2.
Research on the knockout of LMNA gene by CRISPR/Cas9 system in human cell lines
Heng Liu1, Dongming Li1, Lanyu Zhu1, Lejin Lai1, Wanyun Yan1, Yushuang Lu1, Yi Wei1, Yueqi Huang1, Mei Fang1, Yuangang Su1, Fang Yang2, Wei Shu11. 2.
第一联系人:
编委: 谷峰
收稿日期:2018-09-13修回日期:2018-11-21网络出版日期:2019-01-20
| 基金资助: |
Received:2018-09-13Revised:2018-11-21Online:2019-01-20
| Fund supported: |
作者简介 About authors
刘恒,硕士研究生,专业方向:分子生物学与生物化学E-mail:
李东明,硕士研究生,专业方向:分子生物与生物化学E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (854KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
刘恒, 李东明, 朱兰玉, 赖乐锦, 闫婉云, 陆玉双, 韦伊, 黄月琪, 方媚, 苏元港, 杨芳, 舒伟. 利用CRISPR/Cas9敲除人源细胞系中LMNA基因的研究[J]. 遗传, 2019, 41(1): 66-75 doi:10.16288/j.yczz.18-146
Heng Liu, Dongming Li, Lanyu Zhu, Lejin Lai, Wanyun Yan, Yushuang Lu, Yi Wei, Yueqi Huang, Mei Fang, Yuangang Su, Fang Yang, Wei Shu.
细胞核内核膜的核纤层在维持核结构和核稳定方面起到关键作用,也影响到基因的组织和表达[1,2,3]。核纤层由5种中间纤维蛋白组成,包括A型核纤层蛋白(Lamin A)、C型核纤层蛋白(Lamin C)、B1型核纤层蛋白(Lamin B1)、B2型核纤层蛋白(Lamin B2),以及核纤层相关蛋白。其中A型与C型核纤层蛋白由LMNA基因编码表达,LMNA基因的功能包括支撑细胞核膜并调控核膜的分解与重构,稳固染色质和核周转录因子,提供基因组需要的活动场所,还参与细胞骨架到细胞核之间的信号传导。此外,LMNA蛋白直接与核稳定性和细胞可塑性相关[4]。最近的研究表明,核纤层蛋白在人类恶性肿瘤中异常表达[5,6]。在前列腺癌、乳腺癌、结肠癌、卵巢癌和胃癌中,研究人员发现核纤层蛋白表达下调,而且常常与预后不良有关[7]。在结直肠癌、前列腺癌和乳腺癌的研究报告中,Lamin A/C的表达变化与预后存在关联[8]。LMNA基因突变与十几种退行性疾病有关,包括肌肉萎缩症(如Emery-Dreyfus肌肉萎缩症,EDMD)、外周神经病变(如 Charcot-Marie-牙病2B1型, CMT2B1)、脂肪代谢障碍、儿童早老综合征(如 Hutchinson-Gilford早老综合征,HGPS),此外还有非典型沃纳综合征(Atypical Werner syndrome, AWS)、硬皮病(restrictive dermopathy, RD)[9,10,11,12]等。LMNA基因的不同突变能导致同种类型的疾病,而且同一碱基的不同替换也会导致不同的疾病,同样的LMNA突变在一些个体会导致疾病,一些个体却不会有临床症状,这些都表明了LMNA功能复杂而多样[13,14]。人们对于核纤层蛋白病中的基因型和表型之间的联系不甚了解,对LMNA基因的生物学功能也有待进一步研究。
Lamin A和C是由LMNA基因选择性剪切产生,位于细胞核内膜内侧,主要在已分化细胞内表达[15]。Lamin C是由LMNA基因在第10号外显子选择性剪切产生,与Lamin A功能类似,共同在细胞核膜结构和功能中发挥重要作用[16]。为研究LMNA基因在细胞内的功能,本研究利用CRISPR/Cas9技术对不同来源的体外培养细胞(293T和HepG2)中LMNA进行基因编辑,通过敲除LMNA基因使Lamin A/C都不表达,并对敲除后细胞系的形态和增殖情况进行了观察。
1 材料与方法
1.1 材料
293T与HepG2细胞由本实验室保存;PX459质粒购自美国Addgene公司。1.2 gRNA设计与合成
利用美国麻省理工学院 CRISPR Design 软件(http://crispr.mit.edu/)进行gRNA的设计,然后用NCBI的Primer-Blast对所设计的引物进行验证。一共设计4条gRNA,如表1所示。gRNA合成由上海生工生物工程技术服务有限公司负责完成。Table 1
表1
表1 靶向人源LMNA基因敲除的gRNA序列
Table 1
| 名称 | 序列(5°→3°) |
|---|---|
| FO1 | TTCCGCCAGCAGCCGCCGGC |
| RO1 | GCCGGCGGCTGCTGGCGGAA |
| FO2 | AGCGGGAGATGGCCGAGATG |
| RO2 | CATCTCGGCCATCTCCCGCT |
| FO3 | CACGCAGCTCCTGGAAGGGT |
| RO3 | ACCCTTCCAGGAGCTGCGTG |
| FO4 | GCGCCGTCATGAGACCCGAC |
| RO4 | GTCGGGTCTCATGACGGCGC |
新窗口打开|下载CSV
1.3 载体构建
将合成的gRNA正义链和反义链进行复性处理形成双链,反应体系为正、反义链各1 μL,T4连接酶缓冲液(10×) 1 μL,T4多核苷酸激酶(PNK) 0.5 μL,双蒸水6.5 μL,95℃ 5 min,自然冷却至16℃保存10 min。然后利用BbsⅠ内切酶对PX4594℃条件下剪切过夜,电泳切胶回收剪切后的质粒。将寡核苷酸双链与剪切后质粒用T4连接酶进行重组。反应体系为:PX459质粒 50 ng,寡核苷酸双链2 μL,T4连接酶1.5 μL,T4连接酶缓冲液1.5 μL,双蒸水补至15 μL。反应程序:16℃连接10 min后,4℃静置过夜。将重组后的载体悬液产物5~10 μL加入到50 μL的DH5α感受态细胞中,轻弹混匀后冰浴30 min,42℃热激90 s,冰上静置2 min,然后直接涂于氨苄抗性的LB平板。37℃培养箱过夜后挑3~5个白色菌落摇菌培养,提取质粒DNA进行测序验证。测序引物为:上游引物5′-GAGGGCCTATTTCCCATGAT- 3′,下游引物5′-GGGCGTACTTGGCATATGAT-3′。引物由上海生工生物工程技术服务有限公司负责合成。1.4 细胞转染
在6孔板中每孔加入2×106个细胞,12 h后PBS清洗两次;将脂质体(Lipofectamine 3000 Transfection reagent,美国Invitrogen) 10 μL与250 μL无血清培养基混匀,室温放置5 min,同时把5 μg重组质粒与250 μL无血清培养基混匀,室温静置5 min;然后将两种液体混匀,室温静置20 min,最后加入培养孔。在转染6~8 h后换完全培养基,24 h后更换新鲜完全培养基,并加入嘌呤霉素进行筛选(293T的工作浓度为2.0 ug/mL,HepG2的工作浓度为1.5 μg/mL)。1.5 单克隆测序分析
在嘌呤霉素筛选3天后,用胰酶把细胞消化下来,利用有限稀释法稀释细胞至每1个/100 μL,然后在96孔板的每孔中加入100 μL细胞悬液,显微镜观察之后将只有单个细胞的培养孔做好标记;培养五天后更换新鲜培养基,细胞铺板80%以上消化细胞,一半提取DNA测序验证,一半细胞换六孔板扩大培养。收集单克隆细胞,在提取DNA后进行PCR扩增。PCR体系为:DNA 100 ng,上下游引物各0.2 μL,2× Taq mastermix 25 μL,双蒸水补至50 μL;取PCR扩增产物进行1%琼脂糖凝胶,100 V、25 min电泳,得到目的条带后,剩余PCR扩增产物送测序。由于HepG2 gRNA的设计位点(PX-459-gRNA1和PX- 459-gRNA2)与293T gRNA的设计位点(PX-459- gRNA3和PX-459-gRNA4)不同,所以设计了2对不同的的引物。其中Primer1用于293T细胞,Primer2用于HepG2细胞。基因组测序引物如表2所示。由上海生工生物工程技术服务有限公司负责合成。PCR产物电泳切胶回收,在微量离心管中加入T载体1 μL (50 ng),加入等摩尔数PCR产物;加入含ATP的10× Buffer 1 μL,T4 DNA连接酶合适单位,用双蒸水补足至10 μL;低速离心,4℃连接过夜;转化,蓝白斑筛选并菌落PCR测序。
Table 2
表2
表2 LMNA基因敲除位点的测序引物序列
Table 2
| 名称 | 序列(5°→3°) |
|---|---|
| Primer1 | F:TCTGGGGAAGCTCTGATTGC |
| Primer1 | R:AGTGGGGGTCTAGTCAAGGC |
| Primer2 | F:TGATGACAGACTTGGGCTGG |
| Primer2 | R:ACCAATCGAGAGCAAGCACC |
新窗口打开|下载CSV
1.6 免疫荧光检测
将细胞爬片用PBS浸洗;用4%的多聚甲醛固定爬片15 min,然后用PBS浸洗玻片3次,每次3 min;再使用0.5% Triton X-100 (PBS配制)室温通透20 min;PBS浸洗玻片后用吸水纸吸干PBS,用5%脱脂奶粉(PBST)室温封闭30 min;吸水纸吸掉封闭液之后用稀释一抗(Lamin B, AF0219购自中国上海碧云天生物技术有限公司;β-actin, ab8226购自美国Abcam公司) 4℃孵育过夜;然后用PBST浸洗爬片,吸水纸吸干后滴加荧光二抗,室温孵育孵育1 h后用PBST浸洗爬片3次,每次3 min;最后用荧光显微镜观察。1.7 蛋白免疫印记检测
293T与HepG2的野生型和LMNA KO型细胞六孔板铺板24 h后,RIPA裂解液(加pmsf)裂解细胞收集蛋白,聚丙烯酰胺凝胶电泳后转PVDF膜。封闭后,相应一抗(Lamin A,ab8089和Lamin C,ab106682均购自美国Abcam公司) 4℃孵育过夜,二抗室温1 h。凝胶成像系统成像。1.8 细胞增殖实验
把293T与HepG2的野生型和LMNA KO型细胞胰酶消化成单个细胞,在96孔板种板中每孔加入1500个细胞,37℃培养箱中培养;18 h后加入Cell Counting Kit-8,1 h后酶标仪450 nm测吸光度,每种细胞测3个孔,取平均值。每隔一天测一次吸光度,直到其中一种细胞铺板达70%以上就停止细胞增殖实验。1.9 细胞凋亡实验
用无EDTA胰酶消化细胞长满50%~75%状态良好的293T与HepG2的野生型和LMNA KO型细胞,PBS清洗之后1000 r/min 3 min收集细胞。利用Annexin V-FITC/PI双染细胞凋亡试剂盒染色,避光孵育10 min后用流式细胞仪检测。1.10 扫描电镜观察
收集离心细胞后,用3%戊二醛在4℃固定2 h后0.1 mmol/L PPS缓冲液4℃下浸泡清洗3次,每次10 min;然后用四氧化锇在4℃固定1 h,再用0.1 mmol/L PPS缓冲液4℃下浸泡清洗3次,每次15 min;依次从50%、70%、80%、90%、100% (3次)的乙醇浓度浸泡脱水,每次15 min;再使用100%六甲基二硅胺烷浸泡3次,每次10 min,然后放入真空干燥器抽真空干燥;粘样本到样本座,IB3 (IB5)离子溅射仪喷镀后放入电镜(VEGA 3 LMU型,捷克TESCAN公司)观察。1.11 透射电镜观察
收集离心细胞,用3%戊二醛在4℃固定2 h;0.1 mmol/LPPS缓冲液4℃下浸泡清洗3次,每次10 min;用四氧化锇在4℃固定1 h;0.1 mmol/LPPS 缓冲液4℃下浸泡清洗3次,每次15 min;乙醇浓度依次从50%、70%、80%、90%、1∶1 (90%乙醇∶90%丙酮)、90%丙酮、100%丙酮(3次)浸泡脱水,每次15 min;1∶1(丙酮∶包埋剂)室温渗透1 h,纯包埋剂室温浸透2 h;环氧树脂包埋;依次从35℃ 15 h,45℃ 12 h,60℃ 24 h聚合;在修块、超薄切片与染色之后,利用电镜(H-7650型,日本HTACHI公司)观察。2 结果与分析
2.1 PX-459-gRNA(LMNA)重组质粒构建和单克隆测序分析
设计并合成gRNA后,把gRNA与PX459载体连接。PX459具有嘌呤霉素抗性,方便于之后的的细胞单克隆筛选。在转染重组质粒与挑纯之后,提取单克隆细胞的DNA,扩增gRNA两侧400 bp左右序列,测序结果显示在gRNA附近位点开始出现杂峰,经人工比对后,杂峰中不存在原始序列,说明成功的剪切了293T和HepG2的LMNA两个等位基因。然后我们利用TA克隆测序法验证得出两株细胞的LMNA等位都缺失若干碱基(图1A)。将测序结果与LMNA全基因序列进行比对后,发现293T单克隆细胞株在LMNA等位基因分别缺失40和42个碱基,HepG2单克隆细胞株在LMNA等位基因分别缺失35和39个碱基(图1B)。图1
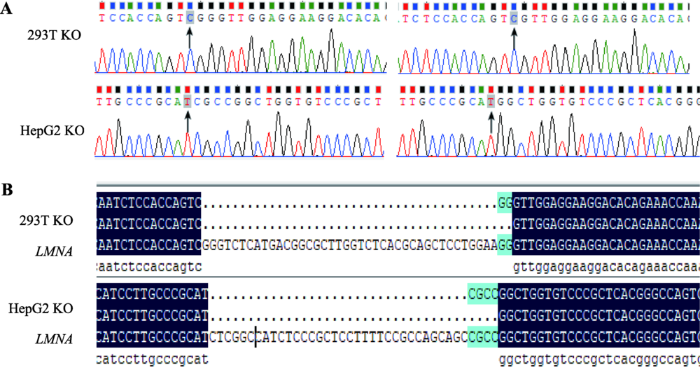 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1LMNA基因敲除单克隆细胞株的TA克隆测序鉴定
A:阳性单克隆细胞的鉴定。黑色箭头指示为CRISPR/Cas9系统开始剪切的位点。B:阳性克隆序列与LMNA全基因序列对比。序列比对显示在gRNA处剪切,293T的LMNA等位基因分别缺失40和42个碱基,HepG2的LMNA等位基因分别缺失35和39个碱基。KO为LMNA敲除细胞。
Fig. 1TA clone sequencing identified the LMNA KO monoclonal cell line
2.2 敲除细胞内LMNA基因表达分析
LMNA基因共有12个外显子,选择性剪切编码Lamin A和Lamin C蛋白。Lamin A 由1-12号外显子编码,Lamin C由1~10号外显子编码(图2A)。PX-459-gRNA1和PX-459-gRNA2(用于HepG2)的识别位点位于第9号外显子;PX-459-gRNA3和PX- 459-gRNA4 (用于293T)的识别位点在第7号外显子。Lamin A蛋白免疫印迹结果显示,293T KO和HepG2 KO不表达Lamin A (图2B)。免疫荧光技术分析结果显示,低倍镜下293T KO和HepG2 KO细胞 Lamin A (绿色)未见荧光信号(图2C)。上述两个结果显示,利用CRISPR/Cas9技术成功获得了敲除LMNA基因293T与HepG2细胞株。同时,Lamin C蛋白免疫印迹结果显示两株LMNA KO细胞也不能表达Lamin C (图2D)。图2
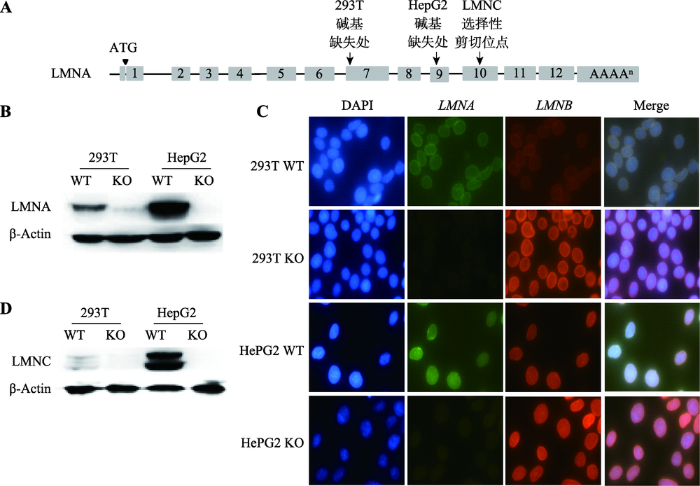 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2LMNA基因敲除细胞内Lamin A和Lamin C表达分析
A:LMNC与LMNA敲除细胞株剪切位点示意图。两株LMNA敲除细胞系的剪切位点都位于LMNC选择性剪切位点之前。B:Lamin A的蛋白免疫印迹。C:LMNA敲除后细胞的免疫荧光比较(400×)。蓝色为DAPI,绿色为Lamin A,红色为Lamin B。D:Lamin C的蛋白免疫印迹。WT为野生型细胞,KO为LMNA敲除细胞。
Fig. 2Analysis of Lamin A and Lamin C expression in LMNA gene knockout cells
2.3 LMNA基因敲除后对细胞增殖和凋亡的影响
CCK-8分析结果显示,LMNA基因敲除之后293T和HepG2两株细胞的增殖受到明显的抑制(图3A),其中对肝癌细胞HepG2的增殖抑制更为明显。同时,利用流式细胞仪对细胞凋亡情况进行检测,Lamin A敲除后,两株细胞的凋亡率都显著上升(图3:B, C)。图3
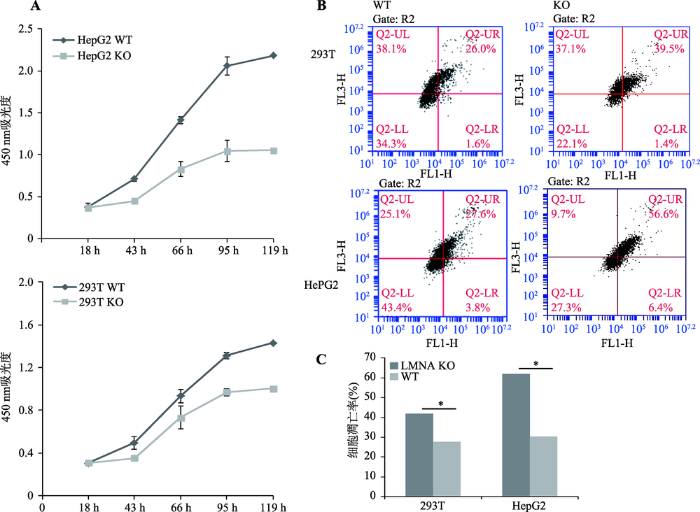 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3LMNA敲除细胞株和野生细胞株的细胞增殖能力和凋亡比较
A:细胞增殖曲线。LMNA敲除后细胞增殖能力都明显下降(n=3)。B:流式细胞仪检测细胞凋亡结果。LMNA敲除后细胞凋亡率都显著上升,FL1-H为绿光通道(Annexin V-FITC),FL3-H为红光通道(PI)。C:细胞凋亡的统计学分析(P<0.05, n=3)。293T WT为293T野生型细胞,293T KO为293T LMNA基因敲除细胞,HepG2 WT为HepG2野生型细胞,HepG2 KO为HepG2 LMNA基因敲除细胞。
Fig. 3Cell proliferation and apoptosis in LMNA knockout cell lines and its wild type
2.4 LMNA基因敲除后细胞核和细胞整体形态的变化
LMNA基因敲除后,与野生型对比,光镜下观察这两株细胞形态上发生了明显改变(图4A)。293T野生型细胞有3~4个突出,在LMNA敲除后,293T细胞胞体变小变圆,突出连接减少变细;HepG2野生型为长梭型,细胞核在正中突出,胞质主要分布在细胞的梭型两端,Lamin A敲除后,细胞变成多边形,胞质较为均匀的分布在核周。DAPI染色后,未发现细胞核发生核凹陷、核畸形等改变(图4B)。为了更清楚的观察细胞形态的改变,利用扫描电镜把细胞放大3000倍(图4C),形态差异更为显著;利用透射电镜将细胞膜放大15 000倍,发现LMNA KO细胞的核膜变得凹凸不平(图4D)。通过免疫荧光技术对细胞骨架(β-actin)进行染色,油镜下也观察到相同的形态变化(图5)。图4
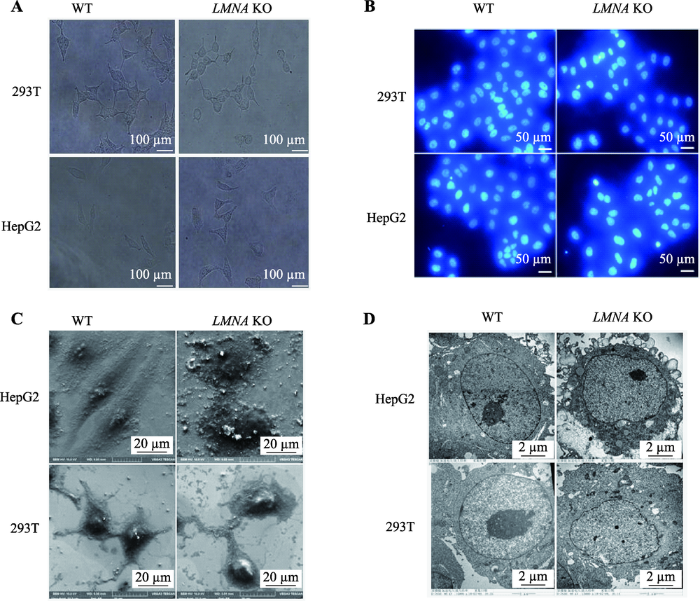 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4LMNA基因敲除后细胞核和细胞整体形态的变化
A:普通光学显微镜下的细胞形态。LMNA敲除后细胞形态发生明显改变。B:DAPI染色后的细胞核形态 未观察到显著差异。C:扫描电镜下的细胞形态。D:透射电镜下的细胞核形态。与野生型对比,LMNA KO细胞核膜凹凸不平WT为野生型细胞,KO为LMNA敲除细胞。
Fig. 4The morphological changes of LMNA KO cells
图5
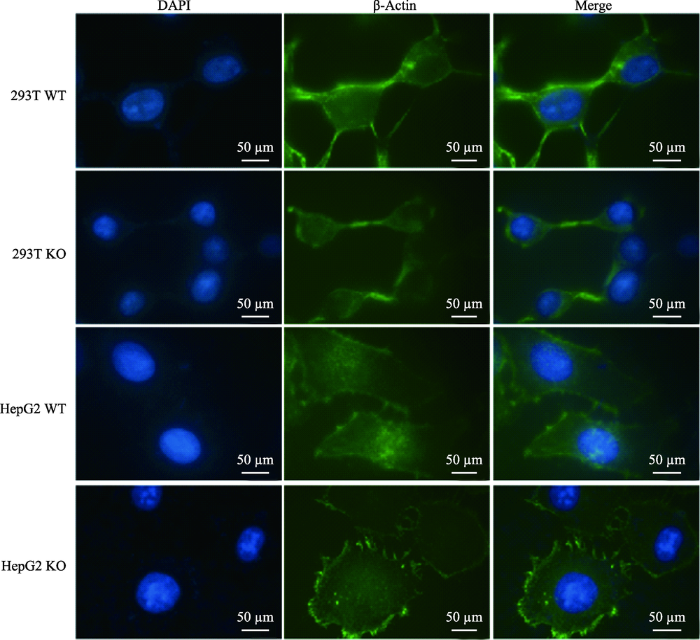 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5免疫荧光技术染色微丝观察LMNA敲除后细胞形态的改变
LMNA敲除后细胞骨架微丝的改变更为明显,蓝色为DAPI染色的细胞核,绿色为β-actin 293T WT为293T野生型细胞,293T KO为293T LMNA基因敲除细胞,HepG2 WT为HepG2野生型细胞,HepG2 KO为HepG2 LMNA基因敲除细胞。
Fig. 5Immunofluorescence staining microfilaments of LMNA knockout cell and its wild type
3 讨论
本文首次利用CRISPR/Cas9系统成功敲除293T和HepG2的LMNA基因。虽然LMNA敲除小鼠早有报道[17],研究人员也获得了敲除小鼠来源的体外培养成纤维细胞系[18],但人源LMNA基因敲除的细胞系未见报道。本研究构建的人源LMNA基因敲除细胞系为进一步基因功能研究奠定基础。LMNA基因不同突变会导致多种疾病,这与核纤层蛋白所发挥的多种细胞功能相一致[9,19,20]。核纤层蛋白病是人类疾病中最耐人寻味的退行性疾病之一,基因型与表型之间的关系仍然不甚清楚[21,22]。有趣的是,尽管核纤层蛋白普遍表达,但在每一种核纤层蛋白病中,只有一种或几种组织受到损害,为了解释这个复杂的谜题,研究人员常常构建不同的LMNA基因突变体(LMNA G608G,R527C和H222P突变等),然后在不同体外培养细胞系中研究突变体的功能[23]。然而,较多的突变体,尤其是隐性遗传的致病突变体,往往在有内源性Lamin A和Lamin C存在的情况下,很难观察到突变体对细胞的影响。本研究构建的敲除细胞系可以避免内源性Lamin A和Lamin C对突变体功能研究的影响,是很好的研究隐性遗传的致病突变体的材料。
CRISPR/Cas9基因编辑技术作为新一代基因编辑技术,尽管具有设计简单,操作方便,效率高,成本低并且可同时进行多点编辑等优势,但也存在敲除效率不高的问题[24,25]。有研究显示,CRISPR/ Cas9系统导致的DNA双链断裂通过同源重组介导的DNA修复后剪切成功比例常不足10%[26,27,28]。前期实验中发现设计两条位点相近的gRNA共同转染能显著提高CRISPR/Cas9系统的双等位基因敲除效率。
在本研究中,构建的两株LMNA KO细胞株与野生型细胞相比增殖能力都明显下降,其中肝癌细胞HepG2的增殖能力下降更为显著,这说明LMNA对肿瘤增殖十分重要。通过流式细胞术检测细胞凋亡情况,我们发现,LMNA KO之后两株细胞的凋亡情况也大幅上升。虽然使用两株细胞特性差异较大的细胞系,293T为胚肾来源,HepG2为肝肿瘤来源。但是在增殖,凋亡与形态上的改变都类似效应(图3)。值得一提的是,高表达LMNA的肿瘤细胞系HepG2,敲除LMNA后其增殖和凋亡变化较为显著。较多的临床样本研究中已经发现的LMNA基因在多种肿瘤中异常表达[29,30],但LMNA异常表达在肿瘤发生发展中的意义未知,本研究观察到LMNA KO对肿瘤细胞增殖与凋亡都有显著影响,这是否与肿瘤的致瘤性相关有待进一步研究。
文献报道在Lamin A缺失的小鼠成纤维细胞中,微管组织中心(MTOC)就会压迫细胞核,形成较多变形的凹型/新月形细胞核[31]。该文献提出假说,细胞核与MTOC相互有一个作用力,只有当这个力度平衡时,细胞核才能维持正常形态[32]。而核纤层蛋白正是细胞核的这种作用力的提供者之一[33]。有趣的是,本研究获得的两株LMNA基因敲除细胞系,却并未发现明显的核凹陷变形(图4和图5)。而只有通过透射电镜才发现核膜由平整光滑变得凹凸不平(图5)。本研究中使用的体外培养的永生化细胞系与文献报道中使用的原代培养的小鼠成纤维细胞不同,由此推测,可能是由于在Lamin A减少或者缺失时,Lamin B的表达相对会增加,而体外培养的永生化细胞内Lamin B的表达大大增加[34]正好调整了这种失衡。
本研究发现两株LMNA KO细胞与野生型相比形态发生明显改变(图4)。LMNA编码核纤层蛋白Lamin A/C,为细胞核提供结构支持。LMNA表达水平与核刚度、组织的硬度和可塑性直接相关[5,35,36]。核刚度对于细胞的生存能力和维持分化细胞的功能都很重要,尤其是在肌肉和皮肤等承受强烈机械张力的组织中[36,37]。
综上所述,LMNA基因功能与细胞增殖、凋亡相关,也影响细胞骨架的分布。本研究成功获得两株体外培养的LMNA KO细胞,为将来研究LMNA基因在肝肿瘤中的作用奠定基础;也为研究LMNA中隐性致病突变是如何对不同细胞产生影响,以及这些突变体如何导致细胞功能的改变提供实验材料。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URL [本文引用: 1]
URLPMID:25747401 [本文引用: 1]

Lamins are proteins that form a scaffold, termed , at the nuclear periphery. A small fraction of lamins also localize throughout the . Lamins bind to a growing number of nuclear protein complexes and are implicated in both nuclear and cytoskeletal organization, mechanical stability, organization, gene regulation, genome stability, differentiation, and tissue-specific functions. The laminbased complexes and their specific functions also provide insights into possible disease mechanisms for laminopathies, ranging from to accelerated , as observed in and atypical . Expected final online publication date for the Annual Review of Biochemistry Volume 84 is June 02, 2015. Please see http://www.annualreviews.org/catalog/pubdates.aspx for revised estimates.
URLPMID:25460776 [本文引用: 1]

Lamins are nuclear intermediate filament (IF) proteins. They assemble to fibrous structures that are positioned between the inner nuclear membrane and the peripheral chromatin. A small fraction of lamins is also present in the nucleoplasm. Lamins are required to maintain the nuclear structure and, together with their associated proteins, are involved in most nuclear activities. Mutations in lamins cause >14 distinct diseases, called laminopathies, that include heart, muscle, fat and early aging diseases. However, it is not clear how lamins are organized in vivo and how the disease mutations affect lamin organization and functions. Here, we will review structural aspects of lamin assembly, discuss differences between peripheral and nucleoplasmic lamins and describe the protein complexes that lamins form.
URLPMID:23990565 [本文引用: 1]

Tissues can be soft like fat, which bears little stress, or stiff like bone, which sustains high stress, but whether there is a systematic relationship between tissue mechanics and differentiation is unknown. Here, proteomics analyses revealed that levels of the nucleoskeletal protein lamin-A scaled with tissue elasticity, E, as did levels of collagens in the extracellular matrix that determine E. Stem cell differentiation into fat on soft matrix was enhanced by low lamin-A levels, whereas differentiation into bone on stiff matrix was enhanced by high lamin-A levels. Matrix stiffness directly influenced lamin-A protein levels, and, although lamin-A transcription was regulated by the vitamin A/retinoic acid (RA) pathway with broad roles in development, nuclear entry of RA receptors was modulated by lamin-A protein. Tissue stiffness and stress thus increase lamin-A levels, which stabilize the nucleus while also contributing to lineage determination.
URLPMID:25436017 [本文引用: 2]

Cell motility plays a critical role in many physiological and pathological settings, ranging from wound healing to cancer metastasis. While cell migration on 2-dimensional (2-D) substrates has been studied for decades, the physical challenges cells face when moving in 3-D environments are only now emerging. In particular, the cell nucleus, which occupies a large fraction of the cell volume and is normally substantially stiffer than the surrounding cytoplasm, may impose a major obstacle when cells encounter narrow constrictions in the interstitial space, the extracellular matrix, or small capillaries. Using novel microfluidic devices that allow observation of cells moving through precisely defined geometries at high spatial and temporal resolution, we determined nuclear deformability as a critical factor in the cells ability to pass through constrictions smaller than the size of the nucleus. Furthermore, we found that cells with reduced levels of the nuclear envelope proteins lamins A/C, which are the main determinants of nuclear stiffness, passed significantly faster through narrow constrictions during active migration and passive perfusion. Given recent reports that many human cancers have altered lamin expression, our findings suggest a novel biophysical mechanism by which changes in nuclear structure and composition may promote cancer cell invasion and metastasis.
URLPMID:28851664 [本文引用: 1]

react-text: 116 We report a case of epithelioid hemangioma in a relatively uncommon location but with typical features in a 55-year-old man. A 55-year-old man was referred to our hospital with an asymptomatic mass of the right third rib, after the mass was incidentally found during a health checkup in April 2014. The mass had been gradually growing in size during the previous 1 year and 8 months. On physical... /react-text react-text: 117 /react-text [Show full abstract]
URLPMID:5136755 [本文引用: 1]

Lamins are the key components of the nuclear lamina and by virtue of their interactions with chromatin and binding partners act as regulators of cell proliferation and differentiation. Of late, the diverse roles of lamins in cellular processes have made them the topic of intense debate for their role in cancer progression. The observations about aberrant localization or misexpression of the nuclear lamins in cancerous tissues have often led to the speculative role of lamins as a cancer risk biomarker. Here we discuss the involvement of lamins in several cancer subtypes and their potential role in predicting the tumor progression.
URLPMID:22795640 [本文引用: 1]

Recent studies have shown that premature cellular senescence and normal organ development and function depend on the type V intermediate filament proteins, the lamins, which are major structural proteins of the nucleus. This review presents an up-to-date summary of the literature describing new findings on lamin functions in various cellular processes and emphasizes the relationship between the lamins and devastating diseases ranging from premature aging to cancer. Recent insights into the structure and function of the A- and B- type lamins in normal cells and their dysfunctions in diseased cells are providing novel targets for the development of new diagnostic procedures and disease intervention. We summarize these recent findings, focusing on data from mice and humans, and highlight the expanding knowledge of these proteins in both healthy and diseased cells.
URLPMID:2701866 [本文引用: 2]

The main function of the , an meshwork lying primarily beneath the inner , is to provide structural scaffolding for the cell . However, the lamina also serves other functions, such as having a role in organization, connecting the to the , gene , and . In somatic cells, the main protein constituents of the are , C, B1, and B2. Interest in the nuclear lamins increased dramatically in recent years with the realization that mutations in , the gene encoding and C, cause a panoply of diseases ("laminopathies"), including , , , and . Here, we review the laminopathies and the long strange trip from basic cell biology to therapeutic approaches for these diseases.
URLPMID:24485450 [本文引用: 1]

Rare diseases are powerful windows into biological processes and can serve as models for the development of therapeutic strategies. The progress made on the premature aging disorder Progeria is a shining example of the impact that studies of rare diseases can have.
URLPMID:26079711 [本文引用: 1]

The integrity of the nuclear lamina has emerged as an important factor in the maintenance of genome stability. In particular, mutations in the LMNA gene, encoding A-type lamins (lamin A/C), alter nuclear morphology and function, and cause genomic instability. LMNA gene mutations are associated with a variety of degenerative diseases and devastating premature aging syndromes such as Hutchinson-Gilford Progeria Syndrome (HGPS) and Restrictive Dermopathy (RD). HGPS is a severe laminopathy, with patients dying in their teens from myocardial infarction or stroke. HGPS patient-derived cells exhibit nuclear shape abnormalities, changes in epigenetic regulation and gene expression, telomere shortening, genome instability, and premature senescence. This review highlights recent advances in identifying molecular mechanisms that contribute to the pathophysiology of HGPS, with a special emphasis on DNA repair defects and genome instability.
URLPMID:26847180 [本文引用: 1]

Hutchinson ilford progeria syndrome (HGPS) is an extremely rare premature aging disease presenting many features resembling the normal aging process. HGPS patients die before the age of 20 years due to cardiovascular problems and heart failure. HGPS is linked to mutations in theLMNAgene encoding the intermediate filament protein lamin A. Lamin A is a major component of the nuclear lamina, a scaffold structure at the nuclear envelope that defines mechanochemical properties of the nucleus and is involved in chromatin organization and epigenetic regulation. Lamin A is also present in the nuclear interior where it fulfills lamina-independent functions in cell signaling and gene regulation. The most commonLMNAmutation linked to HGPS leads to mis-splicing of theLMNAmRNA and produces a mutant lamin A protein called progerin that tightly associates with the inner nuclear membrane and affects the dynamic properties of lamins. Progerin expression impairs many important cellular processes providing insight into potential disease mechanisms. These include changes in mechanosignaling, altered chromatin organization and impaired genome stability, and changes in signaling pathways, leading to impaired regulation of adult stem cells, defective extracellular matrix production and premature cell senescence. In this review, we discuss these pathways and their potential contribution to the disease pathologies as well as therapeutic approaches used in preclinical and clinical tests.
URLPMID:3183053 [本文引用: 1]

Today, there are at least a dozen different genetic disorders caused by mutations within the LMNA gene, and collectively, they are named laminopathies. Interestingly, the same mutation can cause phenotypes with different severities or even different disorders and might, in some cases, be asymptomatic. We hypothesized that one possible contributing mechanism for this phenotypic variability could be the existence of high and low expressing alleles in the LMNA locus. To investigate this hypothesis, we developed an allele-specific absolute quantification method for lamin A and lamin C transcripts using the polymorphic rs4641C/T LMNA coding SNP. The contribution of each allele to the total transcript level was investigated in nine informative human primary dermal fibroblast cultures from Hutchinson-Gilford progeria syndrome (HGPS) and unaffected controls. Our results show differential expression of the two alleles. The C allele is more frequently expressed and accounts for 70% of the lamin A and lamin C transcripts. Analysis of samples from six patients with Hutchinson-Gilford progeria syndrome showed that the c.1824C>T, p.G608G mutation is located in both the C and the T allele, which might account for the variability in phenotype seen among HGPS patients. Our method should be useful for further studies of human samples with mutations in the LMNA gene and to increase the understanding of the link between genotype and phenotype in laminopathies.
URL [本文引用: 1]
URLPMID:29208544 [本文引用: 1]

Mandibuloacral dysplasia (MAD) is a rare genetic condition characterized by bone abnormalities including localized osteolysis and generalized osteoporosis, skin pigmentation, lipodystrophic signs and mildly accelerated ageing. The molecular defects associated with MAD are mutations in LMNA or ZMPSTE24 ( FACE1 ) gene, causing type A or type B MAD, respectively. Downstream of LMNA or ZMPSTE24 mutations, the lamin A precursor, prelamin A, is accumulated in cells and affects chromatin dynamics and stress response. A new form of mandibuloacral dysplasia has been recently associated with mutations in POLD1 gene, encoding DNA polymerase delta, a major player in DNA replication. Of note, involvement of prelamin A in chromatin dynamics and recruitment of DNA repair factors has been also determined under physiological conditions, at the border between stress response and cellular senescence. Here, we review current knowledge on MAD clinical and pathogenetic aspects and highlight aspects typical of physiological ageing.
URLPMID:25283634 [本文引用: 1]

The A-type lamins, lamin A and lamin C, generated from a single gene, LMNA, are major structural components of the nuclear lamina. The two alternative splice products have mostly been studied...
URL [本文引用: 1]
URL [本文引用: 1]
URL [本文引用: 1]
URLPMID:4164485 [本文引用: 1]

Interconnected functional strategies govern chromatin dynamics in eukaryotic cells. In this context, A and B type lamins, the nuclear intermediate filaments, act on diverse platforms involved in tissue homeostasis. On the nuclear side, lamins elicit large scale or fine chromatin conformational changes, affect DNA damage response factors and transcription factor shuttling. On the cytoplasmic side, bridging-molecules, the LINC complex, associate with lamins to coordinate chromatin dynamics with cytoskeleton and extra-cellular signals.
URL [本文引用: 1]
URLPMID:15722103 [本文引用: 1]

Nuclear lamins were identified as core nuclear matrix constituents over 20 years ago. They have been ascribed structural roles such as maintaining nuclear integrity and assisting in nuclear envelope formation after mitosis, and have also been linked to nuclear activities including DNA replication and transcription. Recently, A-type lamin mutations have been linked to a variety of rare human diseases including muscular dystrophy, lipodystrophy, cardiomyopathy, neuropathy and progeroid syndromes (collectively termed laminopathies). Most diseases arise from dominant, missense mutations, leading to speculation as to how different mutations in the same gene can give rise to such a diverse set of diseases, some of which share little phenotypic overlap. Understanding the cellular dysfunctions that lead to laminopathies will almost certainly provide insight into specific roles of A-type lamins in nuclear organization. Here, we compare and contrast the LMNA mutations leading to laminopathies with emphasis on progerias, and discuss possible functional roles for A-type lamins in the maintenance of healthy tissues.
URLPMID:16772334 [本文引用: 1]

A-type lamins are components of the nuclear lamina. Mutations in the gene encoding lamin A are associated with a range of highly degenerative diseases termed laminopathies. To evaluate sensitivity to DNA damage, GFP-tagged lamin A cDNAs with disease-causing mutations were expressed in HeLa cells. The inner nuclear membrane protein emerin was mislocalised upon expression of the muscular dystrophy mutants G232E, Q294P or R386K, which aberrantly assembled into nuclear aggregates, or upon expression of mutants causing progeria syndromes in vivo (lamin A del50, R471C, R527C and L530P). The ability of cells expressing these mutants to form DNA repair foci comprising phosphorylated H2AX in response to mild doses of cisplatin or UV irradiation was markedly diminished, unlike the nearly normal response of cells expressing wild-type GFP-lamin A or disease-causing H222P and R482L mutants. Interestingly, mutants that impaired the formation of DNA repair foci mislocalised ATR (for 'ataxia telangiectasia-mutated and Rad3-related') kinase, which is a key sensor in the response to DNA damage. Our results suggest that a subset of lamin A mutants might hinder the response of components of the DNA repair machinery to DNA damage by altering interactions with chromatin.
URLPMID:25553825 [本文引用: 1]

Gene therapy by engineered nucleases is a genetic intervention being investigated for curing the hereditary disorders by targeting selected genes with specific nucleotides for establishment, suppression, abolishment of a function or correction of mutation. Here, we review the fast developing technology of targeted genome engineering using site specific programmable nucleases zinc finger nucleases (ZFNs), transcription activator like nucleases (TALENs) and cluster regulatory interspaced short palindromic repeat/CRISPR associated proteins (CRISPR/Cas) based RNA-guided DNA endonucleases (RGENs) and their different characteristics including pros and cons of genome modifications by these nucleases. We have further discussed different types of delivery methods to induce gene editing, novel development in genetic engineering other than nucleases and future prospects.
URL [本文引用: 1]

CRISPR/Cas9基因编辑技术在生命科学领域掀起了一场全新的技术革命,该技术可以对基因组特定位点进行靶向编辑,包括缺失、插入、修复等。CRISPR/Cas9比锌指核酸酶(ZFNs)和转录激活因子样效应物核酸酶(TALENs)技术更易于操作,而且更高效。CRISPR/Cas9系统中的向导RNA(Single guide RNA,sg RNA)是一段与目标DNA片段匹配的RNA序列,指导Cas9蛋白对基因组进行识别。研究发现,设计的sg RNA会与非靶点DNA序列错配,引入非预期的基因突变,即脱靶效应(Off-target effects)。脱靶效应严重制约了CRISPR/Cas9基因编辑技术的广泛应用。为了避免脱靶效应,研究者对影响脱靶效应的因素进行了系统研究并提出了许多降低脱靶效应的方法。文章总结了CRISPR/Cas9系统的应用及脱靶效应研究进展,以期为相关领域的工作提供参考。
URL [本文引用: 1]

CRISPR/Cas9基因编辑技术在生命科学领域掀起了一场全新的技术革命,该技术可以对基因组特定位点进行靶向编辑,包括缺失、插入、修复等。CRISPR/Cas9比锌指核酸酶(ZFNs)和转录激活因子样效应物核酸酶(TALENs)技术更易于操作,而且更高效。CRISPR/Cas9系统中的向导RNA(Single guide RNA,sg RNA)是一段与目标DNA片段匹配的RNA序列,指导Cas9蛋白对基因组进行识别。研究发现,设计的sg RNA会与非靶点DNA序列错配,引入非预期的基因突变,即脱靶效应(Off-target effects)。脱靶效应严重制约了CRISPR/Cas9基因编辑技术的广泛应用。为了避免脱靶效应,研究者对影响脱靶效应的因素进行了系统研究并提出了许多降低脱靶效应的方法。文章总结了CRISPR/Cas9系统的应用及脱靶效应研究进展,以期为相关领域的工作提供参考。
URLPMID:27120160 [本文引用: 1]

The bacterial CRISPR/Cas9 system allows sequence-specific gene editing in many organisms and holds promise as a tool to generate models of human diseases, for example, in human pluripotent stem cells. CRISPR/Cas9 introduces targeted double-stranded breaks (DSBs) with high efficiency, which are typically repaired by non-homologous end-joining (NHEJ) resulting in nonspecific insertions, deletions or other mutations (indels). DSBs may also be repaired by homology-directed repair (HDR) using a DNA repair template, such as an introduced single-stranded oligo DNA nucleotide (ssODN), allowing knock-in of specific mutations. Although CRISPR/Cas9 is used extensively to engineer gene knockouts through NHEJ, editing by HDR remains inefficient and can be corrupted by additional indels, preventing its widespread use for modelling genetic disorders through introducing disease-associated mutations. Furthermore, targeted mutational knock-in at single alleles to model diseases caused by heterozygous mutations has not been reported. Here we describe a CRISPR/Cas9-based genome-editing framework that allows selective introduction of mono- and bi-allelic sequence changes with high efficiency and accuracy. We show that HDR accuracy is increased dramatically by incorporating silent CRISPR/Cas-blocking mutations along with pathogenic mutations, and establish a method termed ‘CORRECT’ for scarless genome editing. By characterizing and exploiting a stereotyped inverse relationship between a mutation’s incorporation rate and its distance to the DSB, we achieve predictable control of zygosity. Homozygous introduction requires a guide RNA targeting close to the intended mutation, whereas heterozygous introduction can be accomplished by distance-dependent suboptimal mutation incorporation or by use of mixed repair templates. Using this approach, we generated human induced pluripotent stem cells with heterozygous and homozygous dominant early onset Alzheimer’s disease-causing mutations in amyloid precursor protein (APP) and presenilin 1 (PSEN1) and derived cortical neurons, which displayed genotype-dependent disease-associated phenotypes. Our findings enable efficient introduction of specific sequence changes with CRISPR/Cas9, facilitating study of human disease.
URLPMID:27030102 [本文引用: 1]

Precise genome-editing relies on the repair of sequence-specific nuclease-induced DNA nicking or double-strand breaks (DSBs) by homology-directed repair (HDR). However, nonhomologous end-joining (NHEJ), an error-prone repair, acts concurrently, reducing the rate of high-fidelity edits. The identification of genome-editing conditions that favor HDR over NHEJ has been hindered by the lack of a simple method to measure HDR and NHEJ directly and simultaneously at endogenous loci. To overcome this challenge, we developed a novel, rapid, digital PCR ased assay that can simultaneously detect one HDR or NHEJ event out of 1,000 copies of the genome. Using this assay, we systematically monitored genome-editing outcomes of CRISPR-associated protein 9 (Cas9), Cas9 nickases, catalytically dead Cas9 fused to FokI, and transcription activator ike effector nuclease at three disease-associated endogenous gene loci in HEK293T cells, HeLa cells, and human induced pluripotent stem cells. Although it is widely thought that NHEJ generally occurs more often than HDR, we found that more HDR than NHEJ was induced under multiple conditions. Surprisingly, the HDR/NHEJ ratios were highly dependent on gene locus, nuclease platform, and cell type. The new assay system, and our findings based on it, will enable mechanistic studies of genome-editing and help improve genome-editing technology.
URL [本文引用: 1]

IAA2(Indole Acetic Acid 2)是拟南芥Aux/IAA生长素响应基因大家族中的一员,目前还没有它的突变体的报道,阻碍了对其功能和作用机制的深入研究。在CRISPR/Cas9基因组编辑技术中,1个sgRNA只能靶向基因的1个位点,有时基因敲除的效率并不高。为了提高敲除效率,本文在Golden-Gate克隆技术的基础上,通过两轮PCR扩增,将每3个sgRNA串联到同1个入门载体中,再将入门载体与含Cas9表达框的目标载体LR反应,获得最终的表达载体。结果表明,设计的6个sgRNA有4个发挥了作用,产生了碱基插入突变和大片段缺失突变等多种可遗传的突变。与单个sgRNA相比,多重sgRNA的基因敲除效率高、种系突变多;与其他构建多重sgRNA载体的方法相比,本方法具有快速、高效等优点。本文所得到的5个突变体为后续的IAA2功能研究提供了良好的材料。
URL [本文引用: 1]

IAA2(Indole Acetic Acid 2)是拟南芥Aux/IAA生长素响应基因大家族中的一员,目前还没有它的突变体的报道,阻碍了对其功能和作用机制的深入研究。在CRISPR/Cas9基因组编辑技术中,1个sgRNA只能靶向基因的1个位点,有时基因敲除的效率并不高。为了提高敲除效率,本文在Golden-Gate克隆技术的基础上,通过两轮PCR扩增,将每3个sgRNA串联到同1个入门载体中,再将入门载体与含Cas9表达框的目标载体LR反应,获得最终的表达载体。结果表明,设计的6个sgRNA有4个发挥了作用,产生了碱基插入突变和大片段缺失突变等多种可遗传的突变。与单个sgRNA相比,多重sgRNA的基因敲除效率高、种系突变多;与其他构建多重sgRNA载体的方法相比,本方法具有快速、高效等优点。本文所得到的5个突变体为后续的IAA2功能研究提供了良好的材料。
URLPMID:29637811 [本文引用: 1]

Maintenance of genome integrity is essential to prevent cancer. Genotoxic stress drives damaged DNA out of the nucleus by forming micronuclei. Two studies in Nature reveal how the cytosolic DNA sensor cGAS gains access to the cargo within micronuclei to drive type I IFN responses.
URLPMID:5397038 [本文引用: 1]

Nuclear lamins support the nuclear envelope and provide anchorage sites for chromatin. They are involved in DNA synthesis, transcription, and replication. It has previously been reported that the lack of Lamin A/C expression in lymphoma and leukaemia is due to CpG island promoter hypermethylation. Here, we provide evidence that Lamin A/C is silenced via this mechanism in a subset of neuroblastoma cells. Moreover, Lamin A/C expression can be restored with a demethylating agent. Importantly, Lamin A/C reintroduction reduced cell growth kinetics and impaired migration, invasion, and anchorage-independent cell growth. Cytoskeletal restructuring was also induced. In addition, the introduction of lamin 50, known as Progerin, caused senescence in these neuroblastoma cells. These cells were stiffer and developed a cytoskeletal structure that differed from that observed upon Lamin A/C introduction. Of relevance, short hairpin RNA Lamin A/C depletion in unmethylated neuroblastoma cells enhanced the aforementioned tumour properties. A cytoskeletal structure similar to that observed in methylated cells was induced. Furthermore, atomic force microscopy revealed that Lamin A/C knockdown decreased cellular stiffness in the lamellar region. Finally, the bioinformatic analysis of a set of methylation arrays of neuroblastoma primary tumours showed that a group of patients (around 3%) gives a methylation signal in some of the CpG sites located within the Lamin A/C promoter region analysed by bisulphite sequencing PCR. These findings highlight the importance of Lamin A/C epigenetic inactivation for a subset of neuroblastomas, leading to enhanced tumour properties and cytoskeletal changes. Additionally, these findings may have treatment implications because tumour cells lacking Lamin A/C exhibit more aggressive behaviour.
URLPMID:28557611 [本文引用: 1]

Lamin A (LA) is a critical structural component of the nuclear lamina. Mutations within the LA gene (LMNA) lead to several human disorders, most striking of which is Hutchinson-Gilford Progeria Syndrome (HGPS), a premature aging disorder. HGPS cells are best characterized by an abnormal nuclear morphology known as nuclear blebbing, which arises due to the accumulation of progerin, a dominant mutant form of LA. The microtubule (MT) network is known to mediate changes in nuclear morphology in the context of specific events such as mitosis, cell polarization, nucleus positioning and cellular migration. What is less understood is the role of the microtubule network in determining nuclear morphology during interphase. In this study, we elucidate the role of the cytoskeleton in regulation and misregulation of nuclear morphology through perturbations of both the lamina and the microtubule network. We found that LA knockout cells exhibit a crescent shape morphology associated with the microtubule-organizing center. Furthermore, this crescent shape ameliorates upon treatment with MT drugs, Nocodazole or Taxol. Expression of progerin, in LA knockout cells also rescues the crescent shape, although the response to Nocodazole or Taxol treatment is altered in comparison to cells expressing LA. Together these results describe a collaborative effort between LA and the MT network to maintain nuclear morphology.
URLPMID:15331638 [本文引用: 1]

Mechanical properties of the nuclear envelope have implications for cell and nuclear architecture as well as gene regulation. Using isolated Xenopus oocyte nuclei, we have established swelling conditions that separate the intact nuclear envelope (membranes, pore complexes and underlying lamin filament network) from nucleoplasm and the majority of chromatin. Swelling proves reversible with addition of high molecular mass dextrans. Micropipette aspiration of swollen and unswollen nuclear envelopes is also reversible and yields a network elastic modulus, unaffected by nucleoplasm, that averages 25 mN/m. Compared to plasma membranes of cells, the nuclear envelope is much stiffer and more resilient. Our results suggest that the nuclear lamina forms a compressed network shell of interconnected rods that is extensible but limited in compressibility from the native state, thus acting as a 'molecular shock absorber'. In light of the conservation of B-type lamins in metazoan evolution, the mechanical properties determined in this investigation suggest physical mechanisms by which mutated lamins can either destabilize nuclear architecture or influence nuclear responses to mechanical signals in Emery-Dreifuss muscular dystrophy, cardiomyopathy, progeria syndromes (premature 'aging') and other laminopathies.
URLPMID:5074355 [本文引用: 1]

Lamins are major components of the nuclear lamina, a network of proteins that supports the nuclear envelope in metazoan cells. Over the past decade, biochemical studies have provided support for the view that lamins are not passive bystanders providing mechanical stability to the nucleus but play an active role in the organization of the genome and the function of fundamental nuclear processes. It has also become apparent that lamins are critical for human health, as a large number of mutations identified in the gene that encodes for A-type lamins are associated with tissue-specific and systemic genetic diseases, including the accelerated aging disorder known as Hutchinson-Gilford progeria syndrome. Recent years have witnessed great advances in our understanding of the role of lamins in the nucleus and the functional consequences of disease-associated A-type lamin mutations. Many of these findings have been presented in comprehensive reviews. In this mini-review, we discuss recent breakthroughs in the role of lamins in health and disease and what lies ahead in lamin research.
URLPMID:21346760 [本文引用: 1]

Abstract Hutchinson-Gilford progeria syndrome (HGPS) is a rare and fatal human premature ageing disease, characterized by premature arteriosclerosis and degeneration of vascular smooth muscle cells (SMCs). HGPS is caused by a single point mutation in the lamin A (LMNA) gene, resulting in the generation of progerin, a truncated splicing mutant of lamin A. Accumulation of progerin leads to various ageing-associated nuclear defects including disorganization of nuclear lamina and loss of heterochromatin. Here we report the generation of induced pluripotent stem cells (iPSCs) from fibroblasts obtained from patients with HGPS. HGPS-iPSCs show absence of progerin, and more importantly, lack the nuclear envelope and epigenetic alterations normally associated with premature ageing. Upon differentiation of HGPS-iPSCs, progerin and its ageing-associated phenotypic consequences are restored. Specifically, directed differentiation of HGPS-iPSCs to SMCs leads to the appearance of premature senescence phenotypes associated with vascular ageing. Additionally, our studies identify DNA-dependent protein kinase catalytic subunit (DNAPKcs, also known as PRKDC) as a downstream target of progerin. The absence of nuclear DNAPK holoenzyme correlates with premature as well as physiological ageing. Because progerin also accumulates during physiological ageing, our results provide an in vitro iPSC-based model to study the pathogenesis of human premature and physiological vascular ageing.
URLPMID:4833568 [本文引用: 1]

During cancer metastasis, tumor cells penetrate tissues through tight interstitial spaces, which requires extensive deformation of the cell and its nucleus. Here, we investigated mammalian tumor cell migration in confining microenvironments in vitro and in vivo. Nuclear deformation caused localized loss of nuclear envelope (NE) integrity, which led to the uncontrolled exchange of nucleo-cytoplasmic content, herniation of chromatin across the NE, and DNA damage. The incidence of NE rupture increased with cell confinement and with depletion of nuclear lamins, NE proteins that structurally support the nucleus. Cells restored NE integrity using components of the endosomal sorting complexes required for transport III (ESCRT III) machinery. Our findings indicate that cell migration incurs substantial physical stress on the NE and its content and requires efficient NE and DNA damage repair for cell survival.
URLPMID:24567359 [本文引用: 2]

Cell migration through solid tissue often involves large contortions of the nucleus, but biological significance is largely unclear. The nucleoskeletal protein lamin-A varies both within and between cell types and was shown here to contribute to cell sorting and survival in migration through constraining micropores. Lamin-A proved rate-limiting in 3D-migration of diverse human cells that ranged from glioma and adenocarcinoma lines to primary mesenchymal stem cells (MSCs). Stoichiometry of A- to B-type lamins established an activation barrier, with high lamin-A:B producing extruded nuclear shapes post-migration. Because the juxtaposed A, B polymer assemblies respectively conferred viscous and elastic stiffness to the nucleus, sub-populations with different A:B levels sorted in 3D-migration. However, net migration was also biphasic in lamin-A, as wildtype lamin-A levels protected against stress-induced death, whereas deep knockdown caused broad defects in stress-resistance. In vivo xenografts proved consistent with A:B-based cell sorting, and intermediate A:B enhanced tumor growth. Lamins thus impede 3D migration but also promote survival against migration-induced stresses.
URLPMID:5110382 [本文引用: 1]

Morphological changes in the size and shape of the nucleus are highly prevalent in cancer, but the underlying molecular mechanisms and the functional relevance remain poorly understood. Nuclear envelope proteins, which can modulate nuclear shape and organization, have emerged as key components in a variety of signalling pathways long implicated in tumourigenesis and metastasis. The expression of nuclear envelope proteins is altered in many cancers, and changes in levels of nuclear envelope proteins lamins A and C are associated with poor prognosis in multiple human cancers. In this review we highlight the role of the nuclear envelope in different processes important for tumour initiation and cancer progression, with a focus on lamins A and C. Lamin A/C controls many cellular processes with key roles in cancer, including cell invasion, stemness, genomic stability, signal transduction, transcriptional regulation, and resistance to mechanical stress. In addition, we discuss potential mechanisms mediating the changes in lamin levels observed in many cancers. A better understanding of cause-and-effect relationships between lamin expression and tumour progression could reveal important mechanisms for coordinated regulation of oncogenic processes, and indicate therapeutic vulnerabilities that could be exploited for improved patient outcome.
