 ,1,2, 徐通达
,1,2, 徐通达 ,1,2
,1,2The molecular mechanism of apical hook development in dicot plant
Min Cao ,1,2, Tongda Xu
,1,2, Tongda Xu ,1,2
,1,2通讯作者: 徐通达,教授,博士生导师,研究方向:生长素信号转导途径的研究及其在农作物中的应用。E-mail:tdxu@sibs.ac.cn
编委: 许操
收稿日期:2021-03-22修回日期:2021-06-8网络出版日期:2021-08-20
| 基金资助: |
Received:2021-03-22Revised:2021-06-8Online:2021-08-20
| Fund supported: |
作者简介 About authors

曹珉,博士,研究方向:生长素信号转导途径的分子机制。E-mail:
2014—2019年就读于中国科学院分子植物科学卓越创新中心,在徐通达课题组攻读博士学位,目前在美国加州SalkInstituteforBiologicalStudies进行博士后训练。博士期间的研究方向为生长素信号转导的分子机制。通过研究生长素-TMK1这一非经典生长素信号转导途径调控顶端弯钩发育的分子机制,揭示了顶端弯钩发育过程中内侧细胞中高浓度生长素抑制细胞伸长的原因,阐明了生长素-TMK1-IAA32/34信号通路在顶端弯钩发育过程中与经典的TIR1介导的生长素信号通路的差异性调控机制,为植物生长素信号转导提供了新的研究方向。博士论文《生长素通过类受体激酶TMK1调控植物差异性生长的分子机制》获得2020年中国科学院优秀博士生论文。

摘要
双子叶植物种子在土壤中萌发后,其下胚轴顶端会形成弯钩的特化结构,保护子叶和顶端分生组织在破土过程中不受土壤机械力的破坏,保证幼苗顺利破土。顶端弯钩的发育过程分为弯钩形成、维持及打开3个阶段,其核心在于内外两侧细胞的差异性生长导致弯钩结构。近年来研究表明,植物激素及环境信号对顶端弯钩发育各个过程起着至关重要的调控作用。然而,顶端弯钩两侧细胞不对称生长如何被精准调控的分子机制目前仍不十分清楚。本文综述了近年来顶端弯钩发育调控机制的研究进展,并着重阐述了植物激素生长素在顶端弯钩发育中的关键作用及其分子机制,并对该领域未来的研究方向进行了展望,以期为相关领域的科研人员全面了解植物激素信号相互作用的模式提供参考。
关键词:
Abstract
After the seeds of the dicot model plant Arabidopsis germinate in the soil, the tip of the hypocotyl will form a specialized structure called apical hooks to protect the cotyledons and shoot apical meristems from the mechanical damage during the soil emerging process. The development process of the apical hook is divided into three stages: the apical hook formation, maintenance, and opening. In recent decades, studies have shown that different kinds of plant hormones and environmental signals play a vital role in the development of the apical hook. As the downstream of a variety of signals, the asymmetric distribution of auxin and the signal transduction pathways play a decisive role in the development of the apical hook. However, the detailed mechanism of the asymmetric signal transduction pathway of the cells on both sides of the apical hook is still unclear. In this review, we summarize the molecular mechanisms of the development of apical hook and further refine the role of auxin in the development of apical hook, and prospect for future research directions in this field.
Keywords:
PDF (780KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
曹珉, 徐通达. 双子叶植物顶端弯钩发育的调控机制. 遗传[J], 2021, 43(8): 723-736 doi:10.16288/j.yczz.21-105
Min Cao.
种子的萌发是植物生命周期中至关重要的一步。在吸收土壤中水分之后种子开始萌发,其第一项挑战就是破土而出接收光和空气来进行光合作用从而维持植物自身的正常生长发育。植物在土壤中以黄化苗的状态迅速将下胚轴伸长[1,2],并且采用一定的方式来保护顶端分生组织和子叶避免它们在破土而出的过程中受到损害。单子叶植物和双子叶植物采取了两种截然不同的方式来保护其顶端分生组织和子叶:单子叶植物顶端形成坚硬的胚芽鞘(coleoptile)组织,将顶端分生组织包在其中[3];而双子叶植物在破土过程中,子叶和顶端分生组织及一部分下胚轴组织向下弯曲,形成弯钩状结构,由弯钩处的下胚轴优先接触土壤,人们将这个局部特化的组织称之为顶端弯钩(apical hook)[4]。之前的研究已经表明,具有顶端弯钩缺陷表型的突变体其破土而出的能力显著性降低,这说明顶端弯钩结构对于双子叶植物顺利破土而出非常重要[5,6,7]。
顶端弯钩的发育过程可以分为3个阶段:顶端弯钩形成阶段,维持阶段和打开阶段[8]。目前研究人员已经可以实时观察顶端弯钩的整个发育动态过程[9]。以拟南芥(Arabidopsis thaliana)为例,顶端弯钩的形成从幼苗突破种皮开始,由一部分下胚轴结构持续向下弯曲与生长方向形成180°;形成180°之后的顶端弯钩开始进入维持阶段,在该阶段顶端弯钩持续向下弯曲保持180°并伴随下胚轴的快速生长;这个过程持续一段时间后顶端弯钩重新打开,直到子叶完全直立成0°,伴随子叶完全展开[9,10,11,12]。整个顶端弯钩发育过程受到多种激素及环境信号的调控,从而精准控制顶端弯钩发育的不同阶段,完成破土萌发过程(图1)。
图1
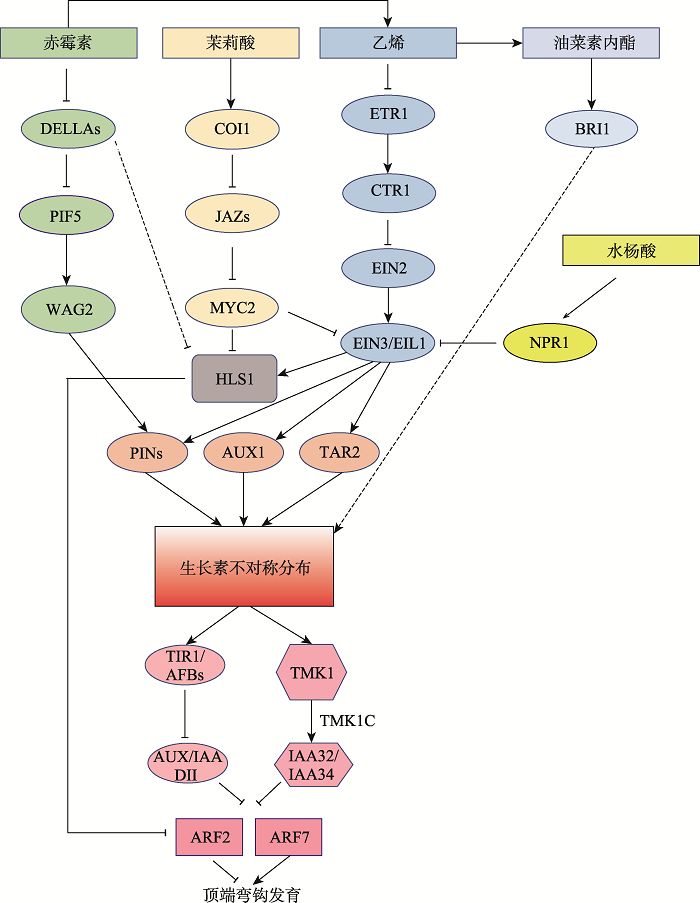 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1多种激素调控双子叶植物顶端弯钩发育
赤霉素、乙烯、茉莉酸、油菜素内酯及水杨酸的信号相互作用并最终作用于生长素的合成及运输,调控顶端弯钩处生长素的不对称分布,由下游TIR1和TMK1介导的生长素信号转导途径最终调控顶端弯钩的发育。箭头表示正调控作用,T型箭头表示负调控作用,虚线的箭头表示具体作用机制未知。
Fig. 1Plant hormone signaling network involved in the regulation of apical hook development
1 乙烯信号通路促进并延长顶端弯钩维持过程
1.1 乙烯合成调控顶端弯钩的发育
在顶端弯钩发育过程中施加外源乙烯(ethylene)处理,会导致顶端弯钩呈现270°的弯曲,并延长顶端弯钩的维持过程[11,13],被称为经典的乙烯三重反应之一。研究发现,对野生型外源施加乙烯合成前体1-aminocyclopropane-1-carboxylic acid (ACC)可以促进顶端弯钩加剧弯曲[14],并且乙烯的过量合成突变体eto1和eto2均表现出顶端弯钩呈现270°弯曲的表型[15,16,17],这说明乙烯的合成对顶端弯钩的发育过程至关重要。
1.2 乙烯信号通路调控顶端弯钩的发育
在乙烯受体ETR1 (ethylene receptor 1)突变体etr1中,施加外源乙烯无法促进顶端弯钩进一步弯曲[18,19,20]。ETR1下游的激酶CTR1为乙烯信号通路中的负调控因子,其缺失突变体ctr1-1在不施加外源乙烯的情况下也表现出顶端弯钩加剧至270°的表型[21,22,23]。CTR1下游底物EIN2为乙烯信号通路中的正调控因子,其突变体ein2在施加乙烯的情况下表现出对乙烯不敏感的表型[22,23,24,25]。乙烯信号通路中重要的转录因子EIN3/EIL1也参与乙烯调控的顶端弯钩发育过程,其突变体ein3eil1与etr1和ein2一样,在施加乙烯的情况下表现出对乙烯不敏感的表型[26,27,28,29]。这些研究结果表明,乙烯及其信号通路转导途径对于调控顶端弯钩的发育过程至关重要。前期有研究表明,乙烯可能通过促进生长素合成基因TAR2的表达来提高顶端弯钩处生长素(auxin)的含量,进而影响顶端弯钩两侧生长素分布[30,31];除此之外,有相关报道证明外源施加生长素运输抑制剂NPA (1-naphthylphthalamic acid),可以抑制ctr1及EIN3ox的顶端弯钩加剧的表型[32];同时,乙烯还可以通过增强PIN3、PIN4和PIN7在顶端弯钩处表皮细胞的定位来调控生长素运输以及弯钩两侧生长素的差异化分布[12];乙烯可以增强顶端弯钩内侧细胞中AUX1的循环来使内侧细胞积累更多的生长素,进而调控顶端弯钩的弯曲程度[11]。综上所述,乙烯通过协同调控生长素的局部合成和极性运输来介导顶端弯钩两侧生长素不对称分布,最终影响顶端弯钩的发育过程。
此外,乙烯可以促进HOOKLESS1的表达[32,33],并且促进HOOKLESS1蛋白的积累,进而抑制生长素信号通路中ARF2 (auxin response factor 2)蛋白的积累[33],参与对顶端弯钩发育的调控[33]。
在植物破土过程中,土壤摩擦产生的机械力会诱导下胚轴及顶端弯钩产生乙烯,从而维持顶端弯钩,确保植物成功破土而出[6,7,34]。乙烯信号通路相关元件的遗传学分析也证实了这一观点,在乙烯信号通路持续激活突变体ctr1或过量合成突变体eto1中,两种突变体均可以成功破土而出,而对乙烯不敏感的突变体,例如ein2和ein3eil1,则其破土的概率相比野生型要低[6,7,34]。这些数据表明乙烯信号通路是植物协同土壤环境及植物发育,巧妙利用顶端弯钩帮助幼苗破土萌发的关键机制。
2 赤霉素信号通路调控顶端弯钩发育
2.1 赤霉素合成调控顶端弯钩发育
随着对顶端弯钩研究的不断深入,大家发现赤霉素(gibberellins, GA)也参与植物顶端弯钩的发育过程。对暗下生长的拟南芥幼苗施加赤霉素可以促进顶端弯钩加剧弯曲[32,35,36]。而外源施加赤霉素合成抑制剂PAC (paclobutrazol)后,幼苗无法形成顶端弯钩,且该表型可以被再次施加赤霉素所回复[36]。进一步研究表明,拟南芥中赤霉素合成酶突变体ga1-3表现出顶端弯钩缺失的表型[36],这说明赤霉素的合成调控了顶端弯钩的发育。2.2 赤霉素信号通路调控顶端弯钩发育及机制
有研究表明,赤霉素信号通路中的负调控因子DELLA家族蛋白缺失突变体也表现出顶端弯钩加剧弯曲的表型,而当过量积累DELLA蛋白时,顶端弯钩无法形成弯曲的形状,直接进入顶端弯钩的打开阶段[32,35,36]。对于赤霉素调控顶端弯钩发育的分子机制,有研究表明赤霉素可以通过激活PIN3和PIN7基因的转录来调控生长素的运输[37]。与之相对应,pin3pin7双突变体表现出顶端弯钩对赤霉素不敏感的表型[37]。这些结果说明赤霉素位于PIN3和PIN7的上游,通过调控生长素运输,最终导致顶端弯钩两侧细胞生长素浓度梯度的变化来影响顶端弯钩的发育过程。除此之外,也有证据表明赤霉素可以调控WAG2基因的表达[38]。WAG2属于AGC型激酶(动物中同源的蛋白激酶A,G,C家族的统称),可以磷酸化生长素转运蛋白PIN蛋白[39,40]。有趣的是,WAG2蛋白在顶端弯钩处呈现不对称的表达,在顶端弯钩处内侧表达较高,而外侧表达较低[38]。WAG2在顶端弯钩内侧的积累可能通过磷酸化PIN蛋白来调控PIN蛋白的生长素运输活性,从而维持顶端弯钩内侧的生长素反应来促进顶端弯钩的弯曲状态[39,40]。也有研究表明,WAG2在赤霉素诱导下的表达是受转录因子PIF5调控[41]。赤霉素通过降解下游的转录抑制子DELLA蛋白,使得PIF5激活WAG2的转录,而WAG2蛋白可以磷酸化PIN蛋白来调控生长素在顶端弯钩内侧的积累,最终调控顶端弯钩的发育过程[37,38]。然而WAG2在顶端弯钩处的精准表达调控机制仍不清楚,有待于进一步解析。
赤霉素除了通过PIN蛋白调控生长素运输,同时也通过乙烯信号通路来维持顶端弯钩避免其过早打开。通过遗传学和生物化学研究手段发现,在拟南芥della突变体中,其乙烯含量高于野生型植物,这说明赤霉素信号通路可能调控乙烯的合成途径[35,36,41]。进一步研究证明,乙烯合成相关的酶ACS5/ETO2和ACS8基因的转录受到赤霉素的正调控[35,36,41],其调控机制也是通过DELLA蛋白降解后PIF5直接结合ACS8的启动子区域驱动转录。
赤霉素除了可以调控乙烯的合成,还可以直接调控顶端弯钩发育过程中的重要元件HOOKLESS1的表达[32,37]。研究表明,EIN3可以直接结合HOOKLESS1的启动子区来调控HOOKLESS1的表达,而DELLA蛋白可以直接和EIN3蛋白相互作用,来抑制EIN3的转录活性[32,37],从而调控顶端弯钩的发育。
3 茉莉酸信号通路在顶端弯钩发育中的调控作用
茉莉酸(jasmonic acid,JA)调控顶端弯钩的维持过程主要与乙烯信号通路和光信号通路有关。有研究表明,当对暗下生长的拟南芥幼苗施加外源茉莉酸处理后,顶端弯钩会提前打开[42,43]。同时,茉莉酸处理可以抑制乙烯过量合成突变体eto1及信号激活突变体ctr1的顶端弯钩的表型[42,43],并且该过程受到COI1-JAZ信号通路调控[42,43]。乙烯处理可以促进HOOKLESS1基因的表达,而茉莉酸处理可以抑制乙烯诱导HOOKLESS1表达[42,43]。进一步研究表明,茉莉酸信号通路中的转录因子MYC2、MYC3、MYC4与乙烯信号通路中的转录因子EIN3和EIL1相互作用,进而抑制EIN3和EIL1的转录活性从而抑制乙烯信号通路[42,43]。此外,也有研究表明茉莉酸可以通过抑制光信号通路中转录因子PIF4的转录活性来抑制HOOKLESS1的表达[44],并且该过程是通过MYC2与PIF4直接相互作用介导的[44]。4 水杨酸信号通路在顶端弯钩发育中的调控作用
水杨酸(salicylic acid, SA)是重要的免疫防御相关的植物激素,主要参与植物抗病、叶片衰老等生物学过程[45,46,47]。然而水杨酸对早期植物发育的作用研究较少。最近研究表明,水杨酸信号通路也参与顶端弯钩的发育调控。外源施加水杨酸处理可以促进顶端弯钩的打开,并且抑制了乙烯诱导的顶端弯钩加剧弯曲的表型,这说明乙烯和水杨酸在顶端弯钩发育过程中是互相拮抗的[48]。进一步研究表明,水杨酸受体NPR1的N端可以直接和乙烯信号通路中的EIN3在细胞核内互作。NPR1结合EIN3后抑制EIN3的转录活性,从而抑制HLS1及其他EIN3/EIL1下游基因的表达,从而抑制顶端弯钩的形成[48]。5 油菜素内酯在顶端弯钩发育中的作用
5.1 油菜素内酯的合成在顶端弯钩发育中的作用
在植物暗形态建成发育过程中,油菜素内酯(brassinolides, BR)的合成基因突变体det2表现为顶端弯钩缺失的表型[49]。此外,另一个控制油菜素内酯合成突变体cpd也表现为顶端弯钩缺失的表型[50]。并且外源施加eBL (24-epibrassinolide)可以恢复det2顶端弯钩发育缺陷的表型。进一步研究显示,外源施加油菜素内酯合成抑制剂也会导致顶端弯钩发育缺陷[51]。这表明油菜素内酯对顶端弯钩的发育具有重要作用。5.2 油菜素内酯调控顶端弯钩发育的机制
有报道证明乙烯对顶端弯钩弯曲的促进作用是依赖于油菜素内酯合成及其下游信号通路的。乙烯处理后会促进BR合成报告基因CPD:GUS的表达[51]。在det2突变体中,外源乙烯处理不能促进顶端弯钩的弯曲角度。这说明乙烯会促进植物体BR的合成来调控顶端弯钩的发育[51]。后续的研究表明,bri1 bzr1-1D双突变体表现出类似于野生型顶端弯钩的表型[52]。这说明BR信号通路可能参与到顶端弯钩的发育过程。乙烯处理不仅仅调控BR的合成,也有可能进一步调控BR的信号通路进而最终影响顶端弯钩的发育。6 生长素在顶端弯钩发育过程中的作用
生长素作为植物最重要的激素之一,几乎参与并调控了植物生长发育的各个阶段。生长素的浓度差异在多种组织弯曲生长过程中起着重要的作用[53]。例如在植物向光性生长过程中,生长素在背光侧积累,导致两侧细胞差异性生长,最终使植物向光弯曲。在植物的根向地性生长过程中,生长素在近地侧积累,导致近地侧的细胞伸长受到抑制,导致植物向地生长[54,55,56]。如前所述,其他激素如乙烯、赤霉素等调控顶端弯钩生长,最终都会聚焦到对生长素浓度分布以及下游信号通路的调控。在顶端弯钩处,通过生长素报告基因DR5-GUS等发现生长素内外侧呈现不对称分布[57]。在hookless1突变体中,顶端弯钩不能形成,并且DR5-GUS在两侧的不对称分布在hookless1突变体中也消失了,这说明两侧的生长素浓度差异对顶端弯钩的形成非常重要[57]。这些发现意味着生长素在顶端弯钩内外侧不对称分布从而诱导不同下游信号通路决定内外细胞差异性生长[58],是顶端弯钩发育的核心机制之一。6.1 生长素的合成调控及其在顶端弯钩发育中的作用
生长素合成作为植物体内生长素的重要来源之一,对植物的生长发育起着非常重要的作用。在拟南芥中,吲哚-3-丙酮酸(indole-3-propionic acid, IPA)依赖的合成通路起着主导作用[59,60,61]。植物体内含有一类色氨酸氨基转移酶,可以将色氨酸(Trp)转化为IPA[31,62]。因此该蛋白也被命名为TAA1 (TRYPTOPHAN AMINOTRANSFERASE of ARABIDOPSIS)[62]。后续的研究发现TAA1蛋白在拟南芥中还有两个同源蛋白TAR1和TAR2[63,64]。该家族基因突变后植物生长具有严重缺陷,并且体内生长素含量也比野生型植物显著降低[63,64]。此外,YUC家族蛋白也参与到生长素的合成调控中[65,66,67]。YUC家族在拟南芥中有11个成员,其过表达植株均表现出相似的生长素含量升高的表型。这说明YUC家族中蛋白的功能比较类似[65,66]。研究表明,TAA家族和YUC家族属于同一条生长素合成通路中的两个步骤中的重要酶:色氨酸经过TAA的催化生成IPA,IPA经过YUC家族的催化最终生成IAA[61,63]。前期研究发现当使用生长素极性运输抑制剂NPA或者1-NOA (1-naphthoxyacetic acid)处理植物时,由于生长素无法在细胞之间运输而在生长素合成的部位大量积累,导致植物的顶端弯钩消失,并且子叶中的DR5-GUS活性显著增高[11,12,33]。这说明子叶中的生长素是顶端弯钩处形成生长素不对称分布浓度梯度的一个重要来源。然而,人们也发现了生长素可以在顶端弯钩区域的细胞中合成。例如编码生长素合成通路中重要的两类催化酶YUC1、TAA1/WEI8和TAR2基因被证明在顶端弯钩处表达[31,68],并且遗传学证据证明,wei8-2 tar2-1和yuc1/ 2/4/6突变体的幼苗无法形成正常的顶端弯钩[31,68]。但是,之前的研究表明在顶端弯钩发育过程中,这些调控生长素合成的基因在顶端弯钩处并不呈现不对称表达。只有TAR2基因在乙烯处理的条件下在顶端弯钩维持阶段的内侧细胞中的表达稍微增强[11,31]。这说明生长素的合成虽然是生长素的来源,但并不是形成顶端弯钩处内外侧生长素浓度差的主要原因。
6.2 生长素的运输调控及其在顶端弯钩发育中的作用
之前的研究已经证实,生长素的极性运输对顶端弯钩的发育至关重要。生长素的极性运输不仅仅从子叶中向下运输生长素,并且顶端弯钩内外侧也存在生长素的极性运输。生长素极性运输蛋白分为内运蛋白和外运蛋白两大类,分别负责将生长素运进细胞或者运出细胞[69,70,71,72]。在拟南芥中,生长素内运蛋白主要由4个蛋白组成——AUX1、LAX1、LAX2和LAX3[71]。其中,在顶端弯钩处主要起作用的是AUX1和LAX3,负责将生长素从子叶处向下运输到顶端弯钩处[11,71]。AUX1主要定位于表皮细胞,而LAX3主要定位于维管组织中。因此,生长素内运蛋白家族的作用主要是将子叶和顶端弯钩处合成的生长素向下运输,而对生长素在顶端弯钩内外侧的不对称分布的建立作用较弱[11,71]。在拟南芥中,还有两大类膜蛋白作为生长素外运蛋白。这两个家族分别是PIN基因家族(具有8个成员)以及两个B型ATP结合的转运蛋白ABCB1和ABCB19[69,72,73]。遗传学分析证明abcb1,abcb19双突变体在顶端弯钩形成和打开过程中有缺陷,并且在利用生长素报告元件DR5-GUS来观察双突变体中生长素的信号激活情况时发现在abcb1,abcb19双突变体中DR5-GUS信号显著降低[74,75]。有趣的是,ABCB19被发现在顶端弯钩处有不对称表达的现象。研究表明,ABCB19定位于顶端弯钩内侧处的表皮细胞膜上[75]。
当使用PIN蛋白家族抑制剂NPA来抑制生长素细胞外运途径时,顶端弯钩在形成阶段就会造成重大缺陷,其顶端弯钩直接打开持续保持直立状态[12,69],并且生长素在顶端弯钩内侧处的积累也会受到抑制。PIN蛋白家族在拟南芥中是重要的一类生长素外运蛋白[12,69,72,76,77]。在PIN蛋白家族中,PIN3、PIN4和PIN7在顶端弯钩处均有表达,并且其表达部位有部分重叠[12,58]。有趣的是,PIN3、PIN4和PIN7也被发现在顶端弯钩处有不对称表达的现象,它们在顶端弯钩外侧处细胞表达量更高[12,58]。在pin3功能缺失突变体中,由于生长素运输的缺陷导致植物无法在顶端弯钩内侧积累足够含量的生长素,导致pin3突变体中的顶端弯钩不能完全闭合[12,58]。pin4和pin7单突变体的表型较弱,但pin3pin4或pin3pin7的双突变体表现出较强的顶端弯钩形成缺陷的表型,说明PIN3、PIN4和PIN7在调控顶端弯钩形成过程中存在着功能冗余[12,58]。
6.3 生长素信号转导途径及其在顶端弯钩发育中的作用
生长素在顶端弯钩处建立内侧浓度高,外侧浓度低的不对称浓度差后,必须要通过信号转导途径来使细胞感知不同浓度的生长素信号,最终调控相应的生物学过程[78]。生长素核内受体TRANSPORT INHIBITOR RESPONSE1/AUXIN SIGNALING F- BOX PROTEIN1-3 (TIR1/AFB1-3)在生长素信号转导中起着重要的作用[79,80,81]。生长素与 TIR1 受体结合后,可以促进TIR1蛋白与AUXIN/INDOLE-3- ACETIC ACID (Aux/IAA)蛋白的互作[79,81]。Aux/IAA可以和转录因子AUXIN RESPONSE FACTOR (ARF),在生长素浓度较低的情况下形成二聚体[82,83,84]。当生长素浓度升高激活TIR1信号通路时,TIR1可以使 Aux/IAA蛋白多泛素化使其通过26S蛋白酶体途径降解。Aux/IAA蛋白的降解使得其对ARF的抑制作用解除,来激活ARF的转录活性,调控基因表达[82,83,84]。研究表明,顶端弯钩维持阶段的内外侧生长素浓度差,导致内外侧细胞的不对称伸长,进而维持顶端弯钩弯曲的状态[58,85]。目前的研究已经发现一些参与生长素信号转导途径中的组分也参与到顶端弯钩的调控,例如生长素核内受体TIR1/AFB家族蛋白参与调控顶端弯钩的维持过程,并且tir1afb1afb2afb3多突变体表现出顶端弯钩缺失的表型[79,80,81]。除此之外,Aux/IAA蛋白家族也参与到顶端弯钩的发育过程中。研究证明,当植物表达不受TIR1家族降解的突变形式的Aux/IAA蛋白时(例如SHY2/IAA3、BDL/ IAA12等),顶端弯钩也会出现发育缺陷的表型[86,87,88]。这些证据说明在内侧生长素积累后其对应的信号转导途径对于顶端弯钩的正常发育过程至关重要。除此之外,生长素信号通路中的重要转录因子家族ARF家族蛋白也参与到这一生物学过程中。转录激活子如NPH4/ARF7和ARF19的功能缺失突变体会表现出顶端弯钩发育缺陷的表型,其表型与显性失活的Aux/IAA蛋白突变体表型类似[89,90,91,92]。除此之外,ARF家族中的转录抑制子也参与顶端弯钩的发育过程。如转录抑制子ARF1和ARF2是顶端弯钩发育的负调控因子,arf1arf2双突变体表现出增强弯曲的顶端弯钩表型[90]。有趣的是,拟南芥中这两个亚家族的转录因子均通过激活或者抑制基因转录来促进或者抑制细胞的不对称生长,进而调控顶端弯钩的发育[90,91,93]。然而,目前还没有证据证明ARF家族转录因子在顶端弯钩处有不对称表达的情况。因此,由生长素浓度梯度导致的特异时空间激活下游转录激活型或者转录抑制型ARF蛋白可能最终导致下游的效应蛋白的不对称表达,最终导致顶端弯钩的弯曲过程。
最新的研究发现,在顶端弯钩维持阶段,其内侧细胞的高浓度生长素能促进细胞膜上的类受体激酶蛋白TMK1剪切形成TMK1C末端片段并从细胞膜转运到细胞质和细胞核内,进而调控下游通路[94]。该研究进一步发现,剪切后的TMK1C能特异和两个非经典Aux/IAA家族转录抑制子IAA32和IAA34互作并磷酸化IAA蛋白[94]。然而与TMK1C互作的IAA32/34并不具有与TIR1互作的结构区域,因此不能被TIR1所降解,这意味着TIR1-介导的生长素信号途径和TMK1-介导的生长素途径通过选择不同IAA蛋白来区分下游信号途径[94]。该研究还意外发现,与之前报道的TIR1/AFB介导的生长素对于Aux/IAA蛋白泛素化降解过程相反,生长素通过TMK1剪切后形成的TMK1C来稳定IAA32和IAA34蛋白,最终依然通过ARF转录因子来调控基因表达,在生长素聚集的地方抑制细胞生长,从而导致顶端弯钩内外侧的差异性生长[94]。
7 结语与展望
植物作为一种固着在土壤中生长的生物,其种子破土而出的过程对于植物后续完成光合作用积累营养,以及后期生长发育至关重要。双子叶植物采用顶端弯钩这一特化结构来保护子叶和顶端分生组织在破土过程中不受土壤机械摩擦的损伤。顶端弯钩的发育过程伴随着多种植物激素的协同作用,而植物的发育与对环境的响应是多种激素协同作用的结果,在未来也仍是植物激素领域的研究热点。7.1 其他激素在顶端弯钩发育过程中的作用
目前顶端弯钩发育过程中的植物激素研究主要集中于乙烯、赤霉素、茉莉酸、水杨酸、油菜素内酯和生长素。然而,拟南芥中其他的重要植物激素,如细胞分裂素(cytokinin, CK)、脱落酸(abscisic acid, ABA)和独角金内脂(strigolactones, SLs)在顶端弯钩发育过程中的作用还不清楚。这些激素是否参与顶端弯钩的发育调控且与其他激素信号通路的互作还有待于进一步研究。其中,细胞分裂素与生长素共同参与根的发育[95]。独脚金内脂参与调控叶片形态和地上部分分支发育[96]。脱落酸在调控种子萌发过程起着至关重要的作用[97],但对于脱落酸是否参与种子萌发后顶端弯钩的发育调控却鲜有报道。最新的研究发现PP2C家族参与了种子萌发后顶端弯钩的形成过程[98],这可能暗示脱落酸也参与了顶端弯钩的发育调控。因此,细胞分裂素、独角金内脂和脱落酸对顶端弯钩发育的调控及其分子机制会是顶端弯钩发育领域值得关注的问题。7.2 进一步解析植物不对称生长的分子机制
顶端弯钩的发育是研究植物差异性生长的经典模型,为揭示植物不同发育阶段的差异性生长调控机制提供重要线索。因此,顶端弯钩内外侧差异性生长的调控机制是否对其他差异性生长(如植物向重力性反应)同样适用有待于进一步研究。此外,生长素在植物体内不同组织中调控细胞伸长的分子机制也不尽相同。例如,生长素不对称分布调控细胞不对称伸长,在顶端弯钩和根的向地性生长的生物学过程中,生长素积累的一侧会抑制细胞的伸长;而在下胚轴向光性生长这一生物学过程中,生长素积累的一侧却促进细胞的伸长。这些结果表明生长素在不同组织中调控细胞伸长的过程是多样且复杂的。植物生长素信号通路包括TIR1介导的经典转录调控通路和TMK1介导的非经典信号通路,作为顶端弯钩的核心机制之一,有待于进一步解析其精准调控机制。比如,最初顶端弯钩形成是如何起始的?生长素内外侧不对称分布如何精准被生长素合成、运输和信号转导三者协同调控的?TIR1和TMK1介导的信号通路是如何协调的?最近的研究发现,机械力能调控植物顶端弯钩形成,但该过程依赖于TMK家族介导的非经典信号通路,而不依赖于TIR1介导的信号通路[99],其具体机制也有待于进一步解析。7.3 激素信号通路互作的组织和细胞特异性
植物激素的协同作用参与了多种植物发育的生物学过程,然而在不同的生物学过程中植物激素的协同作用也不尽相同。例如在顶端弯钩发育过程中,乙烯和茉莉酸、水杨酸通过互相拮抗来调控顶端弯钩的发育,而在植物抗病过程中植物会同时激活乙烯、茉莉酸和水杨酸通路来调控植物免疫反应[100]。植物如何在面对不同发育环境下采取不同甚至相反的激素互作调控方式也是今后的研究热点之一。此外,IAA32/34只特异在顶端弯钩处表达,这说明特定组织或细胞类型中存在着特异的蛋白传递信号来调控该组织的生物学功能。因此,通过不同组织的转录组学分析来寻找不同组织在感受环境信号时的特异性组分可能是未来激素信号转导方向的研究热点。福建农林大学徐通达课题组简介
徐通达教授于2014年全职回国,在中国科学院上海逆境中心成立课题组,2017年课题组转到福建农林大学,主要围绕植物生长素的非转录调控机制、植物生长素浓度效应产生的分子机制及生长素局部浓度如何被上游发育和环境信号精准调控的分子机制等领域进行研究。在植物激素发育和环境响应中取得了一系列研究成果,在Nature、Science、Cell、Nature Communications和PNAS等国际知名期刊发表论文20来篇。与此同时,课题组还承担了科技部、国家自然科学基金委、上海市科学基金委和福建省科学协会等课题。课题组网站:

(责任编委: 许操)
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1016/j.cub.2008.10.034URL [本文引用: 1]
DOI:10.1016/j.cub.2015.03.020URL [本文引用: 1]
DOI:10.1007/s00709-016-1023-6URL [本文引用: 1]
[本文引用: 1]
DOI:10.1080/15592324.2017.1330239URL [本文引用: 1]
[本文引用: 3]
DOI:10.1016/j.cub.2015.11.053URL [本文引用: 3]
PMID:10409511 [本文引用: 1]

Arabidopsis seedlings develop a hook-like structure at the apical part of the hypocotyl when grown in darkness. Differential cell growth processes result in the curved hypocotyl hook. Time-dependent analyses of the hypocotyl showed that the apical hook is formed during an early phase of seedling growth and is maintained in a sequential phase by a distinct process. Based on developmental genetic analyses of hook-affected mutants, we show that the hookless mutants (hls1, cop2) are involved in an early aspect of hook development. From time-dependent analyses of ethylene-insensitive mutants, later steps in hook maintenance were found to be ethylene sensitive. Regulation of differential growth was further studied through examination of the spatial pattern of expression of two hormone-regulated genes: an ethylene biosynthetic enzyme and the ethylene receptor ETR1. Accumulation of mRNA for AtACO2, a novel ACC (1-aminocyclopropane-1-carboxylic acid) oxidase gene, occurred within cells predominantly located on the outer-side of the hook and was tightly correlated with ethylene-induced exaggeration in the curvature of the hook. ETR1 expression in the apical hook, however, was reduced by ethylene treatment. Based on the expression pattern of ETR1 and AtACO2 in the hook-affected mutants, a model for hook development and maintenance is proposed.
[本文引用: 2]
DOI:10.1111/nph.2014.202.issue-4URL [本文引用: 1]
DOI:10.1242/dev.040790PMID:20110325 [本文引用: 7]

Dark-grown dicotyledonous seedlings form a hook-like structure at the top of the hypocotyl, which is controlled by the hormones auxin and ethylene. Hook formation is dependent on an auxin signal gradient, whereas hook exaggeration is part of the triple response provoked by ethylene in dark-grown Arabidopsis seedlings. Several other hormones and light are also known to be involved in hook development, but the molecular mechanisms that lead to the initial installation of an auxin gradient are still poorly understood. In this study, we aimed to unravel the cross-talk between auxin and ethylene in the apical hook. Auxin measurements, the expression pattern of the auxin reporter DR5::GUS and the localization of auxin biosynthesis enzymes and influx carriers collectively indicate the necessity for auxin biosynthesis and efficient auxin translocation from the cotyledons and meristem into the hypocotyl in order to support proper hook development. Auxin accumulation in the meristem and cotyledons and in the hypocotyl is increased approximately 2-fold upon treatment with ethylene. In addition, a strong ethylene signal leads to enhanced auxin biosynthesis at the inner side of the hook. Finally, mutant analysis demonstrates that the auxin influx carrier LAX3 is indispensable for proper hook formation, whereas the auxin influx carrier AUX1 is involved in the hook exaggeration phenotype induced by ethylene.
DOI:10.1242/dev.041277PMID:20110326 [本文引用: 9]

The apical hook of dark-grown Arabidopsis seedlings is a simple structure that develops soon after germination to protect the meristem tissues during emergence through the soil and that opens upon exposure to light. Differential growth at the apical hook proceeds in three sequential steps that are regulated by multiple hormones, principally auxin and ethylene. We show that the progress of the apical hook through these developmental phases depends on the dynamic, asymmetric distribution of auxin, which is regulated by auxin efflux carriers of the PIN family. Several PIN proteins exhibited specific, partially overlapping spatial and temporal expression patterns, and their subcellular localization suggested auxin fluxes during hook development. Genetic manipulation of individual PIN activities interfered with different stages of hook development, implying that specific combinations of PIN genes are required for progress of the apical hook through the developmental phases. Furthermore, ethylene might modulate apical hook development by prolonging the formation phase and strongly suppressing the maintenance phase. This ethylene effect is in part mediated by regulation of PIN-dependent auxin efflux and auxin signaling.
[本文引用: 1]
DOI:10.1111/nph.2014.202.issue-4URL [本文引用: 1]
DOI:10.1038/nature02516URL [本文引用: 1]
PMID:9952448 [本文引用: 1]

The Arabidopsis mutants eto1 (ethylene overproducer) and eto3 produce elevated levels of ethylene as etiolated seedlings. Ethylene production in these seedlings peaks at 60 to 96 h, and then declines back to almost wild-type levels. Ethylene overproduction in eto1 and eto3 is limited mainly to etiolated seedlings; light-grown seedlings and various adult tissues produce close to wild-type amounts of ethylene. Several compounds that induce ethylene biosynthesis in wild-type, etiolated seedlings through distinct 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS) isoforms were found to act synergistically with eto1 and eto3, as did the ethylene-insensitive mutation etr1 (ethylene resistant), which blocks feedback inhibition of biosynthesis. ACS activity, the rate-limiting step of ethylene biosynthesis, was highly elevated in both eto1 and eto3 mutant seedlings, even though RNA gel-blot analysis demonstrated that the steady-state level of ACS mRNA was not increased, including that of a novel Arabidopsis ACS gene that was identified. Measurements of the conversion of ACC to ethylene by intact seedlings indicated that the mutations did not affect conjugation of ACC or the activity of ACC oxidase, the final step of ethylene biosynthesis. Taken together, these data suggest that the eto1 and eto3 mutations elevate ethylene biosynthesis by affecting the posttranscriptional regulation of ACS.
DOI:10.1105/tpc.006882URL [本文引用: 1]
PMID:8211181 [本文引用: 1]

Ethylene behaves as a hormone in plants, regulating such aspects of growth and development as fruit ripening, flower senescence, and abscission. Ethylene insensitivity is conferred by dominant mutations in the ETR1 gene early in the ethylene signal transduction pathway of Arabidopsis thaliana. The ETR1 gene was cloned by the method of chromosome walking. Each of the four known etr1 mutant alleles contains a missense mutation near the amino terminus of the predicted protein. Although the sequence of the amino-terminal half of the deduced ETR1 protein appears to be novel, the carboxyl-terminal half is similar in sequence to both components of the prokaryotic family of signal transducers known as the two-component systems. Thus, an early step in ethylene signal transduction in plants may involve transfer of phosphate as in prokaryotic two-component systems. The dominant etr1-1 mutant gene conferred ethylene insensitivity to wild-type Arabidopsis plants when introduced by transformation.
DOI:10.1104/pp.011635URL [本文引用: 1]
PMID:9974395 [本文引用: 1]

The ETR1 receptor from Arabidopsis binds the gaseous hormone ethylene. A copper ion associated with the ethylene-binding domain is required for high-affinity ethylene-binding activity. A missense mutation in the domain that renders the plant insensitive to ethylene eliminates both ethylene binding and the interaction of copper with the receptor. A sequence from the genome of the cyanobacterium Synechocystis sp. strain 6803 that shows homology to the ethylene-binding domain of ETR1 encodes a functional ethylene-binding protein. On the basis of sequence conservation between the Arabidopsis and the cyanobacterial ethylene-binding domains and on in vitro mutagenesis of ETR1, a structural model for this copper-based ethylene sensor domain is presented.
PMID:8431946 [本文引用: 1]

We isolated a recessive Arabidopsis mutant, ctr1, that constitutively exhibits seedling and adult phenotypes observed in plants treated with the plant hormone ethylene. The ctr1 adult morphology can be phenocopied by treatment of wild-type plants with exogenous ethylene and is due, at least in part, to inhibition of cell elongation. Seedlings and adult ctr1 plants show constitutive expression of ethylene-regulated genes. The epistasis of ctr1 and other ethylene response mutants has defined the position of CTR1 in the ethylene signal transduction pathway. The CTR1 gene has been cloned, and the DNA sequences of four mutant alleles were determined. The gene encodes a putative serine/threonine protein kinase that is most closely related to the Raf protein kinase family.
DOI:10.1126/science.1225974URL [本文引用: 2]
DOI:10.1073/pnas.1214848109URL [本文引用: 2]
DOI:10.1016/j.cell.2015.09.037URL [本文引用: 1]
PMID:10381874 [本文引用: 1]

Ethylene regulates plant growth, development, and responsiveness to a variety of stresses. Cloning of the Arabidopsis EIN2 gene identifies a central component of the ethylene signaling pathway. The amino-terminal integral membrane domain of EIN2 shows similarity to the disease-related Nramp family of metal-ion transporters. Expression of the EIN2 CEND is sufficient to constitutively activate ethylene responses and restores responsiveness to jasmonic acid and paraquat-induced oxygen radicals to mutant plants. EIN2 is thus recognized as a molecular link between previously distinct hormone response pathways. Plants may use a combinatorial mechanism for assessing various stresses by enlisting a common set of signaling molecules.
DOI:10.1073/pnas.1103959108URL [本文引用: 1]
DOI:10.1105/tpc.18.00018URL [本文引用: 1]
DOI:10.1105/tpc.110.076588URL [本文引用: 1]
PMID:17307926 [本文引用: 1]

Ethylene signaling in Arabidopsis thaliana converges on the ETHYLENE-INSENSITIVE3 (EIN3)/EIN3-Like (EIL) transcription factors to induce various responses. EIN3 BINDING F-BOX1 (EBF1) and EBF2 were recently shown to function in ethylene perception by regulating EIN3/EIL turnover. In the absence of ethylene, EIN3 and possibly other EIL proteins are targeted for ubiquitination and subsequent degradation by Cullin 1-based E3 complexes containing EBF1 and 2. Ethylene appears to block this ubiquitination, allowing EIN3/EIL levels to rise and mediate ethylene signaling. Through analysis of mutant combinations affecting accumulation of EBF1, EBF2, EIN3, and EIL1, we show that EIN3 and EIL1 are the main targets of EBF1/2. Kinetic analyses of hypocotyl growth inhibition in response to ethylene and growth recovery after removal of the hormone revealed that EBF1 and 2 have temporally distinct but overlapping roles in modulating ethylene perception. Whereas EBF1 plays the main role in air and during the initial phase of signaling, EBF2 plays a more prominent role during the latter stages of the response and the resumption of growth following ethylene removal. Through their coordinated control of EIN3/EIL1 levels, EBF1 and EBF2 fine-tune ethylene responses by repressing signaling in the absence of the hormone, dampening signaling at high hormone concentrations, and promoting a more rapid recovery after ethylene levels dissipate.
DOI:10.1038/nchembio.1178PMID:23377040 [本文引用: 1]

We identify an Arabidopsis pyridoxal-phosphate-dependent aminotransferase, VAS1, whose loss-of-function simultaneously increases amounts of the phytohormone auxin and the ethylene precursor 1-aminocyclopropane-1-carboxylate. VAS1 uses the ethylene biosynthetic intermediate methionine as an amino donor and the auxin biosynthetic intermediate indole-3-pyruvic acid as an amino acceptor to produce L-tryptophan and 2-oxo-4-methylthiobutyric acid. Our data indicate that VAS1 serves key roles in coordinating the amounts of these two vital hormones.
DOI:10.1016/j.cell.2008.01.047PMID:18394997 [本文引用: 5]

Plants have evolved a tremendous ability to respond to environmental changes by adapting their growth and development. The interaction between hormonal and developmental signals is a critical mechanism in the generation of this enormous plasticity. A good example is the response to the hormone ethylene that depends on tissue type, developmental stage, and environmental conditions. By characterizing the Arabidopsis wei8 mutant, we have found that a small family of genes mediates tissue-specific responses to ethylene. Biochemical studies revealed that WEI8 encodes a long-anticipated tryptophan aminotransferase, TAA1, in the essential, yet genetically uncharacterized, indole-3-pyruvic acid (IPA) branch of the auxin biosynthetic pathway. Analysis of TAA1 and its paralogues revealed a link between local auxin production, tissue-specific ethylene effects, and organ development. Thus, the IPA route of auxin production is key to generating robust auxin gradients in response to environmental and developmental cues.
DOI:10.1038/cr.2012.29URL [本文引用: 6]
DOI:10.1016/j.devcel.2004.07.002URL [本文引用: 4]
DOI:10.1016/j.cub.2015.12.003URL [本文引用: 2]
PMID:14756759 [本文引用: 4]

Dark-grown Arabidopsis seedlings develop an apical hook by differential elongation and division of hypocotyl cells. This allows the curved hypocotyl to gently drag the apex, which is protected by the cotyledons, upwards through the soil. Several plant hormones are known to be involved in hook development, including ethylene, which causes exaggeration of the hook. We show that gibberellins (GAs) are also involved in this process. Inhibition of GA biosynthesis with paclobutrazol (PAC) prevented hook formation in wild-type (WT) seedlings and in constitutive ethylene response (ctr)1-1, a mutant that exhibits a constitutive ethylene response. In addition, a GA-deficient mutant (ga1-3) did not form an apical hook in the presence of the ethylene precursor 1-aminocyclopropane-1-carboxylate (ACC). Analysis of transgenic Arabidopsis seedlings expressing a green fluorescent protein (GFP)-repressor of ga1-3 (RGA) fusion protein suggested that ACC inhibits cell elongation in the apical hook by inhibition of GA signaling. A decreased feedback of GA possibly causes an induction of GA biosynthesis based upon the expression of genes encoding copalyl diphosphate synthase (CPS; GA1) and GA 2-oxidase (AtGA2ox1). Furthermore, expression of GASA1, a GA-response gene, suggests that differential cell elongation in the apical hook might be a result of differential GA-sensitivity.
PMID:14963246 [本文引用: 6]

Plants undergo two different developmental programs depending on whether they are growing in darkness (skotomorphogenesis) or in the presence of light (photomorphogenesis). It has been proposed that the latter is the default pathway followed by many plants after germination and before the seedling emerges from soil. The transition between the two pathways is tightly regulated. The conserved COP1-based complex is central in the light-dependent repression of photomorphogenesis in darkness. Besides this control, hormones such as brassinosteroids (BRs), cytokinins, auxins, or ethylene also have been shown to regulate, to different extents, this developmental switch. In the present work, we show that the hormone gibberellin (GA) widely participates in this regulation. Studies from Arabidopsis show that both chemical and genetic reductions of endogenous GA levels partially derepress photomorphogenesis in darkness. This is based both on morphological phenotypes, such as hypocotyl elongation and hook and cotyledon opening, and on molecular phenotypes, such as misregulation of the light-controlled genes CAB2 and RbcS. Genetic studies indicate that the GA signaling elements GAI and RGA participate in these responses. Our results also suggest that GA regulation of this response partially depends on BRs. This regulation seems to be conserved across species because lowering endogenous GA levels in pea (Pisum sativum) induces full de-etiolation in darkness, which is not reverted by BR application. Our results, therefore, attribute an important role for GAs in the establishment of etiolated growth and in repression of photomorphogenesis.
DOI:10.1111/tpj.2011.67.issue-4URL [本文引用: 5]
DOI:10.1242/dev.081240PMID:22992959 [本文引用: 3]

When penetrating the soil during germination, dicotyledonous plants protect their shoot apical meristem through the formation of an apical hook. Apical hook formation is a dynamic process that can be subdivided into hook formation, maintenance and opening. It has previously been established that these processes require the transport and signaling of the phytohormone auxin, as well as the biosynthesis and signaling of the phytohormones ethylene and gibberellin (GA). Here, we identify a molecular mechanism for an auxin-GA crosstalk by demonstrating that the auxin transport-regulatory protein kinase WAG2 is a crucial transcription target during apical hook opening downstream from GA signaling. We further show that WAG2 is directly activated by PHYTOCHROME INTERACTING FACTOR 5 (PIF5), a light-labile interactor of the DELLA repressors of the GA pathway. We find that wag2 mutants are impaired in the repression of apical hook opening in dark-grown seedlings and that this phenotype correlates with GA-regulated WAG2 expression in the concave (inner) side of the apical hook. Furthermore, wag2 mutants are also impaired in the maintenance or formation of a local auxin maximum at the site of WAG2 expression in the hook. WAG2 is a regulator of PIN auxin efflux facilitators and, in line with previous data, we show that this kinase can phosphorylate the central intracellular loop of all PIN-FORMED (PIN) proteins regulating apical hook opening. We therefore propose that apical hook opening is controlled by the differential GA-regulated accumulation of WAG2 and subsequent local changes in PIN-mediated auxin transport.
PMID:21852755 [本文引用: 2]

The analysis of cell polarity in plants is fueled by the discovery and analysis of auxin efflux carrier PIN proteins that show polar localizations in various plant cell types in line with their roles in directional cell to cell auxin transport. As this asymmetry in cellular PIN localization drives directional auxin fluxes, abnormalities in PIN localizations modify auxin transport culminating into range of auxin distribution defective phenotypes. Because of this influence of PIN localization on plant development via changes in auxin distribution, mechanisms establishing, maintaining and altering PIN polarity are of intense interest in the plant field during the recent years. Recent findings suggest that two categories of molecules, namely AGC-3 kinase family members PINOID, WAG1, WAG2 and ARF-GEF family member GNOM predominantly influence the polar localization of PINs. The emerging mechanism for AGC-3 kinases and ARF-GEF action suggest that AGC-3 kinases predominantly phosphorylate PINs at the plasma membrane for eventual PIN internalization and PIN sorting into ARF-GEF GNOM independent polar recycling pathways. In case of mutant for AGC-3 kinases or mutations in AGC-3 kinase-targeted PIN residues, much less phosphorylated PINs are recruited into ARFGEF GNOM-dependent polar recycling pathway. When ARF-GEF GNOM is inactive, the bias is shifted for rerouting less efficiently phosphorylated PINs into GNOM-independent polar recycling pathways that generally prefer efficiently phosphorylated PINs. Thus, balance shifts between the extent of AGC-3 kinase mediated PIN phosphorylation and the functioning of ARFGEF instruct PIN polarity establishment and/or PIN polarity alterations. Recent studies report utilization of this AGC-3 kinase and ARF-GEF PIN polarity regulation module during diverse developmental and response programs including shoot patterning, root growth, phototropism, gravitropism, organogenesis, leaf epidermal cell indentations and fruit valve margin formation. Based on these findings the same theme of phosphorylated PIN sorting into differential polar recycling pathways for PIN polarity establishment and alteration seems to be employed in a context-dependent manner.
DOI:10.1242/dev.052456PMID:20823065 [本文引用: 2]

Polar membrane cargo delivery is crucial for establishing cell polarity and for directional transport processes. In plants, polar trafficking mediates the dynamic asymmetric distribution of PIN FORMED (PIN) carriers, which drive polar cell-to-cell transport of the hormone auxin, thereby generating auxin maxima and minima that control development. The Arabidopsis PINOID (PID) protein kinase instructs apical PIN localization by phosphorylating PINs. Here, we identified the PID homologs WAG1 and WAG2 as new PIN polarity regulators. We show that the AGC3 kinases PID, WAG1 and WAG2, and not other plant AGC kinases, instruct recruitment of PINs into the apical recycling pathway by phosphorylating the middle serine in three conserved TPRXS(N/S) motifs within the PIN central hydrophilic loop. Our results put forward a model by which apolarly localized PID, WAG1 and WAG2 phosphorylate PINs at the plasma membrane after default non-polar PIN secretion, and trigger endocytosis-dependent apical PIN recycling. This phosphorylation-triggered apical PIN recycling competes with ARF-GEF GNOM-dependent basal recycling to promote apical PIN localization. In planta, expression domains of PID, WAG1 and WAG2 correlate with apical localization of PINs in those cell types, indicating the importance of these kinases for apical PIN localization. Our data show that by directing polar PIN localization and PIN-mediated polar auxin transport, the three AGC3 kinases redundantly regulate cotyledon development, root meristem size and gravitropic response, indicating their involvement in both programmed and adaptive plant development.
DOI:10.1105/tpc.107.051508URL [本文引用: 3]
DOI:10.1105/tpc.113.120394URL [本文引用: 5]
DOI:10.1105/tpc.113.122002URL [本文引用: 5]
DOI:10.1105/tpc.18.00018URL [本文引用: 2]
PMID:10972893 [本文引用: 1]

Leaf senescence is a complex process that is controlled by multiple developmental and environmental signals and is manifested by induced expression of a large number of different genes. In this paper we describe experiments that show, for the first time, that the salicylic acid (SA)-signalling pathway has a role in the control of gene expression during developmental senescence. Arabidopsis plants defective in the SA-signalling pathway (npr1 and pad4 mutants and NahG transgenic plants) were used to investigate senescence-enhanced gene expression, and a number of genes showed altered expression patterns. Senescence-induced expression of the cysteine protease gene SAG12, for example, was conditional on the presence of SA, together with another unidentified senescence-specific factor. Changes in gene expression patterns were accompanied by a delayed yellowing and reduced necrosis in the mutant plants defective in SA-signalling, suggesting a role for SA in the cell death that occurs at the final stage of senescence. We propose the presence of a minimum of three senescence-enhanced signalling factors in senescing leaves, one of which is SA. We also suggest that a combination of signalling factors is required for the optimum expression of many genes during senescence.
PMID:17746926 [本文引用: 1]

In an effort to identify the signal compound that mediates systemic acquired resistance (SAR), changes in the content of phloem sap were monitored in cucumber plants inoculated with either tobacco necrosis virus or the fungal pathogen Colletotrichum lagenarium. The concentration of a fluorescent metabolite was observed to increase transiently after inoculation, with a peak reached before SAR was detected. The compound was purified and identified by gas chromatography-mass spectrometry as salicylic acid, a known exogenous inducer of resistance. The data suggest that salicylic acid could function as the endogenous signal in the transmission of SAR in cucumber.
DOI:10.1105/tpc.17.00438URL [本文引用: 1]
DOI:10.1105/tpc.19.00658URL [本文引用: 2]
DOI:10.2307/3869351URL [本文引用: 1]
PMID:8612270 [本文引用: 1]

The cpd mutation localized by T-DNA tagging on Arabidopsis chromosome 5-14.3 inhibits cell elongation controlled by the ecdysone-like brassinosteroid hormone brassinolide. The cpd mutant displays de-etiolation and derepression of light-induced genes in the dark, as well as dwarfism, male sterility, and activation of stress-regulated genes in the light. The CPD gene encodes a cytochrome P450 (CYP90) sharing homologous domains with steroid hydroxylases. The phenotype of the cpd mutant is restored to wild type both by feeding with C23-hydroxylated brassinolide precursors and by ectopic overexpression of the CPD cDNA. Brassinosteroids also compensate for different cell elongation defects of Arabidopsis det, cop, fus, and axr2 mutants, indicating that these steroids play an essential role in the regulation of plant development.
DOI:10.1093/pcp/pci111URL [本文引用: 3]
DOI:10.1093/mp/ssn005PMID:19825546 [本文引用: 1]

We undertook a chemical genetics screen to identify chemical inhibitors of brassinosteroid (BR) action. From a chemical library of 10,000 small molecules, one compound was found to inhibit hypocotyl length and activate the expression of a BR-repressed reporter gene (CPD::GUS) in Arabidopsis, and it was named brassinopride (BRP). These effects of BRP could be reversed by co-treatment with brassinolide, suggesting that BRP either directly or indirectly inhibits BR biosynthesis. Interestingly, the compound causes exaggerated apical hooks, similar to that caused by ethylene treatment. The BRP-induced apical hook phenotype can be blocked by a chemical inhibitor of ethylene perception or an ethylene-insensitive mutant, suggesting that, in addition to inhibiting BR, BRP activates ethylene response. Analysis of BRP analogs provided clues about structural features important for its effects on two separate targets in the BR and ethylene pathways. Analyses of the responses of various BR and ethylene mutants to BRP, ethylene, and BR treatments revealed modes of cross-talk between ethylene and BR in dark-grown seedlings. Our results suggest that active downstream BR signaling, but not BR synthesis or a BR gradient, is required for ethylene-induced apical hook formation. The BRP-related compounds can be useful tools for manipulating plant growth and studying hormone interactions.
DOI:10.1093/jxb/erx463PMID:29309681 [本文引用: 1]
PMID:16096973 [本文引用: 1]

In an attempt to compensate for their sessile nature, plants have developed growth responses to deal with the copious and rapid changes in their environment. These responses are known as tropisms and they are marked by a directional growth response that is the result of differential cellular growth and development in response to an external stimulation such as light, gravity or touch. While the mechanics of tropic growth and subsequent development have been the topic of debate for more than a hundred years, only recently have researchers been able to make strides in understanding how plants perceive and respond to tropic stimulations, thanks in large part to mutant analysis and recent advances in genomics. This paper focuses on the recent advances in four of the best-understood tropic responses and how each affects plant growth and development: phototropism, gravitropism, thigmotropism and hydrotropism. While progress has been made in deciphering the events between tropic stimulation signal perception and each characteristic growth response, there are many areas that remain unclear, some of which will be discussed herein. As has become evident, each tropic response pathway exhibits distinguishing characteristics. However, these pathways of tropic perception and response also have overlapping components - a fact that is certainly related to the necessity for pathway integration given the ever-changing environment that surrounds every plant.
DOI:10.1016/j.cub.2017.05.085URL [本文引用: 1]
DOI:10.3732/ajb.1200591URL [本文引用: 1]
DOI:10.1016/j.devcel.2004.07.002URL [本文引用: 2]
DOI:10.1105/tpc.15.00569URL [本文引用: 6]
DOI:10.1093/jxb/ert080PMID:23580748 [本文引用: 1]

The plant hormone auxin drives plant growth and morphogenesis. The levels and distribution of the active auxin indole-3-acetic acid (IAA) are tightly controlled through synthesis, inactivation, and transport. Many auxin precursors and modified auxin forms, used to regulate auxin homeostasis, have been identified; however, very little is known about the integration of multiple auxin biosynthesis and inactivation pathways. This review discusses the many ways auxin levels are regulated through biosynthesis, storage forms, and inactivation, and the potential roles modified auxins play in regulating the bioactive pool of auxin to affect plant growth and development.
DOI:10.1073/pnas.1108434108URL [本文引用: 1]
DOI:10.1093/mp/ssr104URL [本文引用: 2]
DOI:10.1016/j.cell.2008.01.049PMID:18394996 [本文引用: 2]

Plants grown at high densities perceive a decrease in the red to far-red (R:FR) ratio of incoming light, resulting from absorption of red light by canopy leaves and reflection of far-red light from neighboring plants. These changes in light quality trigger a series of responses known collectively as the shade avoidance syndrome. During shade avoidance, stems elongate at the expense of leaf and storage organ expansion, branching is inhibited, and flowering is accelerated. We identified several loci in Arabidopsis, mutations in which lead to plants defective in multiple shade avoidance responses. Here we describe TAA1, an aminotransferase, and show that TAA1 catalyzes the formation of indole-3-pyruvic acid (IPA) from L-tryptophan (L-Trp), the first step in a previously proposed, but uncharacterized, auxin biosynthetic pathway. This pathway is rapidly deployed to synthesize auxin at the high levels required to initiate the multiple changes in body plan associated with shade avoidance.
DOI:10.1105/tpc.111.231112 [本文引用: 3]
DOI:10.1105/tpc.114.127993URL [本文引用: 2]
DOI:10.1101/gad.1415106URL [本文引用: 2]
DOI:10.1105/tpc.107.053009URL [本文引用: 2]
PMID:11209081 [本文引用: 1]

Although auxin is known to regulate many processes in plant development and has been studied for over a century, the mechanisms whereby plants produce it have remained elusive. Here we report the characterization of a dominant Arabidopsis mutant, yucca, which contains elevated levels of free auxin. YUCCA encodes a flavin monooxygenase-like enzyme and belongs to a family that includes at least nine other homologous Arabidopsis genes, a subset of which appears to have redundant functions. Results from tryptophan analog feeding experiments and biochemical assays indicate that YUCCA catalyzes hydroxylation of the amino group of tryptamine, a rate-limiting step in tryptophan-dependent auxin biosynthesis.
DOI:10.1105/tpc.111.088047URL [本文引用: 2]
DOI:10.1186/gb-2009-10-12-249PMID:20053306 [本文引用: 4]

The PIN-FORMED (PIN) proteins are secondary transporters acting in the efflux of the plant signal molecule auxin from cells. They are asymmetrically localized within cells and their polarity determines the directionality of intercellular auxin flow. PIN genes are found exclusively in the genomes of multicellular plants and play an important role in regulating asymmetric auxin distribution in multiple developmental processes, including embryogenesis, organogenesis, tissue differentiation and tropic responses. All PIN proteins have a similar structure with amino- and carboxy-terminal hydrophobic, membrane-spanning domains separated by a central hydrophilic domain. The structure of the hydrophobic domains is well conserved. The hydrophilic domain is more divergent and it determines eight groups within the protein family. The activity of PIN proteins is regulated at multiple levels, including transcription, protein stability, subcellular localization and transport activity. Different endogenous and environmental signals can modulate PIN activity and thus modulate auxin-distribution-dependent development. A large group of PIN proteins, including the most ancient members known from mosses, localize to the endoplasmic reticulum and they regulate the subcellular compartmentalization of auxin and thus auxin metabolism. Further work is needed to establish the physiological importance of this unexpected mode of auxin homeostasis regulation. Furthermore, the evolution of PIN-based transport, PIN protein structure and more detailed biochemical characterization of the transport function are important topics for further studies.
DOI:10.1242/dev.021071PMID:18787070 [本文引用: 1]

The signalling molecule auxin controls plant morphogenesis via its activity gradients, which are produced by intercellular auxin transport. Cellular auxin efflux is the rate-limiting step in this process and depends on PIN and phosphoglycoprotein (PGP) auxin transporters. Mutual roles for these proteins in auxin transport are unclear, as is the significance of their interactions for plant development. Here, we have analysed the importance of the functional interaction between PIN- and PGP-dependent auxin transport in development. We show by analysis of inducible overexpression lines that PINs and PGPs define distinct auxin transport mechanisms: both mediate auxin efflux but they play diverse developmental roles. Components of both systems are expressed during embryogenesis, organogenesis and tropisms, and they interact genetically in both synergistic and antagonistic fashions. A concerted action of PIN- and PGP-dependent efflux systems is required for asymmetric auxin distribution during these processes. We propose a model in which PGP-mediated efflux controls auxin levels in auxin channel-forming cells and, thus, auxin availability for PIN-dependent vectorial auxin movement.
DOI:10.1105/tpc.112.097766URL [本文引用: 4]
DOI:10.3390/ijms19092759URL [本文引用: 3]
DOI:10.1111/tpj.2009.59.issue-1URL [本文引用: 1]
PMID:11701880 [本文引用: 1]

Arabidopsis possesses several genes related to the multidrug resistance (MDR) genes of animals, one of which, AtMDR1, was shown to be induced by the hormone auxin. Plants having mutations in AtMDR1 or its closest relative, AtPGP1, were isolated by a reverse genetic strategy. Auxin transport activity was greatly impaired in atmdr1 and atmdr1 atpgp1 double mutant plants. Epinastic cotyledons and reduced apical dominance were mutant phenotypes consistent with the disrupted basipetal flow of auxin. The auxin transport inhibitor 1-naphthylphthalamic acid was shown to bind tightly and specifically to AtMDR1 and AtPGP1 proteins. The results indicate that these two MDR-like genes of Arabidopsis encode 1-naphthylphthalamic acid binding proteins that are required for normal auxin distribution and auxin-mediated development.
DOI:10.1111/j.1365-313X.2010.04137.xURL [本文引用: 2]
DOI:10.1038/415806aURL [本文引用: 1]
PMID:16192309 [本文引用: 1]

Plant development displays an exceptional plasticity and adaptability that involves the dynamic, asymmetric distribution of the phytohormone auxin. Polar auxin flow, which requires polarly localized transport facilitators of the PIN family, largely contributes to the establishment and maintenance of the auxin gradients. Functionally overlapping action of PIN proteins mediates multiple developmental processes, including embryo formation, organ development and tropisms. Here we show that PIN proteins exhibit synergistic interactions, which involve cross-regulation of PIN gene expression in pin mutants or plants with inhibited auxin transport. Auxin itself positively feeds back on PIN gene expression in a tissue-specific manner through an AUX/IAA-dependent signalling pathway. This regulatory switch is indicative of a mechanism by which the loss of a specific PIN protein is compensated for by auxin-dependent ectopic expression of its homologues. The compensatory properties of the PIN-dependent transport network might enable the stabilization of auxin gradients and potentially contribute to the robustness of plant adaptive development.
DOI:10.1146/annurev-genet-102108-134148URL [本文引用: 1]
DOI:10.1038/nature03543URL [本文引用: 3]
PMID:15992545 [本文引用: 2]

The plant hormone auxin has been implicated in virtually every aspect of plant growth and development. Auxin acts by promoting the degradation of transcriptional regulators called Aux/IAA proteins. Aux/IAA degradation requires TIR1, an F box protein that has been shown to function as an auxin receptor. However, loss of TIR1 has a modest effect on auxin response and plant development. Here we show that three additional F box proteins, called AFB1, 2, and 3, also regulate auxin response. Like TIR1, these proteins interact with the Aux/IAA proteins in an auxin-dependent manner. Plants that are deficient in all four proteins are auxin insensitive and exhibit a severe embryonic phenotype similar to the mp/arf5 and bdl/iaa12 mutants. Correspondingly, all TIR1/AFB proteins interact with BDL, and BDL is stabilized in triple mutant plants. Our results indicate that TIR1 and the AFB proteins collectively mediate auxin responses throughout plant development.
DOI:10.1038/nature03542URL [本文引用: 3]
DOI:10.1038/nchembio.926PMID:22466420 [本文引用: 2]

The plant hormone auxin regulates virtually every aspect of plant growth and development. Auxin acts by binding the F-box protein transport inhibitor response 1 (TIR1) and promotes the degradation of the AUXIN/INDOLE-3-ACETIC ACID (Aux/IAA) transcriptional repressors. Here we show that efficient auxin binding requires assembly of an auxin co-receptor complex consisting of TIR1 and an Aux/IAA protein. Heterologous experiments in yeast and quantitative IAA binding assays using purified proteins showed that different combinations of TIR1 and Aux/IAA proteins form co-receptor complexes with a wide range of auxin-binding affinities. Auxin affinity seems to be largely determined by the Aux/IAA. As there are 6 TIR1/AUXIN SIGNALING F-BOX proteins (AFBs) and 29 Aux/IAA proteins in Arabidopsis thaliana, combinatorial interactions may result in many co-receptors with distinct auxin-sensing properties. We also demonstrate that the AFB5-Aux/IAA co-receptor selectively binds the auxinic herbicide picloram. This co-receptor system broadens the effective concentration range of the hormone and may contribute to the complexity of auxin response.
DOI:10.1111/tpj.2009.59.issue-1URL [本文引用: 2]
DOI:10.1038/nature05731URL [本文引用: 2]
DOI:10.1038/nplants.2017.105PMID:28714973 [本文引用: 1]

The phytohormone auxin induces or represses growth depending on its concentration and the underlying tissue type. However, it remains unknown how auxin signalling is modulated to allow tissues transiting between repression and promotion of growth. Here, we used apical hook development as a model for growth transitions in plants. A PIN-FORMED (PIN)-dependent intercellular auxin transport module defines an auxin maximum that is causal for growth repression during the formation of the apical hook. Our data illustrate that growth transition for apical hook opening is largely independent of this PIN module, but requires the PIN-LIKES (PILS) putative auxin carriers at the endoplasmic reticulum. PILS proteins reduce nuclear auxin signalling in the apical hook, leading to the de-repression of growth and the onset of hook opening. We also show that the phytochrome (phy) B-reliant light-signalling pathway directly regulates PILS gene activity, thereby enabling light perception to repress nuclear auxin signalling and to control growth. We propose a novel mechanism, in which PILS proteins allow external signals to alter tissue sensitivity to auxin, defining differential growth rates.
DOI:10.1093/pcp/pci111URL [本文引用: 1]
PMID:8624510 [本文引用: 1]

By screening suppressor mutants of the hy2 mutation of Arabidopsis thaliana, two dominant photomorphogenic mutants, shy1-1D and shy2-1D, for two genetic loci designated as SHY1 and SHY2 (suppressor of hy2 mutation) have been isolated. Both of these non-allelic, extragenic suppressor mutations of hy2 are located on chromosome 1 of the Arabidopsis genome. Both mutations suppress the elongated hypocotyl phenotype of hy2 by light-independent inhibition of hypocotyl growth as well as by increasing the effectiveness of light inhibition of hypocotyl elongation. The shy1-1D mutation is partially photomorphogenic in darkness with apical hook opening and reduced hypocotyl elongation. The shy2-1D mutant displays highly photomorphogenic characteristics in darkness such as true leaf development, cotyledon expansion and extremely reduced hypocotyl growth. In regard to hypocotyl elongation, however, the shy2-1D mutation is still light sensitive. Examination of red-far-red light responses shows that the shy1-1D mutation suppresses the hypocotyl elongation of the hy2 mutation effectively in red light but not effectively in far-red light. The shy2-1D suppresses hypocotyl elongation of the hy2 mutation effectively in both red and far-red light. Both mutations can also suppress the early-flowering phenotype of hy2 and have a distinct pleiotropic effect on leaf development such as upward leaf rolling. The data obtained suggest that SHY1 and SHY2 represent a novel class of components involved in the photomorphogenic pathways of Arabidopsis. This is the first report on the identification of dominant mutations in the light signal transduction pathway of plants.
PMID:9744095 [本文引用: 1]

We previously reported a photomorphogenic mutation of Arabidopsis thaliana, shy2-1D, as a dominant suppressor of a hy2 mutation. Here, we report that shy2-1D confers various photo-responsive phenotypes in darkness and the dark phenotypes of the mutant are affected by phytochrome deficiency. Dark-grown seedlings of the mutant developed several photomorphogenic characteristics such as short hypocotyls, cotyledon expansion and opening, and partial differentiation of plastids. When grown further in darkness, the mutant plant underwent most of the developmental stages of a light-grown wild-type plant, including development of foliar leaves, an inflorescence stem with cauline leaves, and floral organs. In addition, two light-inducible genes, the nuclear-encoded CAB and the plastid-encoded PSBA genes, were highly expressed in the dark-grown mutant seedlings. Furthermore, reduced gravitropism, a phytochrome-modulated response, was observed in the mutant hypocotyl in darkness. Thus, shy2-1D is one of the most pleiotropic photomorphogenic mutations identified so far. The results indicate that SHY2 may be a key component regulating photomorphogenesis in Arabidopsis. Surprisingly, double mutants of the shy2-1D mutant with the phytochrome-deficient mutants hy2, hy3(phyB-1) and fre1-1(phyA-201) showed reduced photomorphogenic response in darkness with a longer hypocotyl, a longer inflorescence stem, and a lower level expression of the CAB gene than the shy2-1D single mutant. These results showed that phytochromes function in darkness in the shy2-1D mutant background. The implications of these results are discussed.
DOI:10.1104/pp.105.070987URL [本文引用: 1]
PMID:15960614 [本文引用: 3]

AUXIN RESPONSE FACTORS (ARFs) regulate auxin-mediated transcriptional activation/repression. They are encoded by a gene family in Arabidopsis, and each member is thought to play a central role in various auxin-mediated developmental processes. We have characterized three arf2 mutant alleles, arf2-6, arf2-7 and arf2-8. The mutants exhibit pleiotropic developmental phenotypes, including large, dark green rosette leaves, delayed flowering, thick and long inflorescence, abnormal flower morphology and sterility in early formed flowers, large organ size and delayed senescence and abscission, compared with wild-type plants. In addition, arf2 mutant seedlings have elongated hypocotyls with enlarged cotyledons under various light conditions. The transcription of ACS2, ACS6 and ACS8 genes is impaired in the developing siliques of arf2-6. The phenotypes of all three alleles are similar to those of the loss-of-function mutants obtained by RNA interference or co-suppression. There is no significant effect of the mutation on global auxin-regulated gene expression in young seedlings, suggesting that ARF2 does not participate in auxin signaling at that particular developmental stage of the plant life cycle. Because ARF2 is thought to function as a transcriptional repressor, the prospect arises that its pleiotropic effects may be mediated by negatively modulating the transcription of downstream genes in signaling pathways that are involved in cell growth and senescence.
PMID:15659631 [本文引用: 2]

The AUXIN RESPONSE FACTOR (ARF) gene family products, together with the AUXIN/INDOLE-3-ACETIC ACID proteins, regulate auxin-mediated transcriptional activation/repression. The biological function(s) of most ARFs is poorly understood. Here, we report the identification and characterization of T-DNA insertion lines for 18 of the 23 ARF gene family members in Arabidopsis thaliana. Most of the lines fail to show an obvious growth phenotype except of the previously identified arf2/hss, arf3/ett, arf5/mp, and arf7/nph4 mutants, suggesting that there are functional redundancies among the ARF proteins. Subsequently, we generated double mutants. arf7 arf19 has a strong auxin-related phenotype not observed in the arf7 and arf19 single mutants, including severely impaired lateral root formation and abnormal gravitropism in both hypocotyl and root. Global gene expression analysis revealed that auxin-induced gene expression is severely impaired in the arf7 single and arf7 arf19 double mutants. For example, the expression of several genes, such as those encoding members of LATERAL ORGAN BOUNDARIES domain proteins and AUXIN-REGULATED GENE INVOLVED IN ORGAN SIZE, are disrupted in the double mutant. The data suggest that the ARF7 and ARF19 proteins play essential roles in auxin-mediated plant development by regulating both unique and partially overlapping sets of target genes. These observations provide molecular insight into the unique and overlapping functions of ARF gene family members in Arabidopsis.
PMID:14729917 [本文引用: 1]

We have isolated a dominant, auxin-insensitive mutant of Arabidopsis thaliana, massugu2 (msg2), that displays neither hypocotyl gravitropism nor phototropism, fails to maintain an apical hook as an etiolated seedling, and is defective in lateral root formation. Yet other aspects of growth and development of msg2 plants are almost normal. These characteristics of msg2 are similar to those of another auxin-insensitive mutant, non-phototropic hypocotyl4 (nph4), which is a loss-of-function mutant of AUXIN RESPONSE FACTOR7 (ARF7) (Harper et al., 2000). Map-based cloning of the MSG2 locus reveals that all four mutant alleles result in amino acid substitutions in the conserved domain II of an Auxin/Indole-3-Acetic Acid protein, IAA19. Interestingly, auxin inducibility of MSG2/IAA19 gene expression is reduced by 65% in nph4/arf7. Moreover, MSG2/IAA19 protein binds to the C-terminal domain of NPH4/ARF7 in a Saccharomyces cerevisiae (yeast) two-hybrid assay and to the whole latter protein in vitro by pull-down assay. These results suggest that MSG2/IAA19 and NPH4/ARF7 may constitute a negative feedback loop to regulate differential growth responses of hypocotyls and lateral root formation.
PMID:17900969 [本文引用: 1]

Auxin signaling is key to many plant growth and developmental processes from embryogenesis to senescence. Most, if not all, of these processes are initiated and/or mediated through auxin-regulated gene expression. Two types of transcription factor families are required for controlling expression of auxin response genes. One of these, the auxin response factor (ARF) family, functions by binding to auxin response elements (AuxREs) on promoters of auxin response genes, activating or repressing the auxin response genes, and recruiting a second family of transcription factors, the Aux/IAA repressors, that confer an auxin response to the genes. Recent advances have provided information on regulation of ARF gene expression, ARF roles in growth and developmental processes, and target genes regulated by ARFs.
DOI:10.1038/s41586-019-1069-7URL [本文引用: 4]
DOI:10.1093/aob/mcl027URL [本文引用: 1]
DOI:10.1105/tpc.15.00605URL [本文引用: 1]
DOI:10.3389/fpls.2018.00838URL [本文引用: 1]
DOI:10.3389/fpls.2021.636098PMID:33767720 [本文引用: 1]

During seedling etiolation after germination in the dark, seedlings have closed cotyledons and form an apical hook to protect the meristem as they break through the soil to reach the surface. Once in contact with light, the hook opens and cotyledons are oriented upward and separate. Hook development in the dark after seedling emergence from the seed follows three distinctly timed and sequential phases: formation, maintenance, and eventual opening. We previously identified () as a phytochrome interacting factor (PIF)-repressed gene in the dark necessary for hook development during etiolated growth. encodes the type 2C phosphatase PP2C.D1, and mutants exhibit open hooks in the dark. Recent evidence has described that PP2C.D1 and other PP2C.D members negatively regulate SMALL AUXIN UP RNA (SAUR)-mediated cell elongation. However, the fundamental question of the timing of PP2C.D1 action (and possibly other members of the PP2C.D family) during hook development remains to be addressed. Here, we show that PP2C.D1 is required immediately after germination to form the hook. shows reduced cell expansion in the outer layer of the hook and, therefore, does not establish the differential cell growth necessary for hook formation, indicating that PP2C.D1 is necessary to promote cell elongation during this early stage. Additionally, genetic analyses of single and high order mutants in PP2C.D1, PP2C.D2, and PP2C.D5 demonstrate that the three PP2C.Ds act collectively and sequentially during etiolation: whereas PP2C.D1 dominates hook formation, PP2C.D2 is necessary during the maintenance phase, and PP2C.D5 acts to prevent opening during the third phase together with PP2C.D1 and PP2C.D2. Finally, we uncover a possible connection of PP2C.D1 levels with ethylene physiology, which could help optimize hook formation during post-germinative growth in the dark.Copyright © 2021 Rovira, Sentandreu, Nagatani, Leivar and Monte.
DOI:10.1016/j.devcel.2020.12.008URL [本文引用: 1]
DOI:10.1371/journal.pgen.1006639URL [本文引用: 1]
