 ,浙江大学生命科学学院遗传与再生生物学研究所,浙江省细胞与基因工程重点研究实验室,杭州 310058
,浙江大学生命科学学院遗传与再生生物学研究所,浙江省细胞与基因工程重点研究实验室,杭州 310058Induced pluripotent stem cell technology and its application in disease research
Chenyi Cai, Feilong Meng, Lin Rao, Yunyue Liu, Xiaoli Zhao ,Key Research Laboratory for Cell and Gene Engineering of Zhejiang Provincial, Institute of Genetics and Regenerative Biology, College of Life Sciences, Zhejiang University, Hangzhou 310058, China
,Key Research Laboratory for Cell and Gene Engineering of Zhejiang Provincial, Institute of Genetics and Regenerative Biology, College of Life Sciences, Zhejiang University, Hangzhou 310058, China通讯作者: 赵小立,副教授,硕士生导师,研究方向:干细胞分化。E-mail:zhaoxiaoli@zju.edu.cn
编委: 李大力
收稿日期:2020-08-12修回日期:2020-10-19网络出版日期:2020-11-20
| 基金资助: |
Received:2020-08-12Revised:2020-10-19Online:2020-11-20
| Fund supported: |
作者简介 About authors
蔡晨依,硕士研究生,专业方向:干细胞分化。E-mail:

摘要
自2006年Takahashi和Yamanaka报道生成诱导多能干细胞(induced pluripotent stem cells, iPSCs)以来,多能干细胞领域进入了前所未有的发展状态,在疾病建模、药物发现以及细胞疗法等各方面都发挥重要作用,促进了细胞生物学和再生医学等学科的发展。目前,iPSCs技术已成为研究病理机制的重要工具,利用iPSCs技术筛选的新药物正在研发中,使用iPSCs衍生细胞的临床试验数量也在逐渐增长。iPSCs与基因编辑技术以及3D类器官相结合的最新研究进展促进了iPSCs在疾病研究中的进一步应用。本文介绍了近年来重编程方法的革新,分析了整合病毒载体系统、整合非病毒载体系统、非整合病毒载体系统以及非整合非病毒载体系统四种重编程方法的利弊;同时综述了iPSCs在疾病建模以及临床治疗等方面的最新研究进展,为促进iPSCs各领域的深入研究提供参考。
关键词:
Abstract
Since Takahashi and Yamanaka reported the generation of induced pluripotent stem cells (iPSCs) in 2006, the field of pluripotent stem cells has entered an unprecedented state of development. It plays an important role in disease modeling, drug discovery and cell therapy, and promotes the development of cell biology and regenerative medicine. At present, iPSC technology has become an important tool for studying of pathological mechanisms. New drugs screened by iPSC technology are being developed, and the number of clinical trials using iPSC-derived cells is gradually increasing. The latest research progress of iPSCs, combined with gene editing technology and 3D organoid methodology, promotes the further applications of iPSCs in disease research. In this review, we introduce the innovation of reprogramming methods in recent years, analyze the advantages and disadvantages of four reprogramming methods: integrated virus vector system, integrated non-viral vector system, non-integrated virus vector system and non-integrated non virus vector system. At the same time, we summarize the latest research progress on iPSCs in disease modeling and clinical treatment strategies, so as to provide a reference for further in-depth research in various fields of iPSCs.
Keywords:
PDF (1267KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
蔡晨依, 孟飞龙, 饶琳, 刘云玥, 赵小立. 诱导多能干细胞技术及其在疾病研究中的应用. 遗传[J], 2020, 42(11): 1042-1061 doi:10.16288/j.yczz.20-235
Chenyi Cai.
最初人们认为,动物的成熟体细胞的基因组被永久锁定在体细胞状态,无法恢复到具有完全的多能性状态[1]。然而,1958年Gurdon等[2]将非洲爪蟾(Xenopus laevis)蝌蚪的体细胞核注射到同一物种的去核卵母细胞中,由此产生了一只功能完善的蝌蚪。39年后,Wilmut等[3]利用核移植技术培育了第一个哺乳动物克隆羊——“多莉”。这些克隆研究表明,分化细胞仍然保留着对机体发育至关重要的“遗传”记忆。1981年,Evans等[4]发现胚胎干细胞(embryonic stem cells, ESCs)可以从小鼠囊胚的内细胞团中获得,其后Thomson等[5]从人囊胚内细胞团成功获得了人胚胎干细胞(human embryonic stem cells, hESCs)。ESCs是多能的,它们具有无限制的自我更新能力,同时拥有向所有细胞类型分化的潜能。1987年,Weintraub等[6]发现单个基因MyoD的表达可以将小鼠成纤维细胞转化为骨骼肌细胞。这一发现证明每种细胞都有自身的主调节基因,这些主调节基因起到维持细胞特性的作用。2006年,Takahashi和Yamanaka[7]从24个不同的基因入手,通过逆转录病毒载体向体细胞引入转录因子,结果发现引入4种转录因子Oct4、Sox2、Klf4和c-Myc (OSKM)可以将小鼠成纤维细胞转化为多能状态,从而建立了诱导多能干细胞(induced pluripotent stem cells, iPSCs)技术。
2007年,日本京都大学Takahashi研究小组和美国威斯康星大学Yu研究小组分别报道了从人成纤维细胞中产生人类诱导多能干细胞(hiPSCs)的案例[8,9]。自此,迅速发展起来的hiPSCs技术为疾病建模、药物发现和再生医学领域开启了一个令人兴奋的新时代。鉴于hiPSCs在疾病建模方面相对于传统细胞筛选的优势,将hiPSCs模型用于药效和潜在毒性的药物筛选变得越来越流行。hiPSCs的优点包括它们起源于人类、易获得、可扩展和能够产生几乎所有所需的细胞类型,避免与hESCs相关的伦理问题,以及具有可以利用患者特定的iPSCs开发个性化药物,进行个性化治疗的潜力。随着3D培养技术以及基因编辑技术,特别是CRISPR/Cas9技术的发展,hiPSCs在人类疾病建模和基于干细胞的临床治疗方面展示出巨大的前景。本文主要介绍了重编程方法的革新以及iPSCs在人类遗传疾病建模和细胞治疗方面的最新发展,总结了目前iPSCs技术面临的挑战并探讨了解决这些问题的方法。
1 重编程方法
1.1 OSKM转录因子的作用
Oct4是一种与多能干细胞(PSCs)的多能性维持有关的同源域转录因子。Sox2在控制Oct4的表达中起着至关重要的作用[10],与Nanog和Oct4一起构成了多能性的关键转录网络。c-Myc是一种与多种癌症病因相关的原癌基因,负责招募染色质修饰蛋白,诱发广泛的转录激活。由于c-Myc有一定的致瘤性,后续有研究用无转化活性的L-Myc替代以解决此问题[11]。Klf4作为肿瘤蛋白或肿瘤抑制因子,是白血病抑制因子的下游靶点,能激活Sox2的表达[12]。重组转录因子与体细胞多能性相关序列结合的能力主要受DNA甲基化、组蛋白修饰和ATP依赖性染色质重塑产生的染色质结构变化的调节[13]。据Stadtfeld和Hochedlinger[14]的报道,细胞的多能性被诱导时会激发两个转录波。在第一个转录波中,c-Myc通过甲基化的H3K4me2和H3K4me3与体细胞基因组相结合,标志着染色质的重塑。随后是体细胞相关基因表达的沉默,包括Thy1、Snai1、Snai2、Zeb1和Zeb2等间充质基因[13,15]。第二个转录波更局限于染色质重塑的细胞。OSKM到达早期多能相关基因(pluripotency-associated genes, PAG)的增强子和启动子,并触发其转录和表达。在此转录波中,体细胞被迫改变其形态,加强增殖,并经历间充质样向上皮样的转化(mesenchymal-to-epithelial transition, MET)。这导致了Cdh1、Epcam和Ocln等上皮基因的上调和上皮特征基本状态的建立[16],形成了更大的ES样细胞团。但MET是一个随机和低效的过程,因为多能诱导基因上存在甲基化组蛋白,而甲基化组蛋白负责封闭染色质构象[13]。Klf4在这两个阶段都扮演着重要的角色。首先,在第一阶段Klf4结合并激活包括E-cadherin在内的上皮基因,抑制分化基因[17]。其次,在第二阶段加速内源性Oct4和Sox2的表达,从而建立维持多能状态的自我调节环。Klf4通过控制细胞的发育、增殖、分化和凋亡等过程,在多能干细胞中发挥重要的作用。Klf4与Oct4和Sox2之间超强的相互作用激活一组如Nanog、Esrrb、Klf2、Sall4和ZFP42等转录因子,以及如Smad1和Stat3等信号通路调节因子[18]。
1.2 重编程载体
在Yamanaka的开创性研究之后,陆续有几个研究室使用不同的方法来生成iPSCs,以满足有效治疗应用的安全性和质量标准。这些重编程策略大致分为两组:(1)通过基因整合或非整合转移系统;(2)利用病毒或非病毒方法。两组重编程策略将现有的重编程方法主要分为四种:整合病毒载体转移系统、整合非病毒载体转移系统、非整合病毒载体转移系统以及非整合非病毒载体转移系统,通过4种重编程方法将各类细胞重编程成iPSCs的过程如图1所示。每种方法都有其优缺点,目前并未找到一个无限制或无潜在不良后果的单一的重编程转移系统。图1
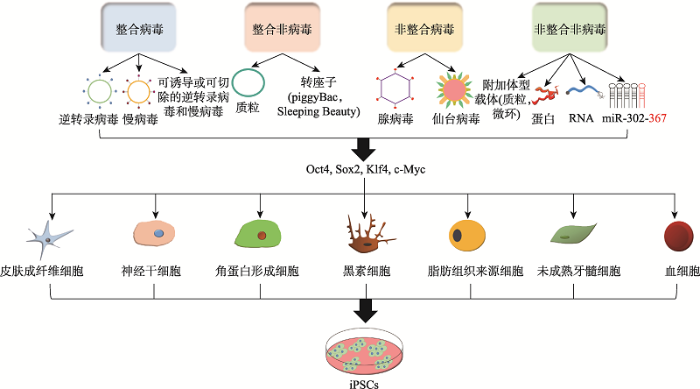 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1产生iPSCs的各种细胞来源和重编程方法
Fig. 1Various cell sources and reprogramming methods for the generation of iPSCs
1.2.1 整合病毒载体转移系统的重编程
iPSCs最初是通过逆转录病毒载体引入重编程因子产生的,逆转录病毒载体已被广泛用作体外和体内研究的基因转移载体[19]。在重编程过程的后期,由于表观遗传修饰[20],转入基因的表达被沉默[21],逆转录病毒载体只提供外源DNA序列的瞬时表达。若未能完全激活与多能性相关的内源基因的表达,通过逆转录病毒载体生成的iPSCs的质量将受到部分损害[22]。此外,一些报道指出,病毒转基因的重新激活及其在产生iPSCs中的残留活性会改变细胞的发育过程,并可能导致嵌合动物肿瘤的形成[23,24]。
慢病毒载体(lentivirus, LV)因其广泛的亲和性而被认为比逆转录病毒载体更加有效[24],因此LV被用来重编程许多体细胞类型。LV基因传递方法如今仍是最有效的重编程策略之一,重编程效率为0.1%~1%[14,25],但科学家们仍在努力改善这一途径的安全性[26]。在设计有效的重编程LV方面取得的进展之一是研制了一种多顺反子LV,它在一个表达系统中携带了由源自口蹄疫病毒的2A“自裂”肽序列连接的一个启动子驱动的四个重编程因子[23,27]。该系统减少了病毒在转基因细胞中整合的拷贝数,降低了转基因沉默的风险,简化了转化过程,并建立了一致的重编程因子的化学计量法[28]。此外,为了消除低效率沉默和转基因再激活的影响,通过引入可切除载体(Cre/loxP系统)[29]和可诱导系统(四环素/强力霉素诱导系统)[26,30]重组多顺反子病毒载体,整合后的转基因可以通过瞬时表达Cre从宿主细胞基因组中移除。但这种策略的转移效率很低[31],并且可能导致iPSCs突变,因为Cre/loxP系统在重新编程后可能会留下loxP的痕迹[32]。
1.2.2 整合非病毒载体转移系统的重编程
由于目前整合病毒转移系统的局限性,科学家们一直在积极研究其他重编程方法,如非病毒转移系统,这些方法对于治疗应用更安全。第一个成功的非病毒性iPSCs是由成熟的胚胎成纤维细胞经两个质粒载体转染产生的:第一个质粒编码c-Myc,而第二个质粒多顺反子载体编码OSKM重编程因子[23]。这些发现表明OSKM的瞬时过表达足以诱导体细胞的多能性,但是整合的风险和重编程的效率低下又成了主要问题[33]。科学家们又设计了一个整合的依赖性基因转移载体,将转录因子整合到重组结构的loxP位点中以解决整合风险的问题[28,29]。然而,短的载体片段可以存在于切除后的基因组细胞中,这可能会影响细胞功能[33]。人们又发现利用如piggyBac(PB)转座子等可移动的基因元件来传递外源多能性基因非常有效,且PB转座系统具有转座效率高、删除精确等优点[34]。此方法通过短暂的转位表达,可以从重新编程的细胞中完全去除该元件的残余[35]。不幸的是,人类基因组中有内源性的类PB转座子元件,在转基因切除后会引起非特异性的基因组改变[36]。为克服PB转座子的局限性,人们又引入了Sleeping Beauty(SB)系统,使其整合频率低于PB转座子,并且人类基因组中没有类SB元件[37]。然而这又导致了新的问题:此方法的重编程效率很低,使用可切除的元件还可能会导致重新整合的风险[33]。
1.2.3 非整合病毒载体转移系统的重编程
Stadtfeld等[38]利用腺病毒等非整合病毒载体成功地建立了人和小鼠iPSCs,从这些研究中获得的iPSCs显示在宿主基因组中没有外源DNA的插入。然而,目前的非整合病毒载体传递方法的重编程效率仅限于0.001%,因此有人认为OSKM的瞬时表达不足以产生完全的表观遗传重塑[14,36]。尽管如此,腺病毒方法在转化医学中的应用仍有很大的前景[24]。另一种方法是使用负单链RNA仙台病毒(Sendai- virus, Se-V),因为它在许多类型的细胞和组织中导入外源基因非常有效,但也遇到了低重编程效率的障碍[39]。尽管如此,人们仍在努力开发一种改良的Se-V[40],因为Se-V在囊性纤维化基因治疗[41]和艾滋病疫苗[42]方面具有巨大的潜力,希望可用于人iPSCs的细胞替代治疗[43]。
1.2.4 非整合非病毒载体转移系统的重编程
为了产生不含载体整合到染色体的iPSCs,可以使用细胞质RNA、附加体型载体(一种自我复制和选择的载体)[44]或多顺反子微环DNA非病毒载体系统[45],将多潜能标记基因直接和瞬时地传递到体细胞中。这些方法相对容易使用,但重编程效率比LV低5~10倍[24]。因此,使用细胞质RNA和微环DNA载体需要广泛的优化以备将来的应用[24]。
小鼠和人成纤维细胞已成功地通过直接转移纯化的重编程蛋白[46]或从ESCs[47]、转基因HEK293细胞中分离的总蛋白[48]提取物进行重编程。然而这种方法存在一定的弊端,因为大量合成这样的蛋白质具有很大的挑战性,且转化效率特别低,细胞重编程过程需要8周。有实验室指出通过化学重编程产生iPSCs也许是可行的,但这一过程可能导致突变,因为细胞基因组易受DNA和组蛋白修饰[14,33]。引入合成RNA或编码重编程因子的信使RNA (mRNA)也许是建立无整合多能干细胞的有力平台,尽管这些方法可能需要多轮转染,但在生成具有更安全的iPSCs方面相对有优势[49]。
1.3 提高重编程效率的方法
为了改进重编程过程,人们采用了诸如microRNA (miRNA)等新方法来提高重编程效率。例如,miR- 291-3p、miR-294和miR-295被用来代替c-Myc以产生均匀的hiPSCs集落[50]。此外,化学化合物如丙戊酸、丁酸钠和组蛋白脱乙酰酶抑制剂等已被证明能促进iPSCs的生成[51,52,53]。培养环境的改变,如缺氧培养也能提高重编程效率[54]。抑制p53途径[55]或抑制NuRD(Mbd3/核小体重塑和去乙酰化抑制因子)复合物的组成成分Mbd3,都可以进一步促进iPSCs的生成[56]。卵母细胞中特异表达的其他因子,如Glis1和H1foo,也能提高重编程效率[57,58]。除了可以提高重编程效率,有些小分子在重编程过程中还能发挥部分OSKM转录因子的作用,甚至能完全代替OSKM转录因子将体细胞重编程为iPSCs。例如,使用组蛋白脱乙酰酶抑制剂和转化生长因子β抑制剂的培养基可以在没有c-Myc或Klf4的情况下提高iPSCs的生成,并且可以替代Oct4来维持细胞的多能性[59]。此外,用Aza(DNA甲基化抑制剂)和TSA(组蛋白脱乙酰酶抑制剂)诱导多能基因Oct4、Nanog、Sox2的表达,可以将小鼠骨髓前体细胞(BPCs)重编程为iPSCs[60]。另一项研究报告表明,尽管重编程效率较低,但转入miRNA-302/367家族可以在没有转入OSKM转录因子的情况下成功地将小鼠和人类体细胞重编程为iPSCs[61]。Hou等[62]使用7种小分子化合物成功将小鼠体细胞重编程成iPSCs,且重编程效率高达0.2%。虽然单用小分子对体细胞进行重编程在临床上有很大的应用价值,但由于重编程不完全,诱导后的多能干细胞容易恢复体细胞特征,这将限制小分子重编程在临床研究中的发展。
2 基于iPSCs的疾病建模
2.1 基因编辑技术
基于iPSCs的疾病模型的建立和使用通常包括以下步骤:收集来源患者的体细胞,通过重编程技术将其重编程成病人特异性iPSCs;利用CRISPR/ Cas9等基因编辑技术建立等基因对照(通常通过编辑已知疾病相关基因获得修复后的健康iPSCs);将细胞分化为各种与疾病相关的特定细胞,并通过比较患病和健康的特定细胞或类器官来定义疾病特异性表型;在分子水平上研究这些表型可以识别新的病理机制,为药物发现和个性化治疗提供新的机会。iPSCs衍生的特定细胞和类器官在疾病建模、药物发现和细胞治疗中的应用如图2所示。图2
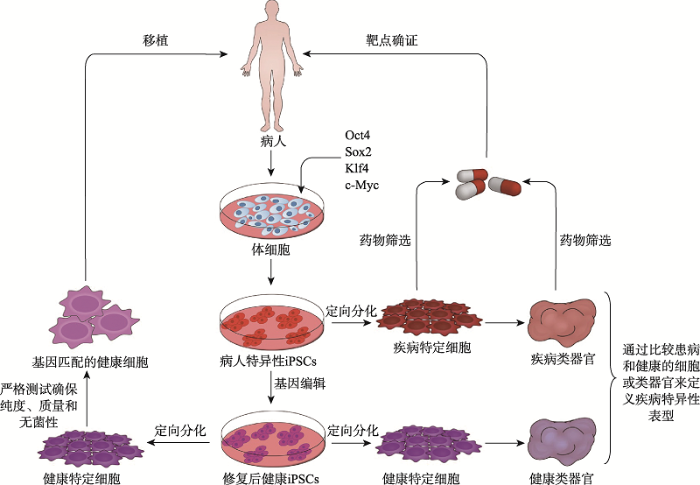 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2来源于iPSCs的特定细胞和类器官在疾病建模、药物发现和细胞治疗中的应用
Fig. 2Application of specific cells and organoids derived from iPSCs in disease modeling, drug discovery and cell therapy
快速发展的基因组编辑技术现在能够在特定位置将基因改变引入iPSCs,包括纠正患者来源的iPSCs中的致病基因突变以及将特定突变引入非疾病野生型iPSCs。这些方法提供以引入的突变作为唯一的变量来产生基因匹配的、等基因的iPS细胞系,确保了对真实病理的可靠鉴定,同时避免了由于可能的细胞系间变异导致的遗传背景差异或偶发现象的混淆。等基因iPSCs控制在模拟散发性或多基因疾病时尤其重要,因为这些疾病的表型差异很小[63]。
可编程位点特异性核酸酶的开发,包括锌指核酸酶(zinc-finger nuclease, ZFN)[64,65]、转录激活物样效应器核酸酶(transcription activator-like effector nucleases, TALENs)[66,67]和CRISPR/Cas9系统[68],通过诱导基因修饰位点的DNA双链断裂,显著提高了人ESCs和iPSCs的基因编辑效率。其中CRISPR/ Cas9技术由于设计简单、使用方便等优点,在人类胚胎干细胞和iPSCs的基因编辑中得到了广泛的应用。这种基因编辑技术允许研究人员将致病突变引入非患者iPSCs或消除患者iPSCs中的致病突变,从而创建基于iPSCs的疾病建模等基因控制平台。尽管使用CRISPR/Cas9技术可能会产生偏离目标的效果,如CRISPR/Cas9在肿瘤细胞系[69]中发现了相对较高水平的非靶基因修饰,但来自多个实验室的全基因组测序(whole genome sequencing, WGS)研究表明,非靶基因修饰在正常人类细胞(包括人iPSCs和ESCs)中很少见[70,71]。基因编辑工具也在不断改进和完善,这可能有助于解决脱靶效应的问题。最初CRISPR/Cas9通过诱导DNA双链断裂来编辑一个基因组位点;后来由成对向导RNA或特异性增强的工程化Cas9核酸酶变体(engineered Cas9 nuclease variants with enhanced specificity, eSpCas9)指导的Cas9核酸内切酶变体(D10A突变体)越来越多地用于基因组编辑[72],因为这两种方法都能在保持对靶点的严格切割的同时显著降低了脱靶效应[73]。此外,催化死亡的Cas9 (dead Cas9, dCas9)通过与转录激活剂或抑制因子融合,用于调节内源基因的转录或通过与荧光蛋白融合来成像基因组位点[74]。对CRISPR/ Cas9系统的改造也能以精确的单等位或双等位基因的方式高效地引入DNA序列的变化[75]。最近在碱基编辑方面的一个新进展是利用CRISPR/Cas9与胞苷脱氨酶融合,使胞苷直接转化为尿苷而不需要双链DNA断裂[76]。这种新方法提高了基因编辑效率,并将进一步促进人类iPSCs的基因编辑发展。用于人类iPSCs的基因编辑技术以及作用机理如表1所示。
Table 1
表1
表1用于人类iPSCs的基因编辑技术
Table 1
| 系统 | 酶 | 作用机理 | 参考文献 |
|---|---|---|---|
| ZFN | 锌指核酸酶 | 定制的锌指蛋白DNA结合模块融合到细菌内切酶FokI的切割域,诱导位点特异性DNA双链断裂(DSB),然后通过NHEJ进行DNA修复以产生小的插入和缺失突变(Indels)或引发HDR以引入精确的核苷酸修饰 | [60,61] |
| TALEN | 转录激活因子样 效应核酸酶 | 定制的TALE蛋白DNA结合模块融合到细菌核酸内切酶FokI中,诱导位点特异性DSB,然后通过NHEJ进行DNA修复引入Indels或通过HDR引入特异性DNA突变 | [62,63] |
| CRISPR/Cas9 | 野生型Cas9,Cas9 核酸内切酶 | RNA引导的位点特异性DNA切割触发NHEJ产生Indels或引发HDR来引入精确的DNA修饰 | [64] |
| Cas9 核酸内切酶 | 结合Cas9核酸内切酶和配对的sgRNA来诱导位点特异性DSB,成对的切割显著减少了脱靶效应(50到1500倍) | [68] | |
| eSpCas9 | 结构导向的蛋白质工程被用来创造spCas9变异体,这种变异体保持对靶点的严格切割的同时显著降低了脱靶效应 | [69] | |
| Cas9-VRER变体 | 这个被称为“CORRECT”的平台允许以精确的单等位基因或双等位基因的方式引入DNA修饰 | [71] | |
| CRISPR/Cas9/ 胞苷脱氨酶 | CRISPR/Cas9与胞 苷脱氨酶的融合体 | 碱基编辑方法可以直接将胞苷转化为尿苷,而不需要DSB和供体DNA模板 | [72] |
新窗口打开|下载CSV
2.2 传统二维细胞建模
识别人类疾病的病理机制对于发现新的治疗策略具有关键作用。虽然利用原代患者来源细胞建立疾病模型有助于研究人类疾病的病因和制定治疗策略,但一个关键的限制因素是无法获得可扩展的患者原代细胞,尤其是脑细胞和心脏细胞。人类iPSCs由于自我更新的内在特性和几乎可以分化为体内任何细胞类型的潜能而成为一个有吸引力的替代品,因为人类疾病(特别是那些有明确遗传原因的疾病)原则上可以使用容易获得的细胞类型(如皮肤成纤维细胞和血细胞)的iPSCs建模。患者特异性iPSCs可以提供大量与疾病相关的细胞和各种以前无法获得的细胞类型,如神经元和心肌细胞。此外,由于iPSCs可以从相关患者自身获得,因此它们可以使个性化疾病建模成为精准医学的核心部分。基于iPSCs的疾病模型被广泛用于研究由单基因突变引起的疾病(单基因疾病)[77],这种方法非常适合于此类疾病,因为iPSCs可以很容易地从这些疾病患者身上获得并分化成如神经元等与疾病相关的细胞。例如,从患者iPSCs中分化出来的神经元被用来模拟脊髓性肌萎缩症(spinal muscular atrophy, SMA),这是一种由SMN1基因突变引起的早发性疾病,SMN1基因突变导致运动神经元变性和随后的肌肉萎缩[77]。Ⅰ 型SMA患者通常在出生后6个月出现症状,疾病进展迅速,在两岁时死亡[78]。在最初的基于iPSCs的疾病模型研究中[77],iPSCs来源于 Ⅰ 型SMA患者的成纤维细胞,并分化为与疾病相关的细胞类型——运动神经元。与未受影响的对照组相比,患者iPSCs分化的运动神经元存活率降低。此外,来源于SMA患者的iPSCs能够对丙戊酸和妥布霉素(已知两种化合物能增加SMN蛋白的表达量)产生反应,且这两种化合物可以提高SMA患者iPSCs的SMN蛋白水平[77]。这项研究提供了一个原则性的证据,病人来源的iPSCs可以用来模拟早发性遗传疾病,并可作为潜在的药物筛选平台。
对发病较晚的疾病进行建模更具挑战性,因为从人类iPSCs分化出来的细胞通常表现出不成熟的表型。例如,理想情况下来源于iPSCs的心肌细胞在用于再生医学或药物发现时应该是成熟的,其成熟度将类似于成年心肌中心肌细胞的成熟度,从而使衍生细胞显示出相似的收缩性、电生理性能和对药物刺激的反应。然而,在现实中,iPSCs来源的心肌细胞是未成熟的,其形态特征等方面更符合胚胎状态下的心肌细胞[79]。未成熟心肌细胞较少显示有组织的肌节结构和钙处理机制,这些特征反映在成熟相关肌节基因(如MYL2、MYH7、TCAP和MYOM2)和离子转运相关基因(如KCNJ2和RYR2)的低表达上[80]。研究人员已在诱导心肌细胞成熟方面做了很多努力:添加甲状腺激素[81],长期培养[82],用三维心肌组织中的机械调节结合电刺激[83]等方法都对iPSCs衍生心肌细胞的成熟有作用。除了要解决iPSCs衍生细胞不成熟表型的问题外,建模晚发性疾病更大的困难在于如何诱导细胞衰老。对人iPSCs衍生的细胞进行诱导衰老的一种方法是用细胞应激源处理细胞,包括如吡咯菌素和MG-132等的靶向线粒体功能或蛋白质降解的化合物[84];另一种诱导细胞衰老的方法是异位表达如早衰蛋白等诱导早衰的基因产物[85]。然而,细胞应激源或早衰蛋白的表达是否能通过一种类似于正常衰老的机制诱导细胞衰老仍有待确定。
iPSCs还为研究散发性疾病提供了一种新的方法(其病因尚未在患者的家族史或基因突变中确定),这一点十分重要,因为许多疾病的大多数患者都属于散发性疾病。例如,在老年痴呆症中,95%的病人属于散发性疾病。研究人员通过对散发性阿尔茨海默病患者iPSCs衍生神经细胞的分析发现,一些散发性病例表现出与特定基因突变的家族性阿尔茨海默病相同的表型[86],这表明使用iPSCs也许可以对散发性疾病进行重新鉴定。然而,使用iPSCs对散发性疾病进行建模通常比单基因疾病更困难,因
为这类疾病的表型变化是由多个小效应遗传风险变异体和环境因素共同诱导的。虽然来源于此类疾病患者的iPSCs可能包含与疾病相关的风险变异体,但使用iPSCs来模拟此类疾病由于遗传和表观遗传背景的逐行变异而变得复杂,而且散发性疾病iPSCs的衍生细胞的表型预计比单基因疾病iPSCs的衍生细胞更为精细。因此,基于iPSCs的散发性疾病模型的一个关键问题是如何产生仅在相关风险变异上存在差异的成对等基因细胞系[63]。利用CRISPR/ Cas9技术产生基因编辑的等基因iPS细胞系的功能可以创造一个良好的控制系统,确保与疾病相关的基因风险变异体成为唯一的变量[87]。这种方法被用于产生帕金森病不同相关风险变异体的等基因iPS细胞系,结合等位基因特异性分析能够对该遗传风险变异体进行强有力的分辨和基因定位[87]。这一实验策略可用于研究与其他疾病相关的遗传危险因素。
此外,基于iPSCs的疾病模型也成为研究肿瘤疾病的发病机制和筛选新的治疗药物的有用工具。在不改变细胞基因组序列的情况下,来源于肿瘤疾病患者的任何细胞都能重编程成iPSCs,再分化为与对应肿瘤疾病相关的细胞类型,以此研究iPSCs向特异性肿瘤细胞转化的过程。例如,青少年骨髓单核细胞白血病(juvenile myelomonocytic leukemia, JMML)是一种侵袭性骨髓增生性肿瘤,其发生的原因是突变导致细胞因子受体信号转导异常。将两名JMML患者的骨髓细胞和外周血细胞重编程成iPSCs,此iPSCs具有与患者体细胞相同的PTPN11基因的p.E76K错义突变,该突变体编码SHP-2 (一种非受体酪氨酸磷酸酶)[88]。与正常iPSCs体外分化产生的髓系细胞相比,JMML-iPSCs衍生的髓系细胞增殖能力增强、粒细胞-巨噬细胞集落刺激因子(GM-CSF)被激活且STAT5/ERK磷酸化增强,这些特征与JMML患者的原代髓系细胞相似,且MEK激酶的药理抑制作用能降低JMML-iPSCs衍生的髓系细胞GM-CSF的活性[88]。这项研究表明iPSCs衍生细胞在人类原发性恶性肿瘤体外建模中的效用,为该疾病潜在的靶向治疗提供理论依据。基于iPSCs的疾病建模在遗传性肿瘤疾病的研究中也展示出巨大的前景。Li-Fraumeni综合征(Li-Fraumeni syndrome, LFS)是一种常见的由p53基因突变引起的恶性肿瘤综合征[89]。来源于LFS患者的iPSCs衍生的成骨细胞显示出分化缺陷性和致瘤性,且H19基因的表达受阻,这些特征与骨肉瘤(osteosarcoma, OS)细胞一致。恢复LFS-iPSCs中H19的表达促进了成骨细胞的分化并抑制了其致瘤性[90]。该研究结果证明了用iPSCs研究遗传性人类肿瘤疾病的可行性。
2.3 类器官:培养皿里的复杂组织
iPSCs衍生产品的临床应用在很大程度上依赖于定向分化、细胞状态转换和组织工程的最新技术。早期利用iPSCs定向分化的研究经常利用单基因疾病中的细胞水平表型,但转化为组织水平和器官水平的疾病需要开发更复杂的3D多细胞系统。随着iPSCs衍生类器官的发展,iPSCs在疾病建模方面取得了重要进展[91]。类器官是由干细胞分化而来的三维多细胞聚集体,能够自我组织,可以表现成熟组织的结构特征和细胞间的相互作用[92]。用于指导类器官与iPSCs分化的可溶性生物物理学方法已逐渐完善,以产生越来越复杂的“人造组织”[91]。患者来源的iPSCs和基因纠正的iPSCs平行分化成类器官,可以将类器官水平的疾病表型归因于特定的分子病变。一旦建立了清晰的类器官水平研究平台,病变的类器官就可以用于药物筛选和验证研究。早期的ESCs和iPSCs研究利用神经分化方法来模拟神经系统疾病[93]。从人iPSCs衍生的3D大脑类器官的创建就是建立在这些基本的神经分化方法的基础上,但随后要在悬浮生物反应器中生长长达70天,另外这些3D大脑类器官还给研究人员带来形态建成的启发线索[94]。这些器官包含脑皮层组织区域以及特定前脑和后脑区域的功能性神经元,甚至包含未成熟视网膜和脉络丛的分化结构[94]。Quadrato和Sloan等[95,96]对3D大脑类器官培养方法的改进还能诱导3D类器官形成大脑中的海马体和小脑等特定区域结构,以及诱导形成皮层折叠结构。神经类器官因为显著的复杂性而被用来模拟各种单基因和多基因神经系统疾病,这些疾病的研究为神经疾病的病理生物学提供了深厚的基础。例如,CDK5RAP2编码一种中心体蛋白,该蛋白在有丝分裂过程中定位于纺锤体极,而CDK5RAP2基因的复合杂合子突变将引起小头畸形。CDK5RAP2基因突变患者iPSCs衍生的神经类器官最初被用来模拟小头畸形,由此发现患者特有的神经类器官经历了神经上皮早期分化,并显示出异常的径向胶质取向和较小的分化神经组织区域[94]。而另一种编码中心体蛋白的CENPJ基因在微管组装和成核的调节中起重要作用,CENPJ的突变将引起小头综合征,该病患者iPSCs衍生的神经类器官中也观察到类似的表型[97]。近年来,来源于人iPSCs的神经类器官还被用作研究脑肿瘤的平台,研究人员使用含有神经干细胞和前体细胞的大脑类器官,通过载体转化以引入常见于脑肿瘤的突变,然后研究突变后细胞的过度生长问题以模拟脑瘤的发生和发展[98]。因此,与2D系统相比,神经类器官的研究让人们增强了对神经系统疾病病理生物学的了解。
在从iPSCs生成肝细胞治疗各种肝脏疾病方面的二维分化方案已被证明是有效的[99]。最近,在三维类器官系统中,从患有肝胆疾病的iPSCs中已经衍生出胆管细胞。目前的分化方案是首先定向分化为内皮细胞和肝母细胞,然后在三维培养中分化为胆管细胞样细胞[100,101]。这些胆管细胞类器官可以流出胆汁酸并具有功能性分泌作用,从而可以模拟Alagille综合征,这是一种由于Notch信号中断而导致胆管形成受损的疾病[101]。在与多囊性肝病患者分化的胆管细胞类器官中,合成生长抑素类似物奥曲肽降低了类器官的大小,与该药物在治疗患者多囊性肝肿大中的作用一致[101]。携带F508del突变的多囊性肝病和囊性纤维化(CF)患者的iPSCs衍生的胆管细胞类器官显示出氯化物转运受损和CFTR蛋白表达量降低,而CF校正药物VX809能稳定该类器官中CFTR蛋白的表达,与该药物在囊性纤维化患者中的作用一致[100,101]。在每一个病例中,复杂疾病表型的建模都是通过三维类器官的发展而实现的。
近年来,心脏类器官的研究取得了极大的进展。iPSCs衍生的心脏类器官具有胎儿样分化的特点,虽已被用于心肌细胞损伤后再生的模型研究,但是这一发现强化了一种观点,即来源于iPSCs的心肌细胞和其他组织经常表现出胎儿样分化,这可能会妨碍成人疾病的建模[102,103]。最近有研究者产生了具有中心空腔的复杂心肌细胞类器官,这让分化方案逐步完善[104]。此外,iPSCs已经被用于构建三维心肌组织,以评估机械力、代谢和细胞外基质对心肌细胞成熟的影响,这些研究包括使用iPSCs的组织工程的各个方面[105]。研究人员还通过3D器官芯片技术将iPSCs用于模拟心肌病,这一策略被应用在许多其他疾病系统中,为模拟血管灌注提供了额外的理论支持[106,107]。
最近的两项研究强调了人iPSCs衍生的肺类器官在疾病建模中的作用[108,109],方法是将人iPSCs定向分化为NKX2-1+的气道祖细胞,该祖细胞能够随后形成近端或远端气道细胞[108]。在低Wnt激活的三维培养中,NKX2-1+祖细胞可重复形成包含各种近端气道细胞的类器官,包括分泌细胞、杯状细胞和基底细胞[108]。用forskolin同时处理来自囊性纤维化F508del-CFTR突变纯合子患者和来自对照的健康人类的iPSCs衍生的近端气道类器官,相比之下,患者衍生的类器官显示forskolin诱导的肿胀受损[108]。研究人员将这一表型归因于CFTR功能障碍,而后iPSCs中F508del的基因纠正改善了forskolin诱导的肿胀[108],进而揭示了基因编辑在iPSCs疾病模型中确认的基因型-表型关系。人iPSCs来源的NKX2-1+肺祖细胞在3D培养中也能分化为远侧肺泡类器官,这些肺泡类器官含有功能性的2型肺泡上皮细胞,这些细胞含有板层小体并分泌表面活性蛋白。而来自缺乏表面活性蛋白B (SFTPB)患者的iPSCs,其衍生的肺泡类器官虽然含有2型肺泡上皮细胞,但这些细胞缺少板层小体或不能合成SFTPB[109]。以上研究结果表明通过基因编辑来纠正SFTPB突变也许能改变该疾病患者iPSCs衍生细胞的表型。
2.4 人-动物嵌合体
尽管基于类器官的疾病模型在复杂性、成熟度和细胞多样性方面有所拓展,但还是局限于组织培养,限制了与体内循环、神经和免疫系统、激素以及其他代谢介质相互作用的探索[110]。此外,类器官还缺少体内存在的许多形态发生信号[92]。例如,在模拟血液疾病时,体外分化系统很少将微环境因子纳入造血分化和造血干细胞(HSCs)的研究中[111,112]。近年来,细胞和类器官异种移植与人iPSCs的研究进展已经克服了这些局限性。鉴于最近许多细胞治疗的临床研究案例呈爆炸性增长之势,而基于iPSCs的异种移植嵌合体疾病模型已经能够更加忠实地模拟人类疾病并成为推动这些研究进展的可靠工具。人类神经组织的异种移植已经用于研究人类疾病几十年了,人胚胎干细胞的分离培养、iPSCs技术的发展、人胚胎干细胞的神经分化倾向以及移植模型生物,都为细胞治疗提供了一种新型的治疗技术[113]。当人类iPSCs分化的神经元异种移植到发育中的小鼠脑中时可以整合、形成功能性突触并整合到神经回路中[114]。iPSCs分化的内源性神经干细胞(NSCs)在大鼠脑内植入后可分化为神经元和星形胶质细胞,并可促进缺血性脑卒模型的恢复[115]。从人类或非人类灵长类动物iPSCs中分化出来的多巴胺能神经元可以植入大鼠的大脑,改善帕金森病模型的功能;非人类灵长类iPSCs的多巴胺能神经元也可以自体移植,并在体内长期植入[116]。这种令人信服的功能恢复的临床前证据为人类帕金森病的临床试验提供了强有力的证明;而采用细胞疗法修复海马体内的神经连接,目前正在作为研究精神分裂症的治疗方法[117]。最近,宿主血管系统灌注和神经活动的电生理学实验描述了将人iPSCs衍生的神经类器官成功植入到成年小鼠脑中,这为神经系统疾病的全方位建模提供了一个新平台[118]。总之,这些实验证明了嵌合模型在用iPSCs模拟人类神经系统疾病中的价值以及基于iPSCs的细胞治疗在这些疾病中的可行性。
研究人员已经将人iPSCs衍生的类器官移植到小鼠体内,以整合血管系统来研究与正常宿主生理的相互作用[119]。来源于人iPSCs的肝内胚层细胞可以与内皮干细胞和间充质干细胞联合培养以形成原始的肝芽,这些肝芽异种移植后与小鼠血管系统形成功能联系,然后将人白蛋白释放到小鼠血清中并产生人类特有的药物代谢产物[119]。研究结果还表明移植这些肝芽可以提高小鼠肝衰竭模型的存活率[119]。从家族性高胆固醇血症患者的iPSCs中分化出的肝细胞已经被异种移植来模拟这种疾病[120]。
将肿瘤异种移植到免疫缺陷小鼠模型中是近年来肿瘤研究的一项关键技术。在人类iPSCs恶性血液肿瘤模型中,已经开发出形成造血嵌合体的案例[121]。急性髓系白血病(acute myeloid leukaemia, AML)细胞在MLL(也称为KMT2A)基因座上发生易位,可以通过阻断异常的MLL驱动的DNA甲基化并有效地将其重新编程为多能性细胞,并能再分化为非造血系细胞[121]。然而,在定向分化为造血祖细胞后,这些细胞在培养和移植过程中表现出异常的自我更新能力,因此该系统可用于模拟人AML的克隆结构,并可用于区分这些iPSCs衍生亚克隆的药物敏感的差异性[121]。基于人类iPSCs的嵌合体也被用来模拟实体肿瘤。人胶质母细胞瘤细胞重编程成iPSCs可消除抑瘤基因启动子的高甲基化功能,当iPSCs定向分化为神经祖细胞时,这些细胞保留了恶性潜能,在异种移植到小鼠纹状体时形成侵袭性肿瘤[122]。从患有Li-Fraumeni综合征的患者中获得的iPSCs衍生的成骨细胞携带有种系TP53突变,这些患者易患多种实体恶性肿瘤和血液系统恶性肿瘤,在免疫缺陷小鼠皮下注射后形成骨肉瘤样肿瘤[123]。今后利用来源于iPSCs的类器官和异种移植到小鼠体内的研究可以为实体瘤的发生和发展提供新的视角。
4 基于iPSCs的临床研究
除了疾病建模之外,iPSCs最大的潜力之一是对许多遗传和退行性疾病进行细胞和基因替代治疗[124]。在这种方法中,病人的体细胞被分离和培养,再通过病毒或非病毒介导的基因转移被重新编程成iPSCs,接着使用基因编辑技术或病毒转导方法对患者来源的iPSCs进行基因纠正,基因纠正筛选出的iPSCs分化为受影响的细胞亚型。在进行细胞鉴定以及纯度、活性和安全性的质量控制试验后,将基因匹配的健康细胞移植到患者体内进行细胞治疗。这种自体移植的方法可以防止严重的并发症,如通常发生在同种异体移植后的移植物抗宿主病。iPSCs在疾病治疗中的应用如图3所示。图3
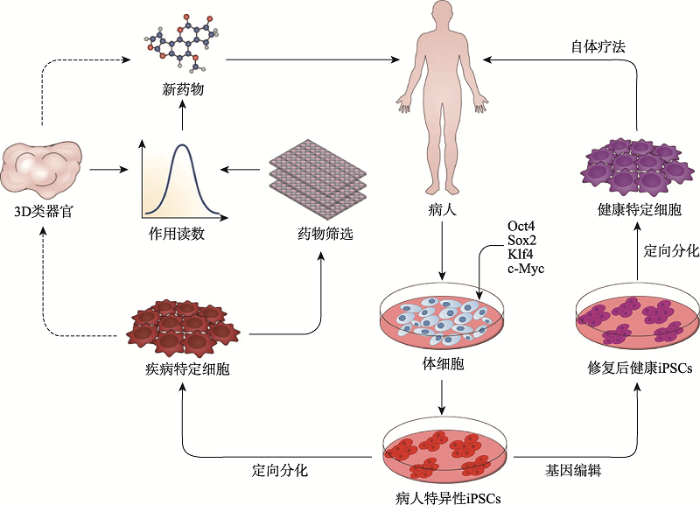 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3iPSCs在疾病治疗中的应用
Fig. 3Application of iPSCs in disease treatment
然而,在常规的临床应用开始之前,基于iPSCs的治疗有几个障碍需要解决,其中一个是iPSCs的致瘤风险[125]。由于iPSCs在培养基中培养了很长时间,它们会积累核型异常、拷贝数变异和杂合性丧失[126]。因此,在临床使用之前,iPSCs衍生产品需要仔细筛选以确定是否存在潜在风险的基因改变[126],并进行严格测试以确保其纯度、质量和无菌性。另一个问题是生成可用于临床的iPSCs的成本很高且耗时较长,解决这个问题需要建立能够储存大量人类白细胞抗原(HLA)纯合超供体iPSCs的细胞库[127]。其中一个例子是2013年日本成立了京都大学iPSCs研究与应用中心(CiRA)。CiRA是一家异基因iPSCs库,收集了50份来自健康志愿者的外周血T淋巴细胞和脐血HLA纯合标本,在HLA的三个主要位点(HLA-A、HLA-B和DRB1)进行匹配[128]。2015年,Cellular Dynamic International, Inc.还成立了一家细胞治疗库,可以满足19%的美国人口需求。人们相信,以可承受的成本为数百万患者提供大量合适细胞的HLA单体型纯合iPS细胞库可能是一种有效的细胞治疗的策略。第三个障碍是iPSCs衍生细胞的异质性和不成熟性。通常,iPSCs衍生的细胞是异质的。例如,在iPSCs衍生神经元细胞的各种方案中,衍生细胞常常包含不同类型的神经元和其他细胞(如胶质细胞)[129]。在临床使用前,必须严格控制这种异质性,以确保植入细胞的一致性。因此,可以通过荧光活化细胞分选法(fluorescence-activated cell sorting, FACS)或磁激活细胞分选法(magnetic- activated cell sorting, MACS)对iPSCs衍生细胞进行分类[130],仅选择临床研究需要的相关衍生细胞。从人类iPSCs分化出的各类细胞都显示出类似胚胎或新生细胞的表型,这表明iPSCs衍生的细胞是不成熟的。添加甲状腺激素[81]、长期培养[82]等方法都能促进iPSCs衍生细胞成熟,将iPSCs衍生的组织作为嵌合体植入动物体内将进一步促进iPSCs衍生的组织成熟[131],但iPSCs衍生细胞仍然与成熟细胞的状态有一定差距。异种移植和类器官技术的进一步发展将有助于克服这个障碍,进而促进iPSCs临床研究的发展。
第一次iPSCs临床试验(第一阶段)于2014年9月在日本进行。来自RIKEN发展生物学中心的高桥雅代证明了基于iPSCs的视网膜色素变性(RP)治疗的安全性,RP的主要特征是眼睛内额外的血管形成导致视力完全丧失。患者在接受自体iPSCs衍生的视网膜色素上皮细胞移植治疗后,视力有所改善[132]。与此相反的是,Kuriyan等[133]报告了使用自体脂肪干细胞治疗老年性黄斑变性(AMD)的灾难性结果,即患者视力迅速丧失,需要紧急护理。因为不需要大量的制备细胞,使用自体iPSCs产品进行个性化药物治疗似乎是单基因疾病的理想选择,但对于更常见的疾病,特别是如心肌梗死等急性常见疾病,自体iPSCs疗法可能不适用于大部分患者,因为仔细检验每个细胞系需要高昂的成本和大量的时间。也因为这些原因,日本iPSC-RPE实验的第二阶段将使用异基因产品[134]。这些事件表明了基于iPSCs的细胞治疗还有很长的一段路要走。
嵌合抗原受体(chimeric antigen receptor, CAR)T细胞治疗策略已被证明能成功地介导血液恶性肿瘤的消退。但大量制备自体T细胞可能成为负担,特别是由于免疫抑制或细胞毒性治疗、体外低淋巴细胞计数和健康T淋巴细胞的无效扩增[135]。解决方案是对患者衍生的iPSCs进行改造,从而生成和HLA无关的可定制抗原识别的抗原特异性的CART细胞。经基因组编辑的完全相同的人类iPSCs (不论其HLA单倍型如何)可以进一步分化为全功能的组织相容性肿瘤靶向T细胞[136]。迄今为止,动物模型的临床前研究表明,iPSCs衍生的工程CART细胞(iCART)细胞具有抗肿瘤活性[137]。2019年,CiRA和Takeda合作宣布,第一个iCART细胞治疗计划将于2021年开始临床试验第一阶段。iCART治疗可以根据需要为患者量身定制现成的免疫疗法[138]。iCART杀死癌细胞的潜在益处,凸显了临床进化和治疗应用的一个重要里程碑。
与T细胞相比,自然杀伤细胞(NK细胞)是一种淋巴细胞,在天然免疫系统介导抗肿瘤和抗病毒活性的能力中发挥着关键作用而无需MHC限制。应用NK细胞免疫疗法治疗急性髓系白血病的临床试验已显示出显著的疗效,而对其他恶性肿瘤的疗效则较低[139]。但NK细胞与T细胞的健康细胞群都很难获得,通常由单核细胞和其他血细胞的异质混合物组成。iPSCs的出现提供了一种独特的解决方案:可以生产同质和明确的NK细胞群,这些NK细胞很容易通过基因改造来提高抗肿瘤活性,用于临床大规模生产“现成”细胞[140]。修饰后的NK细胞可以表达多种受体,如高亲和力DC16-Fc受体或CAR受体,也可以与其他疗法联合应用来提高其抗实体瘤的能力[141]。到目前为止,第一项研究显示iPSCs作为一个平台生产带有靶向肿瘤的CAR受体的NK细胞的可行性和有效性,在卵巢癌异种移植模型中显示出令人印象深刻的抗肿瘤活性,且该细胞在体内的存活扩展毒性较小[142]。2018年11月,人类临床试验(NCT0384110)获得美国食品和药物管理局(FDA)批准,首次使用克隆iPSCs产生的现成NK细胞(命名为FT500)与T细胞协同作用,以更好地治疗实体恶性肿瘤。向三名患者给药(每次给药含1×108细胞,并结合检查点抑制剂和单克隆抗体),初步安全性评估显示在最初28天的观察期内没有严重不良事件。2019年2月,FT500治疗方案在64名不同恶性肿瘤患者中进行了进一步的安全性测试,临床试验目前正在进行中(NCT0384110)。日本京都大学已授权武田制药(Takeda Pharmaceuticals)开发基于iPSCs的现成CART细胞平台并将其商业化,第一次临床试验的目标日期为2021年。将这些临床前研究转化为临床试验的潜力是令人期待的。
5 结语与展望
自iPSCs技术建立以来,尽管人们在研究疾病机制和潜在治疗方面通过将人类iPSCs与其他新技术相结合而取得了很大进展,但仍有几个重要问题有待解决。除iPSCs衍生的如心肌细胞、肾细胞等各类细胞都显示出成熟度的不足外,iPSCs在重编程过程中与细胞特性、年龄、代谢相关的体细胞记忆的存在也格外引人注目。考虑到iPSCs可能保留与肿瘤细胞部分相关的体细胞记忆(癌基因表达、Warburg代谢谱)[143,144],筛选和消除这些记忆以减少移植时肿瘤形成的风险,此类研究更为急迫且意义重大,但是目前还缺乏有效的方法来避免或消除这些体细胞记忆。综上所述,iPSCs技术的发展为定义和治疗疾病提供了一种强有力的新方法,因为iPSCs代表了一种范式的转变:允许人们直接观察和治疗相关的病人细胞,揭示了基因表达的新关系,拓宽和加深了人们对各种疾病发展的理解。以CRISPR/Cas9、3D类器官、动物嵌合体和microRNA分子开关等为代表的新技术的出现和不断革新,将进一步推动基于iPSCs的疾病建模和治疗发展。虽然iPSCs的应用尚处于起步阶段,应用风险较大,但相信在下一个15年,iPSCs的应用会在个体化再生医学和肿瘤免疫治疗方面展现出广阔的前景。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
DOI:10.1038/385810a0URLPMID:9039911 [本文引用: 1]

Fertilization of mammalian eggs is followed by successive cell divisions and progressive differentiation, first into the early embryo and subsequently into all of the cell types that make up the adult animal. Transfer of a single nucleus at a specific stage of development, to an enucleated unfertilized egg, provided an opportunity to investigate whether cellular differentiation to that stage involved irreversible genetic modification. The first offspring to develop from a differentiated cell were born after nuclear transfer from an embryo-derived cell line that had been induced to become quiescent. Using the same procedure, we now report the birth of live lambs from three new cell populations established from adult mammary gland, fetus and embryo. The fact that a lamb was derived from an adult cell confirms that differentiation of that cell did not involve the irreversible modification of genetic material required for development to term. The birth of lambs from differentiated fetal and adult cells also reinforces previous speculation that by inducing donor cells to become quiescent it will be possible to obtain normal development from a wide variety of differentiated cells.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:17515932 [本文引用: 1]
URLPMID:20660764 [本文引用: 1]
DOI:10.1038/nature08113URL [本文引用: 1]
[本文引用: 3]
[本文引用: 4]
DOI:10.1016/j.cell.2012.09.045URLPMID:23159369 [本文引用: 1]

The ectopic expression of transcription factors can reprogram cell fate, yet it is unknown how the initial binding of factors to the genome relates functionally to the binding seen in the minority of cells that become reprogrammed. We report a map of Oct4, Sox2, Klf4, and c-Myc (O, S, K, and M) on the human genome during the first 48 hr of reprogramming fibroblasts to pluripotency. Three striking aspects of the initial chromatin binding events include an unexpected role for c-Myc in facilitating OSK chromatin engagement, the primacy of O, S, and K as pioneer factors at enhancers of genes that promote reprogramming, and megabase-scale chromatin domains spanned by H3K9me3, including many genes required for pluripotency, that prevent initial OSKM binding and impede the efficiency of reprogramming. We find diverse aspects of initial factor binding that must be overcome in the minority of cells that become reprogrammed.
DOI:10.1016/j.cell.2013.02.043URLPMID:23498940 [本文引用: 1]

Reprogramming to induced pluripotent stem cells (iPSCs) proceeds in a stepwise manner with reprogramming factor binding, transcription, and chromatin states changing during transitions. Evidence is emerging that epigenetic priming events early in the process may be critical for pluripotency induction later. Chromatin and its regulators are important controllers of reprogramming, and reprogramming factor levels, stoichiometry, and extracellular conditions influence the outcome. The rapid progress in characterizing reprogramming is benefiting applications of iPSCs and is already enabling the rational design of novel reprogramming factor cocktails. However, recent studies have also uncovered an epigenetic instability of the X chromosome in human iPSCs that warrants careful consideration.
[本文引用: 1]
DOI:10.1128/MCB.00468-06URLPMID:16954384 [本文引用: 1]

Although the POU transcription factor Oct3/4 is pivotal in maintaining self renewal of embryonic stem (ES) cells, little is known of its molecular mechanisms. We previously reported that the N-terminal transactivation domain of Oct3/4 is required for activation of Lefty1 expression (H. Niwa, S. Masui, I. Chambers, A. G. Smith, and J. Miyazaki, Mol. Cell. Biol. 22:1526-1536, 2002). Here we test whether Lefty1 is a direct target of Oct3/4. We identified an ES cell-specific enhancer upstream of the Lefty1 promoter that contains binding sites for Oct3/4 and Sox2. Unlike other known Oct3/4-Sox2-dependent enhancers, however, this enhancer element could not be activated by Oct3/4 and Sox2 in differentiated cells. By functional screening of ES-specific transcription factors, we found that Kruppel-like factor 4 (Klf4) cooperates with Oct3/4 and Sox2 to activate Lefty1 expression, and that Klf4 acts as a mediating factor that specifically binds to the proximal element of the Lefty1 promoter. DNA microarray analysis revealed that a subset of putative Oct3/4 target genes may be regulated in the same manner. Our findings shed light on a novel function of Oct3/4 in ES cells.
[本文引用: 1]
[本文引用: 1]
URLPMID:18371448 [本文引用: 1]
URLPMID:19167336 [本文引用: 1]
URLPMID:18845712 [本文引用: 3]
[本文引用: 5]
URLPMID:20715179 [本文引用: 1]
URLPMID:18786420 [本文引用: 2]
[本文引用: 1]
URLPMID:19252477 [本文引用: 2]
URLPMID:19269371 [本文引用: 2]
DOI:10.1038/nbt1483URLPMID:18594521 [本文引用: 1]

The study of induced pluripotency is complicated by the need for infection with high-titer retroviral vectors, which results in genetically heterogeneous cell populations. We generated genetically homogeneous 'secondary' somatic cells that carry the reprogramming factors as defined doxycycline (dox)-inducible transgenes. These cells were produced by infecting fibroblasts with dox-inducible lentiviruses, reprogramming by dox addition, selecting induced pluripotent stem cells and producing chimeric mice. Cells derived from these chimeras reprogram upon dox exposure without the need for viral infection with efficiencies 25- to 50-fold greater than those observed using direct infection and drug selection for pluripotency marker reactivation. We demonstrate that (i) various induction levels of the reprogramming factors can induce pluripotency, (ii) the duration of transgene activity directly correlates with reprogramming efficiency, (iii) cells from many somatic tissues can be reprogrammed and (iv) different cell types require different induction levels. This system facilitates the characterization of reprogramming and provides a tool for genetic or chemical screens to enhance reprogramming.
[本文引用: 1]
[本文引用: 1]
URLPMID:19345179 [本文引用: 4]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
URLPMID:23933400 [本文引用: 1]
URLPMID:18818365 [本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.ymgme.2013.06.003URLPMID:23810832 [本文引用: 1]

We generated iPS cells from human dermal fibroblasts (HDFs) of Fabry disease using a Sendai virus (SeVdp) vector; this method has been established by Nakanishi et al. for pathogenic evaluation. We received SeVdp vector from Nakanishi and loaded it simultaneously with four reprogramming factors (Klf4, Oct4, Sox2, and c-Myc) to HDFs of Fabry disease; subsequently, we observed the presence of human iPS-like cells. The Sendai virus nucleocapsid protein was not detected in the fibroblasts by RT-PCR analysis. Additionally, we confirmed an undifferentiated state, alkaline phosphatase staining, and the presence of SSEA-4, TRA-1-60, and TRA-1-81. Moreover, ultrastructural features of these iPS cells included massive membranous cytoplasmic bodies typical of HDFs of Fabry disease. Thus, we successfully generated human iPS cells from HDFs of Fabry disease that retained the genetic conditions of Fabry disease; also, these abnormal iPS cells could not be easily differentiated into mature cell types such as neuronal cells, cardiomyocytes, etc. because of a massive accumulation of membranous cytoplasmic bodies in lysosomes, possibly the persistent damages of intracellular architecture.
URLPMID:10973218 [本文引用: 1]
DOI:10.1016/j.vaccine.2008.09.074URLPMID:18930099 [本文引用: 1]

Viral vectors are promising vaccine tools for eliciting antigen-specific T-cell responses. We previously showed the potential of recombinant Sendai virus (SeV) vectors to induce virus-specific T-cell responses in macaque AIDS models. Here, we have evaluated the immunogenicity of replication-competent V-knocked-out and replication-defective F-deleted SeV vectors in macaques. Intranasal replication-competent and replication-defective SeV immunizations both elicited robust systemic antigen-specific T-cell responses, whereas the responses induced by the former were more durable than those by the latter. However, even the latter-induced T-cell responses remained detectable in a local, retropharyngeal lymph node two months after the immunization. These findings are useful for establishment of a vaccine protocol using SeV vectors.
[本文引用: 1]
URLPMID:19325077 [本文引用: 1]
URLPMID:20139967 [本文引用: 1]
DOI:10.1002/stem.201URLPMID:19697349 [本文引用: 1]

Mouse and human fibroblasts have been transformed into induced pluripotent stem (iPS) cells by retroviral transduction or plasmid transfection with four genes. Unfortunately, viral and plasmid DNA incorporation into chromosomes can lead to disruption of gene transcription and malignant transformation. Tumor formation has been found in offspring of mice generated from blastocysts made mosaic with iPS cells. To proceed with iPS cells for human therapy, reprogramming should be done with transient gene expression. Recently, adenoviral vectors have been used to produce mouse iPS cells without viral integration. Here, we report the successful creation of human iPS cells from embryonic fibroblasts using adenoviral vectors expressing c-Myc, Klf4, Oct4, and Sox2. After screening 12 colonies, three stable iPS cell lines were established. Each cell line showed human embryonic stem cell morphology and surface markers. Southern blots and polymerase chain reaction demonstrated that there was no viral DNA integration into iPS cells. Fingerprinting and karyotype analysis confirmed that these iPS cell lines are derived from the parent human fibroblasts. The three human iPS cell lines can differentiate to all three germ layers in vitro, including dopaminergic neurons. After s.c. injection into nonobese diabetic-severe combined immunodeficient mice, each human iPS line produced teratomas within 5 weeks postimplantation. We conclude that adenoviral vectors can reprogram human fibroblasts to pluripotent stem cells for use in individualized cell therapy without the risk for viral or oncogene incorporation.
[本文引用: 1]
DOI:10.1016/j.stem.2009.05.005URLPMID:19481515 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:18849973 [本文引用: 1]
DOI:10.1038/nbt1418URLPMID:18568017 [本文引用: 1]

Reprogramming of mouse and human somatic cells can be achieved by ectopic expression of transcription factors, but with low efficiencies. We report that DNA methyltransferase and histone deacetylase (HDAC) inhibitors improve reprogramming efficiency. In particular, valproic acid (VPA), an HDAC inhibitor, improves reprogramming efficiency by more than 100-fold, using Oct4-GFP as a reporter. VPA also enables efficient induction of pluripotent stem cells without introduction of the oncogene c-Myc.
[本文引用: 1]
URLPMID:19716359 [本文引用: 1]
DOI:10.1038/nature08235URLPMID:19668191 [本文引用: 1]

Induced pluripotent stem (iPS) cells can be generated from somatic cells by the introduction of Oct3/4 (also known as Pou5f1), Sox2, Klf4 and c-Myc, in mouse and in human. The efficiency of this process, however, is low. Pluripotency can be induced without c-Myc, but with even lower efficiency. A p53 (also known as TP53 in humans and Trp53 in mice) short-interfering RNA (siRNA) was recently shown to promote human iPS cell generation, but the specificity and mechanisms remain to be determined. Here we report that up to 10% of transduced mouse embryonic fibroblasts lacking p53 became iPS cells, even without the Myc retrovirus. The p53 deletion also promoted the induction of integration-free mouse iPS cells with plasmid transfection. Furthermore, in the p53-null background, iPS cells were generated from terminally differentiated T lymphocytes. The suppression of p53 also increased the efficiency of human iPS cell generation. DNA microarray analyses identified 34 p53-regulated genes that are common in mouse and human fibroblasts. Functional analyses of these genes demonstrate that the p53-p21 pathway serves as a barrier not only in tumorigenicity, but also in iPS cell generation.
[本文引用: 1]
URLPMID:21654807 [本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.cub.2009.08.025URLPMID:19765992 [本文引用: 1]

Ectopic expression of Oct4, Sox2, cMyc, and Klf4 confers a pluripotent state upon several differentiated cell types, generating induced pluripotent stem cells (iPSCs) [1-8]. iPSC derivation is highly inefficient, and the underlying mechanisms are largely unknown. This low efficiency suggests the existence of additional cooperative factors whose identification is critical for understanding reprogramming. In addition, the therapeutic use of iPSCs relies on the development of efficient nongenetic means of factor delivery, and although a handful of replacement molecules have been identified, their use yields a further reduction to the already low reprogramming efficiency [9-11]. Thus, the identification of compounds that enhance rather than solely replace the function of the reprogramming factors will be of great use. Here, we demonstrate that inhibition of Tgfbbeta signaling cooperates in the reprogramming of murine fibroblasts by enabling faster, more efficient induction of iPSCs, whereas activation of Tgfbeta signaling blocks reprogramming. In addition to exhibiting a strong cooperative effect, the Tgfbeta receptor inhibitor bypasses the requirement for exogenous cMyc or Sox2, highlighting its dual role as a cooperative and replacement factor. The identification of a highly characterized pathway operating in iPSC induction will open new avenues for mechanistic dissection of the reprogramming process.
[本文引用: 2]
DOI:10.1016/j.stem.2011.03.001URLPMID:21474102 [本文引用: 2]

Transcription factor-based cellular reprogramming has opened the way to converting somatic cells to a pluripotent state, but has faced limitations resulting from the requirement for transcription factors and the relative inefficiency of the process. We show here that expression of the miR302/367 cluster rapidly and efficiently reprograms mouse and human somatic cells to an iPSC state without a requirement for exogenous transcription factors. This miRNA-based reprogramming approach is two orders of magnitude more efficient than standard Oct4/Sox2/Klf4/Myc-mediated methods. Mouse and human miR302/367 iPSCs display similar characteristics to Oct4/Sox2/Klf4/Myc-iPSCs, including pluripotency marker expression, teratoma formation, and, for mouse cells, chimera contribution and germline contribution. We found that miR367 expression is required for miR302/367-mediated reprogramming and activates Oct4 gene expression, and that suppression of Hdac2 is also required. Thus, our data show that miRNA and Hdac-mediated pathways can cooperate in a powerful way to reprogram somatic cells to pluripotency.
[本文引用: 2]
URLPMID:27152442 [本文引用: 3]
DOI:10.1038/nbt.1562URLPMID:19680244 [本文引用: 2]

Realizing the full potential of human embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs) requires efficient methods for genetic modification. However, techniques to generate cell type-specific lineage reporters, as well as reliable tools to disrupt, repair or overexpress genes by gene targeting, are inefficient at best and thus are not routinely used. Here we report the highly efficient targeting of three genes in human pluripotent cells using zinc-finger nuclease (ZFN)-mediated genome editing. First, using ZFNs specific for the OCT4 (POU5F1) locus, we generated OCT4-eGFP reporter cells to monitor the pluripotent state of hESCs. Second, we inserted a transgene into the AAVS1 locus to generate a robust drug-inducible overexpression system in hESCs. Finally, we targeted the PITX3 gene, demonstrating that ZFNs can be used to generate reporter cells by targeting non-expressed genes in hESCs and hiPSCs.
DOI:10.1016/j.stem.2009.05.023URLPMID:19540188 [本文引用: 1]

We report here homologous recombination (HR)-mediated gene targeting of two different genes in human iPS cells (hiPSCs) and human ES cells (hESCs). HR-mediated correction of a chromosomally integrated mutant GFP reporter gene reaches efficiencies of 0.14%-0.24% in both cell types transfected by donor DNA with plasmids expressing zinc finger nucleases (ZFNs). Engineered ZFNs that induce a sequence-specific double-strand break in the GFP gene enhanced HR-mediated correction by > 1400-fold without detectable alterations in stem cell karyotypes or pluripotency. Efficient HR-mediated insertional mutagenesis was also achieved at the endogenous PIG-A locus, with a > 200-fold enhancement by ZFNs targeted to that gene. Clonal PIG-A null hESCs and iPSCs with normal karyotypes were readily obtained. The phenotypic and biological defects were rescued by PIG-A transgene expression. Our study provides the first demonstration of HR-mediated gene targeting in hiPSCs and shows the power of ZFNs for inducing specific genetic modifications in hiPSCs, as well as hESCs.
URLPMID:20660643 [本文引用: 1]
DOI:10.1038/nbt.1927URLPMID:21738127 [本文引用: 1]

Targeted genetic engineering of human pluripotent cells is a prerequisite for exploiting their full potential. Such genetic manipulations can be achieved using site-specific nucleases. Here we engineered transcription activator-like effector nucleases (TALENs) for five distinct genomic loci. At all loci tested we obtained human embryonic stem cell (ESC) and induced pluripotent stem cell (iPSC) clones carrying transgenic cassettes solely at the TALEN-specified location. Our data suggest that TALENs employing the specific architectures described here mediate site-specific genome modification in human pluripotent cells with similar efficiency and precision as do zinc-finger nucleases (ZFNs).
DOI:10.1126/science.1231143URLPMID:23287718 [本文引用: 2]

Functional elucidation of causal genetic variants and elements requires precise genome editing technologies. The type II prokaryotic CRISPR (clustered regularly interspaced short palindromic repeats)/Cas adaptive immune system has been shown to facilitate RNA-guided site-specific DNA cleavage. We engineered two different type II CRISPR/Cas systems and demonstrate that Cas9 nucleases can be directed by short RNAs to induce precise cleavage at endogenous genomic loci in human and mouse cells. Cas9 can also be converted into a nicking enzyme to facilitate homology-directed repair with minimal mutagenic activity. Lastly, multiple guide sequences can be encoded into a single CRISPR array to enable simultaneous editing of several sites within the mammalian genome, demonstrating easy programmability and wide applicability of the RNA-guided nuclease technology.
DOI:10.1038/nbt.2623URLPMID:23792628 [本文引用: 2]

Clustered, regularly interspaced, short palindromic repeat (CRISPR) RNA-guided nucleases (RGNs) have rapidly emerged as a facile and efficient platform for genome editing. Here, we use a human cell-based reporter assay to characterize off-target cleavage of CRISPR-associated (Cas)9-based RGNs. We find that single and double mismatches are tolerated to varying degrees depending on their position along the guide RNA (gRNA)-DNA interface. We also readily detected off-target alterations induced by four out of six RGNs targeted to endogenous loci in human cells by examination of partially mismatched sites. The off-target sites we identified harbored up to five mismatches and many were mutagenized with frequencies comparable to (or higher than) those observed at the intended on-target site. Our work demonstrates that RGNs can be highly active even with imperfectly matched RNA-DNA interfaces in human cells, a finding that might confound their use in research and therapeutic applications.
DOI:10.1016/j.stem.2014.06.011URLPMID:24996165 [本文引用: 1]
DOI:10.1016/j.stem.2014.04.020URLPMID:24996167 [本文引用: 2]

Genome editing has attracted wide interest for the generation of cellular models of disease using human pluripotent stem cells and other cell types. CRISPR-Cas systems and TALENs can target desired genomic sites with high efficiency in human cells, but recent publications have led to concern about the extent to which these tools may cause off-target mutagenic effects that could potentially confound disease-modeling studies. Using CRISPR-Cas9 and TALEN targeted human pluripotent stem cell clones, we performed whole-genome sequencing at high coverage in order to assess the degree of mutagenesis across the entire genome. In both types of clones, we found that off-target mutations attributable to the nucleases were very rare. From this analysis, we suggest that, although some cell types may be at risk for off-target mutations, the incidence of such effects in human pluripotent stem cells may be sufficiently low and thus not a significant concern for disease modeling and other applications.
DOI:10.1016/j.cell.2013.08.021URLPMID:23992846 [本文引用: 2]

Targeted genome editing technologies have enabled a broad range of research and medical applications. The Cas9 nuclease from the microbial CRISPR-Cas system is targeted to specific genomic loci by a 20 nt guide sequence, which can tolerate certain mismatches to the DNA target and thereby promote undesired off-target mutagenesis. Here, we describe an approach that combines a Cas9 nickase mutant with paired guide RNAs to introduce targeted double-strand breaks. Because individual nicks in the genome are repaired with high fidelity, simultaneous nicking via appropriately offset guide RNAs is required for double-stranded breaks and extends the number of specifically recognized bases for target cleavage. We demonstrate that using paired nicking can reduce off-target activity by 50- to 1,500-fold in cell lines and to facilitate gene knockout in mouse zygotes without sacrificing on-target cleavage efficiency. This versatile strategy enables a wide variety of genome editing applications that require high specificity.
DOI:10.1126/science.aad5227URLPMID:26628643 [本文引用: 1]

The RNA-guided endonuclease Cas9 is a versatile genome-editing tool with a broad range of applications from therapeutics to functional annotation of genes. Cas9 creates double-strand breaks (DSBs) at targeted genomic loci complementary to a short RNA guide. However, Cas9 can cleave off-target sites that are not fully complementary to the guide, which poses a major challenge for genome editing. Here, we use structure-guided protein engineering to improve the specificity of Streptococcus pyogenes Cas9 (SpCas9). Using targeted deep sequencing and unbiased whole-genome off-target analysis to assess Cas9-mediated DNA cleavage in human cells, we demonstrate that
DOI:10.1016/j.cell.2013.12.001URLPMID:24360272 [本文引用: 1]

The spatiotemporal organization and dynamics of chromatin play critical roles in regulating genome function. However, visualizing specific, endogenous genomic loci remains challenging in living cells. Here, we demonstrate such an imaging technique by repurposing the bacterial CRISPR/Cas system. Using an EGFP-tagged endonuclease-deficient Cas9 protein and a structurally optimized small guide (sg) RNA, we show robust imaging of repetitive elements in telomeres and coding genes in living cells. Furthermore, an array of sgRNAs tiling along the target locus enables the visualization of nonrepetitive genomic sequences. Using this method, we have studied telomere dynamics during elongation or disruption, the subnuclear localization of the MUC4 loci, the cohesion of replicated MUC4 loci on sister chromatids, and their dynamic behaviors during mitosis. This CRISPR imaging tool has potential to significantly improve the capacity to study the conformation and dynamics of native chromosomes in living human cells.
DOI:10.1038/nature17664URLPMID:27120160 [本文引用: 1]

The bacterial CRISPR/Cas9 system allows sequence-specific gene editing in many organisms and holds promise as a tool to generate models of human diseases, for example, in human pluripotent stem cells. CRISPR/Cas9 introduces targeted double-stranded breaks (DSBs) with high efficiency, which are typically repaired by non-homologous end-joining (NHEJ) resulting in nonspecific insertions, deletions or other mutations (indels). DSBs may also be repaired by homology-directed repair (HDR) using a DNA repair template, such as an introduced single-stranded oligo DNA nucleotide (ssODN), allowing knock-in of specific mutations. Although CRISPR/Cas9 is used extensively to engineer gene knockouts through NHEJ, editing by HDR remains inefficient and can be corrupted by additional indels, preventing its widespread use for modelling genetic disorders through introducing disease-associated mutations. Furthermore, targeted mutational knock-in at single alleles to model diseases caused by heterozygous mutations has not been reported. Here we describe a CRISPR/Cas9-based genome-editing framework that allows selective introduction of mono- and bi-allelic sequence changes with high efficiency and accuracy. We show that HDR accuracy is increased dramatically by incorporating silent CRISPR/Cas-blocking mutations along with pathogenic mutations, and establish a method termed 'CORRECT' for scarless genome editing. By characterizing and exploiting a stereotyped inverse relationship between a mutation's incorporation rate and its distance to the DSB, we achieve predictable control of zygosity. Homozygous introduction requires a guide RNA targeting close to the intended mutation, whereas heterozygous introduction can be accomplished by distance-dependent suboptimal mutation incorporation or by use of mixed repair templates. Using this approach, we generated human induced pluripotent stem cells with heterozygous and homozygous dominant early onset Alzheimer's disease-causing mutations in amyloid precursor protein (APP(Swe)) and presenilin 1 (PSEN1(M146V)) and derived cortical neurons, which displayed genotype-dependent disease-associated phenotypes. Our findings enable efficient introduction of specific sequence changes with CRISPR/Cas9, facilitating study of human disease.
DOI:10.1038/nature17946URLPMID:27096365 [本文引用: 1]

Current genome-editing technologies introduce double-stranded (ds) DNA breaks at a target locus as the first step to gene correction. Although most genetic diseases arise from point mutations, current approaches to point mutation correction are inefficient and typically induce an abundance of random insertions and deletions (indels) at the target locus resulting from the cellular response to dsDNA breaks. Here we report the development of 'base editing', a new approach to genome editing that enables the direct, irreversible conversion of one target DNA base into another in a programmable manner, without requiring dsDNA backbone cleavage or a donor template. We engineered fusions of CRISPR/Cas9 and a cytidine deaminase enzyme that retain the ability to be programmed with a guide RNA, do not induce dsDNA breaks, and mediate the direct conversion of cytidine to uridine, thereby effecting a C-->T (or G-->A) substitution. The resulting 'base editors' convert cytidines within a window of approximately five nucleotides, and can efficiently correct a variety of point mutations relevant to human disease. In four transformed human and murine cell lines, second- and third-generation base editors that fuse uracil glycosylase inhibitor, and that use a Cas9 nickase targeting the non-edited strand, manipulate the cellular DNA repair response to favour desired base-editing outcomes, resulting in permanent correction of ~15-75% of total cellular DNA with minimal (typically
URLPMID:19098894 [本文引用: 4]
DOI:10.1016/s0960-8966(06)80015-5URLPMID:1300191 [本文引用: 1]
DOI:10.1161/CIRCRESAHA.114.300558URLPMID:24481842 [本文引用: 1]

The discovery of human pluripotent stem cells (hPSCs), including both human embryonic stem cells and human-induced pluripotent stem cells, has opened up novel paths for a wide range of scientific studies. The capability to direct the differentiation of hPSCs into functional cardiomyocytes has provided a platform for regenerative medicine, development, tissue engineering, disease modeling, and drug toxicity testing. Despite exciting progress, achieving the optimal benefits has been hampered by the immature nature of these cardiomyocytes. Cardiac maturation has long been studied in vivo using animal models; however, finding ways to mature hPSC cardiomyocytes is only in its initial stages. In this review, we discuss progress in promoting the maturation of the hPSC cardiomyocytes, in the context of our current knowledge of developmental cardiac maturation and in relation to in vitro model systems such as rodent ventricular myocytes. Promising approaches that have begun to be examined in hPSC cardiomyocytes include long-term culturing, 3-dimensional tissue engineering, mechanical loading, electric stimulation, modulation of substrate stiffness, and treatment with neurohormonal factors. Future studies will benefit from the combinatorial use of different approaches that more closely mimic nature's diverse cues, which may result in broader changes in structure, function, and therapeutic applicability.
[本文引用: 1]
DOI:10.1016/j.yjmcc.2014.04.005URLPMID:24735830 [本文引用: 2]

BACKGROUND: Cardiomyocytes derived from human induced pluripotent stem cells (hiPSC-CMs) have great potential as a cell source for therapeutic applications such as regenerative medicine, disease modeling, drug screening, and toxicity testing. This potential is limited, however, by the immature state of the cardiomyocytes acquired using current protocols. Tri-iodo-l-thyronine (T3) is a growth hormone that is essential for optimal heart growth. In this study, we investigated the effect of T3 on hiPSC-CM maturation. METHODS AND RESULTS: A one-week treatment with T3 increased cardiomyocyte size, anisotropy, and sarcomere length. T3 treatment was associated with reduced cell cycle activity, manifest as reduced DNA synthesis and increased expression of the cyclin-dependent kinase inhibitor p21. Contractile force analyses were performed on individual cardiomyocytes using arrays of microposts, revealing an almost two-fold higher force per-beat after T3 treatment and also an enhancement in contractile kinetics. This improvement in force generation was accompanied by an increase in rates of calcium release and reuptake, along with a significant increase in sarcoendoplasmic reticulum ATPase expression. Finally, although mitochondrial genomes were not numerically increased, extracellular flux analysis showed a significant increase in maximal mitochondrial respiratory capacity and respiratory reserve capability after T3 treatment. CONCLUSIONS: Using a broad spectrum of morphological, molecular, and functional parameters, we conclude that T3 is a driver for hiPSC-CM maturation. T3 treatment may enhance the utility of hiPSC-CMs for therapy, disease modeling, or drug/toxicity screens.
DOI:10.1253/circj.cj-12-0987URLPMID:23400258 [本文引用: 2]

BACKGROUND: In the short- to mid-term, cardiomyocytes generated from human-induced pluripotent stem cells (hiPSC-CMs) have been reported to be less mature than those of adult hearts. However, the maturation process in a long-term culture remains unknown. METHODS AND RESULTS: A hiPSC clone generated from a healthy control was differentiated into CMs through embryoid body (EB) formation. The ultrastructural characteristics and gene expressions of spontaneously contracting EBs were analyzed through 1-year of culture after cardiac differentiation was initiated. The 14-day-old EBs contained a low number of myofibrils, which lacked alignment, and immature high-density Z-bands lacking A-, H-, I-, and M-bands. Through the long-term culture up to 180 days, the myofibrils became more tightly packed and formed parallel arrays accompanied by the appearance of mature Z-, A-, H-, and I-bands, but not M-bands. Notably, M-bands were finally detected in 360-day-old EBs. The expression levels of the M-band-specific genes in hiPSC-CMs remained lower in comparison with those in the adult heart. Immunocytochemistry indicated increasing number of MLC2v-positive/MLC2a-negative cells with decreasing number of MLC2v/MLC2a double-positive cells, indicating maturing of ventricular-type CMs. CONCLUSIONS: The structural maturation process of hiPSC-CMs through 1-year of culture revealed ultrastructural sarcomeric changes accompanied by delayed formation of M-bands. Our study provides new insight into the maturation process of hiPSC-CMs.
DOI:10.1038/nmeth.2524URLPMID:23793239 [本文引用: 1]

Directed differentiation protocols enable derivation of cardiomyocytes from human pluripotent stem cells (hPSCs) and permit engineering of human myocardium in vitro. However, hPSC-derived cardiomyocytes are reflective of very early human development, limiting their utility in the generation of in vitro models of mature myocardium. Here we describe a platform that combines three-dimensional cell cultivation with electrical stimulation to mature hPSC-derived cardiac tissues. We used quantitative structural, molecular and electrophysiological analyses to explain the responses of immature human myocardium to electrical stimulation and pacing. We demonstrated that the engineered platform allows for the generation of three-dimensional, aligned cardiac tissues (biowires) with frequent striations. Biowires submitted to electrical stimulation had markedly increased myofibril ultrastructural organization, elevated conduction velocity and improved both electrophysiological and Ca(2+) handling properties compared to nonstimulated controls. These changes were in agreement with cardiomyocyte maturation and were dependent on the stimulation rate.
URLPMID:27029645 [本文引用: 1]
DOI:10.1016/j.stem.2013.11.006URLPMID:24315443 [本文引用: 1]

Reprogramming somatic cells to induced pluripotent stem cells (iPSCs) resets their identity back to an embryonic age and, thus, presents a significant hurdle for modeling late-onset disorders. In this study, we describe a strategy for inducing aging-related features in human iPSC-derived lineages and apply it to the modeling of Parkinson's disease (PD). Our approach involves expression of progerin, a truncated form of lamin A associated with premature aging. We found that expression of progerin in iPSC-derived fibroblasts and neurons induces multiple aging-related markers and characteristics, including dopamine-specific phenotypes such as neuromelanin accumulation. Induced aging in PD iPSC-derived dopamine neurons revealed disease phenotypes that require both aging and genetic susceptibility, such as pronounced dendrite degeneration, progressive loss of tyrosine hydroxylase (TH) expression, and enlarged mitochondria or Lewy-body-precursor inclusions. Thus, our study suggests that progerin-induced aging can be used to reveal late-onset age-related disease features in hiPSC-based disease models.
DOI:10.1016/j.stem.2013.01.009URL [本文引用: 1]

Oligomeric forms of amyloid-beta peptide (A beta) are thought to play a pivotal role in the pathogenesis of Alzheimer's disease (AD), but the mechanism involved is still unclear. Here, we generated induced pluripotent stem cells (iPSCs) from familial and sporadic AD patients and differentiated them into neural cells. A beta oligomers accumulated in iPSC-derived neurons and astrocytes in cells from patients with a familial amyloid precursor protein (APP)-E693 Delta mutation and sporadic AD, leading to endoplasmic reticulum (ER) and oxidative stress. The accumulated A beta oligomers were not proteolytically resistant, and docosahexaenoic acid (DHA) treatment alleviated the stress responses in the AD neural cells. Differential manifestation of ER stress and DHA responsiveness may help explain variable clinical results obtained with the use of DHA treatment and suggests that DHA may in fact be effective for a subset of patients. It also illustrates how patient-specific iPSCs can be useful for analyzing AD pathogenesis and evaluating drugs.
URLPMID:27096366 [本文引用: 2]
DOI:10.1182/blood-2013-01-478412URLPMID:23620576 [本文引用: 2]

Juvenile myelomonocytic leukemia (JMML) is an aggressive myeloproliferative neoplasm of young children initiated by mutations that deregulate cytokine receptor signaling. Studies of JMML are constrained by limited access to patient tissues. We generated induced pluripotent stem cells (iPSCs) from malignant cells of two JMML patients with somatic heterozygous p.E76K missense mutations in PTPN11, which encodes SHP-2, a nonreceptor tyrosine phosphatase. In vitro differentiation of JMML iPSCs produced myeloid cells with increased proliferative capacity, constitutive activation of granulocyte macrophage colony-stimulating factor (GM-CSF), and enhanced STAT5/ERK phosphorylation, similar to primary JMML cells from patients. Pharmacological inhibition of MEK kinase in iPSC-derived JMML cells reduced their GM-CSF independence, providing rationale for a potential targeted therapy. Our studies offer renewable sources of biologically relevant human cells in which to explore the pathophysiology and treatment of JMML. More generally, we illustrate the utility of iPSCs for in vitro modeling of a human malignancy.
URLPMID:8500106 [本文引用: 1]
DOI:10.1016/j.cell.2015.02.045URLPMID:25860607 [本文引用: 1]

In vitro modeling of human disease has recently become feasible with induced pluripotent stem cell (iPSC) technology. Here, we established patient-derived iPSCs from a Li-Fraumeni syndrome (LFS) family and investigated the role of mutant p53 in the development of osteosarcoma (OS). LFS iPSC-derived osteoblasts (OBs) recapitulated OS features including defective osteoblastic differentiation as well as tumorigenic ability. Systematic analyses revealed that the expression of genes enriched in LFS-derived OBs strongly correlated with decreased time to tumor recurrence and poor patient survival. Furthermore, LFS OBs exhibited impaired upregulation of the imprinted gene H19 during osteogenesis. Restoration of H19 expression in LFS OBs facilitated osteoblastic differentiation and repressed tumorigenic potential. By integrating human imprinted gene network (IGN) into functional genomic analyses, we found that H19 mediates suppression of LFS-associated OS through the IGN component DECORIN (DCN). In summary, these findings demonstrate the feasibility of studying inherited human cancer syndromes with iPSCs.
URLPMID:28292841 [本文引用: 2]
DOI:10.1016/j.molmed.2017.02.007URL [本文引用: 2]
DOI:10.1038/nature08320URLPMID:19693009 [本文引用: 1]

The isolation of human induced pluripotent stem cells (iPSCs) offers a new strategy for modelling human disease. Recent studies have reported the derivation and differentiation of disease-specific human iPSCs. However, a key challenge in the field is the demonstration of disease-related phenotypes and the ability to model pathogenesis and treatment of disease in iPSCs. Familial dysautonomia (FD) is a rare but fatal peripheral neuropathy, caused by a point mutation in the IKBKAP gene involved in transcriptional elongation. The disease is characterized by the depletion of autonomic and sensory neurons. The specificity to the peripheral nervous system and the mechanism of neuron loss in FD are poorly understood owing to the lack of an appropriate model system. Here we report the derivation of patient-specific FD-iPSCs and the directed differentiation into cells of all three germ layers including peripheral neurons. Gene expression analysis in purified FD-iPSC-derived lineages demonstrates tissue-specific mis-splicing of IKBKAP in vitro. Patient-specific neural crest precursors express particularly low levels of normal IKBKAP transcript, suggesting a mechanism for disease specificity. FD pathogenesis is further characterized by transcriptome analysis and cell-based assays revealing marked defects in neurogenic differentiation and migration behaviour. Furthermore, we use FD-iPSCs for validating the potency of candidate drugs in reversing aberrant splicing and ameliorating neuronal differentiation and migration. Our study illustrates the promise of iPSC technology for gaining new insights into human disease pathogenesis and treatment.
DOI:10.1038/nature12517URL [本文引用: 3]

The complexity of the human brain has made it difficult to study many brain disorders in model organisms, highlighting the need for an in vitro model of human brain development. Here we have developed a human pluripotent stem cell-derived three-dimensional organoid culture system, termed cerebral organoids, that develop various discrete, although interdependent, brain regions. These include a cerebral cortex containing progenitor populations that organize and produce mature cortical neuron subtypes. Furthermore, cerebral organoids are shown to recapitulate features of human cortical development, namely characteristic progenitor zone organization with abundant outer radial glial stem cells. Finally, we use RNA interference and patient-specific induced pluripotent stem cells to model microcephaly, a disorder that has been difficult to recapitulate in mice. We demonstrate premature neuronal differentiation in patient organoids, a defect that could help to explain the disease phenotype. Together, these data show that three-dimensional organoids can recapitulate development and disease even in this most complex human tissue.
DOI:10.1038/nm.4214URLPMID:27783065 [本文引用: 1]

Neuropsychiatric disorders such as autism spectrum disorder (ASD), schizophrenia (SCZ) and bipolar disorder (BPD) are of great societal and medical importance, but the complexity of these diseases and the challenges of modeling the development and function of the human brain have made these disorders difficult to study experimentally. The recent development of 3D brain organoids derived from human pluripotent stem cells offers a promising approach for investigating the phenotypic underpinnings of these highly polygenic disorders and for understanding the contribution of individual risk variants and complex genetic background to human pathology. Here we discuss the advantages, limitations and future applications of human brain organoids as in vitro models of neuropsychiatric disease.
DOI:10.1016/j.neuron.2017.07.035URLPMID:28817799 [本文引用: 1]

There is significant need to develop physiologically relevant models for investigating human astrocytes in health and disease. Here, we present an approach for generating astrocyte lineage cells in a three-dimensional (3D) cytoarchitecture using human cerebral cortical spheroids (hCSs) derived from pluripotent stem cells. We acutely purified astrocyte-lineage cells from hCSs at varying stages up to 20 months in vitro using immunopanning and cell sorting and performed high-depth bulk and single-cell RNA sequencing to directly compare them to purified primary human brain cells. We found that hCS-derived glia closely resemble primary human fetal astrocytes and that, over time in vitro, they transition from a predominantly fetal to an increasingly mature astrocyte state. Transcriptional changes in astrocytes are accompanied by alterations in phagocytic capacity and effects on neuronal calcium signaling. These findings suggest that hCS-derived astrocytes closely resemble primary human astrocytes and can be used for studying development and modeling disease.
DOI:10.15252/embj.201593679URLPMID:26929011 [本文引用: 1]

A mutation in the centrosomal-P4.1-associated protein (CPAP) causes Seckel syndrome with microcephaly, which is suggested to arise from a decline in neural progenitor cells (NPCs) during development. However, mechanisms ofNPCs maintenance remain unclear. Here, we report an unexpected role for the cilium inNPCs maintenance and identifyCPAPas a negative regulator of ciliary length independent of its role in centrosome biogenesis. At the onset of cilium disassembly,CPAPprovides a scaffold for the cilium disassembly complex (CDC), which includes Nde1, Aurora A, andOFD1, recruited to the ciliary base for timely cilium disassembly. In contrast, mutatedCPAPfails to localize at the ciliary base associated with inefficientCDCrecruitment, long cilia, retarded cilium disassembly, and delayed cell cycle re-entry leading to premature differentiation of patientiPS-derivedNPCs. AberrantCDCfunction also promotes premature differentiation ofNPCs in SeckeliPS-derived organoids. Thus, our results suggest a role for cilia in microcephaly and its involvement during neurogenesis and brain size control.
DOI:10.1038/s41592-018-0070-7URLPMID:30038414 [本文引用: 1]

Brain tumors are among the most lethal and devastating cancers. Their study is limited by genetic heterogeneity and the incompleteness of available laboratory models. Three-dimensional organoid culture models offer innovative possibilities for the modeling of human disease. Here we establish a 3D in vitro model called a neoplastic cerebral organoid (neoCOR), in which we recapitulate brain tumorigenesis by introducing oncogenic mutations in cerebral organoids via transposon- and CRISPR-Cas9-mediated mutagenesis. By screening clinically relevant mutations identified in cancer genome projects, we defined mutation combinations that result in glioblastoma-like and central nervous system primitive neuroectodermal tumor (CNS-PNET)-like neoplasms. We demonstrate that neoCORs are suitable for use in investigations of aspects of tumor biology such as invasiveness, and for evaluation of drug effects in the context of specific DNA aberrations. NeoCORs will provide a valuable complement to the current basic and preclinical models used to study brain tumor biology.
DOI:10.1172/JCI43122URLPMID:20739751 [本文引用: 1]

Human induced pluripotent stem (iPS) cells hold great promise for advancements in developmental biology, cell-based therapy, and modeling of human disease. Here, we examined the use of human iPS cells for modeling inherited metabolic disorders of the liver. Dermal fibroblasts from patients with various inherited metabolic diseases of the liver were used to generate a library of patient-specific human iPS cell lines. Each line was differentiated into hepatocytes using what we believe to be a novel 3-step differentiation protocol in chemically defined conditions. The resulting cells exhibited properties of mature hepatocytes, such as albumin secretion and cytochrome P450 metabolism. Moreover, cells generated from patients with 3 of the inherited metabolic conditions studied in further detail (alpha1-antitrypsin deficiency, familial hypercholesterolemia, and glycogen storage disease type 1a) were found to recapitulate key pathological features of the diseases affecting the patients from which they were derived, such as aggregation of misfolded alpha1-antitrypsin in the endoplasmic reticulum, deficient LDL receptor-mediated cholesterol uptake, and elevated lipid and glycogen accumulation. Therefore, we report a simple and effective platform for hepatocyte generation from patient-specific human iPS cells. These patient-derived hepatocytes demonstrate that it is possible to model diseases whose phenotypes are caused by pathological dysregulation of key processes within adult cells.
DOI:10.1038/nbt.3294URLPMID:26167630 [本文引用: 2]

Although bile duct disorders are well-recognized causes of liver disease, the molecular and cellular events leading to biliary dysfunction are poorly understood. To enable modeling and drug discovery for biliary disease, we describe a protocol that achieves efficient differentiation of biliary epithelial cells (cholangiocytes) from human pluripotent stem cells (hPSCs) through delivery of developmentally relevant cues, including NOTCH signaling. Using three-dimensional culture, the protocol yields cystic and/or ductal structures that express mature biliary markers, including apical sodium-dependent bile acid transporter, secretin receptor, cilia and cystic fibrosis transmembrane conductance regulator (CFTR). We demonstrate that hPSC-derived cholangiocytes possess epithelial functions, including rhodamine efflux and CFTR-mediated fluid secretion. Furthermore, we show that functionally impaired hPSC-derived cholangiocytes from cystic fibrosis patients are rescued by CFTR correctors. These findings demonstrate that mature cholangiocytes can be differentiated from hPSCs and used for studies of biliary development and disease.
DOI:10.1038/nbt.3275URLPMID:26167629 [本文引用: 4]

The study of biliary disease has been constrained by a lack of primary human cholangiocytes. Here we present an efficient, serum-free protocol for directed differentiation of human induced pluripotent stem cells into cholangiocyte-like cells (CLCs). CLCs show functional characteristics of cholangiocytes, including bile acids transfer, alkaline phosphatase activity, gamma-glutamyl-transpeptidase activity and physiological responses to secretin, somatostatin and vascular endothelial growth factor. We use CLCs to model in vitro key features of Alagille syndrome, polycystic liver disease and cystic fibrosis (CF)-associated cholangiopathy. Furthermore, we use CLCs generated from healthy individuals and patients with polycystic liver disease to reproduce the effects of the drugs verapamil and octreotide, and we show that the experimental CF drug VX809 rescues the disease phenotype of CF cholangiopathy in vitro. Our differentiation protocol will facilitate the study of biological mechanisms controlling biliary development, as well as disease modeling and drug screening.
DOI:10.1242/dev.143966URLPMID:28174241 [本文引用: 1]

The adult human heart possesses a limited regenerative potential following an ischemic event, and undergoes a number of pathological changes in response to injury. Although cardiac regeneration has been documented in zebrafish and neonatal mouse hearts, it is currently unknown whether the immature human heart is capable of undergoing complete regeneration. Combined progress in pluripotent stem cell differentiation and tissue engineering has facilitated the development of human cardiac organoids (hCOs), which resemble fetal heart tissue and can be used to address this important knowledge gap. This study aimed to characterize the regenerative capacity of immature human heart tissue in response to injury. Following cryoinjury with a dry ice probe, hCOs exhibited an endogenous regenerative response with full functional recovery 2 weeks after acute injury. Cardiac functional recovery occurred in the absence of pathological fibrosis or cardiomyocyte hypertrophy. Consistent with regenerative organisms and neonatal human hearts, there was a high basal level of cardiomyocyte proliferation, which may be responsible for the regenerative capacity of the hCOs. This study suggests that immature human heart tissue has an intrinsic capacity to regenerate.
DOI:10.1016/j.cell.2014.09.040URLPMID:25303535 [本文引用: 1]

The generation of insulin-producing pancreatic beta cells from stem cells in vitro would provide an unprecedented cell source for drug discovery and cell transplantation therapy in diabetes. However, insulin-producing cells previously generated from human pluripotent stem cells (hPSC) lack many functional characteristics of bona fide beta cells. Here, we report a scalable differentiation protocol that can generate hundreds of millions of glucose-responsive beta cells from hPSC in vitro. These stem-cell-derived beta cells (SC-beta) express markers found in mature beta cells, flux Ca(2+) in response to glucose, package insulin into secretory granules, and secrete quantities of insulin comparable to adult beta cells in response to multiple sequential glucose challenges in vitro. Furthermore, these cells secrete human insulin into the serum of mice shortly after transplantation in a glucose-regulated manner, and transplantation of these cells ameliorates hyperglycemia in diabetic mice.
DOI:10.1038/nprot.2018.006URLPMID:29543795 [本文引用: 1]

The creation of human induced pluripotent stem cells (hiPSCs) has provided an unprecedented opportunity to study tissue morphogenesis and organ development through 'organogenesis-in-a-dish'. Current approaches to cardiac organoid engineering rely on either direct cardiac differentiation from embryoid bodies (EBs) or generation of aligned cardiac tissues from predifferentiated cardiomyocytes from monolayer hiPSCs. To experimentally model early cardiac organogenesis in vitro, our protocol combines biomaterials-based cell patterning with stem cell organoid engineering. 3D cardiac microchambers are created from 2D hiPSC colonies; these microchambers approximate an early-development heart with distinct spatial organization and self-assembly. With proper training in photolithography microfabrication, maintenance of human pluripotent stem cells, and cardiac differentiation, a graduate student with guidance will likely be able to carry out this experimental protocol, which requires approximately 3 weeks. We envisage that this in vitro model of human early heart development could serve as an embryotoxicity screening assay in drug discovery, regulation, and prescription for healthy fetal development. We anticipate that, when applied to hiPSC lines derived from patients with inherited diseases, this protocol can be used to study the disease mechanisms of cardiac malformations at an early stage of embryogenesis.
DOI:10.1002/stem.2732URLPMID:29086457 [本文引用: 1]

The ability to differentiate human pluripotent stem cells (hPSCs) into cardiomyocytes (CMs) makes them an attractive source for repairing injured myocardium, disease modeling, and drug testing. Although current differentiation protocols yield hPSC-CMs to >90% efficiency, hPSC-CMs exhibit immature characteristics. With the goal of overcoming this limitation, we tested the effects of varying passive stretch on engineered heart muscle (EHM) structural and functional maturation, guided by computational modeling. Human embryonic stem cells (hESCs, H7 line) or human induced pluripotent stem cells (IMR-90 line) were differentiated to hPSC-derived cardiomyocytes (hPSC-CMs) in vitro using a small molecule based protocol. hPSC-CMs were characterized by troponin(+) flow cytometry as well as electrophysiological measurements. Afterwards, 1.2 x 10(6) hPSC-CMs were mixed with 0.4 x 10(6) human fibroblasts (IMR-90 line) (3:1 ratio) and type-I collagen. The blend was cast into custom-made 12-mm long polydimethylsiloxane reservoirs to vary nominal passive stretch of EHMs to 5, 7, or 9 mm. EHM characteristics were monitored for up to 50 days, with EHMs having a passive stretch of 7 mm giving the most consistent formation. Based on our initial macroscopic observations of EHM formation, we created a computational model that predicts the stress distribution throughout EHMs, which is a function of cellular composition, cellular ratio, and geometry. Based on this predictive modeling, we show cell alignment by immunohistochemistry and coordinated calcium waves by calcium imaging. Furthermore, coordinated calcium waves and mechanical contractions were apparent throughout entire EHMs. The stiffness and active forces of hPSC-derived EHMs are comparable with rat neonatal cardiomyocyte-derived EHMs. Three-dimensional EHMs display increased expression of mature cardiomyocyte genes including sarcomeric protein troponin-T, calcium and potassium ion channels, beta-adrenergic receptors, and t-tubule protein caveolin-3. Passive stretch affects the structural and functional maturation of EHMs. Based on our predictive computational modeling, we show how to optimize cell alignment and calcium dynamics within EHMs. These findings provide a basis for the rational design of EHMs, which enables future scale-up productions for clinical use in cardiovascular tissue engineering. Stem Cells 2018;36:265-277.
DOI:10.1038/s41551-017-0069URLPMID:29038743 [本文引用: 1]

An in vitro model of the human kidney glomerulus - the major site of blood filtration - could facilitate drug discovery and illuminate kidney-disease mechanisms. Microfluidic organ-on-a-chip technology has been used to model the human proximal tubule, yet a kidney-glomerulus-on-a-chip has not been possible because of the lack of functional human podocytes - the cells that regulate selective permeability in the glomerulus. Here, we demonstrate an efficient (> 90%) and chemically defined method for directing the differentiation of human induced pluripotent stem (hiPS) cells into podocytes that express markers of the mature phenotype (nephrin+, WT1+, podocin+, Pax2-) and that exhibit primary and secondary foot processes. We also show that the hiPS-cell-derived podocytes produce glomerular basement-membrane collagen and recapitulate the natural tissue/tissue interface of the glomerulus, as well as the differential clearance of albumin and inulin, when co-cultured with human glomerular endothelial cells in an organ-on-a-chip microfluidic device. The glomerulus-on-a-chip also mimics adriamycin-induced albuminuria and podocyte injury. This in vitro model of human glomerular function with mature human podocytes may facilitate drug development and personalized-medicine applications.
DOI:10.1063/1.4934713URLPMID:26576206 [本文引用: 1]

The blood-brain barrier (BBB) is a critical structure that serves as the gatekeeper between the central nervous system and the rest of the body. It is the responsibility of the BBB to facilitate the entry of required nutrients into the brain and to exclude potentially harmful compounds; however, this complex structure has remained difficult to model faithfully in vitro. Accurate in vitro models are necessary for understanding how the BBB forms and functions, as well as for evaluating drug and toxin penetration across the barrier. Many previous models have failed to support all the cell types involved in the BBB formation and/or lacked the flow-created shear forces needed for mature tight junction formation. To address these issues and to help establish a more faithful in vitro model of the BBB, we have designed and fabricated a microfluidic device that is comprised of both a vascular chamber and a brain chamber separated by a porous membrane. This design allows for cell-to-cell communication between endothelial cells, astrocytes, and pericytes and independent perfusion of both compartments separated by the membrane. This NeuroVascular Unit (NVU) represents approximately one-millionth of the human brain, and hence, has sufficient cell mass to support a breadth of analytical measurements. The NVU has been validated with both fluorescein isothiocyanate (FITC)-dextran diffusion and transendothelial electrical resistance. The NVU has enabled in vitro modeling of the BBB using all human cell types and sampling effluent from both sides of the barrier.
DOI:10.1016/j.stem.2017.03.001URLPMID:28366587 [本文引用: 5]

Effective derivation of functional airway organoids from induced pluripotent stem cells (iPSCs) would provide valuable models of lung disease and facilitate precision therapies for airway disorders such as cystic fibrosis. However, limited understanding of human airway patterning has made this goal challenging. Here, we show that cyclical modulation of the canonical Wnt signaling pathway enables rapid directed differentiation of human iPSCs via an NKX2-1(+) progenitor intermediate into functional proximal airway organoids. We find that human NKX2-1(+) progenitors have high levels of Wnt activation but respond intrinsically to decreases in Wnt signaling by rapidly patterning into proximal airway lineages at the expense of distal fates. Using this directed approach, we were able to generate cystic fibrosis patient-specific iPSC-derived airway organoids with a defect in forskolin-induced swelling that is rescued by gene editing to correct the disease mutation. Our approach has many potential applications in modeling and drug screening for airway diseases.
DOI:10.1016/j.stem.2017.08.014URLPMID:28965766 [本文引用: 2]

Lung alveoli, which are unique to air-breathing organisms, have been challenging to generate from pluripotent stem cells (PSCs) in part because there are limited model systems available to provide the necessary developmental roadmaps for in vitro differentiation. Here we report the generation of alveolar epithelial type 2 cells (AEC2s), the facultative progenitors of lung alveoli, from human PSCs. Using multicolored fluorescent reporter lines, we track and purify human SFTPC+ alveolar progenitors as they emerge from endodermal precursors in response to stimulation of Wnt and FGF signaling. Purified PSC-derived SFTPC+ cells form monolayered epithelial
DOI:10.1242/dev.157628URLPMID:29437830 [本文引用: 1]

Human cleft lip with or without cleft palate (CL/P) is a common craniofacial abnormality caused by impaired fusion of the facial prominences. We have previously reported that, in the mouse embryo, epithelial apoptosis mediates fusion at the seam where the prominences coalesce. Here, we show that apoptosis alone is not sufficient to remove the epithelial layers. We observed morphological changes in the seam epithelia, intermingling of cells of epithelial descent into the mesenchyme and molecular signatures of epithelial-mesenchymal transition (EMT). Utilizing mouse lines with cephalic epithelium-specific Pbx loss exhibiting CL/P, we demonstrate that these cellular behaviors are Pbx dependent, as is the transcriptional regulation of the EMT driver Snail1. Furthermore, in the embryo, the majority of epithelial cells expressing high levels of Snail1 do not undergo apoptosis. Pbx1 loss- and gain-of-function in a tractable epithelial culture system revealed that Pbx1 is both necessary and sufficient for EMT induction. This study establishes that Pbx-dependent EMT programs mediate murine upper lip/primary palate morphogenesis and fusion via regulation of Snail1. Of note, the EMT signatures observed in the embryo are mirrored in the epithelial culture system.
DOI:10.1038/nbt.2915URLPMID:24837661 [本文引用: 1]

Efforts to derive hematopoietic stem cells (HSCs) from human pluripotent stem cells (hPSCs) are complicated by the fact that embryonic hematopoiesis consists of two programs, primitive and definitive, that differ in developmental potential. As only definitive hematopoiesis generates HSCs, understanding how this program develops is essential for being able to produce this cell population in vitro. Here we show that both hematopoietic programs transition through hemogenic endothelial intermediates and develop from KDR(+)CD34(-)CD144(-) progenitors that are distinguished by CD235a expression. Generation of primitive progenitors (KDR(+)CD235a(+)) depends on stage-specific activin-nodal signaling and inhibition of the Wnt-beta-catenin pathway, whereas specification of definitive progenitors (KDR(+)CD235a(-)) requires Wnt-beta-catenin signaling during this same time frame. Together, these findings establish simple selective differentiation strategies for the generation of primitive or definitive hematopoietic progenitors by Wnt-beta-catenin manipulation, and in doing so provide access to enriched populations for future studies on hPSC-derived hematopoietic development.
DOI:10.1182/blood-2004-10-4065URLPMID:15718421 [本文引用: 1]

To date, hematopoietic development of human embryonic stem cells (hESCs) has been limited to cell lines cultured in the presence of either mouse embryonic fibroblasts (MEFs) or MEF-conditioned media (MEF-CM). Anonymous xenogenic factors from MEFs or MEF-CM complicate studies of hESC self-renewal and also raise concerns for the potential clinical applications of generating primitive hematopoietic cells from hESC lines maintained under these ambiguous conditions. Here, we demonstrate that hESCs can be cultured over 30 passages in defined conditions in the absence of MEFs or MEF-CM using only serum replacement (SR) media and high concentrations of basic fibroblast growth factor (SR-bFGF). Similar to hESCs cultured in MEF-CM, hESCs cultured in SR-bFGF sustained characteristics of undifferentiated hESCs, proliferative potential, normal karyotype, in vitro and in vivo 3 germ-layer specification and gave rise to hemogenic-endothelial precursors required for subsequent primitive hematopoietic development. Our report demonstrates that anonymous factors produced by feeder cells are not necessary for hESC maintenance and subsequent hematopoietic specification, thereby providing a defined system for studies of hESC self-renewal and hESC-derived hematopoiesis.
DOI:10.1016/j.stem.2017.09.014URLPMID:29100010 [本文引用: 1]

Stem cell-based therapies for Parkinson's disease are moving into a new and exciting era, with several groups pursuing clinical trials with pluripotent stem cell (PSC)-derived dopamine neurons. As many groups have ongoing or completed GMP-level cell manufacturing, we highlight key clinical translation considerations from our recent fourth GForce-PD meeting.
DOI:10.1016/j.neuron.2012.12.011URLPMID:23395372 [本文引用: 1]

The study of human cortical development has major implications for brain evolution and diseases but has remained elusive due to paucity of experimental models. Here we found that human embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), cultured without added morphogens, recapitulate corticogenesis leading to the sequential generation of functional pyramidal neurons of all six layer identities. After transplantation into mouse neonatal brain, human ESC-derived cortical neurons integrated robustly and established specific axonal projections and dendritic patterns corresponding to native cortical neurons. The differentiation and connectivity of the transplanted human cortical neurons complexified progressively over several months in vivo, culminating in the establishment of functional synapses with the host circuitry. Our data demonstrate that human cortical neurons generated in vitro from ESC/iPSC can develop complex hodological properties characteristic of the cerebral cortex in vivo, thereby offering unprecedented opportunities for the modeling of human cortex diseases and brain repair.
DOI:10.1186/scrt224URLPMID:23769173 [本文引用: 1]

INTRODUCTION: Stroke is a major cause of permanent neurologic damage, with few effective treatments available to restore lost function. Induced pluripotent stem cells (iPSCs) have the potential to generate all cell types in vitro and can be generated from a stroke patient. Therefore, iPSCs are attractive donor sources of genetically identical
DOI:10.1002/stem.1415URLPMID:23666606 [本文引用: 1]

The main motor symptoms of Parkinson's disease are due to the loss of dopaminergic (DA) neurons in the ventral midbrain (VM). For the future treatment of Parkinson's disease with cell transplantation it is important to develop efficient differentiation methods for production of human iPSCs and hESCs-derived midbrain-type DA neurons. Here we describe an efficient differentiation and sorting strategy for DA neurons from both human ES/iPS cells and non-human primate iPSCs. The use of non-human primate iPSCs for neuronal differentiation and autologous transplantation is important for preclinical evaluation of safety and efficacy of stem cell-derived DA neurons. The aim of this study was to improve the safety of human- and non-human primate iPSC (PiPSC)-derived DA neurons. According to our results, NCAM(+) /CD29(low) sorting enriched VM DA neurons from pluripotent stem cell-derived neural cell populations. NCAM(+) /CD29(low) DA neurons were positive for FOXA2/TH and EN1/TH and this cell population had increased expression levels of FOXA2, LMX1A, TH, GIRK2, PITX3, EN1, NURR1 mRNA compared to unsorted neural cell populations. PiPSC-derived NCAM(+) /CD29(low) DA neurons were able to restore motor function of 6-hydroxydopamine (6-OHDA) lesioned rats 16 weeks after transplantation. The transplanted sorted cells also integrated in the rodent brain tissue, with robust TH+/hNCAM+ neuritic innervation of the host striatum. One year after autologous transplantation, the primate iPSC-derived neural cells survived in the striatum of one primate without any immunosuppression. These neural cell grafts contained FOXA2/TH-positive neurons in the graft site. This is an important proof of concept for the feasibility and safety of iPSC-derived cell transplantation therapies in the future.
DOI:10.1016/j.brainres.2016.08.010URLPMID:27544423 [本文引用: 1]

Schizophrenia is a devastating psychiatric disorder characterized by positive, negative and cognitive symptoms. While aberrant dopamine system function is typically associated with the positive symptoms of the disease, it is thought that this is secondary to pathology in afferent regions. Indeed, schizophrenia patients show dysregulated activity in the hippocampus and prefrontal cortex, two regions known to regulate dopamine neuron activity. These deficits in hippocampal and prefrontal cortical function are thought to result, in part, from reductions in inhibitory interneuron function in these brain regions. Therefore, it has been hypothesized that restoring interneuron function in the hippocampus and/or prefrontal cortex may be an effective treatment strategy for schizophrenia. In this article, we will discuss the evidence for interneuron pathology in schizophrenia and review recent advances in our understanding of interneuron development. Finally, we will explore how these advances have allowed us to test the therapeutic value of interneuron transplants in multiple preclinical models of schizophrenia. This article is part of a Special Issue entitled SI:StemsCellsinPsychiatry.
DOI:10.1038/nbt.4127URLPMID:29658944 [本文引用: 1]

Differentiation of human pluripotent stem cells to small brain-like structures known as brain organoids offers an unprecedented opportunity to model human brain development and disease. To provide a vascularized and functional in vivo model of brain organoids, we established a method for transplanting human brain organoids into the adult mouse brain. Organoid grafts showed progressive neuronal differentiation and maturation, gliogenesis, integration of microglia, and growth of axons to multiple regions of the host brain. In vivo two-photon imaging demonstrated functional neuronal networks and blood vessels in the grafts. Finally, in vivo extracellular recording combined with optogenetics revealed intragraft neuronal activity and suggested graft-to-host functional synaptic connectivity. This combination of human neural organoids and an in vivo physiological environment in the animal brain may facilitate disease modeling under physiological conditions.
DOI:10.1038/nature12271URLPMID:23823721 [本文引用: 3]

A critical shortage of donor organs for treating end-stage organ failure highlights the urgent need for generating organs from human induced pluripotent stem cells (iPSCs). Despite many reports describing functional cell differentiation, no studies have succeeded in generating a three-dimensional vascularized organ such as liver. Here we show the generation of vascularized and functional human liver from human iPSCs by transplantation of liver buds created in vitro (iPSC-LBs). Specified hepatic cells (immature endodermal cells destined to track the hepatic cell fate) self-organized into three-dimensional iPSC-LBs by recapitulating organogenetic interactions between endothelial and mesenchymal cells. Immunostaining and gene-expression analyses revealed a resemblance between in vitro grown iPSC-LBs and in vivo liver buds. Human vasculatures in iPSC-LB transplants became functional by connecting to the host vessels within 48 hours. The formation of functional vasculatures stimulated the maturation of iPSC-LBs into tissue resembling the adult liver. Highly metabolic iPSC-derived tissue performed liver-specific functions such as protein production and human-specific drug metabolism without recipient liver replacement. Furthermore, mesenteric transplantation of iPSC-LBs rescued the drug-induced lethal liver failure model. To our knowledge, this is the first report demonstrating the generation of a functional human organ from pluripotent stem cells. Although efforts must ensue to translate these techniques to treatments for patients, this proof-of-concept demonstration of organ-bud transplantation provides a promising new approach to study regenerative medicine.
DOI:10.1016/j.stemcr.2017.01.027URLPMID:28262545 [本文引用: 1]

Familial hypercholesterolemia (FH) causes elevation of low-density lipoprotein cholesterol (LDL-C) in blood and carries an increased risk of early-onset cardiovascular disease. A caveat for exploration of new therapies for FH is the lack of adequate experimental models. We have created a comprehensive FH stem cell model with differentiated hepatocytes (iHeps) from human induced pluripotent stem cells (iPSCs), including genetically engineered iPSCs, for testing therapies for FH. We used FH iHeps to assess the effect of simvastatin and proprotein convertase subtilisin/kexin type 9 (PCSK9) antibodies on LDL-C uptake and cholesterol lowering in vitro. In addition, we engrafted FH iHeps into the liver of Ldlr(-/-)/Rag2(-/-)/Il2rg(-/-) mice, and assessed the effect of these same medications on LDL-C clearance and endothelium-dependent vasodilation in vivo. Our iHep models recapitulate clinical observations of higher potency of PCSK9 antibodies compared with statins for reversing the consequences of FH, demonstrating the utility for preclinical testing of new therapies for FH patients.
DOI:10.1016/j.stem.2016.11.018URLPMID:28089908 [本文引用: 3]

Understanding the relative contributions of genetic and epigenetic abnormalities to acute myeloid leukemia (AML) should assist integrated design of targeted therapies. In this study, we generated induced pluripotent stem cells (iPSCs) from AML patient samples harboring MLL rearrangements and found that they retained leukemic mutations but reset leukemic DNA methylation/gene expression patterns. AML-iPSCs lacked leukemic potential, but when differentiated into hematopoietic cells, they reacquired the ability to give rise to leukemia in vivo and reestablished leukemic DNA methylation/gene expression patterns, including an aberrant MLL signature. Epigenetic reprogramming was therefore not sufficient to eliminate leukemic behavior. This approach also allowed us to study the properties of distinct AML subclones, including differential drug susceptibilities of KRAS mutant and wild-type cells, and predict relapse based on increased cytarabine resistance of a KRAS wild-type subclone. Overall, our findings illustrate the value of AML-iPSCs for investigating the mechanistic basis and clonal properties of human AML.
DOI:10.1101/gad.212662.112URLPMID:23512659 [本文引用: 1]

Epigenetic changes are frequently observed in cancer. However, their role in establishing or sustaining the malignant state has been difficult to determine due to the lack of experimental tools that enable resetting of epigenetic abnormalities. To address this, we applied induced pluripotent stem cell (iPSC) reprogramming techniques to invoke widespread epigenetic resetting of glioblastoma (GBM)-derived neural stem (GNS) cells. GBM iPSCs (GiPSCs) were subsequently redifferentiated to the neural lineage to assess the impact of cancer-specific epigenetic abnormalities on tumorigenicity. GiPSCs and their differentiating derivatives display widespread resetting of common GBM-associated changes, such as DNA hypermethylation of promoter regions of the cell motility regulator TES (testis-derived transcript), the tumor suppressor cyclin-dependent kinase inhibitor 1C (CDKN1C; p57KIP2), and many polycomb-repressive complex 2 (PRC2) target genes (e.g., SFRP2). Surprisingly, despite such global epigenetic reconfiguration, GiPSC-derived neural progenitors remained highly malignant upon xenotransplantation. Only when GiPSCs were directed to nonneural cell types did we observe sustained expression of reactivated tumor suppressors and reduced infiltrative behavior. These data suggest that imposing an epigenome associated with an alternative developmental lineage can suppress malignant behavior. However, in the context of the neural lineage, widespread resetting of GBM-associated epigenetic abnormalities is not sufficient to override the cancer genome.
DOI:10.1016/j.cell.2015.02.045URLPMID:25860607 [本文引用: 1]

In vitro modeling of human disease has recently become feasible with induced pluripotent stem cell (iPSC) technology. Here, we established patient-derived iPSCs from a Li-Fraumeni syndrome (LFS) family and investigated the role of mutant p53 in the development of osteosarcoma (OS). LFS iPSC-derived osteoblasts (OBs) recapitulated OS features including defective osteoblastic differentiation as well as tumorigenic ability. Systematic analyses revealed that the expression of genes enriched in LFS-derived OBs strongly correlated with decreased time to tumor recurrence and poor patient survival. Furthermore, LFS OBs exhibited impaired upregulation of the imprinted gene H19 during osteogenesis. Restoration of H19 expression in LFS OBs facilitated osteoblastic differentiation and repressed tumorigenic potential. By integrating human imprinted gene network (IGN) into functional genomic analyses, we found that H19 mediates suppression of LFS-associated OS through the IGN component DECORIN (DCN). In summary, these findings demonstrate the feasibility of studying inherited human cancer syndromes with iPSCs.
DOI:10.1172/JCI40553URLPMID:20051636 [本文引用: 1]

Induced pluripotent stem (iPS) cells are generated by epigenetic reprogramming of somatic cells through the exogenous expression of transcription factors. These cells, just like embryonic stem cells, are likely to have a major impact on regenerative medicine, because they self-renew and retain the potential to be differentiated into all cell types of the human body. In this Review, we describe the current state of iPS cell technology, including approaches by which they are generated and what is known about their biology, and discuss the potential applications of these cells for disease modeling, drug discovery, and, eventually, cell replacement therapy.
DOI:10.1038/nm.3267URLPMID:23921754 [本文引用: 1]

Human pluripotent stem cells (PSCs) are a leading candidate for cell-based therapies because of their capacity for unlimited self renewal and pluripotent differentiation. These advances have recently culminated in the first-in-human PSC clinical trials by Geron, Advanced Cell Technology and the Kobe Center for Developmental Biology for the treatment of spinal cord injury and macular degeneration. Despite their therapeutic promise, a crucial hurdle for the clinical implementation of human PSCs is their potential to form tumors in vivo. In this Perspective, we present an overview of the mechanisms underlying the tumorigenic risk of human PSC-based therapies and discuss current advances in addressing these challenges.
DOI:10.1038/nrg3271URL [本文引用: 2]

Studies using high-resolution genome-wide approaches have recently reported that genomic and epigenomic alterations frequently accumulate in human pluripotent cells. Detailed characterization of these changes is crucial for understanding the impact of these alterations on self-renewal and proliferation, and particularly on the developmental and malignant potential of the cells. Such knowledge is required for the optimized and safe use of pluripotent cells for therapeutic purposes, such as regenerative cellular therapies using differentiated derivatives of pluripotent cells. In this Review, we summarize the current knowledge of the genomic and epigenomic stability of pluripotent human cells and the implications for stem cell research.
DOI:10.1146/annurev-genet-112414-054926URLPMID:26407033 [本文引用: 1]

The advent of induced pluripotent stem (iPS) cells has opened up numerous avenues of opportunity for cell therapy, including the initiation in September 2014 of the first human clinical trial to treat dry age-related macular degeneration. In parallel, advances in genome-editing technologies by site-specific nucleases have dramatically improved our ability to edit endogenous genomic sequences at targeted sites of interest. In fact, clinical trials have already begun to implement this technology to control HIV infection. Genome editing in iPS cells is a powerful tool and enables researchers to investigate the intricacies of the human genome in a dish. In the near future, the groundwork laid by such an approach may expand the possibilities of gene therapy for treating congenital disorders. In this review, we summarize the exciting progress being made in the utilization of genomic editing technologies in pluripotent stem cells and discuss remaining challenges toward gene therapy applications.
DOI:10.1038/nmeth.1591URLPMID:21460823 [本文引用: 1]

We report a simple method, using p53 suppression and nontransforming L-Myc, to generate human induced pluripotent stem cells (iPSCs) with episomal plasmid vectors. We generated human iPSCs from multiple donors, including two putative human leukocyte antigen (HLA)-homozygous donors who match approximately 20% of the Japanese population at major HLA loci; most iPSCs are integrated transgene-free. This method may provide iPSCs suitable for autologous and allologous stem-cell therapy in the future.
DOI:10.1371/journal.pone.0039715URLPMID:22911689 [本文引用: 1]

Homogeneous culture of neural precursor cells (NPCs) derived from human pluripotent stem cells (hPSCs) would provide a powerful tool for biomedical applications. However, previous efforts to expand mechanically dissected neural rosettes for cultivation of NPCs remain concerns regarding non-neural cell contamination. In addition, several attempts to purify NPCs using cell surface markers have not demonstrated the expansion capability of the sorted cells. In the present study, we show that polysialic acid-neural cell adhesion molecule (PSA-NCAM) is detected in neural rosette cells derived from hPSCs, and employ PSA-NCAM as a marker for purifying expandable primitive NPCs from the neural rosettes. PSA-NCAM-positive NPCs (termed hNPC(PSA-NCAM+)) were isolated from the heterogeneous cell population of mechanically harvested neural rosettes using magnetic-based cell sorting. The hNPC(PSA-NCAM+) extensively expressed neural markers such as Sox1, Sox2, Nestin, and Musashi-1 (80 approximately 98% of the total cells) and were propagated for multiple passages while retaining their primitive characteristics in our culture condition. Interestingly, PSA-NCAM-negative cells largely exhibited characteristics of neural crest cells. The hNPC(PSA-NCAM+) showed multipotency and responsiveness to instructive cues towards region-specific neuronal subtypes in vitro. When transplanted into the rat striatum, hNPC(PSA-NCAM+) differentiated into neurons, astrocytes, and oligodendrocytes without particular signs of tumorigenesis. Furthermore, Ki67-positive proliferating cells and non-neural lineage cells were rarely detected in the grafts of hNPC(PSA-NCAM+) compared to those of neural rosette cells. Our results suggest that PSA-NCAM-mediated cell isolation provides a highly expandable population of pure primitive NPCs from hPSCs that will lend themselves as a promising strategy for drug screening and cell therapy for neurodegenerative disorders.
DOI:10.1016/j.stemcr.2014.01.013URL [本文引用: 1]

Human induced pluripotent stem cells (iPSCs) can provide a promising source of midbrain dopaminergic (DA) neurons for cell replacement therapy for Parkinson's disease. However, iPSC-derived donor cells inevitably contain tumorigenic or inappropriate cells. Here, we show that human iPSC-derived DA progenitor cells can be efficiently isolated by cell sorting using a floor plate marker, CORIN. We induced DA neurons using scalable culture conditions on human laminin fragment, and the sorted CORIN+ cells expressed the midbrain DA progenitor markers, FOXA2 and LMX1A. When transplanted into 6-OHDA-lesioned rats, the CORIN+ cells survived and differentiated into midbrain DA neurons in vivo, resulting in significant improvement of the motor behavior, without tumor formation. In particular, the CORIN+ cells in a NURR1(+) cell-dominant stage exhibited the best survival and function as DA neurons. Our method is a favorable strategy in terms of scalability, safety, and efficiency and may be advantageous for clinical application.
DOI:10.1016/j.stemcr.2015.04.010URL [本文引用: 1]
DOI:10.1056/NEJMoa1608368URLPMID:28296613 [本文引用: 1]

We assessed the feasibility of transplanting a sheet of retinal pigment epithelial (RPE) cells differentiated from induced pluripotent stem cells (iPSCs) in a patient with neovascular age-related macular degeneration. The iPSCs were generated from skin fibroblasts obtained from two patients with advanced neovascular age-related macular degeneration and were differentiated into RPE cells. The RPE cells and the iPSCs from which they were derived were subject to extensive testing. A surgery that included the removal of the neovascular membrane and transplantation of the autologous iPSC-derived RPE cell sheet under the retina was performed in one of the patients. At 1 year after surgery, the transplanted sheet remained intact, best corrected visual acuity had not improved or worsened, and cystoid macular edema was present. (Funded by Highway Program for Realization of Regenerative Medicine and others; University Hospital Medical Information Network Clinical Trials Registry [UMIN-CTR] number, UMIN000011929 .).
DOI:10.1056/NEJMoa1609583URLPMID:28296617 [本文引用: 1]

Adipose tissue-derived
DOI:10.1016/j.stem.2016.01.023URLPMID:26849303 [本文引用: 1]

In an interview, we recently asked four leaders in the field to share their insights with us on the clinical outlook for hiPSCs. We present highlights from their responses here.
DOI:10.1038/nbt.2678URLPMID:23934177 [本文引用: 1]

Progress in adoptive T-cell therapy for cancer and infectious diseases is hampered by the lack of readily available, antigen-specific, human T lymphocytes. Pluripotent stem cells could provide an unlimited source of T lymphocytes, but the therapeutic potential of human pluripotent stem cell-derived lymphoid cells generated to date remains uncertain. Here we combine induced pluripotent stem cell (iPSC) and chimeric antigen receptor (CAR) technologies to generate human T cells targeted to CD19, an antigen expressed by malignant B cells, in tissue culture. These iPSC-derived, CAR-expressing T cells display a phenotype resembling that of innate gammadelta T cells. Similar to CAR-transduced, peripheral blood gammadelta T cells, the iPSC-derived T cells potently inhibit tumor growth in a xenograft model. This approach of generating therapeutic human T cells 'in the dish' may be useful for cancer immunotherapy and other medical applications.
DOI:10.1007/s11899-019-00528-6URLPMID:31243643 [本文引用: 1]

PURPOSE OF REVIEW: In the rapidly developing field of adoptive cell immunotherapy, there is urgent need for discoveries that would improve outcomes, extend the applicability, and reduce the costs. Induced pluripotent stem cells (iPSC) can be a source of broadly applicable cellular immunotherapeutics, which have been manufactured, validated, and banked in advance, and can be applied across HLA barriers. Here, we discuss the recent advances and challenges in the generation of iPSC-derived cellular products for cancer therapy. RECENT FINDINGS: iPSCs can be differentiated to functional tumor-specific T and NK cells in vitro with demonstrable in vitro and in vivo anti-tumor activity. Genetic modifications employed at the iPSC level can deliver desirable immunotherapeutic attributes to the generated immune effectors. iPSC-NK cells are currently evaluated in a clinical setting and pre-clinical testing of iPSC-T cells shows promising results but their production seems more challenging. The use of iPSCs for the generation of tumor-targeting T/NK cells constitutes a feasible strategy to overcome limitations in manufacturing, efficacy, and applicability of cellular therapeutics.
DOI:10.1016/j.stem.2018.10.005URLPMID:30449714 [本文引用: 1]

Limited T cell availability and proliferative exhaustion present major barriers to successful T cell-based immunotherapies and may potentially be overcome through the use of
URL [本文引用: 1]
DOI:10.1038/nrc.2015.5URLPMID:26694935 [本文引用: 1]

Natural killer (NK) cells are the prototype innate lymphoid cells endowed with potent cytolytic function that provide host defence against microbial infection and tumours. Here, we review evidence for the role of NK cells in immune surveillance against cancer and highlight new therapeutic approaches for targeting NK cells in the treatment of cancer.
DOI:10.3390/cancers11060769URL [本文引用: 1]
DOI:10.7150/ijbs.17421URLPMID:27994508 [本文引用: 1]

Cancer development is a multistep process triggered by innate and acquired mutations, which cause the functional abnormality and determine the initiation and progression of tumorigenesis. Gene editing is a widely used engineering tool for generating mutations that enhance tumorigenesis. The recent developed clustered regularly interspaced short palindromic repeats-CRISPR-associated 9 (CRISPR-Cas9) system renews the genome editing approach into a more convenient and efficient way. By rapidly introducing genetic modifications in cell lines, organs and animals, CRISPR-Cas9 system extends the gene editing into whole genome screening, both in loss-of-function and gain-of-function manners. Meanwhile, the system accelerates the establishment of animal cancer models, promoting in vivo studies for cancer research. Furthermore, CRISPR-Cas9 system is modified into diverse innovative tools for observing the dynamic bioprocesses in cancer studies, such as image tracing for targeted DNA, regulation of transcription activation or repression. Here, we view recent technical advances in the application of CRISPR-Cas9 system in cancer genetics, large-scale cancer driver gene hunting, animal cancer modeling and functional studies.
DOI:10.1016/j.stem.2018.06.002URLPMID:30082067 [本文引用: 1]

Chimeric antigen receptors (CARs) significantly enhance the anti-tumor activity of immune effector cells. Although most studies have evaluated CAR expression in T cells, here we evaluate different CAR constructs that improve natural killer (NK) cell-mediated killing. We identified a CAR containing the transmembrane domain of NKG2D, the 2B4 co-stimulatory domain, and the CD3zeta signaling domain to mediate strong antigen-specific NK cell signaling. NK cells derived from human iPSCs that express this CAR (NK-CAR-iPSC-NK cells) have a typical NK cell phenotype and demonstrate improved anti-tumor activity compared with T-CAR-expressing iPSC-derived NK cells (T-CAR-iPSC-NK cells) and non-CAR-expressing cells. In an ovarian cancer xenograft model, NK-CAR-iPSC-NK cells significantly inhibited tumor growth and prolonged survival compared with PB-NK cells, iPSC-NK cells, or T-CAR-iPSC-NK cells. Additionally, NK-CAR-iPSC-NK cells demonstrate in vivo activity similar to that of T-CAR-expressing T cells, although with less toxicity. These NK-CAR-iPSC-NK cells now provide standardized, targeted
DOI:10.1038/nbt.3749URLPMID:27941802 [本文引用: 1]

Induced pluripotent stem cells (iPSCs) are being pursued as a source of cells for autologous therapies, many of which will be aimed at aged patients. To explore the impact of age on iPSC quality, we produced iPSCs from blood cells of 16 donors aged 21-100. We find that iPSCs from older donors retain an epigenetic signature of age, which can be reduced through passaging. Clonal expansion via reprogramming also enables the discovery of somatic mutations present in individual donor cells, which are missed by bulk sequencing methods. We show that exomic mutations in iPSCs increase linearly with age, and all iPSC lines analyzed carry at least one gene-disrupting mutation, several of which have been associated with cancer or dysfunction. Unexpectedly, elderly donors (>90 yrs) harbor fewer mutations than predicted, likely due to a contracted blood progenitor pool. These studies establish that donor age is associated with an increased risk of abnormalities in iPSCs and will inform clinical development of reprogramming technology.
DOI:10.1016/j.cell.2011.02.013URL [本文引用: 1]

The hallmarks of cancer comprise six biological capabilities acquired during the multistep development of human tumors. The hallmarks constitute an organizing principle for rationalizing the complexities of neoplastic disease. They include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis. Underlying these hallmarks are genome instability, which generates the genetic diversity that expedites their acquisition, and inflammation, which fosters multiple hallmark functions. Conceptual progress in the last decade has added two emerging hallmarks of potential generality to this list-reprogramming of energy metabolism and evading immune destruction. In addition to cancer cells, tumors exhibit another dimension of complexity: they contain a repertoire of recruited, ostensibly normal cells that contribute to the acquisition of hallmark traits by creating the "tumor microenvironment." Recognition of the widespread applicability of these concepts will increasingly affect the development of new means to treat human cancer.
