 ,1, 郭磊2, 韩之明
,1, 郭磊2, 韩之明 ,1,3
,1,3Roles of NEK family in cell cycle regulation
Yuanyuan Li ,1, Lei Guo2, Zhiming Han
,1, Lei Guo2, Zhiming Han ,1,3
,1,3通讯作者: 韩之明,博士,副研究员,专业方向:发育生物学。E-mail:hanzm@ioz.ac.cn
编委: 史庆华
收稿日期:2021-03-27修回日期:2021-05-12网络出版日期:2021-07-20
| 基金资助: |
Received:2021-03-27Revised:2021-05-12Online:2021-07-20
| Fund supported: |
作者简介 About authors
李园园,博士,专业方向:发育生物学。E-mail:

摘要
NIMA相关激酶(NIMA-related kinases, NEKs)是丝氨酸/苏氨酸激酶,在细胞周期调控中发挥着重要的作用,参与了中心体分离、纺锤体组装、染色质凝集、核膜破裂、纺锤体组装检验点信号、胞质分裂、纤毛形成及DNA损伤反应等多种细胞活动。本文结合近年来在该激酶家族的相关研究,从NEK家族的组成、结构特征及其在有丝分裂和减数分裂过程中的作用等多个方面展开综述,以期为进一步研究NEKs在细胞周期调控中的作用提供重要基础,也为肿瘤的临床诊断和治疗提供理论依据。
关键词:
Abstract
As a serine/threonine kinase, NIMA-related kinases (NEKs) play important roles in the regulation of cell cycle, and involve in several cellular activities such as centrosome separation, spindle assembly, chromatin condensation, nuclear envelope breakdown, spindle assembly checkpoint signaling, cytokinesis, cilia formation and DNA damage response. In this review, we summarize the component, structural characteristics and functions of NEK family in mitosis and meiosis based on the relevant researches in recent years, providing a reference for the further study on the roles of NEKs in the regulation of cell cycle and a theoretical basis for the clinical diagnosis and treatment of tumors.
Keywords:
PDF (608KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
李园园, 郭磊, 韩之明. NEK家族在细胞周期调控中的作用. 遗传[J], 2021, 43(7): 642-653 doi:10.16288/j.yczz.20-421
Yuanyuan Li.
细胞是生命活动的基本单位。细胞周期是一个非常复杂和精细的调节过程,该过程受到细胞内外各种因素的精密调控,细胞周期的紊乱与许多疾病的发生发展相关。研究显示,许多蛋白激酶家族,如细胞周期蛋白依赖性激酶(cyclin-dependent kinases, CDK)、Aurora激酶、Polo样激酶(polo-like kinase, PLK)和NIMA相关激酶(NIMA-related kinases, NEKs),都参与了细胞周期调控的过程。近年的研究发现,NEK家族蛋白在细胞周期调控的过程中扮演了重要的角色,参与中心体复制和分离、纺锤体形成、染色体在赤道板上的排列、纺锤体检验点(spindle assembly checkpoint,SAC)调控、纤毛形成及DNA损伤反应(DNA damage response, DDR)等多种细胞活动。本文主要综述了NEK家族成员的生物学特性及其在细胞周期调控中的作用,同时对NEK家族的未来研究方向进行了探讨,以期让相关科研人员更充分、更全面地了解NEK家族的研究进展,为进一步研究其在细胞周期调控中的作用提供有力的支撑,也为深入了解肿瘤发生机制及抗肿瘤药物设计提供研究基础。
1 NEK家族及其生物学特性
1.1 NEK家族的发现
NIMA (never in mitosis A)最早是在对曲霉属真菌Aspergillus nidulans的有丝分裂突变体的研究中发现的[1,2]。20世纪80年代中期,Osmani等[3]通过调控nimA基因的mRNA表达水平证明nimA参与了曲霉有丝分裂的G2/M期转换。进一步的研究证明,nimA的过表达可以促进有丝分裂的提前发生,有丝分裂过程中NIMA与CDK1-cyclin B复合体是同等重要的调节因子[4,5]。在对曲霉的研究中发现,NIMA的降解是细胞完成正确的有丝分裂进程所必需的[6]。一系列的研究表明,NIMA激酶在曲霉和酵母(Schizosaccharomyces pombe)中参与了染色质凝集、纺锤体组装和胞质分裂等多个细胞周期过程[7,8,9,10,11]。20世纪90年代初期,Letwin等[12]从小鼠(Mus musculus)中分离出Nek1,发现Nek1编码一种与NIMA相关的蛋白激酶,在结构、组成和表达上与NIMA存在较高的一致性,从而提出了在哺乳动物中可能存在一个Nek基因家族。随后的研究发现了小鼠和人(Homo sapiens)的细胞中均存在与Nek1相关的基因,证明了高等哺乳动物确实存在NEK家族[13,14]。研究已证明,NEKs存在于多种生物体中,从原生生物如衣藻(Chlamydomonas)[15]、疟原虫(Plasmodium falciparum)[16]等到多细胞真核生物如果蝇(Drosophila melanogaster)[17]、非洲爪蟾(Xenopus laevis)[18]、小鼠[13]和人[14]。1.2 NEK家族成员的结构特征
人类NEK家族由11种NIMA相关激酶组成[19,20],这些激酶具有与曲霉NIMA相似的氨基末端催化区域,是含有典型的丝氨酸/苏氨酸激酶序列的高度保守的激酶结构域,其氨基末端和羧基末端的调节结构域在序列组成和长度上有显著差异。一般来讲,NEK家族的氨基末端激酶区域是中度保守的,与NIMA的激酶区域的氨基酸序列有40%~50%的同源性。NEK10的激酶区域位于整个氨基酸序列的中段,与NEK家族典型的氨基末端催化区域不同。在NEK家族中,人NEK2和NIMA的同源性最高,能达到44%[21]。除此之外,NEK6和NEK7的激酶区域的序列一致性达到了85%以上[22]。人类NEK家族的催化区域均含有一个His-Arg-Asp(HRD)基序,在激活环中都有一个丝氨酸/苏氨酸残基,而这个残基很可能是激活修饰的作用位点。在一些NEK家族成员中,这个残基是自磷酸化的,而其他成员则是通过一个上游激酶进行磷酸化修饰的[23,24,25,26]。就磷酸化识别序列而言,NIMA的第3位残基具有对苯丙氨酸的强烈偏好[27],人类NEK家族也具有相似的偏好,例如NEK2和NEK6的第3位残基更喜欢疏水残基,尤其偏爱苯丙氨酸或亮氨酸[28,29]。NEK家族成员具有保守的氨基末端催化区域,而羧基末端区域在长度、序列和结构上都存在很大差异(图1)。其常见的特点就是寡聚化序列,通常是一种卷曲螺旋结构,可通过自磷酸化而被激活。一般而言,自磷酸化通常是在激酶结构域的激活环内进行,但是也可发生在蛋白质的其他区域,例如NEK8和NEK9羧基末端的非催化区域可以通过自磷酸化调控自身的定位和激活[23,30]。研究发现,包括曲霉NIMA和脊椎动物NEK2在内的几种NEKs均显示在非催化区域内存在靶向蛋白质降解的破坏基序[6,31],例如NEK2含有一个KEN (Lys-Glu-Asn)- box和羧基末端MR (methionine-arginine dipeptide)- tail,均能被后期促进复合物/环状体(anaphase- promoting complex/cyclosome, APC/C)所识别,其中MR-tail还可介导NEK2与APC/C的核心亚基CDC20直接作用,从而导致NEK2以一种不依赖于纺锤体组装检验点的方式进行降解[32]。在NEK家族中,NEK6和NEK7仅由一个催化区域和短的氨基末端延伸区域组成[33,34],而后者可能与底物识别有关[35]。NEK6和NEK7是NEK9的下游激酶,可以和NEK9蛋白中RCC1域和coiled-coil域之间的一个序列结合[25]。
图1
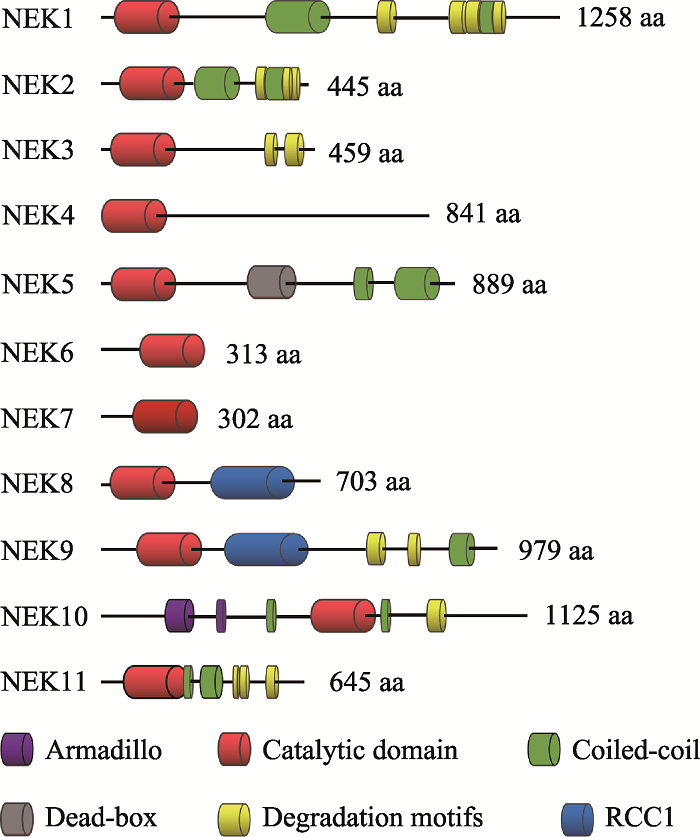 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1人NEK家族的结构特征
Fig. 1The structural features of the human NEK family
2 NEK家族在细胞周期调控中的作用
作为蛋白激酶,NEK家族参与了细胞周期、细胞分裂、纤毛形成和DNA损伤反应等多种细胞活动(表1)。人和哺乳动物NEK家族在细胞有丝分裂和减数分裂过程中的作用主要有以下几个方面。Table 1
表1
表1人和哺乳动物NEK家族的亚细胞定位和功能
Table 1
| NEK | 亚细胞定位 | 功能 |
|---|---|---|
| 1 | 细胞质、中心体、纤毛、DNA损伤部位 | 纤毛发生[36,37]、DNA损伤反应[38,39]、线粒体[39]、纺锤体组装[40] |
| 2 | 中心体 | 有丝分裂[41]、减数分裂[42]、纤毛发生[43,44] |
| 3 | 细胞质 | 催乳素依赖性信号传导[45] |
| 4 | 纤毛基体、纤毛小根 | DNA损伤反应[46]、微管[47]、RNA剪接[48]、纤毛稳定[49] |
| 5 | 细胞质、中心体、纺锤体、线粒体 | 中心体分离[50]、减数分裂恢复[51]、DNA损伤反应[52]、成肌分化[53]、线粒体[54,55] |
| 6 | 细胞质、纺锤体、中心体 | 有丝分裂[24,56,57]、DNA损伤反应[58]、炎症反应[59]、中心体分离[23,60] |
| 7 | 纺锤体两极 | 中心体分离[23,60]、炎症反应[61,62,63]、DNA损伤反应[64,65]、纺锤体组装[56,66,67] |
| 8 | 中心体、纤毛 | 纤毛发生[68,69]、DNA损伤反应[70,71] |
| 9 | 中心体, 纺锤体两极 | 纺锤体形成[72]、中心体成熟和分离[73]、DNA损伤反应[74] |
| 10 | 线粒体 | 细胞周期[75]、DNA损伤反应[75]、线粒体[76]、纤毛发生[77] |
| 11 | 细胞核、核仁、纺锤体 | 细胞周期[78,79]、DNA损伤反应[80]、细胞不对称分裂[81] |
新窗口打开|下载CSV
2.1 NEK家族在有丝分裂中的作用
nimA的过表达可以诱导处于细胞周期任何阶段的曲霉细胞、酵母细胞、非洲爪蟾卵母细胞或人类细胞进入有丝分裂[82,83],研究发现,人类NEK家族参与细胞周期进程和分化过程中的多个事件。在有丝分裂中,NEK2、NEK6、NEK7和NEK9相互配合调控双极纺锤体的形成、染色质凝集、核膜破裂和胞质分裂等。NEK3除参与调控有丝分裂外,还可促进催乳素依赖性信号传导[45],而NEK1、NEK4、NEK5、NEK7、NEK8、NEK10和NEK11均与DNA损伤应答有关。2.1.1 有丝分裂起始
有丝分裂的起始和退出是由CDK1、cyclins、有丝分裂相关激酶和磷酸酶驱动的细胞周期转换。在高等真核生物中,有丝分裂的起始导致多个细胞结构的改变,例如中心体分离、微管生长和收缩、核膜破裂以及染色质凝集等[84]。尽管没有研究证明NEK家族是有丝分裂起始所必需的,但是已确定NEK2、NEK6、NEK7和NEK9参与调控了细胞从间期进入M期的中心体的分离、纺锤体的组装、核孔复合物的去组装和核膜破裂等。
研究发现,一些NEK家族成员在从真菌到人类的微管组织中心均有定位[9,17,85~87]。在人类细胞中,NEK2作为中心体的核心组分,参与调控中心体的分离[41,88,89]。在有丝分裂间期,两个中心粒由一些蛋白质连接体结合在一起,而该连接体是由卷曲螺旋蛋白组成的,包括C-Nap1、rootletin、Cep68、centlein和LRRC45,而NEK2不仅可通过磷酸化连接蛋白[90,91,92,93,94]和中心粒相关蛋白GAS2L1[95,96],还可通过失活驱动蛋白KIFC3[97],共同调控有丝分裂前期的中心体分离和双极纺锤体形成。在有丝分裂间期,NEK2与蛋白激酶MST-2和磷酸酶PP1形成三聚体结构,维持在一个去磷酸化的失活状态。当有丝分裂启动时,PLK1可通过磷酸化MST-2破坏这种结构,导致NEK2的激活。除此之外,NEK2也可通过自磷酸化而被激活[98]。在有丝分裂过程中,NEK5与NEK2的定位模式相似。人NEK5基因的敲降导致分裂间期NEK2减少、中心粒周围物质(pericentriolar material, PCM)缺失、微管生长缓慢以及中心体连接蛋白rootletin被过度募集到中心体上,从而导致中心体的过早分离,分离的中心体之间相对较接近[50],这个现象与过表达人NEK2基因的结果是一致的[41,91],而且同时敲降NEK5和NEK2基因后中心体的过早分离被加重。我们推测,NEK5可能与NEK2协同调控中心体的分离。
研究发现,在有丝分裂的G1期和S期,NEK7可通过调控PCM的募集促进中心体的复制[99]。人NEK7基因的敲降导致PCM组分和原中心粒组装相关蛋白PLK4、CPAP、SAS-6以及STIL不能被募集到中心体,从而调控中心体的复制[100],而人NEK7基因和NEK6基因的过表达能够诱导额外的中心体形成[101]。在有丝分裂中,人NEK6、NEK7和NEK9基因的敲降导致前期中心体的分离失败、分裂中期形成脆弱的纺锤体、纺锤体两极的距离减小以及微管密度降低[23,56,66]。事实上,对于这些纺锤体的缺陷最简单的解释是中心体和纺锤体两极的微管成核作用减少。研究显示,NEK9能与启动微管成核的γ-tubulin环状复合体(γ-tubulin ring complex, γ-TuRC)的多个组分互作,如磷酸化γ-TuRC的衔接蛋白NEDD1[73,102],后者的激活促进了γ-tubulin被募集到中心体上,而Nek9的缺失会导致纺锤体组装延迟、双极纺锤体的形成减少和微管结构异常[102]。此外,NEK6和NEK7均定位到纺锤体两极,NEK6在有丝分裂的中期和后期定位到纺锤体微管上[56],NEK7可将γ-tubulin募集到纺锤体的两极[66]。研究结果提示,这些激酶对微管成核的调控可能不仅是通过纺锤体两极和纺锤体本身,还有可能是通过augmin复合体将γ-TuRCs募集到纺锤体的两极[103]。除此之外,这些激酶调控纺锤体形成的另一种途径可能是通过磷酸化微管相关蛋白进行的,例如Eg5作为一种驱动蛋白,参与了有丝分裂双极纺锤体的形成和维持过程,而Eg5被募集到纺锤体微管上的过程依赖于CDK1对Eg5的磷酸化作用[104,105]。研究发现,NEK6也可磷酸化Eg5[106],这一发现有助于阐明NEK6或NEK9在双极纺锤体的形成和维持中的作用[23,106]。另一项研究显示,EML4作为一种促进微管稳定性的微管相关蛋白参与微管动力学的调控,NEK6和NEK7可通过磷酸化EML4降低其与微管的亲和力,从而促进染色体中板聚合[107]。NEK6和NEK7还可以直接将微管蛋白磷酸化,这一发现提示NEK6和NEK7可能通过磷酸化微管蛋白直接参与微管动力学的调控[56]。这些研究均表明,NEK6、NEK7和NEK9在纺锤体的形成中发挥了重要作用。
NEK2、NEK6、NEK7和NEK9除影响纺锤体形成之外,也发挥其他的功能。例如,NEK2的剪接异构体NEK2C定位在细胞核中,这可能与NEK2在细胞核中的功能有关[108]。研究显示,Nup98是核孔复合体(nuclear pore complexes, NPCs)的组成成分,CDK1和NIMA可磷酸化Nup98,从而促进Nup98从NPCs的解离。CDK1还可磷酸化NEK9的Ser869位点,进而激活NEK9,而NEK6和NEK7可通过与激活的NEK9结合而被激活[23]。因此,我们推测NEKs也可能参与NPCs的解体和核膜破裂[109]。除此之外,NEK9还可与BICD2相互作用。而BICD2作为一种动粒蛋白相关蛋白,在有丝分裂前期可与动力蛋白结合,促进核孔复合体的去组装[110]。这些研究结果均表明,NEK家族在有丝分裂起始中发挥重要作用。
2.1.2 细胞周期检验点
细胞周期阻滞可发生在细胞周期的G1/S、S期和G2/M期,是由内源性因素(如停滞的复制叉)或者外源性因素(包括紫外线(UV)辐射、电离辐射(IR)、活性氧(ROS)和某些化疗药物)所造成的DNA损伤引起的。细胞周期由一系列的检验点所监控,当DNA出现损伤时,这些检验点蛋白被激活,进而导致细胞周期的延迟或阻滞。检验点的激活是由PIKK (phosphatidylinositol-3 kinase-related kinase)家族成员共济失调毛细血管扩张突变(ataxia telangiectasia mutated, ATM)蛋白和共济失调毛细血管扩张突变与Rad3相关(ataxia telangiectasia mutated and Rad3 related, ATR)蛋白及其效应激酶CHK1/2 (checkpoint kinase 1/2)启动的,ERK1/2 (extracellular signal- regulated kinase 1/2)和p38及其下游激酶MK2 (MAPK activated protein kinase 2)在细胞周期阻滞中也发挥重要作用。在NEK家族中,NEK2和NEK6作为DNA损伤反应的靶点,是受DNA损伤抑制的[58,111],而其他的NEK家族成员在DNA损伤修复中发挥重要作用。
在有丝分裂的G1/S和G2/M转换中,NEK1在DNA损伤修复中起作用[112,113,114,115]。当Nek1敲除的细胞暴露于IR和UV辐射时,CHK1和CHK2不能被激活。此外,NEK1的激活不依赖于ATM和ATR。这些研究结果提示,NEK1可能是作为损伤信号的独立传感器发挥作用。
研究发现,NEK2不仅可与SAC蛋白相互作用,还可促进动粒复合蛋白HEC1的Ser165位点磷酸化[116,117,118]。除此之外,在紊乱的染色体动粒上可检测到磷酸化HEC1 (Ser165)的表达,而HEC1可将MPS1和MAD1/MAD2复合体募集到动粒上[119]。由此推测,NEK2可能参与纺锤体组装检验点SAC蛋白完整性的调控。
研究还发现,NEK8可通过RAD51蛋白和DNA损伤修复调控复制叉的稳定性[71],而NEK10和NEK11参与调控G2/M期的DNA损伤反应检验点。当细胞暴露于UV辐射时,NEK10与MEK1、RAF1形成一个三聚体的结构,NEK10可通过促进MEK1的激活,进而导致G2/M期阻滞和ERK1/2的磷酸化[75],敲降人NEK10基因可以抑制MEK1和ERK1/2的磷酸化。当发生DNA损伤和遗传毒性应激时,NEK11活性显著增加,而当抑制ATM和ATR激酶时,NEK11不能被激活[79,80]。当细胞暴露于IR辐射时,ATR和ATM激活CHK1,CHK1的激活促进NEK11和CDC25A的磷酸化,而NEK11的激活可进一步磷酸化CDC25A,这一过程促进SCF泛素连接酶复合物与CDC25A的结合,从而促进CDC25A的降解,最终导致G2/M期阻滞[79],使细胞有充足的时间进行DNA修复,不会过早进入有丝分裂。
2.1.3 胞质分裂
胞质分裂发生在细胞分裂后期姐妹染色单体分离之后, 是细胞周期和生物个体发育过程中的一个重要环节, 直接关系到遗传物质和细胞质组分能否在2个子细胞中正常分配。胞质分裂是由许多亚细胞结构和生物分子相互协调作用的结果。动物细胞胞质分裂过程主要包括分裂沟的定位、胞质分裂结构收缩的组装、分裂沟的产生和收缩、分裂沟膜泡的融合以及中间体的形成和剪切。
在真核生物中,NEK家族也参与胞质分裂的调控。在裂殖酵母中,Grallert等[11]发现FIN1在胞质分裂中起重要作用。在果蝇中,NEK2定位在有丝分裂后期的中体上,它的过表达可导致actin和anillin在卵裂沟的形成部位发生错位[17]。人NEK2剪接异构体NEK2B的敲降可导致细胞无法完成胞质分裂而形成多核细胞[120]。NEK6和NEK7也定位在有丝分裂后期的中体上,在胞质分裂中NEK6的激酶活性达到最大[56,66,106]。人NEK6或NEK7基因敲降的细胞可成功进入中期,但不能完成胞质分裂,而且人NEK6或NEK7的等位基因突变体细胞也经常出现胞质分裂的失败[56,66]。研究还发现,来自小鼠Nek7敲除胚胎的胚胎成纤维细胞也表现出胞质分裂失败的缺陷[121]。除此之外,NEK6和NEK9还可介导与胞质分裂有关的驱动蛋白MKLP2和KIF14的定位和募集[122]。以上证据均表明,NEK家族可能通过胞质分裂相关因子的定位和活性改变调控胞质分裂[56,122]。
2.2 NEK家族在减数分裂中的作用
如上所述,NEK家族在有丝分裂过程中发挥重要的调节作用。减数分裂作为一种特殊的细胞分裂方式,是真核生物和二倍体生物有性生殖和配子产生所必需的。在减数分裂中,染色体的错误分离有可能导致非整倍体受精卵或后代的产生。与有丝分裂相比,人们对NEK家族在减数分裂中的作用了解较少。近些年的研究发现,一些NEK家族成员,如NEK1、NEK2、NEK5、NEK9和NEK11,在减数分裂中也发挥重要的作用。在哺乳动物生殖细胞中,NEK1高表达,并参与减数分裂中纺锤体形成的调控[36]。在Nek1敲除小鼠的精母细胞和卵母细胞中,第一次减数分裂的纺锤体组装和染色体排列异常,调控纺锤体动力相关蛋白-肌球蛋白X (myosin X, MYO10)和α-adducin的定位和表达改变[64,123,124]。我们推测,NEK1可能通过与MYO10和α-adducin的相互作用调控纺锤体的形成。在小鼠卵母细胞中,NEK2是微管组织中心的组成成分,它的敲降导致第一次减数分裂纺锤体两极的异常和染色体排列异常[42],研究证明centrobin/Nip2是NEK2的作用底物,在微管组织中心发挥重要作用[125,126],而且在卵母细胞中敲降centrobin与敲降Nek2的表型是一致的[42]。这些结果提示,NEK2可能通过磷酸化centrobin参与调控卵母细胞减数分裂I中纺锤体组装。在小鼠精母细胞减数分裂过程中,NEK2可磷酸化染色质结构蛋白HMGA2,通过降低后者与DNA的亲和力调控染色质的凝集[127]。我们最近的一项研究发现,NEK5在减数分裂G2/M转换过程中发挥了重要作用,在Nek5敲降的卵母细胞中MPF活性降低,导致了卵母细胞减数分裂恢复的失败[51]。同时,我们还发现NEK5定位在MI~MII期纺锤体上,推测NEK5也可能参与减数分裂纺锤体的组装。在Nek9敲降的小鼠卵母细胞中,纺锤体组装和染色体排列异常,γ-tubulin在纺锤体两极的定位异常,SAC被激活[128]。在小鼠卵母细胞中敲降Nek11影响了MI期纺锤体的迁移,导致卵母细胞的均等分裂[81]。上述研究结果表明,在生殖细胞中NEK1、NEK2、NEK5和NEK9等是保证减数分裂正常进行和染色体正确分离的关键蛋白,其表达的改变会导致纺锤体组装相关因子的定位和活性改变进而干扰纺锤体组装和减数分裂细胞周期进程。
3 结语与展望
自发现以来,NEK家族一直是细胞生物学的研究热点,研究证明NEK家族在细胞周期调控中发挥着关键的作用,但其在减数分裂中的功能和分子机制还有待于进一步深入的研究。细胞周期高度有序的运转是通过G1/S期转换、G2/M转换和中/后期转换等多个过程的调控来实现的。细胞周期紊乱是肿瘤发生的主要原因,细胞周期相关蛋白的表达异常在肿瘤细胞增殖中扮演着重要角色。因此,对NEK家族的生物学功能及其在细胞周期调控中作用的研究,不仅可以更深入地了解细胞周期过程及调控机制,还有助于阐明NEK家族在肿瘤发生发展中的作用机制,对肿瘤的临床诊断和治疗也具有重要意义。(责任编委: 史庆华)
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1017/S0016672300016049URL [本文引用: 1]
PMID:6339527 [本文引用: 1]

In order to develop a method for obtaining mitotic synchrony in aspergillus nidulans, we have characterized previously isolated heat-sensitive nim mutations that block the nuclear division cycle in interphase at restrictive temperature. After 3.5 h at restrictive temperature the mitotic index of a strain carrying one of these mutations, nimA5, was 0, but when this strain was subsequently shifted from restrictive to permissive temperature the mitotic index increased rapidly, reaching a maximum of 78 percent after 7.5 min. When this strain was examined electron-microscopically, mitotic spindles were absent at restrictive temperature. From these data we conclude that at restrictive temperature nimA5 blocks the nuclear division cycle at a point immediately preceding the initiation of chromosomal condensation and mitotic microtubule assembly, and upon shifting to permissive control over the initiation of microtubule assembly and chromosomal condensation in vivo through a simple temperature shift and, consequently, nimA5 should be a powerful tool for studying these processes. Electron-microscopic examination of spindles of material synchronized in this manner reveals that spindle formation, although very rapid, is gradual in the sense that spindle microtubule numbers increase as spindle formation proceeds.
PMID:3294854 [本文引用: 1]

The temperature-sensitive cell cycle mutation nimA5 causes nuclei of Aspergillus nidulans to be blocked in late G2 at restrictive temperature. Under these conditions the spindle pole body divides but does not separate and the mitotic index drops to zero. If nimA5 is blocked for more than one doubling time and then shifted from restrictive to permissive temperature, nuclei immediately enter mitosis, the mitotic spindle forms, and the chromosomes condense (Oakley, B. R., and N. R. Morris, 1983, J. Cell Biol., 96:1155-8). We have cloned the wild-type nimA gene by DNA-mediated complementation of the nimA5 mutant phenotype and have characterized nimA mRNA expression by Northern blot analysis. The transcript is 3.6 kb in length and is under tight nuclear cycle regulation. In synchronously dividing cells, the levels of nimA mRNA become elevated as cells enter mitosis and drop sharply as cells progress through mitosis. Cells blocked in S-phase with hydroxyurea have very low levels of nimA mRNA. Cells blocked in mitosis, either by the antimitotic agent benomyl or by the cell cycle mutation bimE7, maintain elevated levels of the nimA transcript. These data demonstrate not only that nimA is required for entry into mitosis, but because the transcript is normally expressed cyclically and is under tight cell cycle control, they suggest that nimA may play a regulatory role in the initiation of mitosis.
PMID:3359487 [本文引用: 1]

There may be a causal relationship between expression of the G2-specific gene nimA and mitotic regulation in Aspergillus. To test this relationship we have introduced extra inducible copies of nimA into Aspergillus and determined the effect of nimA overproduction on mitotic regulation. The results show that nimA overexpression causes mitotic induction in less than a cell cycle and maintains chromatin in a condensed state. These effects occur even if cells are first blocked in S phase. Sequence analysis shows that the nimA gene encodes a potential protein kinase. These data indicate that there is indeed a causal relationship between expression of nimA and the regulation of mitosis and further implicate protein phosphorylation in mitotic control.
PMID:1913824 [本文引用: 1]

We show that in Aspergillus nidulans, p34cdc2 tyrosine dephosphorylation accompanies activation of p34cdc2 as an H1 kinase at mitosis. However, the nimA5 mutation arrests cells in G2 with p34cdc2 tyrosine dephosphorylated and fully active as an H1 kinase. Activation of NIMA is therefore not required for p34cdc2 activation. Furthermore, mutation of nimT, which encodes a protein with 50% similarity to fission yeast cdc25, causes a G2 arrest and prevents tyrosine dephosphorylation of p34cdc2 but does not prevent full activation of the NIMA protein kinase. Mitotic activation of p34cdc2 by tyrosine dephosphorylation is therefore not required for activation of NIMA. These data suggest that activation of either the p34cdc2 protein kinase or the NIMA protein kinase alone is not sufficient to initiate mitosis. Parallel activation of both cell cycle-regulated protein kinases is required to trigger mitosis.
PMID:7889945 [本文引用: 2]

NIMA is a cell cycle regulated protein kinase required, in addition to p34cdc2/cyclin B, for initiation of mitosis in Aspergillus nidulans. Like cyclin B, NIMA accumulates when cells are arrested in G2 and is degraded as cells traverse mitosis. However, it is stable in cells arrested in mitosis. NIMA, and related kinases, have an N-terminal kinase domain and a C-terminal extension. Deletion of the C-terminus does not completely inactivate NIMA kinase activity but does prevent functional complementation of a temperature sensitive mutation of nimA, showing it to be essential for function. Partial C-terminal deletion of NIMA generates a highly toxic kinase although the kinase domain alone is not toxic. Transient induction experiments demonstrate that the partially truncated NIMA is far more stable than the full length NIMA protein which likely accounts for its toxicity. Unlike full length NIMA, the truncated NIMA is not degraded during mitosis and this affects normal mitotic progression. Cells arrested in mitosis with non-degradable NIMA are able to destroy cyclin B, demonstrating that the arrest is not due to stabilization of p34cdc2/cyclin B activity. The data establish that NIMA degradation during mitosis is required for correct mitotic progression in A. nidulans.
DOI:10.1242/jcs.111.7.967URL [本文引用: 1]
PMID:9647650 [本文引用: 1]

NIMA promotes entry into mitosis in late G2 by some mechanism that is after activation of the Aspergillus nidulans G2 cyclin-dependent kinase, NIMXCDC2/NIMECyclin B. Here we present two independent lines of evidence which indicate that this mechanism involves control of NIMXCDC2/NIMECyclin B localization. First, we found that NIMECyclin B localized to the nucleus and the nucleus-associated organelle, the spindle pole body, in a NIMA-dependent manner. Analysis of cells from asynchronous cultures, synchronous cultures, and cultures arrested in S or G2 showed that NIMECyclin B was predominantly nuclear during interphase, with maximal nuclear accumulation in late G2. NIMXCDC2 colocalized with NIMECyclin B in G2 cells. Although inactivation of NIMA using either the nimA1 or nimA5 temperature-sensitive mutations blocked cells in G2, NIMXCDC2/NIMECyclin B localization was predominantly cytoplasmic rather than nuclear. Second, we found that nimA interacts genetically with sonA, which is a homologue of the yeast nucleocytoplasmic transporter GLE2/RAE1. Mutations in sonA were identified as allele-specific suppressors of nimA1. The sonA1 suppressor alleviated the nuclear division and NIMECyclin B localization defects of nimA1 cells without markedly increasing NIMXCDC2 or NIMA kinase activity. These results indicate that NIMA promotes the nuclear localization of the NIMXCDC2/ NIMECyclin B complex, by a process involving SONA. This mechanism may be involved in coordinating the functions of NIMXCDC2 and NIMA in the regulation of mitosis.
PMID:10975520 [本文引用: 2]

Phosphorylation of histone H3 serine 10 correlates with chromosome condensation and is required for normal chromosome segregation in Tetrahymena. This phosphorylation is dependent upon activation of the NIMA kinase in Aspergillus nidulans. NIMA expression also induces Ser-10 phosphorylation inappropriately in S phase-arrested cells and in the absence of NIMX(cdc2) activity. At mitosis, NIMA becomes enriched on chromatin and subsequently localizes to the mitotic spindle and spindle pole bodies. The chromatin-like localization of NIMA early in mitosis is tightly correlated with histone H3 phosphorylation. Finally, NIMA can phosphorylate histone H3 Ser-10 in vitro, suggesting that NIMA is a mitotic histone H3 kinase, perhaps helping to explain how NIMA promotes chromatin condensation in A. nidulans and when expressed in other eukaryotes.
PMID:12065422 [本文引用: 1]

The Aspergillus nidulans protein kinase NIMA regulates mitotic commitment, while the human and Xenopus equivalents influence centrosome function. Two recessive, temperature-sensitive mutations in the Schizosaccharomyces pombe NIMA homologue, Fin1, blocked spindle formation at 37 degrees C. One of the two spindle pole bodies (SPBs) failed to nucleate microtubules. This phenotype was reduced by accelerating mitotic commitment through genetic inhibition of Wee1 or activation of either Cdc25 or Cdc2. Polo kinase (Plo1) normally associates with the SPB of mitotic, but not interphase cells. cut12.s11 is a dominant mutation in an SPB component that both suppresses cdc25 mutants and promotes Plo1 association with the interphase SPB. Both cut12.s11 phenotypes were abolished by removing Fin1 function. Elevating Fin1 levels promoted Plo1 recruitment to the interphase SPB of wild-type cells and reduced the severity of the cdc25.22 phenotype. These data are consistent with Fin1 regulating Plo1 function during mitotic commitment. The fin1 mitotic commitment and spindle phenotypes resemble distinct nimA phenotypes in different systems and suggest that the function of this family of kinases may be conserved across species.
DOI:10.1101/gad.296204URL [本文引用: 2]
PMID:1382974 [本文引用: 1]

Screening of mouse cDNA expression libraries with antibodies to phosphotyrosine resulted in repeated isolation of cDNAs that encode a novel mammalian protein kinase of 774 amino acids, termed Nek1. Nek1 contains an N-terminal protein kinase domain which is most similar (42% identity) to the catalytic domain of NIMA, a protein kinase which controls initiation of mitosis in Aspergillus nidulans. In addition, both Nek1 and NIMA have a long, basic C-terminal extension, and are therefore similar in overall structure. Despite its identification with anti-phosphotyrosine antibodies, Nek1 contains sequence motifs characteristic of protein serine/threonine kinases. The Nek1 kinase domain, when expressed in bacteria, phosphorylated exogenous substrates primarily on serine/threonine, but also on tyrosine, indicating that Nek1 is a dual specificity kinase with the capacity to phosphorylate all three hydroxyamino acids. Like NIMA, Nek1 preferentially phosphorylated beta-casein in vitro. In situ RNA analysis of nek1 expression in mouse gonads revealed a high level of expression in both male and female germ cells, with a distribution consistent with a role in meiosis. These results suggest that Nek1 is a mammalian relative of the fungal NIMA cell cycle regulator.
DOI:10.1242/jcs.01476URL [本文引用: 2]
PMID:15019993 [本文引用: 2]

The family of human Nek (NIMA Related Kinase) kinases currently contains 11 members. We have identified Nek8 as a new member of the Nek kinase family. For many of the Nek family members, primary tumor expression data and function have been limited. However, all of the Nek family proteins share considerable homology with the Never In Mitosis, gene A (NIMA) kinase from the filamentous fungus Aspergillus nidulans. NIMA, as well as its most closely related human ortholog, Nek2, are required for G(2)/M progression and promote centrosome maturation during mitosis. We isolated Nek8 from a primary human colon cDNA library, and found it to be highly homologous to murine Nek8. Recently, a previously named Nek8 sequence was renamed Nek9/Nercc1 in Genbank due to its lack of homology to murine Nek8 and its high homology to murine Nek9. Interestingly, in our study, phylogenetic analysis suggests that human Nek8 and Nek9 form a subfamily within the Nek family. Nek8 has high homology to the Nek family kinase domain as well as to a regulator of chromosome condensation domain (RCC1), which is also present in Nek9. The open reading frame of human Nek8 encodes a 692 amino-acid protein with a calculated molecular weight of 75 kDa. Nek8 is differently expressed between normal human breast tissue and breast tumors. Overexpression of a mutated kinase domain Nek8 in U2-0S cells led to a decrease in actin protein, and a small increase in the level of cdk1/cyclinB1. Our data demonstrate for the first time that Nek8 is a novel tumor associated gene, and shares considerable sequence homology with the Nek family of protein kinases and may be involved in G(2)/M progression.
[本文引用: 1]
PMID:11322879 [本文引用: 1]

We have cloned Pfnek-1, a gene encoding a novel protein kinase from the human malaria parasite Plasmodium falciparum. This enzyme displays maximal homology to the never-in-mitosis/Aspergillus (NIMA)/NIMA-like kinase (Nek) family of protein kinases, whose members are involved in eukaryotic cell division processes. Similar to other P. falciparum protein kinases and many enzymes of the NIMA/Nek family, Pfnek-1 possesses a large C-terminal extension in addition to the catalytic domain. Bacterially expressed recombinant Pfnek-1 protein is able to autophosphorylate and phosphorylate a panel of protein substrates with a specificity that is similar to that displayed by other members of the NIMA/Nek family. However, the FXXT motif usually found in NIMA/Nek protein kinases is substituted in Pfnek-1 by a SMAHS motif, which is reminiscent of a MAP/ERK kinase (MEK) activation site. Mutational analysis indicates that only one of the serine residues in this motif is essential for Pfnek-1 kinase activity in vitro. We show (a) that recombinant Pfnek-1 is able to specifically phosphorylate Pfmap-2, an atypical P. falciparum MAPK homologue, in vitro, and (b) that coincubation of Pfnek-1 and Pfmap-2 results in a synergistic increase in exogenous substrate labelling. This suggests that Pfnek-1 may be involved in the modulation of MAPK pathway output in malaria parasites. Finally, we demonstrate that recombinant Pfnek-1 can be used in inhibition assays to monitor the effect of kinase inhibitors, which opens the way to the screening of chemical libraries aimed at identifying potential new antimalarials.
PMID:15572022 [本文引用: 3]

The Nima-related kinase 2 (Nek2) has been implicated in the regulation of centrosome integrity and separation in several species and is a candidate for cell transformation. We now show that reduction of levels of the Drosophila Nek2 by RNAi in cultured cells leads to both dispersal of centrosomal antigens and formation of ectopic bodies of centrosomal antigens. Overexpression of the active DmNek2 kinase resulted in an increase in the number of mitotic cells with fragmented centrosomes. The DmNek2 protein kinase is associated with punctuate bodies within the centrosome consistent with its presence on centrioles. In addition, it is present at lower levels on the midbody during cytokinesis. Midbody association was enhanced following overexpression, whereupon the DmNek2 protein kinase also localised to the cell cortex becoming concentrated in the region of the cleavage furrow in late telophase. Many of such cells showed abnormalities in the organisation of anillin and actin in the cleavage furrow that was associated with formation of ectopic membrane protrusions between each daughter cell. We discuss potential roles for DmNek2 in maintaining centrosome integrity in mitosis, during cytokinesis, and consequently for the fidelity of chromosome segregation.
[本文引用: 1]
[本文引用: 1]
DOI:10.1186/1747-1028-6-18URL [本文引用: 1]
DOI:10.1038/sj.onc.1205711URL [本文引用: 1]
PMID:10964517 [本文引用: 1]

Entrance and exit from mitosis in Aspergillus nidulans require activation and proteolysis, respectively, of the NIMA (never in mitosis, gene A) serine/threonine kinase. Four different NIMA-related kinases were reported in mammals (Nek1-4), but none of them has been shown to perform mitotic functions related to those demonstrated for NIMA. We describe here the isolation of two novel murine protein kinase genes, designated nek6 and nek7, which are highly similar to each other (87% amino acid identity in the predicted kinase domain). Interestingly, Nek6 and Nek7 are also highly similar to the F19H6.1 protein kinase of Caenorhabditis elegans (76 and 73% amino acid identity in the kinase domain, respectively), and phylogenetic analysis suggests that these three proteins constitute a novel subfamily within the NIMA family of serine/threonine kinases. In contrast to the other documented NIMA-related kinases, Nek6/7 and F19H6.1 harbor their catalytic domain in the C-terminus of the protein. Immunofluorescence suggests that Nek6 and Nek7 are cytoplasmic. Linkage analysis, using the murine BXD recombinant inbred strain panel, localized nek6 to chromosome 2 at 28 cM. Using a mouse/hamster radiation hybrid panel, we assigned the nek7 gene to chromosome 1 at approximately 73 cM. Copyright 2000 Academic Press.
DOI:10.1038/emboj.2011.179URL [本文引用: 7]
DOI:10.1074/jbc.M303663200URL [本文引用: 2]
DOI:10.1101/gad.972202URL [本文引用: 2]
PMID:17197699 [本文引用: 1]

The dimeric Ser/Thr kinase Nek2 regulates centrosome cohesion and separation through phosphorylation of structural components of the centrosome, and aberrant regulation of Nek2 activity can lead to aneuploid defects characteristic of cancer cells. Mutational analysis of autophosphorylation sites within the kinase domain identified by mass spectrometry shows a complex pattern of positive and negative regulatory effects on kinase activity that are correlated with effects on centrosomal splitting efficiency in vivo. The 2.2-A resolution x-ray structure of the Nek2 kinase domain in complex with a pyrrole-indolinone inhibitor reveals an inhibitory helical motif within the activation loop. This helix presents a steric barrier to formation of the active enzyme and generates a surface that may be exploitable in the design of specific inhibitors that selectively target the inactive state. Comparison of this "auto-inhibitory" conformation with similar arrangements in cyclin-dependent kinase 2 and epidermal growth factor receptor kinase suggests a role for dimerization-dependent allosteric regulation that combines with autophosphorylation and protein phosphatase 1c phosphatase activity to generate the precise spatial and temporal control required for Nek2 function in centrosomal maturation.
PMID:8120013 [本文引用: 1]

NIMA is a cell cycle-regulated protein kinase required for the G2/M transition in the filamentous fungus Aspergillus nidulans. Previous biochemical characterization of the recombinant enzyme indicated that NIMA is a protein serine/threonine specific kinase with beta-casein being the best substrate from the many proteins and peptides tested (Lu, K.P., Osmani, S.A., and Means, A.R. (1993) J. Biol. Chem. 268, 8769-8776). However, substrate specificity or physiologically relevant substrates for NIMA remained unknown. In search for a peptide substrate for this enzyme, we screened an assembled library of synthetic peptides that each contained a phosphorylation site for a known protein kinase and found an excellent peptide substrate for NIMA, phospholemman 42-72 (PLM(42-72)). NIMA kinase phosphorylated PLM(42-72) uniquely and stoichiometrically on Ser63 with a Vmax of 1.4 mumol/min/mg and apparent Km of 20.0 microM. These kinetic constants were about 10-fold higher and 3-fold lower than those for beta-casein, respectively. A detailed analysis of substrate specificity determinants using synthetic peptide analogs of PLM(42-72) indicated that Phe-Arg-Xaa-Ser/Thr represents the optimal primary sequence for NIMA kinase phosphorylation. Replacement of the Arg at P-2 with Ala resulted in a 6-fold increase in Km and 2-fold decrease in Vmax, while substitution of the Phe at P-3 with Ala abolished NIMA phosphorylation. These results reveal the unique nature of substrate recognition by the NIMA kinase and should prove valuable in the search for biologically relevant NIMA substrates.
[本文引用: 1]
PMID:12023960 [本文引用: 1]

The AGC family of protein kinases, which includes isoforms of protein kinase B (also known as Akt), ribosomal S6 protein kinase (S6K), and serum- and glucocorticoid-induced protein kinase (SGK) are activated in response to many extracellular signals and play key roles in regulating diverse cellular processes. They are activated by the phosphorylation of the T loop of their kinase domain by the 3-phosphoinositide-dependent protein kinase-1 and by phosphorylation of a residue located C-terminal to the kinase domain in a region termed the hydrophobic motif. Recent work has implicated the NIMA (never in mitosis, gene A)-related kinase-6 (NEK6) as the enzyme that phosphorylates the hydrophobic motif of S6K1 in vivo. Here we demonstrate that in addition to phosphorylating S6K1 and SGK1 at their hydrophobic motif, NEK6 also phosphorylates S6K1 at two other sites and phosphorylates SGK1 at one other site in vitro. Employing the Jerini pepSTAR method in combination with kinetic analysis of phosphorylation of variant peptides, we establish the key substrate specificity determinants for NEK6. Our analysis indicates that NEK6 has a strong preference for Leu 3 residues N-terminal to the site of phosphorylation. Its mutation to either Ile or Val severely reduced the efficacy with which NEK6-phosphorylated peptide substrates, and moreover, mutation of the equivalent Leu residue in S6K1 or SGK1 prevented phosphorylation of their hydrophobic motifs by NEK6 in vitro. However, these mutants of S6K1 or SGK1 still became phosphorylated at their hydrophobic motif following insulin-like growth factor-1 stimulation of transfected 293 cells. This study provides the first description of the basis for the substrate specificity of NEK6 and indicates that NEK6 is unlikely to be responsible for the IGF1-induced phosphorylation of the hydrophobic motif of S6K, SGK, and protein kinase B isoforms in vivo.
DOI:10.1093/hmg/ddr544URL [本文引用: 1]
PMID:11742988 [本文引用: 1]

Nek2 is a NIMA-related kinase implicated in regulating centrosome structure at the G(2)/M transition. Two splice variants have been identified that exhibit distinct patterns of expression during cell cycle progression and development. Here we show that Nek2A, but not Nek2B, is destroyed upon entry into mitosis coincident with cyclin A destruction and in the presence of an active spindle assembly checkpoint. Destruction of Nek2A is mediated by the proteasome and is dependent upon the APC/C-Cdc20 ubiquitin ligase. Nek2 activity is not required for APC/C activation. Nek2A destruction in early mitosis is regulated by a motif in its extreme C-terminus which bears a striking resemblance to the extended destruction box (D-box) of cyclin A. Complete stabilization of Nek2A requires deletion of this motif and mutation of a KEN-box. Destruction of Nek2A is not inhibited by the cyclin B-type D-box, but the C-terminal domain of Nek2A inhibits destruction of both cyclins A and B. We propose that recognition of substrates by the APC/C-Cdc20 in early mitosis depends upon possession of an extended D-box motif.
DOI:10.1038/ncb1410URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
PMID:11701951 [本文引用: 1]

Neks (NIMA-related kinase) are a group of protein kinases sharing high amino acid sequence identity with NIMA which controls initiation of mitosis in Aspergillus nidulans. We have identified and characterized human NEK7, a novel human gene structurally related to NIMA. Its open reading frame encodes a 302-amino acid protein and is 77% identical to human NEK6 protein. Phylogenetic analysis suggests that NEK6, NEK7, and Caenorhabditis elegans F19H6.1 constitute a subfamily within the NIMA family of protein kinases. Tissue distributions of NEK7 and NEK6 were studied by RT-PCR. NEK7 expression was restricted to a subset of tissues containing lung, muscle, testis, brain, heart, liver, leukocyte, and spleen, but NEK6 transcripts were detected in all tissues studied. The human NEK7 gene was assigned to human chromosome 1 by somatic cell hybrids and 1q31.3 by radiation hybrid mapping. Copyright 2001 S. Karger AG, Basel
DOI:10.1021/pr100562wPMID:20873783 [本文引用: 1]

Physical protein-protein interactions are fundamental to all biological processes and are organized in complex networks. One branch of the kinome network is the evolutionarily conserved NIMA-related serine/threonine kinases (Neks). Most of the 11 mammalian Neks studied so far are related to cell cycle regulation, and due to association with diverse human pathologies, Neks are promising chemotherapeutic targets. Human Nek6 was associated to carcinogenesis, but its interacting partners and signaling pathways remain elusive. Here we introduce hNek6 as a highly connected member in the human kinase interactome. In a more global context, we performed a broad data bank comparison based on degree distribution analysis and found that the human kinome is enriched in hubs. Our networks include a broad set of novel hNek6 interactors as identified by our yeast two-hybrid screens classified into 18 functional categories. All of the tested interactions were confirmed, and the majority of tested substrates were phosphorylated in vitro by hNek6. Notably, we found that hNek6 N-terminal is important to mediate the interactions with its partners. Some novel interactors also colocalized with hNek6 and γ-tubulin in human cells, pointing to a possible centrosomal interaction. The interacting proteins link hNek6 to novel pathways, for example, Notch signaling and actin cytoskeleton regulation, or give new insights on how hNek6 may regulate previously proposed pathways such as cell cycle regulation, DNA repair response, and NF-κB signaling. Our findings open new perspectives in the study of hNek6 role in cancer by analyzing its novel interactions in specific pathways in tumor cells, which may provide important implications for drug design and cancer therapy.
DOI:10.1073/pnas.97.1.217URL [本文引用: 2]
DOI:10.1016/j.ajhg.2010.12.004URL [本文引用: 1]
DOI:10.1038/s41598-017-05325-wPMID:28710492 [本文引用: 1]

NEK family kinases are serine/threonine kinases that have been functionally implicated in the regulation of the disjunction of the centrosome, the assembly of the mitotic spindle, the function of the primary cilium and the DNA damage response. NEK1 shows pleiotropic functions and has been found to be mutated in cancer cells, ciliopathies such as the polycystic kidney disease, as well as in the genetic diseases short-rib thoracic dysplasia, Mohr-syndrome and amyotrophic lateral sclerosis. NEK1 is essential for the ionizing radiation DNA damage response and priming of the ATR kinase and of Rad54 through phosphorylation. Here we report on the structure of the kinase domain of human NEK1 in its apo-and ATP-mimetic inhibitor bound forms. The inhibitor bound structure may allow the design of NEK specific chemo-sensitizing agents to act in conjunction with chemo-or radiation therapy of cancer cells. Furthermore, we characterized the dynamic protein interactome of NEK1 after DNA damage challenge with cisplatin. Our data suggest that NEK1 and its interaction partners trigger the DNA damage pathways responsible for correcting DNA crosslinks.
DOI:10.1080/15384101.2019.1711317URL [本文引用: 2]
DOI:10.1371/journal.pone.0185780URL [本文引用: 1]
PMID:9430639 [本文引用: 3]

Nek2, a mammalian protein kinase of unknown function, is closely related to the mitotic regulator NIMA of Aspergillus nidulans. Here we show by both immunofluorescence microscopy and biochemical fractionation that human Nek2 localizes to the centrosome. Centrosome association occurs throughout the cell cycle, including all stages of mitosis, and is independent of microtubules. Overexpression of active Nek2 induces a striking splitting of centrosomes, whereas prolonged expression of either active or inactive Nek2 leads to dispersal of centrosomal material and loss of a focused microtubule-nucleating activity. Surprisingly, this does not prevent entry into mitosis, as judged by the accumulation of mitotically arrested cells induced by co-expression of a non-destructible B-type cyclin. These results bear on the dynamic function of centrosomes at the onset of mitosis. Moreover, they indicate that one function of mammalian Nek2 relates to the centrosome cycle and thus provide a new perspective on the role of NIMA-related kinases.
DOI:10.1017/S0967199410000183URL [本文引用: 3]
DOI:10.1242/dev.126953PMID:26493400 [本文引用: 1]

Vertebrate left-right (LR) asymmetry originates at a transient left-right organizer (LRO), a ciliated structure where cilia play a crucial role in breaking symmetry. However, much remains unknown about the choreography of cilia biogenesis and resorption at this organ. We recently identified a mutation affecting NEK2, a member of the NIMA-like serine-threonine kinase family, in a patient with congenital heart disease associated with abnormal LR development. Here, we report how Nek2 acts through cilia to influence LR patterning. Both overexpression and knockdown of nek2 in Xenopus result in abnormal LR development and reduction of LRO cilia count and motility, phenotypes that are modified by interaction with the Hippo signaling pathway. nek2 knockdown leads to a centriole defect at the LRO, consistent with the known role of Nek2 in centriole separation. Nek2 overexpression results in premature ciliary resorption in cultured cells dependent on function of the tubulin deacetylase Hdac6. Finally, we provide evidence that the known interaction between Nek2 and Nup98, a nucleoporin that localizes to the ciliary base, is important for regulating cilium resorption. Together, these data show that Nek2 is a switch balancing ciliogenesis and resorption in the development of LR asymmetry. © 2015. Published by The Company of Biologists Ltd.
DOI:10.1083/jcb.201907136URL [本文引用: 1]
DOI:10.1210/me.2004-0443URL [本文引用: 2]
DOI:10.1128/MCB.00436-12URL [本文引用: 1]
DOI:10.1158/0008-5472.CAN-09-2113URL [本文引用: 1]
DOI:10.1186/s12953-015-0065-6URL [本文引用: 1]
DOI:10.1093/hmg/ddr280URL [本文引用: 1]
DOI:10.1083/jcb.201412099URL [本文引用: 2]
DOI:10.1002/mrd.v86.9URL [本文引用: 2]
DOI:10.1002/jcb.28943PMID:31090963 [本文引用: 1]

Cells are daily submitted to high levels of DNA lesions that trigger complex pathways and cellular responses by cell cycle arrest, apoptosis, alterations in transcriptional response, and the onset of DNA repair. Members of the NIMA-related kinase (NEK) family have been related to DNA damage response and repair and the first insight about NEK5 in this context is related to its role in centrosome separation resulting in defects in chromosome integrity. Here we investigate the potential correlation between NEK5 and the DNA damage repair index. The effect of NEK5 in double-strand breaks caused by etoposide was accessed by alkaline comet assay and revealed that NEK5-silenced cells are more sensitive to etoposide treatment. Topoisomerase IIβ (TOPIIβ) is a target of etoposide that leads to the production of DNA breaks. We demonstrate that NEK5 interacts with TOPIIβ, and the dynamics of this interaction is evaluated by proximity ligation assay. The complex NEK5/TOPIIβ is formed immediately after etoposide treatment. Taken together, the results of our study reveal that NEK5 depletion increases DNA damage and impairs proper DNA damage response, pointing out NEK5 as a potential kinase contributor to genomic stability. © 2019 Wiley Periodicals, Inc.
DOI:10.1016/j.febslet.2013.05.049PMID:23727203 [本文引用: 1]

Accumulating evidence suggests that caspase-3-mediated cleavage of protein kinase could be a key event to regulate cell differentiation. In this study, we investigated the role of Nek5 kinase, identified as a novel substrate for caspase-3, in skeletal muscle differentiation. Up-regulation of Nek5 mRNA expression was accompanied by cell differentiation. Myotube formation was promoted in Nek5 expressing cells, and was conversely inhibited in Nek5 knockdown cells. Furthermore, we found that caspase-3 activity, an important factor for myogenic differentiation, was enhanced by Nek5 cleavage. Although caspase-3-cleaved Nek5 partially exerted a promyogenic effect, it tended to induce apoptotic cell death. In summary, our findings suggest that Nek5 promotes myogenic differentiation through up-regulation of caspase activity. Copyright © 2013. Published by Elsevier B.V.
DOI:10.1002/feb4.v11.3URL [本文引用: 1]
DOI:10.1016/j.cellsig.2015.02.021PMID:25725288 [本文引用: 1]

Mitochondria are involved in energy supply, signaling, cell death and cellular differentiation and have been implicated in several human diseases. Neks (NIMA-related kinases) represent a family of mammal protein kinases that play essential roles in cell-cycle progression, but other functions have recently been related. A yeast two-hybrid (Y2H) screen was performed to identify and characterize Nek5 interaction partners and the mitochondrial proteins Cox11, MTX-2 and BCLAF1 were retrieved. Apoptosis assay showed protective effects of stable hNek5 expression from Hek293-T's cell death after thapsigargin treatment (2 μM). Nek5 silenced cells as well as cells expressing a "kinase dead" version of Nek5, displayed an increase in ROS formation after 4 h of thapsigargin treatment. Mitochondrial respiratory chain activity was found decreased upon stable hNek5expression. Cells silenced for hNek5 on the other hand presented 1.7 fold increased basal rates of respiration, especially at the electrons transfer steps from TMPD to cytochrome c and at the complex II. In conclusion, our data suggest for the first time mitochondrial localization and functions for Nek5 and its participation in cell death and cell respiration regulation. Stable expression of hNek5 in Hek293T cells resulted in enhanced cell viability, decreased cell death and drug resistance, while depletion of hNek5by shRNA overcame cancer cell drug resistance and induced apoptosis in vitro. Stable expression of hNek5 also inhibits thapsigargin promoted apoptosis and the respiratory chain complex IV in HEK293T cells. Copyright © 2015 Elsevier Inc. All rights reserved.
DOI:10.1128/MCB.01867-08URL [本文引用: 8]
DOI:10.1074/jbc.M308080200URL [本文引用: 1]
DOI:10.4161/cc.7.17.6551URL [本文引用: 2]
DOI:10.3892/or.2015.4187PMID:26259750 [本文引用: 1]

Ulcerative colitis (UC) is an important risk factor for colorectal cancer (CRC). Histone modifications are one of the epigenetic mechanisms that may have key roles in the carcinogenesis of CRC. At present, there are no studies comparing histone modification patterns of UC and CRC in the literature. Therefore the aim of the present study was to investigate whether genes, particularly those involved in histone modification, have value in patient monitoring with regards to CRC development in UC. Key gene expressions of the histone modification enzyme were assessed and compared in CRC, UC and control groups using the RT-PCR array technique. Patients were divided into subgroups based on the extent and duration of the disease and inflammatory burden, which are considered risk factors for CRC development in UC patients. In UC and CRC groups, a significantly higher overexpression of the NEK6 and AURKA genes compared to the control group was identified. In addition, there was a significantly higher overexpression of HDAC1 and PAK1 genes in the UC group, and of HDAC1, HDAC7, PAK1 and AURKB genes in the CRC group. NEK6, AURKA, HDAC1 and PAK1 were significantly overexpressed in patients with a longer UC duration. Overexpression of AURKA and NEK6 genes was significantly more pronounced in UC patients with more extensive colon involvement. HDAC1, HDAC7, PAK1, NEK6, AURKA and AURKB are important diagnostic and prognostic markers involved in the carcinogenesis of CRC. HDAC1, PAK1, NEK6 and AURKA may be considered as diagnostic markers to be used in CRC screening for UC patients.
DOI:10.4161/cc.10.22.18226PMID:22064517 [本文引用: 2]
DOI:10.1111/j.1600-065X.2011.01046.xPMID:21884173 [本文引用: 1]

An inflammasome is a multiprotein complex that serves as a platform for caspase-1 activation and caspase-1-dependent proteolytic maturation and secretion of interleukin-1β (IL-1β). Though a number of inflammasomes have been described, the NLRP3 inflammasome is the most extensively studied but also the most elusive. It is unique in that it responds to numerous physically and chemically diverse stimuli. The potent proinflammatory and pyrogenic activities of IL-1β necessitate that inflammasome activity is tightly controlled. To this end, a priming step is first required to induce the expression of both NLRP3 and proIL-1β. This event renders the cell competent for NLRP3 inflammasome activation and IL-1β secretion, and it is highly regulated by negative feedback loops. Despite the wide array of NLRP3 activators, the actual triggering of NLRP3 is controlled by integration a comparatively small number of signals that are common to nearly all activators. Minimally, these include potassium efflux, elevated levels of reactive oxygen species (ROS), and, for certain activators, lysosomal destabilization. Further investigation of how these and potentially other as yet uncharacterized signals are integrated by the NLRP3 inflammasome and the relevance of these biochemical events in vivo should provide new insight into the mechanisms of host defense and autoinflammatory conditions. © 2011 John Wiley & Sons A/S.
DOI:S0896-8411(20)30137-2PMID:32703754 [本文引用: 1]

The nucleotide-binding oligomerization domain (NOD)-like receptor containing pyrin domain 3 (NLRP3) inflammasome is a high-molecular-weight complex mediated by the activation of pattern-recognition receptors (PRRs) seed in innate immunity. Once NLRP3 is activated, the following recruitment of the adapter apoptosis-associated speck-like protein containing a caspase recruitment domain (CARD) (ASC) and procaspase-1 would be initiated. Cleavage of procaspase-1 into active caspase-1 then leads to the maturation of the precursor forms of interleukin (IL)-1β and IL-18 into biologically active IL-1β and IL-18. The activation of NLRP3 inflammasome is thought to be tightly associated with a regulator never in mitosis A (NIMA)-related kinase 7 (NEK7), apart from other signaling events such as K efflux and reactive oxygen species (ROS). Plus, the NLRP3 inflammasome has been linked to various metabolic disorders, chronic in?ammation and other diseases. In this review, we firstly describe the cellular/molecular mechanisms of the NEK7-licensed NLRP3 inflammasome activation. Then we detail the potential inhibitors that can selectively and effectively modulate either the NEK7-NLRP3 complex itself or the related molecular/cellular events. Finally, we describe some inhibitors as promising therapeutic strategies for diverse diseases driven by NLRP3 inflammasome. Copyright © 2020. Published by Elsevier Ltd.
DOI:10.3389/fphys.2020.606996URL [本文引用: 1]
DOI:10.1021/pr500437xURL [本文引用: 2]
831.
[本文引用: 1]
DOI:10.1016/j.bbrc.2007.05.206URL [本文引用: 5]
DOI:10.4161/15384101.2014.994988URL [本文引用: 1]
DOI:10.1681/ASN.2006090985URL [本文引用: 1]
DOI:10.1093/hmg/ddr544URL [本文引用: 1]
DOI:10.1016/j.molcel.2013.08.006URL [本文引用: 1]
DOI:10.1080/15384101.2016.1259038URL [本文引用: 2]
DOI:10.1016/j.bbrc.2013.04.105URL [本文引用: 1]
DOI:10.1016/j.cub.2012.06.027URL [本文引用: 2]
DOI:10.1093/nar/gku840URL [本文引用: 1]
DOI:10.1128/MCB.00648-10URL [本文引用: 3]
DOI:10.1186/s12953-020-00160-wURL [本文引用: 1]
DOI:10.1038/s41467-018-03643-9PMID:29581457 [本文引用: 1]

The primary cilium emanates from the cell surface of growth-arrested cells and plays a central role in vertebrate development and tissue homeostasis. The mechanisms that control ciliogenesis have been extensively explored. However, the intersection between GPCR signaling and the ubiquitin pathway in the control of cilium stability are unknown. Here we observe that cAMP elevation promotes cilia resorption. At centriolar satellites, we identify a multimeric complex nucleated by PCM1 that includes two kinases, NEK10 and PKA, and the E3 ubiquitin ligase CHIP. We show that NEK10 is essential for ciliogenesis in mammals and for the development of medaka fish. PKA phosphorylation primes NEK10 for CHIP-mediated ubiquitination and proteolysis resulting in cilia resorption. Disarrangement of this control mechanism occurs in proliferative and genetic disorders. These findings unveil a pericentriolar kinase signalosome that efficiently links the cAMP cascade with the ubiquitin-proteasome system, thereby controlling essential aspects of ciliogenesis.
PMID:15161910 [本文引用: 1]

We previously reported that Nek11, a member of the NIMA (never-in-mitosis A) family of kinases, is activated in G(1)/S-arrested cells. We provide herein several lines of evidence for a novel interaction between Nek11 and Nek2A. Both Nek11 and Nek2A, but not Nek2B, were detected at nucleoli, and the Nek2A-specific C-terminal end (amino acids 399-445) was responsible for nucleolar localization. Endogenous Nek11 coimmunoprecipitated with endogenous Nek2A, and non-catalytic regions of each kinase were involved in the complex formation. Nek11L interacted with phosphorylated Nek2A but barely with the kinase-inactive Nek2A (K37R) mutant. In addition, both Nek2A autophosphorylation activity and the Nek11L-Nek2A complex formation increased in G(1)/S-arrested cells. These results indicate that autophosphorylation of Nek2A could stimulate its interaction with Nek11L at the nucleolus. Moreover, Nek2 directly phosphorylated Nek11 in the C-terminal non-catalytic region and elevated Nek11 kinase activity. The non-catalytic region of Nek11 showed autoinhibitory activity through intramolecular interaction with its N-terminal catalytic domain. Nek2 dissociated this autoinhibitory interaction. Altogether, our studies demonstrate a unique mechanism of Nek11 activation by Nek2A in G(1)/S-arrested cells and suggest a novel possibility for nucleolar function of the NIMA family.
DOI:10.1038/ncb1969PMID:19734889 [本文引用: 3]

DNA damage-induced cell-cycle checkpoints have a critical role in maintaining genomic stability. A key target of the checkpoints is the CDC25A (cell division cycle 25 homologue A) phosphatase, which is essential for the activation of cyclin-dependent kinases and cell-cycle progression. To identify new genes involved in the G2/M checkpoint we performed a large-scale short hairpin RNA (shRNA) library screen. We show that NIMA (never in mitosis gene A)-related kinase 11 (NEK11) is required for DNA damage-induced G2/M arrest. Depletion of NEK11 prevents proteasome-dependent degradation of CDC25A, both in unperturbed and DNA-damaged cells. We show that NEK11 directly phosphorylates CDC25A on residues whose phosphorylation is required for beta-TrCP (beta-transducin repeat-containing protein)-mediated polyubiquitylation and degradation of CDC25A. Furthermore, we demonstrate that CHK1 (checkpoint kinase 1) directly activates NEK11 by phosphorylating it on Ser 273, indicating that CHK1 and NEK11 operate in a single pathway that controls proteolysis of CDC25A. Taken together, these results demonstrate that NEK11 is an important component of the pathway enforcing the G2/M checkpoint, suggesting that genetic mutations in NEK11 may contribute to the development of human cancer.
PMID:12154088 [本文引用: 2]

DNA replication and genotoxic stresses activate various checkpoint-associated protein kinases, and checkpoint dysfunction often leads to cell lethality. Here, we have identified new members of the mammalian NIMA family of kinases, termed Nek11L and Nek11S (NIMA-related kinase 11 Long and Short isoform) as novel DNA replication/damage stresses-responsive kinases. Molecular cloning and biochemical studies showed that the catalytic domain of Nek11 is most similar to Nek4 and Nek3, and substrate specificity of Nek11L is distinguishable from those of NIMA and Nek2. The expression of nek11L mRNA increased through S to G(2)/M phase, and subcellular localization of Nek11 protein altered between interphase and prometaphase, suggesting multiple roles of Nek11. We found an activation of Nek11 kinase activity when cells were treated with various DNA-damaging agents and replication inhibitors, and this activation of Nek11 was suppressed by caffeine in HeLaS3 cells. The transient expression of wild-type Nek11L enhanced the aphidicolin-induced S-phase arrest, whereas the aphidicolin-induced S-phase arrest was reduced in the U2OS cell lines expressing kinase-negative Nek11L (K61R), and these cells were more sensitive to aphidicolin-induced cell lethality. Collectively, these results suggest that Nek11 has a role in the S-phase checkpoint downstream of the caffeine-sensitive pathway.
DOI:10.1016/j.bbrc.2016.05.002URL [本文引用: 2]
PMID:7957060 [本文引用: 1]

The NIMA protein kinase of Aspergillus nidulans is required for the G2/M transition of the cell cycle. Mutants lacking NIMA arrest without morphological characteristics of mitosis, but they do contain an activated p37nimX kinase (the Aspergillus homologue of p34cdc2). To gain a better understanding of NIMA function we have investigated the effects of expressing various NIMA constructs in Aspergillus, fission yeast and human cells. Our experiments have shown that the instability of the NIMA protein requires sequences in the non-catalytic C-terminus of the protein. Removal of this domain results in a stable protein that, once accumulated, promotes a lethal premature condensation of chromatin without any other aspects of mitosis. Similar effects were also observed in fission yeast and human cells accumulating Aspergillus NIMA. This phenotype is independent of cell cycle progression and does not require p34cdc2 kinase activity. As gain of NIMA function by accumulation results in premature chromatin condensation, and loss of NIMA function results in an inability to enter mitosis, we propose that NIMA functions in G2 to promote the condensation of chromatin normally associated with entry into mitosis.
PMID:7736593 [本文引用: 1]

NIMA is essential for entry into mitosis in Aspergillus nidulans. To examine whether there is a NIMA-like pathway in other eukaryotic cell cycles, we expressed NIMA and its dominant negative mutants in two different eukaryotic systems. In Xenopus oocytes, NIMA induced germinal vesicle breakdown without activating Mos, CDC2, or MAP kinase. In HeLa cells, NIMA induced premature mitotic events without activating CDC2, whereas the mutants caused a specific G2 arrest but did not block mutant CDC2T14AY15F-induced premature entry into mitosis. A sequence essential for both these phenotypes was mapped to a region of approximately 100 amino acids lying just after the catalytic domain of NIMA that shows a significant similarity to protein interaction domains in other proteins. These results provide evidence for the existence of a NIMA-like mitotic pathway in vertebrate cells.
DOI:10.1002/bies.201600057PMID:27231150 [本文引用: 1]

Mitotic entry and exit are switch-like transitions that are driven by the activation and inactivation of Cdk1 and mitotic cyclins. This simple on/off reaction turns out to be a complex interplay of various reversible reactions, feedback loops, and thresholds that involve both the direct regulators of Cdk1 and its counteracting phosphatases. In this review, we summarize the interplay of the major components of the system and discuss how they work together to generate robustness, bistability, and irreversibility. We propose that it may be beneficial to regard the entry and exit reactions as two separate reversible switches that are distinguished by differences in the state of phosphatase activity, mitotic proteolysis, and a dramatic rearrangement of cellular components after nuclear envelope breakdown, and discuss how the major Cdk1 activity thresholds could be determined for these transitions. © 2016 The Authors BioEssays Published by WILEY Periodicals, Inc.
DOI:10.1091/mbc.e05-05-0450URL [本文引用: 1]
DOI:10.1093/emboj/21.7.1713URL
[本文引用: 1]
DOI:10.1038/nature02166URL [本文引用: 1]
DOI:10.1186/1747-1028-2-1URL [本文引用: 1]
DOI:10.1083/jcb.200504107URL [本文引用: 1]
PMID:12857871 [本文引用: 2]

Nek2A is a cell cycle-regulated kinase of the never in mitosis A (NIMA) family that is highly enriched at the centrosome. One model for Nek2A function proposes that it regulates cohesion between the mother and daughter centriole through phosphorylation of C-Nap1, a large coiled-coil protein that localizes to centriolar ends. Phosphorylation of C-Nap1 at the G2/M transition may trigger its displacement from centrioles, promoting their separation and subsequent bipolar spindle formation. To test this model, we generated tetracycline-inducible cell lines overexpressing wild-type and kinase-dead versions of Nek2A. Live cell imaging revealed that active Nek2A stimulates the sustained splitting of interphase centrioles indicative of loss of cohesion. However, this splitting is accompanied by only a partial reduction in centriolar C-Nap1. Strikingly, induction of kinase-dead Nek2A led to formation of monopolar spindles with unseparated spindle poles that lack C-Nap1. Furthermore, kinase-dead Nek2A interfered with chromosome segregation and cytokinesis and led to an overall change in the DNA content of the cell population. These results provide the first direct evidence in human cells that Nek2A function is required for the correct execution of mitosis, most likely through promotion of centrosome disjunction. However, they suggest that loss of centriole cohesion and C-Nap1 displacement may be distinct mitotic events.
PMID:9647649 [本文引用: 1]

Nek2 (for NIMA-related kinase 2) is a mammalian cell cycle-regulated kinase structurally related to the mitotic regulator NIMA of Aspergillus nidulans. In human cells, Nek2 associates with centrosomes, and overexpression of active Nek2 has drastic consequences for centrosome structure. Here, we describe the molecular characterization of a novel human centrosomal protein, C-Nap1 (for centrosomal Nek2-associated protein 1), first identified as a Nek2-interacting protein in a yeast two-hybrid screen. Antibodies raised against recombinant C-Nap1 produced strong labeling of centrosomes by immunofluorescence, and immunoelectron microscopy revealed that C-Nap1 is associated specifically with the proximal ends of both mother and daughter centrioles. On Western blots, anti-C-Nap1 antibodies recognized a large protein (>250 kD) that was highly enriched in centrosome preparations. Sequencing of overlapping cDNAs showed that C-Nap1 has a calculated molecular mass of 281 kD and comprises extended domains of predicted coiled-coil structure. Whereas C-Nap1 was concentrated at centrosomes in all interphase cells, immunoreactivity at mitotic spindle poles was strongly diminished. Finally, the COOH-terminal domain of C-Nap1 could readily be phosphorylated by Nek2 in vitro, as well as after coexpression of the two proteins in vivo. Based on these findings, we propose a model implicating both Nek2 and C-Nap1 in the regulation of centriole-centriole cohesion during the cell cycle.
PMID:16339073 [本文引用: 1]

Rootletin, a major structural component of the ciliary rootlet, is located at the basal bodies and centrosomes in ciliated and nonciliated cells, respectively. Here we investigated its potential role in the linkage of basal bodies/centrioles and the mechanism involved in such linkages. We show that rootletin interacts with C-Nap1, a protein restricted at the ends of centrioles and functioning in centrosome cohesion in interphase cells. Their interaction in vivo is supported by their colocalization at the basal bodies/centrioles and coordinated association with the centrioles during the cell cycle. Ultrastructural examinations demonstrate that rootletin fibers connect the basal bodies in ciliated cells and are present both at the ends of and in between the pair of centrioles in nonciliated cells. The latter finding stands in contrast with C-Nap1, which is present only at the ends of the centrioles. Transient expression of C-Nap1 fragments dissociated rootletin fibers from the centrioles, resulting in centrosome separation in interphase. Overexpression of rootletin in cells caused multinucleation, micronucleation, and irregularity of nuclear shape and size, indicative of defects in chromosome separation. These data suggest that rootletin may function as a physical linker between the pair of basal bodies/centrioles by binding to C-Nap1.
[本文引用: 1]
DOI:10.1083/jcb.201909094URL [本文引用: 1]
DOI:10.1016/j.devcel.2016.11.019URL [本文引用: 1]
DOI:10.1038/s41556-019-0382-6URL [本文引用: 1]
DOI:10.4331/wjbc.v5.i2.141PMID:24921005 [本文引用: 1]

Aside from Polo and Aurora, a third but less studied kinase family involved in mitosis regulation is the never in mitosis-gene A (NIMA)-related kinases (Neks). The founding member of this family is the sole member NIMA of Aspergillus nidulans, which is crucial for the initiation of mitosis in that organism. All 11 human Neks have been functionally assigned to one of the three core functions established for this family in mammals: (1) centrioles/mitosis; (2) primary ciliary function/ciliopathies; and (3) DNA damage response (DDR). Recent findings, especially on Nek 1 and 8, showed however, that several Neks participate in parallel in at least two of these contexts: primary ciliary function and DDR. In the core section of this in-depth review, we report the current detailed functional knowledge on each of the 11 Neks. In the discussion, we return to the cross-connections among Neks and point out how our and other groups' functional and interactomics studies revealed that most Neks interact with protein partners associated with two if not all three of the functional contexts. We then raise the hypothesis that Neks may be the connecting regulatory elements that allow the cell to fine tune and synchronize the cellular events associated with these three core functions. The new and exciting findings on the Nek family open new perspectives and should allow the Neks to finally claim the attention they deserve in the field of kinases and cell cycle biology.
DOI:10.1038/ncb1694URL [本文引用: 1]
DOI:10.1091/mbc.e16-09-0643URL [本文引用: 1]
DOI:10.1242/jcs.078089URL [本文引用: 1]
DOI:10.1091/mbc.e05-04-0315URL [本文引用: 2]
DOI:10.1016/j.ceb.2009.11.012PMID:20022736 [本文引用: 1]

The structure, dynamics, and mechanics of mitotic and meiotic spindles have been progressively elucidated through the advancements in microscopic technology, identification of the genes involved, and construction of theoretical frameworks. Here, we review recent works that have utilized quantitative image analysis to advance our understanding of the complex spindle structure of animal cells. In particular, we discuss how microtubules (MTs) are nucleated and distributed inside the spindle. Accumulating evidence supports the presence of MT-dependent MT generation within the spindle. This mechanism would produce dense arrays of intraspindle MTs with various lengths, which may contribute to efficient spindle assembly and stabilize the metaphase spindle. RNA interference (RNAi) screens with quantitative image analysis led to the identification of the augmin complex that plays a key role in this MT generation process. Copyright 2009 Elsevier Ltd. All rights reserved.
PMID:8548803 [本文引用: 1]

We have isolated a human homolog of Xenopus Eg5, a kinesin-related motor protein implicated in the assembly and dynamics of the mitotic spindle. We report that microinjection of antibodies against human Eg5 (HsEg5) blocks centrosome migration and causes HeLa cells to arrest in mitosis with monoastral microtubule arrays. Furthermore, an evolutionarily conserved cdc2 phosphorylation site (Thr-927) in HsEg5 is phosphorylated specifically during mitosis in HeLa cells and by p34cdc2/cyclin B in vitro. Mutation of Thr-927 to nonphosphorylatable residues prevents HsEg5 from binding to centrosomes, indicating that phosphorylation controls the association of this motor with the spindle apparatus. These results indicate that HsEg5 is required for establishing a bipolar spindle and that p34cdc2 protein kinase directly regulates its localization.
DOI:10.1073/pnas.92.10.4289URL [本文引用: 1]
DOI:10.1242/jcs.035360URL [本文引用: 3]
[本文引用: 1]
DOI:10.1074/jbc.M704969200URL [本文引用: 1]
DOI:10.1016/j.cell.2011.01.012PMID:21335236 [本文引用: 1]

Disassembly of nuclear pore complexes (NPCs) is a decisive event during mitotic entry in cells undergoing open mitosis, yet the molecular mechanisms underlying NPC disassembly are unknown. Using chemical inhibition and depletion experiments we show that NPC disassembly is a phosphorylation-driven process, dependent on CDK1 activity and supported by members of the NIMA-related kinase (Nek) family. We identify phosphorylation of the GLFG-repeat nucleoporin Nup98 as an important step in mitotic NPC disassembly. Mitotic hyperphosphorylation of Nup98 is accomplished by multiple kinases, including CDK1 and Neks. Nuclei carrying a phosphodeficient mutant of Nup98 undergo nuclear envelope breakdown slowly, such that both the dissociation of Nup98 from NPCs and the permeabilization of the nuclear envelope are delayed. Together, our data provide evidence for a phosphorylation-dependent mechanism underlying disintegration of NPCs during prophase. Moreover, we identify mitotic phosphorylation of Nup98 as a rate-limiting step in mitotic NPC disassembly. Copyright © 2011 Elsevier Inc. All rights reserved.
PMID:11864968 [本文引用: 1]

We describe the isolation, cloning, and characterization of human Nek8, a new mammalian NIMA-related kinase, and its candidate substrate Bicd2. Nek8 was isolated as a beta-casein kinase activity in rabbit lung and has an N-terminal catalytic domain homologous to the Nek family of protein kinases. Nek8 also contains a central domain with homology to RCC1, a guanine nucleotide exchange factor for the GTPase Ran, and a C-terminal coiled-coil domain. Like Nek2, Nek8 prefers beta-casein over other exogenous substrates, has shared biochemical requirements for kinase activity, and is capable of autophosphorylation and oligomerization. Nek8 activity is not cell cycle regulated, but like Nek3, levels are consistently higher in G(0)-arrested cells. During the purification of Nek8 a second protein co-chromatographed with Nek8 activity. This protein, Bicd2, is a human homolog of the Drosophila protein Bicaudal D, a coiled-coil protein. Bicd2 is phosphorylated by Nek8 in vitro, and the endogenous proteins associate in vivo. Bicd2 localizes to cytoskeletal structures, and its subcellular localization is dependent on microtubule morphology. Treatment of cells with nocodazole leads to dramatic reorganization of Bicd2, and correlates with Nek8 phosphorylation. This may be indicative of a role for Nek8 and Bicd2 associated with cell cycle independent microtubule dynamics.
PMID:15387139 [本文引用: 1]

DNA damage results in cell cycle arrest in G2. Centrosomes also separate in G2, raising the question of whether separation occurs during the DNA damage-induced G2 arrest. Nek2, the mammalian homologue of NIMA, is a cell cycle-regulated serine/threonine protein kinase that regulates centrosome separation during G2. Here we show that damaged cells fail to activate Nek2. Both Nek2 levels and activity are reduced after DNA damage. Radiation inhibits the premature centrosome splitting induced by overexpression of Nek2, indicating that Nek2 is involved in activation of the G2 checkpoint and is not secondary to cell cycle arrest. We confirm using siRNA that centrosome separation and cell growth are impaired in the absence of Nek2. These studies define a previously unreported DNA damage response of inhibition of centrosome separation mechanistically linked to Nek2.
DOI:10.1093/mutage/geq026URL [本文引用: 1]
DOI:10.4161/cc.7.20.6815URL [本文引用: 1]
DOI:10.4161/cc.10.4.14814URL [本文引用: 1]
DOI:10.1158/0008-5472.CAN-04-2243URL [本文引用: 1]
DOI:10.1038/onc.2008.34PMID:18297113 [本文引用: 1]

Loss or gain of whole chromosome, the form of chromosome instability commonly associated with cancers is thought to arise from aberrant chromosome segregation during cell division. Chromosome segregation in mitosis is orchestrated by the interaction of kinetochores with spindle microtubules. Our studies show that NEK2A is a kinetochore-associated protein kinase essential for faithful chromosome segregation. However, it was unclear how NEK2A ensures accurate chromosome segregation in mitosis. Here we show that NEK2A-mediated Hec1 (highly expressed in cancer) phosphorylation is essential for faithful kinetochore microtubule attachments in mitosis. Using phospho-specific antibody, our studies show that NEK2A phosphorylates Hec1 at Ser165 during mitosis. Although such phosphorylation is not required for assembly of Hec1 to the kinetochore, expression of non-phosphorylatable mutant Hec1(S165) perturbed chromosome congression and resulted in a dramatic increase in microtubule attachment errors, including syntelic and monotelic attachments. Our in vitro reconstitution experiment demonstrated that Hec1 binds to microtubule in low affinity and phosphorylation by NEK2A, which prevents aberrant kinetochore-microtubule connections in vivo, increases the affinity of the Ndc80 complex for microtubules in vitro. Thus, our studies illustrate a novel regulatory mechanism in which NEK2A kinase operates a faithful chromosome attachment to spindle microtubule, which prevents chromosome instability during cell division.
DOI:10.1074/jbc.M314205200URL [本文引用: 1]
DOI:10.1091/mbc.e11-01-0012URL [本文引用: 1]
PMID:12351790 [本文引用: 1]

The spindle checkpoint delays sister chromatid separation until all chromosomes have undergone bipolar spindle attachment. Checkpoint failure may result in chromosome mis-segregation and may contribute to tumorigenesis. We showed that the human protein Hec1 was required for the recruitment of Mps1 kinase and Mad1/Mad2 complexes to kinetochores. Depletion of Hec1 impaired chromosome congression and caused persistent activation of the spindle checkpoint, indicating that high steady-state levels of Mad1/Mad2 complexes at kinetochores were not essential for checkpoint signaling. Simultaneous depletion of Hec1 and Mad2 caused catastrophic mitotic exit, making Hec1 an attractive target for the selective elimination of spindle checkpoint-deficient cells.
PMID:15950749 [本文引用: 1]

Two splice variants of Nek2 kinase, a member of the NIMA-related family, have been identified as Nek2A and Nek2B. Nek2A regulates centrosome disjunction, spindle formation checkpoint signaling, and faithful chromosome segregation. A specific role for Nek2B has not yet been identified. Here, we have examined the distinct roles of Nek2A and Nek2B using timelapse video microscopy to follow the fate of cells progressing through the cell cycle in the absence of either Nek2A or Nek2B. We show that the down-regulation of Nek2B leads to a mitotic delay in the majority of cells. Upon exiting mitosis, cells exhibit mitotic defects such as the formation of multinucleated cells. Such phenotypes are not observed in cells that exit mitosis in the absence of Nek2A. These observations suggest that Nek2B may be required for the execution of mitotic exit.
DOI:10.1038/onc.2010.162PMID:20473324 [本文引用: 1]

The mammalian NIMA-related kinases (Neks) are commonly referred to as mitotic kinases, although a definitive in vivo verification of this definition is largely missing. Reduction in the activity of Nek7 or its close paralog, Nek6, has previously been shown to arrest cells in mitosis, mainly at metaphase. In this study, we investigate the developmental and cellular roles of Nek7 kinase through the generation and analysis of Nek7-deficient mice. We show that absence of Nek7 leads to lethality in late embryogenesis or at early post-natal stages and to severe growth retardation. Mouse embryonic fibroblasts (MEFs) derived from Nek7(-/-) embryos show increase tendency for chromosomal lagging, micronuclei formation and cytokinesis failure. Tetraploidy and aneuploidy were commonly observed and their prevalence arises with MEFs passages. The frequency of multicentrosomal cells in the mutant's MEF cells was higher, and it commonly occurred concurrently with a binuclear phenotype, suggesting cytokinesis failure etiology. Lastly, the percentage of mutant MEF cells bearing primary cilia (PC) was low, whereas a cell population having two cilia appeared in the mutant MEFs. Taken together, these results confirm Nek7 as a regulator of cell division, and reveal it as an essential component for mammalian growth and survival. The intimate connection between tetraploidy, aneuploidy and cancer development suggests that Nek7 deregulation can induce oncogenesis.
DOI:10.1083/jcb.201512055URL [本文引用: 2]
DOI:10.1083/jcb.201306083URL [本文引用: 1]
[本文引用: 1]
DOI:10.1242/jcs.03458URL [本文引用: 1]
DOI:10.1007/s10059-009-0097-9URL [本文引用: 1]
PMID:14668482 [本文引用: 1]

The mitogen-activated protein kinase (MAPK) pathway is required for maintaining the chromatin condensed during the two meiotic divisions and to avoid a second round of DNA duplication. However, molecular targets of the MAPK pathway on chromatin have not yet been identified. Here, we show that the architectural chromatin protein HMGA2 is highly expressed in male meiotic cells. Furthermore, Nek2, a serine-threonine kinase activated by the MAPK pathway in mouse pachytene spermatocytes, directly interacts with HMGA2 in vitro and in mouse spermatocytes. The interaction does not depend on the activity of Nek2 and seems constitutive. On progression from pachytene to metaphase, Nek2 is activated and HMGA2 is phosphorylated in an MAPK-dependent manner. We also show that Nek2 phosphorylates in vitro HMGA2 and that this phosphorylation decreases the affinity of HMGA2 for DNA and might favor its release from the chromatin. Indeed, we find that most HMGA2 associates with chromatin in mouse pachytene spermatocytes, whereas it is excluded from the chromatin upon the G2/M progression. Because hmga2-/- mice are sterile and show a dramatic impairment of spermatogenesis, it is possible that the functional interaction between HMGA2 and Nek2 plays a crucial role in the correct process of chromatin condensation in meiosis.
DOI:10.4161/cc.22690URL [本文引用: 1]
