 ,, 曾洁, 薛云新, 赵西林
,, 曾洁, 薛云新, 赵西林 ,厦门大学公共卫生学院,分子疫苗学和分子诊断学国家重点实验室,厦门 361102
,厦门大学公共卫生学院,分子疫苗学和分子诊断学国家重点实验室,厦门 361102Progress on the function and regulatory mechanisms of bacterial Cpx signal transduction system
Liwen Wu ,, Jie Zeng, Yunxin Xue, Xilin Zhao
,, Jie Zeng, Yunxin Xue, Xilin Zhao ,State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, School of Public Health, Xiamen University, Xiamen 361102, China
,State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, School of Public Health, Xiamen University, Xiamen 361102, China通讯作者: 赵西林,博士,教授,博士生导师,研究方向:病原微生物与抗感染治疗。E-mail:zhaox5@xmu.edu.cn
编委: 谢建平
收稿日期:2021-06-10修回日期:2021-07-14网络出版日期:2021-08-20
| 基金资助: |
Received:2021-06-10Revised:2021-07-14Online:2021-08-20
| Fund supported: |
作者简介 About authors
吴丽雯,在读硕士研究生,专业方向:公共卫生。E-mail:

摘要
Cpx (conjugative pilus expression)双组分信号转导系统是革兰阴性细菌中一种复杂的包膜应激系统,能感应从不同信号传输点传入的多种包膜信号。位于胞质中的反应调节子CpxR磷酸化后能够调节众多编码内外膜上相关蛋白基因的表达。Cpx系统的激活还能调节细菌对抗生素和酸等压力的抵抗性。本文介绍了Cpx系统的组成,重点对Cpx系统的信号感应及调控机制进行综述,以期为Cpx系统的调控网络及其调节细菌重要生理过程的研究提供参考依据。
关键词:
Abstract
The Cpx (conjugative pilus expression) two-component signal transduction system is a complex envelope stress response system in Gram-negative bacteria, which can sense a variety of extracellular stimuli that enter the signaling pathway at different points. The phosphorylation of the CpxR, the cytoplasmic cognate response regulator of the Cpx system, can lead to changes in the expression of genes encoding proteins involved in inner and outer membrane functions. Activation of the Cpx system contributes to bacterial resistance/tolerance to certain antibiotics and acidic stress. In this review, we summarize the composition, and the mechanisms of signal detection, and the transcriptional regulation of the Cpx system, with a goal to provide guidance for the study of the regulatory network of the Cpx system and its important regulatory roles in bacterial physiology.
Keywords:
PDF (874KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
吴丽雯, 曾洁, 薛云新, 赵西林. 细菌Cpx信号转导系统的功能及调控机制研究进展. 遗传[J], 2021, 43(8): 747-757 doi:10.16288/j.yczz.21-208
Liwen Wu.
细菌在感染或定植过程中会遇到各种不利环境,例如肠道病原菌在肠道内需要承受各种压力,包括pH值、营养成分的变化以及其他细菌产物和有毒化合物的存在等。革兰阴性细菌的包膜是一个三层的隔室,由内膜(internal membrane, IM)、外膜(outer membrane, OM)和周质空间内的肽聚糖层(peptidoglycan, PG)组成[1]。它是细菌细胞与环境之间的边界,维持着细胞的形状和完整性,也是营养物质和有害产物的运输场所。许多压力环境和抗菌剂会使细菌包膜发生损伤或缺陷,而细菌则通过包膜应激反应(envelope stress responses, ESRs)来感知、响应这些信号从而适应细胞内外环境的变化,维持包膜的功能和完整性。在革兰阴性细菌中已定义了五种ESRs,包括Cpx (conjugative pilus expression)、σE (sigma factor)、Bae (bacterial adaptive response)、Rcs (regulator of capsule synthesis)和Psp (phage shock protein)系统[2]。当它们受到多种包膜压力诱导后,可以通过调控下游基因的转录来减轻包膜损害。其中Cpx是一个典型的双组分信号转导系统,能够响应各种可以引起细菌内膜及周质蛋白质错误折叠的包膜压力[3]。
20世纪80年代初,cpxA基因首次被McEwen等[4]发现。数年后,序列分析将CpxA鉴定为双组分系统中的传感蛋白[5],同时cpxA上游的基因cpxR也被发现[6]。在此之后对Cpx系统的研究逐渐深入,发现Cpx系统的激活可减轻包膜蛋白的错误折叠[7],确立了其作为新型包膜应激反应的观点。90年代至今的研究陆续表明,Cpx系统能被数十种刺激诱导,包括错误折叠蛋白[8]、碱性pH[9]、金属[10,11]等。此外,一些作用于细胞膜的抗生素和抗菌肽在损害包膜后也可能会激活Cpx系统[12,13]。由于这些刺激大多数都会导致蛋白质错误折叠,因此推测出一个信号转导模型,即错误折叠的包膜蛋白激活了Cpx系统,使得CpxR磷酸化后调控一些周质伴侣和蛋白酶的表达上调,进而使这些错误折叠的蛋白质得到重新折叠或降解,最终减轻包膜的压力。近年来许多研究将注意力集中在Cpx反应调控内膜相关基因的表达上[14],这些研究也为Cpx的一些调节表型提供了可能的解释,例如抗生素耐药性等。为了加深人们对这一重要调控系统的理解,本文将对Cpx系统的信号感应和转录调控机制进行综述。
新窗口打开|下载CSV
1 Cpx系统的组成
Cpx系统是由位于细胞质中的反应调节器 (response regulator, RR) CpxR和细胞内膜上的传感器组氨酸激酶 (histidine kinase, HK) CpxA组成的双组分系统。位于细胞周质中的CpxP是一种辅助调节蛋白,既可作为该系统的负反馈调节剂,又可作为应激反应的效应子,因此也属于Cpx系统的一员[15] (图1,A和B)。感应激酶CpxA是Cpx双组分系统中的关键成分。CpxA通常由两个跨膜结构域(two transmembrane domains, TMDs)、一个周质感受域(periplasmic sensory domain, PSD)和一个胞质传输域(transmitter domain)组成,同时具有激酶活性和磷酸酶活性[16,17]。CpxR是位于胞质中的反应调节子,包含一个天冬氨酸(D51)为磷酸化位点的N末端接收域(N-terminal receiver domain, REC)以及一个C末端效应域(C-terminal effector domain, trans_reg_C),该区域作为靶基因的转录调节子介导反应输出[18]。在其磷酸化状态下,以5ʹ-GTAAA(n5)GTAAA-3ʹ作为其共有识别序列与DNA结合[19]。CpxR的失活可通过CpxA的磷酸酶活性或Ser/Thr磷酸酶PrpA实现[16,20]。Cpx系统的第三种成分是周质辅助蛋白CpxP蛋白[21],CpxP是Cpx系统的负反馈调节剂[22]。
2 Cpx系统的信号感应
与许多双组分系统一样,Cpx特定包膜应激信号的分子性质仍然是一个悬而未决的问题。当前Cpx信号转导中最有趣的话题之一是多重信号可以影响该通路,并在信号级联的不同检测点被感知。越来越多的研究表明,在Cpx系统中,至少有四种蛋白参与信号感知:外膜脂蛋白NlpE、周质蛋白CpxP、内膜HK CpxA和细胞质RR CpxR (图1C)。图1
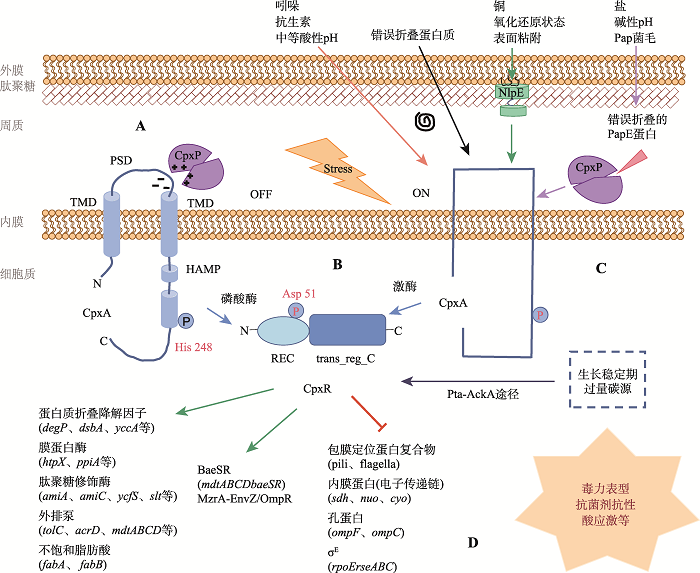 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1Cpx系统信号转导过程及其下游所调控的生命活动
A:CpxA及CpxP的结构及相互作用;B:CpxR的结构;C:Cpx系统感应压力信号;D:CpxR调控网络
Fig. 1The signal transduction of the Cpx system and its regulated downstream life events
2.1 Cpx系统的信号激活机制
1995年Silhavy等[8]首次发现,脂蛋白NlpE的过量表达是影响大肠杆菌(Escherichia coli)野生型中完整Cpx信号级联的激活信号。至今已经识别出许多激活Cpx响应的信号,包括外环境变化如碱性pH[9]、中等酸性pH[23]、高渗透压[24]、疏水表面的附着[25],外源毒性物质如哺乳动物肽聚糖识别蛋白[13]、乙醇[26]、正丁醇[27]、吲哚[28,29]、铜[11]、氨基糖苷和β-内酰胺类抗生素[12,30],内源信号如错误折叠的菌毛蛋白过表达[31,32]、膜磷脂比率改变[33]、外排泵组件的缺失[34]、生长及过量碳源[35]、低cAMP水平[36]等。尽管这些刺激信号的数量庞大且性质广泛,很难对其分子性质下结论,但人们一直认为错误折叠的蛋白质是包膜应激信号的重要部分,因为已证实大肠杆菌中一些受Cpx调控最强的基因编码了周质伴侣和蛋白水解因子[37],并且许多得到深入研究的激活条件都涉及在周质中直接或间接产生错误折叠或聚集的蛋白质。最近一些研究结果提示,Cpx信号通路可能会感应内膜上出现错误折叠蛋白时产生的内源性有毒分子,可能与先前确定的某些诱导Cpx途径的刺激信号有关[34,38],但该假说目前还有待证实。
2.2 Cpx系统的信号传递机制
尽管上述刺激大多数会导致蛋白质错误折叠,但Cpx系统对特定信号的感知具有不同的机制。目前已经确定的CpxA辅助调节因子CpxP和NlpE都通过与CpxA的感知域(直接或间接)之间的交互来起作用,因此CpxA显然是包膜应激信号的枢纽。但是近年来,细胞质信号能够独立于CpxA来改变Cpx信号转导的证据也相继出现。2.2.1 NlpE激活Cpx系统
当大肠杆菌细胞粘附到疏水表面时,外膜脂蛋白NlpE能够作为辅助蛋白向CpxA传递信号,激活Cpx系统[25]。X射线晶体学显示NlpE蛋白是一个双桶状结构,N端锚定在OM中,并且膜结合对NlpE的信号传递功能至关重要。基于此有人提出OM脂蛋白NlpE与IM上的CpxA相互作用的两种可能性[39]:一种是,在表面粘附过程中,N端结构域的构象变化可以使C末端结构域穿过周质与IM接触;另一种是,当周质蛋白折叠机制超负荷时,NlpE无法正确折叠,从而阻碍了负责胞内脂蛋白转运的Lol (localization of lipoprotein)运输系统的识别,导致NlpE到达IM处,激活Cpx反应。最新研究数据显示[40],当NlpE在IM上错误定位时,NlpE的C端结构域对于Cpx的激活是必不可少的,但只有NlpE的N末端域能与CpxA交互作用。此外,OM锚定的N末端结构域似乎不可能跨越周质与CpxA形成复合物。现阶段不能排除NlpE和CpxA复合物中存在其他成分,需要进一步研究阐明NlpE的N端域如何与CpxA交互作用。除了表面粘附之外,NlpE也许可以检测到多种包膜成分,包括脂质、脂多糖或肽聚糖等[39]。长链脂肪酸代谢影响大肠杆菌中二硫键的形成,并且此过程中产生的包膜信号(氧化还原状态)能够激活Cpx系统[41],这可能是通过影响能够感知氧化还原状态的NlpE中二硫键的形成来诱导的[40]。此外,Cpx系统能够通过NlpE感应铜离子水平[11],并且NlpE N末端的CXXC基序可能能够螯合铜离子[39]。
2.2.2 CpxP抑制Cpx系统
CpxP还有一个功能就是参与信号传递。在包膜压力条件下,CpxP被大量诱导,并且其作为CpxA负调节剂的活性消失[42]。目前的研究表明,诸如碱性pH和高盐浓度等条件会导致Cpx系统活化,且有部分依赖于CpxP[24,43]。碱性pH可能引起CpxP构象发生轻微改变,使其无法精确地契合在CpxA的感受域内。高盐浓度则很可能是通过干扰CpxP正电内表面和CpxA负电感受域之间的极性作用来实现的。
德国柏林洪堡大学Sabine Hunke课题组提出,CpxP凹面正电荷表面可能与CpxA负电荷感受域相互作用,而错误折叠的Pap-菌毛亚基暴露的疏水区可能通过与CpxP凸面的疏水区相互作用而使其远离CpxA,CpxP离开CpxA后与错误折叠蛋白结合形成复合体,成为DegP蛋白酶的底物[24]。但在对副溶血性弧菌(Haemophilus parahaemolyticus)的研究中未能鉴定出CpxA感应域与CpxP蛋白之间的相互作用[44],因此,两者相互作用机制还需要进一步研究。CpxP可能与周质伴侣蛋白Spy[45]具有类似分子伴侣活性,但是还不清楚哪些配体可能与CpxP二聚体的相反电荷面结合。也可能存在另外的配体可以结合到CpxA和CpxP表面上形成三元信号复合物,或者CpxP能感知其他尚未被识别的信号。此外,CpxP晶体结构中有锌的存在,也表明CpxP可能与金属结合[43]。因此CpxP在金属感应和蛋白质折叠中的作用是未来研究的重要方向。
2.2.3 CpxA的信号感应机制
组氨酸激酶CpxA是一个重要的信号枢纽。NlpE和CpxP都需要CpxA的周质感受域来发挥诱导和抑制作用[16,22]。CpxA可能能够直接感应到一些错误折叠的包膜蛋白,但其性质尚未识别。大肠杆菌折叠缺陷麦芽糖结合蛋白MalE219突变在体外测定中能增加CpxA至CpxR的磷酸转移[46],这支持了CpxA直接感知错误折叠蛋白的假说。CpxA周质感受域可能感知到的信号还包括磷脂酰乙醇胺的消耗[33]、吲哚[28,29]、乙醇[26]、正丁醇[27]等影响细胞膜物理性质的相关因素[47]。磷脂酰乙醇胺的消耗通过直接影响或改变细胞包膜成分间接激活CpxA[48]。但在大多数情况下,CpxA依赖的信号感应可能涉及其他目前未知的辅助蛋白。最新的研究表明,在肠出血性大肠杆菌(Enterohemorrhagic Escherichia coli)中CpxA是神经递质5-羟色胺的受体,5-羟色胺能够诱导CpxA去磷酸化,使Cpx系统失活[49]。目前,副溶血性弧菌CpxA周质感受域(VpCpxA-peri)的晶体结构已解析[44],并研究了其Per-ARNT-SIM(PAS)域与CpxP的相互作用。加拿大Tracy L. Raivio课题组也解析了大肠杆菌CpxA感受域的结构,遗传分析表明CpxA感受域的突变几乎都能导致该通路的激活,这说明CpxA活性主要通过抑制性信号调控,并且认为重要的抑制信号传递在内膜附近发生[50]。但目前除了CpxP以外,CpxA的抑制剂性质仍然不确定。
2.2.4 CpxR的信号感应机制
众所周知,包膜应激信号是通过细菌双组分系统典型的磷酸转移途径传递的。在压力下,组氨酸激酶CpxA磷酸化其自身保守的His残基以响应环境中的信号。随后,HK的磷酸化基团被转移到RR反应调节子CpxR特定的Asp残基上。激活的RR通常通过调节基因表达来影响细胞生理的变化。目前认为CpxR的磷酸化水平主要取决于CpxA 的磷酸激酶和磷酸酶的活性比值[21],这对于响应外部刺激的特定遗传反应和其持续时间都至关重要(图1D)。
3.1 Cpx系统的转录调控网络
3.1.1 Cpx调控基因的功能分类最初鉴定出的Cpx调控靶基因与包膜蛋白折叠和降解(例如DegP、DsbA和PpiA)相关[19]。后续研究逐渐证实Cpx包膜应激反应具有更广泛的功能,可以调控多种细胞功能分子的表达,例如蛋白质易位基因(secA)、毒力基因(mviM)、氨基酸生物合成基因(aroG和aroK)、磷脂培养基因(psd)、DNA代谢基因(ung)、铁储存和积累基因(efeU和ftnB)等[52,53]。为了区分其靶基因功能,Price等[37]首先分析了大肠杆菌中Cpx系统的转录调控特性,表明受调控最强的为编码包膜蛋白折叠相关蛋白的基因,中度调控的基因功能涉及生物膜形成。随后更多的研究者在大肠杆菌[14]、杜克嗜血杆菌(Haemophilus ducreyi)[54]和霍乱弧菌(Vibrio cholerae)[55]中分别使用转录组学等方法对Cpx调控子进行了详细研究。目前鉴定出的Cpx调节子成员主要分为几个功能类别,包括包膜蛋白复合物、内膜相关蛋白、肽聚糖相关蛋白和其他细胞调节剂[14,56]。许多研究已将Cpx系统与各种复杂的细胞过程相连,如生物膜形成[57]和细菌致病机制[49,58]。
3.1.2 Cpx系统与其他包膜应激系统的联系
作为包膜应激反应的一员,Cpx系统与其他应激调节途径之间也存在联系。目前的研究显示,Cpx应答与其他至少三个包膜应激反应直接相关。包括抑制编码σE的rpoErseABC操纵子的转录[37,53];上调外排泵相关基因mdtABCDbaeSR和acrD以及BaeSR应激反应系统调节子的表达[59];通过正向调控内膜定位的蛋白MzrA来提高其同源信号通路EnvZ/OmpR高渗透压响应系统的活性[60]。Cpx通路不仅通过调节其他调控通路的信号蛋白来建立连接,其活性也受到其他应激反应的影响。这些调控途径之间相互联系从而形成一个广泛网络。
3.2 Cpx系统激活后的压力调控
Cpx系统在调节整体内膜相关过程中的作用以及与其他细胞调节网络的联系使其能够影响多种复杂的细菌行为。Cpx反应对病原菌致病能力的影响仍是当前的研究热点,除了对毒力的调控外,Cpx系统也能够帮助细菌应对感染过程中会接触到的许多有毒物质或一些不利环境,提高病原菌的抗胁迫能力。对Cpx依赖的压力适应调控机制进行研究,将帮助人们更好地了解包膜应激系统的调节子功能,发现更完整的调控网络。3.2.1 Cpx系统对抗生素耐药的调控
细菌接触到临床上使用的抗生素后会发生应激反应,往往可能诱导耐药性的产生。包膜应激系统与抵抗抗生素的能力有关。Cpx反应对氨基糖苷类药物耐药性的影响早在几十年前就被研究者观察到[61,62],随后与β-内酰胺类药物耐药性的关联也得到证实[12,63],但目前的大部分研究并不支持Cpx在所有抗生素耐药性中的广泛作用。随着研究的深入,在多种细菌中都观察到Cpx反应与多种抗生素的耐药性相关(表1)。
Table 1
Table 1Antibiotic resistance mediated by the Cpx system in different bacterial species
| 细菌种类 | 抗生素耐药性 | 啊啊啊啊 |
|---|---|---|
| 大肠杆菌(Escherichia coli) | 氨基糖苷类、β-内酰胺类、杀菌肽聚糖、羟基脲、阳离子抗菌肽、细胞透膜肽(Cell penetrating peptide, CPP) | [13,14,64~67] |
| 肠出血性大肠杆菌O157:H7 (Enterohaemorrhagic Escherichia coli O157:H7) | 磷霉素 | [68] |
| 肺炎克雷伯菌(Klebsiella pneumoniae) | β-内酰胺类、氯霉素、利福平、四环素、链霉素、红霉素 | [63,69] |
| 鼠伤寒沙门氏菌(Salmonella typhimurium) | 氨基糖苷类、β-内酰胺类 | [70,71] |
| 产气克雷伯氏菌(Klebsiella aerogenes) | β-内酰胺类 | [12,72~74] |
| 产气肠杆菌(Enterobacter aerogenes) | ||
| 铜绿假单胞菌(Pseudomonas aeruginosa) | ||
| 霍乱弧菌(Vibrio cholerae) | ||
| 副猪嗜血杆菌(Haemophilus parasuis) | 大环内酯类 | [75] |
新窗口打开|下载CSV
外膜通透性的改变可能会影响某些药物到达其靶点的能力。Cpx位点的突变会改变外膜结构,并且调控OmpF和OmpC孔蛋白的产生[14,77]。孔蛋白的下调可减少渗透,降低对多种抗生素的敏感性。Cpx激活后还可以通过增加细胞壁酰胺酶AmiA和AmiC的表达来恢复外膜完整性,使细菌产生耐药性[78]。最近在产气克雷伯菌的研究中发现,CpxA Y144N突变激活Cpx反应,导致β-内酰胺酶基因ampC过表达,从而引起对β-内酰胺类药物的抗性。这可能与Cpx调节细胞壁修饰酶Slt的表达来调节适应性反应有关[12]。但Cpx反应如何改变外膜完整性以增加对有毒化合物的抗性机制尚不清楚。
一种观点认为,所有杀菌抗生素和其他有毒分子均通过涉及呼吸活动爆发的机制发挥其毒性作用,这种机制最终导致有害的活性氧(reactive oxygen species, ROS)产生,从而杀死细胞[79,80]。并且认为在抗生素存在的情况下,Cpx反应是诱导细胞死亡的原因[81]。随后有多个小组的研究反对了这一模型,其结果证明了Cpx系统的激活不会导致对抗生素的应答而使得细胞死亡,反而会导致耐药性增加[14,64]。随后有研究发现敲除大肠杆菌Cpx系统后,在不同抗生素的处理下一些过氧化物酶相关基因的表达量上调,羟基自由基生成减少,细菌存活率提高,提示Cpx系统介导的细菌抗生素耐药与ROS相关,但该研究中并未明确耐药与Cpx系统活性的关系[82]。转录组数据发现,在大肠杆菌中Cpx反应的激活导致编码琥珀酸和NADH脱氢酶(sdh、nuo、ndh)以及编码电子传递链的细胞色素bo3氧化酶(cyo)基因转录水平降低。这些变化可能导致呼吸减少,也可以解释当Cpx反应被激活时观察到的一些抗生素耐药性,因为这些基因的突变都赋予了对氨基糖苷和羟基脲的耐药性[14]。最近的研究表明,在鼠伤寒沙门氏菌中,ROS的降低只能解释某一些Cpx系统的激活型突变体(如JScpxA92-104)对氨基糖苷类的耐药性,在其他一些激活条件下无法观察到相关基因的转录改变[70]。因此,在不同的Cpx激活条件下,是否存在一个与耐药性有关的共同因子,还有待探究。
Cpx系统除了影响呼吸缺陷和外排泵表达外,对内膜蛋白水解的调节也可能引起氨基糖苷类耐药。Cpx系统激活可能通过增加膜蛋白酶以及蛋白质折叠因子基因(yccA、htpX、ppiA、spy等)的表达来降解翻译错误的膜蛋白,减少对细胞质膜的损伤,从而产生氨基糖苷耐药性[70]。
3.2.2 Cpx系统对酸应激的调控
一些肠道细菌在消化道定植过程中必须对抗酸性环境。大肠杆菌已经进化出不同的酸胁迫反应系统,包括对极端酸胁迫的耐酸反应(acid resistance, AR1~5)系统和对轻中度酸胁迫的耐酸反应(acid tolerance response, ATR)系统。早在1998年就有研究提出,Cpx系统能够被碱性pH诱导[21],但酸性环境与Cpx的关系还不清晰。直到近几年的蛋白质组学研究才提出,极端酸应激可能是Cpx系统新的负调控靶标[56]。该研究发现在Cpx激活后,8种与酸胁迫反应相关的蛋白丰度降低(CydA、CydB、CadA、GadA、GadC、HdeA、HdeB和HdeD)。证明了Cpx对AR2系统的抑制,导致在极端酸胁迫(pH 2.0)下生存能力下降。与之不同的是,最近发表的研究证明了在指数生长期,中等酸性的pH值也是CpxA的激活信号,并提出Cpx是一种新的ATR系统[23]。pH值的降低使CpxA磷酸化,通过激活CpxR提高不饱和脂肪酸UFAs合成基因的转录,使大肠杆菌在pH 4.2下正常生长。这是因为改变细菌膜脂肪酸组成有助于降低膜质子通透性和改善内部pH稳态,提高细菌对酸性环境的适应能力。这与蛋白质组学结果并不矛盾,因为AR2系统负责pH 3.0以下的保护,Cpx介导的对pH 3.0以上指数期AR2的抑制可以保证该系统不会被不适当地诱导,以避免代谢负担。此外,Cpx增加细胞壁的稳定性可能有助于保护大肠杆菌细胞免受酸胁迫[56,78,83]。CpxA也被证明可以与非同源反应调控因子OmpR进行串扰[84],而OmpR本身也参与了大肠杆菌的酸胁迫反应。根据以上数据提出大肠杆菌Cpx系统参与酸应激调控的模型:在指数生长的大肠杆菌中,Cpx系统被中等酸性pH激活,上调UFAs生物合成基因fabA和fabB,以及细胞壁修饰基因ycfS、ycbB、dac、slt、amiA和amiC的转录,并抑制AR2系统基因。
4 结语与展望
目前清楚的是,Cpx包膜应激系统可以通过CpxA依赖与非依赖形式,从多个检测点感应到多种类型的信号。磷酸化后的CpxR调控多种功能的靶基因表达,涉及编码包膜蛋白复合物、内膜蛋白、肽聚糖代谢酶的基因和一些转录因子等,同时与其他包膜应激系统相互联系,形成复杂、广泛的调控网络。最重要的是,Cpx反应调节了许多病原体的毒力、抗生素抗性和酸应激等表型。尽管我们对Cpx信号整合过程和调控基因功能的了解越来越多,但仍有许多问题有待解决:CpxA感应到的信号分子性质是什么?CpxA与已知或未知的辅助蛋白之间的作用机制是什么?是否存在其他直接激活CpxR的信号通路?Cpx调控子中还未知的蛋白功能是什么,参与调节了何种表型?是否存在一类受Cpx影响的共同因子,与所有抗生素抗性或酸应激有关?目前先进的技术有望帮助我们解决这些问题,进一步加深对Cpx反应调节细菌重要生命活动(包括致病、抵抗有毒物质和不利环境等)的理解。这可能帮助促进一些疫苗的开发[85]以及解决抗生素耐药问题,如作为抗菌治疗的药物靶标;该系统在生物生产中也具有潜在的应用前景,如利用酸耐受的重组大肠杆菌进行有机酸的生产。总而言之,阐明Cpx系统信号整合和调控机制具有很大意义。(责任编委: 谢建平)
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
DOI:10.1016/j.mib.2005.02.013URL [本文引用: 1]
DOI:10.1111/j.1574-6968.2011.02436.xURL [本文引用: 1]
DOI:10.1073/pnas.77.1.513URL [本文引用: 1]
DOI:10.1073/pnas.83.20.7850URL [本文引用: 1]
PMID:8294007 [本文引用: 1]

The cpxA gene of Escherichia coli K-12 encodes a membrane-associated sensor element of a two-component signal transduction system in bacteria. The cognate regulator element, however, has not yet been definitively identified. A 2.1-kb segment upstream from cpxA was amplified by polymerase chain reaction, cloned and sequenced. An open reading frame encoding 232 amino acids was found. It showed high homology to the regulator elements of two-component transduction systems. The newly identified gene, designated as cpxR, may encode the cognate protein receiving signals from CpxA.
PMID:8748033 [本文引用: 1]

The processing-defective outer membrane porin protein LamBA23D (Carlson and Silhavy, 1993) and a tripartite fusion protein, LamB-LacZ-PhoA (Snyder and Silhavy, 1995), are both secreted across the cytoplasmic membrane of Escherichia coli, where they exert an extracytoplasmic toxicity. Suppressors of these toxicities map to a previously characterized gene, cpxA, that encodes the sensor kinase protein of a two-component regulatory system. These activated cpxA alleles, designated as cpxA*, stimulate transcription of the periplasmic protease DegP (Danese et al., 1995), which in turn catalyses degradation of the tripartite fusion protein. In contrast, degradation of precursor LamBA23D is not significantly stimulated in a cpxA* suppressor background. In fact, increased levels of DegP in a wild-type background stabilized this protein. While a functional degP gene is required for full cpxA*-mediated suppression of both toxic envelope proteins, residual suppression is seen in cpxA* degP::Tn10 double mutants. Furthermore, cpxA* mutations suppress the toxicity conferred by the LamB-LacZ hybrid protein, which exerts its effects in the cytoplasm, sequestered from DegP. Together, these observations suggest that the activated Cpx pathway regulates additional downstream targets that contribute to suppression. A subset of these targets may constitute a regulon involved in relieving extracytoplasmic and/or secretion-related stress.
PMID:7635808 [本文引用: 2]

The LamB-LacZ-PhoA tripartite fusion protein is secreted to the periplasm, where it exerts a toxicity of unknown origin during high-level synthesis in the presence of the inducer maltose, a phenotype referred to as maltose sensitivity. We selected multicopy suppressors of this toxicity that allow growth of the tripartite fusion strains in the presence of maltose. Mapping and subclone analysis of the suppressor locus identified a previously uncharacterized chromosomal region at 4.7 min that is responsible for suppression. DNA sequence analysis revealed a new gene with the potential to code for a protein of 236 amino acids with a predicted molecular mass of 25,829 Da. The gene product contains an amino-terminal signal sequence to direct the protein for secretion and a consensus lipoprotein modification sequence. As predicted from the sequence, the suppressor protein is labeled with [3H]palmitate and is localized to the outer membrane. Accordingly, the gene has been named nlpE (for new lipoprotein E). Increased expression of NlpE suppresses the maltose sensitivity of tripartite fusion strains and also the extracytoplasmic toxicities conferred by a mutant outer membrane protein, LamBA23D. Suppression occurs by activation of the Cpx two-component signal transduction pathway. This pathway controls the expression of the periplasmic protease DegP and other factors that can combat certain types of extracytoplasmic stress.
PMID:7665485 [本文引用: 2]

In Shigella species, IpaBCD proteins encoded on the virulence plasmid direct the entry of this bacterium into host epithelial cells. Expression of the ipaBCD genes is under the control of several environmental conditions, such as temperature and osmolarity. Extracellular pH also controlled the the expression of the genes, and this regulation occurred mainly at the step of expression of virF, a plasmid-encoded positive regulator of ipaBCD. The expression of virF was activated at high pH (pH 7.4) and repressed at low pH (pH 6.0). We isolated a Tn10 transposon mutant in Escherichia coli K-12 which altered this regulation at the transcriptional level. The Tn10 in the mutant inserted within a reading frame of the cpxA gene, whose product belongs to a family of sensor proteins of two-component signal transduction systems. Complementation analysis showed that cpxA was involved in the pH-dependent regulation of virF gene expression. A gene homologous to cpxA was conserved in Shigella spp. as well as in E. coli. These results may indicate that CpxA senses directly or indirectly a change in extracellular pH and influences the expression of virF in E. coli and Shigella spp.
DOI:10.1128/JB.187.3.1124-1134.2005URL [本文引用: 1]
DOI:10.1271/bbb.60024URL [本文引用: 3]
[本文引用: 4]
DOI:10.1111/mmi.13733PMID:28621879 [本文引用: 2]

Mammalian Peptidoglycan Recognition Proteins (PGRPs) kill both Gram-positive and Gram-negative bacteria through simultaneous induction of oxidative, thiol and metal stress responses in bacteria. However, metabolic pathways through which PGRPs induce these bactericidal stress responses are unknown. We screened Keio collection of Escherichia coli deletion mutants and revealed that deleting genes for respiratory chain flavoproteins or for tricarboxylic acid (TCA) cycle resulted in increased resistance of E. coli to PGRP killing. PGRP-induced killing depended on the production of hydrogen peroxide, which required increased supply of NADH for respiratory chain oxidoreductases from central carbon catabolism (glycolysis and TCA cycle), and was controlled by cAMP-Crp. Bactericidal PGRP induced a rapid decrease in respiration, which suggested that the main source of increased production of hydrogen peroxide was a block in respiratory chain and diversion of electrons from NADH oxidoreductases to oxygen. CpxRA two-component system was a negative regulator of PGRP-induced oxidative stress. By contrast, PGRP-induced thiol stress (depletion of thiols) and metal stress (increase in intracellular free Zn through influx of extracellular Zn ) were mostly independent of oxidative stress. Thus, manipulating pathways that induce oxidative, thiol and metal stress in bacteria could be a useful strategy to design new approaches to antibacterial therapy.© 2017 John Wiley & Sons Ltd.
DOI:10.1128/JB.00105-13PMID:23564175 [本文引用: 6]

The Cpx envelope stress response mediates adaptation to stresses that cause envelope protein misfolding. Adaptation is partly conferred through increased expression of protein folding and degradation factors. The Cpx response also plays a conserved role in the regulation of virulence determinant expression and impacts antibiotic resistance. We sought to identify adaptive mechanisms that may be involved in these important functions by characterizing changes in the transcriptome of two different Escherichia coli strains when the Cpx response is induced. We show that, while there is considerable strain- and condition-specific variability in the Cpx response, the regulon is enriched for proteins and functions that are inner membrane associated under all conditions. Genes that were changed by Cpx pathway induction under all conditions were involved in a number of cellular functions and included several intergenic regions, suggesting that posttranscriptional regulation is important during Cpx-mediated adaptation. Some Cpx-regulated genes are centrally involved in energetics and play a role in antibiotic resistance. We show that a number of small, uncharacterized envelope proteins are Cpx regulated and at least two of these affect phenotypes associated with membrane integrity. Altogether, our work suggests new mechanisms of Cpx-mediated envelope stress adaptation and antibiotic resistance.
DOI:10.1111/j.1365-2958.2009.06982.xURL [本文引用: 1]
PMID:9401031 [本文引用: 3]

Disruption of normal protein trafficking in the Escherichia coli cell envelope (inner membrane, periplasm, outer membrane) can activate two parallel, but distinct, signal transduction pathways. This activation stimulates the expression of a number of genes whose products function to fold or degrade the mislocalized proteins. One of these signal transduction pathways is a two-component regulatory system comprised of the histidine kinase CpxA and the response regulator, CpxR. In this study we characterized gain-of-function Cpx* mutants in order to learn more about Cpx signal transduction. Sequencing demonstrated that the cpx* mutations cluster in either the periplasmic, the transmembrane, or the H-box domain of CpxA. Intriguingly, most of the periplasmic cpx* gain-of-function mutations cluster in the central region of this domain, and one encodes a deletion of 32 amino acids. Strains harboring these mutations are rendered insensitive to a normally activating signal. In vivo and in vitro characterization of maltose-binding-protein fusions between the wild-type CpxA and a representative cpx* mutant, CpxA101, showed that the mutant CpxA is altered in phosphotransfer reactions with CpxR. Specifically, while both CpxA and CpxA101 function as autokinases and CpxR kinases, CpxA101 is devoid of a CpxR-P phosphatase activity normally present in the wild-type protein. Taken together, the data support a model for Cpx-mediated signal transduction in which the kinase/phosphatase ratio is elevated by stress. Further, the sequence and phenotypes of periplasmic cpx* mutations suggest that interactions with a periplasmic signaling molecule may normally dictate a decreased kinase/phosphatase ratio under nonstress conditions.
DOI:10.1111/j.1365-2958.2005.04532.xURL [本文引用: 1]
DOI:10.1007/978-0-387-78885-2_6PMID:18792683 [本文引用: 1]
DOI:10.1101/gad.11.9.1169URL [本文引用: 2]
PMID:9130712 [本文引用: 1]

It is now well established that the sigmaE regulon of Escherichia coli is induced by misfolding of proteins in the periplasm and the outer membrane. htrA belongs to this regulon and encodes a periplasmic protease involved in the degradation of misfolded proteins. htrA transcription is also under the positive control of a two component signal transduction system CpxR CpxA. Closer examination of the putative signal transduction pathway modulating htrA transcription has led us to the identification of two new genes. Biochemical and genetic evidence shows that these two genes encode two phosphoprotein phosphatases, designated PrpA and PrpB. These are the first examples of typical serine/threonine and tyrosine phosphatases described in E. coli. PrpA and PrpB are involved in signaling protein misfolding via the CpxR CpxA transducing system. In addition, both PrpA and PrpB modulate the phosphorylated status of some other phosphoproteins in E. coli. Finally, we show that PrpA is a heat shock protein.
PMID:9473036 [本文引用: 3]

The CpxA/R two-component signal transduction system of Escherichia coli can combat a variety of extracytoplasmic protein-mediated toxicities. The Cpx system performs this function, in part, by increasing the synthesis of the periplasmic protease, DegP. However, other factors are also employed by the Cpx system for this stress-combative function. In an effort to identify these remaining factors, we screened a collection of random lacZ operon fusions for those fusions whose transcription is regulated by CpxA/R. Through this approach, we have identified a new locus, cpxP, whose transcription is stimulated by activation of the Cpx pathway. cpxP specifies a periplasmic protein that can combat the lethal phenotype associated with the synthesis of a toxic envelope protein. In addition, we show that cpxP transcription is strongly induced by alkaline pH in a CpxA-dependent manner and that cpxP and cpx mutant strains display hypersensitivity to growth in alkaline conditions.
PMID:10464196 [本文引用: 2]

In Escherichia coli, the Cpx two-component regulatory system activates expression of protein folding and degrading factors in response to misfolded proteins in the bacterial envelope (inner membrane, periplasm, and outer membrane). It is comprised of the histidine kinase CpxA and the response regulator CpxR. This response plays a role in protection from stresses, such as elevated pH, as well as in the biogenesis of virulence factors. Here, we show that the Cpx periplasmic stress response is subject to amplification and repression through positive and negative autofeedback mechanisms. Western blot and operon fusion analyses demonstrated that the cpxRA operon is autoactivated. Conditions that lead to elevated levels of phosphorylated CpxR cause a concomitant increase in transcription of cpxRA. Conversely, overproduction of CpxP, a small, Cpx-regulated protein of previously unknown function, represses the regulon and can block activation of the pathway. This repression is dependent on an intact CpxA sensing domain. The ability to autoactivate and then subsequently repress allows for a temporary amplification of the Cpx response that may be important in rescuing cells from transitory stresses and cueing the appropriately timed elaboration of virulence factors.
DOI:10.1038/s41467-020-15350-5URL [本文引用: 2]
DOI:10.1074/jbc.M110.194092URL [本文引用: 3]
DOI:10.1073/pnas.042521699URL [本文引用: 2]
DOI:10.1002/bit.22966URL [本文引用: 2]
DOI:10.1128/AEM.02323-09URL [本文引用: 2]
[本文引用: 2]
[本文引用: 2]
DOI:10.1038/nm.2357PMID:21602801 [本文引用: 1]

Mammalian peptidoglycan recognition proteins (PGRPs), similar to antimicrobial lectins, bind the bacterial cell wall and kill bacteria through an unknown mechanism. We show that PGRPs enter the Gram-positive cell wall at the site of daughter cell separation during cell division. In Bacillus subtilis, PGRPs activate the CssR-CssS two-component system that detects and disposes of misfolded proteins that are usually exported out of bacterial cells. This activation results in membrane depolarization, cessation of intracellular peptidoglycan, protein, RNA and DNA synthesis, and production of hydroxyl radicals, which are responsible for bacterial death. PGRPs also bind the outer membrane of Escherichia coli and activate the functionally homologous CpxA-CpxR two-component system, which kills the bacteria. We exclude other potential bactericidal mechanisms, including inhibition of extracellular peptidoglycan synthesis, hydrolysis of peptidoglycan and membrane permeabilization. Thus, we reveal a previously unknown mechanism by which innate immunity proteins that bind the cell wall or outer membrane exploit the bacterial stress defense response to kill bacteria.
PMID:9351822 [本文引用: 1]

The assembly of interactive protein subunits into extracellular structures, such as pilus fibers in the Enterobacteriaceae, is dependent on the activity of PapD-like periplasmic chaperones. The ability of PapD to undergo a beta zippering interaction with the hydrophobic C-terminus of pilus subunits facilitates their folding and release from the cytoplasmic membrane into the periplasm. In the absence of the chaperone, subunits remained tethered to the membrane and were driven off-pathway via non-productive interactions. These off-pathway reactions were detrimental to cell growth; wild-type growth was restored by co-expression of PapD. Subunit misfolding in the absence of PapD was sensed by two parallel pathways: the Cpx two-component signaling system and the sigma E modulatory pathway.
PMID:15629938 [本文引用: 1]

The Cpx envelope stress response mediates adaptation to potentially lethal envelope stresses in Escherichia coli. The two-component regulatory system consisting of the sensor kinase CpxA and the response regulator CpxR senses and mediates adaptation to envelope insults believed to result in protein misfolding in this compartment. Recently, a role was demonstrated for the Cpx response in the biogenesis of P pili, attachment organelles expressed by uropathogenic E. coli. CpxA senses misfolded P pilus assembly intermediates and initiates increased expression of both assembly and regulatory factors required for P pilus elaboration. In this report, we demonstrate that the Cpx response is also involved in the expression of the type IV bundle-forming pili of enteropathogenic E. coli (EPEC). Bundle-forming pili were not elaborated from an exogenous promoter in E. coli laboratory strain MC4100 unless the Cpx pathway was constitutively activated. Further, an EPEC cpxR mutant synthesized diminished levels of bundle-forming pili and was significantly affected in adherence to epithelial cells. Since type IV bundle-forming pili are very different from chaperone-usher-type P pili in both form and biogenesis, our results suggest that the Cpx envelope stress response plays a general role in the expression of envelope-localized organelles with diverse structures and assembly pathways.
DOI:10.1016/j.bbrc.2012.04.003URL [本文引用: 2]
DOI:10.1128/IAI.01316-10URL [本文引用: 2]
DOI:10.1128/JB.01906-07PMID:18223085 [本文引用: 1]

The CpxAR two-component signal transduction system in Escherichia coli and other pathogens senses diverse envelope stresses and promotes the transcription of a variety of genes that remedy these stresses. An important member of the CpxAR regulon is cpxP. The CpxA-dependent transcription of cpxP has been linked to stresses such as misfolded proteins and alkaline pH. It also has been proposed that acetyl phosphate, the intermediate of the phosphotransacetylase (Pta)-acetate kinase (AckA) pathway, can activate the transcription of cpxP in a CpxA-independent manner by donating its phosphoryl group to CpxR. We tested this hypothesis by measuring the transcription of cpxP using mutants with mutations in the CpxAR pathway, mutants with mutations in the Pta-AckA pathway, and mutants with a combination of both types of mutations. From this epistasis analysis, we learned that CpxR integrates diverse stimuli. The stimuli that originate in the envelope depend on CpxA, while those associated with growth and central metabolism depend on the Pta-AckA pathway. While CpxR could receive a phosphoryl group from acetyl phosphate, this global signal was not the primary trigger for CpxR activation associated with the Pta-AckA pathway. On the strength of these results, we contend that the interactions between central metabolism and signal transduction can be quite complex and that successful investigations of such interactions must include a complete epistatic analysis.
DOI:10.1128/JB.187.18.6309-6316.2005URL [本文引用: 1]
DOI:10.1128/JB.00798-08URL [本文引用: 3]
DOI:10.1128/JB.01996-12PMID:23264577 [本文引用: 1]

Escherichia coli has nine inner membrane efflux pumps which complex with the outer membrane protein TolC and cognate membrane fusion proteins to form tripartite transperiplasmic pumps with diverse functions, including the expulsion of antibiotics. We recently observed that tolC mutants have elevated activities for three stress response regulators, MarA, SoxS, and Rob, and we suggested that TolC-dependent efflux is required to prevent the accumulation of stressful cellular metabolites. Here, we used spy::lacZ fusions to show that two systems for sensing/repairing extracytoplasmic stress, BaeRS and CpxARP, are activated in the absence of TolC-dependent efflux. In either tolC mutants or bacteria with mutations in the genes for four TolC-dependent efflux pumps, spy expression was increased 6- to 8-fold. spy encodes a periplasmic chaperone regulated by the BaeRS and CpxARP stress response systems. The overexpression of spy in tolC or multiple efflux pump mutants also depended on these systems. spy overexpression was not due to acetate, ethanol, or indole accumulation, since external acetate had only a minor effect on wild-type cells, ethanol had a large effect that was not CpxA dependent, and a tolC tnaA mutant which cannot accumulate internal indole overexpressed spy. We propose that, unless TolC-dependent pumps excrete certain metabolites, the metabolites accumulate and activate at least five different stress response systems.
PMID:17698001 [本文引用: 3]

NlpE, an outer membrane lipoprotein, functions during envelope stress responses in Gram-negative bacteria. In Escherichia coli, adhesion to abiotic surfaces has been reported to activate the Cpx pathway in an NlpE-dependent manner. External copper ions are also thought to activate the Cpx pathway mediated by NlpE. We determined the crystal structure of NlpE from E. coli at 2.6 A resolution. The structure showed that NlpE consists of two beta barrel domains. The N-terminal domain resembles the bacterial lipocalin Blc, and the C-terminal domain has an oligonucleotide/oligosaccharide-binding (OB) fold. From both biochemical analyses and the crystal structure, it can be deduced that the cysteine residues in the CXXC motif may be chemically active. Furthermore, two monomers in the asymmetric unit form an unusual 3D domain-swapped dimer. These findings indicate that tertiary and/or quaternary structural instability may be responsible for Cpx pathway activation.
[本文引用: 2]
DOI:10.1371/journal.pgen.1009081URL [本文引用: 1]
DOI:10.1371/journal.pone.0107383URL [本文引用: 1]
DOI:10.1128/JB.01296-10PMID:21317318 [本文引用: 2]

CpxP is a novel bacterial periplasmic protein with no homologues of known function. In gram-negative enteric bacteria, CpxP is thought to interact with the two-component sensor kinase, CpxA, to inhibit induction of the Cpx envelope stress response in the absence of protein misfolding. CpxP has also been shown to facilitate DegP-mediated proteolysis of misfolded proteins. Six mutations that negate the ability of CpxP to function as a signaling protein are localized in or near two conserved LTXXQ motifs that define a class of proteins with similarity to CpxP, Pfam PF07813. To gain insight into how these mutations might affect CpxP signaling and/or proteolytic adaptor functions, the crystal structure of CpxP from Escherichia coli was determined to 2.85-Å resolution. The structure revealed an antiparallel dimer of intertwined α-helices with a highly basic concave surface. Each protomer consists of a long, hooked and bent hairpin fold, with the conserved LTXXQ motifs forming two diverging turns at one end. Biochemical studies demonstrated that CpxP maintains a dimeric state but may undergo a slight structural adjustment in response to the inducing cue, alkaline pH. Three of the six previously characterized cpxP loss-of-function mutations, M59T, Q55P, and Q128H, likely result from a destabilization of the protein fold, whereas the R60Q, D61E, and D61V mutations may alter intermolecular interactions.
DOI:10.1002/pro.2120URL [本文引用: 2]
DOI:10.1038/nsmb.2016PMID:21317898 [本文引用: 1]

To optimize the in vivo folding of proteins, we linked protein stability to antibiotic resistance, thereby forcing bacteria to effectively fold and stabilize proteins. When we challenged Escherichia coli to stabilize a very unstable periplasmic protein, it massively overproduced a periplasmic protein called Spy, which increases the steady-state levels of a set of unstable protein mutants up to 700-fold. In vitro studies demonstrate that the Spy protein is an effective ATP-independent chaperone that suppresses protein aggregation and aids protein refolding. Our strategy opens up new routes for chaperone discovery and the custom tailoring of the in vivo folding environment. Spy forms thin, apparently flexible cradle-shaped dimers. The structure of Spy is unlike that of any previously solved chaperone, making it the prototypical member of a new class of small chaperones that facilitate protein refolding in the absence of energy cofactors.
DOI:10.1016/j.resmic.2009.07.002URL [本文引用: 1]
PMID:6360997 [本文引用: 1]

The effects of ethanol on the fluidity of Escherichia coli plasma membranes were examined by using a variety of fluorescent probes: 1,6-diphenyl-1,3,5-hexatriene, perylene, and a set of n-(9-anthroyloxy) fatty acids. The anthroyloxy fatty acid probes were used to examine the fluidity gradient across the width of the plasma membrane and artificial membranes prepared from lipid extracts of plasma membranes. Ethanol caused a small decrease in the polarization of probes primarily located near the membrane surface. In comparison, hexanol decreased the polarization of probes located more deeply in the membrane. Temperature had a large effect on probes located at all depths. The effects of ethanol on E. coli membranes from cells grown with or without ethanol were also examined. Plasma membranes isolated from cells grown in the presence of ethanol were more rigid than those from control cells. In contrast to plasma membranes, artificial membranes prepared from lipid extracts of ethanol-grown cells were more fluid than those from control cells. These differences are explained by analyses of membrane composition. Membranes from cells grown in the presence of ethanol are more rigid than those from control cells due to a decrease in the lipid-to-protein ratio. This change more than compensates for the fluidizing effect of ethanol and the ethanol-induced increase in membrane C18:1 fatty acid which occurs during growth. Our results suggest that the regulation of the lipid-to-protein ratio of the plasma membrane may be an important adaptive response of E. coli to growth in the presence of ethanol.
DOI:10.1093/jb/mvp071URL [本文引用: 1]
DOI:S1931-3128(20)30286-9PMID:32521224 [本文引用: 2]

The gut-brain axis is crucial to microbial-host interactions. The neurotransmitter serotonin is primarily synthesized in the gastrointestinal (GI) tract, where it is secreted into the lumen and subsequently removed by the serotonin transporter, SERT. Here, we show that serotonin decreases virulence gene expression by enterohemorrhagic E. coli (EHEC) and Citrobacter rodentium, a murine model for EHEC. The membrane-bound histidine sensor kinase, CpxA, is a bacterial serotonin receptor. Serotonin induces dephosphorylation of CpxA, which inactivates the transcriptional factor CpxR controlling expression of virulence genes, notably those within the locus of enterocyte effacement (LEE). Increasing intestinal serotonin by genetically or pharmacologically inhibiting SERT decreases LEE expression and reduces C. rodentium loads. Conversely, inhibiting serotonin synthesis increases pathogenesis and decreases host survival. As other enteric bacteria contain CpxA, this signal exploitation may be engaged by other pathogens. Additionally, repurposing serotonin agonists to inhibit CpxA may represent a potential therapeutic intervention for enteric bacteria.Copyright © 2020 Elsevier Inc. All rights reserved.
DOI:10.1016/j.bbamcr.2013.10.018PMID:24184210 [本文引用: 1]

The Cpx envelope stress response (ESR) has been linked to proteins that are integrated into and secreted across the inner membrane for several decades. Initial studies of the cpx locus linked it to alterations in the protein content of both the inner and outer membrane, together with changes in proton motive driven transport and conjugation. Since the mid 1990s, the predominant view of the Cpx envelope stress response has been that it serves to detect and respond to secreted, misfolded proteins in the periplasm. Recent studies in Escherichia coli and other Gram negative organisms highlight a role for the Cpx ESR in specifically responding to perturbations that occur at the inner membrane (IM). It is clear that Cpx adaptation involves a broad suite of changes that encompass many functions in addition to protein folding. Interestingly, recent studies have refocused attention on Cpx-regulated phenotypes that were initially published over 30years ago, including antibiotic resistance and transport across the IM. In this review I will focus on the insights and models that have arisen from recent studies and that may help explain some of the originally published Cpx phenotypes. Although the molecular nature of the inducing signal for the Cpx ESR remains enigmatic, recently solved structures of signaling proteins are yielding testable models concerning the molecular mechanisms behind signaling. The identification of connections between the Cpx ESR and other stress responses in the cell reveals a complex web of interactions that involves Cpx-regulated expression of other regulators as well as small proteins and sRNAs. This article is part of a Special Issue entitled: Protein trafficking and secretion in bacteria. Guest Editors: Anastassios Economou and Ross Dalbey. © 2013.
DOI:10.1111/mmi.2007.65.issue-4URL [本文引用: 1]
[本文引用: 2]
DOI:10.1128/JB.00372-13URL [本文引用: 1]
DOI:10.1128/JB.01957-14URL [本文引用: 1]
DOI:10.1002/mbo3.2016.5.issue-4URL [本文引用: 3]
DOI:10.1128/JB.00938-13URL [本文引用: 1]
[本文引用: 1]
DOI:10.1111/j.1365-2958.2004.04449.xURL [本文引用: 1]
DOI:10.1111/j.1365-2958.2009.06728.xPMID:19432797 [本文引用: 1]

Analysis of suppressors that alleviate the acute envelope stress phenotype of a DeltabamB DeltadegP strain of Escherichia coli identified a novel protein MzrA and pleiotropic envZ mutations. Genetic evidence shows that overexpression of MzrA--formerly known as YqjB and EcfM--modulates the activity of EnvZ/OmpR similarly to pleiotropic EnvZ mutants and alter porin expression. However, porin expression in strains devoid of MzrA or overexpressing it is still sensitive to medium osmolarity, pH and procaine, all of which modulate EnvZ/OmpR activities. Thus, MzrA appears to alter the output of the EnvZ/OmpR system but not its ability to receive and respond to various environmental signals. Localization and topology experiments indicate that MzrA is a type II membrane protein, with its N-terminus exposed in the cytoplasm and C-terminus in the periplasm. Bacterial two-hybrid experiments determined that MzrA specifically interacts with EnvZ but not with OmpR or the related membrane sensor kinase, CpxA. This and additional genetic and biochemical evidence suggest that the interaction of MzrA with EnvZ would either enhance EnvZ's kinase activity or reduce its phosphatase activity, thus elevating the steady state levels of OmpR approximately P. Furthermore, our data show that MzrA links the two-component envelope stress response regulators, CpxA/CpxR and EnvZ/OmpR.
PMID:143238 [本文引用: 1]

Several mutants of Escherichia coli affecting aerobic energy generation and energization of the bacterial membrane have been examined for their effect on streptomycin and gentamicin accumulation and susceptibility. A heme-deficient mutant (K207) and two mutants (CJ-8 [colicin K insensitive] and NR-70) associated with defective aerobic active transport were associated with decreased transport of streptomycin and gentamicin and increased resistance to those antibiotics. These mutants also exhibited increased resistance to several other aminoglycoside antibiotics, but not the aminocyclitol spectinomycin. The same observations were made with a ubiquinone-deficient mutant, but a strA derivative of this mutant was shown additionally to be saturable for streptomycin accumulation at a concentration four or more times lower than that required for saturation of the parent. A mutant uncoupled for adenosine 5'-triphosphate synthesis from electron transport and membrane Mg-adenosine 5'-triphosphatase deficient was hypersensitive to those aminoglycosides tested and spectinomycin, and showed enhanced transport of streptomycin and gentamicin. A variety of compounds structurally related to streptomycin were examined at high concentrations for inhibition of streptomycin uptake in a strA mutant of E. coli K-12 SA 1306, but no evidence for competition was detected, suggesting the absence of a common transport carrier. Four different divalent cations were shown to inhibit streptomycin and gentamicin accumulation in E. coli K-12 SA 1306. Divalent cations were shown to inhibit uptake of these two drugs in two bacterial species with distinct cell wall structures, Pseudomonas aeruginosa and Staphylococcus aureus, and to inhibit streptomycin uptake in spheroplasts of streptomycin-susceptible and -resistant E. coli. However, calcium had almost no inhibitory effect on streptomycin uptake by the ubiquinone-deficient mutant E. coli AN66. These and previous findings have been used to formulate a model for aminoglycoside entry into bacteria using a low-affinity membranous complex involved in membrane energization that includes respiratory quinones, which probably act to bind and transport aminoglycosides across the cell membrane. This phase of transport is associated with the lowest accumulation rate (termed energy-dependent phase I) that is rate limiting for susceptibility. It is further proposed that subsequent association of the membrane-bound aminoglycoside with higher-affinity binding sites on membrane-associated ribosomes carrying out a normal ribosomal cycle and protein synthesis results in a more rapid transport rate (termed energy-dependent phase II). The increased rate could result from a state of membrane energization analogous to that causing enhanced aminoglycoside transport rates seen in the uncoupled mutant, AN120. How this model explains the mechanism by which enzymatically modified aminoglycosides render cells resistant to unmodified aminoglycosides is also discussed.
DOI:10.1007/BF00266619URL [本文引用: 1]
DOI:10.1371/journal.pone.0033777URL [本文引用: 1]
DOI:10.1128/JB.02197-12PMID:23335416 [本文引用: 1]

It has recently been suggested that bactericidal antibiotics, including aminoglycoside antibiotics (AGAs), and toxic small molecules, such as hydroxyurea (HU), kill bacteria the same way, namely, by generating reactive oxygen species (ROS) via a process requiring activation of the Cpx stress response. We suggest an opposite, protective role for Cpx. We have confirmed the initial finding that cpxA null mutations confer resistance to HU. However, the two-component sensor CpxA is both a kinase and a phosphatase, and previous work from our lab has shown that removing CpxA can activate the stress response owing to buildup of the phosphorylated response regulator (CpxR~P) that occurs in the absence of the phosphatase activity. We show that a dominant cpxA* mutation that constitutively activates the Cpx stress response confers a high level of resistance to both HU and AGAs in a CpxR-dependent manner. In contrast, inactivating the CpxR response regulator by mutating the phosphorylation site (D51A) or the putative DNA-binding motif (M199A) does not increase resistance to HU or AGAs. Taken together, these results demonstrate that activation of the Cpx stress response can protect cells from HU and AGAs. However, the Cpx response does not increase resistance to all classes of bactericidal antibiotics, as the cpxA* mutants are not significantly more resistant to fluoroquinolones or β-lactams than wild-type cells. Thus, it seems unlikely that all bactericidal antibiotics kill by the same mechanism.
DOI:10.1074/jbc.M114.565762PMID:25294881

A genome-wide susceptibility assay was used to identify specific CpxR-dependent genes that facilitate Escherichia coli resistance to a model cationic antimicrobial peptide, protamine. A total of 115 strains from the Keio Collection, each of which contained a deletion at a demonstrated or predicted CpxR/CpxA-dependent locus, were tested for protamine susceptibility. One strain that exhibited high susceptibility carried a deletion of tolC, a gene that encodes the outer membrane component of multiple tripartite multidrug transporters. Concomitantly, two of these efflux systems, AcrAB/TolC and EmrAB/TolC, play major roles in protamine resistance. Activation of the CpxR/CpxA system stimulates mar transcription, suggesting a new regulatory circuit that enhances the multidrug resistance cascade. Tripartite multidrug efflux systems contribute to bacterial resistance to protamine differently from the Tat system. DNase I footprinting analysis demonstrated that the CpxR protein binds to a sequence located in the -35 and -10 regions of mar promoter. This sequence resembles the consensus CpxR binding site, however, on the opposite strand. aroK, a CpxR-dependent gene that encodes a shikimate kinase in the tryptophan biosynthesis pathway, was also found to facilitate protamine resistance. Specific aromatic metabolites from this pathway, such as indole, can stimulate expression of well studied CpxR-dependent genes degP and cpxP, which are not components of the tripartite multidrug transporters. Thus, we propose a novel mechanism for E. coli to modulate resistance to protamine and likely other cationic antimicrobial peptides in which the CpxR/CpxA system up-regulates mar transcription in response to specific aromatic metabolites, subsequently stimulating the multidrug resistance cascade. © 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
DOI:10.1128/JB.01151-13PMID:24163343

Although fosfomycin is an old antibiotic, it has resurfaced with particular interest. The antibiotic is still effective against many pathogens that are resistant to other commonly used antibiotics. We have found that fosfomycin resistance of enterohemorrhagic Escherichia coli (EHEC) O157:H7 is controlled by the bacterial two-component signal transduction system CpxAR. A cpxA mutant lacking its phosphatase activity results in constitutive activation of its cognate response regulator, CpxR, and fosfomycin resistance. We have shown that fosfomycin resistance requires CpxR because deletion of the cpxR gene in the cpxA mutant restores fosfomycin sensitivity. We have also shown that CpxR directly represses the expression of two genes, glpT and uhpT, which encode transporters that cotransport fosfomycin with their native substrates glycerol-3-phosphate and glucose-6-phosphate, and repression of these genes leads to a decrease in fosfomycin transport into the cpxA mutant. However, the cpxA mutant had an impaired growth phenotype when cultured with glycerol-3-phosphate or glucose-6-phosphate as a sole carbon substrate and was outcompeted by the parent strain, even in nutrient-rich medium. This suggests a trade-off between fosfomycin resistance and the biological fitness associated with carbon substrate uptake. We propose a role for the CpxAR system in the reversible control of fosfomycin resistance. This may be a beneficial strategy for bacteria to relieve the fitness burden that results from fosfomycin resistance in the absence of fosfomycin.
DOI:10.1128/AAC.02284-12PMID:23836167

Klebsiella pneumoniae has been frequently associated with nosocomial infections. Efflux systems are ubiquitous transporters that also function in drug resistance. Genome analysis of K. pneumoniae strain NTUH-K2044 revealed the presence of ~15 putative drug efflux systems. We discuss here for the first time the characterization of a putative SMR-type efflux pump, an ebrAB homolog (denoted here as kpnEF) with respect to Klebsiella physiology and the multidrug-resistant phenotype. Analysis of hypermucoviscosity revealed direct involvement of kpnEF in capsule synthesis. The ΔkpnEF mutant displayed higher sensitivity to hyperosmotic (~2.8-fold) and high bile (~4.0-fold) concentrations. Mutation in kpnEF resulted in increased susceptibility to cefepime, ceftriaxone, colistin, erythromycin, rifampin, tetracycline, and streptomycin; mutated strains changed from being resistant to being susceptible, and the resistance was restored upon complementation. The ΔkpnEF mutant displayed enhanced sensitivity toward structurally related compounds such as sodium dodecyl sulfate, deoxycholate, and dyes, including clinically relevant disinfectants such as benzalkonium chloride, chlorhexidine, and triclosan. The prevalence of kpnEF in clinical strains broadens the diversity of antibiotic resistance in K. pneumoniae. Experimental evidence of CpxR binding to the efflux pump promoter and quantification of its expression in a cpxAR mutant background demonstrated kpnEF to be a member of the Cpx regulon. This study helps to elucidate the unprecedented biological functions of the SMR-type efflux pump in Klebsiella spp.
DOI:10.3389/fmicb.2021.604079URL [本文引用: 2]
DOI:10.3389/fmicb.2016.00604PMID:27199934

The two-component signal transduction system CpxAR is especially widespread in Gram-negative bacteria. It has been reported that CpxAR contributes to the multidrug resistance (MDR) in Escherichia coli. CpxR is a response regulator in the two-component CpxAR system. The aim of this study was to explore the role of cpxR in the MDR of S. enterica serovar Typhimurium. The minimal inhibitory concentrations (MICs) of various antibiotics commonly used in veterinary medicine for strains JS (a multidrug-susceptible standard strain of S. enterica serovar Typhimurium), JS Delta cpxR, JS Delta cpxR/pcpxR, JS Delta cpxR/pcpxR*, JS Delta cpxR Delta acrB, JS Delta cpxR Delta acrB/pcpxR, JS Delta cpxR Delta acrB/pcpxR*, 9 S. enterica serovar Typhimurium isolates (SH1-9), and SH1-9 Delta cpxR were determined by the 2-fold broth microdilution method. The relative mRNA expression levels of ompF, ompC, ompW, ompD, tolC, acrB, acrD, acrF, mdtA, marA, and soxS in strains JS, JS Delta cpxR, and JS Delta cpxR/pcpxR were detected by real-time PCR. The results showed 2- to 4-fold decreases in the MICs of amikacin (AMK), gentamycin (GEN), apramycin (APR), neomycin (NEO), ceftriaxone (CRO), ceftiofur (CEF), and cefquinome (CEO) for strain JS Delta,cpxR, as compared to those for the parental strain JS. Likewise, SH1-9 Delta cpxR were found to have 2- to 8-fold reduction in resistance to the above antibiotics, except for NEO, as compared to their parental strains SH1-9. Furthermore, 2- to 4-fold further decreases in the MICs of AMK, GEN, APR, and CEF for strain JS Delta cpxR Delta acrB were observed, as compared to those for strain JS Delta acrB. In addition, CpxR overproduction in strain JS Delta cpxR led to significant decreases in the mRNA expression levels of ompF, ompC, ompW, ompD, tolC, acrB, marA, and soxS, and significant increases in those of stm3031 and stm1530. Notably, after all strains were induced simultaneously by GEN to the 15th passage at subinhibitory concentrations, strain JS Delta cpxR/pcpxR showed significant increases in mRNA expression levels of the efflux pump acrD and mdtA genes, as compared to strain JS Delta cpxR. Our results indicate that the two-component regulator CpxR contributes to resistance of S. enterica serovar Typhimurium to aminoglycosides and beta-lactams by influencing the expression level of the MDR-related genes.
DOI:10.1371/journal.pone.0138828URL
DOI:10.1111/mmi.2015.97.issue-5URL
DOI:10.1128/IAI.00025-14PMID:24799626

The Cpx two-component regulatory system has been shown in Escherichia coli to alleviate stress caused by misfolded cell envelope proteins. The Vibrio cholerae Cpx system was previously found to respond to cues distinct from those in the E. coli system, suggesting that this system fulfills a different physiological role in the cholera pathogen. Here, we used microarrays to identify genes that were regulated by the V. cholerae Cpx system. Our observations suggest that the activation of the V. cholerae Cpx system does not induce expression of genes involved in the mitigation of stress generated by misfolded cell envelope proteins but promotes expression of genes involved in antimicrobial resistance. In particular, activation of the Cpx system induced expression of the genes encoding the VexAB and VexGH resistance-nodulation-division (RND) efflux systems and their cognate outer membrane pore protein TolC. The promoters for these loci contained putative CpxR consensus binding sites, and ectopic cpxR expression activated transcription from the promoters for the RND efflux systems. CpxR was not required for intrinsic antimicrobial resistance, but CpxR activation enhanced resistance to antimicrobial substrates of VexAB and VexGH. Mutations that inactivated VexAB or VexGH efflux activity resulted in the activation of the Cpx response, suggesting that vexAB and vexGH and the cpxP-cpxRA system are reciprocally regulated. We speculate that the reciprocal regulation of the V. cholerae RND efflux systems and the Cpx two-component system is mediated by the intracellular accumulation of an endogenously produced metabolic by-product that is normally extruded from the cell by the RND efflux systems. Copyright © 2014, American Society for Microbiology. All Rights Reserved.
DOI:S0944-5013(17)30414-7PMID:29146255

Haemophilus parasuis is an opportunistic pathogen localized in the upper respiratory tracts of pigs, its infection begins from bacterial survival under complex conditions, like hyperosmosis, oxidative stress, phagocytosis, and sometimes antibiotics as well. The two-component signal transduction (TCST) system serves as a common stimulus-response mechanism that allows microbes to sense and respond to diverse environmental conditions via a series of phosphorylation reactions. In this study, we investigated the role of TCST system CpxRA in H. parasuis in response to different environmental stimuli by constructing the ΔcpxA and ΔcpxR single deletion mutants as well as the ΔcpxRA double deletion mutant from H. parasuis serotype 4 isolate JS0135. We demonstrated that H. parasuis TCST system CpxRA confers bacterial tolerance to stresses and bactericidal antibiotics. The CpxR was found to play essential roles in mediating oxidative stress, osmotic stresses and alkaline pH stress tolerance, as well as macrolide resistance (i.e. erythromycin), but the CpxA deletion did not decrease bacterial resistance to abovementioned stresses. Moreover, we found via RT-qPCR approach that HAPS_RS00160 and HAPS_RS09425, both encoding multidrug efflux pumps, were significantly decreased in erythromycin challenged ΔcpxR and ΔcpxRA mutants compared with wild-type strain JS0135. These findings characterize the role of the TCST system CpxRA in H. parasuis conferring stress response tolerance and bactericidal resistance, which will deepen our understanding of the pathogenic mechanism in H. parasuis.Copyright © 2017 Elsevier GmbH. All rights reserved.
DOI:10.1128/AAC.01677-09URL
PMID:16077119 [本文引用: 1]

We performed transposon mutagenesis of a two-color fluorescent reporter strain to identify new regulators of the porin genes ompF and ompC in Escherichia coli. Screening of colonies by fluorescence microscopy revealed numerous mutants that exhibited interesting patterns of porin expression. One mutant harbored an insertion in the gene encoding the histidine kinase CpxA, the sensor for a two-component signaling system that responds to envelope stress. The cpxA mutant exhibited increased transcription of ompC and a very strong decrease in transcription of ompF under conditions in which acetyl phosphate levels were high. Subsequent genetic analysis revealed that this phenotype is dependent on phosphorylation of the response regulator CpxR and that activation of CpxA in wild-type cells results in similar regulation of porin expression. Using DNase I footprinting, we demonstrated that CpxR binds upstream of both the ompF and ompC promoters. It thus appears that two distinct two-component systems, CpxA-CpxR and EnvZ-OmpR, converge at the porin promoters. Within the context of envelope stress, outer membrane beta-barrel proteins have generally been associated with the sigma E pathway. However, at least for the classical porins OmpF and OmpC, our results show that the Cpx envelope stress response system plays a role in regulating their expression.
DOI:10.1074/jbc.M110.200352PMID:21149452 [本文引用: 2]

We demonstrate that the twin arginine translocation (Tat) system contributes to bacterial resistance to cationic antimicrobial peptides (CAMPs). Our results show that a deletion at the tatC gene, which encodes a subunit of the Tat complex, caused Salmonella and Escherichia coli to become susceptible to protamine. We screened chromosomal loci that encode known and predicted Tat-dependent proteins and found that two N-acetylmuramoyl-l-alanine amidases, encoded by amiA and amiC, elevated bacterial resistance to protamine and α-helical peptides magainin 2 and melittin but not to β-sheet defensin HNP-1 and lipopeptide polymyxin B. Genetic analysis suggests that transcription of both amiA and amiC loci in Salmonella is up-regulated by the CpxR/CpxA two-component system when nlpE is overexpressed. A footprinting analysis reveals that CpxR protein can interact with amiA and amiC promoters at the CpxR box, which is localized between the predicted -10 and -35 regions but present on different strands in these two genes. In addition, our results show that activation of the CpxR/CpxA system can facilitate protamine resistance because nlpE overexpression elevates this resistance in the wild-type strain but not the cpxR deletion mutant. Thus, we uncover a new transcriptional regulation pathway in which the Cpx envelope stress response system modulates the integrity of the cell envelope in part by controlling peptidoglycan amidase activity, which confers bacterial resistance to protamine and α-helical CAMPs. Our studies have important implications for understanding transcriptional regulation of peptidoglycan metabolism and also provide new insights into the role of the bacterial envelope in CAMP resistance.
DOI:10.1016/j.molcel.2009.11.024PMID:20005847 [本文引用: 1]

Hydroxyurea (HU) specifically inhibits class I ribonucleotide reductase (RNR), depleting dNTP pools and leading to replication fork arrest. Although HU inhibition of RNR is well recognized, the mechanism by which it leads to cell death remains unknown. To investigate the mechanism of HU-induced cell death, we used a systems-level approach to determine the genomic and physiological responses of E. coli to HU treatment. Our results suggest a model by which HU treatment rapidly induces a set of protective responses to manage genomic instability. Continued HU stress activates iron uptake and toxins MazF and RelE, whose activity causes the synthesis of incompletely translated proteins and stimulation of envelope stress responses. These effects alter the properties of one of the cell's terminal cytochrome oxidases, causing an increase in superoxide production. The increased superoxide production, together with the increased iron uptake, fuels the formation of hydroxyl radicals that contribute to HU-induced cell death.
PMID:17803904 [本文引用: 1]

Antibiotic mode-of-action classification is based upon drug-target interaction and whether the resultant inhibition of cellular function is lethal to bacteria. Here we show that the three major classes of bactericidal antibiotics, regardless of drug-target interaction, stimulate the production of highly deleterious hydroxyl radicals in Gram-negative and Gram-positive bacteria, which ultimately contribute to cell death. We also show, in contrast, that bacteriostatic drugs do not produce hydroxyl radicals. We demonstrate that the mechanism of hydroxyl radical formation induced by bactericidal antibiotics is the end product of an oxidative damage cellular death pathway involving the tricarboxylic acid cycle, a transient depletion of NADH, destabilization of iron-sulfur clusters, and stimulation of the Fenton reaction. Our results suggest that all three major classes of bactericidal drugs can be potentiated by targeting bacterial systems that remediate hydroxyl radical damage, including proteins involved in triggering the DNA damage response, e.g., RecA.
DOI:10.1016/j.cell.2008.09.038PMID:19013277 [本文引用: 1]

Aminoglycoside antibiotics, such as gentamicin and kanamycin, directly target the ribosome, yet the mechanisms by which these bactericidal drugs induce cell death are not fully understood. Recently, oxidative stress has been implicated as one of the mechanisms whereby bactericidal antibiotics kill bacteria. Here, we use systems-level approaches and phenotypic analyses to provide insight into the pathway whereby aminoglycosides ultimately trigger hydroxyl radical formation. We show, by disabling systems that facilitate membrane protein traffic, that mistranslation and misfolding of membrane proteins are central to aminoglycoside-induced oxidative stress and cell death. Signaling through the envelope stress-response two-component system is found to be a key player in this process, and the redox-responsive two-component system is shown to have an associated role. Additionally, we show that these two-component systems play a general role in bactericidal antibiotic-mediated oxidative stress and cell death, expanding our understanding of the common mechanism of killing induced by bactericidal antibiotics.
[本文引用: 1]
[本文引用: 1]
DOI:10.1128/JB.02449-14PMID:25422305 [本文引用: 1]

The Cpx envelope stress response mediates a complex adaptation to conditions that cause protein misfolding in the periplasm. A recent microarray study demonstrated that Cpx response activation led to changes in the expression of genes known, or predicted, to be involved in cell wall remodeling. We sought to characterize the changes that the cell wall undergoes during activation of the Cpx pathway in Escherichia coli. Luminescent reporters of gene expression confirmed that LdtD, a putative l,d-transpeptidase; YgaU, a protein of unknown function; and Slt, a lytic transglycosylase, are upregulated in response to Cpx-inducing conditions. Phosphorylated CpxR binds to the upstream regions of these genes, which contain putative CpxR binding sites, suggesting that regulation is direct. We show that the activation of the Cpx response causes an increase in the abundance of diaminopimelic acid (DAP)-DAP cross-links that involves LdtD and YgaU. Altogether, our data indicate that changes in peptidoglycan structure are part of the Cpx-mediated adaptation to envelope stress and indicate a role for the uncharacterized gene ygaU in regulating cross-linking. Copyright © 2015, American Society for Microbiology. All Rights Reserved.
DOI:10.1111/j.1365-2958.2008.06426.xPMID:18761686 [本文引用: 1]

Many bacteria possess large numbers of two-component signalling systems, which are composed of histidine kinase-response regulator pairs. The high level of sequence similarity between some systems raises the possibility of undesired cross-talk between a histidine kinase and a non-cognate response regulator. Although molecular specificity ensures that phospho-transfer occurs primarily between correct partners, even a low level of inappropriate cross-talk could lead to unacceptable levels of noise or interference in signal transduction. To explore mechanisms that provide insulation against such interference, we have examined cross-talk between the histidine kinase CpxA and non-cognate response regulator OmpR in Escherichia coli. Our results show that there are two mechanisms that suppress cross-talk between these two proteins, which depend on the corresponding cognate partners CpxR and EnvZ and on the bifunctional nature of the histidine kinases CpxA and EnvZ. When cross-talk is detectable, we find it is independent of CpxA stimulus. We also show that cross-talk suppression leads to mutational robustness, i.e. it masks the effects of mutations that would otherwise lead to increased cross-talk. The mechanisms that provide insulation against interference described here may be applicable to many other two-component systems.
DOI:10.1016/j.vaccine.2010.11.039PMID:21115058 [本文引用: 1]

We evaluated a recently developed live fowl typhoid (FT) vaccine candidate, JOL916, the cpxR/lon mutant of Salmonella Gallinarum (SG), for safety and protection efficacy in 5-week-old layer chickens. Intramuscular vaccination with JOL916 revealed no or very few lesions in livers and spleens of the animals until the fourth week post-vaccination (wpv). This candidate clearly induced cellular immune responses in 5 of 5 chickens on the first and second wpv based on the peripheral lymphocyte proliferation assay. Systemic IgG responses were observed in 5 of 5 chickens from the first wpv and dramatic elevations were observed on the second and third wpv. Vaccination of chickens offered efficient protection against challenge by a wild-type SG; only slight anorexia and depression were temporarily observed after challenge in the vaccinated group while 100% mortality was observed in the positive control group. Body weight increases per day were slightly reduced between the 3rd and 6th day post challenge (dpc) compared to the negative control group; it was recovered from the 6th dpc. Collectively, these results demonstrate the safety and protective efficacy of JOL916 as a live vaccine for systemic FT.Copyright © 2010 Elsevier Ltd. All rights reserved.
