 ,1
,1Regulatory signaling pathways in hematopoietic stem cell development
Chunxia Zhang1,2, Feng Liu ,1
,1通讯作者: 刘峰,研究员,博士生导师,研究方向:造血干细胞发育。E-mail:liuf@ioz.ac.cn
编委: 张雷
收稿日期:2021-01-20修回日期:2021-03-1网络出版日期:2021-04-16
| 基金资助: |
Received:2021-01-20Revised:2021-03-1Online:2021-04-16
| Fund supported: |
作者简介 About authors

张春霞,博士,研究方向:造血干细胞发育。E-mail:
2010—2017年就读于中国科学院动物研究所,在血液与心血管发育研究组攻读博士学位,目前在美国哈佛医学院/波士顿儿童医院做博士后。博士期间,主要研究斑马鱼造血干细胞发育的调控机制,阐明了BMP与ERK信号通路在造血干细胞产生过程中的相互关联(NatCommun,2014年),发现了炎性信号通路可以通过Notch信号来调控造血干细胞的产生(Blood,2015年),首次揭示了m6A修饰在胚胎造血干细胞命运决定中的关键作用(Nature,2017年)。这些工作不仅增加了人们对造血干细胞产生机制的认识,而且对体外诱导扩增造血干细胞提供了理论指导。博士论文《BMP-ERK信号通路以及炎性信号调控斑马鱼造血干细胞产生的分子机制》获得2020年中国科学院优秀博士生论文。。

摘要
血液系统是维持机体生命活动最重要的系统之一,为机体提供所需的氧气和营养物质,通过物质交换维持内环境的稳态,同时为机体提供免疫防御与保护。血细胞是血液的重要组成成分,机体中成熟血细胞类型起源于具有自我更新及分化潜能的多能成体干细胞—造血干细胞(hematopoietic stem cells, HSCs)。造血干细胞及各类血细胞产生、发育及成熟的过程称为造血过程,该过程开始于胚胎发育早期并贯穿整个生命过程,任一阶段出现异常都可能导致血液疾病的发生。因此,深入探究造血发育过程及其调控机制对于认识并治疗血液疾病至关重要。近年来,以小鼠(Mus musculus)和斑马鱼(Danio rerio)作为动物模型来研究造血发育取得了一系列的进展。其中,BMP、Notch和Wnt等信号通路对造血干细胞的命运决定和产生发挥了重要作用。本文对这些信号通路在小鼠和斑马鱼造血过程中的调控作用进行系统总结,以期能够完善造血发育过程的调控网络并为临床应用提供指导。
关键词:
Abstract
The blood system provides the body with oxygen and nutrients, maintains the homeostasis of the internal environment through material exchange, and keeps the body with immune defense and protection. Hematopoietic stem cells (HSCs), which are pluripotent adult stem cells with self-renewal and differentiation potential, are the origin of mature blood cells in the body. The production, development and maturation processes of HSCs and their derivatives are the so-called ‘hematopoiesis’, which begins in the early embryonic development and throughout the life course; any abnormality during these processes can cause the occurrence of hematological diseases. Therefore, a deeper understanding of hematopoietic development and its regulation is important to the diagnosis and treatment of blood diseases. In recent years, a series of advances have been made in studying hematopoietic development using mice and zebrafish as animal models. It has been shown that BMP, Notch and Wnt signaling pathways play an important role in the fate determination and generation of HSCs. In this review, we systematically summarize the regulatory roles of these signaling pathways in the hematopoietic process of mice and zebrafish embryos, to improve our understanding on the underlying regulatory network of hematopoietic development and provide guidance for clinical application.
Keywords:
PDF (701KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
张春霞, 刘峰. 造血干细胞发育过程中的信号通路调控. 遗传[J], 2021, 43(4): 295-307 doi:10.16288/j.yczz.21-026
Chunxia Zhang.
造血干细胞(hematopoietic stem cells, HSCs)是一群具有自我更新及分化潜能的多能干细胞,可以产生所有类型的血细胞[1]。血细胞可以分为髓系和淋系两大类,其中髓系细胞包括红系细胞、粒系细胞、巨核系细胞和单核系细胞,淋系细胞主要包括T淋巴细胞、B淋巴细胞和自然杀伤性细胞(NK细胞)。造血干细胞及各类血细胞产生、发育和成熟的过程称为造血过程。脊椎动物的造血过程起始于胚胎期,可以分为初级造血和次级造血两个阶段。造血干细胞以内皮-造血转化的形式产生于次级造血阶段。造血干细胞发育在脊椎动物中是高度保守的过程,该过程受到多种信号通路和调控因子的精密调控。一些信号通路,如ERK、Notch以及β-Catenin介导的Wnt信号通路,在造血干细胞产生过程中的作用是动态变化的,也是浓度和时间依赖的。深入了解这些信号通路在造血干细胞发育过程中的调控网络和作用机制,不仅能够帮助理解体内造血干细胞的产生过程,也能为体外造血提供理论依据。小鼠(Mus musculus)和斑马鱼(Danio rerio)是目前最常用的研究造血发育的动物模型。从胚胎发育早期到成体,尽管发生部位有所不同,造血发育的阶段性过程在两种动物模型中是相似的。本文以BMP、Notch和Wnt信号通路为例,详细介绍信号通路在造血发育过程中的调控作用。
1 脊椎动物造血发育过程
1.1 小鼠造血发育
哺乳动物的造血发生起源于胚胎的腹侧中胚层,其中部分细胞特化为成血成血管细胞(hemangioblast),这类细胞同时具有向血细胞和血管细胞分化的能力[2]。在小鼠中,成血成血管细胞最早出现在卵黄囊的血岛区(blood island in the yolk sac),胚胎期7.5天(E7.5)左右此处可检测到血细胞的产生,这一过程称为初级造血(primitive hematopoiesis),主要产生初级红系(primitive erythroid)和初级髓系(primitive myeloid)血细胞,为胚胎早期发育供氧气和免疫防御[3]。E9.5卵黄囊区能够产生一群具有分化成红细胞、巨核细胞和巨噬细胞等多项潜能的红系-髓系前体细胞(erythro-myeloid progenitors, EMPs)[4,5]。近期研究发现特定组织定居的巨噬细胞,如肝脏的Kupffer细胞、脑部的小胶质细胞、上皮的郎格罕氏细胞及肺部的肺泡巨噬细胞等,都来源于卵黄囊的红系-髓系前体细胞,并不依赖于造血干细胞[6,7]。除红系和髓系细胞之外,在造血干细胞产生之前,卵黄囊还能产生一群具有淋系(lymphoid)分化潜能的前体细胞,可以分化为B淋巴细胞和T淋巴细胞,为机体供免疫防御[8]。初级造血是一个短暂的阶段,其产生的血细胞类型和数目不足以维持胚胎发育和成体需要,因此很快就被次级造血(definitive hematopoiesis)代替[4]。造血干细胞产生于次级造血阶段,主要在E10.5小鼠胚胎的动脉-性腺-中肾区(aorta-gonad-mesonephros, AGM)产生[9],在小鼠的胎盘[10]等区域也能检测到造血干细胞。随着研究的深入,造血干细胞的产生过程已经逐渐明晰。在主动脉腹侧壁的一群内皮细胞可以特化成具有造血潜能的生血内皮(hemogenic endothelium, HE),随后细胞形态发生变化,形成造血簇(hematopoietic clusters),逐渐脱离动脉内皮形成造血干细胞进入主动脉,这一过程称为内皮-造血转化(endothelial-to-hematopoietic transition, EHT)[11]。新产生的造血干细胞在E12左右进入胎肝(fetal liver, FL)并进行大量扩增并逐渐成熟,其中一部分造血干细胞随后进入胸腺,分化为T淋巴细胞。最终,造血干细胞定位到终生造血器官骨髓(bone marrow, BM)。正常状态下骨髓中的造血干细胞处于静息状态,一旦机体需要,造血干细胞将会迅速动员起来并分化成各种血细胞以补充机体需要[1]。
1.2 斑马鱼造血发育
在过去的20年里,斑马鱼凭借其独特的优势,已经成为研究发育的经典动物模型。首先,斑马鱼是体外受精,从受精卵到发育至成体的整个过程都可以在体外直接观察,而且便于进行操控;其次,斑马鱼胚胎是透明的,利用各种转基因或活体标记技术,能够对各组织器官的发育进行实时观察和追踪;再次,斑马鱼产卵的周期比较短,并且较易获得大量胚胎,这样有利于进行高通量筛选实验;最重要的是斑马鱼的基因组与哺乳动物高度保守,斑马鱼中的很多成果可以为哺乳动物的相关研究提供参考 [12]。斑马鱼的造血发育过程与哺乳动物相似,均起源于胚胎的腹侧中胚层。在发育过程中,后部的侧板中胚层(posterior lateral mesoderm, PLM)逐渐向中间迁移形成中间细胞团(intermediate cell mass, ICM),并在此处产生初级红系细胞。同时前部侧板中胚层(anterior lateral mesoderm, ALM)能够产生初级髓系细胞[12,13]。斑马鱼的红系-髓系前体细胞出现在受精后36小时(36 hours post fertilization, 36 hpf)左右的尾部造血组织(caudal hematopoietic tissue, CHT),并且能够在此处进行分化[14]。斑马鱼的造血干细胞也是在AGM区域经过内皮-造血转化过程产生。利用转基因荧光标记的方法对斑马鱼造血干细胞的产生过程进行实时追踪观察发现,主动脉腹侧壁的部分内皮细胞可以特化成为生血内皮,其形态也由扁平逐渐变成圆形,最后以出芽的形式脱离主动脉[14,15]。与小鼠不同的是,斑马鱼的造血干细胞不形成血细胞簇,而是以单个细胞的形式产生,并在产生后直接进入动静脉之间的间充质,随后进入静脉。斑马鱼的内皮-造血转化过程起始于32 hpf,在60 hpf之前结束。新产生的造血干细胞随后会迁移到CHT区域进行扩增和分化,部分细胞会迁移到胸腺分化成T淋巴细胞。造血干细胞最终会进入斑马鱼的终生造血器官—肾脏(Kidney Marrow),为胚胎和成体提供各类血细胞。
2 造血干细胞产生过程的关键转录因子
造血干细胞的发育调控机制精密且有序。在此过程中,任何环节的失调都可能引起血液系统发育缺陷或功能紊乱,最终导致血液疾病的发生。因此,系统了解并深入探究造血干细胞发育的调控机制具有重大意义。在造血发育过程中,转录因子Scl (stem cell leukemia,也称作T-cell acute lymphocytic leukemia 1, TAL1)是成血成血管细胞的标记基因之一,对主动脉和造血干细胞的发育都有重要的调控作用[16,17]。斑马鱼的scl基因有两个转录本—sclα和sclβ,虽然这两个转录本都能够参与调控造血干细胞的产生,但是它们分别调控内皮-造血转化过程的不同阶段。通过构建荧光标记的sclα和sclβ转基因斑马鱼并进行实时观察发现,sclβ在生血内皮细胞中特异表达,对生血内皮的命运决定至关重要,而sclα则在新产生的造血干细胞中特异表达,对造血干细胞在AGM区的维持发挥作用[18]。
作为常用的造血干细胞标记基因之一,转录因子Runx1 (RUNX family transcription factor 1,也称作acute mylogenous leukemia 1, AML1)可以标记所有的胚胎造血干细胞[19]。在小鼠胚胎中进行的功能实验发现,缺失runx1后次级造血过程被阻断[20]。进一步的条件性敲除实验发现,内皮细胞中的Runx1对于造血干细胞的产生必不可少,而造血干细胞产生之后,Runx1对造血维持并不重要[21]。近年来,对于Runx1在造血干细胞产生过程中的作用有了进一步认识。通过观察斑马鱼内皮-造血转化过程发现,缺失runx1后,部分内皮细胞可以发生形变并开始出芽,但在形成造血干细胞过程中这群细胞会发生破裂,导致造血干细胞无法产生[15]。随后的研究发现,Runx1可以保证造血特性的获得,而其下游靶基因Gfi1/Gfi1b (growth factor independent 1A transcription repressor 1)能够在生血内皮细胞中抑制内皮基因的表达,并促进细胞发生形变,进而保证造血干细胞的正常产生[22]。
除此之外,Gata2 (GATA binding protein 2)也是造血干细胞产生的关键调控因子。在小鼠的造血发育过程中,Gata2在主动脉及造血干细胞簇中特异表达[23]。在内皮细胞中特异性敲除Gata2发现,生血内皮可以正常产生,但是内皮-造血转化过程不能发生[24],与Runx1不同的是,在造血干细胞产生之后,血细胞中的Gata2对于造血干细胞的生存和维持也非常重要[24,25]。斑马鱼的gata2有两个同源基因—,gata2a和gata2b。其中,gata2a在受精后11小时就开始表达于成血成血管细胞中[26],而gata2b特异性表达在生血内皮中[27]。在功能上,Gata2a和Gata2b都能通过调控runx1的表达来促进生血内皮的特化,同时gata2b又受到Gata2a的调控[27,28]。
3 调控造血干细胞发育的信号通路
3.1 BMP信号通路
骨形态发生蛋白(bone morphogenesis proteins, BMPs)是转化生长因子(transforming growth factor β, TGF-β)超家族的一员[29]。作为一种形态发生素,BMP信号在胚胎发生、发育以及成体组织稳态的维持中都发挥着重要作用[30]。经典的BMP信号通路是Smads依赖性的。BMP配体先与I型受体(BMPRIs) 结合,形成复合体后再与II型受体(BMPRII)结合。II型受体是持续激活型的,能够磷酸化I型受体。激活的I型受体随后磷酸化下游的Smad蛋白。作为细胞内的信号分子,脊椎动物中的8种Smad蛋白可以分为3类:受体调节型Smads (receptor-regulated Smads, R-Smads)、共配体型Smads (common-mediator Smads, Co-Smads)以及抑制型Smads (inhibitory Smads, I-Smads)。Co-Smads (Smad4)和I-Smads (Smad6和Smad7)在TGF-β超家族中是通用的,而R-Smads中能够参与BMP信号传导的主要包括Smad1、Smad5和Smad8[31]。经受体激活后的R-Smads从细胞膜上脱离,在胞内与Smad4结合并进入细胞核。在细胞核内,Smads可以结合DNA,同时可以招募共同作用因子调控基因表达,如Smad1/5-Smad4与OAZ (Olf-1/ EBF-associated zinc finger)形成复合体后能够招募共激活因子p300/CBP等促进基因表达[32];Smad1- Smad4与Nkx3.2形成复合体后能够招募共抑制因子mSin3/HDAC1等抑制基因表达[33]。作为抑制型Smads,Smad6能够抑制Smad4与磷酸化Smad1的结合从而阻断BMP信号的传递[34];Smad7能够竞争性地结合TGF-β或BMP受体,并能够与Smurf1/2共同作用通过泛素化途径降解受体进而阻断信号的传递[35,36]。
3.1.1 经典的BMP-Smad1/5在HSC发育过程的调控作用
脊椎动物的造血发育起源于腹侧中胚层, BMP蛋白(尤其是BMP4)能够诱导腹侧中胚层的形成[37,38]。为了进一步研究BMP信号通路在造血发育中的作用,研究人员用转基因的方法在时间和空间上来控制BMP信号的活性。首先,为避免BMP信号缺失对早期中胚层发育的影响,研究人员利用造血、血管及前肾前体细胞特异性的lmo2启动子在斑马鱼胚胎侧板中胚层中过表达功能缺失的BMP受体,发现BMP信号在这些前体细胞中的缺失能够促进血管和造血的发育但抑制前肾发育,说明BMP信号在细胞命运决定中起到重要的调控作用[39]。随后,为避免对动脉发育的影响,研究人员利用热激蛋白(heat shock protein 70, hsp70)启动子特异性地在斑马鱼动脉发育完成之后过表达功能缺失的BMP受体来抑制BMP信号通路,结果发现造血干细胞的产生和维持不能正常进行[40],说明BMP信号在造血发育中具有不可缺少的作用。虽然BMP4在24hpf的斑马鱼胚胎中表达于腹侧间充质(ventral mesenchyme)中,但是BMP受体Bmpr2a在主动脉内皮中可以检测到[41],暗示着BMP受体可以将BMP信号传递到主动脉中,进而调控造血干细胞的产生。在小鼠胚胎中,BMP4在腹侧间充质中表达,BMP受体Alk3、Alk6和BmprII,以及BMP下游效应因子Smad1、4、5在AGM区动脉和血细胞中表达用小分子药物抑制BMP信号后,小鼠造血活性明显降低[42]。以上这些研究说明BMP信号对造血干细胞的产生和维持都发挥重要作用。
对于BMP信号通路调控造血发育过程的分子机制,近年来也有深入研究。作为BMP信号通路下游的关键信号分子,Smad1和Smad5对于斑马鱼的初级造血过程发挥着不同的作用。Smad1缺失能够增加初级红细胞的数目,而成熟巨噬细胞的产生受到抑制;Smad5缺失导致初级红细胞的缺陷,巨噬细胞不受影响;但是Smad1或Smad5的缺失都会导致次级造血前体细胞的减少[43,44]。在小鼠成血成血管前体细胞中,缺失Smad1可以促进后续造血基因的表达,同时能够使胞核内的磷酸化Smad2 (pSmad2)信号增强,这也暗示着BMP和TGF-β信号通路在造血发育调控中存在互作[45]。另外,作为TGF-β超家族共用的Co-Smad,Smad4在小鼠内皮细胞而不是血细胞中的缺失能够增加造血动脉内造血簇的产生,也能够促进体外造血前体细胞的产生[46],意味着内皮细胞的Smad4在小鼠造血发育过程中发挥着抑制作用。但是Smad5敲除的小鼠,造血干细胞的产生明显减少,内皮细胞的特性有所增强[44]。体外实验发现Smad1直接结合到runx1启动子区域,促进其表达;同时,Smad6可以在Smurf1的协助下抑制Runx1的活性[47],暗示着BMP信号通路可能通过Smad1直接调控造血基因的表达;但在体内造血过程中是否存在直接调控还有待进一步验证。
3.1.2 造血干细胞发育过程中BMP信号通路与FGF信号通路的相互作用
在脊椎动物早期胚胎发育,包括神经特化、初级造血和胚胎干细胞命运维持等过程中,BMP信号通路和ERK介导的FGF信号通路都发挥作用[48,49,50],共同对细胞命运的选择进行调控。通过hsp70启动子驱动的遗传学控制以及特异性的化学抑制剂处理,研究人员发现在造血干细胞的产生过程中BMP信号通路持续发挥作用,而ERK信号通路的作用是动态变化的。在血管发生过程中,ERK信号通路是动脉分化所必须的,一旦动静脉分化完成,ERK信号就需要下调至阈值范围内以保证造血干细胞的产生。BMP信号通路在Smad1/5介导下调控ERK信号。Smad1/5招募共抑制因子HDAC1到erk2启动子区,使其乙酰化水平下降,从而在转录水平上抑制erk2的表达。进一步研究发现,在斑马鱼造血发育过程中,过度激活的ERK信号能够增强动脉内皮细胞的特性以及内皮细胞间的紧密连接,最终导致生血内皮的产生及内皮-造血转化过程受到抑制,进而影响造血干细胞的产生[44]。在小鼠造血发育过程中,研究人员在内皮细胞内特异性敲除Smad4,发现动脉内皮中的BMP4表达增加,而增加的BMP4能够通过非Smad依赖的方式激活ERK通路,从而增加造血细胞簇的产生[46]。这两项研究说明BMP信号通路可以在蛋白水平和转录水平上通过调控ERK信号的活性来影响造血干细胞的产生,而ERK信号对造血干细胞的调控是动态变化的(图1)。
图1
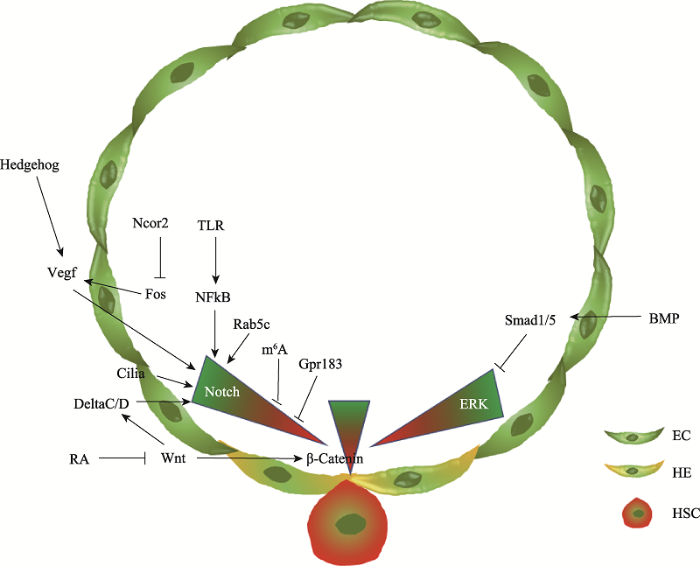 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1斑马鱼造血干细胞发育过程中的信号通路调控示意图
Fig. 1Regulatory signaling pathways during zebrafish hematopoietic stem cell development
3.2 Notch信号通路
Notch蛋白是一个在进化上非常保守的跨膜受体蛋白,能够介导细胞-细胞间的信号传导,对于细胞命运决定非常重要[51]。在哺乳动物和斑马鱼中,Notch信号是由多个蛋白之间的相互关联进行信号传递的,包括信号接收细胞表面的Notch信号的跨膜受体(小鼠Notch1、Notch2、Notch3和Notch4,斑马鱼Notch1a、Notch1b、Notch2和Notch3)与信号释放细胞所释放的Notch配体(Jagged和Delta)结合[52,53]。随后,在ADAM TACE金属蛋白酶(ADAM metalloprotease)的作用下,Notch受体S2位点被切割,在γ-分泌酶(γ-Secretase)的作用下S3位点被切割,释放出Notch细胞内结构域(Notch intracellular domain, NICD)。而在无Notch信号激活的情况下,细胞核内的RBPj能够招募转录共抑制复合物(nuclear corepressor, NCoR)以及组蛋白去乙酰化酶(histone deacetylase, HDACs)抑制Notch信号靶基因的转录。Notch信号激活后,NICD可以入核,替换RBPj并且招募转录共激活复合物从而激活Notch信号靶基因的转录[54]。3.2.1 Notch信号通路在HSC发育过程的调控作用
Notch信号是造血干细胞发育过程中非常重要,也是研究较多的信号通路。Notch信号缺失的突变体中动脉血管不能正常分化[55],作为造血干细胞的来源,动脉血管的缺陷会导致造血干细胞异常产生,这一功能是由Notch1介导的[56]。动脉特异性标记基因ephrinB2作为靶基因,可以直接受Notch1调控[57]。进一步研究发现,Notch信号通路对造血干细胞命运决定及其产生过程也发挥着细胞自主性调控作用。利用hsp70:gal4;uas:NICD转基因斑马鱼品系,在不同时期进行热激处理来控制动脉标记基因的表达,发现Notch通过Runx1调控造血干细胞的产生与动脉发育是两个独立的过程[58]。小鼠中的实验也证明,Jagged1介导的Notch1信号的激活可以通过调节Gata2和Cdca7调控runx1基因的表达,进而调控造血干细胞的产生,这也不依赖于动脉发育[27,59~61]。整体来讲,不同的Notch配体可以介导不同强度的Notch信号,Jagged1能够介导生血内皮细胞中低强度的Notch信号来维持造血干细胞的产生,而Dll4维持内皮细胞中高强度的Notch信号来保证动脉的分化[62]。
近年来,随着研究的深入,越来越多的结果证明Notch信号通路在造血发育过程中的作用是动态变化的,而且也是剂量依赖性的。在斑马鱼中,利用Notch信号的报告基因tp1转基因和造血干细胞标记基因cmyb以及runx1转基因鱼系,追踪斑马鱼造血过程,发现tp1+cmyb+或tp1+runx1+细胞不会发生内皮-造血转化,是因为这些细胞首先要失去Notch活性转变成tp1-细胞后才能产生造血特性,最终转变成造血干细胞。另外,应用转基因鱼系Tg (hsp70:dn- MAML)和Tg (hsp70:GAL4/UAS:NICD)胚胎,通过在不同时期热激处理控制Notch信号强度,研究者发现在动静脉分化之前,激活Notch信号能够促进造血干细胞的产生。在动静脉发育完成后,动脉内皮细胞中的Notch信号的持续激活则会抑制造血干细胞的产生,而降低Notch信号强度,能够促进造血干细胞的产生[63]。在鸡胚造血干细胞产生过程中,随着runx1表达的增加,Notch信号会逐渐降低,而抑制Notch信号后会在一定程度上促进造血干细胞的产生[64]。
3.2.2 Notch信号通路上游调控因子
随着对造血干细胞产生中Notch信号动态调控机制的认识越来越清楚,越来越多的研究阐释了Notch信号通路的上游调控机制(图1)。首先,为保证动脉内皮的分化及造血干细胞的产生,Notch信号需要正确的激活。在动静脉分化阶段,脊索分泌的Hedgehog信号能够促进体节中血管内皮生长因子(Vascular endothelial growth factor, Vegf)的表达,Vegf可以与具有动脉特性的成血管细胞表面的Vegf受体结合,进而激活动脉内皮中的Notch信号,促进动脉的分化[65]。炎性信号也可以激活Notch信号并且特异性地调控造血干细胞的产生,而对动脉发育没有影响。初级中性粒细胞分泌的肿瘤坏死因子a (tumor necrosis factor a, TNFa)和粒细胞集落刺激因子(granulocyte colony-stimulating factor, G-CSF)可以与内皮细胞表面的受体TNFR2,Toll样受体(Toll-like receptor 4, TLR4)结合,通过经典的炎性信号通路使得内皮细胞内的NFkB进入细胞核,进而调控Notch受体Jagged1的表达[66,67]。除此之外,内皮细胞内的初级纤毛发生也能够促进Notch信号在动脉内皮的激活,从而促进生血内皮细胞的特化,保障造血干细胞的正常产生[68]。
另一方面,动脉内皮分化完成后,生血内皮细胞内Notch信号的下降也受到严密的调控。在转录水平上,NcoR2作为一个转录共抑制因子,特异表达在斑马鱼AGM区,通过调控fos启动子的乙酰化水平抑制其转录,进而抑制Vegfd及其下游的Notch信号,以保证内皮-造血转化过程中内皮特性的降低及造血特性的获得[69]。在转录后调控水平上,内皮细胞表达的N6-methyladenosine (m6A)甲基转移酶Mettl3能够在notch1a mRNA的3′UTR区域进行m6A修饰,从而使得notch1a mRNA能被Ythdf2识别并降解。Mettl3或Ythdf2的缺失都会导致notch1a mRNA水平的升高,最终导致内皮细胞特性的增强而阻止造血干细胞的产生[70]。在蛋白水平上,生血内皮细胞中的G蛋白偶联受体183 (G protein- coupled receptor 183, Gpr183)在内皮-造血转化开始之前能够通过招募β-Arrestin1和E3连接酶Nedd4来降解Notch1蛋白从而降低Notch信号的活性,以保证造血干细胞的正常产生[63]。此外,在生血内皮特化过程中,Rab5c通过精密调控Notch信号通路配体和受体的内吞运输,最终促进了造血干细胞的发育[71]。
3.3 Wnt信号通路
Wnt信号通路可以分为β-Catenin依赖的经典Wnt信号通路(canonical pathway),非典型平面细胞极性途径(planar cell polarity pathway)以及非经典Wnt信号/钙通路(Wnt/Ca2+ pathway)。这3种信号通路都依赖于Wnt配体与细胞膜表面受体Frizzled (Fzd)的结合以及细胞内Dishevelled的招募。Wnt信号通路的激活对于胚胎早期形态发生,体轴发育以及干细胞自我更新都至关重要[72]。在β-Catenin依赖的经典Wnt信号通路中,无Wnt配体的情况下,细胞内的β-Catenin被降解复合物(destruction complex, Dishevelled-Axin-GSK3)结合并磷酸化,磷酸化的β-Catenin被β-TrCP识别并泛素化,从而被进一步降解。在Wnt配体与细胞膜上的受体Frizzled (通常为Fzd1或Fzd4)以及共受体LRP5或LRP6结合后使得LRP蛋白发生磷酸化,磷酸化的LRP能够结合降解复合物,阻止β-TrCP对β-Catenin的泛素化及降解,最终细胞浆内的β-Catenin得以稳定存在。部分β-Catenin进入细胞核与TCF/LEF转录因子家族共同调控下游基因的表达,进而调控胚胎体轴建立、组织发生、干细胞的自我更新、增殖和分化,以及成体组织稳态等过程[73]。
在非典型平面细胞极性途径(Wnt/PCP pathway)中,Wnt配体(如Wnt5a、Wnt7、Wnt11等)与受体Frizzled (通常为Fzd3、Fzd6和Fzd7)及共受体(如PTK7、ROR2和RYK)或细胞表面蛋白(CD146、VANGL2、Syndecan和Glypican)结合后,进一步招募Dishevelled与受体复合物结合,激活下游的Rho家族的小G蛋白(如Cdc42、Rac1和RhoA)来调控细胞骨架的形成,并通过JNK通路调控下游基因的转录,进而调控细胞极性及细胞迁移[74]。在非经典Wnt信号/钙通路(Wnt/Ca2+ pathway)中,Wnt配体(如Wnt5a)与受体Frizzled结合后能够通过G蛋白将信号传递给PLCr从而促进Ca2+的释放。Ca2+的增加一方面能通过CaMKII激酶来调控下游信号,另一方面能够通过NFAT来调控下游基因的表达[75]。Wnt信号/钙通路对于胚胎背腹分化和体轴形成以及细胞分化都有重要作用[76]。
3.3.1 Wnt信号通路在HSC发育过程的调控作用
在斑马鱼中,过表达Wnt信号通路下游的抑制因子dkk和axin能够抑制造血干细胞的产生,而前列腺素E2 (prostaglandin E2, PGE2)可以调控Wnt信号通路中β-Catenin的磷酸化进而调控造血发育[77]。
斑马鱼体节中Wnt9a的缺失虽然对造血干细胞的命运决定没有影响,但却导致造血干细胞整体数目减少。进一步的实验说明体节中的Wnt9a能够为造血干细胞的增殖提供有利微环境,从而保证造血干细胞的正常作用。Wnt信号通路可以和BMP信号通路共同激活Cdx-Hox,进而调控造血过程[78]。在小鼠中,利用GSK3的抑制剂来增强Wnt信号能够增加造血干细胞的数目;相反,β-Catenin的小分子拮抗剂处理能够减少造血干细胞的数目。另外,在小鼠的胚胎造血过程中发现,Wnt对造血干细胞产生的作用也是动态变化的[79]。在内皮-造血转化发生之前,β-Catenin在内皮细胞中的激活可以促进造血干细胞的产生,一旦造血的命运决定完成之后,β-Catenin的活性就会下调[79]。近期对于视黄酸(retinoic acid, RA)信号通路的研究发现,它能够下调生血内皮细胞或造血前体细胞中的Wnt信号通路,以促进造血干细胞的产生[80]。
除经典的Wnt/β-Catenin信号通路之外,β-Catenin非依赖性的Wnt信号通路也能够调控造血发育过程(图1)。在受精后14~17小时左右斑马鱼胚胎中,体节中的Wnt16可以促进Notch配体DeltaC和DeltaD的表达,体节中的Notch3能够接受DeltaC和DeltaD传递的信号,进而促进生骨节(sclerotome)和造血干细胞的特化,以细胞非自主性的方式调控造血干细胞的产生[81,82]。鉴于斑马鱼胚胎发育中Wnt16与FGF的活性有时间和空间上的相似性,研究人员对两个信号通路进行检测后发现,Wnt16对DeltaC的调控作用是由FGF信号通路介导的[83]。另外,Rspondin1作为Wnt信号的激活因子,可以通过调控Wnt16的表达来调控造血干细胞的产生[84]。但是目前对于体节如何调控造血干细胞命运决定的分子机制还有待进一步研究。
4 结语与展望
造血干细胞能够自我更新并分化产生各种类型的血细胞,其重建造血的能力是临床上利用骨髓移植治疗血液疾病的基础。因此全面系统地研究造血发育过程的分子机制和复杂的信号调控网络,有助于突破临床上造血干细胞匮乏的瓶颈,对血液疾病的治疗和新药的研发都有非常重要的意义。目前,对于造血干细胞的产生过程已经有清晰的认识,但是该过程的调控机制还有待进一步完善。近年来,随着对造血干细胞产生过程中各个信号通路调控机制的研究越来越多,人们也越来越意识到在内皮-造血转化过程中,调控因子的动态平衡非常重要。例如:ERK和Notch信号的激活在这个过程中的作用都是动态变化的。在内皮细胞中ERK和Notch的激活都是动脉分化所必需的,但是随着内皮-造血转化的开始,内皮细胞若要获得造血特性,细胞内调控内皮特性的ERK和Notch信号活性需要下调,以保证造血特性的获得及维持。一旦这些信号没有及时下调,那么内皮-造血转化过程就不能完成,也不能产生造血干细胞。在体外造血过程中,能否通过调控这些信号通路的活性来促进造血干细胞的产生和维持还需要进一步研究。
致谢
感谢中国科学院动物研究所马东媛博士和中国医学科学院血液病医院(中国医学科学院血液学研究所)王璐博士对本文的仔细阅读和修改。
中国科学院动物研究所血液与心血管发育研究组简介
中国科学院动物研究所膜生物学国家重点实验室血液和心血管发育研究组于2009年组建,课题组长为刘峰研究员。课题组围绕“造血组织及血细胞发育调控”,以斑马鱼和小鼠为主要研究模型,从内源因子、微环境和表观修饰等多角度入手,系统解析造血干细胞命运决定及血细胞发育的调控机制。在造血干细胞产生、维持及分化等方向取得了一系列研究成果,在国际知名学术期刊Nature、Dev Cell、EMBO J、PNAS、J Exp Med、Blood、Nat Commun和Cell Res等发表论文60余篇。与此同时,课题组承担了科技部、国家自然科学基金委以及中国科学院等一系列重大课题。课题组网站:https://liulab.com.cn/。

参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1016/j.cell.2008.01.025URLPMID:18295580 [本文引用: 2]

Establishment and maintenance of the blood system relies on self-renewing hematopoietic stem cells (HSCs) that normally reside in small numbers in the bone marrow niche of adult mammals. This Review describes the developmental origins of HSCs and the molecular mechanisms that regulate lineage-specific differentiation. Studies of hematopoiesis provide critical insights of general relevance to other areas of stem cell biology including the role of cellular interactions in development and tissue homeostasis, lineage programming and reprogramming by transcription factors, and stage- and age-specific differences in cellular phenotypes.
URLPMID:9435292 [本文引用: 1]

Embryonic stem cell-derived embryoid bodies contain a unique precursor population which, in response to vascular endothelial growth factor, gives rise to blast colonies in semi-solid medium. Upon transfer to liquid culture with appropriate cytokines, these blast colonies generate both hematopoietic and adherent, stromal-type cells. Cells within the adherent population display characteristics of endothelial lineage including the expression of CD31, flk-1, flt-1, tie-2, the capacity to take up acetylated LDL and the presence of cytoplasmic Weibel-Palade bodies. Mixing studies demonstrated that the hematopoietic and endothelial precursors within the blast colonies develop from the same cell, the blast colony-forming cell. Kinetic analysis showed that the blast colony-forming cell represents a transient cell population that develops early and is lost quickly during embryoid body development. These findings provide strong evidence that the blast colony-forming cell represents the long-hypothesized hemangioblast, the common precursor of the hematopoietic and endothelial lineages.
URLPMID:10529424 [本文引用: 1]

In this study, we have mapped the onset of hematopoietic development in the mouse embryo using colony-forming progenitor assays and PCR-based gene expression analysis. With this approach, we demonstrate that commitment of embryonic cells to hematopoietic fates begins in proximal regions of the egg cylinder at the mid-primitive streak stage (E7.0) with the simultaneous appearance of primitive erythroid and macrophage progenitors. Development of these progenitors was associated with the expression of SCL/tal-1 and GATA-1, genes known to be involved in the development and maturation of the hematopoietic system. Kinetic analysis revealed the transient nature of the primitive erythroid lineage, as progenitors increased in number in the developing yolk sac until early somite-pair stages of development (E8.25) and then declined sharply to undetectable levels by 20 somite pairs (E9.0). Primitive erythroid progenitors were not detected in any other tissue at any stage of embryonic development. The early wave of primitive erythropoiesis was followed by the appearance of definitive erythroid progenitors (BFU-E) that were first detectable at 1-7 somite pairs (E8.25) exclusively within the yolk sac. The appearance of BFU-E was followed by the development of later stage definitive erythroid (CFU-E), mast cell and bipotential granulocyte/macrophage progenitors in the yolk sac. C-myb, a gene essential for definitive hematopoiesis, was expressed at low levels in the yolk sac just prior to and during the early development of these definitive erythroid progenitors. All hematopoietic activity was localized to the yolk sac until circulation was established (E8.5) at which time progenitors from all lineages were detected in the bloodstream and subsequently in the fetal liver following its development. This pattern of development suggests that definitive hematopoietic progenitors arise in the yolk sac, migrate through the bloodstream and seed the fetal liver to rapidly initiate the first phase of intraembryonic hematopoiesis. Together, these findings demonstrate that commitment to hematopoietic fates begins in early gastrulation, that the yolk sac is the only site of primitive erythropoiesis and that the yolk sac serves as the first source of definitive hematopoietic progenitors during embryonic development.
DOI:10.1016/j.stem.2011.10.003URL [本文引用: 2]

Hematopoietic stem cells (HSCs) and an earlier wave of definitive erythroid/myeloid progenitors (EMPs) differentiate from hemogenic endothelial cells in the conceptus. EMPs can be generated in vitro from embryonic or induced pluripotent stem cells, but efforts to produce HSCs have largely failed. The formation of both EMPs and HSCs requires the transcription factor Runx1 and its non-DNA binding partner core binding factor beta (CBF beta). Here we show that the requirements for CBF beta in EMP and HSC formation in the conceptus are temporally and spatially distinct. Panendothelial expression of CBF beta in Tek-expressing cells was sufficient for EMP formation, but was not adequate for HSC formation. Expression of CBF beta in Ly6a-expressing cells, on the other hand, was sufficient for HSC, but not EMP, formation. The data indicate that EMPs and HSCs differentiate from distinct populations of hemogenic endothelial cells, with Ly6a expression specifically marking the HSC-generating hemogenic endothelium.
DOI:10.1016/j.celrep.2015.05.036URLPMID:26095363 [本文引用: 1]

Hematopoietic potential arises in mammalian embryos before adult-repopulating hematopoietic stem cells (HSCs). At embryonic day 9.5 (E9.5), we show the first murine definitive erythro-myeloid progenitors (EMPs) have an immunophenotype distinct from primitive hematopoietic progenitors, maturing megakaryocytes and macrophages, and rare B cell potential. EMPs emerge in the yolk sac with erythroid and broad myeloid, but not lymphoid, potential. EMPs migrate to the fetal liver and rapidly differentiate, including production of circulating neutrophils by E11.5. Although the surface markers, transcription factors, and lineage potential associated with EMPs overlap with those found in adult definitive hematopoiesis, they are present in unique combinations or proportions that result in a specialized definitive embryonic progenitor. Furthermore, we find that embryonic stem cell (ESC)-derived hematopoiesis recapitulates early yolk sac hematopoiesis, including primitive, EMP, and rare B cell potential. EMPs do not have long-term potential when transplanted in immunocompromised adults, but they can provide transient adult-like RBC reconstitution.
DOI:10.1126/science.1219179URLPMID:22442384 [本文引用: 1]

Macrophages and dendritic cells (DCs) are key components of cellular immunity and are thought to originate and renew from hematopoietic stem cells (HSCs). However, some macrophages develop in the embryo before the appearance of definitive HSCs. We thus reinvestigated macrophage development. We found that the transcription factor Myb was required for development of HSCs and all CD11b(high) monocytes and macrophages, but was dispensable for yolk sac (YS) macrophages and for the development of YS-derived F4/80(bright) macrophages in several tissues, such as liver Kupffer cells, epidermal Langerhans cells, and microglia--cell populations that all can persist in adult mice independently of HSCs. These results define a lineage of tissue macrophages that derive from the YS and are genetically distinct from HSC progeny.
DOI:10.1038/nature13989URLPMID:25470051 [本文引用: 1]

Most haematopoietic cells renew from adult haematopoietic stem cells (HSCs), however, macrophages in adult tissues can self-maintain independently of HSCs. Progenitors with macrophage potential in vitro have been described in the yolk sac before emergence of HSCs, and fetal macrophages can develop independently of Myb, a transcription factor required for HSC, and can persist in adult tissues. Nevertheless, the origin of adult macrophages and the qualitative and quantitative contributions of HSC and putative non-HSC-derived progenitors are still unclear. Here we show in mice that the vast majority of adult tissue-resident macrophages in liver (Kupffer cells), brain (microglia), epidermis (Langerhans cells) and lung (alveolar macrophages) originate from a Tie2(+) (also known as Tek) cellular pathway generating Csf1r(+) erythro-myeloid progenitors (EMPs) distinct from HSCs. EMPs develop in the yolk sac at embryonic day (E) 8.5, migrate and colonize the nascent fetal liver before E10.5, and give rise to fetal erythrocytes, macrophages, granulocytes and monocytes until at least E16.5. Subsequently, HSC-derived cells replace erythrocytes, granulocytes and monocytes. Kupffer cells, microglia and Langerhans cells are only marginally replaced in one-year-old mice, whereas alveolar macrophages may be progressively replaced in ageing mice. Our fate-mapping experiments identify, in the fetal liver, a sequence of yolk sac EMP-derived and HSC-derived haematopoiesis, and identify yolk sac EMPs as a common origin for tissue macrophages.
DOI:10.1016/j.stem.2013.08.012URL [本文引用: 1]

In jawed vertebrates, development of an adaptive immune-system is essential for protection of the born organism against otherwise life-threatening pathogens. Myeloid cells of the innate immune system are formed early in development, whereas lymphopoiesis has been suggested to initiate much later, following emergence of definitive hematopoietic stem cells (HSCs). Herein, we demonstrate that the embryonic lymphoid commitment process initiates earlier than previously appreciated, prior to emergence of definitive HSCs, through establishment of a previously unrecognized entirely immune-restricted and lymphoid-primed progenitor. Notably, this immune-restricted progenitor appears to first emerge in the yolk sac and contributes physiologically to the establishment of lymphoid and some myeloid components of the immune-system, establishing the lymphomyeloid lineage restriction process as an early and physiologically important line-age-commitment step in mammalian hematopoiesis.
DOI:10.1016/1074-7613(94)90081-7URLPMID:7889417 [本文引用: 1]

The precise time of appearance of the first hematopoietic stem cell activity in the developing mouse embryo is unknown. Recently the aorta-gonad-mesonephros region of the developing mouse embryo has been shown to possess hematopoietic colony-forming activity (CFU-S) in irradiated recipient mice. To determine whether the mouse embryo possesses definitive hematopoietic stem cell activity in the analogous AGM region and to determine the order of appearance of stem cells in the yolk sac, AGM region, and liver, we transferred these embryonic tissues into adult irradiated recipients. We report here the long-term, complete, and functional hematopoietic repopulation of primary and serial recipients with AGM-derived cells. We observe potent hematopoietic stem cell activity in the AGM region before the appearance of yolk sac and liver stem cell activity and discuss a model for the maturation of stem cell activity in mouse embryogenesis.
DOI:10.1016/j.devcel.2004.12.016URLPMID:15737932 [本文引用: 1]

The hematopoietic system develops during embryogenesis at temporally and anatomically restricted sites. The anatomical origin of definitive HSCs is not fully resolved, and little is known about how the different fetal hematopoietic microenvironments direct HSC development. Here, we show that the mouse placenta functions as a hematopoietic organ that harbors a large pool of pluripotent HSCs during midgestation. The onset of HSC activity in the placenta parallels that of the AGM (aorta-gonad-mesonephros) region starting at E10.5-E11.0. However, the placental HSC pool expands until E12.5-E13.5 and contains >15-fold more HSCs than the AGM. The expansion of the CD34(+)c-kit(+) HSC pool in the placenta occurs prior to and during the initial expansion of HSCs in the fetal liver. Importantly, the placental HSC pool is not explained by rare circulating HSCs, which appear later. These data support an important, but unappreciated, role for the placenta in establishing the mammalian definitive hematopoietic system.
DOI:10.1038/nature08764URLPMID:20154729 [本文引用: 1]

Haematopoietic stem cells (HSCs), responsible for blood production in the adult mouse, are first detected in the dorsal aorta starting at embryonic day 10.5 (E10.5). Immunohistological analysis of fixed embryo sections has revealed the presence of haematopoietic cell clusters attached to the aortic endothelium where HSCs might localize. The origin of HSCs has long been controversial and several candidates of the direct HSC precursors have been proposed (for review see ref. 7), including a specialized endothelial cell population with a haemogenic potential. Such cells have been described both in vitro in the embryonic stem cell (ESC) culture system and retrospectively in vivo by endothelial lineage tracing and conditional deletion experiments. Whether the transition from haemogenic endothelium to HSC actually occurs in the mouse embryonic aorta is still unclear and requires direct and real-time in vivo observation. To address this issue we used time-lapse confocal imaging and a new dissection procedure to visualize the deeply located aorta. Here we show the dynamic de novo emergence of phenotypically defined HSCs (Sca1(+), c-kit(+), CD41(+)) directly from ventral aortic haemogenic endothelial cells.
DOI:10.1002/jcb.22251URLPMID:19565566 [本文引用: 2]

Within the past two decades, the zebrafish (Danio rerio) has become an excellent model to study the development of hematopoietic stem cells (HSCs). All vertebrates including zebrafish have primitive and definitive waves of hematopoiesis, but self-renewing pluripotent HSCs are only produced by the definitive wave. The primitive wave occurs in two intraembryonic locations called the intermediate cell mass (ICM) and the anterior lateral mesoderm (ALM). Primitive erythropoiesis is in the ICM, whereas myelopoiesis initiates in the ALM. After circulation starts at 24 h post-fertilization, hematopoiesis shifts to the posterior blood island (PBI) for a brief period. The definitive wave starts in the aorta-gonad-mesonephros (AGM). There are three different HSC migration and colonization events that begin 2 days post-fertilization: AGM progenitor cells migrate to (1) the caudal hematopoietic tissue (CHT), which is an intermediate site of blood development; (2) the thymus, which is a site of lymphocyte maturation; and (3) the developing kidney marrow, which is the larval and adult location for production of all hematopoietic cell types, and is comparable to the bone marrow of mammals. Many of the transcription factors and signaling pathways that regulate the formation of HSCs in a zebrafish are conserved with mammals. Large-scale forward and reverse genetic screens have identified zebrafish blood and HSC mutants that represent models for known human diseases. Along with the technological advancements in the field of zebrafish research, future HSC studies in zebrafish will help us illuminate the genetic network controlling the development and function of stem cells in all vertebrates.
DOI:10.1073/pnas.92.23.10713URLPMID:7479870 [本文引用: 1]

Vertebrate hematopoietic stem cells are derived from vental mesoderm, which is postulated to migrate to both extra- and intraembryonic positions during gastrula and neurula stages. Extraembryonic migration has previously been documented, but the origin and migration of intraembryonic hematopoietic cells have not been visualized. The zebrafish and most other teleosts do not form yolk sac blood islands during early embryogenesis, but instead hematopoiesis occurs solely in a dorsal location known as the intermediate cell mass (IM) or Oellacher. In this report, we have isolated cDNAs encoding zebrafish homologs of the hematopoietic transcription factors GATA-1 and GATA-2 and have used these markers to determine that the IM is formed from mesodermal cells in a posterior-lateral position on the yolk syncytial layer of the gastrula yolk sac. Surprisingly, cells of the IM then migrate anteriorly through most of the body length prior to the onset of active circulation and exit onto the yolk sac. These findings support a hypothesis in which the hematopoietic program of vertebrates is established by variations in homologous migration pathways of extra- and intraembryonic progenitors.
DOI:10.1242/dev.012385URLPMID:17959717 [本文引用: 2]

Shifting sites of blood cell production during development is common across widely divergent phyla. In zebrafish, like other vertebrates, hematopoietic development has been roughly divided into two waves, termed primitive and definitive. Primitive hematopoiesis is characterized by the generation of embryonic erythrocytes in the intermediate cell mass and a distinct population of macrophages that arises from cephalic mesoderm. Based on previous gene expression studies, definitive hematopoiesis has been suggested to begin with the generation of presumptive hematopoietic stem cells (HSCs) along the dorsal aorta that express c-myb and runx1. Here we show, using a combination of gene expression analyses, prospective isolation approaches, transplantation, and in vivo lineage-tracing experiments, that definitive hematopoiesis initiates through committed erythromyeloid progenitors (EMPs) in the posterior blood island (PBI) that arise independently of HSCs. EMPs isolated by coexpression of fluorescent transgenes driven by the lmo2 and gata1 promoters exhibit an immature, blastic morphology and express only erythroid and myeloid genes. Transplanted EMPs home to the PBI, show limited proliferative potential, and do not seed subsequent hematopoietic sites such as the thymus or pronephros. In vivo fate-mapping studies similarly demonstrate that EMPs possess only transient proliferative potential, with differentiated progeny remaining largely within caudal hematopoietic tissue. Additional fate mapping of mesodermal derivatives in mid-somitogenesis embryos suggests that EMPs are born directly in the PBI. These studies provide phenotypic and functional analyses of the first hematopoietic progenitors in the zebrafish embryo and demonstrate that definitive hematopoiesis proceeds through two distinct waves during embryonic development.
DOI:10.1038/nature08761URLPMID:20154732 [本文引用: 2]

The ontogeny of haematopoietic stem cells (HSCs) during embryonic development is still highly debated, especially their possible lineage relationship to vascular endothelial cells. The first anatomical site from which cells with long-term HSC potential have been isolated is the aorta-gonad-mesonephros (AGM), more specifically the vicinity of the dorsal aortic floor. But although some authors have presented evidence that HSCs may arise directly from the aortic floor into the dorsal aortic lumen, others support the notion that HSCs first emerge within the underlying mesenchyme. Here we show by non-invasive, high-resolution imaging of live zebrafish embryos, that HSCs emerge directly from the aortic floor, through a stereotyped process that does not involve cell division but a strong bending then egress of single endothelial cells from the aortic ventral wall into the sub-aortic space, and their concomitant transformation into haematopoietic cells. The process is polarized not only in the dorso-ventral but also in the rostro-caudal versus medio-lateral direction, and depends on Runx1 expression: in Runx1-deficient embryos, the exit events are initially similar, but much rarer, and abort into violent death of the exiting cell. These results demonstrate that the aortic floor is haemogenic and that HSCs emerge from it into the sub-aortic space, not by asymmetric cell division but through a new type of cell behaviour, which we call an endothelial haematopoietic transition.
DOI:10.1182/blood-2004-09-3547URLPMID:15644413 [本文引用: 1]

Blood and endothelial cells arise in close association in developing embryos, possibly from a shared precursor, the hemangioblast, or as hemogenic endothelium. The transcription factor, Scl/Tal1 (stem cell leukemia protein), is essential for hematopoiesis but thought to be required only for remodeling of endothelium in mouse embryos. By contrast, it has been implicated in hemangioblast formation in embryoid bodies. To resolve the role of scl in endothelial development, we knocked down its synthesis in zebrafish embryos where early precursors and later phenotypes can be more easily monitored. With respect to blood, the zebrafish morphants phenocopied the mouse knockout and positioned scl in the genetic hierarchy. Importantly, endothelial development was also clearly disrupted. Dorsal aorta formation was substantially compromised and gene expression in the posterior cardinal vein was abnormal. We conclude that scl is especially critical for the development of arteries where adult hematopoietic stem cells emerge, implicating scl in the formation of hemogenic endothelium.
DOI:10.1182/blood-2009-09-244640URLPMID:20185582 [本文引用: 1]

Recent lineage studies suggest that hematopoietic stem cells (HSCs) may be derived from endothelial cells. However, the genetic hierarchy governing the emergence of HSCs remains elusive. We report here that zebrafish ets1-related protein (etsrp), which is essential for vascular endothelial development, also plays a critical role in the initiation of definitive hematopoiesis by controlling the expression of 2 stem cell leukemia (scl) isoforms (scl-alpha and scl-beta) in angioblasts. In etsrp morphants, which are deficient in endothelial and HSC development, scl-alpha alone partially rescues angioblast specification, arterial-venous differentiation, and the expression of HSC markers, runx1 and c-myb, whereas scl-beta requires angioblast rescue by fli1a to restore runx1 expression. Interestingly, when vascular endothelial growth factor (Vegf) signaling is inhibited, HSC marker expression can still be restored by scl-alpha in etsrp morphants, whereas the rescue of arterial ephrinb2a expression is blocked. Furthermore, both scl isoforms partially rescue runx1 but not ephrinb2a expression in embryos deficient in Vegf signaling. Our data suggest that downstream of etsrp, scl-alpha and fli1a specify the angioblasts, whereas scl-beta further initiates HSC specification from this angioblast population, and that Vegf signaling acts upstream of scl-beta during definitive hematopoiesis.
DOI:10.1242/dev.097071URL [本文引用: 1]

Recent studies have shown that nascent hematopoietic stem cells (HSCs) derive directly from the ventral aortic endothelium (VAE) via endothelial to hematopoietic transition (EHT). However, whether EHT initiates from a random or predetermined subpopulation of VAE, as well as the molecular mechanism underlying this process, remain unclear. We previously reported that different zebrafish stem cell leukemia (scl) isoforms are differentially required for HSC formation in the ventral wall of the dorsal aorta. However, the exact stage at which these isoforms impact HSC development was not defined. Here, using in vivo time-lapse imaging of scl isoformspecific reporter transgenic zebrafish lines, we show that prior to EHT scl-beta is selectively expressed in hemogenic endothelial cells, a unique subset of VAE cells possessing hemogenic potential, whereas scl-alpha is expressed later in nascent HSCs as they egress from VAE cells. In accordance with their expression, loss-of-function studies coupled with in vivo imaging analysis reveal that scl-beta acts earlier to specify hemogenic endothelium, which is later transformed by runx1 into HSCs. Our results also reveal a previously unexpected role of scl-alpha in maintaining newly born HSCs in the aorta-gonads-mesonephros. Thus, our data suggest that a defined hemogenic endothelial population preset by scl-beta supports the deterministic emergence of HSCs, and unravel the cellular mechanisms by which scl isoforms regulate HSC development.
DOI:10.1038/nature05883URLPMID:17581586 [本文引用: 1]

Haematopoietic stem cell (HSC) homeostasis is tightly controlled by growth factors, signalling molecules and transcription factors. Definitive HSCs derived during embryogenesis in the aorta-gonad-mesonephros region subsequently colonize fetal and adult haematopoietic organs. To identify new modulators of HSC formation and homeostasis, a panel of biologically active compounds was screened for effects on stem cell induction in the zebrafish aorta-gonad-mesonephros region. Here, we show that chemicals that enhance prostaglandin (PG) E2 synthesis increased HSC numbers, and those that block prostaglandin synthesis decreased stem cell numbers. The cyclooxygenases responsible for PGE2 synthesis were required for HSC formation. A stable derivative of PGE2 improved kidney marrow recovery following irradiation injury in the adult zebrafish. In murine embryonic stem cell differentiation assays, PGE2 caused amplification of multipotent progenitors. Furthermore, ex vivo exposure to stabilized PGE2 enhanced spleen colony forming units at day 12 post transplant and increased the frequency of long-term repopulating HSCs present in murine bone marrow after limiting dilution competitive transplantation. The conserved role for PGE2 in the regulation of vertebrate HSC homeostasis indicates that modulation of the prostaglandin pathway may facilitate expansion of HSC number for therapeutic purposes.
DOI:10.1073/pnas.93.8.3444URLPMID:8622955 [本文引用: 1]

The CBFA2 (AML1) gene encodes a DNA-binding subunit of the heterodimeric core-binding factor. The CBFA2 gene is disrupted by the (8;21), (3;21), and (12;21) chromosomal translocations associated with leukemias and myelodysplasias in humans. Mice lacking a CBF alpha 2 protein capable of binding DNA die between embryonic days 11.5 and 12.5 due to hemorrhaging in the central nervous system (CNS), at the nerve/CNS interfaces of cranial and spinal nerves, and in somitic/intersomitic regions along the presumptive spinal cord. Hemorrhaging is preceded by symmetric, bilateral necrosis in these regions. Definitive erythropoiesis and myelopoiesis do not occur in Cbfa2-deficient embryos, and disruption of one copy of the Cbfa2 gene significantly reduces the number of progenitors for erythroid and myeloid cells.
DOI:10.1038/nature07619URLPMID:19129762 [本文引用: 1]

Haematopoietic stem cells (HSCs) are the founder cells of the adult haematopoietic system, and thus knowledge of the molecular program directing their generation during development is important for regenerative haematopoietic strategies. Runx1 is a pivotal transcription factor required for HSC generation in the vascular regions of the mouse conceptus-the aorta, vitelline and umbilical arteries, yolk sac and placenta. It is thought that HSCs emerge from vascular endothelial cells through the formation of intra-arterial clusters and that Runx1 functions during the transition from 'haemogenic endothelium' to HSCs. Here we show by conditional deletion that Runx1 activity in vascular-endothelial-cadherin-positive endothelial cells is indeed essential for intra-arterial cluster, haematopoietic progenitor and HSC formation in mice. In contrast, Runx1 is not required in cells expressing Vav1, one of the first pan-haematopoietic genes expressed in HSCs. Collectively these data show that Runx1 function is essential in endothelial cells for haematopoietic progenitor and HSC formation from the vasculature, but its requirement ends once or before Vav is expressed.
DOI:10.1182/blood-2011-10-386094URL [本文引用: 1]

Recent studies have established that during embryonic development, hematopoietic progenitors and stem cells are generated from hemogenic endothelium precursors through a process termed endothelial to hematopoietic transition (EHT). The transcription factor RUNX1 is essential for this process, but its main downstream effectors remain largely unknown. Here, we report the identification of Gfi1 and Gfi1b as direct targets of RUNX1 and critical regulators of EHT. GFI1 and GFI1B are able to trigger, in the absence of RUNX1, the down-regulation of endothelial markers and the formation of round cells, a morphologic change characteristic of EHT. Conversely, blood progenitors in Gfi1- and Gfi1b-deficient embryos maintain the expression of endothelial genes. Moreover, those cells are not released from the yolk sac and disseminated into embryonic tissues. Taken together, our findings demonstrate a critical and specific role of the GFI1 transcription factors in the first steps of the process leading to the generation of hematopoietic progenitors from hemogenic endothelium. (Blood. 2012; 120(2): 314-322)
DOI:10.1084/jem.20170807URLPMID:29217535 [本文引用: 1]

Cell fate is established through coordinated gene expression programs in individual cells. Regulatory networks that include the Gata2 transcription factor play central roles in hematopoietic fate establishment. Although Gata2 is essential to the embryonic development and function of hematopoietic stem cells that form the adult hierarchy, little is known about the in vivo expression dynamics of Gata2 in single cells. Here, we examine Gata2 expression in single aortic cells as they establish hematopoietic fate in Gata2Venus mouse embryos. Time-lapse imaging reveals rapid pulsatile level changes in Gata2 reporter expression in cells undergoing endothelial-to-hematopoietic transition. Moreover, Gata2 reporter pulsatile expression is dramatically altered in Gata2(+/-) aortic cells, which undergo fewer transitions and are reduced in hematopoietic potential. Our novel finding of dynamic pulsatile expression of Gata2 suggests a highly unstable genetic state in single cells concomitant with their transition to hematopoietic fate. This reinforces the notion that threshold levels of Gata2 influence fate establishment and has implications for transcription factor-related hematologic dysfunctions.
DOI:10.1084/jem.20130751URL [本文引用: 2]

Knowledge of the key transcription factors that drive hematopoietic stem cell (HSC) generation is of particular importance for current hematopoietic regenerative approaches and reprogramming strategies. Whereas GATA2 has long been implicated as a hematopoietic transcription factor and its dysregulated expression is associated with human immunodeficiency syndromes and vascular integrity, it is as yet unknown how GATA2 functions in the generation of HSCs. HSCs are generated from endothelial cells of the major embryonic vasculature (aorta, vitelline, and umbilical arteries) and are found in intra-aortic hematopoietic clusters. In this study, we find that GATA2 function is essential for the generation of HSCs during the stage of endothelial-to-hematopoietic cell transition. Specific deletion of Gata2 in Vec (Vascular Endothelial Cadherin)-expressing endothelial cells results in a deficiency of long-term repopulating HSCs and intra-aortic cluster cells. By specific deletion of Gata2 in Vav-expressing hematopoietic cells (after HSC generation), we further show that GATA2 is essential for HSC survival. This is in contrast to the known activity of the RUNX1 transcription factor, which functions only in the generation of HSCs, and highlights the unique requirement for GATA2 function in HSCs throughout all developmental stages.
DOI:10.1084/jem.20130733URL [本文引用: 1]

The generation of hematopoietic stem cells (HSCs) from hemogenic endothelium within the aorta, gonad, mesonephros (AGM) region of the mammalian embryo is crucial for development of the adult hematopoietic system. We described a deletion of a Gata2 cis-element (+9.5) that depletes fetal liver HSCs, is lethal at E13-14 of embryogenesis, and is mutated in an immunodeficiency that progresses to myelodysplasia/leukemia. Here, we demonstrate that the +9.5 element enhances Gata2 expression and is required to generate long-term repopulating HSCs in the AGM. Deletion of the +9.5 element abrogated the capacity of hemogenic endothelium to generate HSC-containing clusters in the aorta. Genomic analyses indicated that the +9.5 element regulated a rich ensemble of genes that control hemogenic endothelium and HSCs, as well as genes not implicated in hematopoiesis. These results reveal a mechanism that controls stem cell emergence from hemogenic endothelium to establish the adult hematopoietic system.
DOI:10.1182/blood-2006-02-003087URLPMID:17090656 [本文引用: 1]

The transcription factors Scl and Lmo2 are crucial for development of all blood. An important early requirement for Scl in endothelial development has also been revealed recently in zebrafish embryos, supporting previous findings in scl(-/-) embryoid bodies. Scl depletion culminates most notably in failure of dorsal aorta formation, potentially revealing a role in the formation of hemogenic endothelium. We now present evidence that the requirements for Lmo2 in zebrafish embryos are essentially the same as for Scl. The expression of important hematopoietic regulators is lost, reduced, or delayed, panendothelial gene expression is down-regulated, and aorta-specific marker expression is lost. The close similarity of the phenotypes for Scl and Lmo2 suggest that they perform these early functions in hemangioblast development within a multiprotein complex, as shown for erythropoiesis. Consistent with this, we find that scl morphants cannot be rescued by a non-Lmo2-binding form of Scl but can be rescued by non-DNA-binding forms, suggesting tethering to target genes through DNA-binding partners linked via Lmo2. Interestingly, unlike other hematopoietic regulators, the Scl/Lmo2 complex does not appear to autoregulate, as neither gene's expression is affected by depletion of the other. Thus, expression of these critical regulators is dependent on continued expression of upstream regulators, which may include cell-extrinsic signals.
DOI:10.1242/dev.119180URLPMID:25758220 [本文引用: 3]

The adult blood system is established by hematopoietic stem cells (HSCs), which arise during development from an endothelial-to-hematopoietic transition of cells comprising the floor of the dorsal aorta. Expression of aortic runx1 has served as an early marker of HSC commitment in the zebrafish embryo, but recent studies have suggested that HSC specification begins during the convergence of posterior lateral plate mesoderm (PLM), well before aorta formation and runx1 transcription. Further understanding of the earliest stages of HSC specification necessitates an earlier marker of hemogenic endothelium. Studies in mice have suggested that GATA2 might function at early stages within hemogenic endothelium. Two orthologs of Gata2 exist in zebrafish: gata2a and gata2b. Here, we report that gata2b expression initiates during the convergence of PLM, becoming restricted to emerging HSCs. We observe Notch-dependent gata2b expression within the hemogenic subcompartment of the dorsal aorta that is in turn required to initiate runx1 expression. Our results indicate that Gata2b functions within hemogenic endothelium from an early stage, whereas Gata2a functions more broadly throughout the vascular system.
DOI:10.1038/s42003-020-0798-3URLPMID:32054973 [本文引用: 1]

Gata2 is a key transcription factor required to generate Haematopoietic Stem and Progenitor Cells (HSPCs) from haemogenic endothelium (HE); misexpression of Gata2 leads to haematopoietic disorders. Here we deleted a conserved enhancer (i4 enhancer) driving pan-endothelial expression of the zebrafish gata2a and showed that Gata2a is required for HE programming by regulating expression of runx1 and of the second Gata2 orthologue, gata2b. By 5 days, homozygous gata2a(Deltai4/Deltai4) larvae showed normal numbers of HSPCs, a recovery mediated by Notch signalling driving gata2b and runx1 expression in HE. However, gata2a(Deltai4/Deltai4) adults showed oedema, susceptibility to infections and marrow hypo-cellularity, consistent with bone marrow failure found in GATA2 deficiency syndromes. Thus, gata2a expression driven by the i4 enhancer is required for correct HE programming in embryos and maintenance of steady-state haematopoietic stem cell output in the adult. These enhancer mutants will be useful in exploring further the pathophysiology of GATA2-related deficiencies in vivo.
DOI:10.1146/annurev.biochem.67.1.753URL [本文引用: 1]
DOI:10.1016/j.gendis.2014.07.005URLPMID:25401122 [本文引用: 1]

Bone Morphogenetic Proteins (BMPs) are a group of signaling molecules that belongs to the Transforming Growth Factor-beta (TGF-beta) superfamily of proteins. Initially discovered for their ability to induce bone formation, BMPs are now known to play crucial roles in all organ systems. BMPs are important in embryogenesis and development, and also in maintenance of adult tissue homeostasis. Mouse knockout models of various components of the BMP signaling pathway result in embryonic lethality or marked defects, highlighting the essential functions of BMPs. In this review, we first outline the basic aspects of BMP signaling and then focus on genetically manipulated mouse knockout models that have helped elucidate the role of BMPs in development. A significant portion of this review is devoted to the prominent human pathologies associated with dysregulated BMP signaling.
DOI:10.1101/gad.1350705URLPMID:16322555 [本文引用: 1]

Smad transcription factors lie at the core of one of the most versatile cytokine signaling pathways in metazoan biology-the transforming growth factor-beta (TGFbeta) pathway. Recent progress has shed light into the processes of Smad activation and deactivation, nucleocytoplasmic dynamics, and assembly of transcriptional complexes. A rich repertoire of regulatory devices exerts control over each step of the Smad pathway. This knowledge is enabling work on more complex questions about the organization, integration, and modulation of Smad-dependent transcriptional programs. We are beginning to uncover self-enabled gene response cascades, graded Smad response mechanisms, and Smad-dependent synexpression groups. Our growing understanding of TGFbeta signaling through the Smad pathway provides general principles for how animal cells translate complex inputs into concrete behavior.
DOI:10.1016/s0092-8674(00)81561-5URLPMID:10660046 [本文引用: 1]

We have identified the 30-zinc finger protein OAZ as a DNA-binding factor that associates with Smads in response to BMP2, forming a complex that transcriptionally activates the homeobox regulator of Xenopus mesoderm and neural development, Xvent-2. OAZ contains a BMP signaling module formed by two clusters of fingers that bind Smads and the Xvent-2 BMP response element, respectively. Previously implicated as a transcriptional partner of Olf-1/EBF in olfactory epithelium and lymphocyte development in the rat, OAZ fulfills this role through clusters of fingers that are separate from the BMP signaling module. The mutually exclusive use of OAZ by the BMP-Smad and Olf pathways illustrates the dual role of a multi-zinc finger protein in signal transduction during development.
DOI:10.1128/mcb.23.23.8704-8717.2003URLPMID:14612411 [本文引用: 1]

We have previously shown that Nkx3.2, a transcriptional repressor that is expressed in the sclerotome and developing cartilage, can activate the chondrocyte differentiation program in somitic mesoderm in a bone morphogenetic protein (BMP)-dependent manner. In this work, we elucidate how BMP signaling modulates the transcriptional repressor activity of Nkx3.2. We have found that Nkx3.2 forms a complex, in vivo, with histone deacetylase 1 (HDAC1) and Smad1 and -4 in a BMP-dependent manner. The homeodomain and NK domain of Nkx3.2 support the interaction of this transcription factor with HDAC1 and Smad1, respectively, and both of these domains are required for the transcriptional repressor activity of Nkx3.2. Furthermore, the recruitment of an HDAC/Sin3A complex to Nkx3.2 requires that Nkx3.2 interact with Smad1 and -4. Indeed, Nkx3.2 both fails to associate with the HDAC/Sin3A complex and represses target gene transcription in a cell line lacking Smad4, but it performs these functions if exogenous Smad4 is added to these cells. While prior work has indicated that BMP-dependent Smads can support transcriptional activation, our findings indicate that BMP-dependent Smads can also potentiate transcriptional repression, depending upon the identity of the Smad-interacting transcription factor.
DOI:10.1101/gad.12.2.186URLPMID:9436979 [本文引用: 1]

Bone morphogenetic protein (BMP) receptors signal by phosphorylating Smad1, which then associates with Smad4; this complex moves into the nucleus and activates transcription. Here we report the existence of a natural inhibitor of this process, Smad6, a longer version of the previously reported JV15-1. In Xenopus embryos and in mammalian cells, Smad6 specifically blocks signaling by the BMP/Smad1 pathway. Smad6 inhibits BMP/Smad1 signaling without interfering with receptor-mediated phosphorylation of Smad1. Smad6 specifically competes with Smad4 for binding to receptor-activated Smad1, yielding an apparently inactive Smad1-Smad6 complex. Therefore, Smad6 selectively antagonizes BMP-activated Smad1 by acting as a Smad4 decoy.
DOI:10.1038/39369URLPMID:9335507 [本文引用: 1]

TGF-beta signals from the membrane to the nucleus through serine/threonine kinase receptors and their downstream effectors, termed SMAD proteins. The activated TGF-beta receptor induces phosphorylation of two such proteins, Smad2 and Smad3, which form hetero-oligomeric complex(es) with Smad4/DPC4 that translocate to the nucleus, where they then regulate transcriptional responses. However, the mechanisms by which the intracellular signals of TGF-beta are switched off are unclear. Here we report the identification of Smad7, which is related to Smad6. Transfection of Smad7 blocks responses mediated by TGF-beta in mammalian cells, and injection of Smad7 RNA into Xenopus embryos blocks activin/TGF-beta signalling. Smad7 associates stably with the TGF-beta receptor complex, but is not phosphorylated upon TGF-beta stimulation. TGFbeta-mediated phosphorylation of Smad2 and Smad3 is inhibited by Smad7, indicating that the antagonistic effect of Smad7 is exerted at this important regulatory step. TGF-beta rapidly induces expression of Smad7 mRNA, suggesting that Smad7 may participate in a negative feedback loop to control TGF-beta responses.
DOI:10.1016/s1097-2765(00)00134-9URLPMID:11163210 [本文引用: 1]

Ubiquitin-mediated proteolysis regulates the activity of diverse receptor systems. Here, we identify Smurf2, a C2-WW-HECT domain ubiquitin ligase and show that Smurf2 associates constitutively with Smad7. Smurf2 is nuclear, but binding to Smad7 induces export and recruitment to the activated TGF beta receptor, where it causes degradation of receptors and Smad7 via proteasomal and lysosomal pathways. IFN gamma, which stimulates expression of Smad7, induces Smad7-Smurf2 complex formation and increases TGF beta receptor turnover, which is stabilized by blocking Smad7 or Smurf2 expression. Furthermore, Smad7 mutants that interfere with recruitment of Smurf2 to the receptors are compromised in their inhibitory activity. These studies thus define Smad7 as an adaptor in an E3 ubiquitin-ligase complex that targets the TGF beta receptor for degradation.
DOI:10.1634/stemcells.22-4-457URLPMID:15277693 [本文引用: 1]

Blood formation occurs throughout the life of an individual in a process driven by hematopoietic stem cells (HSCs). The ability of bone marrow (BM) and cord blood (CB) HSC to undergo self-renewal and develop into multiple blood lineages has made these cells an important clinical resource. Transplantation with BM- and CB-derived HSCs is now used extensively for treatment of hematological disorders, malignancies, and immunodeficiencies. An understanding of the embryonic origin of HSC and the factors regulating their generation and expansion in vivo will provide important information for the manipulation of these cells ex vivo. This is critical for the further development of CB transplantation, the potential of which is limited by small numbers of HSC in the donor population. Although the origins of HSCs have become clearer and progress has been made in identifying genes that are critical for the formation and maintenance of HSCs, less is known about the signals that commit specific populations of mesodermal precursors to hematopoietic cell fate. Critical signals acting on these precursor cells are likely to be derived from visceral endoderm in yolk sac and from underlying stroma in the aorta-gonad-mesonephros region. Here we summarize briefly the origin of yolk sac and embryonic HSCs before detailing evidence that bone morphogenic protein-4 (BMP4) has a crucial role in Xenopus and mammalian HSC development. We discuss evidence that BMP4 acts as a hematopoietic growth factor and review its potential to modulate HSC in ex vivo expansion cultures from cord blood.
DOI:10.1242/dev.01806URLPMID:15829520 [本文引用: 1]

Bone morphogenetic protein (Bmp) signaling is crucial for the formation and patterning of zebrafish ventral and posterior mesoderm. Mutants defective in the Bmp pathway have expanded trunk muscle, abnormal tails and severely impaired development of ventral mesodermal derivatives such as vasculature, blood and pronephros. As Bmps continue to be expressed in the ventral and posterior mesoderm after gastrulation, it is likely that Bmp signaling continues to play an important developmental role during outgrowth of the posterior body. However, because Bmp signaling plays an essential role during the gastrula stages, it has not been possible with mutants or standard disruption techniques to determine the later functions of the Bmp pathway. To study the role of Bmp signaling in the ventral and posterior mesoderm during trunk and tail outgrowth, we generated a transgenic zebrafish line containing a heatshock-inducible dominant-negative Bmp receptor-GFP fusion. Our data show that Bmps are important for tail organizer formation and for patterning the ventral mesoderm during early gastrulation. However, from mid-gastrulation to the early somitogenesis stages, Bmp signaling is important for ventral tail fin development and for preventing secondary tail formation. We conclude that the role of Bmp signaling in the ventral and posterior mesoderm changes as gastrulation proceeds.
DOI:10.1242/dev.02386URLPMID:16672337 [本文引用: 1]

The bone morphogenetic protein (BMP) signaling pathway is essential during gastrulation for the generation of ventral mesoderm, which makes it a challenge to define functions for this pathway at later stages of development. We have established an approach to disrupt BMP signaling specifically in lateral mesoderm during somitogenesis, by targeting a dominant-negative BMP receptor to Lmo2+ cells in developing zebrafish embryos. This results in expansion of hematopoietic and endothelial cells, while restricting the expression domain of the pronephric marker pax2.1. Expression of a constitutively active receptor and transplantation experiments were used to confirm that BMP signaling in lateral mesoderm restricts subsequent hemato-vascular development. The results show that the BMP signaling pathway continues to function after cells are committed to a lateral mesoderm fate, and influences subsequent lineage decisions by restricting hemato-vascular fate in favor of pronephric development.
DOI:10.1016/j.devcel.2009.04.014URLPMID:19531361 [本文引用: 1]

Hematopoietic stem cells (HSCs) are first detected in the floor of the embryonic dorsal aorta (DA), and we investigate the signals that induce the HSC program there. We show that while continued Hedgehog (Hh) signaling from the overlying midline structures maintains the arterial program characteristic of the DA roof, a ventral Bmp4 signal induces the blood stem cell program in the DA floor. This patterning of the DA by Hh and Bmp is the mirror image of that in the neural tube, with Hh favoring dorsal rather than ventral cell types, and Bmp favoring ventral rather than dorsal. With the majority of current data supporting a model whereby HSCs derive from arterial endothelium, our data identify the signal driving this conversion. These findings are important for the study of the production of HSCs from embryonic stem cells and establish a paradigm for the development of adult stem cells.
DOI:10.1016/j.ydbio.2007.11.038URLPMID:18222420 [本文引用: 1]

Ligands of the transforming growth factor beta (TGFbeta) superfamily, like Nodal and bone morphogenetic protein (BMP), are pivotal to establish left-right (LR) asymmetry in vertebrates. However, the receptors mediating this process are unknown. Here we identified two new type II receptors for BMPs in zebrafish termed bmpr2a and bmpr2b that induce a classical Smad1/5/8 response to BMP binding. Morpholino-mediated knockdown of bmpr2a and bmpr2b showed that they are required for the establishment of concomitant cardiac and visceral LR asymmetry. Expression of early laterality markers in morphants indicated that bmpr2a and bmpr2b act upstream of pitx2 and the nodal-related southpaw (spaw), which are expressed asymmetrically in the lateral plate mesoderm (LPM), and subsequently regulate lefty2 and bmp4 in the left heart field. We demonstrated that bmpr2a is required for lefty1 expression in the midline at early segmentation while bmpr2a/bmpr2b heteromers mediate left-sided spaw expression in the LPM. We propose a mechanism whereby this differential interpretation of BMP signalling through bmpr2a and bmpr2b is essential for the establishment of LR asymmetry in the zebrafish embryo.
DOI:10.1073/pnas.0706923105URLPMID:18087045 [本文引用: 1]

Hematopoietic stem cell (HSC) self-renewal and differentiation is regulated by cellular and molecular interactions with the surrounding microenvironment. During ontogeny, the aorta-gonad-mesonephros (AGM) region autonomously generates the first HSCs and serves as the first HSC-supportive microenvironment. Because the molecular identity of the AGM microenvironment is as yet unclear, we examined two closely related AGM stromal clones that differentially support HSCs. Expression analyses identified three putative HSC regulatory factors, beta-NGF (a neurotrophic factor), MIP-1gamma (a C-C chemokine family member) and Bmp4 (a TGF-beta family member). We show here that these three factors, when added to AGM explant cultures, enhance the in vivo repopulating ability of AGM HSCs. The effects of Bmp4 on AGM HSCs were further studied because this factor acts at the mesodermal and primitive erythropoietic stages in the mouse embryo. In this report, we show that enriched E11 AGM HSCs express Bmp receptors and can be inhibited in their activity by gremlin, a Bmp antagonist. Moreover, our results reveal a focal point of Bmp4 expression in the mesenchyme underlying HSC containing aortic clusters at E11. We suggest that Bmp4 plays a relatively late role in the regulation of HSCs as they emerge in the midgestation AGM.
DOI:10.1182/blood-2007-04-085753URLPMID:17761518 [本文引用: 1]

The bone morphogenetic protein (BMP) signaling pathway regulates multiple steps of hematopoiesis, mediated through receptor-regulated Smads, including Smad1 and Smad5. Here, we use loss-of-function approaches in zebrafish to compare the roles of Smad1 and Smad5 during embryonic hematopoiesis. We show that knockdown of Smad1 or Smad5 generates distinct and even opposite hematopoietic phenotypes. Embryos depleted for Smad1 have an increased number of primitive erythrocytes, but fail to produce mature embryonic macrophages. In contrast, Smad5-depleted embryos are defective in primitive erythropoiesis, yet have normal numbers of macrophages. Loss of either Smad1 or Smad5 causes a failure in the generation of definitive hematopoietic progenitors. To investigate the mechanism behind these phenotypes, we used rescue experiments and found that Smad5 is unable to rescue the Smad1 loss-of-function phenotype, indicating that the 2 highly related proteins have inherently distinct activities. Microarray experiments revealed that the 2 proteins redundantly regulate the key initiators of the hemato-vascular program, including scl, lmo2, and gfi1. However, each also regulates a remarkably distinct genetic program, with Smad5 uniquely regulating the BMP signaling pathway itself. Our results suggest that specificity of BMP signaling output, with respect to hematopoiesis, can be explained by differential functions of Smad1 and Smad5.
DOI:10.1038/ncomms4431URLPMID:24614941 [本文引用: 3]

The earliest HSCs are derived from haemogenic endothelium via endothelial-to-haematopoietic transition during vertebrate embryogenesis; however, the underlying mechanism is largely unclear. Here we show that interplay of Smad1/5 and ERK signalling is essential for haemogenic endothelium-based HSC emergence. Smad1/5 directly inhibits erk expression through recruiting HDAC1 to and inducing de-acetylation of the erk promoter in endothelial cells. Over-activated ERK signalling conferred by inhibition of Smad1/5 promotes the arterial endothelial cell fate and constitutively strengthens the tight junction between endothelial cells, thereby repressing the specification of haemogenic endothelium and the following endothelial-to-haematopoietic transition process. These findings provide new insights into the in vitro generation of transplantable HSCs for potential clinical applications.
DOI:10.1182/blood-2010-10-312389URL [本文引用: 1]

Bone morphogenetic protein (BMP) signaling regulates embryonic hematopoiesis via receptor-mediated activation of downstream SMAD proteins, including SMAD1. In previous work, we showed that Smad1 expression is sufficient to enhance commitment of mesoderm to hemangioblast fate. We also found indirect evidence to support a subsequent repressive function for Smad1 in hematopoiesis. To test this hypothesis directly, we developed a novel system allowing temporal control of Smad1 levels by conditional knockdown in embryonic stem cell derivatives. Depletion of Smad1 in embryoid body cultures before hemangioblast commitment limits hematopoietic potential because of a block in mesoderm development. Conversely, when Smad1 is depleted in FlK1(+) mesoderm, at a stage after hemangioblast commitment, the pool of hematopoietic progenitors is expanded. This involves enhanced expression levels for genes specific to hematopoiesis, including Gata1, Runx1 and Eklf, rather than factors required for earlier specification of the hemangioblast. The phenotype correlates with increased nuclear SMAD2 activity, indicating molecular cross-regulation between the BMP and TGF-beta signaling pathways. Consistent with this mechanism, hematopoiesis was enhanced when Smad2 was directly expressed during this same developmental window. Therefore, this study reveals a temporally defined function for Smad1 in restricting the expansion of early hematopoietic progenitors. (Blood. 2011;117(24):6489-6497)
DOI:10.1182/blood-2013-09-526053URL [本文引用: 2]

In mouse mid-gestational embryos, definitive hematopoietic stem progenitor cells are derived directly from a very small proportion of the arterial endothelium. However, the physiological mechanisms restraining excessive endothelial-hematopoietic transition remain elusive. We show here that genetic deletion of Smad4 from the endothelium stage (using Tie2-Cre), but not from embryonic hematopoietic cells (using Vav-Cre), leads to a strikingly augmented emergence of intra-arterial hematopoietic clusters and an enhanced in vitro generation of hematopoietic progenitors, with no increase in the proliferation and survival of hematopoietic cluster cells. This finding indicates a temporally restricted negative effect of Smad4 on the endothelial to hematopoietic progenitor transition. Furthermore, the absence of endothelial Smad4 causes an increased expression of subaortic bone morphogenetic protein 4 and an activation of aortic extracellular signal-regulated kinase, thereby resulting in the excessive generation of blood cells. Collectively, our data for the first time identify a physiological suppressor that functions specifically during the transition of endothelial cells to hematopoietic progenitors and further suggest that endothelial Smad4 is a crucial modulator of the subaortic microenvironment that controls the hematopoietic fate of the aortic endothelium.
DOI:10.1073/pnas.0607196104URLPMID:17213321 [本文引用: 1]

Hematopoietic stem cell (HSC) development is regulated by several signaling pathways and a number of key transcription factors, which include Scl/Tal1, Runx1, and members of the Smad family. However, it remains unclear how these various determinants interact. Using a genome-wide computational screen based on the well characterized Scl +19 HSC enhancer, we have identified a related Smad6 enhancer that also targets expression to blood and endothelial cells in transgenic mice. Smad6, Bmp4, and Runx1 transcripts are concentrated along the ventral aspect of the E10.5 dorsal aorta in the aorta-gonad-mesonephros region from which HSCs originate. Moreover, Smad6, an inhibitor of Bmp4 signaling, binds and inhibits Runx1 activity, whereas Smad1, a positive mediator of Bmp4 signaling, transactivates the Runx1 promoter. Taken together, our results integrate three key determinants of HSC development; the Scl transcriptional network, Runx1 activity, and the Bmp4/Smad signaling pathway.
DOI:10.1006/dbio.1999.9205URLPMID:10191050 [本文引用: 1]

In adult vertebrates, fibroblast growth factor (FGF) synergizes with many hematopoietic cytokines to stimulate the proliferation of hematopoietic progenitors. In vertebrate development, the FGF signaling pathway is important in the formation of some derivatives of ventroposterior mesoderm. However, the function of FGF in the specification of the embryonic erythropoietic lineage has remained unclear. Here we address the role of FGF in the specification of the erythropoietic lineage in the Xenopus embryo. We report that ventral injection of embryonic FGF (eFGF) mRNA at as little as 10 pg at the four-cell stage suppresses ventral blood island (VBI) formation, whereas expression of the dominant negative form of the FGF receptor in the lateral mesoderm, where physiologically no blood tissue is formed, results in a dramatic expansion of the VBI. Similar results were observed in isolated ventral marginal zones and animal caps. Bone morphogenetic protein-4 (BMP-4) is known to induce erythropoiesis in the Xenopus embryo. Therefore, we examined how the BMP-4 and FGF signaling pathways might interact in the decision of ventral mesoderm to form blood. We observed that eFGF inhibits BMP-4-induced erythropoiesis by differentially regulating expression of the BMP-4 downstream effectors GATA-2 and PV.1. GATA-2, which stimulates erythropoiesis, is suppressed by FGF. PV.1, which we demonstrate to inhibit blood development, is enhanced by FGF. Additionally, PV.1 and GATA-2 negatively regulate transcription of each other. Thus, BMP-4 induces two transcription factors which have opposing effects on blood development. The FGF and BMP-4 signaling pathways interact to regulate the specification of the erythropoietic lineage.
DOI:10.1101/gad.1153603URLPMID:14701872 [本文引用: 1]

How do very diverse signaling pathways induce neural differentiation in Xenopus? Anti-BMP (Chordin), FGF8, and IGF2 signals are integrated in the embryo via the regulation of Smad1 phosphorylation. Neural induction results from the combined inhibition of BMP receptor serine/threonine kinases and activation of receptor tyrosine kinases that signal through MAPK and phosphorylate Smad1 in the linker region, further inhibiting Smad1 transcriptional activity. This hard-wired molecular mechanism at the level of the Smad1 transcription factor may help explain the opposing activities of IGF, FGF, and BMP signals not only in neural induction, but also in other aspects of vertebrate development.
DOI:10.1016/j.stem.2011.01.001URL [本文引用: 1]

Here, we show that as human embryonic stem cells (ESCs) exit the pluripotent state, NANOG can play a key role in determining lineage outcome. It has previously been reported that BMPs induce differentiation of human ESCs into extraembryonic lineages. Here, we find that FGF2, acting through the MEK-ERK pathway, switches BMP4-induced human ESC differentiation outcome to mesendoderm, characterized by the uniform expression of T (brachyury) and other primitive streak markers. We also find that MEK-ERK signaling prolongs NANOG expression during BMP-induced differentiation, that forced NANOG expression results in FGF-independent BMP4 induction of mesendoderm, and that knockdown of NANOG greatly reduces T induction. Together, our results demonstrate that FGF2 signaling switches the outcome of BMP4-induced differentiation of human ESCs by maintaining NANOG levels through the MEK-ERK pathway.
DOI:10.1016/j.cell.2009.03.045URLPMID:19379690 [本文引用: 1]

Notch signaling regulates many aspects of metazoan development and tissue renewal. Accordingly, the misregulation or loss of Notch signaling underlies a wide range of human disorders, from developmental syndromes to adult-onset diseases and cancer. Notch signaling is remarkably robust in most tissues even though each Notch molecule is irreversibly activated by proteolysis and signals only once without amplification by secondary messenger cascades. In this Review, we highlight recent studies in Notch signaling that reveal new molecular details about the regulation of ligand-mediated receptor activation, receptor proteolysis, and target selection.
DOI:10.1002/bies.20004URLPMID:14988924 [本文引用: 1]

Vascular development entails multiple cell-fate decisions to specify a diverse array of vascular structures. Notch proteins are signaling receptors that regulate cell-fate determination in a variety of cell types. The finding that Notch genes are robustly expressed in the vasculature suggests roles for Notch in guiding endothelial and associated mural cells through the myriad of cell-fate decisions needed to form the vasculature. In fact, mice with defects in genes encoding Notch, Notch ligands, and components of the Notch signaling cascade invariably display vascular defects. Human Notch genes are linked to Alagille's Syndrome, a developmental disorder with vascular defects, and CADASIL, a cerebral arteriopathy. Studies in zebrafish, mice and humans indicate that Notch works in conjunction with other angiogenic pathways to pattern and stabilize the vasculature. Here, we will focus on established functions for Notch in vascular remodeling and arterial/venous specification and more speculative roles in vascular homeostasis and organ-specific angiogenesis.
DOI:10.1002/dvdy.230URLPMID:32996661 [本文引用: 1]

The hematopoietic stem cell (HSC) is able to give rise to all blood cell lineages in vertebrates. HSCs are generated in the early embryo after two precedent waves of primitive hematopoiesis. Canonical Notch signaling is at the center of the complex mechanism that controls the development of the definitive HSC. The successful in vitro generation of hematopoietic cells from pluripotent stem cells with the capacity for multilineage hematopoietic reconstitution after transplantation requires the recapitulation of the most important process that takes place in the hemogenic endothelium during definitive hematopoiesis, that is the endothelial-to-hematopoietic transition (EHT). To meet this challenge, it is necessary to thoroughly understand the molecular mechanisms that modulate Notch signaling during the HSC differentiation process considering different temporal and spatial dimensions. In recent years, there have been important advances in this field. Here, we review relevant contributions describing different genes, factors, environmental cues, and signaling cascades that regulate the EHT through Notch interactions at multiple levels. The evolutionary conservation of the hematopoietic program has made possible the use of diverse model systems. We describe the contributions of the zebrafish model and the most relevant ones from transgenic mouse studies and from in vitro differentiated pluripotent cells.
DOI:10.1016/j.ydbio.2015.11.008URLPMID:26586199 [本文引用: 1]

Hematopoietic stem cells are formed during embryonic development, and serve as the foundation of the definitive blood program for life. Notch signaling has been well established as an essential direct contributor to HSC specification. However, several recent studies have indicated that the contribution of Notch signaling is complex. HSC specification requires multiple Notch signaling inputs, some received directly by hematopoietic precursors, and others that occur indirectly within neighboring somites. Of note, proinflammatory signals provided by primitive myeloid cells are needed for HSC specification via upregulation of the Notch pathway in hemogenic endothelium. In addition to multiple requirements for Notch activation, recent studies indicate that Notch signaling must subsequently be repressed to permit HSC emergence. Finally, Notch must then be reactivated to maintain HSC fate. In this review, we discuss the growing understanding of the dynamic contributions of Notch signaling to the establishment of hematopoiesis during development.
URLPMID:11585794 [本文引用: 1]

Recent evidence indicates that acquisition of artery or vein identity during vascular development is governed, in part, by genetic mechanisms. The artery-specific expression of a number of Notch signaling genes in mouse and zebrafish suggests that this pathway may play a role in arterial-venous cell fate determination during vascular development. We show that loss of Notch signaling in zebrafish embryos leads to molecular defects in arterial-venous differentiation, including loss of artery-specific markers and ectopic expression of venous markers within the dorsal aorta. Conversely, we find that ectopic activation of Notch signaling leads to repression of venous cell fate. Finally, embryos lacking Notch function exhibit defects in blood vessel formation similar to those associated with improper arterial-venous specification. Our results suggest that Notch signaling is required for the proper development of arterial and venous blood vessels, and that a major role of Notch signaling in blood vessels is to repress venous differentiation within developing arteries. Movies available on-line
DOI:10.1016/S1074-7613(03)00117-1URL [本文引用: 1]

Abstract
Hematopoietic stem cells (HSCs) are thought to arise in the aorta-gonad-mesonephros (AGM) region of embryo proper, although HSC activity can be detected in yolk sac (YS) and paraaortic splanchnopleura (P-Sp) when transplanted in newborn mice. We examined the role of Notch signaling in embryonic hematopoiesis. The activity of colony-forming cells in the YS from Notch1−/− embryos was comparable to that of wild-type embryos. However, in vitro and in vivo definitive hematopoietic activities from YS and P-Sp were severely impaired in Notch1−/− embryos. The population representing hemogenic endothelial cells, however, did not decrease. In contrast, Notch2−/− embryos showed no hematopoietic deficiency. These data indicate that Notch1, but not Notch2, is essential for generating hematopoietic stem cells from endothelial cells.DOI:10.1016/j.devcel.2006.12.011URLPMID:17336907 [本文引用: 1]

Ventricular chamber morphogenesis, first manifested by trabeculae formation, is crucial for cardiac function and embryonic viability and depends on cellular interactions between the endocardium and myocardium. We show that ventricular Notch1 activity is highest at presumptive trabecular endocardium. RBPJk and Notch1 mutants show impaired trabeculation and marker expression, attenuated EphrinB2, NRG1, and BMP10 expression and signaling, and decreased myocardial proliferation. Functional and molecular analyses show that Notch inhibition prevents EphrinB2 expression, and that EphrinB2 is a direct Notch target acting upstream of NRG1 in the ventricles. However, BMP10 levels are found to be independent of both EphrinB2 and NRG1 during trabeculation. Accordingly, exogenous BMP10 rescues the myocardial proliferative defect of in vitro-cultured RBPJk mutants, while exogenous NRG1 rescues differentiation in parallel. We suggest that during trabeculation Notch independently regulates cardiomyocyte proliferation and differentiation, two exquisitely balanced processes whose perturbation may result in congenital heart disease.
DOI:10.1101/gad.1337005URLPMID:16166372 [本文引用: 1]

Identifying the molecular pathways regulating hematopoietic stem cell (HSC) specification, self-renewal, and expansion remains a fundamental goal of both basic and clinical biology. Here, we analyzed the effects of Notch signaling on HSC number during zebrafish development and adulthood, defining a critical pathway for stem cell specification. The Notch signaling mutant mind bomb displays normal embryonic hematopoiesis but fails to specify adult HSCs. Surprisingly, transient Notch activation during embryogenesis via an inducible transgenic system led to a Runx1-dependent expansion of HSCs in the aorta-gonad-mesonephros (AGM) region. In irradiated adults, Notch activity induced runx1 gene expression and increased multilineage hematopoietic precursor cells approximately threefold in the marrow. This increase was followed by the accelerated recovery of all the mature blood cell lineages. These data define the Notch-Runx pathway as critical for the developmental specification of HSC fate and the subsequent homeostasis of HSC number, thus providing a mechanism for amplifying stem cells in vivo.
DOI:10.1038/emboj.2008.113URLPMID:18528438 [本文引用: 1]

Specific deletion of Notch1 and RBPjkappa in the mouse results in abrogation of definitive haematopoiesis concomitant with the loss of arterial identity at embryonic stage. As prior arterial determination is likely to be required for the generation of embryonic haematopoiesis, it is difficult to establish the specific haematopoietic role of Notch in these mutants. By analysing different Notch-ligand-null embryos, we now show that Jagged1 is not required for the establishment of the arterial fate but it is required for the correct execution of the definitive haematopoietic programme, including expression of GATA2 in the dorsal aorta. Moreover, successful haematopoietic rescue of the Jagged1-null AGM cells was obtained by culturing them with Jagged1-expressing stromal cells or by lentiviral-mediated transduction of the GATA2 gene. Taken together, our results indicate that Jagged1-mediated activation of Notch1 is responsible for regulating GATA2 expression in the AGM, which in turn is essential for definitive haematopoiesis in the mouse.
DOI:10.1242/dev.01660URLPMID:15689374

Definitive hematopoiesis in the mouse embryo originates from the aortic floor in the P-Sp/AGM region in close association with endothelial cells. An important role for Notch1 in the control of hematopoietic ontogeny has been recently established, although its mechanism of action is poorly understood. Here, we show detailed analysis of Notch family gene expression in the aorta endothelium between embryonic day (E) 9.5 and E10.5. Since Notch requires binding to RBPjkappa transcription factor to activate transcription, we analyzed the aorta of the para-aortic splanchnopleura/AGM in RBPjkappa mutant embryos. We found specific patterns of expression of Notch receptors, ligands and Hes genes that were lost in RBPjkappa mutants. Analysis of these mutants revealed the absence of hematopoietic progenitors, accompanied by the lack of expression of the hematopoietic transcription factors Aml1/Runx1, Gata2 and Scl/Tal1. We show that in wild-type embryos, a few cells lining the aorta endothelium at E9.5 simultaneously expressed Notch1 and Gata2, and demonstrate by chromatin immunoprecipitation that Notch1 specifically associated with the Gata2 promoter in E9.5 wild-type embryos and 32D myeloid cells, an interaction lost in RBPjkappamutants. Consistent with a role for Notch1 in regulating Gata2, we observe increased expression of this gene in 32D cells expressing activated Notch1. Taken together, these data strongly suggest that activation of Gata2 expression by Notch1/RBPjkappa is a crucial event for the onset of definitive hematopoiesis in the embryo.
DOI:10.1084/jem.20131857URL [本文引用: 1]

Hematopoietic stem cell (HSC) specification occurs in the embryonic aorta and requires Notch activation; however, most of the Notch-regulated elements controlling de novo HSC generation are still unknown. Here, we identify putative direct Notch targets in the aorta-gonad-mesonephros (AGM) embryonic tissue by chromatin precipitation using antibodies against the Notch partner RBPj. By ChIP-on-chip analysis of the precipitated DNA, we identified 701 promoter regions that were candidates to be regulated by Notch in the AGM. One of the most enriched regions corresponded to the Cdca7 gene, which was subsequently confirmed to recruit the RBPj factor but also Notch1 in AGM cells. We found that during embryonic hematopoietic development, expression of Cdca7 is restricted to the hematopoietic clusters of the aorta, and it is strongly up-regulated in the hemogenic population during human embryonic stem cell hematopoietic differentiation in a Notch-dependent manner. Down-regulation of Cdca7 mRNA in cultured AGM cells significantly induces hematopoietic differentiation and loss of the progenitor population. Finally, using loss-of-function experiments in zebrafish, we demonstrate that CDCA7 contributes to HSC emergence in vivo during embryonic development. Thus, our study identifies Cdca7 as an evolutionary conserved Notch target involved in HSC emergence.
DOI:10.1038/ncomms9510URLPMID:26465397 [本文引用: 1]

Acquisition of the arterial and haemogenic endothelium fates concurrently occur in the aorta-gonad-mesonephros (AGM) region prior to haematopoietic stem cell (HSC) generation. The arterial programme depends on Dll4 and the haemogenic endothelium/HSC on Jag1-mediated Notch1 signalling. How Notch1 distinguishes and executes these different programmes in response to particular ligands is poorly understood. By using two Notch1 activation trap mouse models with different sensitivity, here we show that arterial endothelial cells and HSCs originate from distinct precursors, characterized by different Notch1 signal strengths. Microarray analysis on AGM subpopulations demonstrates that the Jag1 ligand stimulates low Notch strength, inhibits the endothelial programme and is permissive for HSC specification. In the absence of Jag1, endothelial cells experience high Dll4-induced Notch activity and select the endothelial programme, thus precluding HSC formation. Interference with the Dll4 signal by ligand-specific blocking antibodies is sufficient to inhibit the endothelial programme and favour specification of the haematopoietic lineage.
DOI:10.1038/cr.2015.109URLPMID:26358189 [本文引用: 2]

In vertebrates, embryonic hematopoietic stem and progenitor cells (HSPCs) are derived from a subset of endothelial cells, the hemogenic endothelium (HE), through the endothelial-to-hematopoietic transition (EHT). Notch signaling is essential for HSPC development during embryogenesis across vertebrates. However, whether and how it regulates EHT remains unclear. Here, we show that G protein-coupled receptor 183 (Gpr183) signaling serves as an indispensable switch for HSPC emergence by repressing Notch signaling before the onset of EHT. Inhibition of Gpr183 significantly upregulates Notch signaling and abolishes HSPC emergence. Upon activation by its ligand 7alpha-25-OHC, Gpr183 recruits beta-arrestin1 and the E3 ligase Nedd4 to degrade Notch1 in specified HE cells and then facilitates the subsequent EHT. Importantly, 7alpha-25-OHC stimulation promotes HSPC emergence in vivo and in vitro, providing an attractive strategy for enhancing the in vitro generation of functional HSPCs.
DOI:10.1016/j.devcel.2013.02.011URLPMID:23537631 [本文引用: 1]

Hematopoietic stem cells (HSCs) are produced by a small cohort of hemogenic endothelial cells (ECs) during development through the formation of intra-aortic hematopoietic cell (HC) clusters. The Runx1 transcription factor plays a key role in the EC-to-HC and -HSC transition. We show that Runx1 expression in hemogenic ECs and the subsequent initiation of HC formation are tightly controlled by the subaortic mesenchyme, although the mesenchyme is not a source of HCs. Runx1 and Notch signaling are involved in this process, with Notch signaling decreasing with time in HCs. Inhibiting Notch signaling readily increases HC production in mouse and chicken embryos. In the mouse, however, this increase is transient. Collectively, we show complementary roles of hemogenic ECs and mesenchymal compartments in triggering aortic hematopoiesis. The subaortic mesenchyme induces Runx1 expression in hemogenic-primed ECs and collaborates with Notch dynamics to control aortic hematopoiesis.
DOI:10.1161/CIRCRESAHA.108.188805URLPMID:19286613 [本文引用: 1]

The major arteries and veins of the vertebrate circulatory system are formed early in embryonic development, before the onset of circulation, following de novo aggregation of
DOI:10.1182/blood-2014-09-601542URLPMID:25540193 [本文引用: 1]

Inflammatory signaling has been shown to be essential for stress hematopoiesis in adult bone marrow, either through increasing proliferation or by directing differentiation of hematopoietic stem and progenitor cells (HSPCs) toward myeloid or lymphoid lineages. However, its role in embryonic normal hematopoiesis has been unknown. Here, we demonstrate that in both zebrafish and mouse embryos, inflammatory signaling is necessary and sufficient for HSPC emergence, in the absence of infection or pathological inflammation. Mechanistically, inflammatory signaling regulates hemogenic endothelium-derived HSPC development through a conserved Toll-like receptor 4 (TLR4)-nuclear factor kappa-light-chain enhancer of activated B core (NF-kappaB) signaling, which then promotes Notch activity, a well-known signal required for HSPC specification in vertebrates. Our findings establish a previously unrecognized link between inflammatory signaling and HSPC emergence, and provide new insights into regenerative medicine and novel therapies to treat innate immune-related diseases.
DOI:10.1016/j.cell.2014.10.031URL [本文引用: 1]

Hematopoietic stemcells (HSCs) underlie the production of blood and immune cells for the lifetime of an organism. In vertebrate embryos, HSCs arise from the unique transdifferentiation of hemogenic endothelium comprising the floor of the dorsal aorta during a brief developmental window. To date, this process has not been replicated in vitro from pluripotent precursors, partly because the full complement of required signaling inputs remains to be determined. Here, we show that TNFR2 via TNF alpha activates the Notch and NF-kappa B signaling pathways to establish HSC fate, indicating a requirement for inflammatory signaling in HSC generation. We determine that primitive neutrophils are the major source of TNF alpha, assigning a role for transient innate immune cells in establishing the HSC program. These results demonstrate that proinflammatory signaling, in the absence of infection, is utilized by the developing embryo to generate the lineal precursors of the adult hematopoietic system.
DOI:10.1038/s41467-019-09403-7URLPMID:31015398 [本文引用: 1]

Hematopoietic stem and progenitor cells (HSPCs) are capable of producing all mature blood lineages, as well as maintaining the self-renewal ability throughout life. The hairy-like organelle, cilium, is present in most types of vertebrate cells, and plays important roles in various biological processes. However, it is unclear whether and how cilia regulate HSPC development in vertebrates. Here, we show that cilia-specific genes, involved in primary cilia formation and function, are required for HSPC development, especially in hemogenic endothelium (HE) specification in zebrafish embryos. Blocking primary cilia formation or function by genetic or chemical manipulations impairs HSPC development. Mechanistically, we uncover that primary cilia in endothelial cells transduce Notch signal to the earliest HE for proper HSPC specification during embryogenesis. Altogether, our findings reveal a pivotal role of endothelial primary cilia in HSPC development, and may shed lights into in vitro directed differentiation of HSPCs.
DOI:10.1182/blood-2013-11-541391URL [本文引用: 1]

Nuclear receptor corepressors (Ncors) are important for developmental and homeostatic processes in vertebrates, which exert transcriptional repression by coordinating with histone deacetylases. However, little is known about their roles in definitive hematopoiesis. In this study, we show that in zebrafish, ncor2 is required for hematopoietic stem cell (HSC) development by repressing fos-vegfd signaling. ncor2 is specifically expressed in the aorta-gonad-mesonephros (AGM) region in zebrafish embryos. ncor2 deficiency reduced the population of HSCs in both the AGM region and T cells in the thymus. Mechanistically, ncor2 knockdown upregulated fos transcription by modulating the acetylation level in the fos promoter region, which then enhanced Vegfd signaling. Consequently, the augmented Vegfd signaling induced Notch signaling to promote the arterial endothelial fate, therefore, possibly repressing the hemogenic endothelial specification, which is a prerequisite for HSC emergence. Thus, our findings identify a novel regulatory mechanism for Ncor2 through Fos-Vegfd-Notch signaling cascade during HSC development in zebrafish embryos.
DOI:10.1038/nature23883URLPMID:28869969 [本文引用: 1]

N(6)-methyladenosine (m(6)A) has been identified as the most abundant modification on eukaryote messenger RNA (mRNA). Although the rapid development of high-throughput sequencing technologies has enabled insight into the biological functions of m(6)A modification, the function of m(6)A during vertebrate embryogenesis remains poorly understood. Here we show that m(6)A determines cell fate during the endothelial-to-haematopoietic transition (EHT) to specify the earliest haematopoietic stem/progenitor cells (HSPCs) during zebrafish embryogenesis. m(6)A-specific methylated RNA immunoprecipitation combined with high-throughput sequencing (MeRIP-seq) and m(6)A individual-nucleotide-resolution cross-linking and immunoprecipitation with sequencing (miCLIP-seq) analyses reveal conserved features on zebrafish m(6)A methylome and preferential distribution of m(6)A peaks near the stop codon with a consensus RRACH motif. In mettl3-deficient embryos, levels of m(6)A are significantly decreased and emergence of HSPCs is blocked. Mechanistically, we identify that the delayed YTHDF2-mediated mRNA decay of the arterial endothelial genes notch1a and rhoca contributes to this deleterious effect. The continuous activation of Notch signalling in arterial endothelial cells of mettl3-deficient embryos blocks EHT, thereby repressing the generation of the earliest HSPCs. Furthermore, knockdown of Mettl3 in mice confers a similar phenotype. Collectively, our findings demonstrate the critical function of m(6)A modification in the fate determination of HSPCs during vertebrate embryogenesis.
DOI:10.1371/journal.pbio.3000696URLPMID:32275659 [本文引用: 1]

It is well known that various developmental signals play diverse roles in hematopoietic stem and progenitor cell (HSPC) production; however, how these signaling pathways are orchestrated remains incompletely understood. Here, we report that Rab5c is essential for HSPC specification by endocytic trafficking of Notch and AKT signaling in zebrafish embryos. Rab5c deficiency leads to defects in HSPC production. Mechanistically, Rab5c regulates hemogenic endothelium (HE) specification by endocytic trafficking of Notch ligands and receptor. We further show that the interaction between Rab5c and Appl1 in the endosome is required for the survival of HE in the ventral wall of the dorsal aorta through AKT signaling. Interestingly, Rab5c overactivation can also lead to defects in HSPC production, which is attributed to excessive endolysosomal trafficking inducing Notch signaling defect. Taken together, our findings establish a previously unrecognized role of Rab5c-mediated endocytic trafficking in HSPC development and provide new insights into how spatiotemporal signals are orchestrated to accurately execute cell fate transition.
DOI:10.1242/dev.164988URLPMID:29724757 [本文引用: 1]

Asymmetric division is crucial for embryonic development and stem cell lineages. In the one-cell Caenorhabditis elegans embryo, a contractile cortical actomyosin network contributes to asymmetric division by segregating partitioning-defective (PAR) proteins to discrete cortical domains. In the current study, we found that the plasma membrane lipid phosphatidylinositol 4,5-bisphosphate (PIP2) localizes to polarized dynamic structures in C. elegans zygotes, distributing in a PAR-dependent manner along the anterior-posterior (A-P) embryonic axis. PIP2 cortical structures overlap with F-actin, and coincide with the actin regulators RHO-1 and CDC-42, as well as ECT-2. Particle image velocimetry analysis revealed that PIP2 and F-actin cortical movements are coupled, with PIP2 structures moving slightly ahead of F-actin. Importantly, we established that PIP2 cortical structure formation and movement is actin dependent. Moreover, we found that decreasing or increasing the level of PIP2 resulted in severe F-actin disorganization, revealing interdependence between these components. Furthermore, we determined that PIP2 and F-actin regulate the sizing of PAR cortical domains, including during the maintenance phase of polarization. Overall, our work establishes that a lipid membrane component, PIP2, modulates actin organization and cell polarity in C. elegans embryos.
DOI:10.1016/j.cell.2012.05.012URL [本文引用: 1]

The WNT signal transduction cascade controls myriad biological phenomena throughout development and adult life of all animals. In parallel, aberrant Wnt signaling underlies a wide range of pathologies in humans. In this Review, we provide an update of the core Wnt/beta-catenin signaling pathway, discuss how its various components contribute to disease, and pose outstanding questions to be addressed in the future.
DOI:10.1016/j.trecan.2017.01.001URLPMID:28718442 [本文引用: 1]

Cancer cells are addicted to a large spectrum of extracellular cues implicated in initiation, stem cell renewal, tumor growth, dissemination in the body, and resistance to treatment. Wingless/Int-1 (Wnt) ligands and their associated signaling cascades contribute to most of these processes, paving the way for opportunities in therapeutic development. The developmental Wnt/planar cell polarity (PCP) pathway is the most recently described branch of Wnt signaling strongly implicated in cancer development at early and late stages. We describe here some of the latest knowledge accumulated on this pathway and the pending questions, present the most convincing findings about its role in cancer, and review the most promising strategies currently designed to target its components.
DOI:10.1093/abbs/gmr079URL [本文引用: 1]
DOI:10.1016/j.ceca.2005.06.022URLPMID:16099039 [本文引用: 1]

Wnt signaling is a complex pathway in which beta-catenin is typically viewed as a central mediator. However, within the past 15 years, at least three Wnt-mediated pathways have been proposed that function independent of beta-catenin. One pathway involves activation of calcium/calmodulin-dependent kinase II (CamKII) and protein kinase C (PKC). Another includes recruitment of heterotrimeric GTP-binding proteins to activate phospholipase C (PLC) and phosphodiesterase (PDE). Lastly, a pathway similar to the planar cell polarity (PCP) pathway in Drosophila has been identified that activates the Jun-N-terminal kinase (JNK) and, perhaps, small GTP-binding proteins. Calcium has been implicated as an important second messenger in all of these pathways. This review will focus on the role of calcium in Wnt signaling and, as a consequence, provide a limited overview of beta-catenin-independent Wnt signaling.
DOI:10.1016/j.cell.2009.01.015URLPMID:19303855 [本文引用: 1]

Interactions between developmental signaling pathways govern the formation and function of stem cells. Prostaglandin (PG) E2 regulates vertebrate hematopoietic stem cells (HSC). Similarly, the Wnt signaling pathway controls HSC self-renewal and bone marrow repopulation. Here, we show that wnt reporter activity in zebrafish HSCs is responsive to PGE2 modulation, demonstrating a direct interaction in vivo. Inhibition of PGE2 synthesis blocked wnt-induced alterations in HSC formation. PGE2 modified the wnt signaling cascade at the level of beta-catenin degradation through cAMP/PKA-mediated stabilizing phosphorylation events. The PGE2/Wnt interaction regulated murine stem and progenitor populations in vitro in hematopoietic ES cell assays and in vivo following transplantation. The relationship between PGE2 and Wnt was also conserved during regeneration of other organ systems. Our work provides in vivo evidence that Wnt activation in stem cells requires PGE2, and suggests the PGE2/Wnt interaction is a master regulator of vertebrate regeneration and recovery.
DOI:10.1016/j.stem.2007.10.022URLPMID:18371423 [本文引用: 1]

The formation of blood in the embryo is dependent on bone morphogenetic protein (BMP), but how BMP signaling intersects with other regulators of hematopoietic development is unclear. Using embryonic stem (ES) cells, we show that BMP4 first induces ventral-posterior (V-P) mesoderm and subsequently directs mesodermal cells toward blood fate by activating Wnt3a and upregulating Cdx and Hox genes. When BMP signaling is blocked during this latter phase, enforced expression of either Cdx1 or Cdx4 rescues hematopoietic development, thereby placing BMP4 signaling upstream of the Cdx-Hox pathway. Wnt signaling cooperates in BMP-induced hemogenesis, and the Wnt effector LEF1 mediates BMP4 activation of Cdx genes. Our data suggest that BMP signaling plays two distinct and sequential roles during blood formation, initially as an inducer of mesoderm, and later to specify blood via activation of Wnt signaling and the Cdx-Hox pathway.
DOI:10.1084/jem.20120225URL [本文引用: 2]

Understanding how hematopoietic stem cells (HSCs) are generated and the signals that control this process is a crucial issue for regenerative medicine applications that require in vitro production of HSC. HSCs emerge during embryonic life from an endothelial-like cell population that resides in the aorta-gonad-mesonephros (AGM) region. We show here that beta-catenin is nuclear and active in few endothelial nonhematopoietic cells closely associated with the emerging hematopoietic clusters of the embryonic aorta during mouse development. Importantly, Wnt/beta-catenin activity is transiently required in the AGM to generate long-term HSCs and to produce hematopoietic cells in vitro from AGM endothelial precursors. Genetic deletion of beta-catenin from the embryonic endothelium stage (using VE-cadherin-Cre recombinase), but not from embryonic hematopoietic cells (using Vav1-Cre), precludes progression of mutant cells toward the hematopoietic lineage; however, these mutant cells still contribute to the adult endothelium. Together, those findings indicate that Wnt/beta-catenin activity is needed for the emergence but not the maintenance of HSCs in mouse embryos.
DOI:10.1016/j.cell.2013.08.055URL [本文引用: 1]

Hematopoietic stem cells (HSCs) develop from a specialized subpopulation of endothelial cells known as hemogenic endothelium (HE). Although the HE origin of HSCs is now well established in different species, the signaling pathways that control this transition remain poorly understood. Here, we show that activation of retinoic acid (RA) signaling in aorta-gonad-mesonephros-derived HE ex vivo dramatically enhanced its HSC potential, whereas conditional inactivation of the RA metabolizing enzyme retinal dehydrogenase 2 in VE-cadherin expressing endothelial cells in vivo abrogated HSC development. Wnt signaling completely blocked the HSC inductive effects of RA modulators, whereas inhibition of the pathway promoted the development of HSCs in the absence of RA signaling. Collectively, these findings position RA and Wnt signaling as key regulators of HSC development and in doing so provide molecular insights that will aid in developing strategies for their generation from pluripotent stem cells.
DOI:10.1038/nature10107URLPMID:21654806 [本文引用: 1]

Haematopoietic stem cells (HSCs) are a self-renewing population of cells that continuously replenish all blood and immune cells during the lifetime of an individual. HSCs are used clinically to treat a wide array of diseases, including acute leukaemias and congenital blood disorders, but obtaining suitable numbers of cells and finding immune-compatible donors remain serious problems. These difficulties have led to an interest in the conversion of embryonic stem cells or induced pluripotent stem cells into HSCs, which is not possible using current methodologies. To accomplish this goal, it is critical to understand the native mechanisms involved in the specification of HSCs during embryonic development. Here we demonstrate in zebrafish that Wnt16 controls a novel genetic regulatory network required for HSC specification. Non-canonical signalling by Wnt16 is required for somitic expression of the Notch ligands deltaC (dlc) and deltaD (dld), and these ligands are, in turn, required for the establishment of definitive haematopoiesis. Notch signalling downstream of Dlc and Dld is earlier than, and distinct from, known cell-autonomous requirements for Notch, strongly suggesting that novel Notch-dependent relay signal(s) induce the first HSCs in parallel to other established pathways. Our results demonstrate that somite-specific gene expression is required for the production of haemogenic endothelium.
DOI:10.15252/embj.201488784URL [本文引用: 1]

Hematopoietic stem cells (HSCs) require multiple molecular inputs for proper specification, including activity of the Notch signaling pathway. A requirement for the Notch1 and dispensability of the Notch2 receptor has been demonstrated in mice, but the role of the remaining Notch receptors has not been investigated. Here, we demonstrate that three of the four Notch receptors are independently required for the specification of HSCs in the zebrafish. The orthologues of the murine Notch1 receptor, Notch1a and Notch1b, are each required intrinsically to fate HSCs, just prior to their emergence from aortic hemogenic endothelium. By contrast, the Notch3 receptor is required earlier within the developing somite to regulate HSC emergence in a non-cell-autonomous manner. Epistatic analyses demonstrate that Notch3 function lies downstream of Wnt16, which is required for HSC specification through its regulation of two Notch ligands, dlc and dld. Collectively, these findings demonstrate for the first time that multiple Notch signaling inputs are required to specify HSCs and that Notch3 performs a novel role within the somite to regulate the neighboring precursors of hemogenic endothelium.
DOI:10.1038/ncomms6583URLPMID:25428693 [本文引用: 1]

Haematopoietic stem cells (HSCs) derive from haemogenic endothelial cells of the primitive dorsal aorta (DA) during vertebrate embryogenesis. The molecular mechanisms governing this unique endothelial to haematopoietic transition remain unclear. Here, we demonstrate a novel requirement for fibroblast growth factor (FGF) signalling in HSC emergence. This requirement is non-cell-autonomous, and acts within the somite to bridge the Wnt and Notch signalling pathways. We previously demonstrated that Wnt16 regulates the somitic expression of two Notch ligands, deltaC (dlc) and deltaD (dld), whose combined function is required for HSC fate. How Wnt16 connects to Notch function has remained an open question. Our current studies demonstrate that FGF signalling, via FGF receptor 4 (Fgfr4), mediates a signal-transduction pathway between Wnt16 and Dlc, but not Dld, to regulate HSC specification. Our findings demonstrate that FGF signalling acts as a key molecular relay within the developmental HSC niche to instruct HSC fate.
DOI:10.1242/dev.139956URLPMID:28087636 [本文引用: 1]

Hematopoietic stem cells (HSCs) are the therapeutic component of bone marrow transplants, but finding immune-compatible donors limits treatment availability and efficacy. Recapitulation of endogenous specification during development is a promising approach to directing HSC specification in vitro, but current protocols are not capable of generating authentic HSCs with high efficiency. Across phyla, HSCs arise from hemogenic endothelium in the ventral floor of the dorsal aorta concurrent with arteriovenous specification and intersegmental vessel (ISV) sprouting, processes regulated by Notch and Wnt. We hypothesized that coordination of HSC specification with vessel patterning might involve modulatory regulatory factors such as R-spondin 1 (Rspo1), an extracellular protein that enhances beta-catenin-dependent Wnt signaling and has previously been shown to regulate ISV patterning. We find that Rspo1 is required for HSC specification through control of parallel signaling pathways controlling HSC specification: Wnt16/DeltaC/DeltaD and Vegfa/Tgfbeta1. Our results define Rspo1 as a key upstream regulator of two crucial pathways necessary for HSC specification.
