示蛋白能有效运输到鞭毛末端,但未能成功组装到鞭毛轴丝。
IFT88蛋白不存在成熟精子细胞中。免疫荧光染色未能在附睾尾精子中检测IFT88的阳性信号,类似的分子还有IFT20、IFT57和IFT140等。在精子形成过程中,IFT需要替换在鞭毛末端发生转换的轴丝成分,并将转化产物移出鞭毛,同时还在鞭毛和胞体之间运送信号蛋白、受体蛋白等膜成分。哺乳动物成熟精子不同于发育中的精子细胞,它几乎没有细胞质,没有蛋白质的合成,所以成熟精子可能不需要IFT介导的蛋白质周转,但不排除由其他小分子而非IFT介导的蛋白质运输。
2.6 IFT20调控鞭毛形成并参与细胞自噬
IFT20是最小的IFT蛋白,在脑、肺、肾脏、肝、脾和睾丸等不同的组织中表达。小鼠出生后的第16天首次在睾丸组织中检测到IFT20蛋白,在第30天和第42天表达量显著增加,此时生殖细胞在第一波精子发生中进入凝结/伸长阶段。在生殖细胞中,IFT20分布于晚期精母细胞高尔基体、圆形精子细胞顶体以及长形精子细胞鞭毛[21]。在活细胞内,荧光标记的IFT20是高度动态的,在高尔基复合体和鞭毛之间沿着微管移动,将鞭毛膜蛋白从高尔基复合体运送到鞭毛。Ift20基因敲除可使胚胎致死,表明IFT20对小鼠发育是必需的[24]。生殖细胞特异性Ift20敲除小鼠中,6周龄突变雄性小鼠有37.5%不育,成年后均不育。在条件性敲除Ift20小鼠中,很少有生殖细胞完成精子发生,且附睾尾腔精子数量显著减少。突变小鼠精子细胞数量和活力明显降低,还有圆形肿胀的头部、短小扭曲的精子尾等多种异常形态。扫描电镜发现精子中胞质小泡增多、纤维样结构、线粒体异常堆积、成熟溶酶体减少,这表明在IFT20缺失情况下,用以鞭毛组装的成分不能顺利进入精子尾部,无法到达装配位点[25]。进一步研究发现在精子形成过程中,IFT20不仅通过IFT机制参与鞭毛形成,还参与调节自噬,它与自噬核心蛋白ATG16L相互作用,协助调节清除多余细胞质成分所需的正常自噬过程[26]。ODF2和SPAG16L (一种轴状中央装置蛋白)的表达量未受到Ift20缺失的影响,但这两种精子鞭毛蛋白最终却未能成功组装到精子尾部[25]。IFT20还可与GMAP210、CCDC41、SPATA1、SPEF2、BLOC-1、SPAG17等其他蛋白相互作用。IFT20分布在高尔基体,GMAP210蛋白作为膜受体将IFT20锚定在高尔基复合体,协助IFT20将鞭毛装配所必需的蛋白由高尔基复合体运输到精子尾部。GMAP210丢失将导致精子细胞顶体和线粒体鞘发育异常,IFT20蛋白表达和定位也将受到影响,但它不是鞭毛形成所必需的[21]。CCDC41是参与早期鞭毛形成的母中心粒成分,协助母中心粒募集来自高尔基复合体的IFT20,调控鞭毛装配所需蛋白质的分选和运输[27]。SPATA1定位于顶体,是精子头部形成的重要因素之一,作为IFT20的相互作用蛋白,为IFT20在顶体区域提供一个停靠位点[28]。此外,钙离子是衣藻和哺乳动物纤毛装配和拆卸的关键调节因子,钙浓度过高,将影响IFT逆向运输;钙离子缺失将导致IFT20在鞭毛末端堆积。钙介导的蛋白质磷酸化可以通过调节IFT亚基-驱动蛋白的相互作用,来调控IFT进出鞭毛和在鞭毛内的运输速率[29]。
2.7 IFT172通过IMT机制调控精子发生
IFT172是最大的IFT亚基,小鼠Ift172翻译两种主要蛋白:全长170 kDa的IFT172可以在睾丸和体细胞中表达,130 kDa的IFT172只存在于生殖细胞中。生殖细胞特异性IFT172是完成精子发生所必需的,分布在长形精子细胞的精子领中,含量丰富。小鼠出生后第12天首次检测到130 kDa的IFT172,此时生殖细胞开始进入减数分裂阶段。IFT172参与雄性生殖细胞的发育,虽然生殖细胞条件性敲除Ift172基因阻碍了正常精子发生,但仍有40%小鼠具有生育能力。附睾尾精子数显著减少,大多形态异常且活力下降[30]。IFT172仅分布于长形精子的精子领,可能通过调控IMT过程参与精子发生。精子领是精子形成中独特的必需结构,通过IMT机制在精子头部塑形和运输精子鞭毛发育所需蛋白方面发挥作用[31]。一些暂存在精子领中的货物蛋白通过IMT机制运输到作为成核位点的中心体后,通过IFT运输到发育中的精子鞭毛[32]。鉴于Ift172缺失的精子头部形态异常,精子领形态变长,货物蛋白ODF2和AKAP4在附睾精子中的信号显著减弱,中心体缺失或受到破坏,部分“9+2”轴丝结构紊乱甚至缺失,部分精子尾内出现空泡,据此推测IFT172是精子鞭毛附属结构的关键调节因子,直接或间接参与中心体的分裂与迁移,“9+2”核心轴丝结构缺陷和运动能力减弱可能是由附属结构破坏引起。此外,IFT25和IFT57在突变小鼠中表达显著下调,表明IFT172也能调节雄性生殖细胞中的其他IFT蛋白成分[30]。IFT172是一种膜相互作用蛋白,含有7个β-螺旋桨的WD结构域和α-螺线管的四肽重复序列(tetrapeptide repeat sequence, TPR),与COP具有相似的结构域结构,具有作为膜变形蛋白的潜力,能够将膜重塑为小囊泡,IFT172两种不同的构象可以被脂质调控,IFT57可以减弱IFT172与膜两者间的结合能力,表明IFT172在IFT中具有多种功能[33]。
3 IFT-A复合体调控精子鞭毛发生
IFT-A复合体由6个亚单位组成:IFT121、IFT122、IFT139/TTC21、IFT140、IFT43和IFT144/ WDR19。其中,IFT-122/140/144是IFT-A的核心亚复合体,IFT43/121/139是其外周亚复合体。相比于IFT-B,目前对IFT-A的了解相对较少,主要原因之一是后者的亚单位比大多数IFT-B亚单位大。其中IFT140、IFT144、IFT139在人类男性不育相关的病例中已有报道,下文介绍了这3种蛋白与雄性小鼠和人类男性精子发生的关系。3.1 IFT140通过介导其他IFT相关蛋白的运输参与鞭毛形成
人IFT140基因在睾丸、一些内分泌组织(例如垂体、甲状腺、肾上腺)和中枢神经组织(例如小脑、尾状、海马)中均有高水平的RNA表达,IFT140蛋白位于细胞纤毛基体和中心体。IFT140定位在染色体16p13.3,有40个外显子。IFT140蛋白由1462个氨基酸组成,包含5个WD重复卷曲螺旋序列和9个TPR结构域。据报道,IFT140突变和人类纤毛病有关,在锥虫(Try panosoma)和衣藻体内,Ift140缺陷会导致短鞭毛的形成[34,35]。通过对一名因严重少弱畸精子症而患有原发性不育症的患者进行高通量全外显子组测序,确定该患者编码IFT140蛋白的基因存在两个点突变:c.1837G>A和c.4247G>A,患者的哥哥是IFT140杂合子携带者(c.4247G>A),和配偶自然生育一个健康的孩子。这两种突变分别来自父亲和母亲,这也表明该家系的变异和表型的共分离符合常染色体隐性遗传,这是首例被报道的IFT140突变引起人类不育的病例。与健康成年男性相比,患者精子头部形态异常,顶体与细胞核分离,细胞核形态不规则,尾部短且局部膨大,精子尾部线粒体分布异常。IFT140蛋白分布在正常精子颈段和中段,在患者精子中完全缺失[36]。
生殖细胞特异性敲除Ift140小鼠附睾尾仅有极少精子,其中只有10%的畸形精子具有运动性,活力明显弱于正常精子[37]。突变小鼠精子头部形态异常,鞭毛短而肿胀,形状扭曲。凸起的鞭毛末端是逆行运输缺陷的典型表型[38],推测可能是Ift140缺陷而缺乏驱动逆行运输的IFT-马达动力蛋白,导致精子鞭毛组装的中间产物的堆积。透射电镜发现,Ift140突变小鼠的附睾精子出现了紊乱排列的轴丝微管、分布错误的ODF、FS和线粒体,被破坏的“9+2”微管结构和异常的染色质,睾丸精子也观察到和附睾精子相似的异常[37]。此外,Ift140敲除小鼠附睾内观察到多种未降解的残存细胞质成分,这表明IFT140可能与精子发生过程中的自噬过程有关。IFT140存在于正常精子细胞的精子领中,表明该蛋白可能参与IMT过程。人类不育患者和突变小鼠的精子细胞中IFT140丢失可能影响IMT过程,货物蛋白不能被输送到基底部进行IFT过程,因此精子发生被破坏[36]。
Ift140突变小鼠生精功能障碍、精子形态异常和精子活力缺陷,最终导致雄性不育,这些观察结果与人类不育患者的临床表现相一致。人类IFT140与小鼠Ift140具有同源性,突变表型基本相同,因而可以推测IFT140在哺乳动物精子发生过程中发挥着关键作用。
3.2 IFT144/WDR19突变引起精子鞭毛微管结构紊乱
WDR19编码一个由1342个氨基酸组成的IFT144蛋白,是IFT-A核心亚复合体的组成成分。该蛋白含有6个WD40重复序列、3个TPR重复序列、1个COG5290结构域和1个双锌带结构域(double zinc ribbon, DZR)。目前,有研究报道1例男性不育患者具有IFT144纯合错义突变:c.A3811G,光镜下患者精子呈短尾、卷尾或无鞭毛等异常形态,仅13.5%的精子形态正常,鞭毛运动性完全消失。电镜超微结构观察发现精子轴丝排列紊乱、“9+2”微管结构被破坏、存在空泡和残余胞质成分。免疫荧光显示在健康对照组中,IFT144沿着精子细胞颈部和鞭毛的轴丝呈点状分布,表达量非常丰富,然而在WDR19突变患者精子中,IFT144异常聚集在头部和颈部[39]。生物信息学分析表明,IFT140和IFT88与IFT144之间存在相互作用。在正常精子细胞中,IFT140分布于精子头部和鞭毛,IFT88位于精子领和鞭毛。WDR19突变患者中,IFT140异常聚集在精子头部和颈部,IFT88异常定位于精子颈。与IFT88相比,突变患者IFT140的表达水平和定位差异更为显著,这表明IFT144与IFT140之间存在更为紧密的相互作用。此外,SPAG6定位于精子鞭毛,是“9+2”轴丝中央装置的一个组成部分,对尾部结构完整性和鞭毛的运动具有重要作用[40],然而该成分在患者精子细胞中完全丢失[39]。因此,WDR19缺陷可能会导致其他精子成分异常表达,尤其是IFT成分,从而导致微管结构的破坏和紊乱。
以往研究发现,人类WDR19基因突变可导致多种涉及纤毛的组织和器官的病变,例如视网膜色素变性、肾结核、颅骨外胚层发育不良、窒息性胸廓发育不良和先天性肝内胆管囊性扩张症等。前文提及的WDR19纯合突变患者仅表现为由弱畸精子症引起的不育,与以往报道中由WDR19突变导致的多发性异常综合征相比,该患者精子鞭毛异常是一种较温和的遗传缺陷。结合WDR19上强等位基因和弱等位基因对轴丝结构的不同影响[41],推测可能因为疾病表型受基因变异类型和位置影响,该患者WDR19错义突变发生在C末端的DZR结构域,由于该结构域是WDR19的最后一个结构域,因此对蛋白质的结构和功能影响较小。其次,精子鞭毛的形成与体细胞纤毛的形成不完全一致[42]。
3.3 IFT139/TTC21A突变引起精子头尾连接异常
精子鞭毛多发性形态异常(multiple morphological abnormalities of the sperm flagella, MMAF)是一种遗传缺陷导致的畸形精子症。MMAF有很强的遗传异质性,迄今为止,仅60%MMAF的致病基因已知,仍存在许多与人类MMAF疾病相关的未知基因。为了研究MMAF的未知遗传因素,Liu等[43]对65名患有原发性不育症的汉族男性进行全外显子测序和生物信息学分析,结果发现有3例患者具有TTC21A双等位基因突变。第一例患者为TTC21A剪接供体位点纯合突变:c.716þ1G>A,该突变使内含子6中下游隐性剪接位点替代了正常的剪接供体位点,使翻译提前终止;第二例患者为TTC21A双等位基因突变:c.2329C>T,c.341A>G;第三例患者为TTC21A纯合错义突变:c.2563del。TTC21A上存在TPR结构域,这是一个由34个氨基酸组成的重复序列,通常串行排列,通过形成特殊空间结构,介导蛋白质相互作用。TPR结构域常存在于IFT蛋白中,对纤毛/鞭毛的功能十分重要,三例患者的突变位点都位于TPR保守位点区域[43]。TTC21A编码的IFT139蛋白特异性定位于前细线期精母细胞、粗线期精母细胞、圆形精子细胞和长形精子细胞。IFT139参与精子鞭毛正常组装,在鞭毛弯曲与搏动中发挥着重要作用。临床常规精液分析显示,TTC21A突变的男性患者精子运动能力弱,无进行性运动,但是精液量和精子浓度无明显差异。3例TTC21A突变患者几乎没有正常形态的精子,以头尾连接异常和鞭毛过短最为常见。透射电镜下,精子颈部和尾部存在残余细胞质成分和散乱无序的轴丝成分。动物实验显示,与野生型小鼠相比,Ttc21a基因移码突变的雄性小鼠mRNA相对表达水平显著降低了26%。野生型和杂合子Ttc21a突变小鼠没有明显的生殖表型差异,但78%的纯合突变雄性小鼠不能生育[43]。
精子的颈段由小头(capitulum)、节柱(segmented column)和近端中心粒组成,在核凝聚时小头与核后端相接触,起连接作用,节柱的前端包围近端中心粒,节柱末端与中段的ODF相连。中心粒是节柱和轴丝的组织中心,精子尾部的微管由此长出并延伸,从而使精子尾部延长。ODF围绕轴丝,与精子尾部的弹性回缩有关。线粒体成螺旋状包绕在ODF外形成中段结构,在线粒体鞘末端有一层致密的板状结构,称为环。对Ttc21a突变小鼠睾丸和附睾尾腔的精子形态学分析发现,圆形精子细胞在变形早期没有明显异常,但在核凝聚和后期伸长阶段,精子颈段形成或维持失败,颈部节柱和环分散,没有线粒体的包围和环的重新定位使中段形成失败,进一步导致头尾连接异常。除此之外,明显可见鞭毛内轴丝存在多种异常形态,例如外周微管排列紊乱、中央微管对丢失、动力蛋白臂丢失和异常肿胀,这些超微结构特征和光镜下观察到的精子头尾连接异常相吻合,从人类不育患者和小鼠模型的研究结果可以得出,IFT139基因缺陷可导致生精功能障碍从而引起不育[43]。
TTC21B是TTC21A的同源物,两者编码的氨基酸序列相似性约为50%,并且有11个相似的TPR结构域[44]。TTC21B在各种组织中普遍存在,其突变与各种纤毛病有关,例如局灶节段性肾小球硬化、Joubert综合征、Bardet-Bied1综合征、NPHP和ADT,但未见与男性不育相关的报道[45,46]。然而,TTC21A在睾丸组织中特异性高表达。TTC21B信号可以在生精小管细胞和睾丸间质细胞中被检测到,TTC21A特异性分布在前细线期精母细胞、粗线期精母细胞、圆形精子细胞和长形精子细胞中[43],TTC21A和TTC21B的差异表达更有力地说明TTC21A在男性精子发生中具有特异性功能。
4 结语与展望
本文综述了部分通过IFT机制调控精子发生的IFT蛋白,其基因敲除小鼠有许多共同的表型:精子数量与活力明显下降,精子细胞形态多种异常,超微结构排列紊乱甚至丢失,生育能力减弱甚至完全不育,表明IFT蛋白对雄性小鼠精子发生和生育能力至关重要,相似的表型提示它们在精子发生中以不同方式发挥着相似的作用。随着对精子发生基因调控机制的深入研究,遗传变异在男性不育病因诊断中的价值越来越受到重视,人类男性和小鼠雄性的IFT140(Ift40)、IFT144(Ift144)和IFT139(Ift139)相似的突变表型表明,小鼠模型为研究IFT在人类男性生殖中的作用,以及为男性不育的基因诊断和靶向治疗提供了实验基础和理论依据。IFT复合体由22个亚基组成,本文对其中10个成员在哺乳动物精子发生中的作用进行了介绍,其余12个IFT成员调控机制还有待进一步研究。自IFT被发现以来,人们对其进行的大量研究丰富了对IFT的认知,但对IFT复合体的整体结构和各个亚单位的具体功能仍然所知甚少。IFT仅是一个用以运输蛋白质的传送带,还是更加复杂的系统?在精子发生阶段,IFT蛋白间通过何种方式相互结合发挥作用,具有相似功能的IFT蛋白之间能否代偿,IFT蛋白靶向运输货物的机制等尚待进一步研究。
(责任编委: 刘默芳)
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1007/978-1-60761-376-3_11PMID:19768432 [本文引用: 2]

The growth and maintenance of almost all cilia and flagella are dependent on the proper functioning of the process of intraflagellar transport (IFT). This includes the primary cilia of most cells in humans that are in interphase or the G(o) phase of the cell cycle. The model system for the study of IFT is the flagella of the bi-flagellate green alga Chlamydomonas. It is in this organism that IFT was first discovered, and genetic data from a Chlamydomonas mutant first linked the process of IFT to polycystic kidney disease in humans. The information given in this chapter addresses procedures to purify IFT particles from flagella and localize these particles, and their associated motor proteins, in flagella using light and electron microscopic approaches.
DOI:10.1016/j.diff.2011.11.001URL [本文引用: 1]
DOI:10.1016/j.mce.2020.110987PMID:32810575 [本文引用: 1]

Centrioles are eukaryotic subcellular structures that produce and regulate massive cytoskeleton superstructures. They form centrosomes and cilia, regulate new centriole formation, anchor cilia to the cell, and regulate cilia function. These basic centriolar functions are executed in sperm cells during their amplification from spermatogonial stem cells during their differentiation to spermatozoa, and finally, after fertilization, when the sperm fuses with the egg. However, sperm centrioles exhibit many unique characteristics not commonly observed in other cell types, including structural remodeling, centriole-flagellum transition zone migration, and cell membrane association during meiosis. Here, we discuss five roles of sperm centrioles: orchestrating early spermatogenic cell divisions, forming the spermatozoon flagella, linking the spermatozoon head and tail, controlling sperm tail beating, and organizing the cytoskeleton of the zygote post-fertilization. We present the historic discovery of the centriole as a sperm factor that initiates embryogenesis, and recent genetic studies in humans and other mammals evaluating the current evidence for the five functions of sperm centrioles. We also examine information connecting the various sperm centriole functions to distinct clinical phenotypes. The emerging picture is that centrioles are essential sperm components with remarkable functional diversity and specialization that will require extensive and in-depth future studies.Copyright © 2020 Elsevier B.V. All rights reserved.
DOI:S0968-0004(15)00176-0PMID:26498262 [本文引用: 1]

The motile and sensory functions of cilia and flagella are indispensable for human health. Cilia assembly requires a dedicated protein shuttle, intraflagellar transport (IFT), a bidirectional motility of multi-megadalton protein arrays along ciliary microtubules. IFT functions as a protein carrier delivering hundreds of distinct proteins into growing cilia. IFT-based protein import and export continue in fully grown cilia and are required for ciliary maintenance and sensing. Large ciliary building blocks might depend on IFT to move through the transition zone, which functions as a ciliary gate. Smaller, freely diffusing proteins, such as tubulin, depend on IFT to be concentrated or removed from cilia. As I discuss here, recent work provides insights into how IFT interacts with its cargoes and how the transport is regulated. Copyright © 2015 Elsevier Ltd. All rights reserved.
DOI:10.1101/cshperspect.a028092URL [本文引用: 1]
DOI:10.1093/biolre/iox029URL [本文引用: 3]
DOI:10.1016/j.devcel.2012.04.009URL [本文引用: 1]
DOI:10.1002/jemt.10320URL [本文引用: 1]
DOI:10.1007/s10495-006-0486-xURL [本文引用: 1]
DOI:10.1016/j.jmb.2016.08.022PMID:27575334 [本文引用: 1]

Since its initial formalization nearly 20 years ago, the concept of lipid rafts has generated a tremendous amount of attention and interest and nearly as much controversy. The controversy is perhaps surprising because the notion itself is intuitive: compartmentalization in time and space is a ubiquitous theme at all scales of biology, and therefore, the partitioning of cellular membranes into lateral subdivision should be expected. Nevertheless, the physicochemical principles responsible for compartmentalization and the molecular mechanisms by which they are functionalized remain nearly as mysterious today as they were two decades ago. Herein, we review recent literature on this topic with a specific focus on the major open questions in the field including: (1) what are the best tools to assay raft behavior in living membranes? (2) what is the function of the complex lipidome of mammalian cells with respect to membrane organization? (3) what are the mechanisms that drive raft formation and determine their properties? (4) how can rafts be modulated? (5) how is membrane compartmentalization integrated into cellular signaling? Despite decades of intensive research, this compelling field remains full of fundamental questions.Copyright © 2016 Elsevier Ltd. All rights reserved.
DOI:10.1002/mrd.21382URL [本文引用: 1]
PMID:15955805 [本文引用: 3]

Required for the assembly and maintenance of eukaryotic cilia and flagella, intraflagellar transport (IFT) consists of the bidirectional movement of large protein particles between the base and the distal tip of the organelle. Anterograde movement of particles away from the cell body is mediated by kinesin-2, whereas retrograde movement away from the flagellar tip is powered by cytoplasmic dynein 1b/2. IFT particles contain multiple copies of two distinct protein complexes, A and B, which contain at least 6 and 11 protein subunits, respectively. In this study, we have used increased ionic strength to remove four peripheral subunits from the IFT complex B of Chlamydomonas reinhardtii, revealing a 500-kDa core that contains IFT88, IFT81, IFT74/72, IFT52, IFT46, and IFT27. This result demonstrates that the complex B subunits, IFT172, IFT80, IFT57, and IFT20 are not required for the core subunits to stay associated. Chemical cross-linking of the complex B core resulted in multiple IFT81-74/72 products. Yeast-based two-hybrid and three-hybrid analyses were then used to show that IFT81 and IFT74/72 directly interact to form a higher order oligomer consistent with a tetrameric complex. Similar analysis of the vertebrate IFT81 and IFT74/72 homologues revealed that this interaction has been evolutionarily conserved. We hypothesize that these proteins form a tetrameric complex, (IFT81)2(IFT74/72)2, which serves as a scaffold for the formation of the intact IFT complex B.
DOI:S0012-1606(17)30463-3PMID:28964737 [本文引用: 2]

Intraflagellar transport (IFT) is an evolutionarily conserved mechanism essential for the assembly and maintenance of most eukaryotic cilia and flagella. In mice, mutations in IFT proteins have been shown to cause several ciliopathies including retinal degeneration, polycystic kidney disease, and hearing loss. However, little is known about its role in the formation of the sperm tail, which has the longest flagella of mammalian cells. IFT27 is a component of IFT-B complex and binds to IFT25 directly. In mice, IFT27 is highly expressed in the testis. To investigate the role of IFT27 in male germ cells, the floxed Ift27 mice were bred with Stra8-iCre mice so that the Ift27 gene was disrupted in spermatocytes/spermatids. The Ift27: Stra8-iCre mutant mice did not show any gross abnormalities, and all of the mutant mice survived to adulthood. There was no difference between testis weight/body weight between controls and mutant mice. All adult homozygous mutant males examined were completely infertile. Histological examination of the testes revealed abnormally developed germ cells during the spermiogenesis phase. The epididymides contained round bodies of cytoplasm. Sperm number was significantly reduced compared to the controls and only about 2% of them remained significantly reduced motility. Examination of epididymal sperm by light microscopy and SEM revealed multiple morphological abnormalities including round heads, short and bent tails, abnormal thickness of sperm tails in some areas, and swollen tail tips in some sperm. TEM examination of epididymal sperm showed that most sperm lost the "9+2″ axoneme structure, and the mitochondria sheath, fibrous sheath, and outer dense fibers were also disorganized. Some sperm flagella also lost cell membrane. Levels of IFT25 and IFT81 were significantly reduced in the testis of the conditional Ift27 knockout mice, and levels of IFT20, IFT74, and IFT140 were not changed. Sperm lipid rafts, which were disrupted in the conditional Ift25 knockout mice, appeared to be normal in the conditional Ift27 knockout mice. Our findings suggest that like IFT25, IFT27, even though not required for ciliogenesis in somatic cells, is essential for sperm flagella formation, sperm function, and male fertility in mice. IFT25 and IFT27 control sperm formation/function through many common mechanisms, but IFT25 has additional roles beyond IFT27.Published by Elsevier Inc.
DOI:10.1016/j.devcel.2017.05.016 [本文引用: 1]

Highly conserved intraflagellar transport (IFT) protein complexes direct both the assembly of primary cilia and the trafficking of signaling molecules. IFT complexes initially accumulate at the base of the cilium and periodically enter the cilium, suggesting an as-yet-unidentified mechanism that triggers ciliary entry of IFT complexes. Using affinity-purification and mass spectrometry of interactors of the centrosomal and ciliopathy protein, CEP19, we identify CEP350, FOP, and the RABL2B GTPase as proteins organizing the first known mechanism directing ciliary entry of IFT complexes. We discover that CEP19 is recruited to the ciliary base by the centriolar CEP350/ FOP complex and then specifically captures GTP-bound RABL2B, which is activated via its intrinsic nucleotide exchange. Activated RABL2B then captures and releases its single effector, the intraflagellar transport B holocomplex, from the large pool of pre-docked IFT-B complexes, and thus initiates ciliary entry of IFT.
DOI:10.1091/mbc.E17-01-0017PMID:28428259 [本文引用: 1]

Proteins localized to the basal body and the centrosome play crucial roles in ciliary assembly and function. Although RABL2 and CEP19 are conserved in ciliated organisms and have been implicated in ciliary/flagellar functions, their roles are poorly understood. Here we show that RABL2 interacts with CEP19 and is recruited to the mother centriole and basal body in a CEP19-dependent manner and that CEP19 is recruited to the centriole probably via its binding to the centrosomal protein FGFR1OP. Disruption of the gene in results in the nonflagellated phenotype, suggesting a crucial role of RABL2 in ciliary/flagellar assembly. We also show that RABL2 interacts, in its GTP-bound state, with the intraflagellar transport (IFT)-B complex via the IFT74-IFT81 heterodimer and that the interaction is disrupted by a mutation found in male infertile mice ( mice) with a sperm flagella motility defect. Intriguingly, RABL2 binds to CEP19 and the IFT74-IFT81 heterodimer in a mutually exclusive manner. Furthermore, exogenous expression of the GDP-locked or -type RABL2 mutant in human cells results in mild defects in ciliary assembly. These results indicate that RABL2 localized to the basal body plays crucial roles in ciliary/flagellar assembly via its interaction with the IFT-B complex.© 2017 Nishijima, Hagiya, et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
DOI:10.1093/biolre/ioz071URL [本文引用: 3]
DOI:10.1074/jbc.M110.106997PMID:20435895 [本文引用: 1]

Intraflagellar transport (IFT) particles of Chlamydomonas reinhardtii contain two distinct protein complexes, A and B, composed of at least 6 and 15 protein subunits, respectively. As isolated from C. reinhardtii flagella, IFT complex B can be further reduced to a approximately 500-kDa core that contains IFT88, 2x IFT81, 2x IFT74/72, IFT52, IFT46, IFT27, IFT25, and IFT22. In this study, yeast-based two-hybrid analysis was combined with bacterial coexpression to show that three of the core B subunits, IFT88, IFT52, and IFT46, interact directly with each other and, together, are capable of forming a ternary complex. Chemical cross-linking results support the IFT52-IFT88 interaction and provide additional evidence of an association between IFT27 and IFT81. With previous studies showing that IFT81 and IFT74/72 interact to form a (IFT81)(2)(IFT74/72)(2) heterotetramer and that IFT27 and IFT25 form a heterodimer, the architecture of complex B is revealing itself. Last, electroporation of recombinant IFT46 was used to rescue flagellar assembly of a newly identified ift46 mutant and to monitor in vivo localization and movement of the IFT particles.
DOI:10.1371/journal.pone.0005384URL [本文引用: 1]
DOI:10.1016/j.cub.2015.04.060URL [本文引用: 1]
DOI:10.1152/ajpcell.00450.2019URL [本文引用: 2]
DOI:10.1152/ajpcell.00517.2018URL [本文引用: 3]
DOI:10.1002/dvdy.22563URL [本文引用: 1]
DOI:10.1091/mbc.E15-08-0578URL [本文引用: 1]
DOI:10.1091/mbc.e10-09-0792URL [本文引用: 1]
DOI:10.1091/mbc.e16-05-0318URL [本文引用: 2]
DOI:10.1038/nature12639URL [本文引用: 1]
DOI:10.1073/pnas.1220927110URL [本文引用: 1]
DOI:10.1002/dvdy.v249.4URL [本文引用: 1]
DOI:10.1016/j.devcel.2014.07.019URL [本文引用: 1]
[本文引用: 2]
[本文引用: 1]
DOI:10.1530/REP-15-0310URL [本文引用: 1]
DOI:10.3389/fcell.2019.00352URL [本文引用: 1]
DOI:10.1091/mbc.e07-08-0749URL [本文引用: 1]
DOI:10.1371/journal.pgen.1006627URL [本文引用: 1]
[本文引用: 2]
DOI:10.1002/cm.21427PMID:29236364 [本文引用: 2]

Intraflagellar transport (IFT) is a conserved mechanism essential for the assembly and maintenance of most eukaryotic cilia and flagella. However, little is known about its role in sperm flagella formation and male fertility. IFT140 is a component of IFT-A complex. In mouse, it is highly expressed in the testis. Ift140 gene was inactivated specifically in mouse spermatocytes/spermatids. The mutant mice did not show any gross abnormalities, but all were infertile and associated with significantly reduced sperm number and motility. Multiple sperm morphological abnormalities were discovered, including amorphous heads, short/bent flagella and swollen tail tips, as well as vesicles along the flagella due to spermiogenesis defects. The epididymides contained round bodies of cytoplasm derived from the sloughing of the cytoplasmic lobes and residual bodies. Knockout of Ift140 did not significantly affect testicular expression levels of selective IFT components but localization of IFT27 and IFT88, two components of IFT-B complex, was changed. Our findings demonstrate that IFT140 is a key regulator for male fertility and normal spermiogenesis in mice. It not only plays a role in sperm flagella assembling, but is also involved in critical assembly of proteins that interface between the germ cell plasma and the Sertoli cell.© 2017 Wiley Periodicals, Inc.
DOI:10.1091/mbc.e16-11-0813URL [本文引用: 1]
DOI:10.1007/s10815-020-01770-1URL [本文引用: 2]
DOI:10.1038/srep16506URL [本文引用: 1]
DOI:10.1083/jcb.201110049URL [本文引用: 1]
DOI:10.1073/pnas.0402354101URL [本文引用: 1]
DOI:10.1016/j.ajhg.2019.02.020URL [本文引用: 5]
DOI:10.1038/ng.105URL [本文引用: 1]
DOI:10.1681/ASN.2013101126URL [本文引用: 1]
DOI:10.1038/ng.756URL [本文引用: 1]
