 ,内蒙古民族大学动物科学技术学院,通辽 028043
,内蒙古民族大学动物科学技术学院,通辽 028043The role of EGF-like factor signaling pathway in granulosa cells in regulation of oocyte maturation and development
Xinyu Yang, Zhenwei Jia ,College of Animal Science and Technology, Inner Mongolia University for the Nationalities, Tongliao 028043, China
,College of Animal Science and Technology, Inner Mongolia University for the Nationalities, Tongliao 028043, China通讯作者:
编委: 史庆华
收稿日期:2018-10-7修回日期:2019-01-10网络出版日期:2019-02-25
| 基金资助: |
Editorial board:
Received:2018-10-7Revised:2019-01-10Online:2019-02-25
| Fund supported: |
作者简介 About authors
杨鑫宇,在读硕士,专业方向:配子与胚胎生物技术E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (405KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
杨鑫宇, 贾振伟. 颗粒细胞EGF类因子信号通路在调控卵母细胞成熟和发育中的作用[J]. 遗传, 2019, 41(2): 137-145 doi:10.16288/j.yczz.18-193
Xinyu Yang, Zhenwei Jia.
哺乳动物体内卵母细胞生长、成熟和排卵过程十分复杂,涉及多种激素和生长因子以内分泌或旁分泌的方式作用于卵泡颗粒细胞参与此过程的调控。动物卵泡颗粒细胞包括壁层颗粒细胞和卵丘颗粒细胞,普遍认为,排卵前促黄体素(luteinizing hormone, LH)作用于卵泡颗粒细胞后激活下游信号通路诱导了卵母细胞生长后期细胞质和细胞核成熟、排卵以及排卵后黄体细胞的形成。目前研究认为,卵母细胞不表达LH受体,卵丘颗粒细胞在卵母细胞分泌因子的抑制下也不表达LH受体,但卵泡壁层颗粒细胞表达LH受体,这说明LH作用于卵泡壁层颗粒细胞后将可能激活一些信号分子,将LH的生物作用传递至卵母细胞[1,2]。值得注意的是,目前研究已明确LH作用于卵泡壁层颗粒细胞后将产生表皮生长因子(epidermal growth factor, EGF)类因子,这些细胞因子介导了LH的作用,调控卵母细胞的成熟和发育[3]。
家畜卵母细胞体外成熟培养是一项重要的繁殖生物技术,在畜牧业生产和科学研究上广泛应用,是体外受精、性别控制、转基因动物生产及动物克隆等技术开展的前提和关键。由于体外成熟的卵丘颗粒细胞不表达LH受体,但表达促卵泡生成素(follicle-stimulating hormone, FSH)受体,因此,卵母细胞体外成熟期间通过添加FSH而激活卵丘颗粒细胞内调控卵母细胞恢复减数分裂的信号通路。但目前普遍认为,相对于体内成熟的卵母细胞,体外成熟的卵母细胞发育能力较差,影响了其利用效率。研究发现,卵母细胞在卵泡内生长后期,LH将诱导EGF类因子的产生,并激活EGF类因子信号系统,促进卵母细胞成熟[4]。由于体外成熟的卵母细胞脱离了卵泡环境,而且这些卵母细胞来源于生长卵泡,
提示体外成熟的卵母细胞,卵丘颗粒细胞的EGF类生长因子信号系统可能不完善,不利于卵母细胞成熟,这可能是导致体外成熟的卵母细胞发育能力较差的一个重要因素。因此,本文综述了颗粒细胞EGF类因子信号系统、EGF类因子在调控卵母细胞成熟中的作用及对卵母细胞发育能力的影响,为优化卵母细胞体外成熟培养体系,完善卵丘颗粒细胞的EGF类因子的信号系统,提高卵母细胞体外成熟效率提供理论参考。
1 颗粒细胞EGF类因子信号系统
1.1 EGF类因子及其受体
哺乳动物表皮生长因子(epidermal growth factor, EGF)类因子,与EGF分子结构和生物学功能相似,因此被称为EGF类蛋白家族。目前研究认为,动物卵巢颗粒细胞表达的EGF类因子主要包括:特指双调蛋白(amphiregulin, AREG)、β细胞素(betacellulin, BTC)和表皮调节素(epiregulin, EREG)[5]。这些EGF类因子来源于非活性的跨膜前体糖蛋白,包括信号序列、跨膜结构域和胞外EGF结构域。EGF类因子的前体蛋白在细胞膜外被蛋白酶水解而释放功能性的肽,通过与受体结合而发挥生物学功能。EGF类因子的受体与EGF受体(epidermal growth factor- receptor, EGFR)相同,是一种分子量为170 kDa的细胞膜糖蛋白,属于酪氨酸激酶受体,由细胞外的配体结合区,疏水跨膜区和细胞内的酪氨酸激酶信号活化区3部分组成。目前研究表明,体内卵泡发育和卵母细胞成熟期间,排卵前高水平的LH促进了壁层颗粒细胞表达EGF类因子,且通过旁分泌的方式作用于卵丘颗粒细胞EGFR,使其表达EGF类因子。FSH促进了壁层和卵丘颗粒细胞表达EGFR,而卵母细胞分泌因子促进了卵丘颗粒细胞表达EGFR[4,6,7](图1)。而且,研究认为,颗粒细胞EGF类因子与EGFR胞外区结合后,使其细胞内的部分发生特定酪氨酸残基磷酸化,从而激活细胞内ERK1/2、PI3K以及JAK/STAT等下游信号通路,其中EGF类因子与EGFR结合后通过RAS/cRAF/MEK1级联反应而激活ERK1/2,激活的ERK1/2与CEBPB、c-myc和AP-1等转录因子结合而促进基因表达,因此,ERK1/2被认为是调控卵母细胞减数分裂的恢复、卵丘颗粒细胞的扩散和排卵的关键信号分子[8,9,10]。
1.2 颗粒细胞EGF类因子信号系统功能的完善
近年来,许多****认为卵巢小有腔卵泡来源的卵丘卵母细胞复合体不能对EGF类生长因子发生反应,随着卵泡发育,卵丘颗粒细胞逐渐获得功能性EGF信号网络,这与EGF类生长因子是介导卵母细胞成熟和排卵的中心调控因子的观点一致[11,12]。优势卵泡为了排卵,排卵前卵丘颗粒细胞能够对EGF类生长因子发生反应,而次要卵泡缺乏这种能力。为了了解生长阶段卵泡的颗粒细胞对EGF类生长因子反应能力较低的原因,许多****开展了相关研究。其中一种观点认为,相对于大卵泡,小卵泡的颗粒细胞EGF类生长因子受体基因mRMA表达量较低,导致其对EGF类生长因子反应能力较低[13,14,15]。另外一种观点认为,小卵泡的颗粒细胞EGF类生长因子受体基因mRMA表达量与大卵泡的颗粒细胞一致,但其翻译能力及翻译后蛋白磷酸化水平较低,导致这些小有腔卵泡对EGF类生长因子反应和颗粒细胞扩散能力较低[12]。这些结果说明,体内随着卵泡的生长,卵丘颗粒细胞EGF类因子信号系统功能逐渐完善,并获得支持卵母细胞成熟和发育的能力。同时提示,小的有腔卵泡颗粒细胞EGF类因子信号系统功能不健全,这可能是小卵泡来源卵丘卵母细胞复合体(cumulus-oocyte complex, COCs)体外培养后,导致卵母细胞发育能力较低的一个重要因素。2 EGF类因子在调控卵母细胞成熟的作用
2.1 EGF类因子介导了LH信号
EGF主要由卵泡内膜细胞和颗粒细胞产生。研究表明,体外卵母细胞成熟期间,EGF促进多种哺乳动物卵丘颗粒细胞扩散和卵母细胞成熟[16,17,18]。研究认为,EGF通过卵丘颗粒细胞调控卵母细胞成熟,因为成熟有腔卵泡来源的卵母细胞表达EGF受体数量很少,而卵丘颗粒细胞拥有大量的EGF受体[19,20]。而且,已有的研究证明,LH促进了排卵前卵泡颗粒细胞EGF的表达,这说明EGF通过介导LH信号,并作用于颗粒细胞而调控卵母细胞成熟和发育[18]。然而,Inoue等[21]研究LH调控排卵前卵泡发育机制时发现,LH峰值后EGF表达以及在卵泡液积累的量没有发生显著变化,暗示EGF可能不是介导LH作用的关键因子。特别重要的是,在多种哺乳动物上的研究发现,排卵前EGF家族成员的EGF类生长因子(AREG, EREG和BTC)不表达,而LH峰启动后,这些EGF类生长因子表达量迅速增加[21,22,23]。研究已证明,在LH刺激下,EGF类生长因子首先在卵泡壁层颗粒细胞上表达,然后分别以自分泌或旁分泌的方式作用于壁层颗粒细胞和卵丘颗粒细胞EGFR,增强了前列腺素-过氧化物酶合成酶2 (prostaglandin- peroxidase synthase 2, PTGS2)表达,且促进了卵丘颗粒细胞表达EGF类生长因子。壁颗粒细胞和卵丘颗粒细胞PTGS2表达增加后,促进了前列腺素E2 (prostaglandin E2, PGE2)的合成,PGE2作用于卵丘颗粒细胞上的受体,激活p38MAPK而进一步促进EGF类生长因子表达,这些EGF类生长因子与卵丘颗粒细胞EGF受体结合后,激活了ERK1/2、PI3K等信号通路,促进了调控卵母细胞恢复减数分裂和卵丘颗粒细胞扩散基因的表达,进而放大LH信号,使LH刺激信号从卵泡颗粒细胞外围向内传递至卵母细胞[24,25]。综上所述,EGF类生长因子介导了排卵前LH信号,由于EGF类生长因子受体和EGF受体相同,因此EGF体外可能与其受体结合而模拟了EGF类生长因子的功能,而体内EGF可能不是LH信号的主要介导者。
图1
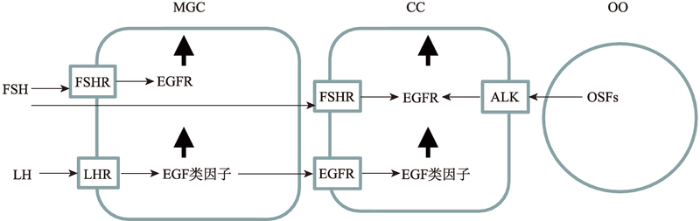 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1EGF类因子及其受体在壁层和卵丘颗粒细胞上的表达
MGC:壁层颗粒细胞;CC:卵丘颗粒细胞;OO:卵母细胞;FSH:促卵泡素;FSHR:促卵泡素受体;LH:促黄体激素;LHR:促黄体激素受体;EGFR:表皮生长因子受体;OSFs:卵母细胞分泌因子;ALK:激活素受体样激酶;↑:表达增加。
Fig. 1The expression of EGF like factors and their receptor in C-type natriuretic peptide and natriuretic peptide receptor 2 expression in mural granulosa cells and cumulus cells
2.2 EGF类因子调控卵母细胞减数分裂
体内有腔卵泡生长期间,颗粒细胞为卵母细胞提供cAMP/cGMP而使其减数分裂阻滞在生发泡阶段。目前,研究已明确,C型尿钠肽(C-type natriuretic peptide, CNP)激活卵丘颗粒细胞上的受体(NPR2)产生cGMP,进入卵母细胞通过抑制磷酸二酯酶(PDE3A)的活性,降低cAMP的水解,高水平的cAMP激活蛋白激酶A而抑制MPF的活性,进而将抑制减数分裂[26]。体内LH峰刺激后,降低了颗粒细胞cGMP的产生,使cGMP进入卵母细胞的量减少,激活了PDE3A,使cAMP水平下降,卵母细胞恢复减数分裂。目前研究表明,LH峰刺激后,AREG和EREG等EGF类因子表达量增加,激活其受体后,抑制CNP表达量,导致cGMP生产水平下降,同时增强间隙连接蛋白磷酸化,进而关闭间隙连接,最终导致cGMP进入卵母细胞的量减少,解除其对卵母细胞减数分裂的阻滞[27](图2)。图2
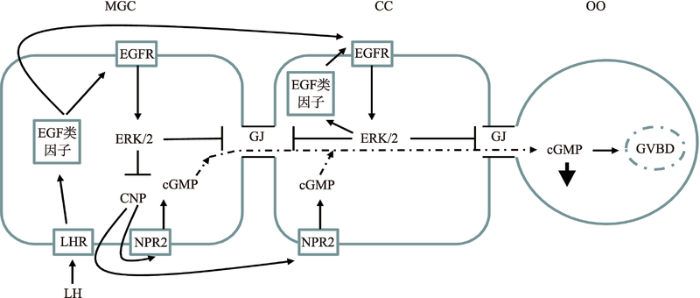 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2EGF类因子对卵母细胞细胞减数分裂恢复的调控作用
MGC:壁层颗粒细胞;CC:卵丘颗粒细胞;OO:卵母细胞;LH:促黄体激素;LHR:促黄体激素受体;EGFR:表皮生长因子受体;CNP:C型钠肽;NPR2:钠肽2型受体;ERK1/2:细胞外调节蛋白激酶1/2;↓:cGMP水平下降;GJ:细胞间隙连接。GVBD:生发泡破裂。
Fig. 2The regulatory roles of EGF like factors in oocyte meiotic resumption
此外,体外研究表明EGF信号网络的激活也参与了哺乳动物卵母细胞成熟的调控。例如,体外cAMP调控剂维持了卵母细胞减数分裂阻滞,而添加EGF诱导了其恢复减数分裂[28]。研究发现,FSH体外由EGF类生长因子介导,诱导了卵母细胞恢复减数分裂和卵丘颗粒细胞扩散[29]。cAMP是FSH信号通路的下游信号分子,高水平的cAMP也能够通过促进EGF类生长因子表达而诱导卵母细胞恢复减数分裂。另外,外源的AREG、EREG和EGF也能诱导多种哺乳动物卵母细胞恢复减数分裂和卵丘颗粒细胞扩散。特别注意的是,在小鼠(Mus musculus)上的研究发现,利用含有AREG的卵泡液培养卵母细胞诱导了卵母细胞成熟和卵丘颗粒细胞扩散,而免疫耗竭AREG后其作用消失[30]。在小鼠中,COC体外培养期间,使用EGF类生长因子受体抑制剂,阻滞了AREG诱导的卵母细胞成熟,但培养前去除卵丘颗粒细胞后,并没有干扰卵母细胞成熟,揭示EGF类生长因子不是直接作用卵母细胞,而是通过作用卵丘颗粒细胞而影响卵母细胞成熟[31]。综上所述,排卵时EGF信号网络降低了卵丘颗粒细胞的cGMP进入卵母细胞,同时体内LH或体外FSH激活EGF信号网络,导致卵丘颗粒细胞发生一系列的生理变化而诱导卵母细胞成熟。
2.3 EGF类因子对卵母细胞代谢的调控
COC代谢是影响卵母细胞成熟和发育的一个重要因素。研究表明,相对于FSH,EGF类生长因子增强了牛(Bos taurus)卵丘颗粒细胞的糖酵解[32]。另有研究发现,相对于FSH,EGF类生长因子诱导了小鼠卵母细胞线粒体较高水平的膜电位,增强了卵母细胞氧化磷酸化,促进了ATP的产生,而且,AREG和EREG提高了卵丘卵母细胞复合体氨基己糖通 路代谢活性,进而促进透明质酸的合成,增强卵丘颗粒细胞扩散,同时促进了卵丘颗粒细胞的糖基 化[33,34]。对牛、小鼠和猪(Sus scrofa)的研究表明,颗粒细胞增强糖基化与体外成熟的卵母细胞发育能力相关[35,36,37],但AREG和EREG等EGF类生长因子诱导的颗粒细胞糖基化是否会影响卵母细胞发育能力,目前尚不确定,仍需进行深入研究。综上所述,EGF类生长因子在调控卵丘颗粒细胞的糖酵解和卵母细胞氧化磷酸化方面发挥了重要作用,这可能是影响卵母细胞成熟和发育能力的关键因素。3 EGF类因子对卵母细胞发育能力的影响
3.1 EGF类因子对卵母细胞发育能力的影响
由于EGF信号系统在诱导卵母细胞成熟和颗粒细胞扩散方面发挥了重要作用,因此,将EGF纳入体外成熟培养体系,有可能提高卵母细胞发育能力。目前许多研究已证明,相对于体内成熟的COC,体外成熟的COC颗粒细胞EGF类生长因子表达量较低。例如,Richani等[38]研究发现,相对于小鼠体内成熟的COC,FSH诱导体外成熟的COC,颗粒细胞AREG基因mRNA表达量及其蛋白的分泌较低。而且,基因芯片研究数据表明,相对于小鼠体内成熟的COC,FSH诱导体外成熟的COC,颗粒细胞基因表达差异最显著的是AREG,EREG和BTC这3个EGF类生长因子[39]。体内LH峰刺激后EGF类因子主要由卵泡壁层颗粒细胞产生,是COC接受的诱导卵母细胞减数分裂的早期信号因子,导致卵丘颗粒细胞自动放大EGF信号网络。由于卵母细胞体外成熟培养期间,脱离了卵泡环境,COC缺少来自于壁层颗粒细胞传递的EGF类因子的作用,不能激活EGF类因子受体,因此,颗粒细胞不能接受ERK1/2- PGE2-p38MAPK自动放大的信号。体外成熟的COC一般来自于小的生长卵泡,其卵丘颗粒细胞EGF信号系统尚处于发育阶段,体外培养期间,培养液内添加FSH可能部分克服EGF信号系统的缺陷。尽管如此,卵母细胞体外成熟培养期间,添加EGF类因子仍有利于卵母细胞成熟和发育。鉴于体外成熟的COC颗粒细胞EGF信号系统缺陷,许多研究检验了体外成熟期间补充EGF类因子对卵母细胞发育能力的影响(表1)。例如,在小鼠上的研究发现,卵母体外成熟期间,相对于FSH和EGF,培养液添加EREG显著提高了卵母细胞成熟和受精后囊胚率,而且EREG和AREG结合使用显著提高了囊胚内细胞团细胞数,并增加了胎儿的数量[38]。Prochazka等[10]研究报道,猪卵母细胞体外成熟期间,补充EREG或AREG,相对于FSH和LH,显著提高孤雌激活后的胚胎数量。这些结果揭示,生物活性的EGF类因子作为卵母细胞体外成熟的添加因子可能比FSH和EGF更适合,但具体的分子机制仍不清楚。这些研究说明,体内FSH和EGF不是诱导卵母细胞成熟的直接生理性因子,而体外成熟培养体系使用EREG和/或AREG可能模拟了体内卵母细胞成熟期间的发生的生理事件,进而提高了卵母细胞发育能力。另外,Richani等[38]发现,EREG和AREG体内刺激了EGF类生长因子的表达,而FSH促进这些因子的表达能力有限。而且在卵母细胞体外培养期间,相对于FSH,EREG和AREG显著提高了EGF类生长因子的表达量,这些研究结果进一步证明EGF类因子是体内影响卵母细胞成熟的关键因子[40]。
Table 1
表1
表1 EGF类因子对卵母细胞发育的影响
Table 1
| EGF类因子 | 功能 | 参考文献 |
|---|---|---|
| AREG | 促进了牛卵丘颗粒细胞的糖酵解 | [32] |
| AREG和EREG | 诱导了小鼠卵母细胞线粒体较高水平的膜电位,增强了卵母细胞氧化磷酸化,增强了COCs氨基己糖通路代谢活性,进而促进透明质酸的合成,同时促进了卵丘颗粒细胞的糖基化 | [33,34] |
| EREG和AREG | EREG显著提高了小鼠卵母细胞成熟和受精后囊胚率,而且EREG和AREG结合使用显著提高了囊胚内细胞团细胞数,并增加了胎儿的数量 | [38] |
| EREG和AREG | 显著提高了猪卵母细胞孤雌激活后的胚胎数量 | [29] |
| AREG | 作用于小鼠卵丘颗粒细胞产生的信号分子,介导了FSH 调控卵母细胞中一些对胚胎发育起关键作用的基因mRNA的翻译 | [41] |
| AREG | AREG与BMP15结合使用提高了体外成熟的牛卵细胞发育能力 | [32] |
| REG 和EREG | cAMP调控剂前成熟处理小鼠COCs后进行体外成熟,体外成熟液中添加AREG 和EREG显著提高了卵母细胞的发育能力 | [42] |
| AREG | 双丁酰环腺甘酸与GDF9和BMP15蛋白前体结合使用,增强了AREG诱导的猪卵母细胞成熟和囊胚形成 | [43] |
| AREG | CNP和GDF9结合使用增强了COCs对AREG的反应能力,提高了腔前小鼠卵泡来源的卵母细胞发育能力 | [44] |
新窗口打开|下载CSV
一般认为EGF类因子对颗粒细胞基因表达、信号通路和细胞功能影响的能力低于促性腺激素,但其调控卵细胞发育的能力却高于促性腺激素的潜在机制是什么?目前研究认为,EGF类因子的优势作用可能是影响了卵丘颗粒细胞和卵母细胞代谢。例如,用AREG处理牛COC,相对于FSH,能显著提高葡萄糖消耗量、乳酸的产量以及乳酸的产量/葡萄糖吸收量的比率[32]。对小鼠的研究发现,相对于FSH和EGF,EGF类因子增强了线粒体的活性,氨基己糖生物合成通路代谢活性,进而提高了透明质酸的产量和蛋白质β-O-连接糖基化的水平[33]。另外,EGF类因子可能通过调控卵母细胞翻译而影响其发育能力。最近研究发现,EGF或 AREG作用于卵丘颗粒细胞产生的信号分子,可能介导了FSH调控卵母细胞中一些对胚胎发育起关键作用的基因mRNA的翻译[41]。但目前关于EGF类因子激活卵丘颗粒细胞何种信号通路而调控卵母细胞mRNA的翻译仍不确定,尚需进行深入研究。
3.2 EGF类因子协同多种信号分子提高了卵母细胞发育能力
目前人们普遍认为,卵母细胞成熟期间,促性腺激素激活了COC多种信号通路,可能与EGF类因子信号通路协作影响卵母细胞减数分裂成熟和发育能力。这些受促性腺激素调控的众多信号通路,其中被卵母细胞分泌因子激活的信号通路能够延长卵丘颗粒细胞和卵母细胞间隙连接的通讯。GDF9和BMP15是主要的卵母细胞分泌因子,能够增强COC对EGF类因子的敏感性而提高卵母细胞的发育能力(表1)。例如,在牛上研究发现,AREG与BMP15结合使用提高了体外成熟的卵细胞发育能力,BMP15增强了颗粒细胞之间的间隙连接功能,AREG增强了卵丘颗粒细胞的糖酵解,BMP15维持了细胞间隙的通讯,进而使颗粒细胞产生的代谢物进入卵母细胞,提高其发育能力[32]。另外,促性腺激素作用于COC后将产生大量的cAMP,cAMP水平的增加不仅能够延长卵丘颗粒细胞和卵母细胞间的通讯,也能增强对COC对EGF类因子的敏感性。例如,研究发现,cAMP调控剂(forskolin+IBMX)前成熟处理COC后进行体外成熟,体外成熟液中添加AREG 和EREG显著提高了卵母细胞的发育能力[42]。
此外,发育能力较低的猪卵母细胞,双丁酰环腺甘酸与GDF9和BMP15蛋白前体结合使用,增强了AREG诱导的卵母细胞成熟、囊胚形成以及颗粒细胞扩散相关基因(HAS2, TNFAIP6和PTGS2)的表达,而且,相对于促性腺激素,这些因子的结合使用促进了EGF类因信号通路的下游因子ERK1/2的磷酸化[43]。另有研究发现,CNP和GDF9结合使用增强了小鼠COC对AREG的反应能力,提高了腔前卵泡来源的卵母细胞发育能力,这些结果进一步说明EGF类因信号系统在调控卵母细胞成熟和发育过程中的重要作用[44]。综上所述,体外成熟期间,EGF类因子刺激卵母细胞恢复减数分裂,过早的使卵母细胞和卵丘颗粒细胞失去间隙连接功能,结合使用卵母细胞分泌因子和cAMP调控剂能够维持卵母细胞和颗粒细胞之间的间隙连接通讯功能,并能增强EGF信号系统功能,进而提高卵母细胞发育能力。
4 结语与展望
EGF类因子信号网络是体内是排卵级联反应的关键参与者,卵母细胞从卵泡排出期间,EGF类因子将LH信号从卵泡周边颗粒细胞传递至卵母细胞,调控了卵丘卵母细胞代谢,促进了卵母细胞成熟,而且,体外卵母细胞成熟期间,添加外源的EGF类因子能够提高卵母细胞发育能力,这说明其在优化卵母细胞体外成熟培养体系方面具有重要的利用价值。目前研究认为EGF类因子增强了卵丘颗粒细胞糖酵解和卵母细胞氧化磷酸化功能,这可能是其提高卵母细胞发育能力的一个重要原因。而且,EGF类因子也增强了卵丘颗粒细胞的糖基化,但其诱导颗粒细胞的糖基化是否会影响卵母细胞发育能力尚不确定,仍需深入研究。另外,EGF类因子通过作用于卵丘颗粒细胞产生的信号分子,调控卵母细胞中一些对胚胎发育起关键作用的基因mRNA的翻译,这也可能是其提高卵母细胞发育能力的另一个原因,但EGF类因子激活卵丘颗粒细胞何种信号通路而调控卵母细胞mRNA的翻译亦不确定,尚需深入研究。
此外,卵母细胞成熟期间,EGF类因子信号通路可能和多种信号通路协作影响卵母细胞减数分裂成熟和发育能力。而且,体外成熟期间,EGF类因子刺激卵母细胞恢复减数分裂,过早的使卵母细胞和卵丘颗粒细胞失去间隙连接功能,通过添加卵母细胞分泌因子和cAMP水平的调控剂(dbcAMP或CNP)维持了间隙连接功能,增强了EGF类因子在调控卵母细胞成熟和发育方面的作用。因此,为了模拟体内的环境,在完善卵丘颗粒细胞EGF类因子的信号系统的同时,使用间隙连接功能的增强剂,将可能建立理想的家畜卵母细胞体外成熟体系。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URLPMID:9491385 [本文引用: 1]

Abstract Mouse oocytes suppress follicle-stimulating hormone (FSH)–induced luteinizing hormone receptor (LHR) messenger ribonucleic acid (mRNA) expression in cultured granulosa cells. The objective of this study was to assess the mechanism by which oocytes suppress FSH-induced LHR expression. The effect of cumulus cell–denuded, germinal-vesicle-stage oocytes, isolated from antral follicles, on FSH-induced cyclic adenosine monophosphate (cAMP) production by cultured granulosa cells was determined by radioimmunoassays. In addition, the effect of oocytes on 8Br-cAMP–induced LHR mRNA steady-state expression by granulosa cells was assessed by RNase protection assays. Oocytes had no detectable effect on FSH-induced cAMP production. However, oocytes dramatically suppressed 8Br-cAMP–induced LHR mRNA steady-state expression by granulosa cells. It was concluded that the mechanism by which oocytes suppress FSH-induced steady-state expression of LHR mRNA is not by inactivating FSH, preventing functional interactions of FSH with its granulosa cell receptors, or by interfering with the signal-transduction mechanisms required for FSH-dependent cAMP production. In addition, since oocytes suppressed the 8Br-cAMP–induced increase in steady-state expression of mRNA for LHR, oocyte-derived factors probably suppress expression by acting downstream of FSH-induced elevation of granulosa cell cAMP. Mol. Reprod. Dev. 49:327–332, 1998. 08 1998 Wiley-Liss, Inc.
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
URLPMID:15459120 [本文引用: 1]

Abstract Previous studies showed that epidermal growth factor (EGF) and TGFalpha mimic the action of LH on the resumption of oocyte maturation. We tested whether EGF-like agents, such as amphiregulin (AR), epiregulin (ER), and betacellulin (BTC), also mediate the LH stimulation of the ovulatory response in the rat. LH induced transient follicular expression of AR, ER, and BTC mRNA, reaching a maximum after 3-h incubation. Furthermore, the addition of ER, AR, and BTC to the culture medium could mimic some of LH actions. AR and ER fully simulated LH-induced resumption of meiosis in vitro, whereas BTC was less effective. To study the putative involvement of EGF-like factors in mediation of LH signal, the effect of the EGF receptor kinase inhibitor AG1478 was tested. When added with LH, AG1478, but not its inactive analog AG43, reduced EGF receptor phosphorylation and oocyte maturation compared with follicles treated with LH only. In addition to the inhibition of resumption of meiosis, AG1478 administration into the bursa (3 microg/bursa) resulted in 51% (P < 0.0005) inhibition of ovulation in the treated ovaries, compared with the untreated contralateral ones, as well as to the vehicle-treated ovaries (P < 0.02). LH, as well as ER, induced the expression of genes associated with the ovulatory response like rat hyaluronan synthase-2, cyclooxygenase-2, and TNFalpha-stimulated gene 6 mRNA, whereas AG1478 inhibited this effect of LH. Release of EGF-like factors from the membrane is dependent on activated metalloproteases. Indeed, Galardin, a broad-spectrum metalloprotease inhibitor, but not a specific matrix metalloprotease 2 and 9 inhibitor, suppressed meiotic maturation induced by LH. Conversely, meiotic maturation induced by ER was not affected by Galardin, thus, supporting the notion that LH releases follicular membrane-bound EGF-like agents. In summary, EGF-like factors such as ER, AR, and BTC seem to mediate, at least partially, the LH stimulation of oocyte maturation, ovulatory enzyme expression, and ovulation.
URLPMID:25385589Magsci [本文引用: 1]

Abstract Fertility depends on the precise coordination of multiple events within the ovarian follicle to ensure ovulation of a fertilizable egg. FSH promotes late follicular development, including expression of luteinizing hormone (LH) receptor by the granulosa cells. Expression of its receptor permits the subsequent LH surge to trigger the release of ligands that activate EGF receptors (EGFR) on the granulosa, thereby initiating the ovulatory events. Here we identify a previously unknown role for FSH in this signaling cascade. We show that follicles of Fshb(-/-) mice, which cannot produce FSH, have a severely impaired ability to support two essential EGFR-regulated events: expansion of the cumulus granulosa cell layer that encloses the oocyte and meiotic maturation of the oocyte. These defects are not caused by an inability of Fshb(-/-) oocytes to produce essential oocyte-secreted factors or of Fshb(-/-) cumulus cells to respond. In contrast, although expression of both Egfr and EGFR increases during late folliculogenesis in Fshb(+/-) females, these increases fail to occur in Fshb(-/-) females. Remarkably, supplying a single dose of exogenous FSH activity to Fshb(-/-) females is sufficient to increase Egfr and EGFR expression and to restore EGFR-dependent cumulus expansion and oocyte maturation. These studies show that FSH induces an increase in EGFR expression during late folliculogenesis and provide evidence that the FSH-dependent increase is necessary for EGFR physiological function. Our results demonstrate an unanticipated role for FSH in establishing the signaling axis that coordinates ovulatory events and may contribute to the diagnosis and treatment of some types of human infertility.
URLPMID:28592334 [本文引用: 1]

Abstract Oocyte-secreted factors (OSFs) play an important role in the acquisition of oocyte developmental competence through bidirectional cross-talk between oocyte and cumulus cells via gap junctions. Thus, the present study was designed to investigate the effect of two OSFs, growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15), on the developmental competence of buffalo oocytes derived from two different follicle sizes. Cumulus-oocyte complexes (COCs) from large follicles (LF, >6 mm) or small follicles (SF, 0.05) between DOs and combination groups. Relative mRNA analysis revealed significantly higher (P > 0.05) expression of the cumulus cell marker genes EGFR, HAS2, and CD44 in LF-derived than SF-derived oocyte; the expression of these markers was significantly higher (P > 0.05) in DOs and combination groups, irrespective of the follicle size. These results suggested that LF-derived oocytes have a higher developmental competence than SF-derived oocytes and that supplementation of GDF9 and BMP15 modulates the developmental competence of buffalo oocytes by increasing the relative abundance of cumulus-enabling factors and thereby increasing cleavage and the quality of blastocyst production.
[本文引用: 1]
URLPMID:2847890 [本文引用: 1]

A surge of luteinizing hormone (LH) from the pituitary gland triggers ovulation, oocyte maturation, and luteinization for successful reproduction in mammals. Because the signaling molecules RAS and ERK1/2 (extracellular signal-regulated kinases 1 and 2) are activated by an LH surge in granulosa cells of preovulatory follicles, we disrupted Erk1/2 in mouse granulosa cells and provide in vivo evidence that these kinases are necessary for LH-induced oocyte resumption of meiosis, ovulation, and luteinization. In addition, biochemical analyses and selected disruption of the Cebpb gene in granulosa cells demonstrate that C/EBPβ (CCAAT/Enhancer-binding protein-β) is a critical downstream mediator of ERK1/2 activation. Thus, ERK1/2 and C/EBPβ constitute an in vivo LH-regulated signaling pathway that controls ovulation- and luteinization-related events.
URLPMID:4685214 [本文引用: 2]

In vivo, resumption of oocyte meiosis occurs in large ovarian follicles after the preovulatory surge of luteinizing hormone (LH). The LH surge leads to the activation of a broad signaling network in mural granulosa cells equipped with LH receptors. The signals generated in the mural granulosa cells are further augmented by locally produced peptides or steroids and transferred to the cumulus cell compartment and the oocyte itself. Over the last decade, essential progress has been made in the identification of molecular events associated with the final maturation and ovulation of mammalian oocytes. All new evidence argues for a multiple roles of mitogen-activated protein kinase 3/1 (MAPK3/1) in the gonadotropin-induced ovulation processes. However, the knowledge of gonadotropin-induced signaling pathways leading to MAPK3/1 activation in follicular cells seems limited. To date, only the LH-induced transactivation of the epidermal growth factor receptor/MAPK3/1 pathway has been described in granulosa/cumulus cells even though other mechanisms of MAPK3/1 activation have been detected in other types of cells. In this review, we aimed to summarize recent advances in the elucidation of gonadotropin-induced mechanisms leading to the activation of MAPK3/1 in preovulatory follicles and cultured cumulus-oocyte complexes and to point out a specific role of this kinase in the processes accompanying final maturation of the mammalian oocyte.
URLPMID:16908014 [本文引用: 1]

The transition of preantral to antral follicles is one of the major steps in follicular development, yet little is known about the molecular and functional changes that occur as preantral granulosa cells differentiate into cumulus cells. The cumulus oophorus of large antral follicles undergoes expansion in response to the preovulatory surge of gonadotropins, but preantral granulosa cells do not. The objective of this project was to determine the molecular mechanisms underlying this differential response. Cumulus expansion in vitro requires secretion of cumulus-expansion enabling factors (CEEFs) by the oocyte and stimulation by a ligand, epidermal growth factor (EGF) or follicle-stimulating hormone (FSH). This combined stimulation results in activation of MAPKs (MAPK3/1 (formerly ERK1/2) and MAPK14 (formerly p38)) and increased Has2, Ptgs2, Tnfaip6 and Ptx3 mRNA levels, all of which are required for cumulus expansion. Only fully-grown oocytes from antral follicles were competent to enable expansion and increases in expansion-related transcripts in cumulus cells, whereas growing oocytes of preantral follicles did not. To assess the competence of preantral granulosa cells to generate responses associated with expansion, they were treated with FSH or EGF and co-cultured with fully-grown oocytes secreting CEEFs. MAPKs were activated by EGF in preantral granulosa cells to essentially the same levels as in cumulus cells. Preantral granulosa cells treated with EGF, but not those treated with FSH increased Has2, Ptgs2 and Ptx3 mRNAs to 17-96% of the levels observed in cumulus cells. In contrast, the level of Tnfaip6 mRNA was minimally stimulated in preantral granulosa cells. Therefore, preantral granulosa cells do not undergo expansion for two fundamental reasons. First, the growing oocytes of preantral follicles do not secrete active CEEFs. Second, activation of MAPKs alone in preantral granulosa cells, even in the presence of CEEFs, is not sufficient to increase the expression of essential transcripts, particularly Tnfaip6 mRNA. Thus, preantral granulosa cells differ from cumulus cells in CEEF-dependent processes downstream of the activation of MAPKs.
URLPMID:25849729 [本文引用: 2]

Abstract Oocytes progressively acquire the competence to support embryo development as oogenesis proceeds with ovarian folliculogenesis. The objectives of this study were to investigate oocyte-secreted factor (OSF) participation in the development of somatic cell epidermal growth factor (EGF) responsiveness associated with oocyte developmental competence. A well-established porcine model was employed using oocytes from small (4 mm) antral follicles, representing low vs moderate developmental competence, respectively. Cumulus-oocyte complexes (COCs) were treated in vitro with inducers of oocyte maturation, and cumulus cell functions and oocyte developmental competence were assessed. COCs from small follicles responded to FSH but, unlike COCs from larger follicles, were incapable of responding to EGF family growth factors known to mediate oocyte maturation in vivo, exhibiting perturbed cumulus expansion and expression of associated transcripts (HAS2 and TNFAIP6). Low and moderate competence COCs expressed equivalent levels of EGF receptor (EGFR) mRNA; however, the former had less total EGFR protein leading to failed activation of phospho-EGFR and phospho-ERK1/2, despite equivalent total ERK1/2 protein levels. Native OSFs from moderate, but not from low, competence oocytes established EGF responsiveness in low competence COCs. Four candidate recombinant OSFs failed to mimic the actions of native OSFs in regulating cumulus expansion. Treatment with OSFs and EGF enhanced oocyte competence but only of the low competence COCs. These data suggest that developmental acquisition by the oocyte of capacity to regulate EGF responsiveness in the oocyte's somatic cells is a major milestone in the oocyte's developmental program and contributes to coordinated oocyte and somatic cell development.
URLPMID:7598904 [本文引用: 1]

Abstract The present study was undertaken to determine the expression of genes for epidermal growth factor (EGF) and its receptor (EGF-R) in various components of medium-sized porcine ovarian follicles by reverse transcription-polymerase chain reaction (RT-PCR), and to localize their peptides during folliculogenesis by immunocytochemistry. A strong band for EGF mRNA transcript was detected in the oocyte, whereas the signal in cumulus, granulosa, and theca cells was very weak but detectable. In contrast, a very strong EGF-R mRNA signal was observed in cumulus, granulosa, and theca cells, whereas the signal in the oocyte was very weak. EGF peptide was localized in the oocyte, cumulus, and granulosa cells of all stages of follicle. In the oocyte, the intensity of immunostaining was more pronounced in primordial and primary follicles, compared to antral follicles. In large antral follicles, immuno-staining was pronounced in granulosa cells, whereas theca cells showed little or no detectable staining for EGF. EGF staining was also observed in the cumulus and granulosa cells of follicles undergoing atresia. EGF-R immunostaining was observed in the oocytes of primordial and primary follicles, and in cumulus, granulosa, and theca cells of all stages of follicle, including atretic follicles. In large antral follicles, the intensity of immunostaining was more pronounced in theca cells than in granulosa cells, and the oocyte showed little or no detectable staining. No immunostaining was observed when the primary antibody was replaced with preimmune serum (EGF), or preabsorbed with the control peptide (EGF-R), confirming the specificity of the staining procedures. These results suggest a local follicular production of EGF and its receptor in the porcine ovary, and thus a role for EGF of follicular origin in the regulation of follicular development in autocrine/paracrine fashion. 1995 Wiley-Liss, Inc.
URLPMID:12604628 [本文引用: 1]

We have recently shown that epidermal growth factor (EGF) strongly stimulates expansion of porcine oocyte-cumulus complexes (OCCs) isolated from large follicles (>6 mm) and does not promote expansion of OCCs from small (3-4-mm) follicles. In order to elucidate the role of EGF in OCCs expansion, in the present study, we first examined the presence of EGF receptors (EGFRs) in cumulus cells isolated from follicles of different sizes. Surprisingly, immunoblotting showed that cumulus cells obtained from all follicular size categories contained similar amounts of EGFR protein. On the other hand, we found a dramatic difference in the pattern of protein tyrosine phosphorylation in a comparison of cumulus cells isolated from small and large follicles treated by EGF. Furthermore, tyrosine-phosphorylated EGFR was specifically immunoprecipitated with antiphosphotyrosine antibodies from EGF-treated cumulus cells isolated from the large follicles. This result strongly indicates that only OCCs from the large follicles contain mature EGFRs that are capable of becoming activated by EGF. Remarkably, preincubation of cumulus cells from small follicles (3-4 mm) with FSH strongly increased EGF-stimulated tyrosine phosphorylation to levels comparable with OCCs from large follicles. The FSH-dependent activation of EGFRs was beneficial for expansion of OCCs isolated from the small follicles since OCCs treated sequentially by FSH (3 h) and EGF (1 h) underwent expansion significantly better then OCCs cultured in FSH or EGF alone. We conclude that a FSH-dependent pathway has an important role in the maturation of the EGFR in cumulus cells and that activation of EGFR-dependent signaling is sufficient to induce expansion.
[本文引用: 1]
URLPMID:7932371 [本文引用: 1]

The aim of this investigation was to determine the influence of epidermal growth factor (EGF) on follicular growth and steroidogenesis in mice. Follicles were cultured in medium containing human recombinant EGF at concentrations of 1-20 ng ml-1. Oestradiol production was assayed immunoenzymatically, and growth was measured by recording follicle diameter daily and by analysing the total DNA content of follicles. The effect of EGF on cumulus-oocyte complexes isolated from cultured follicles was also assessed. Results showed that EGF inhibited oestradiol production in a dose-dependent manner, but had no mitogenic effect. Despite almost complete inhibition of oestradiol production at concentrations of EGF > or = 10 ng ml-1, follicles were still able to achieve preovulatory size and morphology, although the incidence of atresia was increased over controls. Conversely, at a concentration of only 1 ng EGF ml-1, a significantly greater number of follicles reached the Graafian stage compared with control follicles. Cumulus expansion and meiotic maturation by isolated cumulus-oocyte complexes from cultured follicles was dramatically stimulated in the presence of EGF and FSH, but not by FSH alone. These findings suggest that EGF may have a modulatory effect on oestradiol production in vivo, and that follicular growth and differentiation may be uncoupled from steroidogenesis. Finally, ovulatory changes in the cumulus-oocyte complex may require the presence of this factor.
URL [本文引用: 1]

react-text: 325 Biochemical blood parameters generally show fluctuations during the day. The aim of this study is to determine the variations of the following parameters: hemoglobin, glucose, triglycerides, ASAT, ALAT, urea and creatinine in New Zealand white (NZW) rabbits, kept under five different circadian rhythms (12/12, 14/10, 10/14, 16/8, 8/16, light/dark). These parameters were analyzed by using a... /react-text react-text: 326 /react-text [Show full abstract]
URLPMID:10737968 [本文引用: 2]

Abstract <p>Epidermal growth factor (EGF) efficiently stimulates expansion of mouse and rat oocyte-umulus complexes (OCC). Contradictory data have been published by several laboratories about the ability of EGF to stimulate expansion of porcine OCC. We assumed that these contradictions may have resulted from heterogeneous conditions used for isolation, culture, and assessment of OCC. The present experiments were designed to test the hypothesis that porcine OCC acquire the ability to synthesize hyaluronic acid (HA) and undergo expansion following EGF-stimulation gradually during the growth of follicles. For this reason, we isolated OCC from follicles of different sizes and assessed quantity of produced HA and proportions of expanding OCC after stimulation by EGF. In addition, we assessed in those OCC changes in morphology of cumulus cells and assembly of F-actin microfilaments, which are necessary for expansion to occur. Finally, nuclear maturation of EGF-stimulated OCC was assessed and its relationship with occurrence of expansion was evaluated. In all experiments, OCC stimulated with FSH were used as positive controls. The results showed that EGF did not stimulate production of HA, rearrangement of F-actin and expansion in OCC isolated from small follicles (<4 mm in diameter). OCC isolated from large preovulatory follicles (6-7 mm in diameter and PMSG-stimulated follicles) underwent efficient expansion when stimulated by EGF (93% and 100%, respectively). EGF dramatically stimulated total production of HA in these OCC and its retention in extracellular matrix of the expanding cumulus. Cumulus cells of the large OCC underwent essential changes of their morphology and extensive rearrangement of F-actin microfilaments following stimulation with EGF. Interestingly, EGF enhanced nuclear maturation of OCC isolated from both small and large follicles, which suggest diversity of signaling pathways controlling maturation and expansion. FSH caused cumulus expansion, F-actin remodeling, and enhancement of oocyte nuclear maturation in OCC originated from both small and large follicles. We conclude that EGF can stimulate expansion of porcine OCC in vitro; however, only of those isolated from large follicles. This indicates that EGF may have a physiological role in regulation of porcine cumulus expansion in preovulatory follicles, presumably as a mediator of signals elicited by the LH surge. Mol. Reprod. Dev. 56:63-73, 2000. 2000 Wiley-Liss, Inc.</p>
URLPMID:12390893 [本文引用: 1]

Abstract Spatiotemporal expression, endocrine regulation, and activation of epidermal growth factor receptor (EGFR) in the hamster ovary were evaluated by immunofluorescence and in situ hybridization localization. Whereas granulosa cells (GC) of primordial through large preantral (stage 6, 7-8 layers GC) follicles had low immunoreactivity, granulosa cells of antral follicles, theca, and interstitial cells had intense EGFR immunoreactivity. EGFR expression in GC of primordial and small preantral follicles increased progressively from estrous through proestrous, but a significant increase occurred in mural GC of antral follicles following the gonadotropin surge. Interstitial cells around small preantral follicles had strong immunofluorescence, and the intensity increased significantly in fully differentiated thecal cells. Distinct EGFR protein was localized in the nucleus of the oocytes and granulosa cells. FSH significantly stimulated EGFR expression in the GC, especially the mural GC, theca, and interstitial cells in hypophysectomized hamster. Estrogen stimulated EGFR expression in preantral GC as well as in interstitial cells. Progesterone and hCG effect was limited to theca and interstitial cells. EGFR expression correlated well with EGFR activation following endogenous or exogenous gonadotropin exposure. Receptor mRNA expression closely followed the protein expression, with increased mRNA expression in mural GC of antral follicles. These results suggest that low levels of EGF signal as a consequence of low levels of receptors in preantral GC may be critical for cell proliferation, but higher receptor density may evoke increased signal intensity due to activation of other intracellular signal pathways, which activate cellular processes related to granulosa, theca, and interstitial cell differentiation. The spatiotemporal cell type and follicle stage-specific expression of receptor mRNA and protein and EGFR activation is critically regulated by gonadotropins and ovarian steroids, primarily estradiol.
URLPMID:20663235Magsci [本文引用: 1]

Ovarian stimulation with exogenous follicle stimulating hormone (FSH) has been used to increase the number of viable oocytes for laparoscopic oocyte recovery (LOR) in goats. The aim of this study was to evaluate the effect of two FSH protocols for ovarian stimulation in goats on the expression pattern of epidermal growth factor (EGF) receptor (EGFR) in cumulus oocyte complexes (COCs) recovered by LOR. After real-time qRT-PCR analysis, expression profiles of morphologically graded COCs were compared prior to and after in vitro maturation (IVM) on a FSH protocol basis. The use of a protocol with higher number of FSH injections at a shorter interval resulted in GI/GII COCs with a higher level of EGFR expression in cumulus cells, but not in the oocyte, which was correlated with an elevated meiotic competence following IVM. Based on the maturation profile and EGFR expression patterns observed between groups, the morphological selection of COCs prior to IVM was not a good predictor of oocyte meiotic competence. Therefore, EGFR may be a good candidate marker for indirect prediction of goat oocyte quality. The IVM process of goat COCs increased the EGFR expression in oocytes and cumulus cells, which seemed to be strongly associated with the resumption of meiosis. In summary, differential EGFR expression in goat cumulus cells was associated with the in vivo prematuration process, and in turn, the upregulation in the entire COC was associated with IVM. Cause-and-effect relationships between such increased expression levels, particularly in the oocyte, and oocyte competence itself still need to be further investigated.
URLPMID:18325497 [本文引用: 2]

To identify the most important epidermal growth factor (EGF) receptor ligand in the LH or hCG signal pathway in human ovary. A retrospective clinical study. Tertiary university hospital. Ninety-eight infertile patients who underwent IVF-mbryo transfer. Sera and follicular fluid were collected at the time of oocyte retrieval. The levels of EGF, transforming growth factor-alpha (TGF), and amphiregulin (AR) were measured in follicular fluid and sera by using ELISA. The relationships between the level of AR and level of hCG, fertilization rate, and embryo quality. Amphiregulin was abundantly expressed in follicular fluid after hCG stimulation. Although large differences were found between AR and both EGF and TGF in follicular fluid, no significant difference was detected in the levels of the three EGF receptor ligands in sera. The level of AR was inversely correlated with the fertilization rate and hCG level, whereas little significant association was observed between the level of AR and embryo quality. Amphiregulin was expressed most dominantly among EGF receptor ligands tested and may mediate the hCG signal in human oocyte maturation. Elaborate interaction between AR and hCG may be required for an optimal oocyte maturation.
URLPMID:12444039 [本文引用: 1]

In recent years, there have been a number of efforts to identify genes that are expressed in mature ovarian follicles in response to an ovulatory dose of LH or its homologue hCG. This review keys on 20 ovulation-specific genes that we have identified by the molecular procedure known as differential display. The objective is to use this sampling of genes to illustrate the diversity in the temporal and spatial patterns of expression of genes in the ovary following the stimulus of this gonadal target tissue by a single glycoprotein hormone. The specific genes that are surveyed include 5-aminolevulinate synthase; early growth response protein-1; glutamylcysteine synthetase; cyclooxygenase-2; epiregulin; pituitary adenylate cyclase-activating polypeptide; tumor necrosis factor-stimulated gene-6; regulator of G-protein signaling protein-2; adrenodoxin; steroidogenic acute regulatory protein; 3hydroxysteroid dehydrogenase; CD63, a disintegrin and metalloproteinase with thrombospondin motifs; tissue inhibitor of metalloproteinase-1; carbonyl reductase, a G-protein-coupled receptor; pancreatitis-associated protein-III; glutathione S-transferase; and metallothionein-1. The ovulatory expression of these different genes is predominantly within the granulosa layer of mature follicles. However, there were also instances of expression in the thecal and stromal tissue of the ovary, as well as in vascular endothelial cells and in luteal tissue. The overwhelming impression is that the molecular events of ovulation are far more complex, and therefore more highly ordered, than originally imagined.
URLPMID:19225042 [本文引用: 1]

Abstract LH acts on periovulatory granulosa cells by activating the PKA pathway as well as other cell signaling cascades to increase the transcription of specific genes necessary for ovulation and luteinization. Collectively, these cell signaling responses occur rapidly (within minutes); however, presently no high throughput studies have reported changes before 4 h after the LH surge. To identify early response genes that are likely critical for initiation of ovulation and luteinization, mouse granulosa cells were collected before and 1 h after hCG. Fifty-seven gene transcripts were significantly (P<0.05) upregulated and three downregulated following hCG. Twenty-four of these transcripts were known to be expressed after the LH/hCG surge at later time points, while 36 were unknown to be expressed by periovulatory granulosa cells. Temporal expression of several transcripts, including the transcription factors Nr4a1, Nr4a2, Egr1, Egr2, Btg1, and Btg2, and the epidermal growth factor (EGF)-like ligands Areg and Ereg, were analyzed by quantitative RT-PCR, and their putative roles in granulosa cell function are discussed. Epigen (Epgn), another member of the family of EGF-like ligands was identified for the first time in granulosa cells as rapidly induced by LH/hCG. We demonstrate that Epgn initiates cumulus expansion, similar to the other EGF-receptor ligands Areg and Ereg. These studies illustrate that a number of changes in gene expression occur in vivo in response to LH, and that many of the differentially expressed genes are transcription factors that we would predict in turn modulate granulosa cell gene expression to ultimately impact the processes of ovulation and luteinization.
[本文引用: 1]
URLPMID:18187604 [本文引用: 1]

LH activates a cascade of signaling events that are propagated throughout the ovarian preovulatory follicle to promote ovulation of a mature egg. Critical to LH-induced ovulation is the induction of epidermal growth factor (EGF)-like growth factors and transactivation of EGF receptor (EGFR) signaling. Because the timing of this transactivation has not been well characterized, we investigated the dynamics of LH regulation of the EGF network in cultured follicles. Preovulatory follicles were cultured with or without recombinant LH and/or specific inhibitors. EGFR and MAPK phosphorylation were examined by immunoprecipitation and Western blot analyses. By semiquantitative RT-PCR, increases in amphiregulin and epiregulin mRNAs were detected 30 min after recombinant LH stimulation of follicles and were maximal after 2 h. LH-induced EGFR phosphorylation also increased after 30 min and reached a maximum at 2 h. EGFR activation precedes oocyte maturation and is cAMP dependent, because forskolin similarly activated EGFR. LH-induced EGFR phosphorylation was sensitive to AG1478, an EGFR kinase inhibitor, and to inhibitors of matrix metalloproteases GM6001 and TNFalpha protease inhibitor-1 (TAPI-1), suggesting the involvement of EGF-like growth factor shedding. LH- but not amphiregulin-induced oocyte maturation and EGFR phosphorylation were sensitive to protein synthesis inhibition. When granulosa cells were cultured with a combination of neutralizing antibodies against amphiregulin, epiregulin, and betacellulin, EGFR phosphorylation and MAPK activation were inhibited. In cultured follicles, LH-induced MAPK activation was partially inhibited by AG1478 and GM6001, indicating that this pathway is regulated in part by the EGF network but also involves additional pathways. Thus, complex mechanisms are involved in the rapid amplification and propagation of the LH signal within preovulatory follicles and include the early activation of the EGF network.
[本文引用: 1]
URLPMID:22987720Magsci [本文引用: 1]

Oocyte meiosis is arrested at prophase I by factors secreted from surrounding somatic cells after oocytes acquire meiotic competence at an early antral stage, and meiosis resumes in preovulatory follicles as a result of the luteinizing hormone (LH) surge. Recently, signaling by C-type natriuretic peptide (CNP) through its receptor, natriuretic peptide receptor 2 (NPR2), was found to be essential for meiotic arrest at the late antral stage. Whether or not CNP/NPR2 signaling maintains oocyte meiotic arrest in earlier follicular stages and how it is associated with meiotic resumption induced by the LH surge is unclear. In this study, we examined the expression of Nppc and Npr2, respectively encoding CNP and NPR2, in the ovaries of immature mice. Nppc and Npr2 mRNA were specifically expressed in the outer and inner granulosa cell layers, respectively, in early antral follicles. Histological analysis of mice with a mutation in Npr2 revealed precocious resumption of oocyte meiosis in early antral follicles. Ovaries of mice treated with excess human chorionic gonadotropin (hCG) exhibited markedly decreased Nppc mRNA levels in granulosa cells of preovulatory follicles. Moreover, we found that amphiregulin, a mediator of LH/hCG activity through epidermal growth factor receptor (EGFR), suppressed Nppc mRNA levels in cultured granulosa cells. These results suggest that CNP/NPR2 signaling is essential for oocyte meiotic arrest in early antral follicles and that activated LH/amphiregulin/EGFR signaling pathway suppresses this signal by downregulating Nppc expression. Mol. Reprod. Dev. 79: 795-802, 2012. 2012 Wiley Periodicals, Inc.
[本文引用: 1]
URLPMID:21239527 [本文引用: 1]

The aim of this work was to assess the FSH-stimulated expression of epidermal growth factor (EGF)-like peptides in cultured cumulus-oocyte complexes (COCs) and to find out the effect of the peptides on cumulus expansion, oocyte maturation, and acquisition of developmental competence in vitro. FSH promptly stimulated expression of amphiregulin (AREG) and epiregulin (EREG), but not betacellulin (BTC) in the cultured COCs. Expression of AREG and EREG reached maximum at 2 or 4 h after FSH addition respectively. FSH also significantly stimulated expression of expansion-related genes (PTGS2, TNFAIP6, and HAS2) in the COCs at 4 and 8 h of culture, with a significant decrease at 20 h of culture. Both AREG and EREG also increased expression of the expansion-related genes; however, the relative abundance of mRNA for each gene was much lower than in the FSH-stimulated COCs. In contrast to FSH, AREG and EREG neither stimulated expression of CYP11A1 in the COCs nor an increase in progesterone production by cumulus cells. AREG and EREG stimulated maturation of oocytes and expansion of cumulus cells, although the percentage of oocytes that had reached metaphase II was significantly lower when compared to FSH-induced maturation. Nevertheless, significantly more oocytes stimulated with AREG and/or EREG developed to blastocyst stage after parthenogenetic activation when compared to oocytes stimulated with FSH alone or combinations of FSH/LH or pregnant mares serum gonadotrophin/human chorionic gonadotrophin. We conclude that EGF-like peptides do not mimic all effects of FSH on the cultured COCs; nevertheless, they yield oocytes with superior developmental competence.
URLPMID:20719813 [本文引用: 1]

Abstract BACKGROUND: The LH surge promotes ovulation via activation of multiple signaling networks in the ovarian follicle. Studies in animal models have shown the importance of LH-induced activation of the epidermal growth factor (EGF)signaling network in critical peri-ovulatory events. We investigated the biological significance of regulatory mechanisms mediated by EGF-like growth factors during LH stimulation in humans. METHODS: We characterized the EGF signaling network in mature human ovarian follicles using in vivo and in vitro approaches. Amphiregulin (AREG) levels were measured in 119 follicular fluid (FF) samples from IVF/ICSI patients. Biological activity of human FF was assessed using in vitro oocyte maturation, cumulus expansion and cell mitogenic assays. RESULTS: AREG is the most abundant EGF-like growth factor accumulating in the FF of mature follicles of hCG-stimulated patients. No AREG was detected before the LH surge or before hCG stimulation of granulosa cells in vitro, demonstrating that the accumulation of AREG requires gonadotrophin stimulation. Epiregulin and betacellulin mRNA were detected in both human mural and cumulus granulosa cells, although at significantly lower levels than AREG. FF from stimulated follicles causes cumulus expansion and oocyte maturation in a reconstitution assay. Immunodepletion of AREG abolishes the ability of FF to stimulate expansion (P < 0.0001) and oocyte maturation (P < 0.05), confirming the biological activity of AREG. Conversely, mitogenic activity of FF remained after depletion of AREG, indicating that other mitogens accumulate in FF. FF from follicles yielding an immature germinal vesicle oocyte or from an oocyte that develops into an aberrant embryo contains lower AREG levels than that from follicles yielding a healthy oocyte (P = 0.008). CONCLUSIONS: EGF-like growth factors play a role in critical peri-ovulatory events in humans, and AREG accumulation is a useful marker of gonadotrophin stimulation and oocyte competence.
URLPMID:17549700 [本文引用: 1]

This study was carried out to examine the participation of epidermal growth factor (EGF)-like peptides in the induction of germinal vesicle breakdown (GVB) in mouse cumulus cell-enclosed oocytes (CEO). The EGF-like peptide, amphiregulin (AR), dose-dependently stimulated meiotic resumption in CEO, but not denuded oocytes (DO) maintained in meiotic arrest with 300 碌M dbcAMP. The EGF receptor (EGFR) kinase inhibitor, AG1478, blocked meiotic resumption induced by FSH and AR in CEO, but had no effect in DO. FSH-induced maturation was also suppressed by antisera to both EGFR and EGF. Maturation occurred with slightly faster kinetics in AR-stimulated CEO when compared to FSH-stimulated CEO. When CEO were maintained in meiotic arrest with a low level of dbcAMP, FSH was initially inhibitory to maturation and later stimulatory; the stimulatory phase was prevented by AG1478, indicating mediation by EGF-like peptides. Pulsing CEO with high levels of dbcAMP also stimulated GVB and could be blocked by AG1478. Treatment of arrested CEO with PKC agonists stimulated maturation and this was prevented with AG1478 as well as antibodies to EGFR. FSH-induced maturation of dbcAMP-arrested CEO was blocked by bisindolylmaleimide I (BIM-I), an inhibitor of PKC, implicating PKC in FSH action. EGF-stimulated CEO failed to resume maturation in the presence of glycerrhetinic acid, a gap junction inhibitor, suggesting transfer of positive signal through the cell-cell coupling pathway. These data support the idea that EGF-like peptides provide a common pathway mediating the meiosis-inducing influence of FSH, cAMP pulsing, and PKC activation in mouse CEO by a gap junction-dependent process. Mol. Reprod. Dev. 75: 105-114, 2008. 2007 Wiley-Liss, Inc.
URLPMID:24557840Magsci [本文引用: 3]

This study assessed the participation of () and () during maturation of cumulus-oocyte complexes (COCs) on cumulus function and their impact on subsequent . treatment of COCs enhanced and quality only when in the presence of . Expression of was enhanced by (FSH) but not by , which was reflected in the level of cumulus expansion. Although both FSH and stimulated glycolysis, -treated COCs had higher consumption, production and ratio of production to . Autofluorescence levels in oocytes, indicative of NAD(P)H and FAD(++), were increased with combined and treatment of COCs. In contrast, these treatments did not alter autofluorescence levels when cumulus were removed from oocytes, even in the presence of other COCs, suggesting that oocyte-cumulus gap-junctional communication (GJC) is required. FSH contributed to maintaining GJC for an extended period of time. Remarkably, was equally effective at maintaining GJC even in the presence of . Hence, stimulation of COC glycolysis and preservation of GJC may facilitate efficient transfer of metabolites from cumulus to the oocyte thereby enhancing oocyte developmental competence. These results have implications for improving in vitro systems.
[本文引用: 2]
URLPMID:24488930Magsci [本文引用: 1]

SUMMARY<P>Recent studies have independently shown that cyclic adenosine 3′5′-monophosphate (cAMP) modulation prior to in vitro maturation (IVM) and epidermal growth factor (EGF)-like peptide supplementation during IVM improve subsequent oocyte developmental outcomes. This study investigated the effects of an IVM system that incorporates these two concepts. Cumulus–oocyte complexes (COCs) were collected from pre-pubertal mice either 4665hr post-equine chorionic gonadotropin (eCG) (IVM) or post-eCG65+65post-human chorionic gonadotropin (hCG) stimulation (in vivo maturation; IVV). IVM COCs were treated with the cAMP modulators forskolin and IBMX for 1, 2, or 465hr (pre-IVM phase) prior to IVM. COCs then underwent IVM with the EGF-like peptides amphiregulin or epiregulin, or with the common IVM stimulants follicle-stimulating hormone (FSH) or EGF. A pre-IVM phase increased the size of the subsequent blastocysts' inner-cell-mass compared to standard IVM, regardless of IVM treatment (P65<650.05). Unlike FSH or EGF, amphiregulin or epiregulin significantly increased blastocyst quality (trophectoderm and total cell numbers) and/or yield (P65<650.01) compared to standard IVM, and were the only treatments that produced blastocysts comparable to IVV-derived blastocysts. Forskolin acutely up-regulated EGF-like peptide mRNA expression after a 2-hr pre-IVM phase (P65<650.001), although EGF receptor and ERK1/2 activities were not significantly different than control. IVV-like levels of EGF-like peptide mRNA expression during IVM were maintained only by supplementing with EGF-like peptides and EGF, since expression levels induced by FSH were significantly lower in vitro than during IVV. However, EGF receptor and ERK1/2 phosphorylation levels were not significantly different across treatment groups. In conclusion, a pre-IVM phase in conjunction with IVM in the presence of EGF-like peptides endows high oocyte developmental competence, as evidenced by increased embryo yield and/or quality relative to FSH and EGF. Mol. Reprod. Dev. 81: ???–???, 2014. 08 2014 Wiley Periodicals, Inc.</P>
URLPMID:16436527 [本文引用: 1]

Glucose concentration during cumulus-oocyte complex (COC) maturation influences several functions, including progression of oocyte meiosis, oocyte developmental competence, and cumulus mucification. Glucosamine (GlcN) is an alternative hexose substrate, specifically metabolized through the hexosamine biosynthesis pathway, which provides the intermediates for extracellular matrix formation during cumulus cell mucification. The aim of this study was to determine the influence of GlcN on meiotic progression and oocyte developmental competence following in vitro maturation (IVM). The presence of GlcN during bovine IVM did not affect the completion of nuclear maturation and early cleavage, but severely perturbed blastocyst development. This effect was subsequently shown to be dose-dependent and was also observed for porcine oocytes matured in vitro. Hexosamine biosynthesis upregulation using GlcN supplementation is well known to increase O-linked glycosylation of many intracellular signaling molecules, the best-characterized being the phosphoinositol-3-kinase (PI3K) signaling pathway. We observed extensive O-linked glycosylation in bovine cumulus cells, but not oocytes, following IVM in either the presence or the absence of GlcN. Inhibition of O-linked glycosylation significantly reversed the effect of GlcN-induced reduction in developmental competence, but inhibition of PI3K signaling had no effect. Our data are the first to link hexosamine biosynthesis, involved in cumulus cell mucification, to oocyte developmental competence during in vitro maturation.
URLPMID:2842489 [本文引用: 1]

Maternal hyperglycemia is believed to be the metabolic derangement associated with both early pregnancy loss and congenital malformations in a diabetic pregnancy. Using an in vitro model of embryo exposure to hyperglycemia, this study questioned if increased flux through the hexosamine signaling pathway (HSP), which results in increased embryonic O-linked glycosylation (O-GlcNAcylation), underlies the glucotoxic effects of hyperglycemia during early embryogenesis. Mouse zygotes were randomly allocated to culture treatment groups that included no glucose (no flux through HSP), hyperglycemia (27 mM glucose, excess flux), 0.2 mM glucosamine (GlcN) in the absence of glucose (HSP flux alone), and O-GlcNAcylation levels monitored immunohistochemically. The impact of HSP manipulation on the first differentiation in development, blastocyst formation, was assessed, as were apoptosis and cell number in individual embryos. The enzymes regulating O-GlcNAcylation, and therefore hexosamine signaling, are the beta-linked-O-GlcNAc transferase (OGT) and an O-GlcNAc-selective beta-N-acetylglucosaminidase (O-GlcNAcase). Inhibition of these enzymes has a negative impact on blastocyst formation, demonstrating the importance of this signaling system to developmental potential. The ability of the OGT inhibitor benzyl-2-acetamido-2-deoxy-alpha-D-galactopyranoside (BADGP) to reverse the glucotoxic effects of hyperglycemia on these parameters was also sought. Excess HSP flux arising from a hyperglycemic environment or glucosamine supplementation reduced cell proliferation and blastocyst formation, confirming the criticality of this signaling pathway during early embryogenesis. Inhibition of OGT using BADGP blocked the negative impact of hyperglycemia on blastocyst formation, cell number, and apoptosis. Our results suggest that dysregulation of HSP and O-GlcNAcylation is the mechanism by which the embryotoxic effects of hyperglycemia are manifested during preimplantation development.
URLPMID:24713123 [本文引用: 1]

What is the effect of beta-O-linked glycosylation (O-GlcNAcylation) on specific proteins in the cumulus-oocyte complex (COC) under hyperglycaemic conditions?Heat shock protein 90 (HSP90) was identified and confirmed as being O-GlcNAcylated in mouse COCs under hyperglycaemic conditions (modelled using glucosamine), causing detrimental outcomes for embryo development.O-GlcNAcylation of proteins occurs as a result of increased activity of the hexosamine biosynthesis pathway, which provides substrates for cumulus matrix production during COC maturation, and also for O-GlcNAcylation. COCs matured under hyperglycaemic conditions have decreased developmental competence, mediated at least in part through the mechanism of increased O-GlcNAcylation.This study was designed to examine the effect of hyperglycaemic conditions (using the hyperglycaemic mimetic, glucosamine) on O-GlcNAc levels in the mouse COC, and furthermore to identify potential candidate proteins which are targets of this modification, and their roles in oocyte maturation.COCs from 21-day-old superovulated CBA C57BL6 F1 hybrid female mice were matured in vitro (IVM). Levels of O-GlcNAcylated proteins, HSP90 and O-GlcNAc transferase (OGT, the enzyme responsible for O-GlcNAcylation) in COCs were measured using western blot, and localization observed using immunocytochemistry. For glycosylated HSP90 levels, and to test OGT-HSP90 interaction, immunoprecipitation was performed prior to western blotting. Embryo development was assessed using in vitro fertilization and embryo culture post-maturation.Addition of the hyperglycaemic mimetic glucosamine to IVM medium for mouse COCs increased detectable O-GlcNAcylated protein levels (by western blot and immunocytochemistry), and this effect was reversed using an OGT inhibitor (P < 0.05). HSP90 was identified as a target of O-GlcNAcylation in the COC, and inhibition of HSP90 during IVM reversed glucosamine-induced decreases in oocyte developmental competence (P < 0.05). We also demonstrated the novel finding of an association between HSP90 and OGT in COCs, suggesting a possible client-chaperone relationship.In vitro maturation of COCs was used so that treatment time could be limited to the 17 h of maturation prior to ovulation. Additionally, glucosamine, a hyperglycaemic mimetic, was used because it specifically activates the hexosamine pathway which provides the O-GlcNAc moieties. The results in this study should be confirmed using in vivo models of hyperglycaemia and different HSP90 inhibitors.This study leads to a new understanding of how diabetes influences oocyte competence and provides insight into possible therapeutic interventions based on inhibiting HSP90 to improve oocyte quality.This work was supported by a programme grant from the National Health and Medical Research Council, Australia, ID 453556. J.G.T. is a recipient of funding from and a consultant to Cook Medical Pty Ltd. The other authors have no conflicts of interest to declare.
URLPMID:23594928Magsci [本文引用: 3]

The function and impact of epidermal growth factor (EGF)-like peptide signalling during ovulation and in vivo oocyte maturation (IVV) has been recently characterized, however, little is currently known about the effect of oocyte in vitro maturation (IVM) on this pathway. The aim of this study was to examine expression and functional aspects of three EGF-like peptides (amphiregulin, epiregulin and betacellulin) and their common receptor (EGFR) in cumulus cells during mouse oocyte IVM compared with IVV. Cumulusoocyte complexes (COCs) were collected from prepubertal mice either 46 h post-eCG (IVM) or 46 h post-eCG plus 0.512 h post-hCG (IVV). Time course experiments showed mRNA expression of all three EGF-like peptides and amphiregulin protein in IVM media were significantly lower for the majority of FSH-supplemented IVM compared with IVV. The supplementation of EGF during IVM yielded EGF-like peptide expression levels comparable with IVV and amphiregulin/epiregulin supplemented IVM. However, despite this, EGF activation of the COC EGFR remained significantly lower at 3 and 6 h of IVM than in vivo, and levels were similar to those observed during FSH-supplemented IVM. The addition of exogenous epiregulin during IVM significantly increased blastocyst rates, and epiregulin and amphiregulin improved blastocyst quality, compared with FSH or EGF. In conclusion, findings from this study suggest that the widely used IVM additives, FSH and EGF, are inadequate propagators of the essential EGF-like peptide signalling cascade. In contrast, the use of epiregulin and/or amphiregulin during IVM leads to improved oocyte developmental competence and therefore may be preferable IVM additives than FSH or EGF.
URLPMID:22950951 [本文引用: 1]

The IVM of mammalian cumulus-oocyte complexes (COCs) yields reduced oocyte developmental competence compared with oocytes matured in vivo. Altered cumulus cell function during IVM is implicated as one cause for this difference. We have conducted a microarray analysis of cumulus cell mRNA following IVM or in vivo maturation (IVV). Mouse COCs were sourced from ovaries of 21-day-old CBAB6F1 mice 46 h after equine chorionic gonadotrophin (5 IU, i.p.) or from oviducts following treatment with 5 IU eCG (61 h) and 5 IU human chorionic gonadotrophin (13 h). IVM was performed in alpha-Minimal Essential Medium with 50 mIU FSH for 17 h. Three independent RNA samples were assessed using the Affymetrix Gene Chip Mouse Genome 430 2.0 array (Affymetrix, Santa Clara, CA, USA). In total, 1593 genes were differentially expressed, with 811 genes upregulated and 782 genes downregulated in IVM compared with IVV cumulus cells; selected genes were validated by real-time reverse transcription-polymerase chain reaction (RT-PCR). Surprisingly, haemoglobin alpha (Hba-a1) was highly expressed in IVV relative to IVM cumulus cells, which was verified by both RT-PCR and western blot analysis. Because haemoglobin regulates O-2 and/or nitric oxide availability, we postulate that it may contribute to regulation of these gases during the ovulatory period in vivo. These data will provide a useful resource to determine differences in cumulus cell function that are possibly linked to oocyte competence.
URLPMID:4596359 [本文引用: 1]

The gonadotropin-induced resumption of oocyte meiosis in preovulatory follicles is preceded by expression of epidermal growth factor (EGF)-like peptides, amphiregulin (AREG) and epiregulin (EREG), in mural granulosa and cumulus cells. Both the gonadotropins and the EGF-like peptides possess the capacity to stimulate resumption of oocyte meiosis in vitro via activation of a broad signaling network in cumulus cells. To better understand the rapid genomic actions of gonadotropins (FSH) and EGF-like peptides, we analyzed transcriptomes of cumulus cells at 3h after their stimulation. We hybridized aRNA from cumulus cells to a pig oligonucleotide microarray and compared the transcriptomes of FSH- and AREG/EREG-stimulated cumulus cells with untreated control cells and vice versa. The identified over- and underexpressed genes were subjected to functional genomic analysis according to their molecular and cellular functions. The expression pattern of 50 selected genes with a known or potential function in ovarian development was verified by real-time qRT-PCR. Both FSH and AREG/EREG increased the expression of genes associated with regulation of cell proliferation, cell migration, blood coagulation and extracellular matrix remodeling. FSH alone induced the expression of genes involved in inflammatory response and in the response to reactive oxygen species. Moreover, FSH stimulated the expression of genes closely related to some ovulatory events either exclusively or significantly more than AREG/EREG (AREG, ADAMTS1, HAS2, TNFAIP6, PLAUR, PLAT, and HSD17B7). In contrast to AREG/EREG, FSH also increased the expression of genes coding for key transcription factors (CEBPB, FOS, ID1/3, and NR5A2), which may contribute to the differing expression profiles of FSH- and AREG/EREG-treated cumulus cells. The impact of FSH on cumulus cell gene transcription was higher than the impact of EGF-like factors in terms of the number of cell functions affected as well as the number of over- and underexpressed genes. Both FSH and EGF-like factors overexpressed genes involved in the post-ovulatory switch in steroidogenesis and tissue remodelling. However, FSH was remarkably more efficient in the up-regulation of several specific genes essential for ovulation of matured oocytes and also genes that been reported to play an important role in maturation of cumulus-enclosed oocytes in vitro.
URLPMID:26653334 [本文引用: 1]

Abstract A major challenge in assisted reproductive technology is to develop conditions for in vitro oocyte maturation yielding high-quality eggs. Efforts are underway to assess whether known hormonal and local factors play a role in oocyte developmental competence and to identify the molecular mechanism involved. Here we have tested the hypothesis that FSH improves oocyte developmental competence by regulating the translational program in the oocyte. Accumulation of oocyte proteins (targeting protein for the Xenopus kinesin xklp2 and IL-7) associated with improved oocyte quality is increased when cumulus-oocyte complexes are incubated with FSH. This increase is due to enhanced translation of the corresponding mRNAs, as indicated by microinjection of constructs in which the 3' untranslated region of the Tpx2 or Il7 transcripts is fused to the luciferase reporter. A transient activation of the phosphatidyl-inositol 3-phosphate/AKT cascade in the oocyte preceded the increase in translation. When the epidermal growth factor (EGF) receptor is down-regulated in follicular cells, the FSH-induced rate of maternal mRNA translation and AKT activation were lost, demonstrating that the effects of FSH are indirect and require EGF receptor signaling in the somatic compartment. Using Pten(fl/fl):Zp3cre oocytes in which the AKT is constitutively activated, translation of reporters was increased and was no longer sensitive to FSH stimulation. More importantly, the oocytes lacking the phosphate and tensin homolog gene showed increased developmental competence, even when cultured in the absence of FSH or growth factors. Thus, we demonstrate that FSH intersects with the follicular EGF network to activate the phosphatidyl-inositol 3-phosphate/AKT cascade in the oocyte to control translation and developmental competence. These findings provide a molecular rationale for the use of FSH to improve egg quality.
URLPMID:27422885 [本文引用: 1]

The cyclic nucleotides, cAMP and cGMP, are the key molecules controlling mammalian oocyte meiosis. Their roles in oocyte biology have been at the forefront of oocyte research for decades, and many of the long-standing controversies in relation to the regulation of oocyte meiotic maturation are now resolved. It is now clear that the follicle prevents meiotic resumption through the actions of natriuretic peptides and cGMP – inhibiting the hydrolysis of intra-oocyte cAMP – and that the pre-ovulatory gonadotrophin surge reverses these processes. The gonadotrophin surge also leads to a transient spike in cAMP in the somatic compartment of the follicle. Research over the past two decades has conclusively demonstrated that this surge in cAMP is important for the subsequent developmental capacity of the oocyte. This is important, as oocyte maturation (IVM) systems practised clinically do not recapitulate this cAMP surge , possibly accounting for the lower efficiency of IVM compared with clinical IVF. This review particularly focuses on this latter aspect – the role of cAMP/cGMP in the regulation of oocyte quality. We conclude that clinical practice of IVM should reflect this new understanding of the role of cyclic nucleotides, thereby creating a new generation of ART and fertility treatment options.
URLPMID:26254468 [本文引用: 1]

Growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) are oocyte-specific growth factors with central roles in mammalian reproduction, regulating species-specific fecundity, ovarian follicular somatic cell differentiation, and oocyte quality. In the human, GDF9 is produced in a latent form, the mechanism of activation being an open question. Here, we produced a range of recombinant GDF9 and BMP15 variants, examined their in silico and physical interactions and their effects on ovarian granulosa cells (GC) and oocytes. We found that the potent synergistic actions of GDF9 and BMP15 on GC can be attributed to the formation of a heterodimer, which we have termed cumulin. Structural modeling of cumulin revealed a dimerization interface identical to homodimeric GDF9 and BMP15, indicating likely formation of a stable complex. This was confirmed by generation of recombinant heterodimeric complexes of pro/mature domains (pro-cumulin) and covalent mature domains (cumulin). Both pro-cumulin and cumulin exhibited highly potent bioactivity on GC, activating both SMAD2/3 and SMAD1/5/8 signaling pathways and promoting proliferation and expression of a set of genes associated with oocyte-regulated GC differentiation. Cumulin was more potent than pro-cumulin, pro-GDF9, pro-BMP15, or the two combined on GC. However, on cumulus-oocyte complexes, pro-cumulin was more effective than all other growth factors at notably improving oocyte quality as assessed by subsequent day 7 embryo development. Our results support a model of activation for human GDF9 dependent on cumulin formation through heterodimerization with BMP15. Oocyte-secreted cumulin is likely to be a central regulator of fertility in mono-ovular mammals.
URLPMID:27488026 [本文引用: 1]

Abstract C-type natriuretic peptide (CNP) and its receptor Natriuretic peptide receptor 2 (NPR2) play a paramount role in the maintenance of oocyte meiotic arrest in antral follicles via the regulation of the intra-oocyte levels of cyclic guanosine monophosphate and cyclic adenosine monophosphate. We investigated the potential of CNP 1) to maintain oocyte meiotic arrest during a prolonged prematuration culture, and 2) to sustain acquisition of developmental competence of immature cumulus-oocyte complexes (COCs). Compact COCs were collected from small antral follicles of pre-pubertal unprimed mice and placed in prematuration culture under different CNP-supplemented media conditions. A preliminary analysis showed a dose-dependent effect of CNP on the maintenance of meiotic arrest. A dose of 25nM maintained oocytes under meiotic arrest for 24 h, and this period was extended to 48 h in presence of Estradiol. Analysis of transzonal projections of COCs cultured with CNP indicated that oocyte-cumulus connections were well preserved after the prolonged prematuration culture. Furthermore, CNP medium supplemented with FSH and GDF9 promoted oocyte growth and induced a shift in oocyte chromatin configuration from a predominant disperse- to a condensed configuration. Following IVM, oocytes cultured under CNP were capable to extrude the first polar body at a high rate (around 80%). Blastocyst formation was significantly improved when oocytes were cultured under CNP-supplemented medium containing FSH and GDF9. This study reports for the first time a prolonged prematuration culture system, having CNP as pivotal factor, which can efficiently maintain oocytes retrieved from unprimed prepubertal mice under meiotic arrest while promoting their acquisition of developmental competence. Copyright 2016 by The Society for the Study of Reproduction.
