 ,中国农业科学院北京畜牧兽医研究所,北京 100193
,中国农业科学院北京畜牧兽医研究所,北京 100193Research progress of stem cells in agricultural animals
Bingyuan Wang, Yulian Mu, Kui Li, Zhiguo Liu ,Institute of Animal Sciences, Chinese Academy of Agricultural Sciences, Beijing 100193, China
,Institute of Animal Sciences, Chinese Academy of Agricultural Sciences, Beijing 100193, China通讯作者: 刘志国,博士研究生,助理研究员,研究方向:动物遗传育种与繁殖。E-mail:liuzhiguo@caas.cn
编委: 李明洲
收稿日期:2020-06-16修回日期:2020-09-8网络出版日期:2020-11-20
| 基金资助: |
Received:2020-06-16Revised:2020-09-8Online:2020-11-20
| Fund supported: |
作者简介 About authors
王冰源,博士研究生,助理研究员,研究方向:动物遗传育种与繁殖。E-mail:

摘要
干细胞技术是近年来被广泛应用于生命科学领域的重要技术。获取具有无限增殖能力和分化能力的干细胞系主要有3种途径:(1)从胚胎分离胚胎干细胞;(2)从成体组织分离成体干细胞;(3)通过体外诱导体细胞重编程为诱导多能干细胞。在农业领域中,畜禽干细胞的分离、培养、建系有望显著提升体细胞克隆和细胞水平基因修饰的效率;干细胞体外诱导配子技术能够极大简化基因编辑畜禽的制备流程,提升制备效率。同时,通过结合基因编辑、显微注射、干细胞移植、胚胎移植等技术,干细胞技术在基因编辑动物的生产、组织和器官的供体制备、体外配子诱导及遗传重组胚胎制备、疾病治疗靶点的筛选,以及新药药理研究等方面都具有极大的应用潜力,对农业动物的遗传改良、疾病防治具有重要意义。本文综述了干细胞相关研究在农业动物包括猪(Sus scrofa)、牛(Bos taurus)、鸡(Gallus gallus)、山羊(Capra hircus)和绵羊(Ovis aries)中的新进展,以期为农业动物干细胞领域的相关研究提供参考。
关键词:
Abstract
As an important biological technology, stem cell technology has been being widely used in the life sciences for a long time. There are three major ways to obtain stem cells with unlimited proliferation and differentiation capabilities, including 1) isolating embryonic stem cells (ESCs) from embryos, 2) isolating adult stem cells from adult tissues, and 3) in vitro reprogramming of differentiated somatic cells into induced pluripotent stem cells (iPSCs). In the field of agriculture, the efficient purification, culture and establishment of livestock and poultry stem cell lines are expected to significantly improve the efficiency of somatic cell cloning and genetic modification of cells. The technology of stem cell induced-gamete production will greatly simplify the generation process, and consequently improve the generation efficiency of genetically modified animals. In addition, by combining with gene editing, microinjection, stem cell transplantation, and embryo transfer, stem cell technology has great potential in the production of genetically modified animals, tissue and organ donors, in vitro induced gametes and genetically reconstructed embryos, in the screening of disease treatment targets, and in the research of new drug pharmacology, which is of great significance to the genetic improvement, disease prevention and treatment for agricultural animals. In this review, we summarize the current research progress of stem cells in agricultural animals, including pig (Sus scrofa), cattle (Bos taurus), chicken (Gallus gallus), goat (Capra hircus) and sheep (Ovis aries), to provide information for the studies in the field of stem cells in agricultural animals.
Keywords:
PDF (539KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
王冰源, 牟玉莲, 李奎, 刘志国. 农业动物干细胞研究进展. 遗传[J], 2020, 42(11): 1073-1080 doi:10.16288/j.yczz.20-180
Bingyuan Wang.
干细胞是一类具有自我更新和分化能力的细胞,在一定条件下可以分化为多种功能的细胞。根据干细胞在体内所处的发育阶段可将其分类为胚胎干细胞(embryonic stem cells, ESCs)和成体干细胞[1,2]。而通过向体外培养的体细胞中转染重编程因子可诱导体细胞重编程为多能干细胞,即诱导多能干细胞(induced pluripotent stem cells, iPSCs),iPSCs的出现极大地丰富了干细胞的来源[3,4]。干细胞具有体外可操作性、能够减少实验动物的使用数量、提供有效和持久的疾病治疗方法以及丰富的实验素材等优点。在干细胞的体外培养过程中,通过基因编辑等操作获得稳定传代的基因编辑干细胞系,能够用以制备基因编辑动物、定向诱导组织修复或器官生成、诱导配子生成、筛选治疗靶点以及研究新药药理等[5,6],因此具有广阔的应用前景。
近几十年来畜禽胚胎干细胞系的建立进展十分缓慢,直至近些年猪(Sus scrofa)、山羊(Capra hircus)、绵羊(Ovis aries)和鸡(Gallus gallus)的ESCs研究才获得初步进展[7,8,9]。而相比其他农业动物,猪的干细胞相关研究更加丰富,涉及猪ESCs、iPSCs、精原干细胞、肠道干细胞、牙胚干细胞、胰腺干细胞、骨髓间充质干细胞和皮肤干细胞等。牛(Bos taurus)、山羊、绵羊和鸡的相关研究主要集中在ESCs、iPSCs、间充质干细胞和生殖干细胞等。因此,本文总结了猪、牛、山羊、绵羊和鸡等农业动物ESCs、iPSCs以及成体干细胞的相关研究进展,重点综述了猪干细胞的研究进展,以期为相关理论和应用研究提供参考。
1 猪干细胞研究进展
1.1 猪胚胎干细胞
对畜禽干细胞进行基因编辑可用于制备基因编辑农业动物,从而获得具有生长快、抗病力强、高产等优良性状的畜禽品种。尽管已经能够成功分离一些农业动物的ESCs,但由于这些ESCs缺乏种系嵌合能力,即难以形成包括生殖系在内的嵌合体,并且在经过有限的传代后很容易分化或死亡,使得农业动物干细胞建系相较模式动物进展缓慢[7,8,9]。此外,在将干细胞疗法应用于人类之前,有必要用可接受的动物模型验证这些方法的安全性和有效性。而猪被用作临床前实验动物模型,日益受到关注,因此迫切需要建立猪胚胎干细胞系。近年来,猪ESCs的相关研究取得了一定的进展。2009年,Yang等[7]通过电穿孔转染策略,将绿色荧光蛋白(green fluorescent protein, GFP)转入第44代猪胚胎干细胞系,获得了稳定表达GFP并能持续体外增殖90代的GFP-ESCs。这些GFP-ESCs具有正常的核型和胚胎干细胞的典型特征,包括表达标志基因OCT4、碱性磷酸酶阳性、能够形成类胚体、诱导条件下能够分化为神经细胞和心肌细胞谱系。2012年,Haraguchi等[10]将猪内细胞团在添加GSK-3β抑制剂和MAPKK1抑制剂的人ESCs培养基中扩大培养,获得了连续培养100多代的猪ESCs。这些ESCs具有碱性磷酸酶活性,表达标志基因OCT4和NANOG,多次传代后未发生形态改变。此后,Siriboon等[11]尝试使用高质量克隆猪囊胚的内细胞团分离ESCs,在培养过程中发现这些囊胚具有更好的附着力、生长能力和原始克隆形成能力。分离培养出的细胞经过25次传代未发生分化,表达ESCs标志基因OCT4、NANOG、SOX2和REX1,能体外分化为表达三胚层标志基因的类胚体。综上所述,猪ESCs的相关研究仍处于探索能够获得长期稳定传代培养的建系阶段,亟需加快猪ESCs的分化和生殖系嵌合等技术及理论的相关研究。1.2 猪诱导多能干细胞
近10年,猪iPSCs的相关研究取得了较大的进展。2009年,Esteban等[12]将小鼠(Mus musculus) OCT4、SOX2、KLF4、c-MYC或人OCT4、SOX2、KLF4、c-MYC通过逆转录病毒载体转入藏猪的成纤维细胞中,成功诱导出iPSCs。这些藏猪iPSCs具有和人ESCs相似的形态,核型正常,碱性磷酸酶阳性,表达ESCs标志基因NANOG、REX1、LIN28和SSEA4,并能够分化为具有三胚层的畸胎瘤。Chakritbudsabong等[13]使用人源的OCT4、SOX2、KLF4、c-MYC和LIN28通过逆转录病毒转导方法将猪的胚胎成纤维细胞诱导为iPSCs,这些猪iPSCs经碱性磷酸酶染色呈阳性,表达干细胞标志基因OCT4、SOX2、NANOG和SSEA1,能够分化形成类胚体和畸胎瘤。进一步向心肌方向诱导分化后,产生了持续跳动并表达标志基因心脏肌钙蛋白T的心肌细胞。Gallegos- Cdsabong等[14]将猪iPSCs分化为神经玫瑰状细胞,模拟人iPSCs的神经分化,为人类神经细胞疗法提供相关数据。此后,Webb等[15]证明了猪iPSCs分化得到的神经玫瑰状细胞可以进一步分化为神经嵴细胞和与周围神经系统相关的其他类型细胞。该研究用骨形态发生蛋白4或胎牛血清处理神经玫瑰状细胞可使其进一步分化为BRN3A阳性的感觉细胞,利用这些分化获得的猪的感觉神经细胞来检测其对有害刺激、镇痛药和修复机制的反应,可平行比较人和猪的感觉神经细胞的相似性。同时,猪感觉神经元的体外分化为神经细胞亚型的形成提供了一个新的模型系统,也为再生疗法的研究提供了一个新的平台。此外,Liao等[16]将猪iPSCs诱导分化为成骨样细胞,将这些成骨样细胞移植到兰屿猪的左胫骨后可显著改善移植部位的小梁骨结构,证实了猪iPSCs来源的成骨样细胞的治疗潜力。而Talbot等[17,18]将猪iPSCs诱导分化为肝脏干细胞系,此细胞系能在无饲养层条件下持续培养,并能够在体外分化为胆管或单层肝细胞。2019年Xu等[19]将猪的周细胞通过逆转录病毒载体转染OCT4、SOX2、KLF4和c-MYC诱导产生iPSCs,将iPSCs培养在含重组人LIF、CHIR 99021、(S)-(+)-马来酸二甲茚酯((S)-(+)-dimethindene maleate)和盐酸米诺环素(minocycline hydrochloride) 的基础培养基中,这些iPSCs表达标志基因OCT4、SOX2和NANOG,核型正常,能够分化为类胚体并表达外胚层、中胚层和内胚层这3个胚层的标志基因。最重要的是,这些带有GFP的iPSCs显微注射到猪核移植胚胎4~8细胞中并培养至囊胚期后,内细胞团和滋养外胚层细胞均有绿色GFP的表达。进一步将注射了GFP-iPSCs的核移植胚胎移植到代孕母猪后,在胚胎25~30天产生了4只存活的胎儿,尽管荧光显微镜下未见绿色的GFP,但在胚胎和胚外组织中通过巢式PCR可以检测到GFP序列的表达。综上所述,近年来猪iPSCs的研究取得了较大进展,也为猪ESCs的相关研究提供了技术参考。1.3 猪精原干细胞
精原干细胞(spermatogonial stem cells, SSCs)能够自我更新并分化为成熟的功能性精子,是雄性哺乳动物体内唯一可以将遗传信息传递给下一代的成体干细胞。猪SSCs在制备基因编辑猪及建立用于再生医学的模型猪方面均具有重要价值。但一直以来,由于猪SSCs数量少以及缺乏理想的培养体系,猪SSCs的相关研究和应用受到了极大的阻碍。然而,令人激动的是,2020年,Zheng等[20]将表达猿猴病毒40(SV40)大T抗原的质粒通过慢病毒转导到猪的原代SSCs中,建立了具有猪SSCs属性的永生化细胞系。这些永生化细胞表达SSCs和生殖细胞的标志基因,对视黄酸处理有分化反应,在移植后能够定植于受体小鼠睾丸而无肿瘤形成。同时,这些细胞具有无限的增殖潜能,体外培养7个多月,传35代以上,仍未出现形态异常,标志着首次建立了猪SSCs细胞系。而猪SSCs细胞系的成功建立,能够为猪SSCs相关研究提供丰富的细胞来源,促进猪SSCs培养体系的开发及其在畜牧业中的应用。1.4 猪其他类型成体干细胞
猪间充质干细胞(mesenchymal stem cells,MSCs)来源广泛,具有增殖和多种分化潜能,且与人的MSCs具有很高的相似性,因此具有重要的研究和应用价值。Huang等[21]分离获得猪骨髓MSCs和脐带MSCs后,首次发现猪骨髓MSCs相比脐带MSCs具有更强的迁移能力。进行蛋白质组比较分析,发现了95个差异蛋白,其中VIMENTIN具有正向调控MSCs迁移的作用。随后的研究还发现半乳糖凝集素3通过抑制RhoA-GTP活性而促进猪骨髓MSCs的迁移[22]。猪胰腺干细胞在II型糖尿病的移植治疗中具有重要价值,Han等[23]建立了动态表达WNT3A的猪胰腺干细胞系,并发现WNT3A能够促进猪胰腺干细胞的增殖潜力,为进一步研究猪胰腺干细胞的发育和分化提供了重要的工具。牙齿来源的干细胞作为组织再生的一种新来源,其优势为非侵入性的收集过程,以及在获得和使用方面不涉及伦理问题。Gurel等[24]从6月龄猪的下颌第三磨牙牙胚中分离出牙胚干细胞,这是首次报道的猪牙胚干细胞分离和鉴定研究。此外,Lermen等[25]首次分离扩增了成年猪皮肤来源的干细胞样细胞,在体外维持超过120天的增殖活性,表达多能因子SOX2和OCT4,并能向神经、肌肉和脂肪样细胞分化。肠道干细胞位于Lieberkek隐窝的底部,负责体内稳态和损伤后的肠道修复。Stieler等[26]报道了一种从肠道缺血环中分离肠道干细胞及培养的方法,可用于研究肠道干细胞在体外进行的上皮修复过程。综上所述,猪的各类成体干细胞的分离和培养的成功,为体外相关机理的研究提供了丰富的实验材料,也为组织修复及器官生成提供了素材,在农业和医学领域具有重要的应用价值。2 其他农业动物干细胞研究进展
2.1 牛干细胞
牛ESCs的相关研究也处于建立稳定长期传代的胚胎干细胞系阶段。Bogliotti等[27]使用含有成纤维细胞生长因子2和经典Wnt信号通路抑制剂的培养系统,获得了形态稳定、表达多能性标记基因并具有表观遗传学特征的牛ESCs。这些ESCs可快速建立克隆(3~4周)且易于增殖传代。当用作核移植的供体时,能获得与对照组相似的囊胚率。牛MSCs因其再生潜力和可塑性,受到了广泛关注。同时,MSCs易于分离并且具有抗炎和血管生成能力。在分离培养或复苏培养后,可将MSCs用于自体或同种异体治疗,使得干细胞治疗在临床上更具吸引力。目前已知牛MSCs的标志基因包括CD29、CD166、CD105、CD73、CD44和CD90等。牛MSCs可从骨髓、脂肪组织、脐带、胎盘、子宫和肺等组织分离获得。MSCs疗法在生产中可应用到乳腺炎、繁殖、骨损伤、关节损伤、糖尿病等疾病[28],可见牛MSCs具有广阔的应用前景。
将动物体细胞重编程为iPSCs的机制并不是高度保守的,Pillai等[29]在诱导牛iPSCs形成的过程中发现,优化的小鼠和人的iPSCs诱导条件并不能有效诱导牛体细胞产生iPSCs,使用能够改进小鼠和人体细胞重编程效率的方法也对诱导牛体细胞产生iPSCs没有影响。尽管使用编码牛OCT4、SOX2、KLF4、c-MYC和NANOG的逆转录病毒载体似乎能诱导成纤维细胞产生iPSCs样细胞,但这些克隆不能维持生长,说明牛iPSCs的培养条件和重编程还不完全,仍需进一步的改进。
牛奶是新生婴儿发育、营养和免疫保护所需的重要且复杂的液态物质。Pipino等[30]试图分离牛奶中潜在的多能干细胞样细胞群体,并研究其特征。发现分离出的牛奶干细胞表达典型的间充质表面抗原(CD90、CD73和CD105)、干细胞标记基因(SOX2和OCT4),并能分化为成骨细胞、软骨细胞和脂肪细胞。这些结果提示牛奶也可以作为分化为多种细胞谱系的多能干细胞来源,进一步丰富了牛成体干细胞的来源。
2.2 山羊和绵羊干细胞
Pawar等[8]从体外受精的山羊囊胚中分离出内细胞团,并培养出了ESCs样细胞,这些细胞中碱性磷酸酶和OCT4的表达均为阳性。此后,De等[31]对体外生产的处于桑椹胚、囊胚和孵化囊胚期的山羊胚胎进行干细胞分离培养,发现孵化囊胚分离效率相对更高。获得的克隆保持未分化状态培养至第15代,且具有干细胞形态特征、正常核型,并表达干细胞特异性标志基因。长时间培养后能分化成神经元样和上皮样细胞。很快,Garg等[32]也从体外受精的山羊胚胎分离了ESCs,使用饲养层和LIF培养至第22代仍保持未分化状态。诱导分化后形成类胚体,并在30天后观察到心肌细胞的节律性跳动。雄性生殖干细胞的自我更新与分化之间的平衡对于哺乳动物精子发生的启动和维持至关重要,对山羊雄性生殖干细胞的研究发现miR-544通过靶向早幼粒细胞白血病锌指蛋白(promyelocytic leukemia zinc finger,PLZF)来调节奶山羊雄性生殖系干细胞的自我更新[33]。对来自同一个供体绵羊的MSCs包括牙周膜MSCs、牙髓MSCs和骨髓MSCs等进行蛋白质组分析,获得的差异蛋白为相应组织的生长发育提供了分子基础[34]。Fadel等[35]通过比较不同的分离和培养方法,初步获得了脐带血和肾周脂肪组织来源的MSCs。目前的研究现状提示山羊和绵羊的干细胞相关研究仍需进一步深入。2.3 鸡干细胞
鸡ESCs和SSCs相关研究近年来也取得了较大进展。Zhang等[9]从X期的鸡胚中分离获得ESCs,这些ESCs经碱性磷酸酶染色呈阳性并表达干细胞标志基因SSEA1,通过优化电转染方法、孵化方法、显微注射方法将其注射到鸡胚腔后获得了2只生殖系嵌合鸡。用DF-1成纤维细胞做饲养层,在基础培养基中添加人类碱性成纤维细胞生长因子(human basic fibroblast growth factor, hbFGF)、小鼠干细胞因子(mouse stem cell factor, mSCF)和人白血病抑制因子(human leukaemia inhibitory factor, hLIF)后,体外培养的鸡ESCs能够长期维持干细胞样特性,即具有典型的ESCs形态、表达干细胞标志基因、具有相对稳定的增殖速率和端粒酶活性,可体外诱导分化为心肌细胞、平滑肌细胞、神经细胞、成骨细胞和脂肪细胞。用该培养体系培养了25代的鸡ESCs能够制备出嵌合体鸡,提示DF-1成纤维细胞是鸡ESCs能长期培养优选饲养层细胞,hbFGF是维持鸡ESCs多能性的重要因素[36]。因SSCs可体外获取,并不涉及伦理问题等优势,已日益成为体外干细胞研究的热点。经过改进酶消化、差速贴壁、流式或磁珠富集等现有的鸡SSCs分离和纯化方法,在添加生长因子并覆以饲养层细胞的培养基中培养,可获得体外培养的鸡SSCs[37]。同时,利用建系的鸡ESCs诱导分化为SSCs,也为SSCs的获得提供了新途径。在鸡ESCs向SSCs分化的研究中发现,CRISPR/Cas9介导的C1EIS缺失抑制鸡ESCs分化为SSCs样细胞[38];WNT通路通过WNT5A促进鸡ESCs向SSCs分化[39];成纤维细胞生长因子8 (fibroblast growth factor 8, FGF8)通过动态调控生殖细胞自我更新和分化进而负向调控鸡ESCs向SSCs分化[40];3β羟基类固醇脱氢酶2 (3β-hydroxysteroid dehydrogenase2, HSD3B2)则通过调节类固醇激素合成途径正向调控鸡ESCs向SSCs分化[41];作为鸡SSCs的特异性标记,LBC基因通过转录因子HOXA5促进鸡ESCs向SSCs分化[42]。综上可见,鸡ESCs和SSCs的相关研究已进入新的阶段,即建系后的机理研究和分化研究阶段,可为其他农业动物干细胞提供方法和理论参考。
3 结语与展望
自开展农业动物干细胞相关研究以来,虽然对多种畜禽干细胞的认识和利用取得了很大的进步,但在分离、培养、建系、分化以及应用等方面仍存在许多未知的问题。因此,对品种改良、生产基因编辑动物、研制新兽药、研发疾病新疗法以及组织修复和器官移植等应用,都需要对畜禽干细胞进行更多更深的探索和研究,同时也需要长时间的不懈努力。目前,猪已经成为生物医学研究中最受欢迎的大型动物模型之一,亦被认为是比啮齿类动物模型更好的选择。在进行人体实验之前,使用猪的干细胞及其衍生物进行研究可作为评价药物或治疗的安全性和有效性的实验平台。阐明如何将干细胞分化成精子样和卵母细胞样细胞,将这些精子样和卵母细胞样细胞进行体外受精,以创建拥有新的遗传组合的重构胚胎,再通过这些重构胚胎分离出更多的干细胞。即可以利用这种“干细胞-精子样和卵母细胞样细胞-重构胚胎-干细胞”循环,在体外快速获得大量具有特定遗传效应的胚胎,进行胚胎移植后获得特定个体(图1)。该策略能够实现缩短世代间隔和加速改善后代性状的目的,也意味着等待动物妊娠的时间变短,同时被消耗的动物变少,因此具有广阔的应用前景。综上所述,干细胞技术是未来农业科技快速进步不可缺少的重要技术,畜禽干细胞的成功和发展有望显著提高动物克隆和基因编辑的效率,畜禽干细胞成功诱导分化成配子将极大简化畜禽基因工程和合成生物技术,为畜禽种质创新技术提供重要支撑。
图1
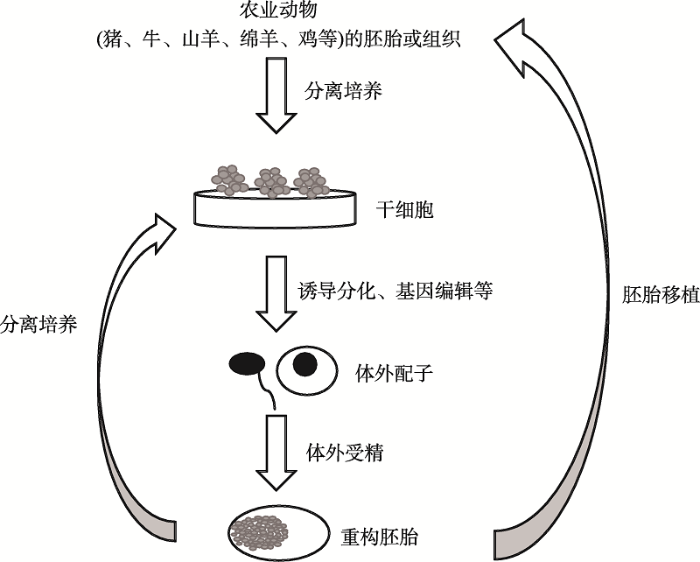 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1农业动物干细胞技术展望
建立猪、牛、山羊、绵羊、鸡等农业动物来源的“干细胞-精子样和卵母细胞样细胞-重构胚胎-干细胞”循环,可快速高效地获得具有特定基因或目标性状的个体。
Fig. 1Perspectives on stem cell technology in agricultural animals
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1038/292154a0URLPMID:7242681 [本文引用: 1]
DOI:10.1016/S0753-3322(01)00057-9URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/s11259-019-9744-6URLPMID:30656543 [本文引用: 1]

Stem cells are undifferentiated and self-renewable cells that present new possibilities for both regenerative medicine and the understanding of early mammalian development. Adult multipotent stem cells are already widely used worldwide in human and veterinary medicine, and their therapeutic signalling, particularly with respect to immunomodulation, and their trophic properties have been intensively studied. The derivation of embryonic stem cells (ESCs) from domestic species, however, has been challenging, and the poor results do not reflect the successes obtained in mouse and human experiments. More recently, the generation of induced pluripotent stem cells (iPSCs) via the forced expression of specific transcription factors has been demonstrated in domestic species and has introduced new potentials in regenerative medicine and reproductive science based upon the ability of these cells to differentiate into a variety of cells types in vitro. For example, iPSCs have been differentiated into primordial germ-like cells (PGC-like cells, PGCLs) and functional gametes in mice. The possibility of using iPSCs from domestic species for this purpose would contribute significantly to reproductive technologies, offering unprecedented opportunities to restore fertility, to preserve endangered species and to generate transgenic animals for biomedical applications. Therefore, this review aims to provide an updated overview of adult multipotent stem cells and to discuss new possibilities introduced by the generation of iPSCs in domestic animals, highlighting the possibility of generating gametes in vitro via PGCL induction.
DOI:10.1089/clo.2008.0050URLPMID:19508116 [本文引用: 3]

The purpose of this study was to establish transgenic porcine embryonic stem (pES) cell lines that can stably express report gene. Established pES cell line at passage 44 was transfected with pAAV-hrGFP Control Plasmid by electroporation-mediated, viral vector-mediated, and liposome-mediated strategies. Although there were several pES colonies expressing green fluorescent protein (GFP) obtained from the retrovirus-mediated and liposome-mediated transfection methods, no stable GFP-expressing pES cell line was then derived. A total of 28 GFP-expressing pES cell colonies were obtained following electroporation with two DC pulses of 150 V/cm for 10 msec and three GFP-expressing pES (pES/GFP(+)) cell lines were established. These pES/GFP(+) cell lines stably expressed exogenous GFP and continuously proliferated in vitro for more than 90 passages in 20 months. They maintained normal karyotype of 36 + XX and typical characteristics of pluripotent stem cells, including expression of pluripotent markers Oct-4, AP, SSEA-4, TRA-1-60, and TRA-1-81, formation of embryoid bodies under suspension culture. They were able to differentiate in vitro into neural and cardiomyocytic lineage, respectively, under suitable induction. To our knowledge, there has been no report of establishing GFP-expressing pES cell lines. These novel pES/GFP(+) cell lines established in this study might serve as a nonrodent model and would benefit to the studies involving ES cell transplantation, cell replacement therapy, and tissue regeneration due to their traceable capacity.
URLPMID:19775069 [本文引用: 3]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1089/scd.2015.0025URLPMID:25826126 [本文引用: 1]

For diseases of the brain, the pig (Sus scrofa) is increasingly being used as a model organism that shares many anatomical and biological similarities with humans. We report that pig induced pluripotent stem cells (iPSC) can recapitulate events in early mammalian neural development. Pig iPSC line (POU5F1(high)/SSEA4(low)) had a higher potential to form neural rosettes (NR) containing neuroepithelial cells than either POU5F1(low)/SSEA4(low) or POU5F1(low)/SSEA4(high) lines. Thus, POU5F1 and SSEA4 pluripotency marker profiles in starting porcine iPSC populations can predict their propensity to form more robust NR populations in culture. The NR were isolated and expanded in vitro, retaining their NR morphology and neuroepithelial molecular properties. These cells expressed anterior central nervous system fate markers OTX2 and GBX2 through at least seven passages, and responded to retinoic acid, promoting a more posterior fate (HOXB4+, OTX2-, and GBX2-). These findings offer insight into pig iPSC development, which parallels the human iPSC in both anterior and posterior neural cell fates. These in vitro similarities in early neural differentiation processes support the use of pig iPSC and differentiated neural cells as a cell therapy in allogeneic porcine neural injury and degeneration models, providing relevant translational data for eventual human neural cell therapies.
[本文引用: 1]
DOI:10.1371/journal.pone.0202155URLPMID:30157199 [本文引用: 1]

The application of appropriate animal models and techniques for the study of osteoporosis is important. Lanyu pigs, a local miniature breed, have been widely used in various biomedical studies in Taiwan. This study aimed to induce bone loss in Lanyu pigs and to examine whether porcine induced pluripotent stem cell (piPSC)-derived osteoblast-like cells could recover bone mass of tibiae via local cell transplantation. piPSCs were directed to differentiate into osteoblast-like cells using osteogenic medium, and differentiated cells expressed osteogenic markers and phenotypes. Twenty mature female Lanyu pigs were divided into four groups, including control (C, 1% calcium diet), treatment 1 (T1, ovariectomy + 1% calcium diet), treatment 2 (T2, ovariectomy + 0.5% calcium diet), and treatment 3 (T3, ovariectomy + 0.5% calcium diet + 1 mg/kg of prednisolone) and were subjected to bone loss induction for twelve months. Micro-CT images revealed that the lowest trabecular bone parameters, such as trabecular bone volume, thickness, separation, number, and total porosity, were detected in the T3 group. The lowest proportions of cortical bone in the proximal metaphysis, proximal diaphysis, and distal diaphysis were also found in the T3 group. These results indicate that ovariectomy, calcium restriction, and prednisolone administration can be applied to induce proper bone loss in Lanyu pigs. After bone loss induction, pigs were subjected to cell transplantation in the left tibiae and were maintained for another six months. Results showed that transplanted piPSC-derived osteoblast-like cells significantly improved trabecular bone structures at transplanted sites and maintained cortical bone structures in the proximal metaphysis. In conclusion, the therapeutic potential of piPSC-derived osteoblast-like cells was confirmed via cell transplantation in the left tibiae of Lanyu pigs. These findings reveal the therapeutic potential of piPSCs for glucocorticoid-induced bone loss in pig models.
[本文引用: 1]
URLPMID:23397443 [本文引用: 1]
[本文引用: 1]
DOI:10.1186/s40104-020-00439-0URLPMID:32308978 [本文引用: 1]

Background: Spermatogonial stem cells (SSCs) are capable of both self-renewal and differentiation to mature functional spermatozoa, being the only adult stem cells in the males that can transmit genetic information to the next generation. Porcine SSCs hold great value in transgenic pig production and in establishment of porcine models for regenerative medicine. However, studies and applications of porcine SSCs have been greatly hampered by the low number of SSCs in the testis as well as the lack of an ideal stable long-term culture system to propagate porcine SSCs perpetually. Results: In the present study, by lentiviral transduction of plasmids expressing the simian virus 40 (SV40) large T antigen into porcine primary SSCs, we developed two immortalized cell lines with porcine SSC attributes. The established cell lines, with the expression of porcine SSC and germ cell markers UCHL1, PLZF, THY1, VASA and DAZL, could respond to retinoic acid (RA), and could colonize the recipient mouse testis without tumor formation after transplantation. The cell lines displayed infinite proliferation potential, and have now been cultured for more than 7 months and passaged for over 35 times without morphological abnormalities. Conclusions: We have for the first time established porcine SSC lines that could provide abundant cell sources for mechanistic studies on porcine SSC self-renewal and differentiation, thereby facilitating development of an optimal long-term culture system for porcine primary SSCs and their application to animal husbandry and medicine.
[本文引用: 1]
URLPMID:27215170 [本文引用: 1]
[本文引用: 1]
DOI:10.1080/21691401.2017.1332637URLPMID:28562085 [本文引用: 1]

Stem cells of dental origin emerged as a new source for the regeneration of tissues with advantages mainly including non-invasive collection procedures and lack of ethical contraversies with their harvest or use. In this study, porcine TGSCs (pTGSCs) were isolated from mandibular third molar tooth germs of 6-month-old domestic pigs. This is the first study that reports the isolation and characterization of TGSCs from porcine third molars and their differentiation depending on STRO-1 expression. PTGSCs were sorted according to their STRO-1 expression as STRO-1(+) and STRO-1(-). Sorted and unsorted heterogenous cells (US) were characterized by their osteogenic, chondrogenic and adipogenic differentiation capabilities. STRO-1(+) cells exhibited a higher proliferation rate owing to their clonogenic properties. All three groups of cells were found differentiated into osteogenic lineage as shown by ALP activity, calcium deposition assay, detection of osteogenic mRNAs and, proteins and mineralization staining. According to differentiation analysis, STRO-1(+) cells did not show a better performance for osteogenesis compared to STRO-1(-) and US cells. This might indicate that STRO-1(+) cells might require a heterogeneous population of cells including STRO-1(-) in their niche to perform their proposed role in osteogenesis.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1111/asj.13272URLPMID:31322312 [本文引用: 1]

Mechanisms that direct reprogramming of differentiated somatic cells to induced pluripotent stem cells (iPSCs), albeit incomplete in understanding, are highly conserved across all mammalian species studied. Equally, proof of principle that iPSCs can be derived from domestic cattle has been reported in several publications. In our efforts to derive and study bovine iPSCs, we encountered inadequacy of methods to generate, sustain, and characterize these cells. Our results suggest that iPSC protocols optimized for mouse and human somatic cells do not effectively translate to bovine somatic cells, which show some refractoriness to reprogramming that also affects sustenance. Moreover, methods that enhance reprogramming efficiency in mouse and human cells had no effect on improving bovine cell reprogramming. Although use of retroviral vectors coding for bovine OCT4, SOX2, KLF4, cMYC, and NANOG appeared to produce consistent iPSC-like cells from both fibroblasts and cells from the Wharton's jelly, these colonies could not be sustained. Use of bovine genes could successfully reprogram both mouse and human cells. These findings indicated either incomplete reprogramming and/or discordant/inadequate culture conditions for bovine pluripotent stem cells. Therefore, additional studies that advance core knowledge of bovine pluripotency are necessary before any anticipated iPSC-driven bovine technologies can be realized.
DOI:10.1089/scd.2018.0114URLPMID:30142991 [本文引用: 1]

Milk is a complex fluid required for development, nutrition and immunological protection to the newborn offspring. Interestingly, latest finding proved the presence of novel stem cell population in human milk with multilineage differentiation potential. Given that little is known about cellular milk content in other mammalian species such as bovine, the purpose of our study was to isolate and characterize a potential stem cell-like population in bovine milk. In detail, we first analyzed the phenotype of the isolated cells able to grow in plastic adherence and then their capability to differentiate into osteogenic, chondrogenic, and adipogenic lineages. Bovine milk stem cells (bMSCs) resulted plastic adherent and showed a heterogeneous population with epithelial and spindle-shaped cells. Successively, their immunophenotype indicated that bovine milk cells were positive for the typical epithelial markers E-cadherin, cytokeratin-14, cytokeratin-18, and smooth muscle actin. Notably, a subset (30%-40%), constantly observed in purified milk cells, showed the typical mesenchymal surface antigens CD90, CD73, and CD105. Furthermore, the same percentage of bMSCs expressing CD90, CD73, and CD105 presented the stemness markers SOX2 and OCT4 translocated in their nuclei. Finally, our data showed that bMSCs were able to differentiate into osteoblasts, chondroblasts, and adipocytes. In addition, the flow cytometry analysis revealed the presence of a subpopulation of events characterized by typical extracellular vesicles (EVs, size 0.1-1 mum), which did not contain nuclei and were positive for the same markers identified on the surface of bMSCs (CD73, CD90, and CD105), and thus might be considered milk cell-derived EVs. In conclusion, our data suggest that bovine milk is an easily available source of multipotent stem cells able to differentiate into multiple cell lineages. These features can open new possibilities for development biology and regenerative medicine in veterinary area to improving animal health.
DOI:10.1080/10495398.2011.622189URL [本文引用: 1]

The aim of the present study was to isolate and characterize goat embryonic stem cell-like cells from in vitro produced goat embryos. Inner cell mass (ICM) cells were isolated either mechanically or by enzymatic digestion from 150 blastocysts and 35 hatched blastocysts whereas 100 morulae were used for blastomeres isolation mechanically. The ICM derived cells or blastomeres were cultured on a feeder layer. The primary colony formation was significantly higher (P < 0.01) for hatched blastocysts (77.14%) than early/expanded blastocysts (54%) or morula (14%). When ICMs were isolated mechanically the primary colony formation for hatched blastocysts (90%) as well as blastocysts (66%) were significantly more than when ICMs were isolated by enzymatic digestion (60% and 30%, respectively). The colonies were disaggregated either mechanically or by enzymatic digestion for further subculture. When mechanical method was followed, the colonies remained undifferentiated up to 15 passages and three ES cell-like cell lines were produced (gES-1, gES-2, and gES-3). However, enzymatic disaggregation resulted in differentiation. The undifferentiated cells showed stem cell like morphological features, normal karyotype, and expressed stem cell specific surface markers like alkaline phosphatase, TRA-1-61, TRA-1-81, and intracellular markers Oct4, Sox2, and Nanog. Following prolonged culture of the ES cell-like cells were differentiated into several types of cells including neuron like and epithelium-like cells. In conclusion, goat embryonic stem cell-like cells can be isolated from in vitro produced goat embryos and can be maintained for long periods in culture.
DOI:10.1016/j.theriogenology.2011.05.029URL [本文引用: 1]

The aim of present investigation was isolation, characterization and differentiation into cardiomyocytes of putative goat embryonic stem cells produced from in vitro fertilized goat embryos. Goat blastocysts were produced in vitro by standard methods of in vitro maturation (IVM), in vitro fertilization (IVF) and in vitro culture (IVC) techniques. The ICMs isolated from IVF blastocysts were cultured on 10 mu l/ml mitomycin-C inactivated fetal fibroblast feeder layer with LIF. The putative ES colonies were characterized for extracellular markers like alkaline phosphatase, TRA-1-60, TRA-1-81, SSEA-1, SSEA-4 by immunocytochemistry and intracellular markers like Oct4, Sox2 and Nanog with reverse-transcription-PCR. The ES cells were successfully subcultured up to 22nd passage with feeder layer and LIF and up to 12th passage without feeder layer with LIF only. They exhibited normal karyotyping (20th passage) and maintained the expression of specific surface markers like alkaline phosphatase, SSEA-4, TRA-1-61, TRA-1-81 and intracellular markers Oct4, Sox2 and Nanog. The embryoid bodies (EBs) were generated from goat ES cells of 20th passage and were analyzed with markers like Gata4, BMP4 and Nestin. Differentiation was induced by medium containing 100 ng/ml Activin A, 10 ng/ml FGF-2 and 100 ng/ml BMP-4. The embryoid bodies were analyzed with markers like Gata4, BMP4 and Nestin. The rhythmic beating of cardiomyocytes was observed after 30 d and the beating was still continuing even after 160 d of culturing. Similarly, 2nd and 3rd batches of EBs were also beating and the beating continues after 75 d and on. The beating cells were observed positive for cardiac specific markers like a Actinin, C-Troponin and alpha-Myosin heavy chain. Histological studies also revealed morphology similar to cardiomyocytes. Prominent contractions typical of cardiac tissue have been maintained in the differentiated cells up to 160 d and stilt continuing beating at the rate of 30 beats/min. It could be concluded that ES cells generated from goat embryos were maintained undifferentiated up to 22nd passage on feeder layer and to 12th passage without feed layer using LIF and that the differentiation protocol induced rhythmic beating cells. (C) 2012 Elsevier Inc.
DOI:10.1002/jcb.25172URLPMID:25808723 [本文引用: 1]

The balance between the self-renewal and differentiation of male germline stem cells (mGSCs) is critical for the initiation and maintenance of mammalian spermatogenesis. The promyelocytic leukemia zinc finger (PLZF), a zinc finger protein, is a critical factor for maintaining the self-renewal of mGSCs, so, evaluation of the PLZF pathway in mGSCs may provide a deeper insight into mammalian spermatogenesis. miRNA was also an important regulating factor for the self-renewal and differentiation of mGSCs; however, there is currently no data indicating that which miRNA regulate the self-renewal and differentiation of mGSCs via PLZF. Here, we predicted the prospective miRNA targeting to PLZF using the online Bioinformatics database-Targetscan, and performed an analysis of the dual-luciferase recombinant vector, psiCHCEKTM-2-PLZF-3'UTR. miR-544 mimics (miR-544m), miR-544 inhibitors (miR-544i), Control (NC, scrambled oligonucleotides transfection), pPLZF-IRES2-EGFP or PLZF siRNA were transfected into mGSCs; the cells proliferation was evaluated by BRDU incorporation assay and flow cytometry, and the mGSC marker, GFRa1, PLZF, KIT, DAZL, and VASA expression were analyzed by RT-qPCR, immunofluorescence and Western blot. The results showed that miR-544 regulates dairy goat male germline stem cell self-renewal via targeting PLZF. Our study identifies a new regulatory pathway for PLZF and expands upon the PLZF regulatory network in mGSCs.
[本文引用: 1]
[本文引用: 1]
URLPMID:29861740 [本文引用: 1]
DOI:10.1080/00071668.2017.1365354URLPMID:28840744 [本文引用: 1]

1. The avian embryo is an excellent model for studying embryology and the production of pharmaceutical proteins in transgenic chickens. Furthermore, chicken stem cells have the potential for proliferation and differentiation and emerged as an attractive tool for various cell-based technologies. 2. The objective of these studies is the derivation and culture of these stem cells is the production of transgenic birds for recombinant biomaterials and vaccine manufacture, drug and cytotoxicity testing, as well as to gain insight into basic science, including cell tracking. 3. Despite similarities among the established chicken stem cell lines, fundamental differences have been reported between their culture conditions and applications. Recent conventional protocols used for expansion and culture of chicken stem cells mostly depend on feeder cells, serum-containing media and static culture. 4. Utilising chicken stem cells for generation of cell-based transgenic birds and a variety of vaccines requires large-scale cell production. However, scaling up the conventional adherent chicken stem cells is challenging and labour intensive. Development of a suspension cell culture process for chicken embryonic stem cells (cESCs), chicken primordial germ cells (PGCs) and chicken induced pluripotent stem cells (ciPSCs) will be an important advance for increasing the growth kinetics of these cells. 6. This review describes various approaches and suggestions to achieve optimal cell growth for defined chicken stem cells cultures and use in future manufacturing applications.
[本文引用: 1]
URLPMID:28786525 [本文引用: 1]
[本文引用: 1]
URLPMID:28703914 [本文引用: 1]
URLPMID:32918333 [本文引用: 1]
