 ,1
,1Culturing of human umbilical cord mesenchymal stem cells via a 3D suspension culture system using the microcarrier
Xia Li1,2,3, Huijuan Hua2,3, Jie Hao2,3, Liu Wang2,3, Zhonghua Liu ,1
,1通讯作者:
编委: 高绍荣
收稿日期:2018-06-15修回日期:2018-09-3网络出版日期:2018-12-20
| 基金资助: |
Received:2018-06-15Revised:2018-09-3Online:2018-12-20
| Fund supported: |
作者简介 About authors
李夏,硕士研究生,专业方向:发育生物学E-mail:lixia_bjscb@foxmail.com。

摘要
关键词:
Abstract
Keywords:
PDF (770KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
李夏, 滑慧娟, 郝捷, 王柳, 刘忠华. 利用微载体悬浮培养人脐带间充质干细胞[J]. 遗传, 2018, 40(12): 1120-1128 doi:10.16288/j.yczz.18-123
Xia Li, Huijuan Hua, Jie Hao, Liu Wang, Zhonghua Liu.
间充质干细胞(mesenchymal stem cells, MSCs)是一种来源于中胚层的成体干细胞,最早发现于骨髓组织,但广泛分布在骨髓、脐带、脂肪等间质组织中[1,2,3,4,5]。该类细胞具有自我更新、贴壁生长、能够分化为多种成体细胞的特性[6,7]。同时,MSCs具有免疫调节作用,其主要组织相容性复合体(major histocompatibility complex,MHC)表达较低,具有较低的免疫原性,不易引发免疫排斥反应[8,9]。MSCs还具有旁分泌特性,能够分泌多种调节因子,并作用于炎症区域,促进组织修复[10,11,12]。基于以上特性,MSCs已经成为干细胞临床治疗的理想种子细胞,在再生医学等领域具有巨大的科学和应用前景。随着干细胞临床转化的发展,利用干细胞移植已经能够治疗多种常规医疗手段无法解决的重大疾病,如癌症、免疫疾病、心梗、脊髓损伤、子宫内膜损伤等[13,14,15,16]。在美国国立卫生研究院临床试验网站上已经有6500多项注册的干细胞临床试验,并且国际上已经有多种干细胞产品获批上市(www.fda.gov)。但是,不论是干细胞功能分化研究,还是干细胞治疗及器官再生研究,往往需要大量的干细胞,如使用干细胞移植治疗肝、肾和心脏等器官损伤时,需要109~1010级别细胞量才可实现组织修复和功能重建[17,18,19]。目前,国内常用的常规实验室干细胞制备体系仍为贴壁培养体系,细胞产量很大程度上受到培养容器的限制,若要获得大量细胞则需要增加培养容器的数量。以实验室常用的细胞培养瓶为例,一个T225细胞培养瓶中的细胞量约为1×107,一次细胞治疗所需的细胞量就需要约100个T225培养瓶,不仅效率低,而且耗时耗力,在制备过程中污染风险大,批次间的稳定性差,大大增加了干细胞治疗的成本[20,21]。因此,用于干细胞治疗的功能细胞需要标准化、批量化、规模化生产制备,才能满足未来细胞治疗的需求。
本研究利用微载体和旋转瓶,通过优化细胞接种量及搅拌转速等影响因素,初步建立了一种高效的人脐带间充质干细胞(human umbilical cord mesenchymal stem cells, hUCMSCs)悬浮培养体系。通过此体系悬浮培养的hUCMSCs,具有较高的细胞活率,且正常表达hUCMSCs特异性标记物[22]。在3D转2D培养后,能够维持典型的hUCMSCs形态,并且与平面培养的hUCMSCs具有相似的增殖能力。该方法可以用于大量制备hUCMSCs,为将来标准化、规模化制备hUCMSCs提供种子细胞和技术基础。
1 材料和方法
1.1 MSCs平面培养材料与方法
本研究中使用的hUCMSCs来源于北京干细胞库。hUCMSCs复苏:在15 mL离心管中加入3 mL预热好的MSCs培养基,将冻存管从液氮中取出,37℃快速解冻细胞至固液混合态,将细胞悬液加入离心管中,1200 r/min离心3 min,弃上清。加入适量hUCMSCs培养液重悬并计数,根据细胞量将悬液接种到10 cm培养皿中,补足hUCMSCs培养基至10 mL,轻轻摇匀后放入5%CO2的37℃培养箱中培养,隔天换液。hUCMSCs传代:吸弃培养皿中的培养液,用磷酸盐缓冲溶液(phosphate buffer saline, PBS)洗一遍,加入37℃预热的0.05%胰蛋白酶约3 mL,放入37℃培养箱中消化3 min,在镜下观察细胞飘起变圆后加入等量培养基终止消化,1200 r/min离心3 min,弃上清,加入适量MSCs培养液重悬并计数,根据细胞量将悬液接种到10 cm培养皿中,补足MSCs培养基至10 mL,轻轻摇匀后放入5% CO2的37℃培养箱中培养,隔天换液。hUCMSCs冻存:吸弃培养皿中的培养液,PBS洗一遍,加入37℃预热的0.05%胰蛋白酶约3 mL,放入37℃培养箱中消化3 min,在镜下观察细胞飘起变圆后加入等量培养基终止消化,1200 r/min离心3 min,弃上清,加入适量4℃预冷的冻存液,混匀后以500 μL/管分装至冻存管中,将冻存管放入程序降温盒,-80℃过夜,第二天将冻存管转移至液氮中保存。1.2 hUCMSCs微载体悬浮培养
微载体CytodexTM1制备:计算并称量实验所需微载体使用量,微载体浓度为2 mg/mL,用50~ 100 g/mL的PBS浸泡3 h,使微载体充分膨胀,弃上清,用PBS洗涤微载体2遍,弃上清,加入新PBS,高压灭菌,4℃保存,使用前用培养液洗涤微载体;hUCMSCs微载体悬浮培养:消化平面的培养hUCMSCs (方法同hUCMSCs传代)并计数,根据细胞数量,按照细胞密度为1.5×105/mL接种于1 L Wheaton旋转瓶中,并补充微载体至2 mg/mL,将整个装置放置于5% CO2的37℃培养箱中,调整转速至30 r/min,第二天观察微载体是否沉降,提高调速至35~40 r/min,隔天换液,并每天取样观察细胞生长情况,消化计数。1.3 细胞生长曲线绘制
将培养瓶中液体移至50 mL离心管中,待微载体沉降后,弃上清液,加入新的培养液,移至瓶中,不要吹打,以免微载体破碎;使用新的培养液重悬后,吸取1 mL至皿中,拍照并记录;将皿中培养液移至离心管中,用PBS冲洗皿中微载体,一并移至离心管中;待微载体沉降后,弃液,用PBS冲洗3遍,至管中无血清成分;加0.25%胰酶1 mL,移至皿中消化3 min;加等量MSCs培养液终止消化,计数。1.4 细胞活性检测
使用Live-Dead试剂盒对贴附在微载体上的hUCMSCs进行活性检测,取1 mL PBS加入0.5 μL钙黄绿素及2 μL黄溴乙啡锭二聚体,吸取1 mL含细胞的培养液至3.5 cm皿中,弃培养基,PBS洗一遍,将含有Live-Dead试剂的PBS加入皿中,室温孵育30 min,荧光显微镜下观察细胞存活情况。1.5 流式细胞分析
将细胞悬液转移至离心管中,待微载体沉降后,吸弃离心管中的培养液,PBS洗一遍,加入适量37℃预热的0.05%胰蛋白酶,放入37℃培养箱中消化3 min,在镜下观察细胞飘起变圆后,加入等量培养基终止消化。1200 r/min离心3 min,弃上清;加入2 mL PBS将细胞重悬,1200 r/min离心3 min,弃上清;加入2 mL PBS将细胞重悬,过40 μm细胞筛;根据需要鉴定的直标抗体数,将细胞悬液均匀分装至离心管中,其中包括空白对照;向离心管中加入1:100稀释后的抗体,总体积200~400 μL即可; 37℃避光孵育30 min;上样进行流式细胞仪检测。2 结果与分析
2.1 培养体系优化
在本研究中,首先以2D培养的hUCMSCs作为悬浮培养的种子细胞,并使用Wheaton磁力搅拌系统进行后续的悬浮培养(图1A)。此外,选用Cytodex 1作为悬浮培养的载体(图1B)。然后对实验进行分组:微载体密度分别为2 g/L、5 g/L、10 g/L,细胞接种密度分别为5×104个/mL、1×105个/mL, 2×105个/mL,培养体积为100 mL,每天半量换液,培养5天后进行细胞计数检测。基于前人研究的结果,多数的MSCs悬浮培养的初始转速在30 r/min左右[23,24,25],所以,本研究首先选择了30 r/min的转速进行悬浮培养。从初步的实验结果可以看出,细胞接种量为1×105个/mL及2×105个/mL的实验组,其扩增倍数相似,而5×104个/mL实验组的细胞扩增倍数最低 (图1C)。不同的微载体密度对细胞扩增倍数的影响差别不大。基于经济性和操作性方面的考虑,在后续的实验中选用2 g/L的微载体密度。此外,缩小了细胞接种量的范围,选择4组不同的接种密度:5× 104个/mL,1×105个/mL,1.2×105个/mL和1.5× 105个/mL,培养5 d,每天进行细胞计数并绘制生长曲线。其中,5×104个/mL组在培养1 d后,发现有大量细胞死亡。培养3 d后,因细胞量过少而终止实验。而1×105个/mL,1.2×105个/mL,1.5× 105个/mL组的细胞扩增良好,1.5×105个/mL组在培养4 d后,细胞量可以达到7×107个/mL (图1D)。图1
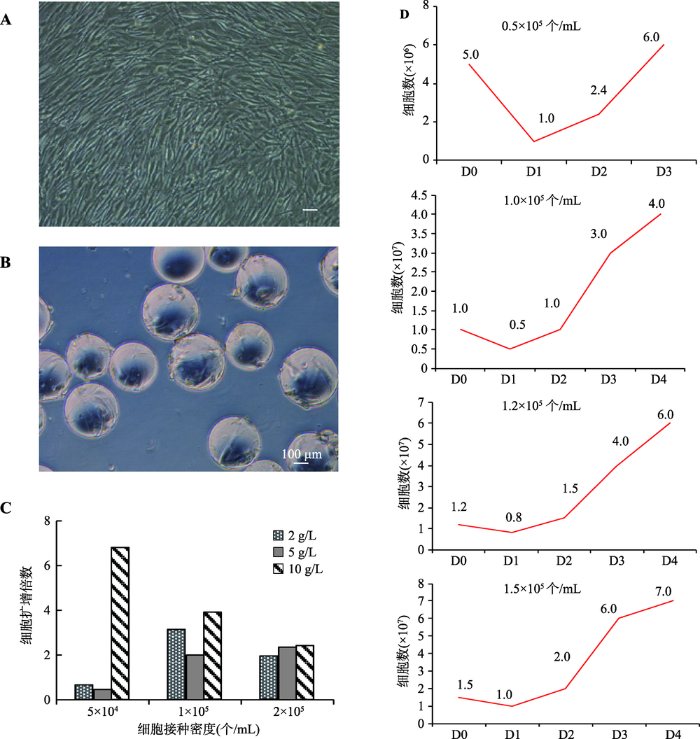 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1人脐带间充质干细胞微载体悬浮培养体系的筛选与优化
A:平面培养的人脐带间充质干细胞形态;B:在Cytodex 1上培养的人脐带间充质干细胞形态;C:不同细胞密度及微载体浓度对细胞扩增倍数的影响;D:不同细胞接种密度下hUCMSCs悬浮培养生长曲线。D0,D1,D2,D3,D4指接种后培养天数。标尺= 100 μm。
Fig. 1Optimization of a 3D hUCMSCs suspension culture system
2.2 培养体系扩大
在上述100 mL培养体系的基础上,将培养体系扩大为300 mL。为防止细胞/微载体沉降[26],本研究提高搅拌转速至35 r/min,以2×105个/mL的较大细胞密度接种,每天计数,培养6天后,细胞量可达到3.9×108个/L。通过比较不同天数细胞形态和生长曲线,发现培养3 d后,细胞已经在微载体上长满,细胞量为3.0×108(图2,A和B)。为获得更大扩增倍数,在此基础上,进一步降低初始接种细胞密度,在1 L培养体系中(图2C),以1.5×105个/mL细胞密度接种,转速提高至39 r/min,培养3 d后,细胞量可达到7×108个/L (图3,A和B)。值得注意的是,在已发表的多篇研究报道中,随着培养天数和细胞量的增加,为防止微载体沉降,都采用适当提高了初始转速的改进策略[24,26~28],因此本研究在100 mL培养体系中使用30 r/min,而在300 mL和1 L培养体系中,分别使用35 r/min和39 r/min。图2
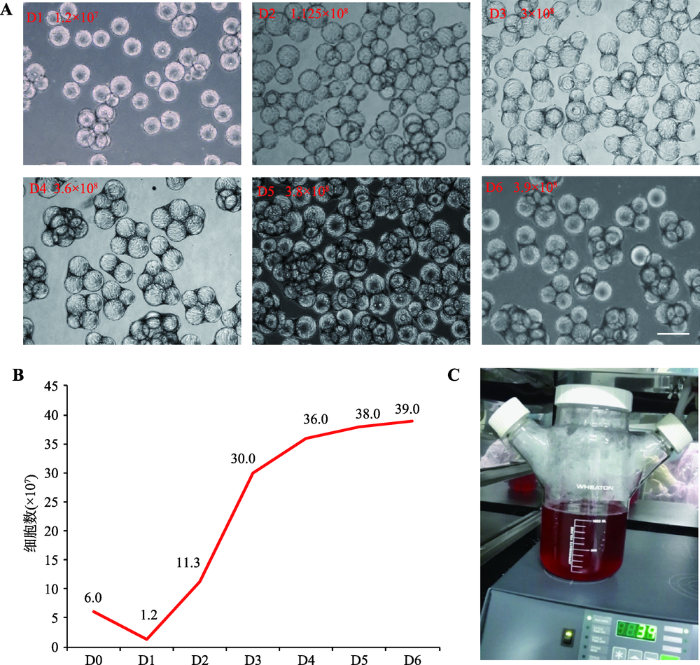 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2人脐带间充质干细胞培养体系放大
A:300 mL体系下不同培养天数的细胞形态;B:300 mL体系细胞悬浮培养生长曲线;C:1 L培养体系细胞培养瓶及磁力搅拌装置外观。标尺= 200 μm。
Fig. 2Scaling up of hUCMSCs in the 3D suspension culture system
图3
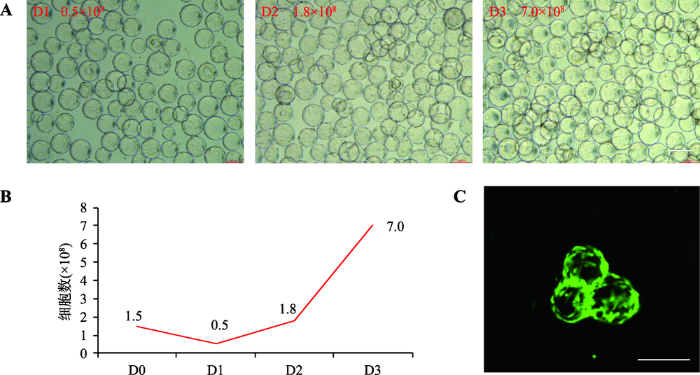 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3悬浮培养的hUCMSCs细胞生长、形态和活性检测
A:1 L培养体系下不同培养天数的细胞形态;B:1 L体系细胞悬浮培养生长曲线,D0,D1,D2,D3指接种后培养天数;C:Live-Dead染色观察细胞存活情况。标尺= 100 μm。
Fig. 3Growth, morphology and variability of cultured hUCMSCs
2.3 hUCMSCs细胞生长、形态和活性鉴定
对上述1 L培养体系获得的hUCMSCs进行Dead-Live染色,结果表明,细胞活性良好(图3C)。将悬浮培养的细胞转为平皿培养后,细胞呈现经典的MSCs细胞形态,消化细胞进行流式细胞分析,其HLA (human lymphocyte antigen)、CD34和CD45为阴性,并且高表达CD29、CD73、CD90和CD105,符合MSCs特征[29]。MTT检测细胞增殖能力与正常2D培养的细胞相似(图4,A和B)。图4
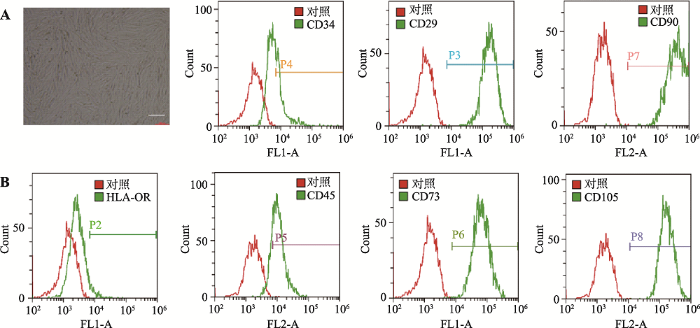 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4悬浮培养的hUCMSCs转平面培养后细胞形态和特异性标记物的流式细胞分析
A:3D转2D间充质干细胞的细胞形态;B:流式细胞分析检测间充质干细胞特异性标记物。标尺= 200 μm。
Fig. 4Morphology of hUCMSCs and flow cytometry of hUCMSC-specific markers after transferred into a 2D culture system
3 讨 论
在对培养体系优化的过程中,本研究发现培养1 d后,所有组别的细胞量都会存在不同程度的下降,这是由于部分细胞在培养第一天时没有贴壁所致,而细胞是否贴壁与细胞接种量及搅拌转速有关。当细胞数量过低时,较多细胞未贴壁会直接导致细 胞量过少无法扩增。就转速而言,当转速过高时(如70 r/min),细胞不易与微载体结合。而转速过低则会导致形成大量团块,可能会导致细胞分化或死亡。因此,如何优化培养条件,提高细胞在培养初期(第1 d)的贴壁率,是MSCs悬浮培养的关键问题。目前,国际上已有多篇使用微载体培养MSCs的报道[30],例如,Yang等[31]在2007年使用多种微载体对大鼠MSCs进行了悬浮培养。Eibes等[32]对人骨髓间充质干细胞在微载体上在摇瓶中培养进行了探索,发现微载体上有骨髓间充质干细胞生长,且保持间充质干细胞的特性。Schop等[33]在2010年使用人骨髓间充质干细胞对多种微载体进行了筛选,发现Cytodex 1组表现出最高的接种效率与增殖速率,经过9 d培养扩增4.8倍并达到了6×105个/mL的细胞密度,但其培养体系较小。Heathman等[34]在2015年使用100 mL的旋转瓶在无血清、无动物源性成分的体系内对MSCs进行悬浮培养,细胞6 d内扩增10倍,并且保持了良好的细胞状态。Rafiq等[35]利用5 L的自动化生物反应器培养人间充质干细胞, 12天细胞扩增6倍达到了1.7×105个 / mL的细胞密度。但是,国内该项研究尚未有较为成功的人脐带间质干细胞大规模培养报道。吴清法等[36]于2003年曾尝试利用旋转瓶并添加CultSpher G明胶多孔为载体,进行人骨髓间充质干细胞的悬浮培养,在100 mL的体系中以5×104 个 /mL的细胞密度接种,转速30 r/min,经过7 d的培养达到5.15×105/ mL的最大细胞量。该研究主要测定了悬浮培养细胞的生长曲线和代谢产物的变化,未进行更大规模的培养,且该研究使用多孔微载体,不利于后续的细胞消化等操作,限制了其在临床使用上的发展。韩宝三等[37]也曾尝试在培养瓶中使用Cytodex 3微载体对人间充质干细胞进行了小体积(T25细胞培养瓶)的培养,验证了微载体培养人间充质干细胞的可行性。本研究通过优化初始接种密度和转速等培养条件,初步建立了一种基于微载体和旋转瓶的高效hUCMSCs悬浮培养体系。在1 L培养体系中,选用2 g/L的微载体密度, 以1.5×105个/mL细胞初始密度接种,转速39 r/min,培养3 d后,细胞产量可达到7×108个/L,细胞扩增量可高达4.67倍, 并且所获得的干细胞活性良好,符合hUCMSCs特征。恢复平面培养后,仍能维持hUCMSCs的正常细胞形态和增殖能力。该培养体系的优势在于简单、经济、高效,无需复杂昂贵的设备就可以进行操作,培养时程短,并且细胞培养量大,一个批次就可以满足一个病人细胞治疗的需求。人脐带间充质干细胞悬浮培养体系的初步建立,为人间充质干细胞功能分化研究和临床应用奠定了一定的技术基础。后续实验将使用可降解的微载体,采用自动化的生物反应器,对培养条件进行进一步的优化,如pH、溶氧等,并扩大培养规模至2~5 L,为临床应用提供足够的种子细胞,满足MSCs临床应用的需求。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URLPMID:15625118 [本文引用: 1]

Current sources of stem cells include embryonic stem cells (ESCs) and adult stem cells (ASCs). However, concerns exist with either source: ESCs, with their significant ethical considerations, tumorigenicity, and paucity of cell lines; and ASCs, which are possibly more limited in potential. Thus, the search continues for an ethically conducive, easily accessible, and high-yielding source of stem cells. We have isolated a population of multipotent cells from the human term placenta, a temporary organ with fetal contributions that is discarded postpartum. These placenta-derived multipotent cells (PDMCs) exhibit many markers common to mesenchymal stem cells - including CD105/endoglin/SH-2, SH-3, and SH-4 - and they lack hematopoietic-, endothelial-, and trophoblastic-specific cell markers. In addition, PDMCs exhibit ESC surface markers of SSEA-4, TRA-1-61, and TRA-1-80. Adipogenic, osteogenic, and neurogenic differentiation were achieved after culturing under the appropriate conditions. PDMCs could provide an ethically uncontroversial and easily accessible source of multipotent cells for future experimental and clinical applications.
URLPMID:16410387 [本文引用: 1]

Mesenchymal stem cells (MSCs) represent a promising tool for new clinical concepts in supporting cellular therapy. Bone marrow (BM) was the first source reported to contain MSCs. However, for clinical use, BM may be detrimental due to the highly invasive donation procedure and the decline in MSC number and differentiation potential with increasing age. More recently, umbilical cord blood (UCB), attainable by a less invasive method, was introduced as an alternative source for MSCs. Another promising source is adipose tissue (AT). We compared MSCs derived from these sources regarding morphology, the success rate of isolating MSCs, colony frequency, expansion potential, multiple differentiation capacity, and immune phenotype. No significant differences concerning the morphology and immune phenotype of the MSCs derived from these sources were obvious. Differences could be observed concerning the success rate of isolating MSCs, which was 100% for BM and AT, but only 63% for UCB. The colony frequency was lowest in UCB, whereas it was highest in AT. However, UCB-MSCs could be cultured longest and showed the highest proliferation capacity, whereas BM-MSCs possessed the shortest culture period and the lowest proliferation capacity. Most strikingly, UCB-MSCs showed no adipogenic differentiation capacity, in contrast to BM- and AT-MSCs. Both UCB and AT are attractive alternatives to BM in isolating MSC: AT as it contains MSCs at the highest frequency and UCB as it seems to be expandable to higher numbers.
URLPMID:21854621 [本文引用: 1]

pAbstract/p pBackground/p pDeveloping efficient methods to isolate and identify human adipose-derived mesenchymal stem cells (hADSCs) remains to be one of the major challenges in tissue engineering./p pMethods/p pWe demonstrate here a method by isolating hADSCs from abdominal subcutaneous adipose tissue harvested during caesarian section. The hADSCs were isolated from human adipose tissue by collagenase digestion and adherence to flasks./p pResults/p pThe yield reached around 1 10sup6 /suphADSCs per gram adipose tissue. The following comprehensive identification and characterization illustrated pronounced features of mesenchymal stem cells (MSCs). The fibroblast-like hADSCs exhibited typical ultrastructure details for vigorous cell activities. Karyotype mapping showed normal human chromosome. With unique immunophenotypes they were positive for CD29, CD44, CD73, CD105 and CD166, but negative for CD31, CD34, CD45 and HLA-DR. The growth curve and cell cycle analysis revealed high capability for self-renewal and proliferation. Moreover, these cells could be functionally induced into adipocytes, osteoblasts, and endothelial cells in the presence of appropriate conditioned media./p pConclusion/p pThe data presented here suggest that we have developed high efficient isolation and cultivation methods with a systematic strategy for identification and characterization of hADSCs. These techniques will be able to provide safe and stable seeding cells for research and clinical application./p
URLPMID:14576065 [本文引用: 1]

Abstract It is well accepted that umbilical cord blood has been a source for hematopoietic stem cells. However, controversy exists as to whether cord blood can serve as a source of mesenchymal stem cells, which can differentiate into cells of different connective tissue lineages such as bone, cartilage, and fat, and little success has been reported in the literature about the isolation of such cells from cord blood. Here we report a novel method to obtain single cell-derived, clonally expanded mesenchymal stem cells that are of multilineage differentiation potential by negative immunoselection and limiting dilution. The immunophenotype of these clonally expanded cells is consistent with that reported for bone marrow mesenchymal stem cells. Under appropriate induction conditions, these cells can differentiate into bone, cartilage, and fat. Surprisingly, these cells were also able to differentiate into neuroglial- and hepatocyte-like cells under appropriate induction conditions and, thus, these cells may be more than mesenchymal stem cells as evidenced by their ability to differentiate into cell types of all 3 germ layers. In conclusion, umbilical cord blood does contain mesenchymal stem cells and should not be regarded as medical waste. It can serve as an alternative source of mesenchymal stem cells to bone marrow.
URLPMID:12529557 [本文引用: 1]

Mesenchymal stem cells (MSCs) have the capability for renewal and differentiation into various lineages of mesenchymal tissues. These features of MSCs attract a lot of attention from investigators in the context of cell-based therapies of several human diseases. Despite the fact that bone marrow represents the main available source of MSCs, the use of bone marrow-derived cells is not always acceptable due to the high degree of viral infection and the significant drop in cell number and proliferative/differentiation capacity with age. Thus, the search for possible alternative MSC sources remains to be validated. Umbilical cord blood is a rich source of hematopoietic stem/progenitor cells and does not contain mesenchymal progenitors. However, MSCs circulate in the blood of preterm fetuses and may be successfully isolated and expanded. Where these cells home at the end of gestation is not clear. In this investigation, we have made an attempt to isolate MSCs from the subendothelial layer of umbilical cord vein using two standard methodological approaches: the routine isolation of human umbilical vein endothelial cell protocol and culture of isolated cells under conditions appropriate for bone-marrow-derived MSCs. Our results suggest that cord vasculature contains a high number of MSC-like elements forming colonies of fibroblastoid cells that may be successfully expanded in culture. These MSC-like cells contain no endothelium- or leukocyte-specific antigens but express alpha-smooth muscle actin and several mesenchymal cell markers. Therefore, umbilical cord/placenta stroma could be regarded as an alternative source of MSCs for experimental and clinical needs.
URL [本文引用: 1]
URL [本文引用: 1]
URLPMID:14550804 [本文引用: 1]

Mesenchymal stem cells (MSC) do not elicit alloreactive lymphocyte responses due to immune modulations. We investigated the immunologic properties of MSC after differentiation along three lineages: bone, cartilage, and adipose. Flow cytometry showed that undifferentiated MSC express HLA class I but not class II, although HLA class II was present intracellularly as detected by Western blot. Addition of interferon γ (IFN-γ) for 48 hours induced greater than 90% of cells to express HLA class II. No lymphocyte response was induced by allogeneic irradiated MSC as stimulators. Results were similar using MSC pretreated with IFN-γ. After growth of cells in medium to induce differentiation to bone, cartilage, or adipose for 6 or 12 days, the expression of HLA class I increased but no class II was detected on the cell surface. The ability to upregulate HLA class II on the cell surface after exposure to IFN-γ for 48 hours was clearly diminished after the cells had been cultured in differentiation medium for 6 or 12 days, with only 10% of cells expressing HLA class II. Using MSC grown in osteogenic, chondrogenic, or adipogenic medium as stimulator cells, no lymphocyte alloreactivity was seen, even if differentiated MSC had been pretreated with IFN-γ. MSC inhibit mixed lymphocyte cultures, particularly after osteogenic differentiation. This suppression was further enhanced by IFN-γ. Undifferentiated and differentiated MSC do not elicit alloreactive lymphocyte proliferative responses and modulate immune responses. The findings support that MSC can be transplantable between HLA-incompatible individuals.
URLPMID:15864738 [本文引用: 1]

Human mesenchymal stem cells (MSCs) were evaluated for their ability to activate allogeneic T cells in cell mixing experiments. Phenotypic characterization of MSCs by flow cytometry showed expression of MHC Class I alloantigens, but minimal expression of Class II alloantigens and costimulatory molecules, including CD80 (B7-1), CD86 (B7-2), and CD40. T cells purified from peripheral blood mononuclear cells (PBMCs) did not proliferate to allogeneic MSCs. Lack of response was not due to a deficiency of costimulation, since retroviral transduction of MSCs with either B7-1 or B7-2 costimulatory molecules did not result in lymphoproliferation. Although these results suggested that MSCs were immunologically inert or potentially tolerogenic, T cells cultured with MSCs produced IFN- and displayed secondary kinetics to restimulation with PBMCs, indicating alloantigen priming rather than tolerance induction by the MSCs. To determine whether MSCs suppressed alloreactive T cells, MSCs were added to primary mixed lymphocyte reaction (MLR) cultures. MSCs suppressed cell proliferation when added at the initiation of culture or when added to an ongoing MLR culture. Suppression was dose-dependent, genetically unrestricted, and occurred whether or not MSCs were pretreated with IFN- . MSCs in transwell chambers suppressed primary MLR cultures, indicating that suppression was mediated by soluble molecules. Analysis of cytokines in suppressed MLR cultures demonstrated up-regulation of IFN- and IL-10, and down-regulation of TNF- production relative to control cultures. We conclude that MSCs can initiate activation of alloreactive T cells, but do not elicit T cell proliferative responses due to active suppressive mechanisms.
URLPMID:3930331 [本文引用: 1]

78 Induction of EMT in mammary epithelial cells depends on collaborating pathways 78 Pathways that induce EMT also maintain the resultant cellular state 78 Autocrine signaling maintains the mesenchymal and stem-cell traits induced by EMT 78 Similar signals maintain both normal and neoplastic mammary stem cells
URLPMID:3182886 [本文引用: 1]

There is currently much interest in adult mesenchymal stem cells (MSCs) and their ability to differentiate into other cell types, and to partake in the anatomy and physiology of remote organs. It is now clear these cells may be purified from several organs in the body besides bone marrow. MSCs take part in wound healing by contributing to myofibroblast and possibly fibroblast populations, and may be involved in epithelial tissue regeneration in certain organs, although this remains more controversial. In this review, we examine the ability of MSCs to modulate liver, kidney, heart and intestinal repair, and we update their opposing qualities of being less immunogenic and therefore tolerated in a transplant situation, yet being able to contribute to xenograft models of human tumour formation in other contexts. However, such observations have not been replicated in the clinic. Recent studies showing the clinical safety of MSC in several pathologies are discussed. The possible opposing powers of MSC need careful understanding and control if their clinical potential is to be realised with long-term safety for patients.
URLPMID:21726829 [本文引用: 1]

Now that mesenchymal stem cells (MSCs) have been shown to be perivascular in02vivo, the existing traditional view that focuses on the multipotent differentiation capacity of these cells should be expanded to include their equally interesting role as cellular modulators that brings them into a broader therapeutic scenario. We discuss existing evidence that leads us to propose that during local injury, MSCs are released from their perivascular location, become activated, and establish a regenerative microenvironment by secreting bioactive molecules and regulating the local immune response. These trophic and immunomodulatory activities suggest that MSCs may serve as site-regulated “drugstores” in02vivo.
URLPMID:15494428 [本文引用: 1]

Mesenchymal stem cells (MSCs) are multipotent cells found in several adult tissues. Transplanted allogeneic MSCs can be detected in recipients at extended time points, indicating a lack of immune recognition and clearance. As well, a role for bone marrow-derived MSCs in reducing the incidence and severity of graft-versus-host disease (GVHD) during allogeneic transplantation has recently been reported; however, the mechanisms remain to be investigated. We examined the immunomodulatory functions of human MSCs (hMSCs) by coculturing them with purified subpopulations of immune cells and report here that hMSCs altered the cytokine secretion profile of dendritic cells (DCs), naive and effector T cells (T helper 1 [TH1] and TH2), and natural killer (NK) cells to induce a more anti-inflammatory or tolerant phenotype. Specifically, the hMSCs caused mature DCs type 1 (DC1) to decrease tumor necrosis factor (TNF- ) secretion and mature DC2 to increase interleukin-10 (IL-10) secretion; hMSCs caused TH1 cells to decrease interferon (IFN- ) and caused the TH2 cells to increase secretion of IL-4; hMSCs caused an increase in the proportion of regulatory T cells (TRegs) present; and hMSCs decreased secretion of IFN- from the NK cells. Mechanistically, the hMSCs produced elevated prostaglandin E2 (PGE2) in co-cultures, and inhibitors of PGE2 production mitigated hMSC-mediated immune modulation. These data offer insight into the interactions between allogeneic MSCs and immune cells and provide mechanisms likely involved with the in vivo MSC-mediated induction of tolerance that could be therapeutic for reduction of GVHD, rejection, and modulation of inflammation. (Blood. 2005;105:1815-1822)
URLPMID:22785564 [本文引用: 1]

Abstract Critical limb ischemia (CLI) is commonly caused by atherosclerotic arterial obstruction or stenosis in the leg, as demonstrated by rest pain, skin ulcers and gangrene (Fontaine III or IV), often fails to respond to conservative treatments, and carries a high risk for limb amputation, with a particularly dismal prognosis. Although surgical revascularization techniques may be used for certain CLI patients, such techniques are not indicated for most CLI patients due to the diffuse nature of the responsible lesions, distal location of the obstruction, or coexisting systemic comorbidities. For such CLI patients with no alternative treatments, the potential utility of cell therapies has been investigated. Indeed many clinical trials are being carried out by academic sectors, and their achievements will facilitate clinical development by pharmaceutical companies.In order to understand the situation regarding competitive international R&D of revascularization seeds for CLI, we surveyed the status of clinical trials. As a result, we identified 58 clinical trials on revascularization for CLI, with the majority in the early phase (<phase II: 82.7%). Revascularization seeds for CLI are in the development and competition phase, and promising seeds are expected to appear in the near future.In this review, we discuss how to develop optimal regenerative medicine concerning the selection of cell origin, cell type, combination with growth factor, and the influence of concomitant disease.
URLPMID:28185615 [本文引用: 1]

Regeneration of damaged neurons and recovery of sensation and motor function after complete spinal cord injury (SCI) are challenging. We previously developed a collagen scaffold, NeuroRegen scaffold, to promote axonal growth along collagen fibers and inhibit glial scar formation after SCI. When functionalized with multiple biomolecules, this scaffold promoted neurological regeneration and functional recovery in animals with SCI. In this study, eight patients with chronic complete SCI were enrolled to examine the safety and efficacy of implanting NeuroRegen scaffold with human umbilical cord mesenchymal stem cells (MSCs). Using intraoperative neurophysiological monitoring, we identified and surgically resected scar tissues to eliminate the inhibitory effect of glial scarring on nerve regeneration. We then implanted NeuroRegen scaffold loaded with MSCs into the resection sites. No adverse events (infection, fever, headache, allergic reaction, shock, perioperative complications, aggravation of neurological status, or cancer) were observed during 1 year of follow up. Primary efficacy outcomes, including expansion of sensation level and motor-evoked potential (MEP)-responsive area, increased finger activity, enhanced trunk stability, defecation sensation, and autonomic neural function recovery, were observed in some patients. Our findings suggest that combined application of NeuroRegen scaffold and MSCs is safe and feasible for clinical therapy in patients with chronic SCI. Our study suggests that construction of a regenerative microenvironment using a scaffold-based strategy may be a possible future approach to SCI repair.
URLPMID:26140604 [本文引用: 1]

Clinical investigations using stem cell products are addressing a wide spectrum of conditions using many different stem cell types. Trounson and McDonald review the clinical trials in which data have now been published and highlight areas where progress is being made as well as failures and areas of concern.
URLPMID:19603344 [本文引用: 1]

Low CD34 + cell doses increase allograft-related mortality and very high doses increase the risk of graft-versus-host disease. The optimum CD34 + cell dose remains undefined. The effect of the CD34 + cell dose based on ideal weight was analyzed in 130 patients with hematologic malignancies undergoing reduced-intensity allogeneic blood cell transplantation in the context of factors known to affect the outcome: chemosensitivity, donor age, lactate dehydrogenase (LDH), human leukocyte antigen (HLA) match, performance status, and platelet count. The survival of patients receiving >8 0103 106/kg CD34 + cells was not significantly different from those receiving <6. The outcome of those receiving 60900098 0103 106/kg CD34 + cells was significantly better than the rest. This superiority was confirmed in multivariable analysis. Among patients receiving 0909¤8 0103 106/kg CD34 + cells, an increasing number of infused cells was associated with higher overall survival in a continuous fashion (Risk ratio (RR) 0.8759; p = 0.045). Cell dose based on actual weight did not correlate with survival. The number of CD34 + cells infused, a potentially modifiable factor, affects survival after reduced-intensity allogeneic transplantation. We recommend a CD34 + cell dose of 60900098 0103 106 per kg ideal body weight to optimize outcome. The possible adverse effect of higher cell doses (>8) needs further confirmation.
URLPMID:19739936 [本文引用: 1]

Abstract Advances in stem cell biology have afforded promising results for the generation of various cell types for therapies against devastating diseases. However, a prerequisite for realizing the therapeutic potential of stem cells is the development of bioprocesses for the production of stem cell progeny in quantities that satisfy clinical demands. Recent reports on the expansion and directed differentiation of human embryonic stem cells (hESCs) in scalable stirred-suspension bioreactors (SSBs) demonstrated that large-scale production of therapeutically useful hESC progeny is feasible with current state-of-the-art culture technologies. Stem cells have been cultured in SSBs as aggregates, in microcarrier suspension and after encapsulation. The various modes in which SSBs can be employed for the cultivation of hESCs and human induced pluripotent stem cells (hiPSCs) are described. To that end, this is the first account of hiPSC cultivation in a microcarrier stirred-suspension system. Given that cultured stem cells and their differentiated progeny are the actual products used in tissue engineering and cell therapies, the impact of bioreactor's operating conditions on stem cell self-renewal and commitment should be considered. The effects of variables specific to SSB operation on stem cell physiology are discussed. Finally, major challenges are presented which remain to be addressed before the mainstream use of SSBs for the large-scale culture of hESCs and hiPSCs.
URLPMID:20921206 [本文引用: 1]

Abstract BACKGROUND AND AIM: Mesenchymal stromal cells (MSCs) are pluripotent cells that have immunosuppressive effects both in vitro and in experimental colitis. Promising results of MSC therapy have been obtained in patients with severe graft versus host disease of the gut. Our objective was to determine the safety and feasibility of autologous bone marrow derived MSC therapy in patients with refractory Crohn's disease. PATIENTS AND INTERVENTION: 10 adult patients with refractory Crohn's disease (eight females and two males) underwent bone marrow aspiration under local anaesthesia. Bone marrow MSCs were isolated and expanded ex vivo. MSCs were tested for phenotype and functionality in vitro. 9 patients received two doses of 1-2 10(6) cells/kg body weight, intravenously, 7 days apart. During follow-up, possible side effects and changes in patients' Crohn's disease activity index (CDAI) scores were monitored. Colonoscopies were performed at weeks 0 and 6, and mucosal inflammation was assessed by using the Crohn's disease endoscopic index of severity. RESULTS: MSCs isolated from patients with Crohn's disease showed similar morphology, phenotype and growth potential compared to MSCs from healthy donors. Importantly, immunomodulatory capacity was intact, as Crohn's disease MSCs significantly reduced peripheral blood mononuclear cell proliferation in vitro. MSC infusion was without side effects, besides a mild allergic reaction probably due to the cryopreservant DMSO in one patient. Baseline median CDAI was 326 (224-378). Three patients showed clinical response (CDAI decrease 70 from baseline) 6 weeks post-treatment; conversely three patients required surgery due to disease worsening. CONCLUSIONS: Administration of autologous bone marrow derived MSCs appears safe and feasible in the treatment of refractory Crohn's disease. No serious adverse events were detected during bone marrow harvesting and administration.
URLPMID:22541338 [本文引用: 1]

Human pluripotent stem cells (hPSCs), including embryonic and induced pluripotent stem cells, constitute an extremely attractive tool for cell therapy. However, flexible platforms for the large-scale production and storage of hPSCs in tightly controlled conditions are necessary to deliver high-quality cells in relevant quantities to satisfy clinical demands. Here we discuss the main principles for the bioprocessing of hPSCs, highlighting the impact of environmental factors, novel 3D culturing approaches and integrated bioreactor strategies for controlling hPSC culture outcome. Knowledge on hPSC bioprocessing accumulated during recent years provides important insights for the establishment of more robust production platforms and should potentiate the implementation of novel hPSC-based therapies.
URLPMID:23081828 [本文引用: 1]

Abstract Current practices to maintain human pluripotent stem cells (hPSCs), which include induced pluripotent stem cells and embryonic stem cells, in an undifferentiated state typically depend on the support of feeder cells such as mouse embryonic fibroblasts (MEFs) or an extracellular matrix such as Matrigel. Culture conditions that depend on these undefined support systems limit our ability to interpret mechanistic studies aimed at resolving how hPSCs interact with their extracellular environment to remain in a unique undifferentiated state and to make fate-changing lineage decisions. Likewise, the xenogeneic components of MEFs and Matrigel ultimately hinder our ability to use pluripotent stem cells to treat debilitating human diseases. Many of these obstacles have been overcome by the development of synthetic coatings and bioreactors that support hPSC expansion and self-renewal within defined culture conditions that are free from xenogeneic contamination. The establishment of defined culture conditions and synthetic matrices will facilitate studies to more precisely probe the molecular basis of pluripotent stem cell self-renewal and differentiation. When combined with three-dimensional cultures in bioreactors, these systems will also enable large-scale expansion for future clinical applications. S TEM C ells 2013;31:1 7
URLPMID:16923606 [本文引用: 1]

The considerable therapeutic potential of human multipotent mesenchymal stromal cells (MSC) has generated markedly increasing interest in a wide variety of biomedical disciplines. However, investigators report studies of MSC using different methods of isolation and expansion, and different approaches to characterizing the cells. Thus it is increasingly difficult to compare and contrast study outcomes, which hinders progress in the field. To begin to address this issue, the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy proposes minimal criteria to define human MSC. First, MSC must be plastic-adherent when maintained in standard culture conditions. Second, MSC must express CD105, CD73 and CD90, and lack expression of CD45, CD34, CD14 or CD11b, CD79alpha or CD19 and HLA-DR surface molecules. Third, MSC must differentiate to osteoblasts, adipocytes and chondroblasts in vitro. While these criteria will probably require modification as new knowledge unfolds, we believe this minimal set of standard criteria will foster a more uniform characterization of MSC and facilitate the exchange of data among investigators.
URLPMID:26971681 [本文引用: 1]

The selection of medium and associated reagents for human mesenchymal stromal cell (hMSC) culture forms an integral part of manufacturing process development and must be suitable for multiple process scales and expansion technologies. In this work, we have expanded BM-hMSCs in fetal bovine serum (FBS)- and human platelet lysate (HPL)-containing media in both a monolayer and a suspension-based microcarrier process. The introduction of HPL into the monolayer process increased the BM-hMSC growth rate at the first experimental passage by 0.049 day and 0.127 day for the two BM-hMSC donors compared with the FBS-based monolayer process. This increase in growth rate in HPL-containing medium was associated with an increase in the inter-donor consistency, with an inter-donor range of 0.406 cumulative population doublings after 18 days compared with 2.013 in FBS-containing medium. Identity and quality characteristics of the BM-hMSCs are also comparable between conditions in terms of colony-forming potential, osteogenic potential and expression of key genes during monolayer and post-harvest from microcarrier expansion. BM-hMSCs cultured on microcarriers in HPL-containing medium demonstrated a reduction in the initial lag phase for both BM-hMSC donors and an increased BM-hMSC yield after 6 days of culture to 1.2065±650.1765×65105and 1.0265±650.00565×65105cells/mL compared with 0.7965±650.0565×65105and 0.3665±650.0465×65105cells/mL in FBS-containing medium. This study has demonstrated that HPL, compared with FBS-containing medium, delivers increased growth and comparability across two BM-hMSC donors between monolayer and microcarrier culture, which will have key implications for process transfer during scale-up.
URL [本文引用: 2]
URLPMID:20887551 [本文引用: 1]

Objectives: For reasons of provision of highly-specific surface area and three-dimensional culture, microcarrier culture (MC) has garnered great interest for its potential to expand anchorage-dependent stem cells. This study utilizes MC for in vitro expansion of human bone marrow mesenchymal stem cells (BMMSCs) and analyses its effects on BMMSC proliferation and differentiation.Materials and methods: Effects of semi-continuous MC compared to control plate culture (PC) and serial bead-to-bead transfer MC (MC bead-T) on human BMMSCs were investigated. Cell population growth kinetics, cell phenotypes and differentiation potential of cells were assayed.Results: Maximum cell density and overall fold increase in cell population growth were similar between PCs and MCs with similar starting conditions, but lag period of BMMSC growth differed substantially between the two; moreover, MC cells exhibited reduced granularity and higher CXCR4 expression. Differentiation of BMMSCs into osteogenic and adipogenic lineages was enhanced after 3 days in MC. However, MC bead-T resulted in changes in cell granularity and lower osteogenic and adipogenic differentiation potential.Conclusions: In comparison to PC, MC supported expansion of BMMSCs in an up-scalable three-dimensional culture system using a semi-continuous process, increasing potential for stem cell homing ability and osteogenic and adipogenic differentiation.
URLPMID:26977155 [本文引用: 2]

The great properties of human mesenchymal stromal cells (hMSCs) make these cells an important tool in regenerative medicine. Because of the limitations of hMSCs derived from the bone marrow during isolation and expansion, hMSCs derived from the umbilical cord stroma are a great alternative to overcome these issues. For a large expansion of these cells, we performed a process transfer from static culture to a dynamic system. For this reason, a microcarrier selection out of five microcarrier types was made to achieve a suitable growth surface for the cells. The growth characteristics and metabolite consumption and production were used to compare the cells growth in 12-well plate and spinner flask. The goal to determine relevant process parameters to transfer the expansion process into a stirred tank bioreactor was achieved.
URLPMID:3226421

Abstract The immunomodulatory properties of mesenchymal stem cells (MSCs) make them attractive therapeutic agents for a wide range of diseases. However, the highly demanding cell d..
URLPMID:3620494 [本文引用: 1]

Stirred microcarrier (MC) culture has been suggested as the method of choice for supplying large volumes of mesenchymal stem cells (MSCs) for bone tissue engineering. In this study, we show that in addition to the improvement in cell expansion capacity, MSCs propagated and harvested from MC culture also demonstrate higher osteogenic potency when differentiatedin vivoorin vitroin three-dimensional (3D) scaffold cultures as compared with traditional monolayer (MNL) cultures. Cytodex 3 microcarrier-expanded human fetal MSC (hfMSC) cultures (MC-hfMSCs) achieved 12- to 16-fold expansion efficiency (6×105–8×105cells/mL) compared to 4- to 6-fold (1.2×105–1.8×105cells/mL) achieved by traditional MNL-expanded hfMSC culture (MNL-hfMSCs;p<0.05). Both MC-hfMSCs and MNL-hfMSCs maintained similar colony-forming capacity, doubling times, and immunophenotype postexpansion. However, when differentiated underin vitrotwo-dimensional (2D) osteogenic conditions, MC-hfMSCs exhibited a 45-fold reduction in alkaline phosphatase level and a 37.5% decrease in calcium deposition compared with MNL-hfMSCs (p<0.05). Surprisingly, when MC-hfMSCs and MNL-hfMSCs were seeded on 3D macroporous scaffold culture or subcutaneously implanted into nonobese diabetic/severe combined immunodeficient mice, MC-hfMSCs deposited 63.5% (p<0.05) more calcium and formed 47.2% (p<0.05) more bone volume, respectively. These results suggest that the mode of hfMSC growth in the expansion phase affects the osteogenic potential of hfMSCs differently in various differentiation platforms. In conclusion, MC cultures are advantageous over MNL cultures in bone tissue engineering because MC-hfMSCs have improved cell expansion capacity and exhibit higher osteogenic potential than MNL-hfMSCs when seededin vitrointo 3D scaffolds or implantedin vivo.
URLPMID:26384580 [本文引用: 1]

Mesenchymal stem cells (MSCs) are multipotent stem cells. Although they were originally identified in bone marrow and described as arrow stromal cells , they have since been identified in many other anatomical locations in the body. MSCs can be isolated from bone marrow, adipose tissue, umbilical cord and other tissues but the richest tissue source of MSCs is fat. Since they are adherent to plastic, they may be expandedin vitro. MSCs have a distinct morphology and express a specific set of CD (cluster of differentiation) molecules. The phenotypic pattern for the identification of MSCs cells requires expression of CD73, CD90, and CD105 and lack of CD34, CD45, and HLA-DR antigens. Under appropriate micro-environmental conditions MSCs can proliferate and give rise to other cell types. Therefore, they are ideally suited for the treatment of systemic inflammatory and autoimmune conditions. They have also been implicated as key players in regenerating injured tissue following injury and trauma. MSC populations isolated from adipose tissue may also contain regulatory T (Treg) cells, which have the capacity for modulating the immune system. The immunoregulatory and regenerative properties of MSCs make them ideal for use as therapeutic agentsin vivo. In this paper we review the literature on the identification, phenotypic characterization and biological properties of MSCs and discuss their potential for applications in cell therapy and regenerative medicine. We also discuss strategies for biomaterial micro-engineering of the stem cell niche.
URL [本文引用: 1]

Human mesenchymal stem cell (hMSC)-based therapies are of increasing interest in the field of regenerative medicine. As economic considerations have shown, allogeneic therapy seems to be the most cost-effective method. Standardized procedures based on instrumented single-use bioreactors have been shown to provide billion of cells with consistent product quality and to be superior to traditional expansions in planar cultivation systems. Furthermore, under consideration of the complex nature and requirements of allogeneic hMSC-therapeutics, a new equipment for downstream processing (DSP) was successfully evaluated. This mini-review summarizes both the current state of the hMSC production process and the challenges which have to be taken into account when efficiently producing hMSCs for the clinical scale. Special emphasis is placed on the upstream processing (USP) and DSP operations which cover expansion, harvesting, detachment, separation, washing and concentration steps, and the regulatory demands.
URLPMID:17433434 [本文引用: 1]

Bone marrow mesenchymal stromal cells (BM-MSC) are attractive candidates for connective tissue regeneration. Currently, their use is limited by poor overall cell survival and high apoptosis rates upon transplantation Sprague Dawley rat BM-MSC expansion. Bead-expanded BM-MSC could still be differentiated along the chondrogenic, osteogenic and adipogenic lineages. In the short term, direct subcutaneous transplantation of cells expanded on microcarriers was associated with significantly less apoptosis than trypsinized control cells. In the long term, BM-MSC expanded on microcarrier beads induced de novo trabecular bone formation in vivo. This novel approach present several advantages over current expansion ransplantation protocols for mesenchymal tissue regeneration.
URLPMID:20188771 [本文引用: 1]

Bioreactor systems have been developed as alternatives to standard culture flasks due to their homogeneous nature, easiness of monitoring and increased cell production. Here we investigated the in vitro expansion of bone marrow (BM) mesenchymal stem cells (MSC) in spinner flasks, using gelatin microcarriers ( Cultispher S) to support cell adhesion and proliferation. MSC expansion was performed using a low-serum containing medium (2% of fetal bovine serum, FBS). A strategy was defined for the maximization of cell expansion: microcarriers were pre-coated with FBS in order to increase cell seeding efficiency and an adequate feeding regime was established (25% medium exchange everyday). The maximum cell density, 4.2 10 5 cells/mL, was obtained at day 8, corresponding to a fold increase in total cell number of 8.4 0.8. Expanded MSC retained their differentiation potential into adipogenic and osteogenic lineages, as well as their clonogenic ability. Harvested cells expressed >90% of CD73, CD90 and CD105 markers. These results demonstrated that a microcarrier-based stirred culture system is adequate for human MSC expansion, using a low-serum containing medium, allowing the generation of significant cell numbers for potential applications in regenerative medicine.
URLPMID:19842106 [本文引用: 1]

Adult stem cells, or mesenchymal stromal cells (MSCs), are of great potential for cell therapy and tissue-engineering applications. However, for therapeutic use, these cells need to be isolated from tissue or a biopsy and efficiently expanded, as they cannot be harvested in sufficient quantities from the body. In our opinion, efficient expansion of MSCs can be achieved in a microcarrier-based cultivation system. This study selected a suitable microcarrier for human bone marrow-derived stromal cells (HBMSCs), optimized cell-seeding strategies by varying serum concentrations, and optimized dynamic expansion of the HBMSCs in a microcarrier-based spinner flask cultivation system by applying various feeding regimes. Cytodex 1 microcarriers in combination with a low-serum concentration (0-5%) in the medium resulted in the highest seeding efficiency for the HBMSCs. Subsequently, significant expansion of the HBMSCs on these carriers has been observed. The highest number of HBMSCs population doublings (4.8 doublings) was obtained by a combination of 50% medium refreshment combined with addition of 30% medium containing microcarriers every 3 days. Exponential cell growth was observed for at least 9 days after seeding, provided that sufficient nutrients (such as glucose) were present, metabolite concentrations (such as ammonia) were kept below growth-inhibitory concentrations and adequate surface area was present for the cells. After dynamic expansion of the HBMSCs, the cells retained their differentiation potential and their cell surface markers, indicating that HBMSCs expansion on Cytodex 1 microcarriers did not alter the phenotypic properties of the cells. Copyright 2009 John Wiley & Sons, Ltd.
URLPMID:25727395 [本文引用: 1]

ABSTRACT Human mesenchymal stem cell (hMSC) therapies are currently progressing through clinical development, driving the need for consistent, and cost effective manufacturing processes to meet the lot-sizes required for commercial production. The use of animal-derived serum is common in hMSC culture but has many drawbacks such as limited supply, lot-to-lot variability, increased regulatory burden, possibility of pathogen transmission, and reduced scope for process optimization. These constraints may impact the development of a consistent large-scale process and therefore must be addressed. The aim of this work was therefore to run a pilot study in the systematic development of serum-free hMSC manufacturing process. Human bone-marrow derived hMSCs were expanded on fibronectin-coated, non-porous plastic microcarriers in 10065mL stirred spinner flasks at a density of 365×6510565cells.mL611 in serum-free medium. The hMSCs were successfully harvested by our recently-developed technique using animal-free enzymatic cell detachment accompanied by agitation followed by filtration to separate the hMSCs from microcarriers, with a post-harvest viability of 99.6365±650.03%. The hMSCs were found to be in accordance with the ISCT characterization criteria and maintained hMSC outgrowth and colony-forming potential. The hMSCs were held in suspension post-harvest to simulate a typical pooling time for a scaled expansion process and cryopreserved in a serum-free vehicle solution using a controlled-rate freezing process. Post-thaw viability was 75.865±651.4% with a similar 365h attachment efficiency also observed, indicating successful hMSC recovery, and attachment. This approach therefore demonstrates that once an hMSC line and appropriate medium have been selected for production, multiple unit operations can be integrated to generate an animal component-free hMSC production process from expansion through to cryopreservation. Biotechnol. Bioeng. 2015;112: 1696–1707. 08 2015 The Authors. Biotechnology and Bioengineering Published by Wiley Periodicals, Inc.
URLPMID:23609232 [本文引用: 1]

For the first time, fully functional human mesenchymal stem cells (hMSCs) have been cultured at the litre-scale on microcarriers in a stirred-tank 5 l bioreactor, (2.5 l working volume) and were harvested via a potentially scalable detachment protocol that allowed for the successful detachment of hMSCs from the cell-microcarrier suspension. Over 12 days, the dissolved O-2 concentration was > 45 % of saturation and the pH between 7.2 and 6.7 giving a maximum cell density in the 5 l bioreactor of 1.7 x 10(5) cells/ml; this represents > sixfold expansion of the hMSCs, equivalent to that achievable from 65 fully-confluent T-175 flasks. During this time, the average specific O-2 uptake of the cells in the 5 l bioreactor was 8.1 fmol/cell h and, in all cases, the 5 l bioreactors outperformed the equivalent 100 ml spinner-flasks run in parallel with respect to cell yields and growth rates. In addition, yield coefficients, specific growth rates and doubling times were calculated for all systems. Neither the upstream nor downstream bioprocessing unit operations had a discernible effect on cell quality with the harvested cells retaining their immunophenotypic markers, key morphological features and differentiation capacity.
.
URL [本文引用: 1]

本研究采用微载体旋转培养系统和常规静止培养系统对成人骨髓间充质干细胞 (mesenchymalstemcell,MSC)的培养进行比较。MSC是贴壁依赖性细胞 ,旋转培养系统采用CultiSpherG大孔微载体 ,浓度为 1g L ,常规静止培养在 12孔培养板中进行。两系统细胞接种密度均为 5× 10 4 cells ml。结果 :旋转培养 7天后达到最大活细胞密度5 .15 0× 10 5cells ml,常规静止培养第 5天就达到最大活细胞密度 1.6 75× 10 5cells ml。在微载体旋转培养中生成乳酸12 .0 6mmol L ,而常规静止培养中生成乳酸 13.10mmol L ,葡萄糖消耗分别为 7.38mmol L和 5 .37mmol L。在微载体旋转培养中平均乳酸产率为 1.6 3,远低于常规静止培养的平均乳酸产率 2 .4 4。这些表明 ,在微载体悬浮培养条件下 ,MSC生长更为旺盛 ,细胞产量更高 ,葡萄糖消耗和能量利用率优于常规静止培养。微载体悬浮培养 12天后 ,MSC依然保持其干细胞特性。结论 :微载体培养系统是扩增组织工程种子细胞的有效方法
URL [本文引用: 1]

本研究采用微载体旋转培养系统和常规静止培养系统对成人骨髓间充质干细胞 (mesenchymalstemcell,MSC)的培养进行比较。MSC是贴壁依赖性细胞 ,旋转培养系统采用CultiSpherG大孔微载体 ,浓度为 1g L ,常规静止培养在 12孔培养板中进行。两系统细胞接种密度均为 5× 10 4 cells ml。结果 :旋转培养 7天后达到最大活细胞密度5 .15 0× 10 5cells ml,常规静止培养第 5天就达到最大活细胞密度 1.6 75× 10 5cells ml。在微载体旋转培养中生成乳酸12 .0 6mmol L ,而常规静止培养中生成乳酸 13.10mmol L ,葡萄糖消耗分别为 7.38mmol L和 5 .37mmol L。在微载体旋转培养中平均乳酸产率为 1.6 3,远低于常规静止培养的平均乳酸产率 2 .4 4。这些表明 ,在微载体悬浮培养条件下 ,MSC生长更为旺盛 ,细胞产量更高 ,葡萄糖消耗和能量利用率优于常规静止培养。微载体悬浮培养 12天后 ,MSC依然保持其干细胞特性。结论 :微载体培养系统是扩增组织工程种子细胞的有效方法
.
URL [本文引用: 1]

目的建立一种体外分离、大量扩增培养成人骨髓间充质干细胞(hMSCs)的方法。方法用Percoll梯度离心结合贴壁法分离hMSCs,用微载体cytodex 3培养hMSCs,以普通单层聚苯乙烯(TCPS)培养作对照,用流式细胞仪(FCM)和MTT法对其细胞表型和增殖活性检测。结果①梯度离心结合早期换液是分离hMSCs的较好方法,FCM检测表明hMSCs表面表达CD29、CD44和CD105,而不表达CD14、CD34、CD45、VLA-1和HLA-DR。hMSCs细胞周期分布显示:G0/G1期(86.4±3.8)%,S+G2+M期(13.6±4.2)%;②hMSCs与cytodex 3有良好的相容性,MTT法表明hMSCs在cytodex 3表面悬浮生长时具有比普通单层TCPS培养时更高的数量和增殖活性,FCM分析表明两者的细胞表型和细胞周期分布相同,无显著性差异(P>0.05)。结论微载体cytodex 3培养方法是扩增组织工程种子细胞hMSCs的有效方法。
URL [本文引用: 1]

目的建立一种体外分离、大量扩增培养成人骨髓间充质干细胞(hMSCs)的方法。方法用Percoll梯度离心结合贴壁法分离hMSCs,用微载体cytodex 3培养hMSCs,以普通单层聚苯乙烯(TCPS)培养作对照,用流式细胞仪(FCM)和MTT法对其细胞表型和增殖活性检测。结果①梯度离心结合早期换液是分离hMSCs的较好方法,FCM检测表明hMSCs表面表达CD29、CD44和CD105,而不表达CD14、CD34、CD45、VLA-1和HLA-DR。hMSCs细胞周期分布显示:G0/G1期(86.4±3.8)%,S+G2+M期(13.6±4.2)%;②hMSCs与cytodex 3有良好的相容性,MTT法表明hMSCs在cytodex 3表面悬浮生长时具有比普通单层TCPS培养时更高的数量和增殖活性,FCM分析表明两者的细胞表型和细胞周期分布相同,无显著性差异(P>0.05)。结论微载体cytodex 3培养方法是扩增组织工程种子细胞hMSCs的有效方法。
