 ,1,2, 张珍珍1,2, 高焕1,21
,1,2, 张珍珍1,2, 高焕1,21 2
Progress on the left-right asymmetry patterning in amphioxus
Hu Guangwei ,1,2, Zhang Zhenzhen1,2, Gao Huan1,21
,1,2, Zhang Zhenzhen1,2, Gao Huan1,21 2
第一联系人:
收稿日期:2020-07-28
| 基金资助: |
Received:2020-07-28
| Fund supported: |

摘要
两侧对称动物左右体轴建立机制研究是发育生物学领域重要的基础科学问题之一。文昌鱼(amphioxus)由于其特殊的进化地位以及与脊椎动物相似的胚胎发育模式和身体构筑方式,是研究动物左右体轴建立机制的理想模式物种。近年来随着文昌鱼室内全人工繁育技术、高效显微注射技术和基因敲除技术的建立,国内外****在左右体轴建立机制研究上取得了丰硕的成果。本文从文昌鱼胚胎左右不对称发育特点出发,总结了近期文昌鱼左右体轴建立方面取得的研究进展,并提出了文昌鱼左右体轴调控网络图:纤毛运动导致Hh蛋白在文昌鱼中不对称分布(L<R),从而导致Hh信号通路的不对称激活以及Cer的不对称表达(L<R),Cer对Nodal具有抑制作用,由此导致依赖于Nodal活性的基因呈左右不对称表达并最终诱导胚胎左右体轴的正确建立;BMP信号可能并不提供左右不对称信号,但却是Cer和Nodal正确表达的必要条件。
关键词:
Abstract
Keywords:
PDF (752KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
胡广伟, 张珍珍, 高焕. 文昌鱼左右体轴建立机制的研究进展. 遗传[J], 2021, 43(2): 134-141 doi:10.16288/j.yczz.20-246
Hu Guangwei.
两侧对称动物(Bilateria)体轴的正确建立是胚胎发育过程中的重要事件,是器官发生与形态建成的重要基础[1]。体轴的建立主要包括前后轴(anterior- posterior axis)、背腹轴(dorsal-ventral axis)和左右轴(left-right axis)。左右体轴的建立一般始于胚胎发育早期,其过程主要包括胚胎原始对称的打破、左右体轴建立相关基因的不对称激活和不对称器官的形态建成[2]。在成体中,两侧对称动物外部形态呈两侧对称,但这种对称是相对的,其内部器官的排布往往呈左右不对称排列,从软体动物贝壳的旋转方向到脊椎动物内脏器官的排布都有这种现象。内脏器官的不对称分布不仅能有效地利用躯体空间,而且对于各个器官功能的正常行使也至关重要[3]。
文昌鱼(amphioxus)隶属脊索动物门(Chordata)、头索动物亚门(Cephalochordata),是无脊椎动物向脊椎动物过渡的中间类群,同时也是脊索动物中现存最原始的类群[4]。由于文昌鱼身体构筑方式、基因组成以及胚胎发育模式均与脊椎动物相似,是进化发育与比较基因组学研究的理想模式物种[5,6]。此外,文昌鱼在胚胎学研究上也具有其独特优势。首先,文昌鱼个体小,躯体结构相对简单,繁殖能力强,卵子体外受精、体外发育,胚胎和成体透明,从受精卵的第一次卵裂到神经胚的发育过程都十分清晰[7],非常利于胚胎发育早期的观察和研究;其次,佛罗里达文昌鱼(Branchiostoma floridae)和白氏文昌鱼(Branchiostoma belcheri)全基因组测序工作已经完成[8,9],相比脊椎动物,文昌鱼的基因组没有经历两轮复制,因此与脊椎动物同源基因家族中的多数成员都是以单拷贝的形式存在,为通过基因编辑方法研究基因功能提供了极大的便利;最后,针对文昌鱼研究的相关技术手段也日趋成熟,目前已经实现了文昌鱼室内全人工繁育技术[10,11]、建立了高效的显微注射技术[12]、实现了TALEN介导的基因
敲除技术[13]、建立了文昌鱼转基因技术[14]等。得益于上述技术手段的突破,近年来人们在文昌鱼左右体轴建立方面的研究也取得了很多新成果。本文从文昌鱼胚胎左右不对称发育特点出发,综述了近期文昌鱼左右体轴建立取得的研究进展。
1文昌鱼形态学上的左右不对称
文昌鱼形态学上的左右不对称相比于其他脊索动物较为特殊,在文昌鱼胚胎发育至神经胚中期,体节(somite)首先呈现出左右不对称排布(图1E),其中左侧体节相比右侧体节位置更靠前[15],而且这种不对称会一直持续到成体阶段,最终导致肌节和外周神经元也呈现与体节一致的左右不对称排列特征[16]。文昌鱼在整个胚胎发育过程中的左右不对称在幼体阶段最为明显,尤其以咽区器官(pharyngeal organs)最为显著(图1)。在器官发生时,咽区左右两侧细胞会经历完全不同的命运走向,其中胚胎左侧细胞会分化形成口和前窝(preoral pit),口前窝与脊椎动物垂体前叶(anterior pituitary)同源,由左侧体腔囊(coelomic pouch)发育而来,最终与口前端表皮融合[17]。胚胎右侧咽壁会分化形成内柱(endostyle)和棒状腺(club-shaped gland),内柱与脊椎动物甲状腺同源,位置与口相对。棒状腺是一个管状结构横跨着整个咽区,右侧部分与咽壁相连是具有分泌功能的腺体部,位于内柱的后方;左侧部分是没有分泌功能的管状结构开口于体外,位于口的下方,棒状腺的具体功能以及其在脊椎动物中的同源器官目前尚不清楚[18]。随着发育的进行,咽区器官的不对称程度会随着第一鳃裂的形成而进一步加剧。比较分析发现,不同种类的文昌鱼在胚胎发育过程中虽然会有细微的差异,但是幼体时期的左右不对称特征是这类生物的普遍共性[19]。图1
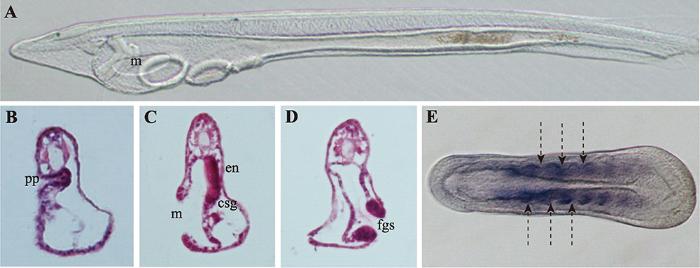 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1文昌鱼左右不对称器官的分布情况
A:文昌鱼三鳃裂时期幼体的侧面观;B~D:文昌鱼咽区器官纵切片结果;E:文昌鱼神经胚期体节的排布情况。pp:口前窝;en:内柱;csg:棒状腺;m:口;fgs:第一鳃裂;箭头表示体节间隔。
Fig. 1Amphiouxs left-right asymmetry organs arrangement
2参与文昌鱼左右体轴建立的信号通路
2.1Nodal信号通路与文昌鱼左右体轴建立
Nodal是TGF-β超家族成员之一,在绝大多数后口动物中均有发现[20]。研究表明,Nodal信号通路通过调控下游基因的不对称表达参与左右体轴建立,在左右体轴建立调控网络中处于核心位置[21]。在小鼠(Mus musculus)中,Nodal基因在节点(node)两侧最初呈对称表达,随后由于Nodal抑制因子Cer在右侧开始表达,使得Nodal的表达主要集中在节点左侧。随着发育的进行,节点处Nodal活性被传递到侧板中胚层[22?~24],并在侧板中胚层诱导其自身以及Lefty和Pitx基因的表达,由于Lefty扩散速度较快,且对Nodal具有抑制作用,从而将Nodal的活性限制在左侧。通过这种自诱导反馈方式(self- enhancement and lateral inhibition, SELL),Nodal、Lefty和Pitx在侧板中胚层中的表达得到进一步强化,最终转录因子Pitx通过激活下游基因的表达参与细胞增殖、分化和不对称器官的发育[25]。在文昌鱼胚胎中,Nodal信号通路的成员在胚胎孵化早期至神经胚中期呈明显的不对称表达,具体体现为Nodal、Gdf1/3(Vg1)、Lefty和Pitx主要在左侧表达(L>R),Nodal的抑制因子Cer主要在右侧表达(L<R)[26??~29]。研究人员在胚胎发育的不同时期用Nodal信号通路抑制剂处理胚胎,通过分析文昌鱼左右不对称器官发育及基因的不对称表达情况,证实从神经胚早期至中期是左右体轴建立的关键时期,并提出了文昌鱼左右体轴调控网络:Cer最早呈现不对称表达(L<R),右侧表达的Cer能够抑制Nodal在右侧表达,从而将Nodal的信号活性限制在左侧,左侧的Nodal一方面可以抑制Cer在左侧的表达,另一方面进一步促进激活Nodal的表达外还可以激活Gdf1/3、Lefty和Pitx在左侧表达,其中Lefty作为Nodal的调节因子将Nodal活性限制在左侧,转录因子Pitx参与不对称器官的形态建成[30]。厦门大学王义权课题组利用基因敲除和过表达等方法肯定了上述调控模型,并且对不同基因之间的调控关系进行了系统地分析,证实Nodal信号通路在脊椎动物和文昌鱼左右体轴建立中功能相似,敲除Cer能够导致Nodal,Lefty和Pitx在右侧异位表达,过表达Cer则导致上述基因在左侧的表达消失;过表达Nodal会抑制右侧Cer的表达,但导致Lefty和Nodal在右侧的异位表达,相反如果抑制Nodal信号活性则会导致Cer在两侧表达,同时会使Lefty和Pitx表达消失[31]。上述一系列研究证实了Cer-Nodal- Lefty-Pitx级联信号通路在脊椎动物和文昌鱼左右体轴建立过程中的功能是保守的,但是在文昌鱼中Pitx可以反馈激活Nodal的表达,这一点在脊椎动物和棘皮动物中均未发现。
2.2BMP信号通路与文昌鱼左右体轴建立
BMP (bone morphogenetic protein)是一类多功能的分泌蛋白,同属于TGF-β超家族,在胚胎发育和器官形态发生中发挥重要功能[32]。已有研究表明,BMP信号通路可与Nodal信号通路相互作用参与动物左右体轴的建立,如在小鼠中BMP信号活性受Nodal信号调控[33],与此同时,BMP信号也可通过调控Nodal信号参与左右体轴建立,敲除Bmp4导致小鼠节点及侧板中胚层中Nodal表达下降,进而影响左右体轴的正常建立[34]。同样在鸡(Gallus gallus)和海胆(Ciona intestinalis)中,Nodal信号通路成员的不对称表达也依赖于BMP信号活性[35??~38]。最新研究显示,在文昌鱼中BMP信号通路通过调控Nodal信号通路参与左右不对称发育,在原肠胚晚期到神经胚期抑制BMP信号活性会使Nodal、Gdf1/3、Pitx和Lefty在左侧的表达消失,Cer和FoxH呈现异位表达。表型分析发现,抑制BMP信号活性导致文昌鱼左右体轴建立异常,原来位于左侧的口和口前窝消失,取而代之的是原本仅出现在右侧的内柱和棒状腺,胚胎左右两侧均为右侧结构(right isomerism)[39],相关特征与抑制Nodal信号通路产生的结果相一致,说明BMP信号通路与Nodal信号通路在文昌鱼左右体轴调控上具有一致性,同时也表明BMP信号通路在左右体轴建立方面功能的保守性。2.3Hedgehog信号通路与文昌鱼左右体轴建立
Hedgehog (Hh)基因最早是由Nusslein-Volhard等[40]在果蝇(Drosophila)发育相关基因突变体筛选过程中发现的,由于该基因突变导致果蝇身体节段(segment)后部消失,使整个胚胎看起来像一个“刺猬”,因此将这一基因命名为Hedgehog。1993年Forbe等[41]首先提出Hh信号通路概念。随后Hh信号通路核心成员在果蝇中相继被发现,后来又在多种脊椎动物中发现了Hh信号通路的同源基因,证实Hh信号通路在动物中普遍存在,广泛参与细胞增殖、分化及多种组织器官的发生[42]。英国雷丁大学Shimeld[43]最初将Hh信号通路与文昌鱼左右体轴形成建立联系,通过表达谱分析发现,Hh基因在文昌鱼神经胚中期(5体节)开始出现不对称表达,这与文昌鱼胚胎开始出现不对称表型的时期一致,考虑到Hh信号通路在鸡胚左右体轴建立中的功能[44],Shimeld推测文昌鱼中Hh的不对称表达极有可能与左右体轴建立相关。但是这一观点并未得到大多数****的认可,主要原因是Hh在文昌鱼中不对称表达出现的时间是在神经胚中期,远远晚于左右体轴调控基因Nodal开始不对称表达的时期[28]。直到2014年,随着基因敲除技术的兴起,研究人员利用TALEN技术构建了Hh基因敲除突变体文昌鱼,发现突变体文昌鱼左右不对称器官发育异常,证明Hh信号通路在文昌鱼左右不对称发育过程中发挥重要功能[45]。在这一结果的基础上,Hu等[46]通过组织切片、扫描电镜系统地分析了Hh基因敲除后的表型,发现Hh基因敲除后文昌鱼咽区器官及体节的不对称性均受到影响,主要表现在右侧器官消失,左右两侧均为左侧特征,整个胚胎呈现左右对称的表型;此外原位杂交结果也显示,在Hh突变体中Cer表达消失,Nodal及其下游调控基因Lefty和Pitx表达由左侧表达变为左右对称表达,这些结果证明了Hh信号通路在文昌鱼中通过调控Cer参与左右不对称建立。最新研究结果显示,Hh信号活性在文昌鱼中存在左右差异(L<R),而且不对称的信号活性对于Cer (原文中使用Dand5来表示Cer,本文统一用Cer)的不对称表达至关重要,在野生型胚胎左侧上调Hh可以异位激活Cer的表达,同时在Hh敲除纯合突变体胚胎中单侧恢复Hh表达可以在Hh表达一侧诱导Cer的表达,如果用甲基纤维素抑制纤毛运动则可以导致Hh蛋白分布异常,从而导致Cer表达随机化,最终影响左右体轴的正常建立[47]。
3左右组织者结构与左右对称的打破
在大多数脊椎动物中,胚胎原始对称的打破与原肠背部一群短暂存在的特殊细胞团密切相关,这群细胞称为左右组织者(LR organizer, LRO),如小鼠中的节点(node)[48]、非洲爪蟾(Xenopus laevis)中的原肠顶板(gastrocoel roof plate, GRP)[49]和斑马鱼(Danio rerio)中的库氏囊泡(Kupffer’s Vesicle, KV)[50]。尽管左右组织者结构在不同物种中形态结构差异较大但都具有如下特点:都存在动纤毛,能够以固定的方式运动产生单向的液流[51,52],液流导致LRO两侧产生差异,并诱导相关基因的左右不对称表达,最终打破左右对称[1]。文昌鱼胚胎发育模式及身体构筑方式与脊椎动物高度相似,因此有****认为文昌鱼中也应存在类似于脊椎动物的LRO结构[3,53],并对文昌鱼中左右组织者存在的部位和特点进行了推测:首先,位置上应该位于原肠腔的背壁,可在脊索形成时整合到脊索中;其次,应该存在极性细胞,具有动纤毛能够定向摆动产生单向的液流,并且能够激活相关信号通路;最后,LRO结构出现的时期应该与不对称基因开始表达的时间相一致。根据这一推测,研究人员一直在试图寻找文昌鱼的“LRO”。但事实上,文昌鱼胚胎在原肠胚时期绝大多数细胞都具有纤毛[54],而且在文昌鱼原肠胚期也没有检测到动纤毛标记基因Fox J1的表达[55],敲除纤毛形成相关基因也不影响文昌鱼左右体轴的正常建立[47]。由此看来,有必要对文昌鱼中是否存在LRO结构这一问题进行重新讨论。需要指出的是,最新的研究结果发现纤毛运动对文昌鱼左右对称的打破至关重要,纤毛运动会导致Hh信号的不对称激活,进而通过调控Cer的表达调控文昌鱼左右不对称发育,用甲基纤维素抑制纤毛运动会导致Hh蛋白分布异常、Cer表达随机化,最终导致左右体轴建立异常[47]。但是由于甲基纤维素只起到抑制纤毛运动的作用,不能确定动纤毛的着生位置,因此依然不确定在文昌鱼中是否有LRO这一结构。
4结语与展望
文昌鱼是人们对头索动物亚门动物的统称,与尾索动物亚门和脊椎动物亚门共同组成脊索动物门,是脊索动物中最早分化出来的一支。与以海鞘为代表的尾索动物不同,文昌鱼与脊椎动物具有更多相似的特征,是了解脊椎动物祖先的现生动物代表;此外,文昌鱼是与步带动物(mbulacrarians)亲缘关系最近的脊索动物,因此揭示文昌鱼左右体轴建立机制并将其与步带动物体轴建立机制进行比较对于全面理解两侧对称动物左右体轴建立机制以及背腹轴转换(dorso-ventral inversion)都具有十分重要的意义。在现存脊索动物中,文昌鱼幼体时期形态学上的左右不对称发育最为特殊,是研究左右体轴建立的绝佳材料。长期以来由于技术手段的限制,使得文昌鱼的研究相比其他模式物种较为滞后,近年来随着相关研究技术手段的建立,尤其是胚胎显微注射技术以及基因敲除技术的应用,以文昌鱼为实验材料,在左右体轴发育机制方面的研究正在迅速展开。研究人员先后阐释了Nodal信号通路、Cer- Nodal-Pitx-Lefty级联信号通路、Hedgehog信号通路、BMP信号通路在文昌鱼左右体轴建立中的功能,且证实纤毛运动为打破左右对称的原始动力。总结现有的文昌鱼左右体轴调控相关研究,本文将文昌鱼左右体轴建立的调控网络总结如下(图2):在文昌鱼中纤毛运动导致Hh蛋白的不对称分布(L<R),由此产生Hh信号活性的左右差异(L<R),右侧高Hh信号活性诱导Cer基因的表达,右侧表达的Cer能够抑制右侧Nodal信号活性,左侧由于没有Cer的抑制作用,Nodal得以正常表达,进而诱导Gdf1/3、Lefty、Pitx和Bmp4的表达。此外,BMP信号活性是Cer、Nodal信号成员以及Bmp4正常表达的必要前提。通过Hh信号通路、Cer-Nodal-Lefty-Pitx级联信号以及BMP信号的分工协作最终保证文昌鱼左右体轴的正确建立。
图2
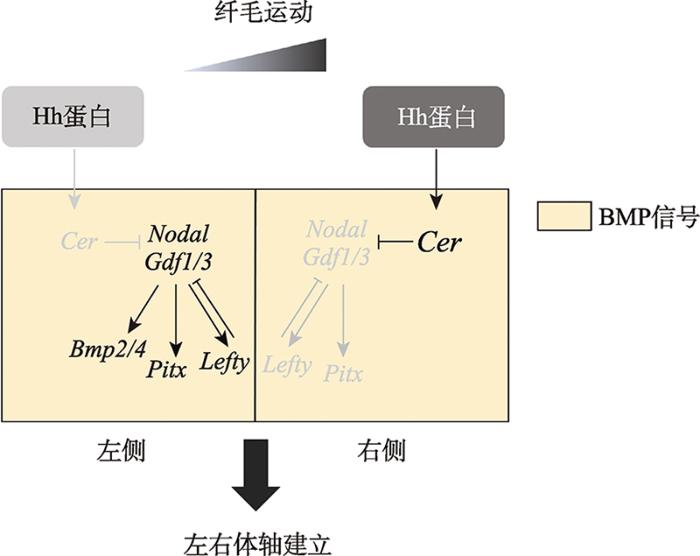 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2文昌鱼左右体轴建立调控网络图
纤毛运动导致Hh蛋白在文昌鱼中不对称分布(L<R),从而导致Hh信号通路不对称激活以及Cer的不对称表达(L<R)。Cer对Nodal信号具有抑制作用,由此导致依赖于Nodal活性的基因Gdf1/3、Lefty、Pitx和Bmp2/4的不对称表达,左右体轴建立相关基因的不对称表达最终诱导文昌鱼胚胎左右体轴的正确建立。
Fig. 2Model of the regulation of left-right asymmetry in amphioxus
尽管文昌鱼左右体轴建立机制越来越清晰,但是文昌鱼左右体轴建立和分化是一个复杂的动态过程,需要多条信号通路精确调控,目前仍有很多问题有待阐明,如Hh信号通路如何决定Cer动态表达进而参与左右体轴建立?在脊椎动物中参与左右体轴建立的Delta/Notch、Wnt/β-catenin和Wnt/PCP信号通路在文昌鱼中是否参与左右体轴建立?此外,有研究发现用质子泵(proton pumps)抑制剂处理会导致文昌鱼胚胎左右体轴建立异常[53],说明离子流(ion flux)参与文昌鱼左右体轴建立,但是其具体功能还不清楚。最后,文昌鱼中是否存在LRO结构还有待进行深入研究。对于上述科学问题的回答将有助于人们全面阐释文昌鱼左右体轴建立的机制。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 2]
[本文引用: 1]
[本文引用: 2]
T Oceanol Limnol, 2007, (
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/s00018-004-4075-2URLPMID:15378201 [本文引用: 1]

The cephalochordate amphioxus is the closest living invertebrate relative of the vertebrates. It is vertebrate-like in having a dorsal, hollow nerve cord, notochord, segmental muscles, pharyngeal gill slits and a post-anal tail that develops from a tail bud. However, amphioxus is less complex than vertebrates, lacking neural crest and having little or no mesenchyme. The genetic programs patterning the amphioxus embryo are also similar to those patterning vertebrate embryos, although the amphioxus genome lacks the extensive gene duplications characteristic of vertebrates. This relative structural and genomic simplicity in a vertebrate-like organism makes amphioxus ideal as a model organism for understanding mechanisms of vertebrate development.
[本文引用: 1]
URLPMID:18563158 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
.
[本文引用: 1]
URLPMID:24780619 [本文引用: 1]
[本文引用: 1]
DOI:10.1006/dbio.2002.0660URLPMID:12051829 [本文引用: 1]

Mox genes are members of the
[本文引用: 1]
URLPMID:9636084 [本文引用: 1]
[本文引用: 1]
URLPMID:20972258 [本文引用: 1]
[本文引用: 1]
URLPMID:16838294 [本文引用: 1]
DOI:10.1101/gad.1016202URLPMID:12231623 [本文引用: 1]

Nodal is expressed at the lateral edges of the mouse node, but its function in this
DOI:10.1016/j.ydbio.2011.03.009URL [本文引用: 1]
DOI:10.1016/s0012-1606(02)00121-5URLPMID:12654299 [本文引用: 1]

Initial determination of left-right (L-R) polarity in mammalian embryos takes place in the node. However, it is not known how asymmetric signals are generated in the node and transferred to the lateral plate mesoderm (LPM). Mice homozygous for a hypomorphic Nodal allele (Nodal(neo)) were generated and found to exhibit L-R defects, including right isomerism. Although the mutant embryos express Nodal at gastrulation stages, the subsequent expression of this gene in the node and left LPM is lost. A transgene that conferred Nodal expression specifically in the node rescued the L-R defects of the Nodal(neo/neo) embryos. Conversely, ectopic expression of the Nodal inhibitor Lefty2 in the node of Nodal(neo/+) embryos resulted in a phenotype similar to that of the Nodal(neo/neo) mutant. These results indicate that Nodal produced in the node is required for expression of Nodal and other left side-specific genes in the LPM.
DOI:10.1016/j.tig.2017.06.004URLPMID:28720483 [本文引用: 1]

Vertebrates exhibit striking left-right (L-R) asymmetries in the structure and position of the internal organs. Symmetry is broken by motile cilia-generated asymmetric fluid flow, resulting in a signaling cascade - the Nodal-Pitx2 pathway - being robustly established within mesodermal tissue on the left side only. This pathway impinges upon various organ primordia to instruct their side-specific development. Recently, progress has been made in understanding both the breaking of embryonic L-R symmetry and how the Nodal-Pitx2 pathway controls lateralized cell differentiation, migration, and other aspects of cell behavior, as well as tissue-level mechanisms, that drive asymmetries in organ formation. Proper execution of asymmetric organogenesis is critical to health, making furthering our understanding of L-R development an important concern.
[本文引用: 1]
[本文引用: 1]
URLPMID:12492142 [本文引用: 2]
[本文引用: 1]
DOI:10.1186/2041-9139-6-5URLPMID:25954501 [本文引用: 1]

BACKGROUND: Nodal is an important determinant of the left-right (LR) body axis in bilaterians, specifying the right side in protostomes and non-chordate deuterostomes as opposed to the left side in chordates. Amphioxus represents an early-branching chordate group, rendering it especially useful for studying the character states that predate the origin of vertebrates. However, its anatomy, involving offset arrangement of axial structures, marked asymmetry of the oropharyngeal region, and, most notably, a mouth positioned on the left side, contrasts with the symmetric arrangement of the corresponding regions in other chordates. RESULTS: We show that the Nodal signaling pathway acts to specify the LR axis in the cephalochordate amphioxus in a similar way as in vertebrates. At early neurula stages, Nodal switches from initial bilateral to the left-sided expression and subsequently specifies the left embryonic side. Perturbation of Nodal signaling with small chemical inhibitors (SB505124 and SB431542) alters expression of other members of the pathway and of left/right-sided, organ-specific genes. Upon inhibition, larvae display loss of the innate alternation of both somites and axons of peripheral nerves and loss of left-sided pharyngeal structures, such as the mouth, the preoral pit, and the duct of the club-shaped gland. Concomitantly, the left side displays ectopic expression of otherwise right-sided genes, and the larvae exhibit bilaterally symmetrical morphology, with duplicated endostyle and club-shaped gland structures. CONCLUSIONS: We demonstrate that Nodal signaling is necessary for establishing the LR embryonic axis and for developing profound asymmetry in amphioxus. Our data suggest that initial symmetry breaking in amphioxus and propagation of the pathway on the left side correspond with the situation in vertebrates. However, the organs that become targets of the pathway differ between amphioxus and vertebrates, which may explain the pronounced asymmetry of its oropharyngeal and axial structures and the left-sided position of the mouth.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:18550712 [本文引用: 1]
URLPMID:12091313 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:23055827 [本文引用: 1]
[本文引用: 1]
DOI:10.1038/287795a0URLPMID:6776413 [本文引用: 1]

In systematic searches for embryonic lethal mutants of Drosophila melanogaster we have identified 15 loci which when mutated alter the segmental pattern of the larva. These loci probably represent the majority of such genes in Drosophila. The phenotypes of the mutant embryos indicate that the process of segmentation involves at least three levels of spatial organization: the entire egg as developmental unit, a repeat unit with the length of two segments, and the individual segment.
URLPMID:1982529 [本文引用: 1]

The methylation status of the c-H-ras-1, insulin and retinoblastoma genes was determined in human sperm, hydatidiform mole, fetal tissues, adult lymphocytes and adult kidney. Individual alleles of c-H-ras-1 and insulin were distinguishable due to presence of endogenous variable number of tandem repeat (VNTR) polymorphisms. Both alleles of the latter two genes were extensively methylated in sperm compared to the other tissues. Several sites within these genes were less methylated in fetal tissues and the two alleles were differentially methylated in some cases. The retinoblastoma gene was highly methylated in all tissues examined, with the exception of a single site that was under-methylated in sperm only. The sperm-specific methylation patterns in all three genes could represent imprinting of the parental chromosomes. Since 5-methylcytosine is inherently mutagenic, it is possible that methylation imprinting could alter the susceptibilities of human genes to point mutations.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:7671308 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1242/dev.157172URLPMID:29122841 [本文引用: 1]

Correct patterning of left-right (LR) asymmetry is essential during the embryonic development of bilaterians. Hedgehog (Hh) signaling is known to play a role in LR asymmetry development of mouse, chicken and sea urchin embryos by regulating Nodal expression. In this study, we report a novel regulatory mechanism for Hh in LR asymmetry development of amphioxus embryos. Our results revealed that Hh(-/-) embryos abolish Cerberus (Cer) transcription, with bilaterally symmetric expression of Nodal, Lefty and Pitx In consequence, Hh(-/-) mutants duplicated left-side structures and lost right-side characters, displaying an abnormal bilaterally symmetric body plan. These LR defects in morphology and gene expression could be rescued by Hh mRNA injection. Our results indicate that Hh participates in amphioxus LR patterning by controlling Cer gene expression. Curiously, however, upregulation of Hh signaling failed to alter the Cer expression pattern or LR morphology in amphioxus embryos, indicating that Hh might not provide an asymmetric cue for Cer expression. In addition, Hh is required for mouth opening in amphioxus, hinting at a homologous relationship between amphioxus and vertebrate mouth development.
DOI:10.1242/dev.182469URLPMID:31826864 [本文引用: 3]
URLPMID:18629866 [本文引用: 1]
DOI:10.1016/j.cub.2006.10.067URLPMID:17208188 [本文引用: 1]

Determination of the vertebrate left-right body axis during embryogenesis results in asymmetric development and placement of most inner organs. Although the asymmetric Nodal cascade is conserved in all vertebrates, the mechanism of symmetry breakage has remained controversial. In mammalian and fish embryos, a cilia-driven leftward flow of extracellular fluid is required for initiation of the Nodal cascade. This flow is localized at the posterior notochord (
[本文引用: 1]
URLPMID:19056505 [本文引用: 1]
DOI:10.1016/j.cell.2006.03.002URLPMID:16615888 [本文引用: 1]

The establishment of left-right asymmetry in mammals is a good example of how multiple cell biological processes coordinate in the formation of a basic body plan. The leftward movement of fluid at the ventral node, called nodal flow, is the central process in symmetry breaking on the left-right axis. Nodal flow is autonomously generated by the rotation of cilia that are tilted toward the posterior on cells of the ventral node. These cilia are built by transport via the KIF3 motor complex. How nodal flow is interpreted to create left-right asymmetry has been a matter of debate. Recent evidence suggests that the leftward movement of membrane-sheathed particles, called nodal vesicular parcels (NVPs), may result in the activation of the non-canonical Hedgehog signaling pathway, an asymmetric elevation in intracellular Ca(2+) and changes in gene expression.
DOI:10.1387/ijdb.170251vsURLPMID:29319110 [本文引用: 2]

Extant bilaterally symmetrical animals usually show asymmetry in the arrangement of their inner organs. However, the exaggerated left-right (LR) asymmetry in amphioxus represents a true peculiarity among them. The amphioxus larva shows completely disparate fates of left and right body sides, so that organs associated with pharynx are either positioned exclusively on the left or on the right side. Moreover, segmented paraxial structures such as muscle blocks and their neuronal innervation show offset arrangement between the sides making it difficult to propose any explanation or adaptivity to larval and adult life. First LR asymmetries can be traced back to an early embryonic period when morphological asymmetries are preceded by molecular asymmetries driven by the action of the Nodal signaling pathway. This review sums up recent advances in understanding LR asymmetry specification in amphioxus and proposes upstream events that may regulate asymmetric Nodal signaling. These events include the presence of the vertebrate-like LR organizer and a cilia-driven fluid flow that may be involved in the breaking of bilateral symmetry. The upstream pathways comprising the ion flux, Delta/Notch, Wnt/beta-catenin and Wnt/PCP are hypothesized to regulate both formation of the LR organizer and expression of the downstream Nodal signaling pathway genes. These suggestions are in line with what we know from vertebrate and ambulacrarian LR axis specification and are directly testable by experimental manipulations. Thanks to the phylogenetic position of amphioxus, the proposed mechanisms may be helpful in understanding the evolution of LR axis specification across deuterostomes.
DOI:10.1002/jmor.1052070106URLPMID:29865496 [本文引用: 1]

The gastrulae of amphioxus were investigated by means of scanning and transmission electron microscopy (SEM and TEM) during 7 arbitrary stages that were seen about 4 to 10 hr after fertilization. Throughout gastrulation, SEM revealed subtle differences in cells of the blastoporal lip. In fractured specimens at early and middle stages, two opposing zones different in shape, size, and connection of the component cells were found: one which consists of columnar smaller cells in close contact in animal region and the other which is composed of round or polygonal larger cells in looser association in vegetal region. The polar body was found unexpectedly on the concave vegetal surface of the early gastrula in about 25% of cases. This might be the result of migration of the polar body. A short cilium that later elongated was recognized on each cell at mid-gastrula stage. The cilia on the dorsal surface (the neural ectoderm) of the final-stage gastrula became shorter than those on the epidermal ectoderm. TEM of thin sections demonstrated that the cytoplasmic components of gastrula cells are essentially the same as those of cleavage cells. But, the homogeneous nucleus seen during cleavage changed into a heterogeneous structure in which a nucleolus and dense particles were seen. Until the late stage, regional characteristics of the gastrulae indicating definitively the anterior-posterior and dorso-ventral polarity were not detected in the present SEM and TEM study.
[本文引用: 1]
