 ,1, 刘自广2, 张楠1, 严容1, 张楠1, 何鑫淼2, 王文涛2, 刘娣2, 吴娟
,1, 刘自广2, 张楠1, 严容1, 张楠1, 何鑫淼2, 王文涛2, 刘娣2, 吴娟 ,1
,1Stress response of ABI5 to BR stress and its regulation on hypocotyls growth in Arabidopsis thaliana
Jianhui Lin ,1, Ziguang Liu2, Nan Zhang1, Rong Yan1, Nan Zhang1, Xinmiao He2, Wentao Wang2, Di Liu2, Juan Wu
,1, Ziguang Liu2, Nan Zhang1, Rong Yan1, Nan Zhang1, Xinmiao He2, Wentao Wang2, Di Liu2, Juan Wu ,1
,1通讯作者: 吴娟,博士,硕士生导师,研究方向:植物生理学和RNA分子生物学。E-mail:wuj1970@163.com
编委: 储成才
收稿日期:2021-06-7修回日期:2021-08-16
| 基金资助: |
Received:2021-06-7Revised:2021-08-16
| Fund supported: |
作者简介 About authors
林建辉,在读硕士研究生,专业方向:生物化学与分子生物学。E-mail:

摘要
植物脱落酸不敏感蛋白5 (abscisic acid-insensitive 5, ABI5)是种子中大量表达的碱性亮氨酸拉链类型(basic leucine zipper, bZIP)转录因子,在调节种子萌发和幼苗早期生长的脱落酸(abscisic acid, ABA)信号中起着核心作用。油菜素内酯(brassinosteroid, BR)是一种新型植物内源激素,具有调节植物生长发育和逆境胁迫响应等诸多生理功能。近期研究发现,油菜素内酯胁迫条件下,BR信号通路中BIN2 (BRASSINOSTEROID INSENSITIVE2)和BES1 (BRI1-EMS-SUPPRESSOR 1)通过抑制ABI5表达,促进拟南芥(Arabidopsis thaliana)种子萌发。为进一步探究BR胁迫下ABI5功能,本研究分析了种子萌发期ABI5表达特性,鉴定出拟南芥ABI5基因缺失突变体abi5-1并对BR胁迫下其功能进行解析。结果表明:ABI5在拟南芥干种子中大量表达并响应萌发期BR胁迫;正常条件下,abi5-1与野生型幼苗下胚轴无明显差异;BR胁迫下,abi5-1幼苗下胚轴明显长于野生型。本研究结果揭示了ABI5调控BR胁迫下拟南芥下胚轴生长,为深入了解ABI5调节植物发育的分子机制提供了依据。
关键词:
Abstract
Abscisic acid-insensitive 5 (ABI5) is a basic leucine zipper (bZIP) transcription factor that is abundantly expressed in seeds. It plays a central role in regulating the abscisic acid (ABA) signal of seed germination and early seedling growth. Brassinosteroid (BR) is a new type of plant endogenous hormone, which has many physiological functions such as regulating plant growth and development and response to adversity stress. It has recently been discovered that under brassinolide stress, BIN2 (BRASSINOSTEROID INSENSITIVE2) and BES1 (BRI1-EMS-SUPPRESSOR 1) in the BR signaling pathway can inhibit the expression of ABI5 and promote Arabidopsis thaliana seed germination. In order to further explore the function of ABI5 under BR stress, this study analyzed the ABI5 expression characteristics during seed germination, identified Arabidopsis ABI5 gene deletion mutant abi5-1 and analyzed its function under BR stress, the results of which indicated that ABI5 was abundantly expressed in Arabidopsis dry seeds and responded to BR stress during germination. Under normal conditions, there was no significant difference between the hypocotyls of abi5-1 and wild-type seedlings; but under BR stress, the hypocotyls of abi5-1 seedlings were significantly longer than those of wild-type seedlings. These results reveal that ABI5 regulates the growth of Arabidopsis hypocotyls under BR stress, thereby providing a basis for in-depth understanding of the molecular mechanism of ABI5 regulation on plant development.
Keywords:
PDF (652KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
林建辉, 刘自广, 张楠, 严容, 张楠, 何鑫淼, 王文涛, 刘娣, 吴娟. 拟南芥ABI5基因对BR胁迫响应及其对下胚轴生长的调节作用. 遗传[J], 2021, 43(9): 901-909 doi:10.16288/j.yczz.21-199
Jianhui Lin.
植物脱落酸不敏感蛋白5 (abscisic acid-insensitive 5,ABI5)是成熟种子和花朵中大量表达的一类bZIP转录因子[1],干旱、高盐等胁迫环境和脱落酸(abscisic acid, ABA)条件诱导ABI5表达[2],多种转录因子和酶通过调节ABI5表达影响了拟南芥(Arabidopsis thaliana)种子萌发和幼苗早期发育[3]。MYBs是非生物胁迫相关转录因子,MYB7通过抑制ABI5表达,促进ABA介导的盐和渗透胁迫下种子萌发[4]。非生物胁迫条件下植物体内ABA含量升高,转录因子HY5 (ELONGATED HYPOCOTYL 5)激活ABI5表达并与其互作促进ABI5下游ABA应答基因表达,改变了拟南芥萌发种子ABA敏感性[5]。拟南芥核因子Y家族蛋白(NUCLEAR FACTOR Y FAMILY PROTEIN)NF-YC9直接与ABI5结合,进而激活靶基因EM6 (EARLY METHIONINE-LABELED 6)表达,参与种子萌发对ABA响应[6]。此外,ABI5也参与了生长素(auxin, IAA)、赤霉素(gibberellin, GA)、细胞分裂素(cytokinin, CTK)和油菜素内酯(brassinosteroid, BR)等信号传导和代谢途径并发挥重要作用。PIN1编码植物IAA转运蛋白,ABI5通过抑制PIN1积累引起根中IAA水平降低,进而参与蔗糖介导的初生根生长抑制过程 [7]。CTK通过AHP2、AHP3、AHP5和AHK4等基因促进ABI5的26S蛋白酶体降解,从而拮抗ABA介导的种子萌发后生长和子叶绿化的抑制作用[8]。
BR是植物特有的甾醇类激素,在种子萌发、光形态建成、衰老[9]以及环境胁迫响应等生理过程中发挥重要作用[10]。BR信号激酶BSK5 (BRASSINOSTEROID-SIGNALING KINASE 5)显著抑制ABA合成基因ABA3和NCED3表达,增强盐胁迫和ABA介导的干旱胁迫耐受性[11]。BR激活的转录因子BES1和BZR1 (BRASSINAZOLE-RESISTANT 1)直接与AGL15 (AGAMOUS-LIKE15)启动子结合,抑制其表达,进而抑制种子成熟[12]。光照条件下,BIN2通过磷酸化GLK1 (GOLDEN2-LIKE 1)整合了BR和光信号,抑制GLK1泛素化降解,使幼苗叶绿体正常发育[13]。此外,GA和BR诱导的HBI1和BEE2基因通过调节GASA6 (GA-STIMULATED ARABIDOPSIS 6)基因表达促进了胚乳破裂和种子萌发[14]。近期研究发现,拟南芥BR信号转录因子BIN2和BES1通过抑制ABA信号核心成员ABI5表达从而促进种子萌发[15,16],表明ABI5可以整合ABA与BR信号的拮抗作用并促进种子萌发,这为不同信号协作调控种子萌发过程的复杂机制提供了新见解。
为了进一步探究BR胁迫下ABI5功能,本研究分析了种子萌发期ABI5表达特性,鉴定了拟南芥ABI5基因缺失突变体abi5-1,对BR胁迫下abi5-1表型进行观察并对BR信号通路相关基因表达进行了分析。本研究为深入探究BR胁迫下ABI5调控植物生长发育的作用机制提供了依据。
1 材料与方法
1.1 实验材料及培养条件
野生型拟南芥由东北林业大学生命科学学院东北盐碱植被恢复与重建教育部重点实验室提供,拟南芥abi5-1突变体(CS_8105,该突变体为单碱基突变,缺失了DNA结合的碱性亮氨酸拉链结构域,不能发挥正常功能)购自ABRC (Arabidopsis Biological Resource Center)。24-表油菜素内酯(EBR)购自上海源叶生物科技有限公司,用乙醇配成50 mg/L母液,-20℃保存。pMDTM 18-T Vector Cloning Kit购自日本TaKaRa公司;大肠杆菌克隆表达菌株Top 10感受态细胞购自哈尔滨海基生物科技有限公司。拟南芥种子经0.1% (V/V)次氯酸钠和75% (V/V)乙醇消毒后用无菌水反复冲洗4~6次,播种在1/2MS固体培养基(2.37 g/L MS粉、3%蔗糖、0.8%琼脂、pH5.8)和含有5 µmol/L BR的1/2MS固体培养基上,黑暗中4℃春化3 d后,于培养箱中22℃、16 h明/8 h暗进行培养。
1.2 RNA提取及cDNA合成
使用Trizol试剂(美国Invitrogen公司)从0.1 g拟南芥干种子、吸涨阶段(1 d、2 d、3 d)、萌发阶段(1 d、2 d、3 d)和7 d幼苗中提取总RNA。使用反转录试剂盒(PrimerScriptRT Reagent Kit with gDNA Eraser,日本TaKaRa公司)进行反转录,2 μg总RNA合成第一链cDNA。1.3 突变体筛选与鉴定
1.3.1 植物基因组DNA提取选取3~4片嫩叶于液氮中研磨后,加入750 µL DNA提取液(10 mmol/L EDTA、50 mmol/LTris-HCl、100 mmol/L NaCl、10 mmol/L β-巯基乙醇、1% (W/V) SDS),65℃水浴10 min;加入150 µL 5 mol/L乙酸钾,冰浴20 min,4℃、13,000 r/min离心10 min,取上清;加入等体积异丙醇,4℃、13,000 r/min离心2 min;弃上清,向沉淀中加入75%(V/V)乙醇800 µL,4℃、13,000 r/min离心2 min;向沉淀物中加入200 µL灭菌水,离心取上清约180 µL;加入20 µL 3 mol/L乙酸钾和500 µL无水乙醇,-20℃静止30 min,离心得沉淀;向沉淀中加入75% (V/V)乙醇800 µL,离心得沉淀;向沉淀中加入20 µL灭菌水充分溶解,-20℃保存。
1.3.2 纯合突变体筛选及单碱基突变位点鉴定
拟南芥abi5-1突变体种子播种于含卡那霉素(50 μg/mL)的1/2MS培养基上培养10 d,将正常生长幼苗移栽到土中继续培养3周后,提取叶片基因组DNA为模板,利用上下游引物(表1)扩增ABI5基因。经电泳分离及胶回收后连接到pMD18-T克隆载体,转化大肠杆菌top10感受态细胞,挑取单克隆,经鉴定后由吉林省库美生物科技有限公司测序并进行序列分析。
1.3.3 纯合突变体RNA水平鉴定
使用Trizol试剂(美国Invitrogen公司)从7 d幼苗中提取总RNA,用反转录试剂盒(PrimerScriptRT Reagent Kit with gDNA Eraser,日本TaKaRa公司)合成cDNA。以合成的cDNA为模板,用特异引物qRT-PCR扩增突变体相应的ABI5基因,以Actin2 (At3g18780)为内参(表1)。使用Stratagene Mx3000P荧光定量PCR仪(美国安捷伦科技有限公司)进行qRT-PCR,根据样品特有Ct值,采用2-ΔΔCt法计算ABI5基因在突变体中的相对表达量。
1.4 实时荧光定量PCR
使用Stratagene Mx3000P荧光定量PCR仪(美国安捷伦科技有限公司)进行qRT-PCR扩增。以合成好的cDNA为模板,用ABI5、BIN2、BES1、BZR1、PIN7、EXP3的定量特异性引物进行PCR扩增,以Actin2 (At3g18780)为内参(表1)。qRT-PCR扩增体系包括:2×UltraSYBR Mixture (Low ROX) 10 μL,正向引物和反向引物(20 μmol/L)各0.5 μL,cDNA模板(约50 ng/μL) 1 μL,ddH2O 8 μL。采用3步法反应程序进行扩增:95℃ 15 min;95℃ 10 s,60℃ 30 s,72℃ 30 s,40个循环。根据各样品特有Ct值,采用2-ΔΔCt法计算目的基因在不同处理条件下的相对表达量。1.5 植株表型观测及统计分析
随机选取30株正常生长和5 µmol/L BR胁迫处理7 d的野生型和abi5-1拟南芥植株,使用实体显微镜(SZX9,日本OLYMPUS公司)观测并记录下胚轴长度。每组实验重复3次,通过t检验确定统计学上的显著性差异,其中*P<0.05、**P<0.01和***P<0.001为显著性阈值。1.6 BR胁迫下野生型和abi5-1中BR信号通路相关基因表达分析
将1/2MS培养基上萌发4 d的野生型和abi5-1幼苗移入1/2MS培养基和含有5 µmol/L BR的1/2MS培养基中分别培养24 h后,提取总RNA。使用Trizol试剂(美国Invitrogen公司)从7 d幼苗中提取总RNA,用反转录试剂盒(PrimerScriptRT Reagent Kit with gDNA Eraser,日本TaKaRa公司)合成cDNA。以合成的cDNA为模板,用BIN2、BES1、BZR1、PIN7、EXP3的定量特异性引物进行PCR扩增,以Actin2 (At3g18780)为内参(表1)。使用Stratagene Mx3000P荧光定量PCR仪(美国安捷伦科技有限公司)进行qRT-PCR,根据样品特有Ct值,采用2-ΔΔCt法计算目的基因在不同处理条件下的相对表达量。Table 1
表1
表1本研究所用引物信息
Table 1
| 引物名称 | 引物序列(5ʹ→3ʹ) | 复性温度(℃) | 用途 |
|---|---|---|---|
| Actin2-F | GGTAACATTGTGCTCAGTGGTGG | 58 | qRT-PCR |
| Actin2-R | AACGACCTTAATCTTCATGCTGC | ||
| abi5-1-F | CGAGGGTGGTGTTGGTGTCTTTA | 60 | 突变体鉴定 |
| abi5-1-R | CTACTCCATACTGACCTCCTA | ||
| ABI5-F(q) | AACCTAATCCAACCCGAACC | 60 | qRT-PCR |
| ABI5-R(q) | ACCCTCCTCCTCCTGTCC | ||
| BIN2-F(q) | ACAAAAGGATGCCCCCAGAA | 59 | qRT-PCR |
| BIN2-R(q) | TGAAGTTGAAGAGAGGCGGG | ||
| BES1-F(q) | GCAATTGTCTCCAAACACAGCAG | 61 | qRT-PCR |
| BES1-R(q) | CTCCAATCCTTCCTTCCGACATG | ||
| BZR1-F(q) | GCAGATGTCTCCAAATACTGCTG | 61 | qRT-PCR |
| BZR1-R(q) | GACATGCCATTTGGGTTTGCCTAG | ||
| PIN7-F(q) | GTGGGATGTGGCAATGCCTAA | 60 | qRT-PCR |
| PIN7-R(q) | TCCAATAGCCATTGCTGCCAC | ||
| EXP3-F(q) | GGAACTTGTACAGCCAAGGATA | 57 | qRT-PCR |
| EXP3-R(q) | AATAGATGGATTTCCCGGAACA |
新窗口打开|下载CSV
2 结果与分析
2.1 拟南芥生长发育过程中ABI5 mRNA表达特性分析
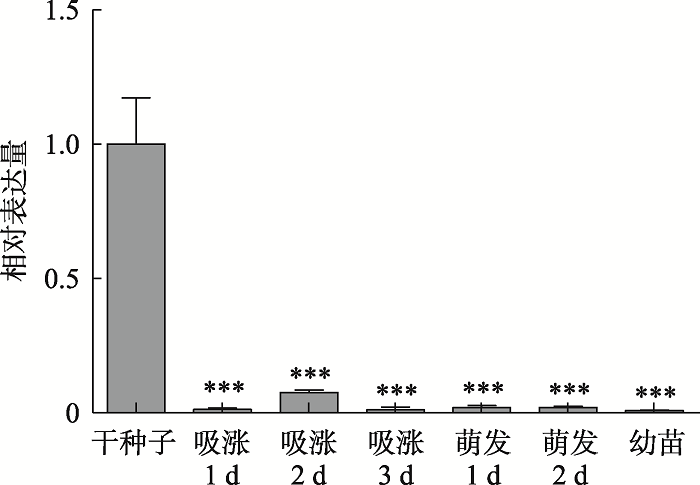
为研究拟南芥生长发育过程中ABI5 mRNA表达特性,分别提取干种子、吸涨阶段(1 d、2 d、3 d)、萌发阶段(1 d、2 d)和7 d幼苗总RNA,使用ABI5特异性引物进行qRT-PCR分析。结果表明,ABI5 mRNA大量积累于干种子中,此后随着萌发进行积累量逐渐降低(图1)。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1拟南芥生长发育过程中ABI5 mRNA表达特性分析
提取不同生长阶段野生型拟南芥总RNA,反转录成cDNA,以Actin2为内参,使用ABI5 mRNA特异引物进行qRT-PCR分析。数值为3次独立实验平均值,误差为标准误差;***P<0.001。
Fig. 1Expression analysis of ABI5 mRNA in the development of Arabidopsis
2.2 BR胁迫条件下,拟南芥幼苗中ABI5 mRNA表达特性分析
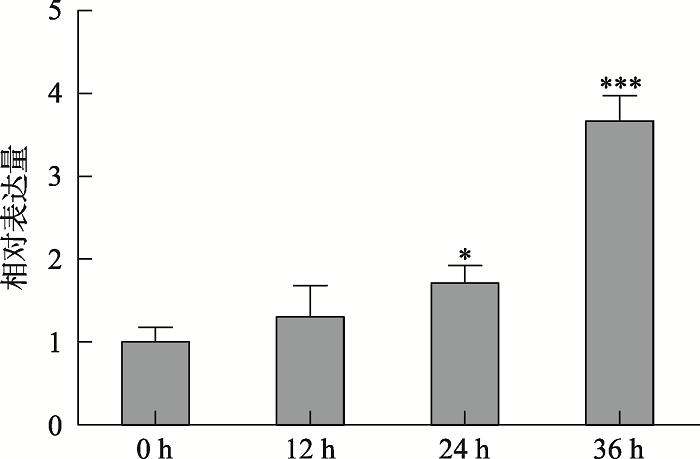
为了探究BR胁迫条件下ABI5 mRNA表达特性,将1/2MS培养基上萌发4 d的野生型拟南芥幼苗移到含有5 µmol/L BR的1/2MS培养基上分别培养0 h、12 h、24 h、36 h后,提取总RNA进行qRT-PCR分析。结果表明,5 µmol/L BR胁迫处理下,随着胁迫时间增加,拟南芥幼苗中ABI5 mRNA开始积累,并在36 h时达到最高值(图2)。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2BR胁迫条件下拟南芥萌发早期幼苗中ABI5 mRNA表达特性分析
提取BR胁迫处理0 h、12 h、24 h和36 h萌发4 d的野生型拟南芥总RNA,反转录成cDNA,以Actin2为内参,使用ABI5特异引物进行qRT-PCR分析。数值为3次独立实验平均值,误差为标准误差;*P<0.05,***P<0.001。
Fig. 2Expression analysis of ABI5 mRNA in Arabidopsis germination early seedlings under BR stress
2.3 ABI5缺失突变体鉴定
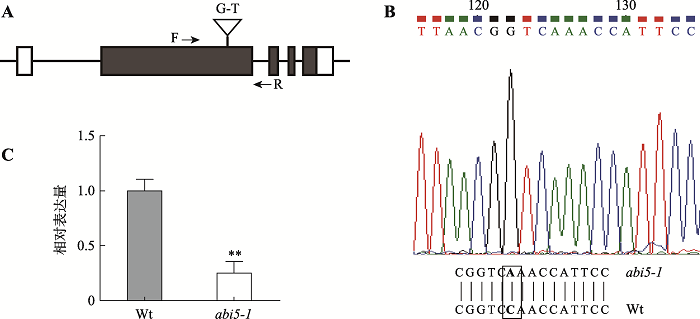
提取生长3周abi5-1突变体叶片基因组DNA,通过DNA测序和Blast序列比对,结果表明abi5-1的编码序列中发生了单碱基变化:G→T (图3:A,B),可用于后续功能分析。同时提取正常生长7 d的abi5-1幼苗总RNA,使用ABI5特异性引物,对突变体中ABI5表达量进行了qRT-PCR分析,结果表明abi5-1突变体中ABI5表达水平显著降低(图3C)。2.4 BR胁迫条件下ABI5缺失对拟南芥幼苗下胚轴生长的影响
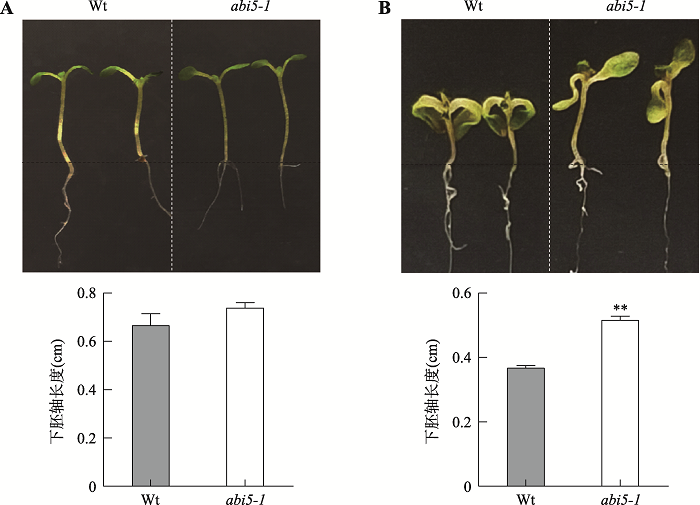
为了探究BR胁迫下ABI5功能,将野生型拟南芥(Wt)和abi5-1种子播种在含有0 µmol/L和5 µmol/L BR的1/2MS培养基上,4℃吸胀处理3 d后,于22℃(16 h明/8 h暗)培养箱中培养7 d,进行表型观察。结果发现,正常条件下,野生型拟南芥和abi5-1幼苗生长状态无明显差异(图4A);但5 µmol/L BR条件下,abi5-1幼苗下胚轴长度明显大于野生型拟南芥(图4B),这表明ABI5缺失促进了BR胁迫条件下拟南芥下胚轴的生长。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3ABI5缺失突变体鉴定
A:ABI5基因结构和突变位点。白框表示UTR区域,黑框表示外显子,线条表示内含子。B:ABI5基因DNA测序和Blast鉴定结果。突变位点加粗并用黑框标记。C:abi5-1突变体中ABI5表达量分析。分析数值为3次独立实验的平均值,误差为标准误差;**P<0.01;Wt:野生型。
Fig. 3Identification of ABI5 homozygous mutant
图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4BR胁迫条件下ABI5调控拟南芥幼苗下胚轴生长分析
A:Wt和abi5-1种子在1/2 MS培养基上生长7 d后下胚轴长度统计分析;B:Wt和abi5-1种子在含有5 µmol/L BR的1/2 MS培养基上生长7 d后下胚轴长度统计分析。数值为3次独立实验的平均值,误差为标准误差;** P<0.01;Wt:野生型。
Fig. 4Analysis of ABI5 regulation on hypocotyl growth of Arabidopsis seedlings under BR stress
2.5 BR胁迫条件下abi5-1中BR信号通路相关基因表达变化
为深入了解BR胁迫条件下ABI5缺失和拟南芥下胚轴伸长相关性,本研究对3个BR信号通路基因(BZR1[17]、BES1[17]和BIN2[18]),1个下胚轴向光性弯曲相关基因PIN7[19]和1个细胞扩增相关基因EXP3[20]在BR胁迫条件下的表达响应进行了研究。将1/2MS培养基上萌发4 d的野生型和abi5-1幼苗移入1/2MS培养基和含有5 µmol/L BR的1/2MS培养基中分别培养24 h后,提取总RNA,使用特异性引物进行qRT-PCR分析。结果表明,在正常和5 µmol/L BR胁迫处理下ABI5缺失都抑制了EXP3表达,同时5 µmol/L BR胁迫处理下ABI5缺失明显抑制了BIN2、BES1表达(图5)。这些结果证明EXP3、BIN2和BES1响应BR胁迫条件下ABI5缺失导致的拟南芥下胚轴伸长。图5
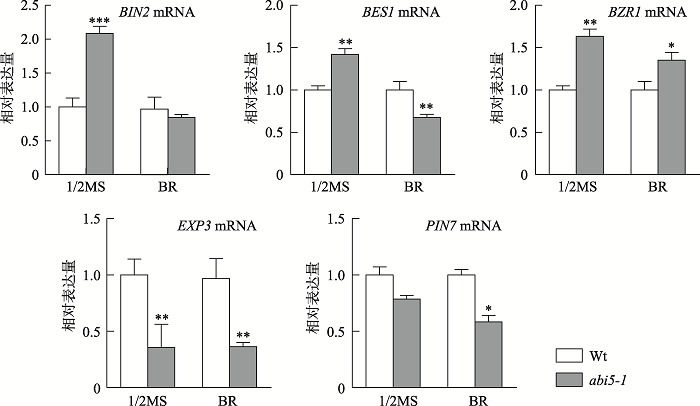 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图51/2MS和BR胁迫条件下BR信号通路相关基因表达分析
1/2MS和BR胁迫条件下,BIN2、BES1、BZR1、EXP3和PIN7 mRNA在Wt和abi5-1突变体中相对表达量分析。数值为3次独立实验的平均值,误差为标准误差;*P<0.05,**P<0.01,***P<0.001;Wt:野生型。
Fig. 5Expression analysis of genes related to the BR signaling pathway under 1/2MS and BR stress
3 讨论
下胚轴是连接子叶和根的胚性部分,由成熟胚中位于顶端和基部分生组织之间的细胞发育而成。种子萌发过程中,下胚轴伸长有利于将胚根推出种皮,吸收环境中的水分及营养物质,加快种子萌发[21]。种子萌发后下胚轴伸长是幼苗早期光形态建成过程中一个重要过程。温度、光及水分作为植物赖以生存的环境条件调控了下胚轴伸长,但作用机制不尽相同[22]。高温促进下胚轴伸长,而光和低温抑制下胚轴伸长[23]。下胚轴伸长也受外界环境和多种植物激素(CTK、BR和GA等)的协同调控。CTK能抑制黑暗诱导的下胚轴伸长但在光下不影响下胚轴伸长[24]。BR与可溶性碳水化合物协同上调BZR1与BES1的表达促进下胚轴伸长[25]。光照条件下乙烯通过促进EIN3/EIL1介导的PIF3转录活性促进下胚轴伸长[26]。光通过下调GA含量促进了DELLAs积累,从而抑制PIF3/4/5转录活性并促进下胚轴伸长[27]。此外,光照条件下HY5通过增强BR信号转导的关键阻遏物GSK3激酶BIN2活性,从而促进BIN2介导的磷酸化和BZR1的降解以抑制下胚轴伸长[28]。目前,植物激素和外界环境因素调控下胚轴伸长的详细机制尚不清楚。ABA和BR信号通路之间存在复杂的拮抗交互作用。然而,ABA是否或如何与植物中BR协同相互作用仍有待阐明。本研究发现,ABA信号核心成员ABI5响应BR胁迫,并在拟南芥幼苗体内大量积累。正常培养条件下,野生型拟南芥和abi5-1下胚轴生长状态无明显差异;BR胁迫下,abi5-1幼苗下胚轴明显长于野生型,这表明,BR胁迫下ABI5基因通过调控拟南芥下胚轴伸长影响了幼苗早期构建。该发现为进一步研究植物如何调控自身生长发育的过程提供了一个全新的视角。BR胁迫处理下ABI5缺失明显抑制了BR信号通路中BIN2、BES1表达,进一步揭示了该作用可能依赖ABI5与BR信号通路的分子机制,为阐明ABA与BR间复杂交互作用提供了新的切入点。此外,本研究也有其他发现,正常和BR胁迫处理下ABI5缺失都抑制了EXP3表达,EXP3作为一种扩展蛋白在细胞伸长生长中起促进作用,但也有研究表明,多种扩展蛋白基因表现出差异表达和受激素调节的重叠表达,扩展蛋白的过度表达可能会导致生长发育的有害缺陷[29]。因此,本研究推测单一的EXP3可能执行不同于其他扩增蛋白基因的细胞功能,导致abi5-1对环境刺激的不同敏感性。
接下来,我们将制备ABI5过表达植株,深入挖掘BR胁迫下ABI5作用的靶基因,阐明ABI5调控下胚轴伸长的分子机制,为深化理解拟南芥ABI5的调控机制和BR信号传导途径提供依据,更有助于了解植物激素BR和ABA之间复杂的网络串联。同时也在作物逆境耐受性的改良中有必要考虑对不同胁迫的差异化适应机制,以确保获得适度逆境耐受性的同时兼顾有利于生产的作物生长发育。
(责任编委: 储成才)
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
PMID:10760247 [本文引用: 1]

The Arabidopsis abscisic acid (ABA)-insensitive abi5 mutants have pleiotropic defects in ABA response, including decreased sensitivity to ABA inhibition of germination and altered expression of some ABA-regulated genes. We isolated the ABI5 gene by using a positional cloning approach and found that it encodes a member of the basic leucine zipper transcription factor family. The previously characterized abi5-1 allele encodes a protein that lacks the DNA binding and dimerization domains required for ABI5 function. Analyses of ABI5 expression provide evidence for ABA regulation, cross-regulation by other ABI genes, and possibly autoregulation. Comparison of seed and ABA-inducible vegetative gene expression in wild-type and abi5-1 plants indicates that ABI5 regulates a subset of late embryogenesis-abundant genes during both developmental stages.
DOI:10.3389/fpls.2016.01884PMID:28018412 [本文引用: 1]

ABA Insensitive 5 (ABI5) is a basic leucine zipper transcription factor that plays a key role in the regulation of seed germination and early seedling growth in the presence of ABA and abiotic stresses. ABI5 functions in the core ABA signaling, which is composed of PYR/PYURCAR receptors, PP2C phosphatases and SnRK2 kinases, through the regulation of the expression of genes that contain the ABSCISIC ACID RESPONSE ELEMENT (ABRE) motif within their promoter region. The regulated targets include stress adaptation genes, e.g., LEA proteins. However, the expression and activation of ABI5 is not only dependent on the core ABA signaling. Many transcription factors such as ABI3, ABI4, MYB7 and WRKYs play either a positive or a negative role in the regulation of ABI5 expression. Additionally, the stability and activity of ABI5 are also regulated by other proteins through post-translational modifications such as phosphorylation, ubiquitination, sumoylation and S-nitrosylation. Moreover, ABI5 also acts as an ABA and other phytohormone signaling integrator. Components of auxin, cytokinin, gibberellic acid, jasmonate and brassinosteroid signaling and metabolism pathways were shown to take part in ABI5 regulation and/or to be regulated by ABI5. Monocot orthologs of AtABI5 have been identified. Although their roles in the molecular and physiological adaptations during abiotic stress have been elucidated, knowledge about their detailed action still remains elusive. Here, we describe the recent advances in understanding the action of ABI5 in early developmental processes and the adaptation of plants to unfavorable environmental conditions. We also focus on ABI5 relation to other phytohormones in the abiotic stress response of plants.
DOI:10.1016/j.tplants.2015.05.004URL [本文引用: 1]
DOI:10.1111/pce.2015.38.issue-3URL [本文引用: 1]
DOI:10.1111/nph.v230.2URL [本文引用: 1]
DOI:10.1007/s11103-017-0661-1URL [本文引用: 1]
DOI:10.1111/pce.2014.37.issue-6URL [本文引用: 1]
DOI:10.1104/pp.113.234740URL [本文引用: 1]
DOI:10.1242/dev.060590URL [本文引用: 1]
DOI:10.1105/tpc.19.00335URL [本文引用: 1]
DOI:10.1016/j.bbrc.2012.08.118URL [本文引用: 1]
DOI:10.1093/plphys/kiab089URL [本文引用: 1]
DOI:10.1016/j.devcel.2020.12.001URL [本文引用: 1]
DOI:10.1093/jxb/erab192URL [本文引用: 1]
DOI:10.1105/tpc.114.130849URL [本文引用: 1]
DOI:10.1111/nph.2019.221.issue-2URL [本文引用: 1]
DOI:10.1016/j.pbi.2013.08.002URL [本文引用: 2]
DOI:10.1016/j.molp.2017.12.013URL [本文引用: 1]
PMID:7770519 [本文引用: 1]

Cytokinins have profound effects on seedling development in Arabidopsis thaliana. Benzyladenine (BA) inhibits root elongation in light- or dark-grown seedlings, and in dark-grown seedlings BA inhibits hypocotyl elongation and exaggerates the curvature of apical hooks. The latter are characteristic ethylene responses and, therefore, the possible involvement of ethylene in BA responses was examined in seedlings. It was found that the inhibitory effects of BA on root and hypocotyl elongation were partially blocked by the action of ethylene inhibitors or ethylene-resistant mutations (ein1-1 and ein2-1). Ethylene production was stimulated by submicromolar concentrations of BA and could account, in part, for the inhibition of root and hypocotyl elongation. It was demonstrated further that BA did not affect the sensitivity of seedlings to ethylene. Thus, the effect of cytokinin on root and hypocotyl elongation in Arabidopsis appears to be mediated largely by the production of ethylene. The coupling between cytokinin and ethylene responses is further supported by the discovery that the cytokinin-resistant mutant ckr1 is resistant to ethylene and is allelic to the ethylene-resistant mutant ein2.
DOI:10.1104/pp.16.01099URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1038/ncb2545URL [本文引用: 1]
DOI:10.1007/s00425-015-2328-yURL [本文引用: 1]
DOI:10.1073/pnas.1402491111URL [本文引用: 1]
DOI:10.1271/bbb.100586URL [本文引用: 1]
DOI:10.1038/s41467-020-15394-7URL [本文引用: 1]
DOI:10.1111/jipb.v62.10URL [本文引用: 1]
DOI:10.1007/s10529-008-9678-5PMID:18317696 [本文引用: 1]

Expansins are cell wall loosening proteins that appear to permit the microfibril matrix network to slide in growing plant cell walls, thereby enabling the wall to expand. To scrutinize possible impacts on plant growth and development when expansins are over-expressed, we characterized phenotypic alterations of the transgenic plants that constitutively expressed AtEXP3 or AtEXP-beta1 under control of 35S-CaMV promoter. Our results suggest that both AtEXP3-OX and AtXPbeta1-OX are very sensitive to salt stress. However, the mechanisms underlying their enhanced salt sensitivity appear to be different.
