 ,1
,1Progress on signal pathways related to bone metabolism in animals
Jingying Zhao1, Xiaohua Duan1,2, Qiuting Wang1, Ying Huang1, Junjing Jia1, Tengfei Dou ,1
,1通讯作者: 豆腾飞,讲师,研究方向:动物遗传育种与繁殖。E-mail:tengfeidou@sina.com
编委: 张雷
收稿日期:2020-03-11修回日期:2020-06-14网络出版日期:2020-10-20
| 基金资助: |
Received:2020-03-11Revised:2020-06-14Online:2020-10-20
| Fund supported: |
作者简介 About authors
赵净颖,在读硕士研究生,专业方向:动物营养与饲料科学。E-mail:

摘要
骨骼是组成脊椎动物内骨骼的坚硬器官,对机体起着运动、支撑和保护的作用。骨骼处于骨形成和骨吸收两种活动所组成的骨代谢的动态平衡状态,这种平衡对于维持骨量和矿物质稳态至关重要。在动物骨代谢过程中,存在着众多调节骨形成和骨吸收的信号通路,如BMP (bone morphogenetic protein)/SMADs、TGF-β (transforming growth factor β)、Wnt/β-catenin、OPG (osteoprotegerin)/RANKL (receptor activator of NF-κB ligand)/ RANK (receptor activator of NF-κB)、FGF (fibroblast growth factor)和Notch信号通路等。这些信号通路具有复杂的调控机制,参与骨代谢过程的调节。本文综述了在动物骨代谢过程中起关键调节作用的相关信号通路的作用机制及研究进展,以期为动物骨代谢研究奠定基础。
关键词:
Abstract
Bone is a hard organ that makes up vertebrate endoskeleton, which plays a role in movement, support and protection for the body. The normal growth and development of bone is in the dynamic balance of bone metabolism, which is composed of bone formation and bone absorption. This balance is very important for maintaining bone mass and mineral homeostasis. In the process of bone growth and metabolism, there are many signaling pathways regulating bone formation and absorption, such as BMP (bone morphogenetic protein)/SMADs, TGF-β (transforming growth factor β), Wnt/β-catenin, OPG (osteoprotegerin)/RANKL (receptor activator of NF-κB ligand)/RANK (receptor activator of NF-κB), FGF (fibroblast growth factor) and Notch signaling pathway. These signaling pathways have complex regulatory mechanisms and are involved in the regulation of bone metabolism. In this review, we summarize the mechanism and research progress of signal pathways that play key regulatory roles in the process of animal bone metabolism, thereby laying a foundation for research in animal bone metabolism.
Keywords:
PDF (632KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
赵净颖, 段小花, 王秋婷, 黄英, 贾俊静, 豆腾飞. 动物骨代谢相关信号通路研究进展. 遗传[J], 2020, 42(10): 979-992 doi:10.16288/j.yczz.20-066
Jingying Zhao.
骨骼是脊椎动物机体重要的刚性组织,主要由磷酸钙矿物质和I型胶原组成,对动物机体起到支撑、保护以及运动等作用。骨骼系统的发育和维持受遗传及环境等因素的影响[1],其中遗传因素是影响骨骼系统发育的重要因素,其对骨骼生长发育的影响在动物的整个生命过程中均有体现,包括骨相关信号通路调控机制、基因作用的发育时机、骨相关基因产物的表观遗传修饰等[2]。
骨骼发育过程包括膜内骨化和软骨内骨化两种方式[3]。在膜内骨化过程中,间充质干细胞(mesenchymal stem cell, MSC)直接分化成成骨细胞(osteoblast, OB)。膜内骨化主要发生在颅顶骨部位。在软骨内骨化过程中,间充质干细胞发生增殖分化形成软骨,随后被矿化骨所取代。软骨内骨化发生在颅底和颅骨后部、轴向骨和四肢骨。发育结束进入稳态维持的骨骼处于骨形成和骨吸收所组成的骨代谢动态平衡状态,其中骨形成过程是由MSC衍生的OB介导,骨吸收过程是由造血干细胞(hematopoietic stem cell, HSC)衍生的破骨细胞(osteoclast,OC)介导。骨吸收与骨形成之间的平衡对于维持骨量和维持全身矿物质稳态至关重要,因此可以保持骨骼健康。一旦这种平衡状态被破坏,骨骼便会处于病理状态,也称骨代谢性疾病,如高破骨细胞活性或低成骨细胞活性导致的低骨量(骨质减少),以及低破骨细胞活性或高成骨细胞活性导致的高骨量(骨硬化)[4]。
在动物骨骼生长发育和骨代谢过程中,存在着生长因子、细胞因子、酶等众多调控因子,而这些调控因子通过相关的信号通路参与骨形成和骨吸收过程,如BMP (bone morphogenetic protein)/Smads、TGF-β (transforming growth factor β)、Wnt/β-catenin、OPG (osteoprotegerin)/RANKL (receptor activator of NF-κB ligand)/RANK (receptor activator of NF-κB)、FGF (fibroblast growth factor)和Notch信号通路等。其中,BMP/Smads信号通路影响成骨细胞分化及骨形成,TGF-β信号通路参与调控成骨过程中细胞的活动和代谢,Wnt/β-catenin信号通路调控骨代谢的平衡,OPG/RANKL/RANK信号通路可以维持骨骼稳态,FGF信号通路和Notch信号通路参与成骨细胞分化。本文介绍了参与动物骨代谢的信号通路相关研究进展,阐明这些信号通路在调节骨形成和骨吸收方面的作用机制,可以为临床有效防治骨异常性疾病提供理论依据。
1 影响动物骨代谢的关键信号通路
动物骨代谢的调控是一个非常复杂的过程,有众多的调节因子及信号途径参与。这些途径各自具有复杂的调控机制,单独或共同参与动物骨代谢的调控。近年来众多研究表明,动物骨代谢的平衡主要由BMP/Smads、TGF-β、Wnt/β-catenin、OPG/RANKL/ RANK和FGF等多条关键信号通路参与调控。1.1 BMP/Smads信号通路
在骨骼发育过程中,多条信号通路参与调节成骨与破骨过程的平衡,而最早发现和确认的最重要的一条通路是影响成骨细胞分化及骨形成的关键信号通路——BMP/Smads信号通路[5,6]。骨形态发生蛋白(bone morphogenetic protein, BMPs)是转化生长因子β(transform growth factor β, TGF-β)超家族的成员,是骨骼发育的重要生长因子,具有增强骨髓干细胞向成骨细胞分化的能力,从而促进骨骼的生长发育[5]。在已发现的20余种亚型中,BMP2、4、5、6、7、9等均对成骨细胞分化有调节作用[6,7,8]。其中BMP2因具有增强骨形成及代谢的作用而成为研究热点。BMP2能够诱导间充质干细胞的增殖、迁移以及向成骨细胞的分化[8,9],且能够协同其他成骨因子共同刺激成骨细胞增殖,增强成骨细胞活性,加速骨重建[10]。
BMP/Smads信号通路由BMPs及其受体、Smad蛋白和相关转录因子组成。BMP2通过自分泌或旁分泌形式释放后,其单体可通过二硫键连接形成二聚体,再结合BMPs受体。BMPs受体是丝氨酸/苏氨酸激酶受体,包括I型受体(BMPR-IA、BMPR-IB和ACVR-I)和II型受体(BMPR-II、ActR-IIA和ActR-IIB)[11]。BMP2先结合BMPR-II,发生自身磷酸化而被激活,继而磷酸化BMPR-I,使BMPR-I激活[12,13,14],下游Smads 信号Smad1/5/8被BMPR-I受体激活并和受体形成短暂复合物,使其与Smad4结合,转移至核内,转录激活成骨分化基因,如上调核心结合蛋白因子2 (Runx2)[15,16]、成骨细胞特异性转录因子Osterix (OSX)[17]和同源盒基因HoxC-8[18]等,促进成骨细胞分化。而Smad6和Smad7则参与了骨形态发生蛋白信号传导的负调控[19]。
Dallari等[20]和Yang等[21]在人类骨骼疾病的临床研究中发现骨质疏松性骨折患者常伴有碱性磷酸酶活性下降的现象,而导致碱性磷酸酶活性下降的重要原因是BMP2等成骨基因表达不足,并且存在抑制软骨细胞向成骨细胞的分化并伴有成骨细胞凋亡的增加现象。Zappitelli等[22]在小鼠(Mus musculus)细胞实验中的研究显示,上调BMP2/4基因的表达可提高成骨细胞特定标志物的表达。Li等[19]和Zarrinkalam等[23]在山羊(Capra hircus)的活体实验研究中发现,注射人重组的BMP2可提高骨密度,并可以治疗骨质疏松山羊腰椎骨缺损处;Cipitria等[24]研究表明注射人重组的BMP7可提高山羊胫骨的韧性和强度。Mizrahi等[25]在猪(Capra hircus)的间充质干细胞研究中发现,人重组的BMP6比BMP2更加有效地诱导成骨分化,促进骨骼的形成。在BMP2/ Smads信号通路中,存在众多的相关调控因子。研究发现,泛素化调节因子(Smurf)与BMP2激活的Smad1、Smad5相互作用并调节成骨细胞特异性转录因子Runx2降解[26],并且Smad6与Smurf的协同作用可下调Runx2蛋白水平,负向调控BMP/Smads信号通路[27]。而I型多发性内分泌腺瘤肿瘤抑制基因产物(Menin)可增强BMP2诱导的Smad1/5、Runx2的转录活性。胶原三股螺旋重复蛋白1 (collagen triple helix repeat containing 1, Cthrc1)可正向调节OB骨形成,从而使骨量增加。Takeshita等[28]研究发现,缺失Cthrc1的小鼠在生长初期,碱性磷酸酶(alkaline phosphatase, ALP)和骨钙素(bone gamma- carboxyglutamic-acid-containing proteins, BGP)表达减少。对猪和羊的研究发现,Noggin基因是BMPs的拮抗剂,可调节BMPs对骨骼的作用,调节骨生长发育的重要细胞因子[29,30]。
除BMP/Smads信号通路相关调控因子以外,一些外在因素也可通过调节BMP/Smads信号通路来促进动物骨骼的生长发育。Feng等[31]对大鼠(Rattus norvegicus)细胞的研究显示,辛伐他汀(simvastain)可促进骨质疏松症(osteoporosi, OP)大鼠的关键成骨分化相关因子Runx2,骨保护素(osteoprotegerin, OPG),骨桥蛋白(osteopontin, OPN)和OSX的mRNA表达水平升高,可以通过BMP/Smads信号通路促进OP大鼠模型中MSCs向OB分化。Chai等[32]在去卵巢大鼠的研究中发现,传统中药配方骨疏康处理组通过上调BMP2,磷酸化Smad1和磷酸化Smad5 (p-Smad1/5),升高Osterix和Runx2的表达来显著增强BMP/Smads信号通路,促进骨形成。Yu等[33]研究显示,大豆(Glycine max)苷元通过激活BMP/Smads途径促进成骨细胞增殖和分化,具体表现为上调ALP、Runx2、Smad1基因的表达以及Runx2和Smad1蛋白的表达。可见,BMP/Smads信号通路的激活可以促进动物成骨细胞的增殖、分化及骨形成。
1.2 TGF-β信号通路
骨骼中TGF-β家族成员参与了整个成骨过程中细胞活动和代谢的调控。TGF-β家族由3种亚型组成:TGF-β1、TGF-β2和TGF-β3[34],均在骨中表达。TGF-β与其结合蛋白(LTBP)结合形成复合物,由OB分泌,可以与细胞外基质(extracellular matrix, ECM)成分(纤维连接蛋白、纤颤蛋白1和整合素等)相互作用,增强成骨细胞分化,促进骨骼发育[35]。Dünker等[36]研究表明,TGF-β2-/-和TGF-β3-/-双基因敲除小鼠远端部分骨量丢失,特别是TGF-β2缺失的小鼠在膜内和软骨内骨化方面表现出严重的骨骼异常。在骨基质合成终止后,成骨细胞发生凋亡或分化为骨细胞(骨衬细胞)。成熟的TGF-β通过阻止成骨细胞凋亡来调控成骨细胞的存活。骨髓巨噬细胞是破骨细胞的前体,而TGF-β直接作用于骨髓巨噬细胞,促进破骨细胞生成。Yasui等[37]研究表明,TGF-β诱导Smad2/3与泛素连接酶TRAF6 (TNF receptor associated factor 6)之间的分子相互作用,对于RANKL诱导的破骨细胞信号通路至关重要。此外,成骨细胞可产生RANKL和巨噬细胞集落刺激因子(macrophage colony stimulating factor, M-CSF)。因此,TGF-β对破骨细胞的作用也来源于成骨细胞。TGF-β刺激成骨细胞不仅表达I型胶原、ALP、BGP等成骨功能蛋白,还表达M-CSF、RANKL、OPG等破骨细胞调控基因[38]。TGF-β对破骨细胞生成的影响与剂量有关,低剂量TGF-β通过增加M-CSF的表达和前列腺素的产生,以及RANKL与OPG的比值来增强破骨细胞的生成[39],而高浓度TGF-β则通过增加OPG的表达来抑制M-CSF和RANKL的表达[40]。由于OPG是RANKL的高亲和力配体,是成骨细胞产生的RANKL的可溶性抑制剂,因此TGF-β对成骨细胞介导的破骨细胞的作用可能是骨重建的负反馈作用。
Shi等[41]研究发现,用TGF-β信号抑制剂SB431542处理的骨髓间充质干细胞(mesenchymal stem cells, MSC),移植到猪上颌骨缺损处,可以促进成骨分化增加,成功修复小型猪严重的颌面部骨缺损。Zeng等[42]通过细胞实验发现miR-23a簇(miR-23a-24-2-27a簇)通过靶向TGF-β途径的负调节剂Prdm16来调节TGF-β信号通路,从而促进小鼠骨细胞的分化。Xu等[43]研究表明,系统或局部阻断软骨中TGF-β活性可减轻类风湿关节炎(rheumatoid arthritis, RA)关节软骨退变,表明软骨中TGF-β的异常激活与RA关节软骨退变的发生有关。可见,TGF-β对动物成骨分化起着负反馈作用。
1.3 Wnt/β-catenin信号通路
Wnt信号通路已成为骨代谢平衡的关键调控信号通路[44]。Wnt信号控制胚胎发育和细胞增殖、分化、迁移等多个过程。β-catenin是调控Wnt信号的必要调控因子。一旦Wnt蛋白与Frizzled受体、低密度脂蛋白相关蛋白受体(Lrp5或Lrp6)结合,β-catenin将逃脱降解机制,转移到细胞核并与转录调节因子Tcf/Lef相互作用,激活Wnt靶基因的转录。在骨质疏松/高骨密度综合征中,Lrp5的功能发生缺陷[45,46]。β-catenin信号对成骨细胞发挥细胞环境相关的功能取决于分化的阶段。在早期阶段,β-catenin在前成骨细胞中失活,导致成骨细胞分化受阻,从而致使骨骼中缺乏成熟的成骨细胞[47,48,49,50]。然而,在后期阶段,β-catenin在成熟的成骨细胞和骨细胞中失活并不影响成骨细胞分化和骨形成,而是通过增加破骨细胞分化和骨吸收导致骨量降低[51,52,53]。在Wnt/β-catenin信号通路中,甲状旁腺激素和机械负荷等因素可下调骨细胞中Sost基因并增强β-catenin信号[54,55,56]。Sost编码硬化蛋白,是一种有效的骨形成抑制剂,通过与Lrp5/6结合来拮抗Wnt信号,而硬化蛋白表达的缺失是导致高骨量疾病范·巴克病(van Buchem)和硬化性骨化病的原因[57,58]。因此,Wnt信号的激活对动物骨代谢的调节起重要作用。Tu等[43]研究表明,激活β-catenin的小鼠表现出四肢骨骼骨矿物质密度增加,骨小梁数量、骨松质密度、骨形成标记物显著增加,且骨膜骨形成率明显升高;骨骼中Wnt信号靶向基因、成骨细胞和骨细胞标记、原骨细胞因子和抗破骨细胞因子含量升高。因此,激活骨细胞中的Wnt/β-catenin信号可增加成骨细胞的增殖、分化,从而促进骨量增加。可见,β-catenin可激活骨细胞和成骨细胞等骨细胞的合成代谢是骨骼中Wnt/β-catenin信号促进骨形成的重要原因。Wang等[59]研究发现,脂联素(adiponectin)转基因的BMSCs中的β-catenin和细胞周期蛋白D1 (cyclinD1)基因及其蛋白表达水平较高,且脂联素治疗组小鼠观察到更多新骨形成,表明脂联素可促进BMSCs成骨分化和成骨,而Wnt/β-catenin途径参与脂联素的成骨作用。Zhu等[60]在体外培养的BMSCs中发现,梓醇(catalpol)可显著增强成骨细胞特异性基因表达、碱性磷酸酶活性和钙沉积,可通过激活Wnt/β-catenin途径促进BMSCs的成骨分化。Molagoda等[61]发现太平洋牡蛎(Crassostrea gigas)提取物可以在斑马鱼(Brachydanio rerio var)中通过诱导Wnt/β-catenin途径来促进幼体骨矿化及尾鳍再生。因此,在动物骨骼生长发育中可通过激活Wnt/ β-catenin信号通路来促进成骨分化,促进骨量增加。
1.4 OPG/RANKL/RANK信号通路
骨转换(bone turnover)取决于成骨细胞的骨形成和破骨细胞的骨吸收之间的平衡。骨量丢失和骨质疏松症的发生是骨吸收大于骨形成导致的。OPG、细胞核因子-κB受体活化因子(RANK)和RANK配体(RANKL)是偶联成骨细胞、基质细胞和破骨细胞分化、活化及生物活性的3种主要细胞因子,OPG/ RANKL/RANK信号通路是破骨细胞生物学的基础,在骨代谢中起十分重要的作用[62]。大量研究表明,人类及动物代谢性骨病与这一系统的改变有关[63,64,65]。OPG是肿瘤坏死因子(tumor necrosis factor, TNF)受体家族成员,又称破骨细胞生成抑制因子或TNF受体样分子,是一种通过抑制破骨细胞分化和活化来调节骨量的分泌蛋白。RANKL是肿瘤坏死因子超家族的一员,已被证明既能介导破骨细胞生成,又能激活成熟破骨细胞。RANK是RANKL的受体,结合RANKL发挥生物学功能。在骨组织中,RANKL由多种细胞表达,包括成骨细胞、骨细胞和免疫细胞,尤其在成骨细胞和骨细胞中的表达较高[66]。RANKL结合并激活其位于破骨细胞祖细胞和成熟破骨细胞上的受体RANK。RANK刺激导致前破骨细胞分化为活性破骨细胞,活性破骨细胞重新吸收矿化骨基质。RANKL的活化优先表达于成骨前细胞的细胞膜上,而其特异性受体RANK则表达于破骨细胞前体细胞的细胞膜上。RANKL与RANK的结合导致破骨前细胞分化、形成、融合和存活[67]。RANK- RANKL相互作用是破骨细胞形成的必要条件。
研究表明,缺失RANKL的动物在缺乏骨基质或成骨细胞的情况下,无法产生破骨细胞,而提供外源性RANKL可刺激体外破骨细胞生成[68]。基质细胞和包括成骨细胞系在内的其他类型细胞分泌的OPG,可竞争性地结合RANKL,阻断RANK对破骨细胞的作用,进而抑制破骨细胞的活化,进而抑制破骨和骨溶解。过表达OPG的小鼠导致严重骨丢失和成熟破骨细胞减少实验,表明OPG可作为破骨细胞生成抑制剂[69],而RANKL缺失的小鼠表现出严重的骨丢失,并且由于不能支持破骨细胞的生成而完全缺乏破骨细胞[70]。然而,Liu等[71]研究表明敲除OPG的小鼠骨质疏松,骨密度降低,骨折发生率高,通过静脉注射重组OPG蛋白可逆转其病理。因此,OPG的存在对维持正常骨量是绝对必要的。另外,有研究表明,作为破骨生成促进因子的RANKL和破骨生成抑制因子的OPG表达水平的平衡决定了骨吸收的程度,RANKL上调和OPG下调都会导致骨质流失[72],且OPG/RANKL比值的失衡可能导致骨量的丢失[73]。
Huang等[74]对家鸡(Gallus gallus)的研究表明,OPG/RANKL平衡的破坏导致骨形成改变,引起鸡胫骨结构改变、胫骨质量降低,导致胫骨软骨发育不良。血清中OPG水平、Ca2+浓度和ALP活性均显著降低,进一步证实了骨代谢受到抑制。Wu等[75]通过人乳腺癌细胞MDA-MB-231和小鼠成骨细胞MC3T3-E1共培养系统的研究结果表明,与未经处理的共培养物相比,马钱子碱(brucine)处理显著提高了共培养物中OPG/RANKL mRNA表达比率和OPG/RANKL蛋白比率,说明马钱子碱可通过调节成骨细胞中OPG和RANKL的表达和分泌来间接控制破骨细胞,从而抑制破骨细胞的分化和骨吸收功能。Hou等[76]将雌性Sprague-Dawley大鼠进行卵巢切除术,分别用10、100、1000和2000 mg/kg /d等剂量的乳铁蛋白(lactoferrin, LF)进行口服治疗,饲喂6个月后发现,LF剂量依赖性地增加了Ovx大鼠骨体积、骨小梁厚度和骨小梁数目,减少了骨小梁分离;此外,与未经处理的Ovx大鼠相比,更高剂量的LF (1000 mg/kg/d和2000 mg/kg/d)显著增加了骨密度,且总体上LF处理显著提高了Ovx大鼠OPG mRNA水平,抑制了RANKL mRNA水平;这些结果表明,口服LF可保留骨质并改善骨骼的微结构,且LF可能通过OPG/RANKL/RANK途径的调节来增强骨形成,减少骨吸收及骨质流失。据Ma等[77]发现220 mg/kg或440 mg/kg的1,6-二磷酸果糖锶(FDP-Sr)治疗能显著提高Ovx大鼠的骨密度,改善骨微结构和骨强度,且用FDP-Sr治疗以剂量依赖性方式降低了血清中RANKL水平,增加了OPG水平,也显著下调了骨髓中的RANKL表达和上调了OPG表达,结果表明,FDP-Sr对绝经后骨质疏松症的有效治疗,其部分作用是通过OPG-RANKL-RANK途径减少破骨细胞的生成完成。可见,OPG/RANKL的比值可调节骨吸收,OPG-RANK-RANKL信号通路在调节动物骨形成和骨吸收的过程中起着至关重要的作用。
1.5 FGF信号通路
成纤维细胞生长因子(fibroblast growth factor, FGF)信号通路已被证实在调节成骨细胞和成纤维细胞的增殖和分化、成骨以及许多其他重要的细胞过程中发挥着重要的作用,包括血管生成和伤口愈合[78]。此外,FGF信号通路在调节骨祖细胞膜内和软骨内骨化的信号传递过程中发挥着至关重要的作用[79]。由于FGF通路对成骨细胞分化的刺激作用以及对成骨细胞分化的抑制作用,表明FGF信号通路对成骨细胞成熟过程的影响是阶段性的[80],且颅骨和长骨生长异常多与FGF信号通路的突变有关[81]。
在23个FGF家族成员中,一些FGF在成骨过程中起着关键作用。例如,FGF2磷酸化激活Runx2,可影响骨形成[82]。此外,已有研究表明FGFR2 (FGF receptor 2)也参与了骨生长的正向调控和成骨细胞的合成代谢功能[83]。FGFR3可通过调节成骨细胞分化来影响骨骼的骨密度和皮质骨厚度[84]。FGF9和FGF18的表达也显著影响胚胎骨形成[85]。
Kanda等[86]发现大鼠骨髓细胞在含碱性成纤维细胞生长因子(basic fibroblast growth factor, bFGF)培养基中培养后,细胞数量显著增加,细胞膨胀,BMP2和骨桥蛋白表达明显增加。Furuya等[87]研究发现水凝胶bFGF治疗骨折缺损后,小鼠骨密度较高,骨矿化率较高,Runx2和BGP基因表达上调。D’mello等[88]研究表明,转染编码FGF2蛋白(PEI-pFGF2)的聚乙烯亚胺纳米复合物(nanoplexes)的骨髓基质细胞(BMSCs)中的FGF2基因高表达。Khorsand等[89]在糖尿病兔(Leporidae)模型中也开发了相同的nanoplex,用于将FGF2和BMP2蛋白递送到缺陷部位。结果表明,与单纯植入胶原支架的PEI-pBMP2相比,植入胶原支架内的PEI-(pBMP2 + pFGF2)可显著改善骨再生。据Charles等[90]报道,在颅骨缺损的年老小鼠中,向BMP2中添加FGF2联合治疗后,小鼠骨缺损中心区域的骨填充增强,且骨体积增加。该研究表明,相对于单独的BMP2或FGF2,低剂量FGF2和低剂量BMP2联合应用有可能增加老年小鼠的骨愈合能力。这些结果表明BMP2和FGF2协同作用可促进骨修复。Yuan等[91]研究发现,单独的BMP4/7显著促进了BMSCs的增殖,同时,它也促进或抑制了BMSCs的成骨分化,而BMP4/7和bFGF的协同作用显著促进了BMSCs的增殖和成骨分化,协同作用的治疗取决于剂量和时间。BMP4/7和bFGF的合理组合可以促进BMSCs的增殖和成骨分化。综上所述,BMP和FGF协同作用对成骨过程的调节起重要作用。
1.6 其他骨代谢重要信号通路
1.6.1 Notch信号通路Notch信号通路作为一种进化上高度保守的配体受体信号通路,在细胞存活、增殖、分化以及发育过程中的命运决定、稳态等方面发挥着重要的机制作用[92]。Notch信号通路在骨骼生长发育中起着对成骨细胞的直接诱导作用。而骨祖细胞中Notch信号通路受到抑制可导致骨髓源性间充质干细胞的损耗,并与各年龄段骨质流失有关[93,94]。Pan等[95]研究发现,活化的B淋巴细胞通过激活Notch信号来抑制BMSC的成骨作用,当B淋巴细胞被灭活或Notch信号被抑制时,BMSC的成骨作用将部分恢复。He等[96]在小鼠细胞实验中发现,抑制Notch1减少了BMSC的增殖并促进其成骨分化。这些结果表明Notch信号通路在BMSC分化过程中受到抑制,说明Notch信号通路对BMSC成骨分化具有抑制作用。另一方面,Fukushima等[97]研究发现,Notch 2是破骨细胞重塑刺激物,它在破骨细胞分化过程中调节活化T细胞核因子c1(NFAT-c1)的启动子并诱导破骨细胞形成。因此,Notch信号在骨骼发育中起着不可或缺的作用。
1.6.2 Hedgehog信号通路
Hedgehog(Hh)信号通路是调节骨骼发育的关键。目前在哺乳动物中已经确定了3种Hh:Sonic Hh(Shh),Indian Hh(Ihh)和Desert Hh(Dhh)[98]。其中,Ihh是在发育中的骨骼内唯一发现的Hh,Ihh由软骨细胞分泌,调节软骨细胞的增殖和分化,对骨骼生长至关重要[99,100]。有研究发现,Hh信号通路可能通过增加Runx2和OSX的表达来诱导MSC的成骨分化,并抑制MSC分化为脂肪细胞[101]。Zaman等[102]研究显示,在内源性抗凋亡蛋白humanin(HN)高表达小鼠、HN处理的野生型小鼠和HN处理的培养小鼠跖骨中,糖皮质激素(glucocorticoid, GC)诱导的骨生长损伤和软骨细胞凋亡被阻止,HN可通过靶向Hh途径使GC诱导的骨生长障碍受到抑制并恢复,而不会干扰GC所发挥的抗炎作用。可见,Hedgehog信号通路在调节成骨细胞分化和骨骼发育中起重要作用。
1.6.3 PI3K/Akt信号通路
PI3K/Akt信号通路是一种重要的有丝分裂信号通路,在生长、存活、增殖和活性等多种细胞过程中发挥着重要作用[103]。在骨骼生长发育中,PI3K信号及其下游靶点在骨形成和骨重塑中起着重要调控功能[104]。Ke等[105]研究表明,川续断皂苷VI (asperosaponin VI, ASA VI)促进了去卵巢小鼠BMSC的增殖,增强了ALP活性,并促进了钙结节的生成;此外,ASA VI增强了ALP、BGP和Runx2的表达,而经LY294002 (阻断PI3K/Akt信号通路的蛋白激酶抑制剂)处理则降低了以上成骨作用,并降低了ASA VI诱导的p-Akt (磷酸化Akt)水平。这些结果表明,ASA VI通过作用于PI3K/Akt信号通路来促进Ovx的BMSC的成骨分化。可见,在动物骨骼生长发育中激活PI3K/Akt信号通路可促进BMSC的成骨分化。
1.6.4 钙离子信号通路
钙离子(Ca2+)在骨骼中起着重要的结构作用,且Ca2+信号在成骨细胞分化过程中起着重要作用。在骨重塑过程中,Ca2+作为矿物相的成分,以游离离子的形式不断释放到细胞外环境中[106]。因此,Ca2+持续可作用于成骨细胞和骨祖细胞发挥生物学功能。Ca2+进入钙通道介导的细胞,Runx2起中介作用,激活磷脂酶C(PLC)和肌醇-1,4,5-三磷酸肌醇(IP3)信号[107],进而促进细胞内存储Ca2+的释放,从而促进骨骼的生长发育。细胞外钙敏感受体(Calcium- Sensing Receptor, CaR)是一种G蛋白偶联受体,在调节细胞外钙稳态中起重要作用,Liu等[108]研究表明,高钙血症的减少在预防CaR缺陷(CaR-/-)小鼠的早期致死性中起关键作用,且CaR-/-小鼠软骨内骨形成缺陷是由于血清钙浓度显著升高、血清磷浓度和骨骼甲状旁腺激素相关蛋白水平降低所致。Chang 等[109]研究表明,骨骼中CaR的缺失会导致严重的骨骼缺陷,而软骨细胞(软骨生成细胞)中CaR的缺失导致胚胎第13天(E13)前死亡,但在E16~E18之间诱导小鼠软骨细胞特异性缺失CaR是可行的,但显示小鼠生长板发育延迟;这些结果显示CaR在早期胚胎发生和骨骼发育中起着关键作用。综上所述,钙离子受体敲除小鼠实验证明了钙离子信号对骨发育非常重要。
1.6.5 Hippo信号通路
Hippo信号是调节器官大小和组织再生的主要因素,近年来的一些研究表明Hippo信号通路在调节骨形成方面发挥作用。Wang等[110]通过体外实验研究表明,小鼠BMSCs包括迁移和成骨的生物学功能与Hippo途径的关键下游效应物YAP(Yes相关蛋白)的表达增强有关。Chen等[111]发现,在成骨培养条件下,BMSCs中Hippo信号的激活抑制了成骨分化。Hippo信号通路对于骨骼发育的具体调控机制还不明确,还需开展更多的相关研究。
2 结语与展望
动物骨骼生长发育及骨代谢受BMP/Smads、TGF-β、Wnt/β-catenin、OPG/RANKL/RANK和FGF等多条关键信号通路调控,这些信号通路通过直接或间接作用于Runx2或β-catenin等关键转录因子而彼此相互联系、相互影响,从而组成了复杂的调控网络(骨代谢调控的关键信号通路图见图1)。该网络协同参与了骨代谢过程的调控,维持骨稳态。尽管目前对骨代谢相关信号通路的研究较多,也取得了一些重要的研究成果,但对于调控动物骨代谢的分子机制仍未阐明清晰。随着生命科学的不断发展,在不远的将来能够解析骨代谢调控的完整分子机制,只有理解了这些信号通路的分子作用机制,并阐明这些信号通路传导途径之间的相互作用,才能为动物病理性骨骼的治疗奠定基础。图1
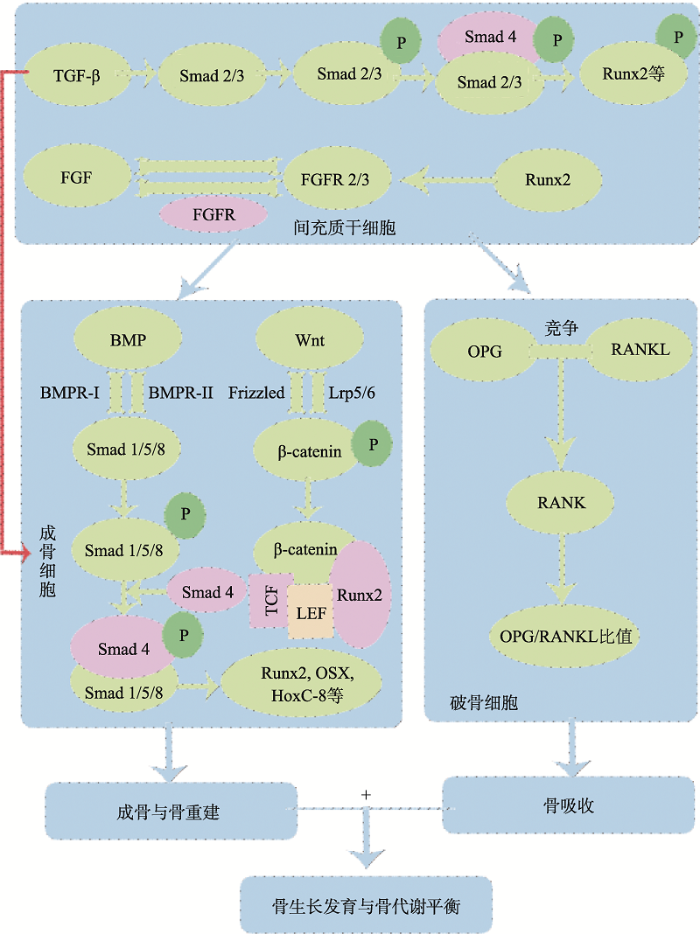 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1骨代谢调控的关键信号通路图
TGF-β信号通路通过Smad2/3路径调节BMSCs的增殖、分化及其成骨细胞分化;FGF信号通路中FGF和FGFR可调节BMSCs的增殖和成骨分化;BMP/Smads信号通路通过激活Smads1/5/8,结合Smad4,再与Runx2和OSX等相互作用,调节成骨细胞分化与骨重建;Wnt/β-catenin信号通路通过Wnt蛋白与Frizzled 和Lrp5/6结合,激活β-catenin并将其转移到细胞核与TCF/LEF等相互作用,激活Wnt靶基因的转录,从而调控成骨细胞的增殖、分化及骨形成;OPG/RANKL/RANK信号通路中OPG和RANKL竞争性结合,阻止RANKL和RANK之间的结合,通过调节OPG/RANKL比值来调控骨吸收过程。该5条关键信号通路共同参与调节动物骨骼的生长发育与骨代谢平衡。
Fig. 1Key signaling pathways for bone metabolism regulation
此外,本文介绍了中草药及其提取物可通过这些信号通路调节骨形成或骨吸收过程,从而维持骨代谢的平衡。目前已有较多研究表明,淫羊藿(Epimedii Folium)、补骨脂(Psoralea corylifolia Linn.)、当归(Angelicasinensis(Oliv.) Diels)、山药(Dioscorea opposita Thunb.)、杜仲(Eucommia ulmoides)等单体及其提取物,或是由多味单剂组成的方剂如更年春、密骨胶囊等均对动物骨骼的生长发育起作用,说明中草药在防治动物病理性骨骼方面具有广阔的前景。但动物骨骼疾病的分子机制复杂,中草药对于骨代谢相关信号通路的调控研究还处于初始阶段,还需进一步深入研究。将中草药应用与动物骨骼发育和骨代谢的分子机制研究相结合,不仅可以深入揭示中草药防治骨骼疾病的机制,还能为动物骨骼疾病的防治提供依据,使中草药的应用前景更广阔。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1002/jbm4.10241URLPMID:31844829 [本文引用: 1]

Our understanding of the genetic control of bone strength has relied mainly on estimates of bone mineral density. Here we have mapped genetic factors that influence femoral and tibial microarchitecture using high-resolution x-ray computed tomography (8-mum isotropic voxels) across a family of 61 BXD strains of mice, roughly 10 isogenic cases per strain and balanced by sex. We computed heritabilities for 25 cortical and trabecular traits. Males and females have well-matched heritabilities, ranging from 0.25 to 0.75. We mapped 16 genetic loci most of which were detected only in females. There is also a bias in favor of loci that control cortical rather than trabecular bone. To evaluate candidate genes, we combined well-established gene ontologies with bone transcriptome data to compute bone-enrichment scores for all protein-coding genes. We aligned candidates with those of human genome-wide association studies. A subset of 50 strong candidates fell into three categories: (1) experimentally validated genes already known to modulate bone function (Adamts4, Ddr2, Darc, Adam12, Fkbp10, E2f6, Adam17, Grem2, Ifi204); (2) candidates without any experimentally validated function in bone (eg, Greb1, Ifi202b), but linked to skeletal phenotypes in human cohorts; and (3) candidates that have high bone-enrichment scores, but for which there is not yet any functional link to bone biology or skeletal system disease (including Ifi202b, Ly9, Ifi205, Mgmt, F2rl1, Iqgap2). Our results highlight contrasting genetic architecture between sexes and among major bone compartments. The alignment of murine and human data facilitates function analysis and should prove of value for preclinical testing of molecular control of bone structure. (c) 2019 The Authors. JBMR Plus published by Wiley Periodicals, Inc. on behalf of American Society for Bone and Mineral Research.
DOI:10.1002/ajpa.22183URL [本文引用: 1]

Growth, development, and decline of the human skeleton are of central importance to physical anthropology. All processes of skeletal growth (longitudinal growth as well as gains and losses of bone mass) are subjected to environmental and genetic influences. These influences, and their relative contributions to the phenotype, can be asserted at any stage of life. We present here the gross phenotypic and genetic landscapes of four skeletal traits, and show how they vary across the life span. Phenotypic sex differences are found in bone diameter and cortical index (a ratio of cortical thickness over bone diameter) at a very early age and continue throughout most of life. Sexual dimorphism in summed cortical thickness and bone length, however, is not evident until shortly after the pubertal growth spurt. Genetic contributions (heritability) to these skeletal phenotypes are generally moderate to high. Bone length and bone diameter (which both scale with body size) tend to have the highest heritability, with heritability of bone length fairly stable across ages (with a notable dip in early childhood) and that of bone diameter peaking in early childhood. The bone traits summed cortical thickness and cortical index that may better reflect bone mass, a more plastic phenomenon, have slightly lower genetic influences, on average. Results from our phenotypic and genetic landscapes serve three key purposes: 1) demonstration of the integrated nature of the genetic and environmental underpinnings of skeletal form, 2) identification of periods of bone's relative sensitivity to genetic and environmental influences, 3) and stimulation of hypotheses predicting the effects of exposure to environmental variables on the skeleton, given variation in the underlying genetic architecture. Am J Phys Anthropol, 2013. © 2012 Wiley Periodicals, Inc.
DOI:10.1016/j.bone.2015.04.035URLPMID:26453494 [本文引用: 1]

The development of the vertebrate skeleton reflects its evolutionary history. Cartilage formation came before biomineralization and a head skeleton evolved before the formation of axial and appendicular skeletal structures. This review describes the processes that result in endochondral and intramembranous ossification, the important roles of growth and transcription factors, and the consequences of mutations in some of the genes involved. Following a summary of the origin of cartilage, muscle, and tendon cell lineages in the axial skeleton, we discuss the role of muscle forces in the formation of skeletal architecture and assembly of musculoskeletal functional units. Finally, ontogenetic patterning of bones in response to mechanical loading is reviewed.This article is part of a Special Issue entitled
DOI:10.1002/wdev.159URLPMID:25270716 [本文引用: 1]

The balance between bone formation and bone resorption controls postnatal bone homeostasis. Research over the last decade has provided a vast amount of evidence that WNT signaling plays a pivotal role in regulating this balance. Therefore, understanding how the WNT signaling pathway regulates skeletal development and homeostasis is of great value for human skeletal health and disease.
[本文引用: 2]
DOI:10.1007/s00223-015-9966-8URLPMID:25694359 [本文引用: 2]

There is no clear evidence to show the direct causal relationship between passive cigarette smoking and osteoporosis. Furthermore, the underlying mechanism is unknown. The objective of this study is to demonstrate the effects of long-term passive cigarette smoking on bone metabolism and microstructure by a mouse model and cell culture systems. BALB/c mice were exposed to 2 or 4 % cigarette smoke for 14 weeks. The bone turnover biochemical markers in urine and serum and also the bone micro-architecture by micro-CT were compared with the control group exposed to normal ambient air. In the cell culture experiments, mouse MC3T3-E1 and RAW264.7 cell lines to be employed as osteoblast and osteoclast, respectively, were treated with the sera obtained from 4 % smoking or control mice. Their actions on cell viability, differentiation, and function on these bone cells were assessed. The urinary mineral and deoxypyridinoline (DPD) levels, and also the serum alkaline phosphatase activity, were significantly higher in the 4 % smoking group when compared with the control group, indicating an elevated bone metabolism after cigarette smoking. In addition, femoral osteopenic condition was observed in the 4 % smoking group, as shown by the decrease of relative bone volume and trabecular thickness. In isolated cell studies, osteoblast differentiation and bone formation were inhibited while osteoclast differentiation was increased. The current mouse smoking model and the isolated cell studies demonstrate that passive cigarette smoke could induce osteopenia by exerting a direct detrimental effect on bone cells differentiation and further on bone remodeling process.
DOI:10.1007/s00264-014-2512-xURL [本文引用: 1]

Purpose Since the original extraction of bone morphogenetic proteins (BMPs) from bovine bone, research interest and clinical use has increased exponentially. With this, a concomitant analysis of BMP expression in bone tumours has been performed. BMP ligands, receptors, and signaling activity have been observed in diverse benign and malignant bone tumours. However, the reported expression, function, and importance of BMPs in bone tumours, and specifically osteosarcomas, have been far from uniform. This review highlights recent advances in understanding the role of BMP signaling in osteosarcoma biology, focusing on the sometimes divergent findings by various researchers and the challenges inherent in the study of osteosarcoma.
Methods We performed a literature review of all studies examining BMP signaling in osteosarcoma.
Results Overall, multiple BMP ligands and receptors are expressed in most osteosarcoma cell lines and subtypes, although BMP signaling may be reduced in comparison with benign bone-forming tumours. Studies suggest that osteosarcomas with different lineages of differentiation may have differential expression of BMP ligands. Although significant disagreement in the literature exists, the presence of BMP signaling in osteosarcoma may impart a worse prognosis. On the cellular level, BMP signaling appears to mediate promigratory effects in osteosarcoma and chondrosarcoma cell types, possibly via interaction and activation of Integrin beta 1.
Conclusions BMP signaling has clear biologic importance in osteosarcoma, although it is not yet fully understood. Future questions for study include assessing the utility of BMP signaling in prognostication of osteosarcoma and the potential modulation of BMP signaling for inhibition of osteosarcomagenesis, growth and invasion.
DOI:10.1177/0022034513518561URL [本文引用: 2]

Bone morphogenetic proteins (BMPs) are members of the TGF-beta superfamily, acting as potent regulators during embryogenesis and bone and cartilage formation and repair. Cell and molecular biology approaches have unveiled the great complexity of BMP action, later confirmed by transgenic animal studies. Genetic engineering allows for the production of large amounts of BMPs for clinical use, but they have systematically been associated with a delivery system, such as type I collagen and calcium phosphate ceramics, to ensure controlled release and to maximize their biological activity at the surgical site, avoiding systemic diffusion. Clinical orthopedic studies have shown the benefits of FDA-approved recombinant human BMPs (rhBMPs) 2 and 7, but side effects, such as swelling, seroma, and increased cancer risk, have been reported, probably due to high BMP dosage. Several studies have supported the use of BMPs in periodontal regeneration, sinus lift bone-grafting, and non-unions in oral surgery. However, the clinical use of BMPs is growing mainly in off-label applications, with robust evidence to ascertain rhBMPs' safety and efficacy through well-designed, randomized, and double-blind clinical trials. Here we review and discuss the critical data on BMP structure, mechanisms of action, and possible clinical applications.
URLPMID:22846268 [本文引用: 1]

Mesenchymal stem cells (MSCs) have the capabilities to proliferate and differentiate into osteoblastic and chondrogenic lineages when stimulated with bone morphogenic proteins (BMPs). BMPs play a vital role in skeletal development. BMPs bind to their cell surface receptors on MSCs and send signals to the cell nucleus which results to the synthesis of macromolecules involved in cartilage and bone formation and then the mesenchymal cell differentiates into chondrocytes or osteoblasts. The objective of this study was to evaluate the effects of BMP-2, BMP-7, and BMP-13 on undifferentiated MSCs for cellular damage markers, alkaline phosphatase, and morphological changes at 24, 72, 120, and 168 hours of culture. MSC viability, proliferation, cellular damage, and cellular morphology were evaluated after each time point. As early as 48 hours MSCs treated with BMPs resulted in morphological changes, and increased cell numbers. At 120 and 168 hours BMP-13 resulted in morphological changes resembling chrondrocytes. It was concluded that BMPs have no adverse effects on proliferation and are non- toxic to undiffetentiated MSCs.
DOI:10.1016/j.gene.2012.09.130URL [本文引用: 1]

The specific role of endogenous Bmp2 gene in chondrocytes and in osteoblasts in fracture healing was investigated by generation and analysis of chondrocyte- and osteoblast-specific Bmp2 conditional knockout (cKO) mice. The unilateral open transverse tibial fractures were created in these Bmp2 cKO mice. Bone fracture callus samples were collected and analyzed by X-ray, micro-CT, histology analyses, biomechanical testing and gene expression assays. The results demonstrated that the lack of Bmp2 expression in chondrocytes leads to a prolonged cartilage callus formation and a delayed osteogenesis initiation and progression into mineralization phase with lower biomechanical properties. In contrast, when the Bmp2 gene was deleted in osteoblasts, the mice showed no significant difference in the fracture healing process compared to control mice. These findings suggest that endogenous BMP2 expression in chondrocytes may play an essential role in cartilage callus maturation at an early stage of fracture healing. Our studies may provide important information for clinical application of BMP2. (c) 2012 Elsevier B.V.
DOI:10.1002/jcb.23287URL [本文引用: 1]

Bone marrow-derived mesenchymal stem cells (MSCs) are multipotent progenitors that can commit to osteoblast, chondrocyte, adipocyte, and several other lineages. The proper utilization of stem cells for clinical applications requires an integrated understanding of multiple signal inputs that control maintenance of stemness, proliferation, commitment, and differentiation. Various signaling pathways have been implicated in the regulation of MSC differentiation; however, complexities of pathway interactions, as well as seemingly contradictory results in the literature, create an often confusing and disjointed knowledge base. Several recent publications explore the integration of signaling pathways such as BMP, Wnt, Notch, Hedgehog, and Fibroblast Growth Factors in MSC osteoblast differentiation. The transcription factor Cbfa1/Runx2 has been implicated in these pathways as a potential focal point for signaling integration. This review will outline the current understanding of these pathways and indicate where both spatiotemporal effects during differentiation and comparable experimental conditions need to be considered in order to clarify the outcome(s) of differing regulatory levels of these signaling pathways. J. Cell. Biochem. 112: 3491-3501, 2011. (C) 2011 Wiley Periodicals, Inc.
DOI:10.1093/jb/mvp148URLPMID:19762341 [本文引用: 1]

Bone morphogenetic proteins (BMPs) exhibit broad spectra of biological activities in various tissues, including bone, cartilage, blood vessels, heart, kidney, neurons, liver and lung. BMPs are members of the transforming growth factor-beta (TGF-beta) family that bind to type II and type I serine-threonine kinase receptors, and transduce signals through Smad and non-Smad signalling pathways. Recent findings have revealed that BMP signalling is finely tuned by various mechanisms in both positive and negative fashions. Perturbations of BMP signalling pathways are linked to a wide variety of clinical disorders, including vascular diseases, skeletal diseases and cancer. Administration of recombinant BMP ligands and increasing endogenous expression of BMPs provide therapeutic effects on some diseases. The recent development of BMP receptor inhibitors may also prove useful for some clinical diseases induced by hyperactivation of the BMP signalling pathways.
DOI:10.1016/j.bbrc.2010.12.099URLPMID:21187071 [本文引用: 1]

This study examined the role of AMPK activation in osteoblast differentiation and the underlining mechanism. An AMPK activator (AICAR or metformin) stimulated osteoblast differentiation with increases in ALP and OC protein production as well as the induction of AMPK phosphorylation in MC3T3E1 cells. In addition, metformin induced the phosphorylation of Smad1/5/8 and expression of Dlx5 and Runx2, whereas compound C or dominant negative AMPK inhibited these effects. Transient transfection studies also showed that metformin increased the BRE-Luc and Runx2-Luc activities, which were inhibited by DN-AMPK or compound C. Down-regulation of Dlx5 expression by siRNA suppressed metformin-induced Runx2 expression. These results suggest that the activation of AMPK stimulates osteoblast differentiation via the regulation of Smad1/5/8-Dlx5-Runx2 signaling pathway.
DOI:10.1146/annurev-biochem-052110-115718URLPMID:22463691 [本文引用: 1]

Excessive caloric intake without a rise in energy expenditure promotes adipocyte hyperplasia and adiposity. The rise in adipocyte number is triggered by signaling factors that induce conversion of mesenchymal stem cells (MSCs) to preadipocytes that differentiate into adipocytes. MSCs, which are recruited from the vascular stroma of adipose tissue, provide an unlimited supply of adipocyte precursors. Members of the BMP and Wnt families are key mediators of stem cell commitment to produce preadipocytes. Following commitment, exposure of growth-arrested preadipocytes to differentiation inducers [insulin-like growth factor 1 (IGF1), glucocorticoid, and cyclic AMP (cAMP)] triggers DNA replication and reentry into the cell cycle (mitotic clonal expansion). Mitotic clonal expansion involves a transcription factor cascade, followed by the expression of adipocyte genes. Critical to these events are phosphorylations of the transcription factor CCATT enhancer-binding protein beta (C/EBPbeta) by MAP kinase and GSK3beta to produce a conformational change that gives rise to DNA-binding activity.
DOI:10.3892/ijmm.2012.1079URL [本文引用: 1]

Icariin, the main active compound of the traditional Chinese medicine, Epimedium, is commonly used for the clinical treatment of osteoporosis. However, the precise molecular mechanism of the therapeutic effect of icariin has not been elucidated. The aim of this study was to examine the effect of icariin on cell viability, alkaline phosphatase (ALP) activity, the amount of calcified nodules, and to delineate the molecular mechanism of icariin-enhanced bone formation by investigating the expression of bone morphogenic protein-2 (BMP-2), Smad4, Cbfa1/Runx2, osteoprotegerin (OPG), receptor activator of nuclear factor kappa-B ligand (RANKL) and the OPG/RANKL ratio in the hFOB 1.19 human osteoblastic cell line. We found that icariin significantly increased the cell viability, the activity of ALP and the amount of calcified nodules in the hFOB 1.19 cells. Furthermore, we observed that icariin upregulated the expression of BMP-2, Smad4, Cbfa1/Runx2, OPG, RANKL and the OPG/RANKL ratio. Our results indicate that icariin can modulate the process of bone formation via the BMP-2/Smad4 signal transduction pathway in hFOB 1.19 cells.
DOI:10.1111/febs.12887URL [本文引用: 1]

Runx2 plays essential roles in bone formation and chondrocyte maturation. Akt promotes osteoblast differentiation induced by the bone morphogenetic proteins BMP2 and enhances the function and transcriptional activity of Runx2. However, the precise molecular mechanism underlying the relationship between Runx2 and Akt is not well understood. In this study, we examined the role of Akt in regulating Runx2 function. We found that Akt increases the stability of Runx2 protein. However, the level of Runx2 mRNA was not affected by Akt, and we did not find any evidence for direct modification of Runx2 by Akt. Instead, we found evidence that Akt induces the phosphorylation of the Smad ubiquitination regulatory factor Smurf2 and decreases the level of Smurf2 protein through ubiquitin/proteasome-mediated degradation of Smurf2. Akt also alleviates Smurf2-mediated suppression of Runx2 transcriptional activity. Taken together, our results suggest that Akt regulates osteoblast differentiation, at least in part, by enhancing the protein stability and transcriptional activity of Runx2 through regulation of ubiquitin/proteasome-mediated degradation of Smurf2.
DOI:10.1242/dev.116228URLPMID:25670797 [本文引用: 1]

The periodontal ligament (PDL) is a mechanosensitive noncalcified fibrous tissue connecting the cementum of the tooth and the alveolar bone. Here, we report that scleraxis (Scx) and osterix (Osx) antagonistically regulate tensile force-responsive PDL fibrogenesis and osteogenesis. In the developing PDL, Scx was induced during tooth eruption and co-expressed with Osx. Scx was highly expressed in elongated fibroblastic cells aligned along collagen fibers, whereas Osx was highly expressed in the perialveolar/apical osteogenic cells. In an experimental model of tooth movement, Scx and Osx expression was significantly upregulated in parallel with the activation of bone morphogenetic protein (BMP) signaling on the tension side, in which bone formation compensates for the widened PDL space away from the bone under tensile force by tooth movement. Scx was strongly expressed in Scx(+)/Osx(+) and Scx(+)/Osx(-) fibroblastic cells of the PDL that does not calcify; however, Scx(-)/Osx(+) osteogenic cells were dominant in the perialveolar osteogenic region. Upon BMP6-driven osteoinduction, osteocalcin, a marker for bone formation was downregulated and upregulated by Scx overexpression and knockdown of endogenous Scx in PDL cells, respectively. In addition, mineralization by osteoinduction was significantly inhibited by Scx overexpression in PDL cells without affecting Osx upregulation, suggesting that Scx counteracts the osteogenic activity regulated by Osx in the PDL. Thus, Scx(+)/Osx(-), Scx(+)/Osx(+) and Scx(-)/Osx(+) cell populations participate in the regulation of tensile force-induced remodeling of periodontal tissues in a position-specific manner.
DOI:10.1371/journal.pone.0008978URLPMID:20126390 [本文引用: 1]

Hox genes encode transcription factors, which regulate skeletal patterning and chondrocyte differentiation during the development of cartilage, the precursor to mature bone. Overexpression of the homeobox transcription factors Hoxc8 and Hoxd4 causes severe cartilage defects due to delay in cartilage maturation. Matrix metalloproteinases (MMPs), bone morphogenetic proteins (BMPs) and fibroblastic growth factors (FGFs) are known to play important roles in skeletal development and endochondral bone formation and remodeling. In order to investigate whether these molecules are aberrantly expressed in Hoxc8- and/or Hoxd4-transgenic cartilage, we performed quantitative RT-PCR on chondrocytes from Hox-transgenic mice. Gene expression levels of Bmp4, Fgf8, Fgf10, Mmp9, Mmp13, Nos3, Timp3, Wnt3a and Wnt5a were altered in Hoxc8-transgenic chondrocytes, and Fgfr3, Ihh, Mmp8, and Wnt3a expression levels were altered in Hoxd4-transgenic chondrocytes, respectively. Notably, Wnt3a expression was elevated in Hoxc8- and reduced in Hoxd4-transgenic cartilage. These results suggest that both transcription factors affect cartilage maturation through different molecular mechanisms, and provide the basis for future studies into the role of these genes and possible interactions in pathogenesis of cartilage defects in Hoxc8- and Hoxd4-transgenic mice.
DOI:10.1007/s11999-010-1321-9URLPMID:20306162 [本文引用: 2]

BACKGROUND: The capacity for bone healing reportedly is limited in osteoporosis with a less than ideal environment for healing of bone grafts. We therefore developed a composite bone substitute with rhBMP-2 loaded gelatin microsphere (GM) and calcium phosphate cement (CPC) to use in osteoporosis. QUESTIONS/PURPOSES: We asked whether (1) controlled release of rhBMP-2 could be improved in this composite bone substitute and (2) increasing factors released from the bone substitute could accelerate osteoporotic bone healing. METHODS: We soaked rhBMP-2/GM/CPC and rhBMP-2/CPC composites in simulated body fluid for 28 days and then determined the amount of rhBMP-2 released. Both composites were implanted in bone defects of osteoporotic goats and left in place for 45 and 140 days; the specimens then were evaluated mechanically (pushout test) and morphologically (CT scanning, histology). RESULTS: The in vitro study showed the new composite released more rhBMP-2 compared with rhBMP-2/CPC. CT showed the defects healed more quickly with new grafts. The bone mineralization rate was greater in rhBMP-2/GM/CPC than in rhBMP-2/CPC after 45 days of implantation and the pushout test was stronger after 45 and 140 days of implantation. CONCLUSIONS: The new graft composite released more loaded factors and appeared to repair osteoporotic bone defects. CLINICAL RELEVANCE: These preliminary data suggest the new composite can be used as a bone substitute to accelerate healing of fractures and bone defects in osteoporosis.
DOI:10.1007/s00167-011-1790-8URLPMID:22113222 [本文引用: 1]

PURPOSE: A male patient suffering from non-union of the femoral diaphysis after a traumatic fracture was treated with deep decortication and grafted with lyophilized bone, platelet gel (PG) and autologous bone marrow stromal cells (BMSCs). After 40 days from surgery, he was re-operated, due to fracture secondary displacement, caused by inappropriate load during sports activity. In addition to radiographs, two bone biopsies were retrieved: this allowed for a histological evaluation of the early response of host bone to the graft. To our knowledge, there is no report describing such early tissue response. METHODS: A clinical-radiographic evaluation of the patient and a histomorphometric analysis of the bone biopsies were performed. RESULTS: An early reparative bone formation was observed adjacent to the osteointegrated graft. Non-resorbed bone chips and large islands of non-vital bone particles, surrounded by fibrous tissue, were observed in a zone of sclerotic diaphyseal bone, that is the process was delayed despite decortication. CONCLUSIONS: These findings support the concept, until now evidenced only by imaging, that bone chips added with PG and BMSCs are effective in shortening the healing time in fracture non-union. The clinical relevance of deep decortication and vascularization is emphasized. LEVEL OF EVIDENCE: Therapeutic studies-investigating the results of treatment, Level V.
DOI:10.1242/jcs.118596URL [本文引用: 1]
DOI:10.1091/mbc.E14-06-1136URLPMID:25568340 [本文引用: 1]

Gja1(Jrt)/+ mice carry a mutation in one allele of the gap junction protein alpha1 gene (Gja1), resulting in a G60S connexin 43 (Cx43) mutant protein that is dominant negative for Cx43 protein production of <50% of wild-type (WT) levels and significantly reduced gap junction formation and function in osteoblasts and other Cx43-expressing cells. Previously we reported that Gja1(Jrt)/+ mice exhibited early-onset osteopenia caused by activation of osteoclasts secondary to activation of osteoblast lineage cells, which expressed increased RANKL and produced an abnormal resorption-stimulating bone matrix high in BSP content. Gja1(Jrt)/+ mice also displayed early and progressive bone marrow atrophy, with a significant increase in bone marrow adiposity versus WT littermates but no increase in adipose tissues elsewhere in the body. BMP2/4 production and signaling were increased in Gja1(Jrt)/+ trabecular bone and osteogenic stromal cell cultures, which contributed to the up-regulated expression of osteoblast-specific markers (e.g., Bsp and Ocn) in Gja1(Jrt)/+ osteoblasts and increased Pparg2 expression in bone marrow-derived adipoprogenitors in vitro. The elevated levels of BMP2/4 signaling in G60S Cx43-containing cells resulted at least in part from elevated levels of cAMP. We conclude that up-regulation of BMP2/4 signaling in trabecular bone and/or stromal cells increases osteoblast-specific marker expression in hyperactive Gja1(Jrt)/+ osteoblasts and may also increase bone marrow adipogenesis by up-regulation of Pparg2 in the Cx43-deficient Gja1(Jrt)/+ mouse model.
DOI:10.1002/jor.22387URLPMID:23737220 [本文引用: 1]

The failure of orthopedic implants in osteoporotic patients is attributed to the lack of sufficient bone stock and regenerative capacity but most treatments for osteoporosis fail to address this issue. rhBMP-2 is known to promote bone formation under normal conditions but has not been used clinically in the osteoporotic condition. Osteoporosis was induced in 19 ewes using ovariectomy, low calcium diet, and steroid injection. After induction, the steroid was withdrawn and pellets containing inert carrier with rhBMP-2 in either slow or fast-release formulation were implanted into the lumbar vertebrae of each animal. After 2, 3, and 6 months the spines were harvested and assessed for changes in BMD and histomorphometric indices. BMD did not change after cessation of steroid treatment. After 2 months BV/TV increased in the vicinity of the pellets containing the fast-release rhBMP-2 and was sustained for the duration of the study. Focal voids surrounding all implants, particularly the slow-release formulation, were observed initially but resolved with time. Increased BV/TV adjacent to rhBMP-2 pellets suggests it could be used for localized treatment of osteoporosis. Refinement of the delivery system and supplementary treatments may be necessary to overcome the initial catabolic effects of rhBMP-2.
DOI:10.1016/j.biomaterials.2013.09.011URL [本文引用: 1]

The transplantation of autologous bone graft as a treatment for large bone defects has the limitation of harvesting co-morbidity and limited availability. This drives the orthopaedic research community to develop bone graft substitutes. Routinely, supra-physiological doses of bone morphogenetic proteins (BMPs) are applied perpetuating concerns over undesired side effects and cost of BMPs. We therefore aimed to design a composite scaffold that allows maintenance of protein bioactivity and enhances growth factor retention at the implantation site. Critical-sized defects in sheep tibiae were treated with the autograft and with two dosages of rhBMP-7, 3.5 mg and 1.75 mg, embedded in a slowly degradable medical grade poly(epsilon-caprolactone) (PCL) scaffold with beta-tricalcium phosphate microparticles (mPCL-TCP). Specimens were characterised by biomechanical testing, microcomputed tomography and histology. Bridging was observed within 3 months for the autograft and both rhBMP-7 treatments. No significant difference was observed between the low and high rhBMP-7 dosages or between any of the rhBMP-7 groups and autograft implantation. Scaffolds alone did not induce comparable levels of bone formation compared to the autograft and rhBMP-7 groups. In summary, the mPCL-TCP scaffold with the lower rhBMP-7 dose led to equivalent results to autograft transplantation or the high BMP dosage. Our data suggest a promising clinical future for BMP application in scaffold-based bone tissue engineering, lowering and optimising the amount of required BMP. Crown Copyright (C) 2013 Published by Elsevier Ltd.
DOI:10.1038/gt.2012.45URLPMID:22717741 [本文引用: 1]

Bone regeneration achieved using mesenchymal stem cells (MSCs) and nonviral gene therapy holds great promise for patients with fractures seemingly unable to heal. Previously, MSCs overexpressing bone morphogenetic proteins (BMPs) were shown to differentiate into the osteogenic lineage and induce bone formation. In the present study, we evaluated the potential of osteogenic differentiation in porcine adipose tissue- and bone marrow-derived MSCs (ASCs and BMSCs, respectively) in vitro and in vivo when induced by nucleofection with rhBMP-2 or rhBMP-6. Our assessment of the in vivo efficiency of this procedure was made using quantitative micro-computed tomography (micro-CT). Nucleofection efficiency and cell viability were similar in both cell types; however, the micro-CT analyses demonstrated that in both ASCs and BMSCs, nucleofection with rhBMP-6 generated bone tissue faster and of higher volumes than nucleofection with rhBMP-2. RhBMP-6 induced more efficient osteogenic differentiation in vitro in BMSCs, and in fact, greater osteogenic potential was identified in BMSCs both in vitro and in vivo than in ASCs. On the basis of our findings, we conclude that BMSCs nucleofected with rhBMP-6 are superior at inducing bone formation in vivo than all other groups studied.
DOI:10.7150/ijbs.2929URLPMID:22298955 [本文引用: 1]

Transforming growth factor-beta (TGF-beta)/bone morphogenic protein (BMP) signaling is involved in a vast majority of cellular processes and is fundamentally important throughout life. TGF-beta/BMPs have widely recognized roles in bone formation during mammalian development and exhibit versatile regulatory functions in the body. Signaling transduction by TGF-beta/BMPs is specifically through both canonical Smad-dependent pathways (TGF-beta/BMP ligands, receptors and Smads) and non-canonical Smad-independent signaling pathway (e.g. p38 mitogen-activated protein kinase pathway, MAPK). Following TGF-beta/BMP induction, both the Smad and p38 MAPK pathways converge at the Runx2 gene to control mesenchymal precursor cell differentiation. The coordinated activity of Runx2 and TGF-beta/BMP-activated Smads is critical for formation of the skeleton. Recent advances in molecular and genetic studies using gene targeting in mice enable a better understanding of TGF-beta/BMP signaling in bone and in the signaling networks underlying osteoblast differentiation and bone formation. This review summarizes the recent advances in our understanding of TGF-beta/BMP signaling in bone from studies of genetic mouse models and human diseases caused by the disruption of TGF-beta/BMP signaling. This review also highlights the different modes of cross-talk between TGF-beta/BMP signaling and the signaling pathways of MAPK, Wnt, Hedgehog, Notch, and FGF in osteoblast differentiation and bone formation.
DOI:10.1002/jcb.22586URLPMID:20512916 [本文引用: 1]

The homologous to the E6-associated protein carboxyl terminus (HECT) domain E3 ubiquitin ligase Smurf1 is the first E3 ligase to be implicated in regulating bone cell function. The involvement of Smurf1 in multiple signaling pathways and pathological conditions is presently an area of extensive scientific interest. This review highlights recent works exploring Smurf-regulated biological processes in bone cells and highlights recent discoveries surrounding the regulatory mechanisms modulating its catalytic activity and substrate recognition capability. Moreover, we discuss the relevance of targeting the HECT E3s through the development of small-molecule inhibitors as an anticancer therapeutic strategy.
DOI:10.1172/JCI69493URL [本文引用: 1]

Bone remodeling is characterized by the sequential, local tethering of osteoclasts and osteoblasts and is key to the maintenance of bone integrity. While bone matrix-mobilized growth factors, such as TGF-beta, are proposed to regulate remodeling, no in vivo evidence exists that an osteoclast-produced molecule serves as a coupling factor for bone resorption to formation. We found that CTHRC1, a protein secreted by mature bone-resorbing osteoclasts, targets stromal cells to stimulate osteogenesis. Cthrc1 expression was robustly induced when mature osteoclasts were placed on dentin or hydroxyapatite, and also by increasing extracellular calcium. Cthrc1 expression in bone increased in a high-turnover state (such as that induced by RANKL injections in vivo), but decreased in conditions associated with suppressed bone turnover (such as with aging and after alendronate treatment). Targeted deletion of Cthrc1 in mice eliminated Cthrc1 expression in bone, whereas its deficiency in osteoblasts did not exert any significant effect. Osteoclast-specific deletion of Cthrc1 resulted in osteopenia due to reduced bone formation and impaired the coupling process after resorption induced by RANKL injections, impairing bone mass recovery. These data demonstrate that CTHRC1 is an osteoclast-secreted coupling factor that regulates bone remodeling.
DOI:10.3945/jn.109.109041URLPMID:19710159 [本文引用: 1]

Relatively few studies have examined the effects of formula feeding relative to breast-feeding on bone in the neonate. Using peripheral quantitative CT scan and histomorphometric analysis, we demonstrated that neonatal piglets fed with soy-based formula (SF) and cow milk-based formula (MF) for 21 or 35 d had greater bone mineral density and content than breast-fed piglets (BF) (P < 0.05). Osteoblast numbers and bone formation rate at postnatal d 35 were greater in SF compared with other groups (P < 0.05), whereas osteoclast numbers were lower in both MF and SF groups than in the BF group (P < 0.05). Osteoblastogenesis was greater in ex vivo bone marrow cell cultures from SF than in MF or BF piglets (P < 0.05). Bone formation markers in serum were greater, whereas bone resorption markers were lower in the MF- and SF-fed groups than in the BF group (P < 0.05). Bone morphogenic protein (BMP) 2 and alkaline phosphatase mRNAs were upregulated in the MF and SF groups compared with the BF group (P < 0.05), whereas receptor activator of NF-kappaB ligand was downregulated (P < 0.05). Extracellular signal-regulated kinase, p38, Smad1/5/8 phosphorylation, and runt-related transcription factor 2 expression were greater in bone from the MF and SF groups compared with the BF group (P < 0.05). In vitro studies showed that 2.5% serum from SF- or MF-fed piglets was able to stimulate osteoblast differentiation but not in the presence of the BMP blocker noggin. Therefore, formula feeding promoted bone growth compared with BF. SF piglets had the highest bone volume over tissue volume. This suggests that SF-fed piglets may have the best quality bone. The anabolic effects of SF on bone appear to be mediated through enhanced BMP signaling.
URLPMID:19678759 [本文引用: 1]
DOI:10.26355/eurrev_202001_19943URLPMID:31957858 [本文引用: 1]

OBJECTIVE: By establishing osteoporosis (OP) model in rats, the specific regulatory effect of simvastatin on promoting the differentiation of mesenchymal stem cells (MSCs) into osteoblasts through the bone morphogenetic protein 2 (BMP-2)/Smads signaling pathway was investigated. MATERIALS AND METHODS: A total of 45 Sprague-Dawley rats were selected to establish the OP model by performing ovariectomy. The rats were divided into OP model group (OP group, n=15), 10-7 mmol/L simvastatin treatment group (SIM group, n=15), and normal control group (Control group, n=15). After the experimental period, the enzyme-linked immunosorbent assay (ELISA) was applied to observe the serum levels of tumor necrosis factor-alpha (TNF-alpha), interleukin-6 (IL-6), and IL-1. Reverse Transcription-Polymerase Chain Reaction (RT-PCR) was adopted to detect the contents of the differentiation-associated genes [runt-related transcription factor 2 (RUNX2) and Osterix (Osx)]. Later, the bone marrow MSCs (BMSCs) were selected and divided into Control group, 10-7 mol/L simvastatin group (SIM group), and osteoinduction medium group (OM group). Cell morphology in each group was observed. The Cell Counting Kit-8 (CCK-8) was performed to determine the proliferation activity of BMSCs. ELISA was performed to measure the level of alkaline phosphatase (ALP). RT-PCR was conducted to examine the levels of key differentiation-associated gene RUNX2 and those in BMP-2/Smads pathway. Moreover, the Western blotting was adopted to analyze the expressions of RUNX2 and genes in BMP-2/Smads pathway. RESULTS: The serum levels of TNF-alpha, IL-6, and IL-1 in OP group were remarkably higher than those in the Control group, and their levels in the SIM group were close to those in the Control group. The elevated messenger ribonucleic acid (mRNA) levels of the key differentiation-associated factors RUNX2, osteoprotegerin (OPG), osteopontin (OPN), and Osx were observed in the SIM group. In vitro cell culture revealed that the cells were in a favorable growth status in the SIM group and OM group, mostly manifesting in fusiform or spindle shape, and proliferated rapidly. In addition, the ALP level notably increased in the two groups compared with that in the Control group (p<0.05). Both SIM group and OM group had evidently higher mRNA expression levels of RUNX2, OPG, OPN, and Osx than those in the Control group (p<0.05), consistent with the expression trends of the genes in BMP-2/Smads pathway. The Western blotting indicated that the expression levels of RUNX2 and genes in BMP-2/Smads pathway in the SIM group were significantly higher than those in the Control group. CONCLUSIONS: Simvastatin can promote the differentiation of MSCs into osteoblasts in the OP rat model through the BMP-2/Smads signaling pathway.
DOI:10.1016/j.phymed.2019.153063URLPMID:31419728 [本文引用: 1]

BACKGROUND: Traditional herbal formula Gushukang (GSK) has been clinically applied to treat primary osteoporosis, which can stimulate osteoblastogenesis and improve calcium homeostasis. However, it remains unknown the mechanism that GSK against ovariectomized (OVX) induced damage. PURPOSE: The aim of this study was to investigate the effect of GSK on BMP-2/Smsds signaling pathway and osteocyte apoptosis which has been reported to play a central role in bone remodeling. STUDY DESIGN: OVX in rat was established and GSK was administered. RESULTS: BMP-2/Smsds signaling pathway was inhibited and the number of apoptotic osteocytes was increased in OVX rats. Treatment with GSK significantly enhanced BMP-2/Smsds signaling pathway by up-regulating the expression of BMP-2, p-Smad1 and p-Smad5, Osterix and Runx2, and inhibited osteocyte apoptosis by up-regulating Bcl-xl and down-regulating Bak, which were consistent with histological changes revealed by ALP, Trap and TUNEL staining. GSK treatment improved bone mass and micro-structure of trabecular bone at distal femur in OVX rats shown by BMD, micro-CT measurement and HE staining. CONCLUSION: These data suggest that GSK exhibited protective effects on promoting bone formation and precluding osteocyte apoptosis. The underlying mechanism may be attributed to its regulation on BMP-2/Smads signaling pathway and Bcl2 family.
DOI:10.1691/ph.2017.6502URLPMID:29441895 [本文引用: 1]

Daidzein, the most widely studied soy phytoestrogen, is not only a potential antiosteoporosis agent owing to its possible osteogenic activity, but also shows anticancer activity. However, the mechanisms through which daidzein affects osteoblast function have not been well understood. Here, we show that daidzein stimulated cell proliferation and differentiation of osteoblasts, demonstrated by upregulation of XTT activity, enhancement of alkaline phosphatase (ALP) activity, and upregulation of osteoblast-specific marker genes, including Runt-related transcription factor 2 (Runx2) and Smad1, as well as up-regulation of Runx2 and Smad1 protein expression. To determine the mechanisms underlying daidzein's effects on osteoblast differentiation, we first tested the role of daidzein in bone morphogenetic protein (BMP)-2 gene expression in OCT1 cells, and found that it significantly upregulated the expression of BMP-2. Furthermore, it significantly enhanced the phosphorylated protein level of Smad1/5/8and protein expression of Osterix (Osx, a direct target gene of BMP signaling) and increased the activity of BMP signaling reporter (12xSBE-OC-Luc). Finally, we demonstrated that daidzein stimulated Col I, Runx2, and ALP expression, while these effects were significantly blocked by the BMP signaling inhibitor noggin. Thus, our data indicate that daidzein acts through stimulating the activation of BMP-2/Smads pathway to promote osteoblast proliferation and differentiation.
DOI:10.1146/annurev.cellbio.21.022404.142018URLPMID:16212511 [本文引用: 1]

The TGF-beta family comprises many structurally related differentiation factors that act through a heteromeric receptor complex at the cell surface and an intracellular signal transducing Smad complex. The receptor complex consists of two type II and two type I transmembrane serine/threonine kinases. Upon phosphorylation by the receptors, Smad complexes translocate into the nucleus, where they cooperate with sequence-specific transcription factors to regulate gene expression. The vertebrate genome encodes many ligands, fewer type II and type I receptors, and only a few Smads. In contrast to the perceived simplicity of the signal transduction mechanism with few Smads, the cellular responses to TGF-beta ligands are complex and context dependent. This raises the question of how the specificity of the ligand-induced signaling is achieved. We review the molecular basis for the specificity and versatility of signaling by the many ligands through this conceptually simple signal transduction mechanism.
DOI:10.1083/jcb.200608167URLPMID:17242066 [本文引用: 1]

We have discovered that fibrillin-1, which forms extracellular microfibrils, can regulate the bioavailability of transforming growth factor (TGF) beta1, a powerful cytokine that modulates cell survival and phenotype. Altered TGFbeta signaling is a major contributor to the pathology of Marfan syndrome (MFS) and related diseases. In the presence of cell layer extracellular matrix, a fibrillin-1 sequence encoded by exons 44-49 releases endogenous TGFbeta1, thereby stimulating TGFbeta receptor-mediated Smad2 signaling. This altered TGFbeta1 bioavailability does not require intact cells, proteolysis, or the altered expression of TGFbeta1 or its receptors. Mass spectrometry revealed that a fibrillin-1 fragment containing the TGFbeta1-releasing sequence specifically associates with full-length fibrillin-1 in cell layers. Solid-phase and BIAcore binding studies showed that this fragment interacts strongly and specifically with N-terminal fibrillin-1, thereby inhibiting the association of C-terminal latent TGFbeta-binding protein 1 (a component of the large latent complex [LLC]) with N-terminal fibrillin-1. By releasing LLC from microfibrils, the fibrillin-1 sequence encoded by exons 44-49 can contribute to MFS and related diseases.
DOI:10.1007/s00429-002-0273-6URL [本文引用: 1]
DOI:10.1002/jbmr.357URLPMID:21305609 [本文引用: 1]

Previous studies have shown that transforming growth factor beta (TGF-beta) promotes receptor activator of nuclear factor-kappaB ligand (RANKL)-induced osteoclastogenesis. However, the underlying molecular mechanisms have not been elucidated. When TGF-beta signals were blocked either by a specific inhibitor of TGF-beta type 1 receptor kinase activity, SB431542, or by introducing a dominant-negative mutant of TGF-beta type 2 receptor, RANKL-induced osteoclastogenesis was almost completely suppressed. Blockade of Smad signaling by overexpression of Smad7 or c-Ski markedly suppressed RANKL-induced osteoclastogenesis, and retroviral induction of an activated mutant of Smad2 or Smad3 reversed the inhibitory effect of SB431542. Immunoprecipitation analysis revealed that Smad2/3 directly associates with the TRAF6-TAB1-TAK1 molecular complex, which is generated in response to RANKL stimulation and plays an essential role in osteoclast differentiation. TRAF6-TAB1-TAK1 complex formation was not observed when TGF-beta signaling was blocked. Analysis using deletion mutants revealed that the MH2 domain of Smad3 is necessary for TRAF6-TAB1-TAK1 complex formation, downstream signal transduction, and osteoclast formation. In addition, gene silencing of Smad3 in osteoclast precursors markedly suppressed RANKL-induced osteoclast differentiation. In summary, TGF-beta is indispensable in RANKL-induced osteoclastogenesis, and the binding of Smad3 to the TRAF6-TAB1-TAK1 complex is crucial for RANKL-induced osteoclastogenic signaling.
DOI:10.1371/journal.pone.0005275URLPMID:19357790 [本文引用: 1]

During development, growth factors and hormones cooperate to establish the unique sizes, shapes and material properties of individual bones. Among these, TGF-beta has been shown to developmentally regulate bone mass and bone matrix properties. However, the mechanisms that control postnatal skeletal integrity in a dynamic biological and mechanical environment are distinct from those that regulate bone development. In addition, despite advances in understanding the roles of TGF-beta signaling in osteoblasts and osteoclasts, the net effects of altered postnatal TGF-beta signaling on bone remain unclear. To examine the role of TGF-beta in the maintenance of the postnatal skeleton, we evaluated the effects of pharmacological inhibition of the TGF-beta type I receptor (TbetaRI) kinase on bone mass, architecture and material properties. Inhibition of TbetaRI function increased bone mass and multiple aspects of bone quality, including trabecular bone architecture and macro-mechanical behavior of vertebral bone. TbetaRI inhibitors achieved these effects by increasing osteoblast differentiation and bone formation, while reducing osteoclast differentiation and bone resorption. Furthermore, they induced the expression of Runx2 and EphB4, which promote osteoblast differentiation, and ephrinB2, which antagonizes osteoclast differentiation. Through these anabolic and anti-catabolic effects, TbetaRI inhibitors coordinate changes in multiple bone parameters, including bone mass, architecture, matrix mineral concentration and material properties, that collectively increase bone fracture resistance. Therefore, TbetaRI inhibitors may be effective in treating conditions of skeletal fragility.
DOI:10.1002/jcp.20036URLPMID:15137062 [本文引用: 1]

To better understand the complex roles of transforming growth factor-beta (TGF-beta) in bone metabolism, we examined the impact of a range of TGF-beta concentrations on osteoclast differentiation. In co-cultures of support cells and spleen or marrow osteoclast precursors, low TGF-beta concentrations stimulated while high concentrations inhibited differentiation. We investigated the influences of TGF-beta on macrophage colony stimulating factor (M-CSF), receptor activator of NF-kappaB ligand (RANKL), and osteoprotegerin (OPG) expression and found a dose dependent inhibition of M-CSF expression. RANKL expression was elevated at low TGF-beta concentrations with a less dramatic increase in OPG. Addition of OPG blocked differentiation at the stimulatory TGF-beta dose. Thus, low TGF-beta concentrations elevated the RANKL/OPG ratio while high concentrations did not, supporting that, at low TGF-beta concentrations, there is sufficient M-CSF and a high RANKL/OPG ratio to stimulate differentiation. At high TGF-beta concentrations, the RANKL/OPG ratio and M-CSF expression were both repressed and there was no differentiation. We examined whether TGF-beta-mediated repression of osteoclasts differentiation is due to these changes by adding M-CSF and/or RANKL and did not observe any impact on differentiation repression. We studied direct TGF-beta impacts on osteoclast precursors by culturing spleen or marrow cells with M-CSF and RANKL. TGF-beta treatment dose-dependently stimulated osteoclast differentiation. These data indicate that low TGF-beta levels stimulate osteoclast differentiation by impacting the RANKL/OPG ratio while high TGF-beta levels repress osteoclast differentiation by multiple avenues including mechanisms independent of the RANKL/OPG ratio or M-CSF expression regulation.
DOI:10.1016/j.bbrc.2009.12.171URLPMID:20059964 [本文引用: 1]

TGFbeta inducible early gene-1 (TIEG) is a member of the Kruppel-like family of transcription factors (KLF10) that plays an important role in TGFbeta mediated Smad signaling. In order to better understand the role of TIEG in bone, we generated TIEG knockout (KO) mice. Calvarial osteoblasts (OBs) isolated from these mice exhibit a reduced ability to support osteoclastogenesis in vitro. Gene expression studies revealed decreased receptor activator of NF-kappaB ligand (RANKL) and increased osteoprotegerin (OPG) expression in TIEG KO OBs, suggesting a potential role for TIEG in regulating the expression of these genes. Since OPG and RANKL are two important regulators of osteoclast (OC) differentiation, we sought to determine if TIEG directly regulates their expression. Luciferase constructs, containing fragments of either the mouse OPG promoter (-1486 to +133 bp) or the RANKL promoter (-2000 to +1 bp) were each cloned into the pGL3 basic reporter vector and transiently transfected into TIEG KO calvarial OBs with and without a TIEG expression vector. No significant changes in the activity of the RANKL promoter were detected in the presence of TIEG. However, OPG promoter activity was inhibited in the presence of TIEG protein suggesting that TIEG directly represses the expression of OPG in OBs. In order to determine the region of this promoter through which TIEG acts, sequential 5'-deletion constructs were generated. Transient transfection of these constructs revealed that the TIEG regulatory element(s) reside within a 200 bp region of the OPG promoter. Transient ChIP analyses, using a TIEG-specific antibody, revealed that TIEG binds to this region of the OPG promoter. Since we have previously shown that TIEG regulates target gene expression through Sp-1 sites, we examined this region of the OPG promoter for potential TIEG binding elements and identified four potential Sp-1 binding sites. Site-directed mutagenesis was used to determine if TIEG utilizes these Sp-1 elements to regulate the activity of the OPG promoter. The data demonstrate that two Sp-1 sites are likely to be involved in TIEG's repression of the OPG promoter. Taken together, these results confirm that TIEG directly binds to and inhibits OPG promoter activity in OBs, partially explaining the inability of TIEG KO OBs to fully support osteoclast differentiation.
DOI:10.1186/s13287-019-1281-2URLPMID:31196174 [本文引用: 1]

BACKGROUND: Clinically, for stem cell-based therapy (SCBT), autologous stem cells are considered better than allogenic stem cells because of little immune rejection and no risk of communicable disease infection. However, severe maxillofacial bone defects restoration needs sufficient autologous stem cells, and this remains a challenge worldwide. Human gingival mesenchymal stem cells (hGMSCs) derived from clinically discarded, easily obtainable, and self-healing autologous gingival tissues, have higher proliferation rate compared with autologous bone marrow mesenchymal stem cells (hBMSCs). But for clinical bone regeneration purpose, GMSCs have inferior osteogenic differentiation capability. In this study, a TGF-beta signaling inhibitor SB431542 was used to enhance GMSCs osteogenesis in vitro and to repair minipig severe maxillofacial bone defects. METHODS: hGMSCs were isolated and cultured from clinically discarded gingival tissues. The effects of SB431542 on proliferation, apoptosis, and osteogenic differentiation of hGMSCs were analyzed in vitro, and then, SB431542-treated hGMSCs composited with Bio-Oss(R) were transplanted into immunocompromised mice subcutaneously to explore osteogenic differentiation in vivo. After that, SB431542-treated autologous pig GMSCs (pGMSCs) composited with Bio-Oss(R) were transplanted into circular confined defects (5 mm x 12 mm) in minipigs maxillary to investigate severe bone defect regeneration. Minipigs were sacrificed at 2 months and nude mice at 8 weeks to retrieve specimens for histological or micro-CT or CBCT analysis. Effects of SB431542 on TGF-beta and BMP signaling in hGMSCs were investigated by Western Blot or qRT-PCR. RESULTS: One micromolar of SB431542 treatment induced a robust osteogenesis of hGMSCs in vitro, without adverse effect on apoptosis and growth. In vivo, 1 muM SB431542 treatment also enabled striking osteogenesis of hGMSCs subcutaneously in nude mice and advanced new bone formation of pGMSCs in minipig maxillary bone defect model. In addition, SB431542-treated hGMSCs markedly increased bone-related proteins expression, and BMP2 and BMP4 gene expression. Conversely, SMAD3 protein-dependent TGF-beta signal pathway phosphorylation was decreased. CONCLUSIONS: Our study show that osteogenic differentiation of GMSCs treated with TGF-beta signaling inhibitor SB431542 was increased, and SB431542-treated autologous pig GMSCs could successfully repair minipig severe maxillofacial bone defects. This preclinical study brings about a promising large bone regeneration therapeutic potential of autologous GMSCs induced by SB431542 in clinic settings.
DOI:10.1038/ncomms15000URLPMID:28397831 [本文引用: 1]

Osteocytes are the terminally differentiated cell type of the osteoblastic lineage and have important functions in skeletal homeostasis. Although the transcriptional regulation of osteoblast differentiation has been well characterized, the factors that regulate differentiation of osteocytes from mature osteoblasts are poorly understood. Here we show that miR-23a approximately 27a approximately 24-2 (miR-23a cluster) promotes osteocyte differentiation. Osteoblast-specific miR-23a cluster gain-of-function mice have low bone mass associated with decreased osteoblast but increased osteocyte numbers. By contrast, loss-of-function transgenic mice overexpressing microRNA decoys for either miR-23a or miR-27a, but not miR24-2, show decreased osteocyte numbers. Moreover, RNA-sequencing analysis shows altered transforming growth factor-beta (TGF-beta) signalling. Prdm16, a negative regulator of the TGF-beta pathway, is directly repressed by miR-27a with concomitant alteration of sclerostin expression, and pharmacological inhibition of TGF-beta rescues the phenotypes observed in the gain-of-function transgenic mice. Taken together, the miR-23a cluster regulates osteocyte differentiation by modulating the TGF-beta signalling pathway through targeting of Prdm16.
DOI:10.1002/jbmr.2550URL [本文引用: 2]
DOI:10.1073/pnas.1409857112URLPMID:25605937 [本文引用: 1]

Osteocytes, >90% of the cells in bone, lie embedded within the mineralized matrix and coordinate osteoclast and osteoblast activity on bone surfaces by mechanisms still unclear. Bone anabolic stimuli activate Wnt signaling, and human mutations of components along this pathway underscore its crucial role in bone accrual and maintenance. However, the cell responsible for orchestrating Wnt anabolic actions has remained elusive. We show herein that activation of canonical Wnt signaling exclusively in osteocytes [dominant active (da)betacat(Ot) mice] induces bone anabolism and triggers Notch signaling without affecting survival. These features contrast with those of mice expressing the same dass-catenin in osteoblasts, which exhibit decreased resorption and perinatal death from leukemia. dasscat(Ot) mice exhibit increased bone mineral density in the axial and appendicular skeleton, and marked increase in bone volume in cancellous/trabecular and cortical compartments compared with littermate controls. dasscat(Ot) mice display increased resorption and formation markers, high number of osteoclasts and osteoblasts in cancellous and cortical bone, increased bone matrix production, and markedly elevated periosteal bone formation rate. Wnt and Notch signaling target genes, osteoblast and osteocyte markers, and proosteoclastogenic and antiosteoclastogenic cytokines are elevated in bones of dasscat(Ot) mice. Further, the increase in RANKL depends on Sost/sclerostin. Thus, activation of osteocytic beta-catenin signaling increases both osteoclasts and osteoblasts, leading to bone gain, and is sufficient to activate the Notch pathway. These findings demonstrate disparate outcomes of beta-catenin activation in osteocytes versus osteoblasts and identify osteocytes as central target cells of the anabolic actions of canonical Wnt/beta-catenin signaling in bone.
DOI:10.1056/NEJMoa013444URLPMID:12015390 [本文引用: 1]

BACKGROUND: Osteoporosis is a major public health problem of largely unknown cause. Loss-of-function mutations in the gene for low-density lipoprotein receptor-related protein 5 (LRP5), which acts in the Wnt signaling pathway, have been shown to cause osteoporosis-pseudoglioma. METHODS: We performed genetic and biochemical analyses of a kindred with an autosomal dominant syndrome characterized by high bone density, a wide and deep mandible, and torus palatinus. RESULTS: Genetic analysis revealed linkage of the syndrome to chromosome 11q12-13 (odds of linkage, >1 million to 1), an interval that contains LRP5. Affected members of the kindred had a mutation in this gene, with valine substituted for glycine at codon 171 (LRP5V171). This mutation segregated with the trait in the family and was absent in control subjects. The normal glycine lies in a so-called propeller motif that is highly conserved from fruit flies to humans. Markers of bone resorption were normal in the affected subjects, whereas markers of bone formation such as osteocalcin were markedly elevated. Levels of fibronectin, a known target of signaling by Wnt, a developmental protein, were also elevated. In vitro studies showed that the normal inhibition of Wnt signaling by another protein, Dickkopf-1 (Dkk-1), was defective in the presence of LRP5V171 and that this resulted in increased signaling due to unopposed Wnt activity. CONCLUSIONS: The LRP5V171 mutation causes high bone density, with a thickened mandible and torus palatinus, by impairing the action of a normal antagonist of the Wnt pathway and thus increasing Wnt signaling. These findings demonstrate the role of altered LRP5 function in high bone mass and point to Dkk as a potential target for the prevention or treatment of osteoporosis.
DOI:10.1086/338450URLPMID:11741193 [本文引用: 1]

Osteoporosis is a complex disease that affects >10 million people in the United States and results in 1.5 million fractures annually. In addition, the high prevalence of osteopenia (low bone mass) in the general population places a large number of people at risk for developing the disease. In an effort to identify genetic factors influencing bone density, we characterized a family that includes individuals who possess exceptionally dense bones but are otherwise phenotypically normal. This high-bone-mass trait (HBM) was originally localized by linkage analysis to chromosome 11q12-13. We refined the interval by extending the pedigree and genotyping additional markers. A systematic search for mutations that segregated with the HBM phenotype uncovered an amino acid change, in a predicted beta-propeller module of the low-density lipoprotein receptor-related protein 5 (LRP5), that results in the HBM phenotype. During analysis of >1,000 individuals, this mutation was observed only in affected individuals from the HBM kindred. By use of in situ hybridization to rat tibia, expression of LRP5 was detected in areas of bone involved in remodeling. Our findings suggest that the HBM mutation confers a unique osteogenic activity in bone remodeling, and this understanding may facilitate the development of novel therapies for the treatment of osteoporosis.
DOI:10.1242/dev.01564URLPMID:15576404 [本文引用: 1]

Signals that govern development of the osteoblast lineage are not well understood. Indian hedgehog (Ihh), a member of the hedgehog (Hh) family of proteins, is essential for osteogenesis in the endochondral skeleton during embryogenesis. The canonical pathway of Wnt signaling has been implicated by studies of Lrp5, a co-receptor for Wnt proteins, in postnatal bone mass homeostasis. In the present study we demonstrate that beta-catenin, a central player in the canonical Wnt pathway, is indispensable for osteoblast differentiation in the mouse embryo. Moreover, we present evidence that Wnt signaling functions downstream of Ihh in development of the osteoblast lineage. Finally Wnt7b is identified as a potential endogenous ligand regulating osteogenesis. These data support a model that integrates Hh and Wnt signaling in the regulation of osteoblast development.
DOI:10.1016/j.devcel.2005.03.016URLPMID:15866164 [本文引用: 1]

Chondrocytes and osteoblasts are two primary cell types in the skeletal system that are differentiated from common mesenchymal progenitors. It is believed that osteoblast differentiation is controlled by distinct mechanisms in intramembranous and endochondral ossification. We have found that ectopic canonical Wnt signaling leads to enhanced ossification and suppression of chondrocyte formation. Conversely, genetic inactivation of beta-catenin, an essential component transducing the canonical Wnt signaling, causes ectopic formation of chondrocytes at the expense of osteoblast differentiation during both intramembranous and endochondral ossification. Moreover, inactivation of beta-catenin in mesenchymal progenitor cells in vitro causes chondrocyte differentiation under conditions allowing only osteoblasts to form. Our results demonstrate that beta-catenin is essential in determining whether mesenchymal progenitors will become osteoblasts or chondrocytes regardless of regional locations or ossification mechanisms. Controlling Wnt/beta-catenin signaling is a common molecular mechanism underlying chondrocyte and osteoblast differentiation and specification of intramembranous and endochondral ossification.
URLPMID:15866163 [本文引用: 1]
DOI:10.1242/dev.02480URLPMID:16854976 [本文引用: 1]

Hedgehog and canonical Wnt/beta-catenin signaling are implicated in development of the osteoblast, the bone matrix-secreting cell of the vertebrate skeleton. We have used genetic approaches to dissect the roles of these pathways in specification of the osteoblast lineage. Previous studies indicate that Ihh signaling in the long bones is essential for initial specification of an osteoblast progenitor to a Runx2+ osteoblast precursor. We show here that this is a transient requirement, as removal of Hh responsiveness in later Runx2+, Osx1+ osteoblast precursors does not disrupt the formation of mature osteoblasts. By contrast, the removal of canonical Wnt signaling by conditional removal of the beta-catenin gene in early osteoblast progenitors or in Runx2+, Osx1+ osteoblast precursors results in a similar phenotype: osteoblasts fail to progress to a terminal osteocalcin+ fate and instead convert to a chondrocyte fate. By contrast, stabilization of beta-catenin signaling in Runx2+, Osx1+ osteoblast precursors leads to the premature differentiation of bone matrix secreting osteoblasts. These data demonstrate that commitment within the osteoblast lineage requires sequential, stage-specific, Ihh and canonical Wnt/beta-catenin signaling to promote osteogenic, and block chondrogenic, programs of cell fate specification.
DOI:10.1016/j.devcel.2005.02.017URLPMID:15866165 [本文引用: 1]

Inactivation of beta-catenin in mesenchymal progenitors prevents osteoblast differentiation; inactivation of Lrp5, a gene encoding a likely Wnt coreceptor, results in low bone mass (osteopenia) by decreasing bone formation. These observations indicate that Wnt signaling controls osteoblast differentiation and suggest that it may regulate bone formation in differentiated osteoblasts. Here, we study later events and find that stabilization of beta-catenin in differentiated osteoblasts results in high bone mass, while its deletion from differentiated osteoblasts leads to osteopenia. Surprisingly, histological analysis showed that these mutations primarily affect bone resorption rather than bone formation. Cellular and molecular studies showed that beta-catenin together with TCF proteins regulates osteoblast expression of Osteoprotegerin, a major inhibitor of osteoclast differentiation. These findings demonstrate that beta-catenin, and presumably Wnt signaling, promote the ability of differentiated osteoblasts to inhibit osteoclast differentiation; thus, they broaden our knowledge of the functions Wnt proteins have at various stages of skeletogenesis.
DOI:10.1074/jbc.M501900200URLPMID:15802266 [本文引用: 1]

Mutations in the Wnt co-receptor LRP5 alter bone mass in humans, but the mechanisms responsible for Wnts actions in bone are unclear. To investigate the role of the classical Wnt signaling pathway in osteogenesis, we generated mice lacking the beta-catenin or adenomatous polyposis coli (Apc) genes in osteoblasts. Loss of beta-catenin produced severe osteopenia with striking increases in osteoclasts, whereas constitutive activation of beta-catenin in the conditional Apc mutants resulted in dramatically increased bone deposition and a disappearance of osteoclasts. In vitro, osteoblasts lacking the beta-catenin gene exhibited impaired maturation and mineralization with elevated expression of the osteoclast differentiation factor, receptor activated by nuclear factor-kappaB ligand (RANKL), and diminished expression of the RANKL decoy receptor, osteoprotegerin. By contrast, Apc-deficient osteoblasts matured normally but demonstrated decreased expression of RANKL and increased osteoprotegerin. These findings suggest that Wnt/beta-catenin signaling in osteoblasts coordinates postnatal bone acquisition by controlling the differentiation and activity of both osteoblasts and osteoclasts.
DOI:10.1128/MCB.01428-09URLPMID:20404086 [本文引用: 1]

Beta-Catenin-dependent canonical Wnt signaling plays an important role in bone metabolism by controlling differentiation of bone-forming osteoblasts and bone-resorbing osteoclasts. To investigate its function in osteocytes, the cell type constituting the majority of bone cells, we generated osteocyte-specific beta-catenin-deficient mice (Ctnnb1(loxP/loxP); Dmp1-Cre). Homozygous mutants were born at normal Mendelian frequency with no obvious morphological abnormalities or detectable differences in size or body weight, but bone mass accrual was strongly impaired due to early-onset, progressive bone loss in the appendicular and axial skeleton with mild growth retardation and premature lethality. Cancellous bone mass was almost completely absent, and cortical bone thickness was dramatically reduced. The low-bone-mass phenotype was associated with increased osteoclast number and activity, whereas osteoblast function and osteocyte density were normal. Cortical bone Wnt/beta-catenin target gene expression was reduced, and of the known regulators of osteoclast differentiation, osteoprotegerin (OPG) expression was significantly downregulated in osteocyte bone fractions of mutant mice. Moreover, the OPG levels expressed by osteocytes were higher than or comparable to the levels expressed by osteoblasts during skeletal growth and at maturity, suggesting that the reduction in osteocytic OPG and the concomitant increase in osteocytic RANKL/OPG ratio contribute to the increased number of osteoclasts and resorption in osteocyte-specific beta-catenin mutants. Together, these results reveal a crucial novel function for osteocyte beta-catenin signaling in controlling bone homeostasis.
DOI:10.1210/en.2005-0239URLPMID:16081646 [本文引用: 1]

Both chronic excess of PTH, as in hyperparathyroidism, and intermittent elevation of PTH (by daily injections) increase the number of osteoblasts; albeit, the former is associated with bone catabolism and the later with bone anabolism. Intermittent PTH increases osteoblast number by attenuating osteoblast apoptosis, an effect that requires the transcription factor Runx2. However, chronic elevation of PTH does not affect osteoblast apoptosis because it stimulates the proteasomal degradation of Runx2. Here, we studied the effects of PTH on Sost, a Runx2 target gene expressed in osteocytes (former osteoblasts embedded in the bone matrix), which antagonizes the pro-osteoblastogenic actions of bone morphogenetic proteins and Wnts. We report that continuous infusion of PTH to mice for 4 d decreased Sost mRNA expression in vertebral bone by 80-90%. This effect was accompanied by a comparable reduction of sclerostin, the product of Sost, in osteocytes, as determined by quantitative immunoblot analysis of bone extracts and by immunostaining. In contrast, a single injection of PTH caused a transient 50% reduction in Sost mRNA at 2 h, but four daily injections had no effect on Sost mRNA or sclerostin. PTH strongly decreased Sost expression in osteocytes formed in primary cultures of neonatal murine calvaria cells as well as in osteocytic MLO-A5 cells, demonstrating a direct effect of PTH on this cell type. These results, together with evidence that sclerostin antagonizes bone morphogenetic proteins and Wnts, strongly suggest that suppression of Sost by PTH represents a novel mechanism for hormonal control of osteoblastogenesis mediated by osteocytes.
DOI:10.1371/journal.pone.0002942URLPMID:18698360 [本文引用: 1]

Osteocytes, former osteoblasts buried within bone, are thought to orchestrate skeletal adaptation to mechanical stimuli. However, it remains unknown whether hormones control skeletal homeostasis through actions on osteocytes. Parathyroid hormone (PTH) stimulates bone remodeling and may cause bone loss or bone gain depending on the balance between bone resorption and formation. Herein, we demonstrate that transgenic mice expressing a constitutively active PTH receptor exclusively in osteocytes exhibit increased bone mass and bone remodeling, as well as reduced expression of the osteocyte-derived Wnt antagonist sclerostin, increased Wnt signaling, increased osteoclast and osteoblast number, and decreased osteoblast apoptosis. Deletion of the Wnt co-receptor LDL related receptor 5 (LRP5) attenuates the high bone mass phenotype but not the increase in bone remodeling induced by the transgene. These findings demonstrate that PTH receptor signaling in osteocytes increases bone mass and the rate of bone remodeling through LRP5-dependent and -independent mechanisms, respectively.
DOI:10.1016/j.bone.2011.10.025URL [本文引用: 1]

Sclerostin, the Wnt signaling antagonist encoded by the Sost gene, is secreted by osteocytes and inhibits bone formation by osteoblasts. Mechanical stimulation reduces sclerostin expression, suggesting that osteocytes might coordinate the osteogenic response to mechanical force by locally unleashing Wnt signaling. To investigate whether sclerostin downregulation is a pre-requisite for load-induced bone formation, we conducted experiments in transgenic mice (TG) engineered to maintain high levels of SOST expression during mechanical loading. This was accomplished by introducing a human SOST transgene driven by the 8 kb fragment of the DMP1 promoter that also provided osteocyte specificity of the transgene. Right ulnae were subjected to in vivo cyclic axial loading at equivalent strains for 1 min/day at 2 Hz; left ulnae served as internal controls. Endogenous murine Sost mRNA expression measured 24 h after 1 loading bout was decreased by about 50% in TG and wild type (WT) littermates. In contrast, human SOST, only expressed in TG mice, remained high after loading. Mice were loaded on 3 consecutive days and bone formation was quantified 16 days after initiation of loading. Periosteal bone formation in control ulnae was similar in WT and TG mice. Loading induced the expected strain-dependent increase in bone formation in WT mice, resulting from increases in both mineralizing surface (MS/BS) and mineral apposition rate (MAR). In contrast, load-induced bone formation was reduced by 70-85% in TG mice, due to lower MS/BS and complete inhibition of MAR. Moreover, Wnt target gene expression induced by loading in WT mice was absent in TG mice. Thus, downregulation of Sost/sclerostin in osteocytes is an obligatory step in the mechanotransduction cascade that activates Writ signaling and directs osteogenesis to where bone is structurally needed. (C) 2011 Elsevier Inc.
DOI:10.1101/gr.3437105URLPMID:15965026 [本文引用: 1]

Mutations in distant regulatory elements can have a negative impact on human development and health, yet because of the difficulty of detecting these critical sequences, we predominantly focus on coding sequences for diagnostic purposes. We have undertaken a comparative sequence-based approach to characterize a large noncoding region deleted in patients affected by Van Buchem (VB) disease, a severe sclerosing bone dysplasia. Using BAC recombination and transgenesis, we characterized the expression of human sclerostin (SOST) from normal (SOST(wt)) or Van Buchem (SOST(vbDelta) alleles. Only the SOST(wt) allele faithfully expressed high levels of human SOST in the adult bone and had an impact on bone metabolism, consistent with the model that the VB noncoding deletion removes a SOST-specific regulatory element. By exploiting cross-species sequence comparisons with in vitro and in vivo enhancer assays, we were able to identify a candidate enhancer element that drives human SOST expression in osteoblast-like cell lines in vitro and in the skeletal anlage of the embryonic day 14.5 (E14.5) mouse embryo, and discovered a novel function for sclerostin during limb development. Our approach represents a framework for characterizing distant regulatory elements associated with abnormal human phenotypes.
DOI:10.1093/hmg/10.5.537URLPMID:11181578 [本文引用: 1]

Sclerosteosis is a progressive sclerosing bone dysplasia with an autosomal recessive mode of inheritance. Radiologically, it is characterized by a generalized hyperostosis and sclerosis leading to a markedly thickened and sclerotic skull, with mandible, ribs, clavicles and all long bones also being affected. Due to narrowing of the foramina of the cranial nerves, facial nerve palsy, hearing loss and atrophy of the optic nerves can occur. Sclerosteosis is clinically and radiologically very similar to van Buchem disease, mainly differentiated by hand malformations and a large stature in sclerosteosis patients. By linkage analysis in one extended van Buchem family and two consanguineous sclerosteosis families we previously mapped both disease genes to the same chromosomal 17q12-q21 region, supporting the hypothesis that both conditions are caused by mutations in the same gene. After reducing the disease critical region to approximately 1 Mb, we used the positional cloning strategy to identify the SOST gene, which is mutated in sclerosteosis patients. This new gene encodes a protein with a signal peptide for secretion and a cysteine-knot motif. Two nonsense mutations and one splice site mutation were identified in sclerosteosis patients, but no mutations were found in a fourth sclerosteosis patient nor in the patients from the van Buchem family. As the three disease-causing mutations lead to loss of function of the SOST protein resulting in the formation of massive amounts of normal bone throughout life, the physiological role of SOST is most likely the suppression of bone formation. Therefore, this gene might become an important tool in the development of therapeutic strategies for osteoporosis.
DOI:10.1038/s41598-017-03899-zURLPMID:28623357 [本文引用: 1]

Recent studies have demonstrated the stimulatory effects of adiponectin on bone formation, but the mechanism underlying these effects remains unclear. The Wnt/beta-catenin pathway, one of the most important pathways in osteogenesis, has rarely been associated with the osteogenic effects of adiponectin in previous studies. The present study was designed to investigate the effects of adiponectin on bone mesenchymal stem cell (BMSC) osteogenic differentiation and bone formation through the Wnt/beta-catenin pathway. We detected adiponectin receptor expression in BMSCs, constructed a recombinant adenovirus containing the human adiponectin gene, and then used the adenovirus to transfect BMSCs in vitro or injected the adenovirus into bone defect areas in animal models. Wnt/beta-catenin pathway and osteogenesis were detected by real-time PCR, western blotting, immunofluorescence, HE staining and micro-CT. In both our in vivo and in vitro experiments, we detected higher gene and protein expression levels of the Wnt/beta-catenin pathway-related factors beta-catenin and cyclinD1 in adiponectin transgenic BMSCs and rats. Similar results were noted regarding the gene and protein expression levels of osteogenesis-related genes. In addition, more new bone formation was observed in the adiponectin-treated groups. Our results indicate that adiponectin could facilitate BMSC osteogenic differentiation and osteogenesis, and the Wnt/beta-catenin pathway was involved in the osteogenic effect of adiponectin.
URLPMID:30670092 [本文引用: 1]
DOI:10.3390/biom9110711URL [本文引用: 1]
DOI:10.1080/03008207.2017.1290085URLPMID:28324674 [本文引用: 1]

Bone homeostasis depends on the resorption of bones by osteoclasts and formation of bones by the osteoblasts. Imbalance of this tightly coupled process can cause diseases such as osteoporosis. Thus, the mechanisms that regulate communication between osteoclasts and osteoblasts are critical to bone cell biology. It has been shown that osteoblasts and osteoclasts can communicate with each other through direct cell-cell contact, cytokines, and extracellular matrix interaction. Osteoblasts can affect osteoclast formation, differentiation, or apoptosis through several pathways, such as OPG/RANKL/RANK, RANKL/LGR4/RANK, Ephrin2/ephB4, and Fas/FasL pathways. Conversely, osteoclasts also influence formation of bones by osteoblasts via the d2 isoform of the vacuolar (H+) ATPase (v-ATPase) V0 domain (Atp6v0d2), complement component 3a, semaphorin 4D or microRNAs. In addition, cytokines released from the resorbed bone matrix, such as TGF-beta and IGF-1, also affect the activity of osteoblasts. Drugs could be developed by enhancing or restricting some of these interactions. Several reviews have been performed on the osteoblast-osteoclast communication. However, few reviews have shown the research advances in the recent years. In this review, we summarized the current knowledge on osteoblast-osteoclast communication.
URLPMID:17458179 [本文引用: 1]
URLPMID:15615494 [本文引用: 1]

The OPG/RANKL/RANK cytokine system is essential for osteoclast biology. Various studies suggest that human metabolic bone diseases are related to alterations of this system. Here we summarize OPG/RANKL/RANK abnormalities in different forms of osteoporoses and hyperparathyroidism. Skeletal estrogen agonists (including 17beta-estradiol, raloxifene, and genistein) induce osteoblastic OPG production through estrogen receptor-alpha activation in vitro, while immune cells appear to over-express RANKL in estrogen deficiency in vivo. Of note, OPG administration can prevent bone loss associated with estrogen deficiency as observed in both animal models and a small clinical study. Glucocorticoids and immunosuppressants concurrently up-regulate RANKL and suppress OPG in osteoblastic cells in vitro, and glucocorticoids are among the most powerful drugs to suppress OPG serum levels in vivo. As for mechanisms of immobilization-induced bone loss, it appears that mechanical strain inhibits RANKL production through the ERK 1/2 MAP kinase pathway and up-regulates OPG production in vitro. Hence, lack of mechanical strainduring immobilization may favor an enhanced RANKL-to-OPG ratio leading to increased bone loss. As for hyperparathyroidism, chronic PTH exposure concurrently enhances RANKL production and suppresses OPG secretion through activation of osteoblastic protein kinase A in vitro which would favour increased osteoclastic activity. In sum, the capacity for OPG to antagonize the increases in bone loss seen in many rodent models of metabolic bone disease implicates RANKL/OPG imbalances as the likely etiology and supports the potential role for a RANKL antagonist as a therapeutic intervention in these settings.
[本文引用: 1]
DOI:10.1186/s41232-019-0111-3URLPMID:32047573 [本文引用: 1]

Receptor activator of NF-kappaB (RANK) ligand (RANKL) induces the differentiation of monocyte/macrophage-lineage cells into the bone-resorbing cells called osteoclasts. Because abnormalities in RANKL, its signaling receptor RANK, or decoy receptor osteoprotegerin (OPG) lead to bone diseases such as osteopetrosis, the RANKL/RANK/OPG system is essential for bone resorption. RANKL was first discovered as a T cell-derived activator of dendritic cells (DCs) and has many functions in the immune system, including organogenesis, cellular development. The essentiality of RANKL in the bone and the immune systems lies at the root of the field of
DOI:10.1016/j.molmed.2005.11.007URLPMID:16356770 [本文引用: 1]

Hundreds of millions of people worldwide are affected by bone-related diseases, such as osteoporosis and rheumatoid arthritis. Understanding the molecular mechanisms of bone metabolism is crucial for developing novel drugs for treating such diseases. In particular, genetic experiments showing that the receptor activator of NF-kappaB (RANK), its ligand RANKL, and the decoy receptor OPG are essential, central regulators of osteoclast development and osteoclast function were significant turning points in our understanding of bone diseases. RANKL-RANK signaling activates a variety of downstream signaling pathways required for osteoclast development. Moreover, molecular cross-talk between RANKL-RANK and other ligand-receptor systems fine-tunes bone homeostasis in normal physiology and disease. Designing novel drugs that target RANKL-RANK and their signaling pathways in osteoclasts could potentially revolutionize the treatment of many diseases associated with bone loss such as arthritis, tooth loss, cancer metastases or osteoporosis.
DOI:10.1084/jem.20021321URLPMID:12707301 [本文引用: 1]

Osteoclasts are multinucleated cells that resorb bones, and are derived from hematopoietic cells of the monocyte/macrophage lineage. The receptor activator of NF-kappaB ligand (RANKL, also called ODF/TRANCE/OPGL) stimulates both osteoclast differentiation from osteoclast progenitors and activation of mature osteoclasts. To identify genes responsible for osteoclast differentiation, we used a molecular indexing technique. Here, we report a clone of one of these genes whose transcription is induced by soluble RANKL (sRANKL) in both the RAW264.7 cells of the mouse macrophage cell line and the mouse primary bone marrow cells. The predicted protein was found to be a mouse homologue of Jun dimerization protein 2 (JDP2), a member of the AP-1 family of transcription factors, containing a basic region-leucine zipper motif. Transient transfection experiments revealed that overexpression of JDP2 leads to activation of both tartrate-resistant acid phosphatase (TRAP) and cathepsin K gene promoters in RAW264.7 cells. Infection of mouse primary bone marrow cells with retroviruses expressing JDP2-facilitated sRANKL-mediated formation of TRAP-positive multinuclear osteoclasts. Importantly, antisense oligonucleotide to JDP2 strongly suppressed sRANKL-induced osteoclast formation of RAW264.7 cells. Our findings suggest that JDP2 may play an important role in the RANK-mediated signal transduction system, especially in osteoclast differentiation.
DOI:10.1016/s0092-8674(00)80209-3URLPMID:9108485 [本文引用: 1]

A novel secreted glycoprotein that regulates bone resorption has been identified. The protein, termed Osteoprotegerin (OPG), is a novel member of the TNF receptor superfamily. In vivo, hepatic expression of OPG in transgenic mice results in a profound yet nonlethal osteopetrosis, coincident with a decrease in later stages of osteoclast differentiation. These same effects are observed upon administration of recombinant OPG into normal mice. In vitro, osteoclast differentiation from precursor cells is blocked in a dose-dependent manner by recombinant OPG. Furthermore, OPG blocks ovariectomy-associated bone loss in rats. These data show that OPG can act as a soluble factor in the regulation of bone mass and imply a utility for OPG in the treatment of osteoporosis associated with increased osteoclast activity.
DOI:10.1002/jbmr.2082URLPMID:24038209 [本文引用: 1]

Reduced bioavailability of estrogen increases skeletal fracture risk in postmenopausal women, but the mechanisms by which estrogen regulates bone mass are incompletely understood. Because estrogen signaling in bone acts, in part, through estrogen receptor alpha (ERalpha), mice with global deletion of ERalpha (ERalphaKO) have been used to determine the role of estrogen signaling in bone biology. These animals, however, have confounding systemic effects arising from other organs, such as increased estrogen and decreased insulin-like growth factor 1 (IGF-1) serum levels, which may independently affect bone. Mice with tissue-specific ERalpha deletion in chondrocytes, osteoblasts, osteocytes, or osteoclasts lack the systemic effects seen in the global knockout, but show that presence of the receptor is important for the function of each cell type. Although bone mass is reduced when ERalpha is deleted from osteoblasts, no study has determined if this approach reduces whole bone strength. To address this issue, we generated female osteoblast-specific ERalphaKO mice (pOC-ERalphaKO) by crossing mice expressing a floxed ERalpha gene (ERalpha(fl/fl)) with mice transgenic for the osteocalcin-Cre promoter (OC-Cre). Having confirmed that serum levels of estrogen and IGF-1 were unaltered, we focused on relating bone mechanics to skeletal phenotype using whole bone mechanical testing, microcomputed tomography, histology, and dynamic histomorphometry. At 12 and 18 weeks of age, pOC-ERalphaKO mice had decreased cancellous bone mass in the proximal tibia, vertebra, and distal femur, and decreased cortical bone mass in the tibial midshaft, distal femoral cortex, and L5 vertebral cortex. Osteoblast activity was reduced in cancellous bone of the proximal tibia, but osteoclast number was unaffected. Both femora and vertebrae had decreased whole bone strength in mechanical tests to failure, indicating that ERalpha in osteoblasts is required for appropriate bone mass and strength accrual in female mice. This pOC-ERalphaKO mouse is an important animal model that could enhance our understanding of estrogen signaling in bone cells in vivo.
DOI:10.1016/j.joen.2016.07.008URL [本文引用: 1]
DOI:10.1016/j.coph.2005.06.005URL [本文引用: 1]

Osteoprotegerin (OPG) and receptor activator of nuclear factor-κB ligand (RANKL) are dominant regulators of bone resorption. Many hormones, cytokines and growth factors mediate bone resorption by altering the ratio of RANKL to OPG. RANKL and OPG expression is also altered in numerous bone diseases, and these changes can reflect disease etiology or compensatory responses to disease. RANKL stimulates osteoclast formation, function and survival, and each of these effects is inhibited by OPG. OPG suppresses bone resorption and increases the density, area and strength of both cancellous and cortical bone. Denosumab (AMG 162), a fully human monoclonal antibody to RANKL, shares the pharmacologic attributes of OPG but has a significantly longer half-life that allows less frequent administration.
DOI:10.1371/journal.ppat.1004497URLPMID:25393853 [本文引用: 1]

HIV infection is associated with high rates of osteopenia and osteoporosis, but the mechanisms involved are unclear. We recently reported that bone loss in the HIV transgenic rat model was associated with upregulation of B cell expression of the key osteoclastogenic cytokine receptor-activator of NF-kappaB ligand (RANKL), compounded by a simultaneous decline in expression of its physiological moderator, osteoprotegerin (OPG). To clinically translate these findings we performed cross-sectional immuno-skeletal profiling of HIV-uninfected and antiretroviral therapy-naive HIV-infected individuals. Bone resorption and osteopenia were significantly higher in HIV-infected individuals. B cell expression of RANKL was significantly increased, while B cell expression of OPG was significantly diminished, conditions favoring osteoclastic bone resorption. The B cell RANKL/OPG ratio correlated significantly with total hip and femoral neck bone mineral density (BMD), T- and/or Z-scores in HIV infected subjects, but revealed no association at the lumbar spine. B cell subset analyses revealed significant HIV-related increases in RANKL-expressing naive, resting memory and exhausted tissue-like memory B cells. By contrast, the net B cell OPG decrease in HIV-infected individuals resulted from a significant decline in resting memory B cells, a population containing a high frequency of OPG-expressing cells, concurrent with a significant increase in exhausted tissue-like memory B cells, a population with a lower frequency of OPG-expressing cells. These data validate our pre-clinical findings of an immuno-centric mechanism for accelerated HIV-induced bone loss, aligned with B cell dysfunction.
DOI:10.1038/s41598-018-22109-yURLPMID:29487404 [本文引用: 1]

Tibial dyschondroplasia (TD) is the most-prevalent leg disorder in fast-growing chickens; it is intractable and characterized by abnormal endochondral bone formation of proximal tibial growth-plates (TGPs). Previous studies have shown that bone is a highly vascularized tissue dependent on the coordinated coupling between angiogenesis and osteogenesis, but the underlying mechanisms of bone formation and bone remodeling are poorly defined in TD chickens. Here, we observed that inhibition of vasculogenesis and angiogenesis remarkably impaired vascular invasion in the hypertrophic chondrocyte zone of the TGPs, resulting in the massive death of chondrocytes due to a shortage of blood vessels and nutrients. Moreover, the balance of the OPG (osteoprotegerin)/RANKL (receptor activator of nuclear factor-kB ligand) system is also severely disrupted during the osteogenesis process while coupling with angiogenesis, both of which eventually lead to abnormal endochondral bone formation in TD chickens. Thus, the process of vascular formation in endochondral bone appears to initiate the pathological changes in TD, and improvement of this process during coupling with osteogenesis may be a potential therapeutic approach to treat this intractable disease.
DOI:10.1155/2017/1693643URLPMID:29081815 [本文引用: 1]

The present study examined the effects of brucine on the OPG/RANKL/RANK signaling pathway for exploring the mechanism of brucine suppression of bone metastasis in breast cancer. MDA-MB-231 breast cancer cells and mouse osteoblast MC3T3-E1 cells were cocultured to mimic the breast cancer bone metastasis microenvironment in vitro. qRT-PCR and Western blotting were used to detect the expressions of OPG and RANKL at the mRNA and protein levels, respectively, in brucine-treated cultures and they were compared to those in untreated cultures. We aimed to understand the effect of brucine on the entire OPG/RANKL/RANK signaling pathway after analyzing these effects. Results showed that brucine treatment significantly increased both the OPG mRNA/RANKL mRNA expression ratio and the OPG protein/RANKL protein ratio in cocultures compared to those in untreated cocultures (P < 0.01). Brucine, therefore, plays a regulatory role in the OPG/RANKL/RANK signaling pathway, suggesting that it can indirectly control osteoclasts by regulating the expression and secretion of OPG and RANKL in osteoblast cells, thereby inhibiting the differentiation and bone resorption function of osteoclasts.
DOI:10.1038/aps.2012.83URL [本文引用: 1]

Aim: Lactoferrin (LF), an 80-kDa iron-binding glycoprotein, is a pleiotropic factor found in colostrum, milk, saliva and epithelial cells of the exocrine glands. The aim of this study was to evaluate the effects of LF on the bones in ovariectomized (Ovx) rats and to identify the pathways that mediate the anabolic action of LF on the bones.
Methods: Female Sprague-Dawley rats (6-month-old) underwent ovariectomy, and were treated with different doses of LF (10, 100, 1000, and 2000 mg.kg(-1).d(-1), po) or with 7 beta-estradiol (0.1 mg.kg(-1), im, each week) as the positive control. By the end of 6 month-treatments, the bone mass and microstructure in the rats were scanned by micro-computed tomography (micro-CT), and the bone metabolism was evaluated with specific markers, and the mRNA levels of osteoprotegerin (OPG) and the receptor-activator of nuclear factor kappa B ligand (RANKL) in femur were measured using qRT-PCR.
Results: LF treatment dose-dependently elevated the bone volume (BV/TV), trabecular thickness (TbTh) and trabecular number (TbN), and reduced the trabecular separation (TbSp) in Ovx rats. Furthermore, higher doses of LF (1000 and 2000 mg.kg(-1).d(-1)) significantly increased the bone mineral density (BMD) compared with the untreated Ovx rats. The higher doses of LF also significantly increased the serum levels of OC and BALP, and decreased the serum levels of beta-CTx and NTX. LF treatment significantly increased the OPG mRNA levels, and suppressed the RANKL mRNA levels, and the RANKL/OPG mRNA ratio in Ovx rats.
Conclusion: Oral administration of LF preserves the bone mass and improves the bone microarchitecture. LF enhances bone formation, reduces bone resorption, and decreases bone mass loss, possibly through the regulation of OPG/RANKL/RANK pathway.
DOI:10.1038/aps.2011.177URL [本文引用: 1]

Aim: To evaluate the protective effects of strontium fructose 1,6-diphosphate (FDP-Sr), a novel strontium salt that combined fructose 1,6-diphosphate (FDP) with strontium, on bone in an ovariectomy-induced model of bone loss. Methods: Eighty female Sprague-Dawley rats were ovariectomized (OVX) or sham-operated. Three months later, the rats were assigned to six groups (10 for each): sham-operated, OVX control, OVX+FDP-Sr (110, 220, or 440 mg/kg), or OVX+strontium ranelate (SR, 180 mg/kg). Drugs were administered orally for 3 months. When the treatment was terminated, the following parameters were assessed: bone mineral density (BMD), the biomechanical properties of the femur and lumbar vertebrae, trabecular histomorphology, serum phosphorus, calcium, bone-specific alkaline phosphatase (B-ALP), tartrate-resistant acid phosphatase 5b (TRACP5b), N-telopeptide of type I collagen (NTx) and a series of markers for oxidative stress. Receptor activator of NF-KB ligand (RANKL) and osteoprotegerin (OPG) levels in serum were measured using ELISA and their gene expression levels in the bone were measured using R-T PCR. Results: Treatment with FDP-Sr (220 or 440 mg/kg) or SR (180 mg/kg) significantly increased the BMD and improved the bone microarchitecture and bone strength in OVX rats. The treatments also decreased in the levels of H202 and MDA, restored the CAT level in serum and bone marrow, increased the serum B-ALP and decreased NTx and TRACP 5b in OVX rats. Treatment with FDP-Sr decreased the RANKL level, and increased the OPG level in serum in a dose-dependent manner. It also significantly down-regulated the RANKL expression and up-regulated OPG expression in bone marrow. Conclusion: FDP-Sr may be an effectve treatenent for postmenopausal osteoporosis that acts, in part, via a decrease iq osteoclastogenesis through the OPG \RANKL\RANK pathway.
DOI:10.4061/2010/218142URLPMID:21350642 [本文引用: 1]

Fibroblast growth factors (FGFs) that signal through FGF receptors (FGFRs) regulate a broad spectrum of biological functions, including cellular proliferation, survival, migration, and differentiation. The FGF signal pathways are the RAS/MAP kinase pathway, PI3 kinase/AKT pathway, and PLCgamma pathway, among which the RAS/MAP kinase pathway is known to be predominant. Several studies have recently implicated the in vitro biological functions of FGFs for tissue regeneration. However, to obtain optimal outcomes in vivo, it is important to enhance the half-life of FGFs and their biological stability. Future applications of FGFs are expected when the biological functions of FGFs are potentiated through the appropriate use of delivery systems and scaffolds. This review will introduce the biology and cellular functions of FGFs and deal with the biomaterials based delivery systems and their current applications for the regeneration of tissues, including skin, blood vessel, muscle, adipose, tendon/ligament, cartilage, bone, tooth, and nerve tissues.
DOI:10.1101/gad.990702URLPMID:12080084 [本文引用: 1]
[本文引用: 1]
DOI:10.1002/jcp.24083URL [本文引用: 1]

Fibroblast growth factor (FGF)/FGF (FGFR) signaling is an important pathway involved in skeletal development. Missense mutations in FGFs and FGFRs were found clinically to cause multiple congenital skeleton diseases including chondrodysplasia, craniosynostosis, syndromes with dysregulated phosphate metabolism. FGFs/FGFRs also have crucial roles in bone fracture repair and bone regeneration. Understanding the molecular mechanisms for the role of FGFs/FGFRs in the regulation of skeletal development, genetic skeletal diseases, and fracture healing will ultimately lead to better treatment of skeleton diseases caused by mutations of FGFs/FGFRs and fracture. This review summarizes the major findings on the role of FGF signaling in skeletal development, genetic skeletal diseases and bone healing, and discusses issues that remain to be resolved in applying FGF signaling-related measures to promote bone healing. This review has also provided a perspective view on future work for exploring the roles and action mechanisms of FGF signaling in skeletal development, genetic skeletal diseases, and fracture healing. J. Cell. Physiol. 227: 37313743, 2012. (c) 2012 Wiley Periodicals, Inc.
DOI:10.1074/jbc.M206057200URLPMID:12110689 [本文引用: 1]

Fibroblast growth factor 2 (FGF-2) is an important regulator of bone formation and osteoblast activity. However, its mechanism of action on bone cells is largely unknown. A major route for FGF signaling is through the mitogen-activated protein kinase (MAPK) pathway. We showed recently that this pathway is important for activation and phosphorylation of Cbfa1/Runx2, an osteoblast-related transcription factor (Xiao, G., Jiang, D., Thomas, P., Benson, M. D., Guan, K., Karsenty, G., and Franceschi, R. T. (2000) J. Biol. Chem. 275, 4453-4459). The present study examined the mechanism of FGF-2 regulation of the mouse osteocalcin gene in MC3T3-E1 preosteoblastic cells. FGF-2 stimulated osteocalcin mRNA and promoter activity in a dose- and time-dependent manner in MC3T3-E1 preosteoblastic cells. Similar results were obtained in mouse bone marrow stromal cells. This stimulation required Runx2 and its DNA binding site in the osteocalcin promoter. FGF-2 also dramatically increased phosphorylation of extracellular signal-regulated kinase 1 and 2 (ERK1/2) followed by phosphorylation of Runx2. Furthermore, a specific ERK1/2 phosphorylation inhibitor, U0126, completely blocked both FGF-2-stimulated Runx2 phosphorylation and osteocalcin promoter activity, indicating that this regulation requires the MAPK pathway. Deletion studies showed that the C-terminal PST domain of Runx2 is required for the FGF-2 response. This study is the first demonstration that Runx2 is phosphorylated and activated by FGF-2 via the MAPK pathway and suggests that FGF-2 plays an important role in regulation of Runx2 function and bone formation.
DOI:10.1016/j.ydbio.2006.05.031URLPMID:16815385 [本文引用: 1]

Mutations in fibroblast growth factor receptors (Fgfrs) 1-3 cause skeletal disease syndromes in humans. Although these Fgfrs are expressed at various stages of chondrocyte and osteoblast development, their function in specific skeletal cell types is poorly understood. Using conditional inactivation of Fgfr1 in osteo-chondrocyte progenitor cells and in differentiated osteoblasts, we provide evidence that FGFR1 signaling is important for different stages of osteoblast maturation. Examination of osteogenic markers showed that inactivation of FGFR1 in osteo-chondro-progenitor cells delayed osteoblast differentiation, but that inactivation of FGFR1 in differentiated osteoblasts accelerated differentiation. In vitro osteoblast cultures recapitulated the in vivo effect of FGFR1 on stage-specific osteoblast maturation. In immature osteoblasts, FGFR1 deficiency increased proliferation and delayed differentiation and matrix mineralization, whereas in differentiated osteoblasts, FGFR1 deficiency enhanced mineralization. Furthermore, FGFR1 deficiency in differentiated osteoblasts resulted in increased expression of Fgfr3, a molecule that regulates the activity of differentiated osteoblasts. Mice lacking Fgfr1, either in progenitor cells or in differentiated osteoblasts, showed increased bone mass as adults. These data demonstrate that signaling through FGFR1 in osteoblasts is necessary to maintain the balance between bone formation and remodeling through a direct effect on osteoblast maturation.
DOI:10.1111/j.1582-4934.2010.01224.xURL [本文引用: 1]

Introduction;Bone fracture healing and healing problems;Biomaterial scaffolds and tissue engineering in bone formation;Bone tissue engineering;Biomaterial scaffolds;Synthetic scaffolds;Micro- and nanostructural properties of scaffolds;Conclusion;Mesenchymal stem cells and osteogenesis;Bone tissue;Origin of osteoblasts;Isolation and characterization of bone marrow derived MSC;In vitro differentiation of MSC into osteoblast lineage cells;In vivo differentiation of MSC into bone;Factors and pathways controlling osteoblast differentiation of hMSC;Defining the relationship between osteoblast and adipocyte differentiation from MSC;MSC and sex hormones;Effect of aging on osteoblastogenesis;Conclusion;Embryonic, foetal and adult stem cells in osteogenesis;Cell-based therapies for bone;Specific features of bone cells needed to be advantageous for clinical use;Development of therapeutic biological agents;Clinical application concerns;Conclusion;Platelet-rich plasma (PRP), growth factors and osteogenesis;PRP effects in vitro on the cells involved in bone repair;PRP effects on osteoblasts;PRP effects on osteoclasts;PRP effects on endothelial cells;PRP effects in vivo on experimental animals;The clinical use of PRP for bone repair;Non-union;Distraction osteogenesis;Spinal fusion;Foot and ankle surgery;Total knee arthroplasty;Odontostomatology and maxillofacial surgery;Conclusion;Molecular control of osteogenesis;TGF-beta signalling;FGF signalling;IGF signalling;PDGF signalling;MAPK signalling pathway;Wnt signalling pathway;Hedgehog signalling;Notch signalling;Ephrin signalling;Transcription factors regulating osteoblast differentiation;Conclusion;Summary;This invited review covers research areas of central importance for orthopaedic and maxillofacial bone tissue repair, including normal fracture healing and healing problems, biomaterial scaffolds for tissue engineering, mesenchymal and foetal stem cells, effects of sex steroids on mesenchymal stem cells, use of platelet-rich plasma for tissue repair, osteogenesis and its molecular markers. A variety of cells in addition to stem cells, as well as advances in materials science to meet specific requirements for bone and soft tissue regeneration by addition of bioactive molecules, are discussed.
DOI:10.2741/2296URLPMID:17485283 [本文引用: 1]

The major event that triggers osteogenesis is the transition of mesenchymal stem cells into bone forming, differentiating osteoblast cells. Osteoblast differentiation is the primary component of bone formation, exemplified by the synthesis, deposition and mineralization of extracellular matrix. Although not well understood, osteoblast differentiation from mesenchymal stem cells is a well-orchestrated process. Recent advances in molecular and genetic studies using gene targeting in mouse enable a better understanding of the multiple factors and signaling networks that control the differentiation process at a molecular level. Osteoblast commitment and differentiation are controlled by complex activities involving signal transduction and transcriptional regulation of gene expression. We review Wnt signaling pathway and Runx2 regulation network, which are critical for osteoblast differentiation. Many other factors and signaling pathways have been implicated in regulation of osteoblast differentiation in a network manner, such as the factors Osterix, ATF4, and SATB2 and the TGF-beta, Hedgehog, FGF, ephrin, and sympathetic signaling pathways. This review summarizes the recent advances in the studies of signaling transduction pathways and transcriptional regulation of osteoblast cell lineage commitment and differentiation. The knowledge of osteoblast commitment and differentiation should be applied towards the development of new diagnostic and therapeutic alternatives for human bone diseases.
DOI:10.1016/j.reth.2016.06.001URLPMID:31245496 [本文引用: 1]

Dynamic cultivation using a radial flow bioreactor (RFB) has gained increasing interest as a method of achieving bone regeneration. In order to enhance bone generation in large bone defects, it is necessary to use an RFB to expand the primary cells such as bone marrow cells derived from biotissue. The present study aimed to evaluate the cell expansion and osteogenic differentiation of rat bone marrow cells (rBMC) when added to basic fibroblast growth factor containing medium (bFGFM) or osteogenic differentiation factor containing medium (ODM) under dynamic cultivation using an RFB. Cell proliferation was evaluated with a DNA-based cell count method and histological analysis. An alkaline phosphatase (ALP) activity assay and immunohistochemistry staining of osteogenic markers including BMP-2 and osteopontin were used to assess osteogenic differentiation ability. After culture for one week, rBMC cell numbers increased significantly under dynamic cultivation compared with that under static cultivation in all culture media. For different culture media in dynamic cultivation, bFGFM had the highest increase in cell numbers. ALP activity was facilitated by dynamic cultivation with ODM. Furthermore, both BMP-2 and osteopontin were detected in the dynamic cultivation with ODM. These results suggested that bFGFM promotes cell proliferation and ODM promotes osteogenic differentiation of rBMC under dynamic cultivation using an RFB.
DOI:10.1089/ten.TEA.2012.0763URLPMID:24410201 [本文引用: 1]

Biodegradable hydrogels with three different water contents were prepared through the glutaraldehyde crosslinking of gelatin with an isoelectric point of 5.0 under varied reaction conditions. The objective of this study is to investigate the effect of time period of basic fibroblast growth factor (bFGF) release that is modified by the hydrogel water content, on the bone regeneration. A bone fracture was prepared at the femur bone of mice, while gelatin hydrogels incorporating bFGF or bFGF solution was applied at the fracture site. The gelatin hydrogels incorporating bFGF exhibited significantly stronger bone regeneration than the bFGF solution, although the extent depended on the water content of hydrogels. Bone regeneration induced by gelatin hydrogels incorporating bFGF increased with a decrease in their water content. Histological and biochemical examinations indicated that the area of bone tissue newly formed and the level of osteogenic genes were enhanced for the hydrogels incorporating bFGF of lower water content compared with those of higher one. It is possible that the hydrogels that slowly degraded released bFGF for a longer time period, resulting in an enhanced bone regeneration. It is concluded that the time profile of bFGF release is one of the factors contributing to the bFGF-induced bone regeneration.
DOI:10.1016/j.archoralbio.2015.09.005URLPMID:26433191 [本文引用: 1]

BACKGROUND: In this study, we report on the results from the development and early in vitro testing of a gene activated matrix encoding basic human fibroblast growth factor 2 (FGF2) in bone marrow stromal cells (BMSCs). METHODS: Polyethylenimine (PEI), a cationic polymer, was utilized as a gene delivery vector and collagen scaffolds were used as the carrier to deliver the PEI-pDNA nano-sized complexes (nanoplexes) encoding the FGF2 protein. Initially, the BMSCs were transfected in vitro with the PEI-pFGF2 nanoplexes, prepared at a N/P ratio of 10, with cells alone and naked DNA as controls. This was followed by transfection experiments using collagen scaffold containing complexes, with the scaffold alone as a control. The transfection efficacy of the nanoplexes was assessed using ELISA for the determination of FGF2 protein expressed by the transfected cells. The functionality of transfection was assessed by evaluating cellular recruitment, attachment, and proliferation of BMSCs on the scaffold using imaging techniques. RESULTS: BMSCs transfected with the PEI-pFGF2 nanoplexes (either alone or within the scaffold) led to higher expression of FGF2, compared to controls. Scanning electron microscopy and confocal imaging confirmed the recruitment and attachment of BMSCs to scaffolds containing the PEI-pFGF2 nanoplexes. Confocal microscopy showed a significantly higher number of proliferating cells within PEI-pFGF2 nanoplex-loaded scaffolds than with empty scaffolds. CONCLUSIONS: This first in vitro evaluation in BMSCs provides evidence that gene activated matrices (GAMs) encoding the FGF2 protein may have strong translational potential for clinical applications that require enhanced osseous and periodontal tissue regeneration.
DOI:10.1016/j.jconrel.2017.01.008URLPMID:28069556 [本文引用: 1]

Bone fracture healing impairment related to systemic diseases such as diabetes can be addressed by growth factor augmentation. We previously reported that growth factors such as fibroblast growth factor-2 (FGF-2) and bone morphogenetic protein-2 (BMP-2) work synergistically to encourage osteogenesis in vitro. In this report, we investigated if BMP-2 and FGF-2 together can synergistically promote bone repair in a leporine model of diabetes mellitus, a condition that is known to be detrimental to union. We utilized two kinds of plasmid DNA encoding either BMP-2 or FGF-2 formulated into polyethylenimine (PEI) complexes. The fabricated nanoplexes were assessed for their size, charge, in vitro cytotoxicity, and capacity to transfect human bone marrow stromal cells (BMSCs). Using diaphyseal long bone radial defects in a diabetic rabbit model it was demonstrated that co-delivery of PEI-(pBMP-2+pFGF-2) embedded in collagen scaffolds resulted in a significant improvement in bone regeneration compared to PEI-pBMP-2 embedded in collagen scaffolds alone. This study demonstrated that scaffolds loaded with PEI-(pBMP-2+pFGF-2) could be an effective way of promoting bone regeneration in patients with diabetes.
DOI:10.1016/j.exger.2015.02.006URLPMID:25681640 [本文引用: 1]

There is an age-associated reduction in the bone healing activity of bone morphogenetic protein-2 (BMP-2) that is currently addressed by administering higher doses of BMP-2 in elderly patients. The unwanted medical complications from high dose BMP-2 motivated this investigation to determine whether the addition of a low dose of fibroblast growth factor 2 (FGF-2) could enhance the ability of a lower dose of BMP-2 to heal calvarial bone defects in old mice (18-20 months old). FGF-2 (5 ng) and BMP-2 (2 mug) were administered by a controlled release two-phase biomaterial scaffold placed into the bone defect. FGF-2 released more rapidly and completely in vitro than BMP-2 (40% vs 2%). In vivo, both BMP-2 and FGF-2+BMP-2 groups formed more new bone in calvarial defects than scaffold alone (p < 0.001) or FGF-2 only groups (p < 0.01). The overall total volume of new bone was not statistically increased by the addition of FGF-2 to BMP-2 as measured by microCT, but the pattern of bone deposition was different. In old mice, but not young, there was enhanced bony fill in the central bone defect area when the BMP-2 was supplemented with FGF-2. Histological analysis of the center of the defect revealed an increased bone volume (%BV/TV (p = 0.004)) from the addition of FGF-2. These studies suggest that combining a low dose of FGF-2 with a low dose of BMP-2 has the potential to increase bone healing in old mice relative to BMP-2 alone.
[本文引用: 1]
DOI:10.1055/s-0042-115405URL [本文引用: 1]
DOI:10.1038/nm1716URLPMID:18297083 [本文引用: 1]

Postnatal bone marrow houses mesenchymal progenitor cells that are osteoblast precursors. These cells have established therapeutic potential, but they are difficult to maintain and expand in vitro, presumably because little is known about the mechanisms controlling their fate decisions. To investigate the potential role of Notch signaling in osteoblastogenesis, we used conditional alleles to genetically remove components of the Notch signaling system during skeletal development. We found that disruption of Notch signaling in the limb skeletogenic mesenchyme markedly increased trabecular bone mass in adolescent mice. Notably, mesenchymal progenitors were undetectable in the bone marrow of mice with high bone mass. As a result, these mice developed severe osteopenia as they aged. Moreover, Notch signaling seemed to inhibit osteoblast differentiation through Hes or Hey proteins, which diminished Runx2 transcriptional activity via physical interaction. These results support a model wherein Notch signaling in bone marrow normally acts to maintain a pool of mesenchymal progenitors by suppressing osteoblast differentiation. Thus, mesenchymal progenitors may be expanded in vitro by activating the Notch pathway, whereas bone formation in vivo may be enhanced by transiently suppressing this pathway.
DOI:10.1371/journal.pgen.1002577URLPMID:22457635 [本文引用: 1]

Notch signaling between neighboring cells controls many cell fate decisions in metazoans both during embryogenesis and in postnatal life. Previously, we uncovered a critical role for physiological Notch signaling in suppressing osteoblast differentiation in vivo. However, the contribution of individual Notch receptors and the downstream signaling mechanism have not been elucidated. Here we report that removal of Notch2, but not Notch1, from the embryonic limb mesenchyme markedly increased trabecular bone mass in adolescent mice. Deletion of the transcription factor RBPjk, a mediator of all canonical Notch signaling, in the mesenchymal progenitors but not the more mature osteoblast-lineage cells, caused a dramatic high-bone-mass phenotype characterized by increased osteoblast numbers, diminished bone marrow mesenchymal progenitor pool, and rapid age-dependent bone loss. Moreover, mice deficient in Hey1 and HeyL, two target genes of Notch-RBPjk signaling, exhibited high bone mass. Interestingly, Hey1 bound to and suppressed the NFATc1 promoter, and RBPjk deletion increased NFATc1 expression in bone. Finally, pharmacological inhibition of NFAT alleviated the high-bone-mass phenotype caused by RBPjk deletion. Thus, Notch-RBPjk signaling functions in part through Hey1-mediated inhibition of NFATc1 to suppress osteoblastogenesis, contributing to bone homeostasis in vivo.
DOI:10.1155/2019/8150123URLPMID:31281386 [本文引用: 1]

Estrogen is very important to the differentiation of B lymphocytes; B lymphopoiesis induced by OVX was supposedly involved in osteoporosis. But the effects of B lymphocytes on the osteogenic differentiation of bone mesenchymal stem cells (BMSCs) are not clear. In this study, we detected bone quality and bone loss in a trabecular bone by electronic universal material testing machine and microcomputed tomography (micro-CT) in OVX and splenectomized-ovariectomy (SPX-OVX) rats. Additionally, changes in lymphocytes (B lymphocyte, CD4(+) and CD8(+) T lymphocytes, and macrophages) in the bone marrow were analyzed by flow cytometry. The osteogenesis of BMSCs cocultured with normal and LPS-pretreated B lymphocytes was detected by BCIP/NBT and Alizarin red S staining. Measurement of the Notch2, Notch4, Hey1, Hey2, Hes1, and runt-related transcription factor 2 (Runx2) expression in BMSCs cocultured with B lymphocytes was done using real-time PCR. The effects of dexamethasone and DAPT (inhibitor of Notch signaling) on osteogenesis of BMSCs were detected by BCIP/NBT, Alizarin red S staining, and real-time PCR. Osteoporosis happened in OVX rats, more serious in SPX-OVX rats, B lymphocytes increased in OVX rats, and sharply higher in SPX-OVX rats. Osteoporosis did not happen in SPX rats which is still companied with a high increase of B lymphocytes. LPS-pretreated B lymphocytes suppressed the osteogenesis of BMSCs, but the normal B lymphocytes could not. The LPS-pretreated B lymphocytes upregulated the expression of Notch4, Hes1, and Hey2 and downregulated the expression of Runx2 in BMSCs. Dexamethasone and DAPT could downregulate the high expression of Notch4, Hes1, Hey2 and upregulate the low expression of Runx2 in BMSCs which cocultured with LPS treated B lymphocytes, the inhibited ALP and Alizarin red staining in BMSCs which cocultured with LPS treated B lymphocytes also partly restored.
DOI:10.3892/etm.2019.7765URLPMID:31410150 [本文引用: 1]

Low differentiation and high proliferation rates are critical factors affecting bone marrow mesenchymal stem cell (BMSC) tumorigenesis. The present study aimed to investigate the role of the Notch signaling pathway in BMSC proliferation and osteogenic differentiation. Mouse BMSCs were divided into control, vector, Notch1-small interfering (si)RNA, gamma-secretase inhibitor, and Notch1-siRNA + gamma-secretase inhibitor groups. The siRNA-Notch1, gamma-secretase inhibitor, and Notch1-siRNA + gamma-secretase inhibitor groups were treated with Notch1 siRNA and/or gamma-secretase inhibitor. Following treatment, cell proliferation was evaluated using a Cell Counting Kit-8. Tumor-related factors, including transforming growth factor (TGF)-beta1, c-Myc and p53, were detected by reverse transcription-quantitative polymerase chain reaction and western blot analyses. BMSC osteogenic differentiation was induced and the cells were stained with alizarin red at 14 and 21 days. Alkaline phosphatase (AKP) activity was also evaluated. The siRNA-Notch1 and gamma-secretase inhibitor both reduced BMSC proliferation and the expression of TGF-beta1 and c-Myc and increased the expression of p53. Following the induction of osteogenesis and staining with alizarin red, the level of AKP was significantly higher in cells in the siRNA-Notch1 and gamma-secretase inhibitor groups compared with that in the control group. It was found that Notch1 inhibition reduced proliferation and promoted the osteogenic differentiation of BMSCs.
URLPMID:18710934 [本文引用: 1]
DOI:10.1083/jcb.201605119URLPMID:28696225 [本文引用: 1]

Autosomal-recessive omodysplasia (OMOD1) is a genetic condition characterized by short stature, shortened limbs, and facial dysmorphism. OMOD1 is caused by loss-of-function mutations of glypican 6 (GPC6). In this study, we show that GPC6-null embryos display most of the abnormalities found in OMOD1 patients and that Hedgehog (Hh) signaling is significantly reduced in the long bones of these embryos. The Hh-stimulatory activity of GPC6 was also observed in cultured cells, where this GPC increased the binding of Hh to Patched 1 (Ptc1). Consistent with this, GPC6 interacts with Hh through its core protein and with Ptc1 through its glycosaminoglycan chains. Hh signaling is triggered at the primary cilium. In the absence of Hh, we observed that GPC6 is localized outside of the cilium but moves into the cilium upon the addition of Hh. We conclude that GPC6 stimulates Hh signaling by binding to Hh and Ptc1 at the cilium and increasing the interaction of the receptor and ligand.
DOI:10.1101/gad.13.16.2072URLPMID:10465785 [本文引用: 1]

The mechanisms that control cell proliferation and cell differentiation during morphogenesis of the endochondral skeleton of vertebrates are poorly understood. Indian hedgehog (Ihh) signaling from prehypertrophic chondrocytes has been implicated in the control of chondrocyte maturation by way of feedback control of a second secreted factor parathyroid hormone-related peptide (PTHrP) at the articular surfaces. Analysis of an Ihh null mutant suggests a more extensive role for Ihh in skeletal development. Mutants display markedly reduced chondrocyte proliferation, maturation of chondrocytes at inappropriate position, and a failure of osteoblast development in endochondral bones. Together, the results suggest a model in which Ihh coordinates diverse aspects of skeletal morphogenesis through PTHrP-dependent and independent processes.
DOI:10.1126/science.273.5275.613URL [本文引用: 1]
URLPMID:24826487 [本文引用: 1]

Hedgehog signaling pathway is a conserved and important signaling pathway involved in proliferation and differentiation of many types of cells. Latest studies have found that Hedgehog signaling pathway may induce MSCs osteoblast differentiation by increasing the expression of the Runx2 and Osx and inhibit MSCs differentiate to adipocyte. Hedgehog signaling pathway may also promote osteoblast proliferation by regulating cyclin. This review summarizes the mechanism that Hedgehog signaling pathway regulates osteoblast differentiation and proliferation,and concludes that Hedgehog signaling pathway can regulate bone metabolism. It might provide new ideas for the treatment of osteoporosis.
DOI:10.1096/fj.201801741RURLPMID:30657335 [本文引用: 1]

Glucocorticoids (GCs) are frequently used to treat chronic disorders in children, including inflammation and cancer. Prolonged treatment with GCs is well known to impair bone growth, an effect linked to increased apoptosis and suppressed proliferation in growth plate chondrocytes. We hypothesized that the endogenous antiapoptotic protein humanin (HN) may prevent these effects. Interestingly, GC-induced bone growth impairment and chondrocyte apoptosis was prevented in HN overexpressing mice, HN-treated wild-type mice, and in HN-treated cultured rat metatarsal bones. GC-induced suppression of chondrocyte proliferation was also prevented by HN. Furthermore, GC treatment reduced Indian Hedgehog expression in growth plates of wild-type mice but not in HN overexpressing mice or HN-treated wild-type animals. A Hedgehog (Hh) antagonist, vismodegib, was found to suppress the growth of cultured rat metatarsal bones, and this effect was also prevented by HN. Importantly, HN did not interfere with the desired anti-inflammatory effects of GCs. We conclude that HN is a novel regulator of Hh signaling preventing GC-induced bone growth impairment without interfering with desired effects of GCs. Our data may open for clinical studies exploring a new possible strategy to prevent GC-induced bone growth impairment by cotreating with HN.-Zaman, F., Zhao, Y., Celvin, B., Mehta, H. H., Wan, J., Chrysis, D., Ohlsson, C., Fadeel, B., Cohen, P., Savendahl, L. Humanin is a novel regulator of Hedgehog signaling and prevents glucocorticoid-induced bone growth impairment.
DOI:10.3109/07853890.2014.912836URL [本文引用: 1]

Despite development of novel agents targeting oncogenic pathways, matching targeted therapies to the genetic status of individual tumors is proving to be a daunting task for clinicians. To improve the clinical efficacy and to reduce the toxic side effects of treatments, a deep characterization of genetic alterations in different tumors is required. The mutational profile often evidences a gain of function or hyperactivity of phosphoinositide 3-kinases (PI3Ks) in tumors. These enzymes are activated downstream tyrosine kinase receptors (RTKs) and/or G proteins coupled receptors (GPCRs) and, via AKT, are able to induce mammalian target of rapamycin (mTOR) stimulation. Here, we elucidate the impact of class I (p110 alpha, beta, gamma, and delta) catalytic subunit mutations on AKT-mediated cellular processes that control crucial mechanisms in tumor development. Moreover, the interrelation of PI3K signaling with mTOR, ERK, and RAS pathways will be discussed, exploiting the potential benefits of PI3K signaling inhibitors in clinical use.
DOI:10.1002/jcp.20988URLPMID:17226753 [本文引用: 1]

To investigate the molecular mechanism underlying the differentiation of osteoblasts and chondroblasts, we established a clonal cell lines, RD-C6, from Runx2-deficient mouse embryos. RD-C6 cells expressed almost undetectable levels of phenotypes related to osteoblast and chondroblast differentiation at basal culture condition, whereas treatment with recombinant human bone morphogenetic protein-2 (rhBMP-2) or transduction of BMP-2 by adenovirus effectively induced this cell line to express mRNA related to the differentiation of osteoblasts and chondroblasts including alkaline phosphatase, osteocalcin, and osterix. Transduction of Runx2 also induced the expression of these mRNA in RD-C6 cells. BMP-2 transduction increased expression levels of mRNA for Msx2 and Dlx5, but Runx2 transduction induced no significant increases in expression levels of these mRNA. Microarray analysis using RD-C6 cells with or without rhBMP-2 treatment demonstrated that BMP-2 upregulated 66 genes including 13 transcription-related molecules such as Id1, Id2, Id4, Hey1, Smad6, Smad7, and Msx2. To confirm bone and cartilage formation ability of RD-C6 cells, we transplanted RD-C6 cells into the peritoneal cavity of athymic mice using diffusion chambers with rhBMP-2. RD-C6 cells generated unmineralized cartilage but not bone. These results indicate that BMP-2 induces Runx2-deficient cells to express markers related to osteoblast and chondroblast differentiation using a Runx2-independent pathway, but it failed to induce these cells to differentiate into bone-forming osteoblasts and mature chondrocytes.
DOI:10.1038/srep35233URLPMID:27756897 [本文引用: 1]

Asperosaponin VI (ASA VI), a natural compound isolated from the well-known traditional Chinese herb Radix Dipsaci, has an important role in promoting osteoblast formation. However, its effects on osteoblasts in the context of osteoporosis is unknown. This study aimed to investigate the effects and mechanism of ASA VI action on the proliferation and osteogenic differentiation of bone marrow stromal cells isolated from the ovariectomized rats (OVX rBMSCs). The toxicity of ASA VI and its effects on the proliferation of OVX rBMSCs were measured using a CCK-8 assay. Various osteogenic differentiation markers were also analyzed, such as ALP activity, calcified nodule formation, and the expression of osteogenic genes, i.e., ALP, OCN, COL 1 and RUNX2. The results indicated that ASA VI promoted the proliferation of OVX rBMSCs and enhanced ALP activity and calcified nodule formation. In addition, while ASA VI enhanced the expression of ALP, OCN, Col 1 and RUNX2, treatment with LY294002 reduced all of these osteogenic effects and reduced the p-AKT levels induced by ASA VI. These results suggest that ASA VI promotes the osteogenic differentiation of OVX rBMSCs by acting on the phosphatidylinositol-3 kinase/AKT signaling pathway.
DOI:10.1016/j.biomaterials.2012.01.020URL [本文引用: 1]

The response of osteoprogenitors to calcium (Ca2+) is of primary interest for both normal bone homeostasis and the clinical field of bone regeneration. The latter makes use of calcium phosphate-based bone void fillers to heal bone defects, but it is currently not known how Ca2+ released from these ceramic materials influences cells in situ. Here, we have created an in vitro environment with high extracellular Ca2+ concentration and investigated the response of human bone marrow-derived mesenchymal stromal cells (hMSCs) to it. Ca2+ enhanced proliferation and morphological changes in hMSCs. Moreover, the expression of osteogenic genes is highly increased. A 3-fold up-regulation of BMP-2 is observed after only 6 h and pharmaceutical interference with a number of proteins involved in Ca2+ sensing showed that not the calcium sensing receptor, but rather type L voltage-gated calcium channels are involved in mediating the signaling pathway between extracellular Ca2+ and BMP-2 expression. MEK1/2 activity is essential for the effect of Ca2+ and using microarray analysis, we have identified c-Fos as an early Ca2+ response gene. We have demonstrated that hMSC osteogenesis can be induced via extracellular Ca2+, a simple and economic way of priming hMSCs for bone tissue engineering applications. (c) 2012 Elsevier Ltd.
DOI:10.1016/j.bbrc.2006.07.214URLPMID:16930556 [本文引用: 1]

Several in vivo and in vitro studies with different loading regimens showed that mechanical stimuli have an influence on proliferation and differentiation of bone cells. Prerequisite for this influence is the transduction of mechanical signals into the cell, a phenomenon that is termed mechanotransduction, which is essential for the maintenance of skeletal homeostasis in adults. Mechanoreceptors, such as the integrins, cadherins, and stretch-activated Ca2+ channels, together with various signal transduction pathways, are involved in the mechanotransduction process that ultimately regulates gene expression in the nucleus. Mechanotransduction itself is considered to be regulated by hormones, the extracellular matrix of the osteoblastic cells and the mode of the mechanical stimulus.
DOI:10.1371/journal.pgen.1002294URLPMID:21966280 [本文引用: 1]

Patients with neonatal severe hyperparathyroidism (NSHPT) are homozygous for the calcium-sensing receptor (CaR) mutation and have very high circulating PTH, abundant parathyroid hyperplasia, and severe life-threatening hypercalcemia. Mice with homozygous deletion of CaR mimic the syndrome of NSHPT. To determine effects of CaR deficiency on skeletal development and interactions between CaR and 1,25(OH)(2)D(3) or PTH on calcium and skeletal homeostasis, we compared the skeletal phenotypes of homozygous CaR-deficient (CaR(-/-)) mice to those of double homozygous CaR- and 1alpha(OH)ase-deficient [CaR(-/-)1alpha(OH)ase(-/-)] mice or those of double homozygous CaR- and PTH-deficient [CaR(-/-)PTH(-/-)] mice at 2 weeks of age. Compared to wild-type littermates, CaR(-/-) mice had hypercalcemia, hypophosphatemia, hyperparathyroidism, and severe skeletal growth retardation. Chondrocyte proliferation and PTHrP expression in growth plates were reduced significantly, whereas trabecular volume, osteoblast number, osteocalcin-positive areas, expression of the ALP, type I collagen, osteocalcin genes, and serum ALP levels were increased significantly. Deletion of 1alpha(OH)ase in CaR(-/-) mice resulted in a longer lifespan, normocalcemia, lower serum phosphorus, greater elevation in PTH, slight improvement in skeletal growth with increased chondrocyte proliferation and PTHrP expression, and further increases in indices of osteoblastic bone formation. Deletion of PTH in CaR(-/-) mice resulted in rescue of early lethality, normocalcemia, increased serum phosphorus, undetectable serum PTH, normalization in skeletal growth with normal chondrocyte proliferation and enhanced PTHrP expression, and dramatic decreases in indices of osteoblastic bone formation. Our results indicate that reductions in hypercalcemia play a critical role in preventing the early lethality of CaR(-/-) mice and that defects in endochondral bone formation in CaR(-/-) mice result from effects of the marked elevation in serum calcium concentration and the decreases in serum phosphorus concentration and skeletal PTHrP levels, whereas the increased osteoblastic bone formation results from direct effects of PTH.
DOI:10.1126/scisignal.135pe40URLPMID:18765829 [本文引用: 1]

Experiments performed in mice in which expression of the extracellular calcium-sensing receptor (CaSR) was completely nullified specifically in parathyroid cells, chondrocytes, or cells of the osteoblast lineage have identified phenotypes that indicate a key role for the CaSR in embryonic development of the skeleton, postnatal bone formation, and osteoblast differentiation that are independent of the calcitropic hormone axis. These long-awaited studies further clarify the signaling relationships between the parathyroid gland, kidney, and metabolic bone disease in patients with mutations in the gene encoding the CaSR, and they provide new insights into understanding the signaling pathways involving the CaSR in skeletal cells.
DOI:10.1177/0963689719871000URLPMID:31426665 [本文引用: 1]

Alpha-calcitonin gene-related peptide (alphaCGRP) plays a significant pathophysiological role in the regulation of bone metabolism. Our previous research indicated that alphaCGRP might have a potential application in enhancing osseointegration in vivo. To further uncover the intrinsic mechanism of its networks in bone regeneration, here we investigate the impact of alphaCGRP on osteogenic differentiation in bone marrow-derived mesenchymal stem cells (BMSCs) from both wild-type and alphaCGRP(-/-) mice. Considering the half-life of alphaCGRP in plasma is only 10 min, we applied alphaCGRP lentivirus and stably transfected it into BMSCs, followed by transfection identification and cell cycle assay. We further conducted a series of in vitro tests, and the results revealed that biological functions including migratory ability and osteogenicity exhibited positive correlation with BMSCs' alphaCGRP expression. Meanwhile, this phenomenon was associated with an enhanced expression of YAP (Yes-associated protein), the key downstream effector of the Hippo pathway. To sum up, our data together with previous in vivo observations is likely to elucidate the intrinsic mechanism of alphaCGRP in bone remodeling, and alphaCGRP would appear to be a novel treatment to promote bone wound healing.
DOI:10.1038/srep30322URLPMID:27444891 [本文引用: 1]

Microgravity induces observed bone loss in space flight, and reduced osteogenesis of bone mesenchymal stem cells (BMSCs) partly contributes to this phenomenon. Abnormal regulation or functioning of the actin cytoskeleton induced by microgravity may cause the inhibited osteogenesis of BMSCs, but the underlying mechanism remains obscure. In this study, we demonstrated that actin cytoskeletal changes regulate nuclear aggregation of the transcriptional coactivator with PDZ-binding motif (TAZ), which is indispensable for osteogenesis of bone mesenchymal stem cells (BMSCs). Moreover, we utilized a clinostat to model simulated microgravity (SMG) and demonstrated that SMG obviously depolymerized F-actin and hindered TAZ nuclear translocation. Interestingly, stabilizing the actin cytoskeleton induced by Jasplakinolide (Jasp) significantly rescued TAZ nuclear translocation and recovered the osteogenic differentiation of BMSCs in SMG, independently of large tumor suppressor 1(LATS1, an upstream kinase of TAZ). Furthermore, lysophosphatidic acid (LPA) also significantly recovered the osteogenic differentiation of BMSCs in SMG through the F-actin-TAZ pathway. Taken together, we propose that the depolymerized actin cytoskeleton inhibits osteogenic differentiation of BMSCs through impeding nuclear aggregation of TAZ, which provides a novel connection between F-actin cytoskeleton and osteogenesis of BMSCs and has important implications in bone loss caused by microgravity.
