 ,, 楚丹, 颜赛娜, 尹艳飞, 卞桥, 翁波, 陈斌, 冉茂良湖南农业大学动物科学技术学院,畜禽遗传改良湖南省重点实验室,长沙 410128
,, 楚丹, 颜赛娜, 尹艳飞, 卞桥, 翁波, 陈斌, 冉茂良湖南农业大学动物科学技术学院,畜禽遗传改良湖南省重点实验室,长沙 410128MiR-191 promotes the porcine immature Sertoli cell proliferation by targeting the BDNF gene through activating the PI3K/AKT signaling pathway
Xiangwei Tang ,, Dan Chu, Saina Yan, Yanfei Yin, Qiao Bian, Bo Weng, Bin Chen, Maoliang RanHunan Provincial Key Laboratory for Genetic Improvement of Domestic Animal, College of Animal Science and Technology, Hunan Agricultural University, Changsha 410128, China
,, Dan Chu, Saina Yan, Yanfei Yin, Qiao Bian, Bo Weng, Bin Chen, Maoliang RanHunan Provincial Key Laboratory for Genetic Improvement of Domestic Animal, College of Animal Science and Technology, Hunan Agricultural University, Changsha 410128, China通讯作者: 冉茂良,博士,副教授,硕士生导师,研究方向:猪遗传育种。E-mail:ranmaoliang0903@126.com;陈斌,博士,教授,博士生导师,研究方向:猪遗传育种。E-mail:chenbin7586@126.com
编委: 李明洲
收稿日期:2021-04-25修回日期:2021-06-2网络出版日期:2021-07-20
| 基金资助: |
Received:2021-04-25Revised:2021-06-2Online:2021-07-20
| Fund supported: |
作者简介 About authors
唐湘薇,在读硕士研究生,专业方向:猪遗传育种。E-mail:

摘要
睾丸支持细胞数量是影响精子生成能力的主要因素之一,microRNA (miRNA)参与调控猪未成熟支持细胞的发育过程,然而,大多数被鉴定出的miRNA对支持细胞的作用及其机制尚不明确。基于本课题组前期高内涵筛选结果,本文进一步通过流式细胞术、蛋白免疫印迹和双荧光素酶报告基因等方法,研究了miR-191调控猪未成熟支持细胞增殖和凋亡的作用机理。结果表明:过表达miR-191显著促进细胞周期由G1期进入S期和G2期,细胞增殖能力显著增强,细胞凋亡率显著降低;而抑制表达miR-191则与之相反。双荧光素酶报告基因系统验证miR-191直接靶向BDNF基因3′-UTR。抑制表达BDNF基因促进细胞周期进入S期,并促进细胞增殖而抑制细胞凋亡,与过表达miR-191的作用一致。共转染试验结果显示,BDNF基因可以拮抗miR-191对细胞增殖和凋亡的调控作用。此外,过表达miR-191和抑制表达BDNF基因均可显著促进PI3K/AKT信号通路中关键蛋白PI3K和AKT的磷酸化水平,且BDNF基因同样拮抗miR-191对PI3K和AKT蛋白的调控作用。本研究结果证实miR-191靶向BDNF基因,通过激活PI3K/AKT信号通路促进猪未成熟支持细胞增殖且抑制其凋亡,为进一步解析miR-191调控猪精子生成的生物学功能提供了理论基础。
关键词:
Abstract
The number of Sertoli cells in the testis is a major regulator on the sperm production capacity. MicroRNAs (miRNAs) participate in regulating the proliferation and apoptosis of porcine immature Sertoli cells. However, the functions and mechanisms of action of most identified miRNAs in porcine Sertoli cells remain largely unknown. In the present study, based on our previous results from an EdU-based high-content screening assay, we further studied the mechanism of action of miR-191 on the proliferation and apoptosis of porcine immature Sertoli cells through flow cytometry, Western blotting, and dual-luciferase activity analyses. The results demonstrated that overexpression of miR-191 promoted cell cycle progression from G1 phase to the S and G2 phases, enhanced cell proliferation, and inhibited apoptosis in the porcine immature Sertoli cells, whereasmiR-191 inhibition resulted in the opposite effects. The results from a luciferase reporter assay showed that miR-191 directly targeted the 3′-UTR of theBDNF gene. BDNF knockdown also promoted cell cycle progression to the S phase, cell proliferation and inhibited cell apoptosis, which were consistent with the effects of the miR-191overexpression. A co-transfection experiment showed that BDNF knockdown abolished the effects of miR-191 inhibition. Furthermore, both miR-191 overexpression and BDNFinhibition elevated the phosphorylation of PI3K and AKT, the key components of the PI3K/AKT signaling pathway, whereas BDNFinhibition offset the effects of the miR-191 knockdown. Overall, these data indicated that miR-191 promotes cell proliferation and inhibits apoptosis in porcine immature Sertoli cells by targeting theBDNF gene through activating the PI3K/AKT signaling pathway. This study provides a novel scientific basis for further investigation on the biological functions of miR-191 on porcine spermatogenesis.
Keywords:
PDF (2681KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
唐湘薇, 楚丹, 颜赛娜, 尹艳飞, 卞桥, 翁波, 陈斌, 冉茂良. miR-191靶向BDNF基因通过激活PI3K/AKT信号通路促进猪未成熟支持细胞增殖. 遗传[J], 2021, 43(7): 680-693 doi:10.16288/j.yczz.21-154
Xiangwei Tang.
睾丸支持细胞作为生精小管内一种体细胞,不仅通过细胞间的紧密连接形成血睾屏障,为精子细胞的生长发育提供物理支撑和稳定的微环境,还分泌多种细胞因子以保障和促进精子细胞的成熟。猪睾丸支持细胞增殖活性的高峰期在出生至1月龄与初情期前后两个阶段,随后睾丸支持细胞增殖活性逐渐丧失,公猪性成熟后睾丸组织中支持细胞数量保持相对恒定[1]。然而,每个成熟的支持细胞仅能支撑30~50个精子细胞的生长发育,因此支持细胞数量与成年雄性动物睾丸组织大小、精子密度、精子活力、生精小管中生殖细胞数量和间质细胞数量息息相关[2,3]。这表明猪睾丸支持细胞的增殖活性决定着性成熟后睾丸支持细胞数量,进而影响公猪整个使用年限内的精子发育和成熟以及精液品质。
MicroRNA (miRNA)作为一类长约22 nt的非编码RNA,通过靶向蛋白编码基因3′-非翻译区(3′-un-translated region, 3′-UTR)抑制其翻译过程,从而广泛参与调控细胞增殖、凋亡、分化等多种生物学过程。近年来,利用RNA-seq技术已从不同发育阶段的猪睾丸组织中鉴定出300余个miRNA[4,5,6],且经细胞分离后,相比于生精细胞,18个miRNA高表达于支持细胞[7]。在此基础上,多个miRNA已被证实在猪睾丸支持细胞的生长发育过程中具有重要调控作用。例如:miR-762[8]、miR-196a[9]和miR-499[10]等调控猪睾丸支持细胞增殖;miR-8-3p[11]、miR-375[12]和miR-1285[13]等调控猪睾丸支持细胞氧化应激水平;miR-26a调控猪睾丸支持细胞自噬活性[14]。本课题组前期采用高内涵筛选技术对60个miRNA调控猪未成熟支持细胞增殖的作用进行了筛选,鉴定出包括miR-191在内的27个miRNA对猪未成熟支持细胞具有较强的促增殖效应[15],但miR-191的作用机制尚不清楚。研究表明,miR-191可以促进多种细胞的增殖,例如肝癌细胞[16]、食管鳞状细胞[17]、成纤维细胞[18]等。基于miRNA序列在物种间的高度保守性,我们推测miR-191对猪未成熟支持细胞增殖具有重要的调控作用。因此,本研究利用流式细胞术、CCK-8 (cell counting kit-8)、EdU (5-ethynyl-2′-deoxyuridine)、实时荧光定量PCR (quantitative real time PCR, qRT-PCR)、Western blotting和双荧光素酶报告基因等技术解析了miR-191调控猪未成熟支持细胞增殖和凋亡的靶基因及信号机制,为进一步阐明miR-191调控猪睾丸发育和精子生成的机制奠定理论基础。
1 材料与方法
1.1 细胞培养
猪睾丸细胞系(swine testicular, ST) ATCC®CRL- 1746TM和293T工具细胞购自上海安为生物科技有限公司,其中ST已被证实为猪未成熟支持细胞[19],且广泛应用于相关研究[8,15]。细胞培养基为DMEM高糖培养基(美国Gibco公司):胎牛血清(美国Gibco公司):双抗(Penicillin-Streptomycin,美国Gibco公司)= 10∶1∶0.11,并将细胞置于37℃、5%CO2的培养箱中培养。1.2 细胞转染
设计合成miR-191模拟物(mimic)和抑制剂(inhibitor)序列过表达和抑制表达miR-191,设计合成BDNF基因siRNA (5′-GCCAACTGAAGCAGTACTT- 3′)抑制表达BDNF基因。接种细胞于6孔板,在融合度达到60%~70%时,吸弃培养基,每孔加入1 mL PBS (美国Gibco公司)洗涤,重复操作2次,吸弃PBS。向DMEM高糖基础培养基(每孔250 μL)中加入Lipofectamine 2000试剂(每孔5 μL,美国Invitrogen公司),充分混匀成试剂①,室温静置5 min;再向DMEM 高糖基础培养基(每孔250 μL)中分别加入 miR-191 mimic、mimic NC、miR-191 inhibitor、inhibitor NC、BDNF siRNA、siRNA NC、miR-191 inhibitor + BDNF siRNA、miR-191inhibitor + siRNA NC和inhibitor NC + siRNA NC (每孔5 μL,广州锐博生物科技有限公司),充分混匀成试剂②,室温静置5 min;将试剂①与试剂②混匀,静置30 min后加入细胞液中。每组至少设置3个重复,转染6~8 h后更换为完全培养基继续培养,以备后续实验使用。1.3 流式细胞周期检测
细胞转染24 h后,胰酶(美国HyClone公司)消化并收集细胞于1.5 mL EP管,每管加入250 μL PBS重悬,将细胞悬液逐滴加入750 μL预冷乙醇中,4℃过夜保存,1000 r/min离心5 min,弃上清,每管加入1 mL预冷的PBS,重悬细胞,1000 r/min离心5 min,弃PBS,每管加入150 μL浓度为100 µg/mL的碘化丙啶(propidium iodide,PI)染色液(长沙维尔生物科技有限公司),在4℃避光条件下染色30 min。使用流式细胞仪(FACSCalibur,美国BD公司)检测细胞周期分布情况。1.4 细胞增殖检测
接种细胞于96孔板,采用CCK-8(东仁化学科技(上海)有限公司)和EdU试剂盒(广州锐博生物科技有限公司)检测细胞增殖情况。CCK-8检测方法:分别转染24 h和48 h后,每孔加入10 μL CCK-8试剂,继续置于37℃、5%CO 2条件下孵育1 h,使用酶标仪于450 nm波长下检测每孔细胞的吸光度值,将24 h作为对照,计算48 h的相对增殖率。EdU检测方法:转染24 h后,每孔加入100 μL EdU培养基,继续置于37℃、5%CO 2条件下孵育2 h,并严格按照EdU试剂盒使用说明书进行细胞固定、Apollo染色和DNA染色,置于荧光显微镜(德国ZEISS公司)下拍照,并使用Image J软件对图片中的所有活细胞和有丝分裂细胞计数。1.5 流式细胞凋亡检测
接种细胞于6孔板,采用Annexin V-FITC/PI染色法(南京凯基生物科技发展有限公司)于流式细胞仪上检测细胞凋亡情况。流式细胞凋亡检测:转染24 h后,胰酶消化并收集细胞,每管加100 μL 1× Binding Buffer (上海碧云天生物技术有限公司)重悬洗涤,加入5 μL Annexin V-APC试剂孵育15 min,加5 μL PI染色液重悬细胞,置于4℃避光,使用流式细胞仪检测细胞凋亡情况。1.6 细胞ATP水平检测
接种细胞于6孔板,转染24 h后,弃去培养基,PBS清洗2次,每孔加入200 μL ATP裂解液(上海碧云天生物技术有限公司),静置裂解5 min,收集细胞于1.5 mL EP管,4℃、15,000 r/min离心5 min,使用ATP试剂盒(上海碧云天生物技术有限公司)按照使用说明检测细胞ATP水平。1.7 Western blotting检测
收集细胞于1.5 mL EP管,采用RIPA lysis buffer (上海碧云天生物技术有限公司)提取细胞总蛋白,采用BCA蛋白定量试剂盒(上海碧云天生物技术有限公司)在酶标仪上对总蛋白进行定量。每孔加10 μL煮沸的蛋白样品于10%SDS-PAGE的分离胶和5%SDS-PAGE的浓缩胶(上海碧云天生物技术有限公司)中,先60~80 V、30 min,然后调整为100~ 120 V、60 min进行电泳,观察溴酚蓝至胶板底部即可停止。使用电流300 mA、40 min条件进行转膜,随后加入含5%脱脂奶粉的TBST封闭液(北京索莱宝科技有限公司),摇床室温封闭条带1.5 h,加入一抗于4℃孵育过夜。一抗包括:Bcl2 (1∶1000,美国Protein Tech Group公司)、BAX (1∶2000,美国Protein Tech Group公司)、Caspase-3 (1∶100,英国Abcam公司)、p-PI3K (1∶1000,phospho-Tyr458,美国Cell Signaling Technology公司)、p-AKT (1∶1000,phosphoSer473,美国Affinity Biosciences公司)和β-actin (1∶2000,美国Protein Tech Group公司)。最后,加入适量二抗稀释液,室温摇床2 h。二抗包括:辣根过氧化物酶标记山羊抗兔IgG (1∶1000,上海碧云天生物技术有限公司)和辣根过氧化物酶标记山羊抗小鼠IgG (1∶1000,上海碧云天生物技术有限公司)。二抗洗涤后,使用ECL化学发光液(上海翊圣生物科技有限公司)与膜孵育1 min,采用保鲜膜完全包裹印迹膜,在暗盒内与X胶片曝光10 min,显影冲洗。1.8 实时荧光定量PCR
采用TRIzol试剂盒(美国Thermo公司)提取细胞总RNA,采用核酸/蛋白浓度测定仪(NanDrop ND- 2000,美国Thermo Scientific公司)检测总RNA质量,合格的总RNA使用PrimeScriptTM RT试剂盒(日本TaKaRa公司)进行cDNA逆转录。采用Oligo 6.0软件设计实时荧光定量PCR (qRT-PCR)引物,由上海生工生物工程股份有限公司合成,引物信息见表1。构建25 μL qRT-PCR反应体系,包括12.5 μL SYBR Premix Ex Taq (2×,日本TaKaRa公司)、1 μL上游引物、1 μL下游引物、2 μL cDNA模板和8.5 μL ddH2O。反应体系置于IQ-5荧光定量PCR仪(美国Bio-Rad公司)上采用如下程序进行qRT-PCR反应:95℃ 30 s;95℃ 5 s,60℃ 30 s,40个循环;55℃~95℃溶解30 s,81个循环。pig-TBP基因作为内参基因,每组设置3个重复。采用2-ΔΔCt公式计算各基因的相对表达水平。Table 1
表1
表1本研究所用的引物序列
Table 1
| 基因 | 引物序列(5′→ 3′) |
|---|---|
| CCND1 | F: TACACCGACAACTCCATCCG |
| R: GCCGCCAGGTTCCACTT | |
| CCNE1 | F: CCTGCTGAAGATGCCCATAAC |
| R: TGCTCTGCTTCTTACTGCTCG | |
| CDK-4 | F: GTGGCCCTCAAGAGCGTAAG |
| R: CAGACATCCATCAGCCGGAC | |
| c-MYC | F: AACCCTTGGCTCTCCACGAG |
| R: ATTCCGACCTTTTGGCAGGG | |
| FGF2 | F: ACCAGGTCACTGAGATCCATCCAC |
| R: TTCGGCAACAGCACACCAATCC | |
| BMP4 | F: ACCAATCATGCCATCGTTCAGACC |
| R: TGATTCAGCGGCAACCACATCC | |
| GDNF | F: GAGACCGCTGTGTATCGCATTCC |
| R: GCCTTCTTCCTCTTCCTCCTCCTC | |
| PCNA | F: ATTTGGCCATGGGCGTGAAC |
| R: CTAGTGCCAAGGTGTCTGCAT | |
| EGF | F: GCGAGCGATGTCAGCACAGAG |
| R: AGGAGCAGCAGCAGGACCAG | |
| TBP | F: GCGATTTGCTGCTGTAATCA |
| R: CCCCACCATGTTCTGAATCT |
新窗口打开|下载CSV
1.9 双荧光素酶活性检测
将合成的BDNF (brain-derived neurotrophic factor)基因3′-UTR-WT和3′-UTR-MUT序列(广州市锐博生物科技有限公司)连接至psiCHECK2载体,筛选阳性克隆并测序确认后,与miR-191 mimic和mimic NC在DMEM high glucose基础培养基条件下两两组合共转染至293 T细胞,48 h后收集细胞,每孔加35 μL PBS、35 μL luciferase reagent (美国Promega公司),震荡10 min,移至96孔白色细胞培养板中,采用多功能酶标仪(Spectra max m5e,美国Molecular Devices公司)测定荧光值;每孔加30 μL Stop reagent (美国Promega公司),震荡10 min后,测定荧光值。1.10 统计分析
采用IBM SPSS 22.0软件进行统计分析,使用One-Way ANOVA进行单因素方差分析,并采用Duncan氏法进行多重比较以评估各组之间的差异。数据均以平均值±标准差的形式表示,*P<0.05,**P<0.01。2 结果与分析
2.1 miR-191促进猪未成熟支持细胞增殖
为明确miR-191对猪未成熟支持细胞增殖的作用,分别转染miR-191 mimic、mimic NC、miR-191 inhibitor和inhibitor NC。流式细胞周期结果表明,过表达miR-191后,处于G1期的细胞比例极显著降低(P<0.01),S期和G2期的细胞比例显著增加(P< 0.05) (图1A),抑制表达miR-191后,处于S期和G2期的细胞比例则显著降低(P<0.05) (图1C)。采用qRT-PCR技术检测细胞周期相关基因的表达,结果表明,过表达miR-191极显著促进c-MYC、CCNE1、CCND1和CDK4基因的表达水平(P<0.01) (图1B),而抑制表达miR-191则显著抑制以上基因的表达水平(P<0.05) (图1D)。以上结果表明,miR-191促进猪未成熟支持细胞周期进程。图1
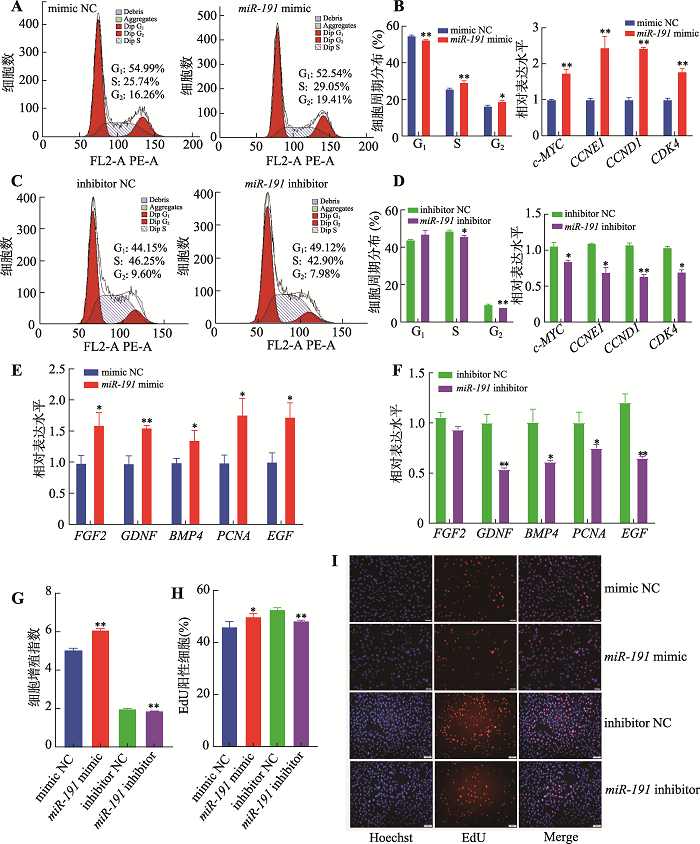 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1miR-191促进猪未成熟支持细胞增殖
A:转染miR-191 mimic和mimic NC,流式细胞仪检测流式周期;B:转染miR-191 mimic和mimic NC,qRT-PCR检测周期相关基因(c-MYC、CCNE1、CCND1和CDK4) mRNA相对表达水平;C:转染miR-191 inhibitor和inhibitor NC,流式细胞仪检测流式周期;D:转染miR-191 inhibitor和inhibitor NC,qRT-PCR检测周期相关基因(c-MYC、CCNE1、CCND1和CDK4) mRNA相对表达水平;E:转染miR-191 mimic和mimic NC,qRT-PCR检测增殖相关基因(FGF2、GDNF、BMP4、PCNA和EGF) mRNA相对表达水平;F:转染miR-191 inhibitor和inhibitor NC,qRT-PCR检测增殖相关基因(FGF2、GDNF、BMP4、PCNA和EGF) mRNA相对表达水平;G:转染miR-191 mimic、mimic NC、miR-191 inhibitor和inhibitor NC,CCK-8试剂检测细胞增殖指数;H:转染miR-191 mimic、mimic NC、miR-191inhibitor和inhibitor NC,EdU试剂盒检测增殖细胞比例;I:细胞EdU实验染色图(蓝色细胞为Hoechst染色,红色细胞为EdU染色,Merge为Hoechst和EdU染色合并图,标尺:50 μm)。* P<0.05表示差异显著,**P<0.01表示差异极显著。
Fig. 1miR-191 promotes the porcine immature Sertoli cell proliferation
采用qRT-PCR技术检测细胞增殖相关基因的表达水平。结果表明,过表达miR-191显著增加FGF2、GDNF、BMP4、PCNA和EGF基因的表达水平(P< 0.05) (图1E),而抑制表达miR-191则显著降低细胞增殖相关基因的表达(P<0.05) (图1F)。CCK-8试剂盒检测细胞增殖情况,结果表明过表达miR-191极显著提高细胞增殖能力(P<0.01),而抑制表达miR-191则极显著降低细胞增殖能力(P<0.01) (图1G)。此外,EdU染色结果表明,过表达miR-191显著促进细胞增殖活性(P<0.05),而抑制表达miR-191则极显著降低细胞增殖活性(P<0.01) (图1:H和I)。
2.2 miR-191抑制猪未成熟支持细胞凋亡
为进一步明确miR-191对猪未成熟支持细胞凋亡的影响,本研究采用Annexin V-FIT/PI方法检测细胞凋亡情况。结果表明,过表达miR-191显著降低细胞凋亡率(P<0.05) (图2:A和B),而抑制表达miR-191则显著增加细胞凋亡率(P<0.05) (图2:A和D)。细胞ATP水平检测结果表明,过表达miR-191极显著增加细胞ATP水平(P<0.01) (图2C),而抑制表达miR-191则显著降低细胞ATP水平(P<0.05) (图2E)。采用Western blotting技术检测细胞凋亡相关基因蛋白水平的表达,结果显示,过表达miR-191增加Bcl2蛋白表达水平而抑制BAX和Caspase-3蛋白表达水平(图2F),而抑制表达miR-191则与之相反(图2G)。综上所述,miR-191抑制猪未成熟支持细胞凋亡。图2
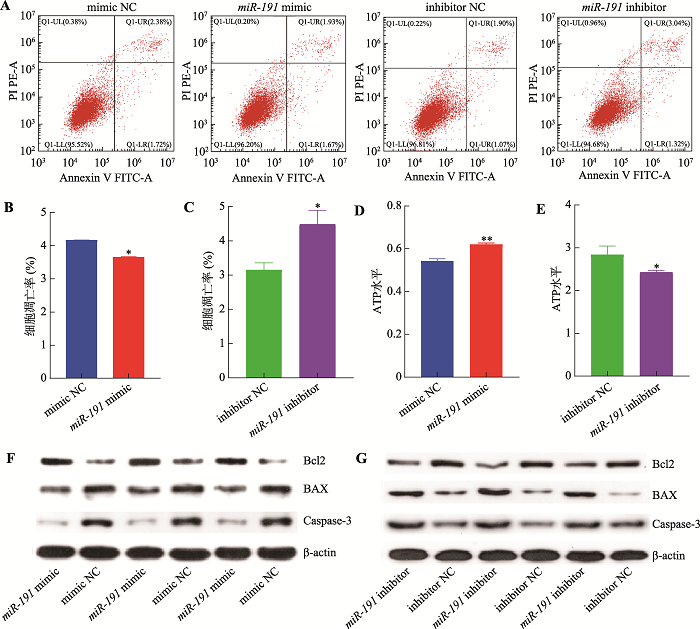 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2miR-191抑制猪未成熟支持细胞凋亡
A:转染miR-191 mimic、mimic NC、miR-191inhibitor和inhibitor NC,流式细胞仪检测细胞凋亡情况;B:转染miR-191 mimic和mimic NC,细胞凋亡比例统计结果;C:转染miR-191 inhibitor和inhibitor NC,细胞凋亡比例统计结果;D:转染miR-191 mimic和mimic NC,细胞ATP水平;E:转染miR-191 inhibitor和inhibitor NC,细胞ATP水平;F:转染miR-191mimic和mimic NC,Western blotting 检测细胞凋亡标志基因Bcl2、BAX和Caspase-3蛋白表达水平;G:转染miR-191 inhibitor和inhibitor NC,Western blotting 检测细胞凋亡标志基因Bcl2、BAX和Caspase-3蛋白表达水平。*P<0.05表示差异显著,**P<0.01表示差异极显著。
Fig. 2miR-191 impedes immature porcine Sertoli cell apoptosis
2.3 miR-191靶向BDNF基因3′-UTR
本研究利用miRwalk、TargetScan和miRanda在线软件预测了不同物种中miR-191的靶基因,根据结合位点保守性分析,初步筛选出BDNF基因作为候选靶基因(图3A)。为进一步确定miR-191与基因BDNF之间的靶向关系,本研究构建了BDNF-WT和BDNF-MUT双荧光素酶报告基因载体,并与miR-191 mimic和mimic NC两两组合共转染至293T细胞。双荧光素酶活性检测结果表明,BDNF-WT + miR-191 mimic共转染组的双荧光素酶活性显著低于其他3组(P<0.05) (图3B)。采用qRT-PCR技术检测miR-191对BDNF基因mRNA表达的影响,结果表明,过表达miR-191极显著降低BDNF基因mRNA表达水平(P<0.01),而抑制表达miR-191极显著增加BDNF基因mRNA表达水平(P<0.01)。综上所述,miR-191靶向BDNF基因3′-UTR并抑制其表达水平。图3
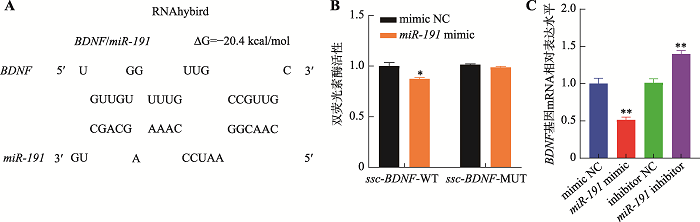 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3miR-191靶向BDNF基因3′-UTR
A:miR-191与BDNF基因3′-UTR的潜在结合位点;B:共转染BDNF-WT + mimic NC、BDNF-WT + miR-191 mimic、BDNF-MUT + mimic NC和BDNF-MUT + miR-191 mimic后,检测双荧光素酶活性;C:转染mimic NC、miR-191 mimic、inhibitor NC和miR-191 inhibitor,qRT-PCR检测BDNF基因mRNA相对表达水平。*P<0.05表示差异显著,**P<0.01表示差异极显著。
Fig. 3miR-191 targets the 3'-UTR region of theBDNF gene
2.4 BDNF基因抑制猪未成熟支持细胞增殖而促进其凋亡
为解析BDNF基因对猪未成熟支持细胞增殖和凋亡的影响,针对BDNF基因序列设计了BDNF siRNA和siRNA NC,并转染至细胞。结果表明,相较于对照组,转染BDNF siRNA后,处于G1期和G2期的细胞比例显著下降(P<0.05),S期细胞比例极显著上升(P<0.01) (图4A),c-MYC、CCND1和CDK4基因的表达水平显著增加(P<0.05) (图4B)。此外,抑制BDNF基因的表达后,细胞增殖相关基因FGF2、GDNF、BMP4、PCNA和EGF基因表达水平显著增加(P<0.05) (图4C);CCK-8和EdU检测结果表明,抑制BDNF基因极显著促进猪未成熟支持细胞增殖(P<0.01) (图4:D,E和F)。细胞凋亡检测结果显示,抑制BDNF基因的表达可极显著抑制细胞的凋亡率(P<0.01) (图5A),促进Bcl2蛋白的表达而抑制BAX和Caspase-3蛋白的表达(图5B),增加细胞ATP水平(图5C)。以上结果表明,抑制表达BDNF基因促进猪未成熟支持细胞增殖而抑制其凋亡,与过表达miR-191的结果一致。图4
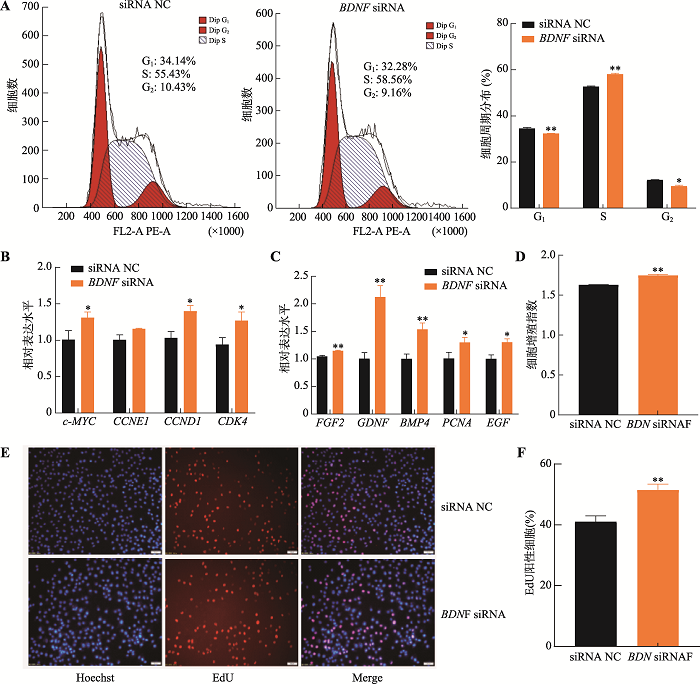 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4抑制表达BDNF基因促进猪未成熟支持细胞增殖
A:流式细胞术检测流式周期分布情况;B:qRT-PCR检测周期相关基因c-MYC、CCNE1、CCND1和CDK4的mRNA相对表达水平;C: qRT-PCR检测增殖相关基因FGF2、GDNF、BMP4、PCNA和EGF的mRNA相对表达水平;D:CCK-8试剂检测细胞增殖指数;E:细胞EdU实验染色图(蓝色细胞为Hoechst染色,红色细胞为EdU染色,Merge为Hoechst和EdU染色合并图,标尺:50 μm);F:EdU细胞染色统计结果。* P<0.05表示差异显著,**P<0.01表示差异极显著。
Fig. 4BDNF inhibition promotes porcine immature Sertoli cell proliferation
图5
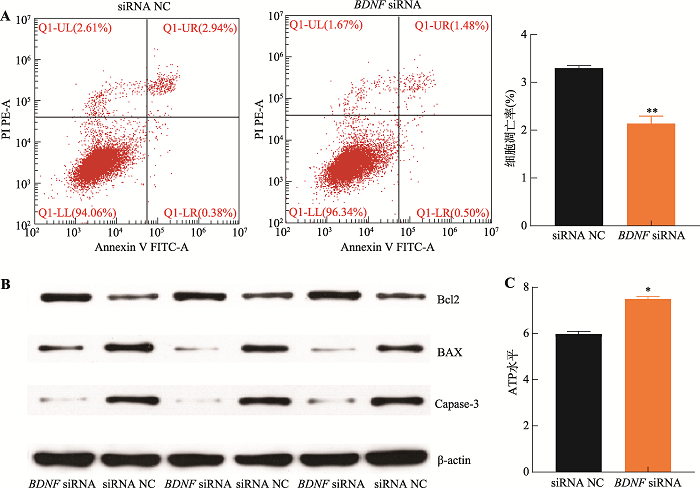 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5抑制表达BDNF基因促进猪未成熟支持细胞凋亡
A:流式细胞仪检测不同凋亡期细胞分布及细胞数统计;B:Western blotting 检测细胞凋亡标志基因Bcl2、BAX和Caspase-3蛋白表达水平;C:细胞内ATP水平检测。*P<0.05表示差异显著,**P<0.01表示差异极显著。
Fig. 5BDNF knockdown inhibits porcine immature Sertoli cell apoptosis
2.5 BDNF基因拮抗miR-191的调控作用
为进一步明确miR-191是否通过靶向BDNF进而调控猪未成熟支持细胞增殖和凋亡,本研究将miR-191 inhibitor + BDNF siRNA、miR-191 inhibitor + siRNA NC和inhibitor NC + siRNA NC三组共转染于猪未成熟支持细胞。EdU和CCK-8结果表明,相较于inhibitor NC + siRNA NC对照组,miR-191 inhibitor + siRNA NC组的细胞增殖活性显著降低(P<0.05),而miR-191 inhibitor + BDNF siRNA组的细胞增殖活性则极显著升高(P<0.01) (图6:A和B)。同时,相较于inhibitor NC + siRNA NC对照组,miR-191 inhibitor + siRNA NC组的细胞增殖相关基因FGF2、GDNF、BMP4、PCNA和EGF的表达水平显著降低(P<0.05),而miR-191 inhibitor + BDNF siRNA组的BMP4、PCNA和EGF基因表达水平显著增加(P<0.05) (图6C)。此外,相比于inhibitor NC + siRNA NC对照组,miR-191 inhibitor + siRNA NC组的Bcl2蛋白表达水平降低且BAX和Caspase-3蛋白表达水平增加,而miR-191 inhibitor + BDNF siRNA组的Bcl2蛋白表达水平升高且BAX和Caspase-3蛋白表达水平降低(图6D)。以上结果表明,抑制表达BDNF基因可以拮抗miR-191对猪未成熟支持细胞增殖和凋亡的作用。
图6
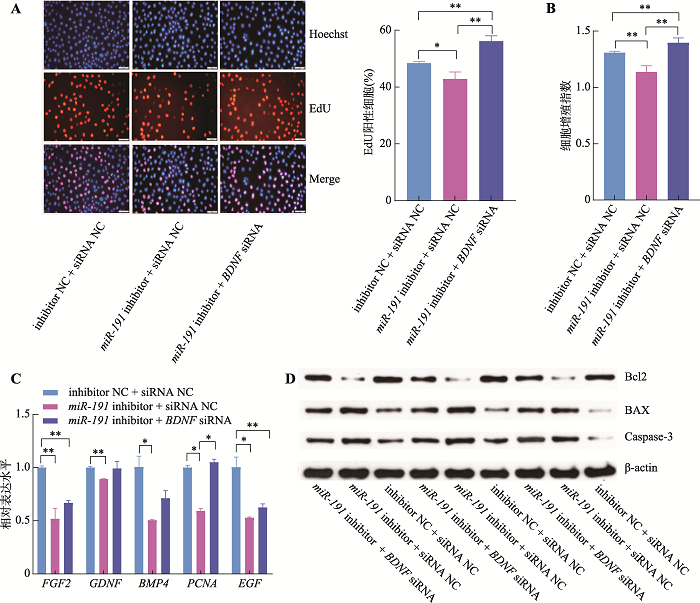 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6BDNF基因拮抗miR-191对猪未成熟支持细胞增殖和凋亡的调控作用
A:细胞EdU实验染色图(蓝色细胞为Hoechst染色,红色细胞为EdU染色,Merge为Hoechst和EdU染色合并图,标尺:50 μm);B:CCK-8试剂检测细胞增殖指数;C:qRT-PCR检测增殖相关基因 FGF2、GDNF、BMP4、PCNA和EGF的mRNA相对表达水平;D:Western blotting 检测细胞凋亡标志基因Bcl2、BAX和Caspase-3蛋白表达水平。*P<0.05表示差异显著,**P<0.01表示差异极显著。
Fig. 6BDNF gene offset the effects of miR-191 on the proliferation and apoptosis of porcine immature Sertoli cells
2.6 miR-191靶向BDNF基因3′-UTR激活PI3K/AKT信号通路
生物信息学分析结果表明,miR-191和BDNF基因可能作用于PI3K/AKT信号通路,因此,采用Western blotting技术检测了二者对PI3K/AKT信号通路中p-PI3K和p-AKT蛋白的磷酸化水平。结果表明,过表达miR-191极显著增加p-PI3K和p-AKT蛋白的磷酸化水平(P<0.01) (图7A),而抑制表达miR-191极显著抑制p-PI3K和p-AKT蛋白的磷酸化水平(P<0.01)(图7B);抑制表达BDNF基因极显著增加p-PI3K和p-AKT蛋白的磷酸化水平(P<0.01) (图7C),与过表达miR-191一致;同时,相比于inhibitor NC + siRNA NC对照组,miR-191 inhibitor + siRNA NC组的p-PI3K和p-AKT蛋白的磷酸化水平极显著降低(P<0.01),而miR-191 inhibitor + BDNF siRNA组的p-PI3K和p-AKT蛋白的磷酸化水平则显著增加(P<0.05) (图7D)。以上结果表明,miR-191靶向BDNF基因通过激活PI3K/AKT信号通路促进猪未成熟支持细胞的增殖而抑制其凋亡。图7
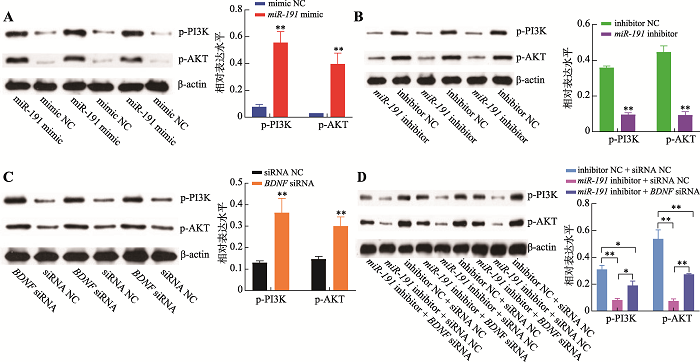 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7miR-191靶向BDNF基因激活PI3K/AKT信号
A:转染miR-191 mimic和mimic NC,Western blotting检测p-PI3K和p-AKT蛋白相对表达水平及结果分析;B:转染miR-191 inhibitor和inhibitor NC,Western blotting 检测p-PI3K和p-AKT蛋白相对表达水平及结果分析;C:转染BDNF siRNA和siRNA NC,Western blotting检测p-PI3K和p-AKT蛋白相对表达水平及结果分析;D:共转染inhibitor NC + siRNA NC、miR-191 inhibitor + siRNA NC和miR-191 inhibitor + BDNF siRNA,Western blotting 检测p-PI3K和p-AKT蛋白相对表达水平及结果分析。*P<0.05表示差异显著,**P<0.01表示差异极显著。
Fig. 7miR-191 activates PI3K/AKT signaling pathway by targeting the BDNF gene
3 讨论
支持细胞数量是影响精子生成能力的主要因素,而提高公猪的精子产量,对实际生产中提高猪的生产效率至关重要。但是,目前关于支持细胞增殖的分子机制尚不清楚[20]。研究表明,miRNA在发育过程中调控许多基因的表达,且一些特异性的miRNA参与了未成熟支持细胞增殖的调控过程,例如miR-130a[15]、miR-499[10]、miR-762[8]和miR-1285[13]等。然而,分析表明,现有的关于miRNA调控猪未成熟支持细胞增殖的研究,还不足以解析其潜在的分子调控机制[21]。本课题组前期采用高内涵筛选技术鉴定出包括miR-191在内的27个miRNA具有促进猪未成熟支持细胞增殖的潜在作用,但其作用的特定分子信号途径有待进一步研究。目前,关于miR-191的研究主要集中于医学领域,例如,促进肝癌细胞[16]、食管鳞状细胞[17]和成纤维细胞[18]等的增殖,抑制胆管癌细胞[22]、乳腺癌细胞(MCF7和ZR-75)[23]和子宫内膜样癌细胞[24]等的凋亡。本研究综合采用流式细胞术、CCK-8、EdU和Western blotting等方法在细胞水平明确了miR-191促进猪未成熟支持细胞增殖而抑制其凋亡,表明miR-191广泛参与调控细胞增殖和凋亡,并有可能是调控猪未成熟支持细胞的一个特异性分子。miRNA通常靶向结合蛋白编码基因3′-UTR以抑制其翻译过程。本研究采用miRwalk、TargetScan和miRanda软件预测miR-191的靶基因,并取结果的交集,再根据结合位点的保守性筛选出BDNF基因为其潜在靶基因。随后,通过双荧光素酶报告基因实验进一步证实miR-191直接靶向BDNF基因3′-UTR的预测结合位点。但是,目前尚没有猪BDNF基因商业化蛋白抗体,导致无法在现有条件下进一步验证miR-191是否抑制了BDNF基因的蛋白翻译过程。但是,在成肌细胞和海马神经元细胞中已证实miR-191可以靶向BDNF基因并抑制其翻译过程[25,26,27]。基于miR-191与BDNF基因结合位点在物种间的高度保守性,因此我们推测miR-191在猪未成熟支持细胞中同样可以抑制BDNF基因的翻译过程。同时,本研究明确了抑制表达BDNF基因可促进猪未成熟支持细胞增殖并抑制细胞凋亡的,其调控作用与过表达miR-191一致。在此基础上,本文进一步设计了关于miR-191与BDNF基因的共转染试验,结果表明BDNF基因可以拮抗miR-191对猪未成熟支持细胞增殖和凋亡的调控作用。综上所述,本文基本确定miR-191靶向BDNF基因3′-UTR并调控了其表达。
细胞周期是一个受多个因子调控的复杂过程,主要参与生物的生长和发育,c-MYC作为细胞进程中一个重要的调节因子,活化后可以缩短G1期的时间,是从G1期转化至S期必不可少的调节因子之一[28],c-MYC还可以通过活化细胞周期蛋白CCND1、CCNE1和CDK4而促进周期进程,若抑制c-MYC的表达时,细胞周期的G1期向S期的转化将受到抑制,进而抑制细胞周期进程[29]。本研究发现,过表达miR-191和抑制表达BDNF基因后,支持细胞由G1期进入S期的比例均显著增加,且c-MYC、CCND1、CCNE1和CDK4的表达水平显著增加。此外,本研究还发现,过表达miR-191和抑制表达BDNF基因可显著增加FGF2、GDNF、BMP4、PCNA和EGF的表达水平。研究表明,支持细胞本身表达以上细胞增殖相关基因,且EGF和FGF2基因可通过激活MAPK信号通路促进细胞增殖[30],PCNA基因则可以通过调控细胞周期相关基因的表达促进细胞周期的进程以及促进DNA复制进而促进细胞增殖[31],BMP4基因则可以通过激活SMAD基因家族促进细胞增殖[32]。此外,本研究采用CCK-8和EdU实验进一步证实miR-191和BDNF基因可以调控猪睾丸支持细胞增殖。
细胞凋亡是由内源性或外源性通路引起的细胞程序性死亡的过程,多个基因参与到细胞凋亡调控过程中,但Bcl2基因在整个过程起决定性作用,细胞凋亡基因调控的方式分为促进细胞凋亡和抑制细胞凋亡两种[33]。BAX是促细胞凋亡基因,Bcl2是抑细胞凋亡基因,二者都是通过激活下游基因以调节细胞凋亡。Bcl2对各种因素引起的凋亡都有抑制作用,但BAX能与线粒体上的Bcl2结合从而促进细胞凋亡[34]。Caspase-3是Caspase家族中重要的一员,是一类与细胞密切相关的蛋白水解酶,通过自身活化或相互激活形成活性复合物引起瀑布式的级联反应,导致细胞凋亡,在整个过程中发挥着最后的枢纽作用[35]。本研究结果表明,过表达miR-191和抑制表达BDNF基因后,猪睾丸支持细胞中Bcl2蛋白表达水平增加,而BAX和Capase-3蛋白表达水平降低,且细胞ATP水平显著增加,细胞凋亡率显著减少。
为进一步解析miR-191靶向BDNF基因调控猪未成熟支持细胞增殖和凋亡的信号机制,本文对miR-191所有的潜在靶基因进行了功能富集分析,预测出PI3K/AKT信号通路为潜在路径。同时,研究表明,BDNF基因与PI3K/AKT信号通路之间具有调控关系。本研究结果表明,过表达miR-191和抑制表达BDNF基因均可显著促进PI3K/AKT信号通路中关键蛋白PI3K和AKT的磷酸化水平,且BDNF基因同样拮抗miR-191对PI3K和AKT蛋白的调控作用。在我们的前期研究中发现,740Y-P激活剂诱导的PI3K/AKT信号通路激活显著促进猪未成熟支持细胞周期进程和细胞增殖,而抑制细胞凋亡[15],且LY294002抑制剂诱导的PI3K/AKT信号通路减活则显著抑制猪未成熟支持细胞增殖而促进其凋亡[10]。此外,甲状腺激素、促卵泡激素和邻苯二甲酸二丁酯等均可以通过抑制PI3K/AKT信号通路活性抑制支持细胞增殖而促进细胞凋亡[36,37,38],17β-雌二醇则通过激活PI3K/AKT信号通路促进支持细胞增殖[39]。同时,miR-638[40]、miR-499[10]和miR-222[41]等均被报道通过调节PI3K/AKT信号通路活性以调控支持细胞增殖和凋亡。因此,PI3K/AKT信号通路的活性可能对于猪未成熟支持细胞的增殖和凋亡具有重要作用。综上所述,miR-191靶向BDNF基因通过激活PI3K/AKT信号通路促进猪未成熟支持细胞增殖而抑制其凋亡。
(责任编委: 李明洲)
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1111/andr.12165URL [本文引用: 1]
DOI:10.1210/en.2017-00196PMID:28911170 [本文引用: 1]

Sertoli cells regulate differentiation and development of the testis and are essential for maintaining adult testis function. To model the effects of dysregulating Sertoli cell number during development or aging, we have used acute diphtheria toxin-mediated cell ablation to reduce Sertoli cell population size. Results show that the size of the Sertoli cell population that forms during development determines the number of germ cells and Leydig cells that will be present in the adult testis. Similarly, the number of germ cells and Leydig cells that can be maintained in the adult depends directly on the size of the adult Sertoli cell population. Finally, we have used linear modeling to generate predictive models of testis cell composition during development and in the adult based on the size of the Sertoli cell population. This study shows that at all ages the size of the Sertoli cell population is predictive of resulting testicular cell composition. A reduction in Sertoli cell number/proliferation at any age will therefore lead to a proportional decrease in germ cell and Leydig cell numbers, with likely consequential effects on fertility and health.
DOI:10.1016/j.mce.2016.01.001PMID:26772142 [本文引用: 1]

The AMP-activated protein kinase (AMPK) is an important regulator of cellular energy homeostasis which plays a role in fertility. Complete disruption of the AMPK catalytic subunit α1 gene (α1AMPK KO) in male mice results in a decrease in litter size which is associated with the production of altered sperm morphology and motility. Because of the importance of Sertoli cells in the formation of germ cells, we have chosen to selectively disrupt α1AMPK only in the Sertoli cells in mice (Sc-α1AMPK-KO mice). Specific deletion of the α1AMPK gene in Sertoli cells resulted in a 25% reduction in male fertility associated with abnormal spermatozoa with a thin head. No clear alterations in testis morphology or modification in the number of Sertoli cells in vivo were observed, but a dysregulation in energy metabolism in Sertoli cells occurred. We have reported an increase in lactate production, in lipid droplets, and a reduction in ATP production in Sc-α1AMPK-KO Sertoli cells. These perturbations were associated with lower expression of mitochondrial markers (cytochrome c and PGC1-α). In addition another metabolic sensor, the deacetylase SIRT1, had a reduction in expression which is correlated with a decline in deacetylase activity. Finally, expression and localization of junctions forming the blood-testis barrier between Sertoli cells themselves and with germ cells were deregulated in Sc-α1AMPK-KO. In conclusion, these results suggest that dysregulation of the energy sensing machinery exclusively through disruption of α1AMPK in Sertoli cells translates to a reduction in the quality of germ cells and fertility. Copyright © 2016 Elsevier Ireland Ltd. All rights reserved.
DOI:10.1039/C5RA07488FURL [本文引用: 1]
DOI:10.1186/s12864-020-07096-7URL [本文引用: 1]
DOI:10.1016/j.theriogenology.2017.06.023URL [本文引用: 1]
DOI:10.1186/s40104-020-00487-6URL [本文引用: 1]
DOI:10.1038/srep32783URL [本文引用: 3]
DOI:10.1089/dna.2018.4387URL [本文引用: 1]
[本文引用: 4]
DOI:10.1002/mrd.v86.11URL [本文引用: 1]
DOI:10.1016/j.gene.2018.10.086URL [本文引用: 1]
DOI:10.1210/en.2014-1982PMID:26287402 [本文引用: 2]

This study investigated the capacity of 10 μM 17β-estradiol to inhibit immature boar Sertoli cell (SC) proliferation and the involvement of microRNA (miR)-1285 in this process. SC viability and cell cycle progression were investigated using a cell counting kit-8 and flow cytometry, respectively. Expression of AMP-activated protein kinase (AMPK), S phase kinase-associated protein 2 (Skp2), and miR-1285 was analyzed by real-time RT-PCR and Western blotting. 17β-Estradiol (10 μM) reduced SC viability and miR-1285 expression and promoted AMPK phosphorylation. A double-stranded synthetic miR-1285 mimic promoted SC viability, increased levels of ATP, and phosphorylated mammalian target of rapamycin (mTOR) and Skp2 mRNA and protein, whereas p53 and p27 expression decreased, and 17β-estradiol-mediated effects on SCs were significantly attenuated. A single-stranded synthetic miR-1285 inhibitor produced the opposite effects on these measures. Activation of AMPK inhibited SC viability, reduced levels of ATP, phosphorylated mTOR and Skp2 mRNA and protein, and increased p53 and p27 expression. An AMPK inhibitor (compound C) attenuated the effects of 17β-estradiol on SCs. This indicated that 17β-estradiol (10 μM) reduced SC proliferation by inhibiting miR-1285 and thus activating AMPK. Phosphorylated AMPK is involved in the regulation of 17β-estradiol-mediated inhibition of SC viability through increasing p53 and p27 expression and inhibiting mTOR and Skp2 expression. Our findings also implicated Skp2 as the downstream integration point of p53 and mTOR. These findings indicated that miR-1285 may represent a target for the manipulation of boar sperm production.
DOI:10.1111/rda.2018.53.issue-4URL [本文引用: 1]
DOI:10.1096/fsb2.v34.11URL [本文引用: 4]
[本文引用: 2]
DOI:10.3349/ymj.2017.58.6.1101URL [本文引用: 2]
DOI:10.1371/journal.pone.0126535URL [本文引用: 2]
DOI:10.1007/s11626-015-9994-8URL [本文引用: 1]
DOI:10.1038/s41419-019-1782-zURL [本文引用: 1]
DOI:10.1016/j.livsci.2020.103954URL [本文引用: 1]
DOI:10.1159/000493654URL [本文引用: 1]
DOI:10.1261/rna.060657.117URL [本文引用: 1]
[本文引用: 1]
DOI:10.1007/s00018-014-1628-xPMID:24804980 [本文引用: 1]

Brain-derived neurotrophic factor (BDNF) is a secreted protein of the neurotrophin family that regulates brain development, synaptogenesis, memory and learning, as well as development of peripheral organs, such as angiogenesis in the heart and postnatal growth and repair of skeletal muscle. However, while precise regulation of BDNF levels is an important determinant in defining the biological outcome, the role of microRNAs (miRs) in modulating BDNF expression has not been extensively analyzed. Using in silico approaches, reporter systems, and analysis of endogenous BDNF, we show that miR-1, miR-10b, miR-155, and miR-191 directly repress BDNF through binding to their predicted sites in BDNF 3'UTR. We find that the overexpression of miR-1 and miR-10b suppresses endogenous BDNF protein levels and that silencing endogenous miR-10b increases BDNF mRNA and protein levels. Furthermore, we show that miR-1/206 binding sites within BDNF 3'UTR are used in differentiated myotubes but not in undifferentiated myoblasts. Finally, our data from two cell lines suggest that endogenous miR-1/206 and miR-10 family miRs act cooperatively in suppressing BDNF through their predicted sites in BDNF 3'UTR. In conclusion, our results highlight miR-1, miR-10b, miR-155, and miR-191 as novel regulators of BDNF long and short 3'UTR isoforms, supporting future research in different physiological and pathological contexts.
DOI:10.1080/15376516.2021.1886211URL [本文引用: 1]
DOI:S0306-4522(19)30464-6PMID:31279826 [本文引用: 1]

The aim of this study was to investigate the effect of paradoxical sleep deprivation (PSD) on the BDNF-related miRNA expression in ovariectomized (OVX) rats. The animals were randomly divided into eight groups (control, PSD, wide platform, sham surgery, anti-miR-191, anti-miR-191/PSD, scrambled and PSD in intact). Bilateral-ovariectomy was performed one month before the experiment in the OVX rats. For the induction of 72?h of PSD, the multiple platform method was applied. The Morris water maze (MWM) test was carried out 30?min after PSD to test spatial learning and memory. Finally, the rats were euthanized 24?h after the last experiment. We quantified miR-10a-5P, miR-10b-5P, miR-125a-3p, miR-155-5p, miR-191a-5p, BDNF and TMOD-2 level using real-time PCR. BNDF protein was also measured by Western blotting. Hippocampal miR-191a and miR-155 were significantly up-regulated (P ˂.01) and BDNF down-regulated (P ˂.05) in the PSD group. PSD rats with up-regulated miR-191a swam longer distance and spent more time to find a hidden platform (positive correlation) and showed the lowest percentage of time and distance in the target quadrant (negative correlation). The negative correlation between miR-191a and BDNF levels in the PSD condition provided more evidence for the role of miR-191a in cognitive function. Intracerebroventricular (ICV) injection of anti-miR-191a improved the down-regulation of BDNF and attenuated PSD-induced cognitive impairment. Hippocampal BDNF is probably one of the targets of miR-191a in sleep-deprived OVX rats. Our results suggest that miR-191a may be increased in the sleep-deprived OVX rats to regulate BDNF levels. Copyright © 2019 IBRO. Published by Elsevier Ltd. All rights reserved.
DOI:10.1038/414768aURL [本文引用: 1]
DOI:10.1158/1535-7163.MCT-09-0795PMID:20354121 [本文引用: 1]

Colon cancer is the leading cause of cancer death in both men and women worldwide. The deregulated cell cycle control or decreased apoptosis of normal epithelial cells leading to uncontrolled proliferation is one of the major features of tumor progression. We have previously shown that aldose reductase (AR), a NADPH-dependent aldo-keto reductase, has been shown to be involved in growth factor-induced proliferation of colon cancer cells. Herein, we report that inhibition of AR prevents epidermal growth factor (EGF)- and basic fibroblast growth factor (bFGF)-induced HT29 cell proliferation by accumulating cells at G(1) phase of cell cycle. Similar results were observed in SW480 and HCT-116 colon cancer cells. Treatment of HT29 cells with AR inhibitor, sorbinil or zopolrestat, prevented the EGF- and bFGF-induced DNA binding activity of E2F-1 and phosphorylation of retinoblastoma protein. Inhibition of AR also prevented EGF- and bFGF-induced phosphorylation of cyclin-dependent kinase (cdk)-2 and expression of G(1)-S transition regulatory proteins such as cyclin D1, cdk4, proliferating cell nuclear antigen, cyclin E, and c-myc. More importantly, inhibition of AR prevented the EGF- and bFGF-induced activation of phosphoinositide 3-kinase/AKT and reactive oxygen species generation in colon cancer cells. Further, inhibition of AR also prevented the tumor growth of human colon cancer cells in nude mouse xenografts. Collectively, these results show that AR mediates EGF- and bFGF-induced colon cancer cell proliferation by activating or expressing G(1)-S phase proteins such as E2F-1, cdks, and cyclins through the reactive oxygen species/phosphoinositide 3-kinase/AKT pathway, indicating the use of AR inhibitors in the prevention of colon carcinogenesis. Mol Cancer Ther; 9(4); 813-24. (c)2010 AACR.
[本文引用: 1]
DOI:10.1093/aob/mcq243URL [本文引用: 1]
[本文引用: 1]
DOI:10.1038/cdd.2013.153PMID:24162659 [本文引用: 1]

Apoptosis, a mechanism for programmed cell death, has key roles in human health and disease. Many signals for cellular life and death are regulated by the BCL-2 family proteins and converge at mitochondria, where cell fate is ultimately decided. The BCL-2 family includes both pro-life (e.g. BCL-XL) and pro-death (e.g. BAX, BAK) proteins. Previously, it was thought that a balance between these opposing proteins, like a simple 'rheostat', could control the sensitivity of cells to apoptotic stresses. Later, this rheostat concept had to be extended, when it became clear that BCL-2 family proteins regulate each other through a complex network of bimolecular interactions, some transient and some relatively stable. Now, studies have shown that the apoptotic circuitry is even more sophisticated, in that BCL-2 family interactions are spatially dynamic, even in nonapoptotic cells. For example, BAX and BCL-XL can shuttle between the cytoplasm and the mitochondrial outer membrane (MOM). Upstream signaling pathways can regulate the cytoplasmic-MOM equilibrium of BAX and thereby adjust the sensitivity of cells to apoptotic stimuli. Thus, we can view the MOM as the central locale of a dynamic life-death rheostat. BAX invariably forms extensive homo-oligomers after activation in membranes. However, recent studies, showing that activated BAX monomers determine the kinetics of MOM permeabilization (MOMP), perturb the lipid bilayer and form nanometer size pores, pose questions about the role of the oligomerization. Other lingering questions concern the molecular mechanisms of BAX redistribution between MOM and cytoplasm and the details of BAX/BAK-membrane assemblies. Future studies need to delineate how BCL-2 family proteins regulate MOMP, in concert with auxiliary MOM proteins, in a dynamic membrane environment. Technologies aimed at elucidating the structure and function of the full-length proteins in membranes are needed to illuminate some of these critical issues.
[本文引用: 1]
DOI:10.1134/S0006297915110012URL [本文引用: 1]
DOI:10.1152/ajpendo.00477.2011URL [本文引用: 1]
DOI:10.1016/j.theriogenology.2014.08.003PMID:25284282 [本文引用: 1]

Accumulating researches show that thyroid hormone (TH) inhibits Sertoli cells (SCs) proliferation and stimulates their functional maturation in prepubertal rat testis, confirming that TH plays a key role in testicular development. However, the mechanism under the T3 regulation of piglet SC proliferation remains unclear. In the present study, in order to investigate the possible mechanism of T3 on the suppression of SC proliferation, the expression pattern of TRα1 and cell cycle-related molecules, effect of T3 on SC proliferation, and the role of phosphoinositide 3-kinase (PI3K)/Akt signaling pathway on the T3-mediated SC proliferation in piglet testis were explored. Our results demonstrated that TRα1 was expressed in all tested stages of SCs and decreased along with the ages. T3 inhibited the proliferation of SCs in a time- and dose-dependent manner, and T3 treatment downregulated the expressions of cell cycling molecules, such as cyclinA2, cyclinD1, cyclinE1, PCNA, and Skp2, but upregulated the p27 expression in SCs. Most importantly, the suppressive effects of T3 on SC proliferation seemed dependent on the inhibition of PI3K/Akt signaling pathway, and pre-stimulation of PI3K could enhance such suppressive effects. Together, our findings demonstrate that TH inhibits the proliferation of piglet SCs via the suppression of PI3K/Akt signaling pathway. Copyright © 2015 Elsevier Inc. All rights reserved.
DOI:10.1016/j.theriogenology.2019.04.025URL [本文引用: 1]
DOI:10.1177/1933719116649696URL [本文引用: 1]
DOI:10.1080/15384101.2017.1380130URL [本文引用: 1]
DOI:10.3389/fgene.2020.581593URL [本文引用: 1]
