The effect of cgVEGF164 on the growth of murine hair follicles
Hao Zhang1, Zhipeng Zhang2, Xiaodong Guo1, Min Ma1, Yue Ao1, Xu Liu1, Xiaoyan Ma1, Hao Liang1, Xudong Guo1第一联系人:
编委: 卢大儒
收稿日期:2018-10-17修回日期:2018-12-12网络出版日期:2019-01-20
| 基金资助: |
Received:2018-10-17Revised:2018-12-12Online:2019-01-20
| Fund supported: |
作者简介 About authors
张豪,硕士研究生,专业方向:生殖生物学与生物技术E-mail:
张志鹏,硕士研究生,专业方向:生殖生物学与生物技术E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (899KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
张豪, 张志鹏, 郭晓东, 马敏, 敖月, 刘旭, 马小燕, 梁浩, 郭旭东. cgVEGF164基因对小鼠毛囊生长的影响[J]. 遗传, 2019, 41(1): 76-84 doi:10.16288/j.yczz.18-136
Hao Zhang, Zhipeng Zhang, Xiaodong Guo, Min Ma, Yue Ao, Xu Liu, Xiaoyan Ma, Hao Liang, Xudong Guo.
毛囊的性状和结构决定动物毛发的品质,研究毛囊生长调控机制对于提高产绒动物产绒量至关重要。毛囊生长所需的营养物质以及调控生长所需的各类细胞因子均由其周围血管(循环系统)提供,因而充足的血液供应是毛囊细胞生长分化的基础。哺乳动物的毛囊通常呈现周期性变化,包括生长期、退行期和休止期[1,2]。研究显示,毛囊周围的毛细微血管会随着毛囊周期的变化而变化,毛细微血管在毛囊生长期比较发达,进入退行期后逐渐退化[3,4,5]。
血管内皮生长因子(vascular endothelial growth factor, VEGF)是一种由二硫键连接而成的二聚体糖蛋白,能够促进血管再生和调节血管渗透性[6,7]。研究证实,VEGF通过与血管内皮生长因子受体(vascular endothelial growth factor receptor, VEGFR)相互作用,促进血管内皮细胞的增殖和迁移[8,9]。皮肤中VEGF基因的表达水平在毛囊生长期上调,进入退行期后逐渐降低,在休止期则基本消失[4,6]。在小鼠毛囊结构中,VEGF基因主要在外根鞘和毛乳头中表达[10],进而诱导毛囊周围毛细血管的形成,促进外根鞘和毛乳头细胞的增殖[11,12],增大毛囊直径和毛干长度[13]。
由于mRNA剪切方式的不同,在人中已发现7种VEGF mRNA剪接变体,包括VEGF121、VEGF145、VEGF165、VEGF183、VEGF189、VEGF148和VEGF206[14,15,16]。已知cgVEGF164是绒山羊VEGF-A基因的一种主要剪接变体,在绒山羊脑、心脏、睾丸、胰腺、脾、肾和肺中都有表达,与人源VEGF165基因的同源性高达94%[17]。已有研究证实VEGF165基因能够促进毛发生长[18,19,20],而cgVEGF164基因是否具有调控毛囊生长发育的作用目前尚不清楚。本研究旨在通过原核显微注射法,获得cgVEGF164转基因小鼠,比较转基因小鼠与非转基因对照小鼠毛囊的直径和数量以及相关信号蛋白ERK1/2、AKT1、LEF1磷酸化水平的变化,探讨外源基因cgVEGF164对毛囊生长的影响及调节机制,为利用现代生物学技术手段提高产绒动物的产绒量提供实验依据。
1 材料与方法
1.1 材料
BDF1雌鼠、CD-1雄鼠、C57小鼠均由内蒙古大学国家清洁级实验动物繁育室提供。所有研究符合内蒙古大学动物伦理委员会的规定并授权。K14-cgVEGF164载体由内蒙古大学王志钢教授提供[17],其启动子为Keratin 14 (K14),外源基因为cgVEGF164。1.2 注射用外源基因的制备
用限制性内切酶Bgl Ⅱ对K14-cgVEGF164质粒载体进行线性化酶切,获得线性化K14-cgVEGF164质粒载体。用乙醇沉淀法对线性化K14-cgVEGF164质粒载体进行纯化,最后用TE缓冲液溶解并调整浓度为1.5 ng/μL。用分光光度计测定OD值,OD值在1.7~1.9之间才可注射。1.3 原核显微注射
原核显微注射法制备转基因小鼠参考文献[21]中的方法。将6~7周龄性成熟的BDF1母鼠与CD1公鼠合笼,20 h后取见栓母鼠的受精卵。将1×10-6 μL左右含K14-cgVEGF164质粒载体的注射液注入胚胎雄原核,注射完毕后将胚胎移入KSOM培养液,放入CO2培养箱。30 min后选取25~30枚注射后存活胚胎移入经麻醉处理的假孕母鼠输卵管壶腹部。移植后将假孕母鼠放置于保温板上待其苏醒后放回鼠房,怀孕成功母鼠约19天后分娩产仔。1.4 PCR检测
根据K14-cgVEGF164质粒载体的序列信息,设计一对用于检测外源基因整合的PCR引物。引物信息:F:5'-CAGGGTCCGATGGGAAAGTGTA-3';
R:5'-TGCTGGCTTTGG TGAGGTTTGA-3'。
扩增产物为675 bp,产物横跨K14启动子和cgVEGF164基因。待出生幼鼠长至3周时,剪取约0.5 cm尾尖提取基因组DNA,进行PCR鉴定,确定转基因阳性小鼠。PCR扩增条件:95℃预变性10 min,95℃变性30 s,60℃复性42 s,72℃延伸45 s,共30个循环;最后再72℃延伸7 min。扩增产物经1%琼脂糖凝胶电泳检测。
1.5 qRT-PCR分析
取10周龄cgVEGF164转基因小鼠为实验组,保留同窝的阴性小鼠为非转基因对照组。脱毛后剪取背部皮肤组织,液氮研磨后加入800 μL RNAiso Plus,按RNA抽提试剂盒说明书提取总RNA,利用反转录试剂盒(TaKaRa,日本)合成cDNA。qRT-PCR扩增体系为20 μL,包括:SYBR Premix Ex TaqⅡ (2×) 10 μL,上、下游引物各0.4 μL,Rox Reference Dye (50×) 0.4 μL,ddH2O 6.8 μL,cDNA 2 μL。qRT-PCR 扩增条件:95℃预变性30 s;95℃变性10 s,60℃延伸30 s,40个循环,每个样品进行3次重复。根据每个样品与内参基因GAPDH所得的Ct值,利用2-ΔΔCt公式分析目的基因cgVEGF164 mRNA的相对表达量。目的基因cgVEGF164和内参基因GAPDH的扩增引物见表1。Table 1
表1
表1 Real-time PCR扩增引物
Table 1
| 基因 | 引物序列 (5'→3') |
|---|---|
| cgVEGF164 | F:CATTGAGACCCTGGTGGACAT |
| R:CTGGCTTTGGTGAGGTTTGAT | |
| GAPDH | F:GTGAAGCAGGCATCTGAGGG |
| R:TGAAGTCGCAGGAGACAACC |
新窗口打开|下载CSV
1.6 Western Blot检测
取转基因小鼠和同窝非转基因对照小鼠皮肤组织,放于1.5 mL离心管内剪碎,RIPA裂解液裂解细胞,收集蛋白,聚丙烯酰胺凝胶电泳后转PVDF膜。封闭后,相应一抗(VEGF164 ab53465、α-tubulin ab15246、ERK1/2 ab17942、P-ERK1/2 ab50011、AKT1 ab32505、P-AKT1 ab66138、LEF1 ab137872、P-LEF1 ab74067均购自美国Abcam公司)4℃孵育过夜,羊抗兔二抗(ab6721购自美国Abcam公司)室温孵育1 h。最后利用Tanon 5200全自动化学发光成像分析系统进行曝光处理。1.7 HE染色与免疫荧光
选取cgVEGF164转基因小鼠和同窝非转基因对照小鼠的背部组织进行固定、脱水透明、浸蜡包埋、切片与贴片、脱蜡、苏木精与伊红染色。VEGF164一抗(1∶200)过夜孵育,羊抗兔二抗(ab6717购自美国Abcam公司) (1∶1000)孵育1 h进行免疫荧光染色。1.8 数据分析
cgVEGF164 mRNA和VEGF164蛋白的相对表达量,毛囊的直径、密度,ERK1/2、AKT1、LEF1磷酸化水平等数据均以平均数和平均数的标准差表示,采用GraphPad Prism 6软件进行统计学分析,所有数据均进行3次以上重复实验。P>0.05表示没有统计学差异,用“N.S.”(No significance)表示;P<0.05表示差异显著,P<0.01表示差异极显著,具有统计学意义。2 结果与分析
2.1 cgVEGF164转基因小鼠的鉴定
本研究共移植原核注射胚胎296枚,获得59只仔鼠,经PCR法初步鉴定共获得5只转基因小鼠(图1A),其编号分别为878号、890号、1008号、1018号和1021号,阳性率为8.5%。将F0代cgVEGF164转基因小鼠和野生型C57小鼠交配,获得F1代小鼠。将F1代cgVEGF164转基因小鼠自交获得F2代cgVEGF164转基因小鼠。出生仔鼠用与原代小鼠相同的鉴定方法进行鉴定。所有F0代转基因阳性小鼠都能够将外源基因传递给子代(表2)。其中1008号小鼠共获得6只F2代cgVEGF164转基因小鼠,其编号分别为1311、1313、1316、1317、1319和1322 (图1B)。图1
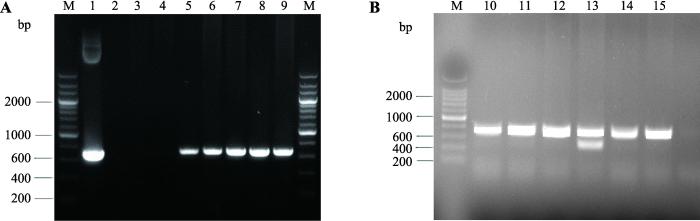 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1cgVEGF164转基因小鼠PCR鉴定结果
A:F0代cgVEGF164转基因小鼠PCR鉴定结果。M:200 bp DNA marker;1:质粒阳性对照(K14-cgVEGF164 plasmids);2:空白对照(H2O);3, 4:非转基因对照小鼠;5~9:F0代转基因小鼠(对应编号分别为878、890、1008、1018、1021)。B:1008号小鼠F2代cgVEGF164转基因小鼠PCR鉴定结果。M:200 bp DNA marker;10~15:F2代转基因小鼠(对应编号分别为1311、1313、1316、1317、1319和1322)。
Fig. 1cgVEGF164 transgenic mice identification by PCR
Table 2
表2
表2 cgVEGF164转基因小鼠传代统计
Table 2
| cgVEGF164 转基因小鼠 (F0)编号 | F1小鼠(只) | F1阳性小鼠(只) | 阳性率(%) |
|---|---|---|---|
| 878♀ | 35 | 12 | 34.3 |
| 890♂ | 38 | 4 | 10.5 |
| 1008♀ | 34 | 4 | 11.8 |
| 1018♀ | 36 | 6 | 16.7 |
| 1021♀ | 32 | 4 | 12.5 |
新窗口打开|下载CSV
2.2 转基因小鼠cgVEGF164 mRNA及蛋白表达水平的检测
利用qRT-PCR、Western Blot和免疫荧光染色检测5只转基因小鼠与同窝非转基因对照小鼠皮肤cgVEGF164 mRNA及蛋白的相对表达量。结果显示,890、1008、1018、1021号转基因阳性小鼠中cgVEGF164 mRNA及VEGF164蛋白的相对表达量均高于同窝非转基因对照小鼠,而878号转基因阳性小鼠中cgVEGF164 mRNA及VEGF164蛋白的相对表达量与其同窝非转基因对照小鼠没有显著性差异(图2,A~D)。890号转基因阳性小鼠cgVEGF164 mRNA相对表达量最高,是同窝对照小鼠886号的6.25倍(图2A);1008号转基因阳性小鼠VEGF164蛋白表达量最高,是同窝对照小鼠1013号的1.68倍(图2,B和C)。经免疫荧光染色,发现VEGF164蛋白主要分布于毛囊球部(图2D)。图2
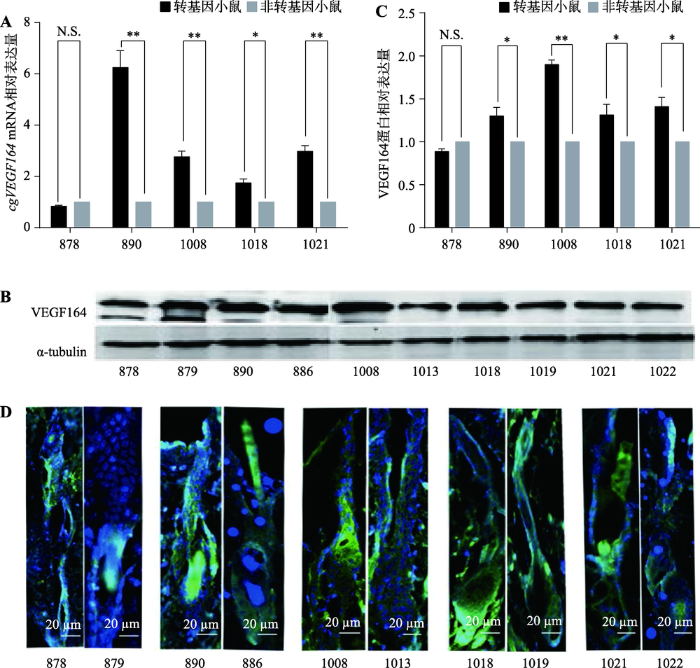 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2转基因小鼠cgVEGF164 mRNA及蛋白表达水平检测
A:转基因小鼠与非转基因对照小鼠cgVEGF164 mRNA表达量的对比;B:转基因小鼠与非转基因对照小鼠Western Blot结果;C:转基因小鼠与非转基因对照小鼠Western Blot结果的灰度值分析;D:转基因小鼠与非转基因对照小鼠VEGF164蛋白免疫荧光染色。图中绿色荧光为VEGF164染色,蓝色荧光为DAPI染色。N.S.表示P>0.05,没有统计学差异;*表示P<0.05,差异显著;**表示P<0.01,差异极显著。
Fig. 2Expression patterns of cgVEGF164 mRNA and protein in transgenic mice
2.3 转基因小鼠cgVEGF164 mRNA组织表达谱检测及表型分析
为探究cgVEGF164在转基因小鼠各组织中的表达水平,本研究将1008号cgVEGF164转基因小鼠F2代作为实验组,其同窝的非转基因小鼠作为对照组,观察实验组与对照组小鼠表型的变化,并利用qRT-PCR检测转基因和非转基因小鼠各组织中cgVEGF164 mRNA表达水平。结果表明,与非转基因对照小鼠相比,8周龄cgVEGF164转基因小鼠的表型无异常变化,生长状况良好(图3A)。转基因小鼠脑、脂肪、肌肉、肝脏、肺、脾脏、肾脏、心脏、胃、大肠、小肠、睾丸、卵巢等组织中cgVEGF164 mRNA的相对表达量均高于皮肤组织(图3B);与非转基因对照小鼠相比,cgVEGF164在转基因小鼠脑、脂肪、肌肉、肝脏、肺、脾脏、肾脏、心脏、胃、大肠、小肠、睾丸、卵巢等组织中的表达量并没有显著升高(图3C),这可能与K14启动子是表皮特异启动子有关,表明外源cgVEGF164基因对转基因小鼠其他组织的影响比较小。图3
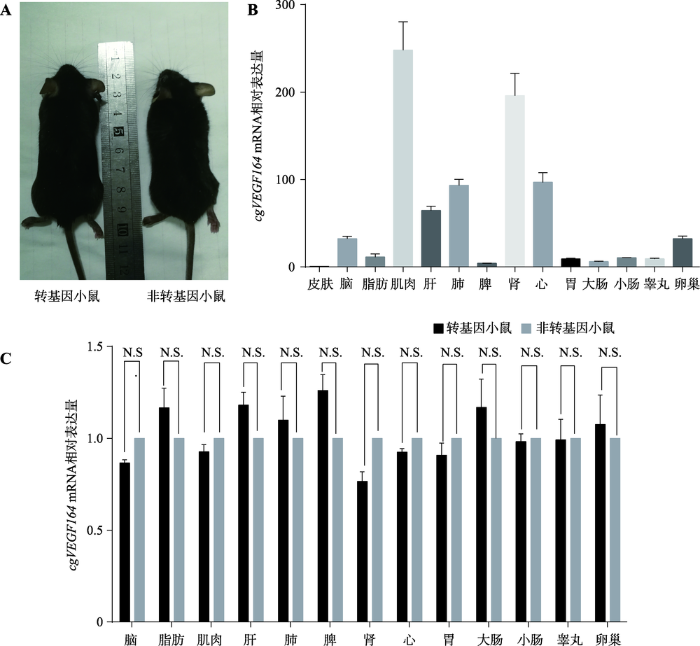 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3转基因小鼠表型观察及各组织中cgVEGF164 mRNA相对表达量
A:转基因小鼠与非转基因小鼠的表型观察;B:转基因小鼠各组织cgVEGF164表达谱;C:转基因小鼠与非转基因小鼠cgVEGF164 mRNA在各组织中相对表达量。N.S.表示P>0.05,没有统计学差异。
Fig. 3Phenotypes and cgVEGF164 mRNA expression levels in transgenic mice
2.4 cgVEGF164基因对小鼠毛囊生长的影响
本研究将1008号cgVEGF164转基因小鼠F2代作为实验组,其同窝非转基因小鼠作为对照组,待小鼠生长到10周龄时,将两组小鼠背部剃毛后,取背部组织制备组织切片,经HE染色后观察。结果显示,与非转基因对照小鼠相比,转基因小鼠的毛囊直径明显增大(图4A),毛囊密度显著升高(图4B)。转基因小鼠毛囊深入真皮层,而非转基因对照小鼠毛囊在真皮层比较浅的位置(图4C)。图4
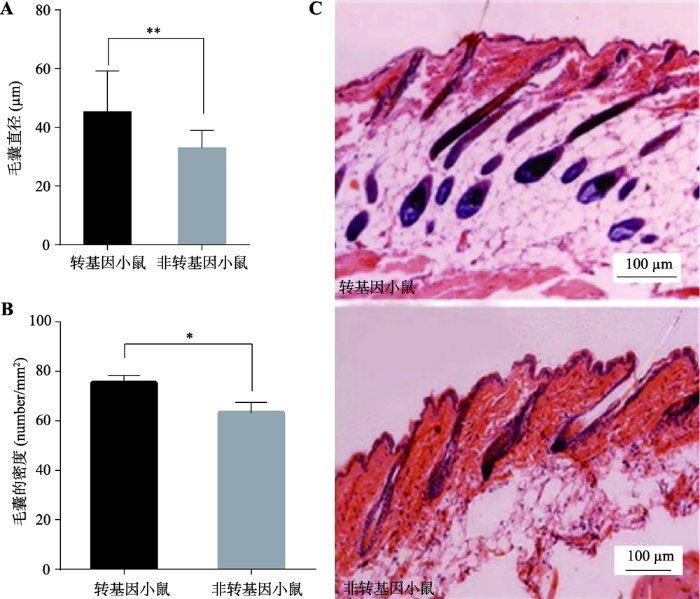 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4转基因小鼠皮肤组织切片观察
A:转基因小鼠与非转基因对照小鼠毛囊直径;B:转基因小鼠与非转基因对照小鼠毛囊密度;C:转基因小鼠和非转基因对照小鼠石蜡切片HE染色图(纵切)。*表示P<0.05,差异显著;**表示P<0.01,差异极显著。
Fig. 4Morphology of skin in transgenic mice
2.5 VEGF下游信号通路蛋白的检测
利用Western Blot方法检测转基因小鼠与非转基因对照小鼠VEGF下游蛋白ERK1/2、P-ERK1/2、AKT1、P-AKT1、LEF1、P-LEF1的相对表达量,α-tubulin作为内参蛋白,结果如图5A所示。通过灰度值计算P-ERK1/2、P-AKT1、P-LEF1分别占ERK1/2、AKT1、LEF1表达量的百分比,结果发现转基因小鼠中P-ERK1/2/ERK1/2、P-AKT1/AKT1、P-LEF1/ LEF1分别为44%、51%、11%,均高于对照组18%、11%、8% (图5B)。
图5
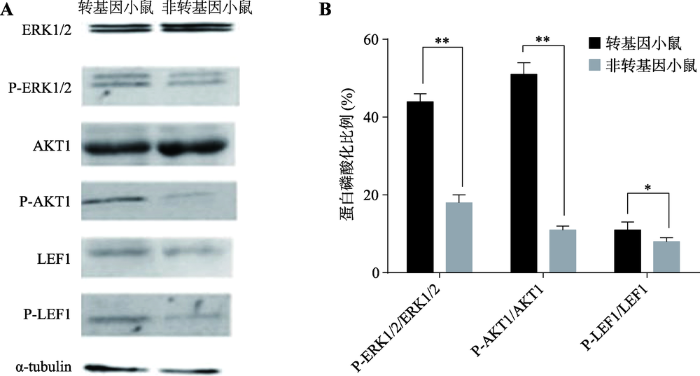 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5VEGF下游信号通路蛋白的检测
A:ERK1/2、P-ERK1/2、AKT1、P-AKT1、LEF1、P-LEF1的Western Blot检测;B:P-ERK1/2、P-AKT1、P-LEF1分别占ERK1/2、AKT1、LEF1表达量的百分比。*表示P<0.05,差异显著;**表示P<0.01,差异极显著。
Fig. 5Detection of the downstream signal proteins of VEGF in transgenic mice
3 讨论
转基因动物是指通过转基因技术将外源基因整合到动物的基因组中,从而使外源基因在动物体内表达并且可以稳定遗传[22]。建立转基因动物模型,对研究外源基因在动物体内的功能和表达调控机制具有至关重要的作用[23,24]。本研究利用原核显微注射技术制作cgVEGF164转基因小鼠模型,旨在研究cgVEGF164基因对小鼠毛囊生长的影响。经PCR初步鉴定,确定5只小鼠成功转入了cgVEGF164基因(编号分别是878、890、1008、1018、1021),阳性率为8.5%。经qRT-PCR和Western Blot进一步检测分析,获得4只高表达cgVEGF164的转基因阳性小鼠(编号分别是890、1008、1018、1021)。878号小鼠虽然成功转入了cgVEGF164基因,但其cgVEGF164 mRNA和蛋白表达量不高于非转基因对照小鼠,推测原因可能是由于本研究中构建的转基因表达载体属于随机整合载体,外源基因虽然发生了基因组整合,但其转录和蛋白质表达效率将受到整合位点的影响而不可预测。由于K14启动子是表皮特异启动子,因而与非转基因对照小鼠相比,cgVEGF164基因在转基因小鼠脑、脂肪、肌肉、肝脏、肺、脾脏、肾脏、心脏、胃、大肠、小肠、睾丸、卵巢等组织中的表达量并没有显著升高。cgVEGF164基因编码一段由190个氨基酸构成的多肽,其N端的26个氨基酸序列与人源VEGF165的N端完全相同,属信号肽序列,推测该序列与其分泌功能有关[25,26]。VEGF是血管再生的关键因子,通过促进毛囊周围毛细血管的生长,进而影响毛发生长和毛囊周期[27]。本研究使用HE染色法对小鼠背部组织切片进行染色观察,结果发现,与非转基因对照小鼠相比cgVEGF164转基因小鼠毛囊的直径和密度均增加,毛囊深入真皮层,表明cgVEGF164基因具有促进毛囊生长的作用。
为进一步探究cgVEGF164基因参与小鼠毛发生长的调节机制,本研究通过检测信号蛋白ERK1/2、AKT、LEF1的磷酸化水平初步确定cgVEGF164与MAPK/ERK1/2、PI-3K-AKT1/PAK和Wnt/β-catenin/ LEF1等信号通路的联系。ERK1/2、AKT1、LEF1是与VEGF信号通路相关的下游信号蛋白,在促进毛发生长和调节毛囊周期中扮演重要角色。ERK1/2是MAPK家族成员之一,参与VEGF调节表皮细胞的增殖[1,12,28,29]。AKT也是MAPK家族成员之一,能够与VEGFR作用促进表皮细胞的存活和增殖[15,30]。LEF1是Wnt途径中主要成员之一,与β-catenin蛋白结合作用于VEGF,调控毛囊周期促进毛发再生[31,32,33]。研究结果显示,cgVEGF164转基因小鼠背部上皮中ERK1/2、AKT1、LEF1磷酸化水平均高于非转基因对照小鼠,由此我们推断cgVEGF164基因通过促进RK1/2、AKT1、LEF1磷酸化调控毛囊的生长。但是,毛囊的生长是多种生物因子综合作用的结果,cgVEGF164基因调控毛囊生长的分子机制还需要进一步深入研究。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URLPMID:1566372 [本文引用: 2]

Abstract The mammalian hair follicle is a treasure waiting to be discovered by more molecular geneticists. How can a tiny cluster of apparently uniform epithelial cells, adjacent to a tiny cluster of uniform mesenchymal cells, give rise to five or six concentric cylinders, each of which is composed of cells of a distinctive type that synthesize their own distinctive set of proteins? There is now evidence that several growth factors, cell adhesion molecules and other molecules play important roles in the regulation of this minute organ.
URLPMID:11152763 [本文引用: 1]

Abstract Nearly 50 years ago, Chase published a review of hair cycling in which he detailed hair growth in the mouse and integrated hair biology with the biology of his day. In this review we have used Chase as our model and tried to put the adult hair follicle growth cycle in perspective. We have tried to sketch the adult hair follicle cycle, as we know it today and what needs to be known. Above all, we hope that this work will serve as an introduction to basic biologists who are looking for a defined biological system that illustrates many of the challenges of modern biology: cell differentiation, epithelial-mesenchymal interactions, stem cell biology, pattern formation, apoptosis, cell and organ growth cycles, and pigmentation. The most important theme in studying the cycling hair follicle is that the follicle is a regenerating system. By traversing the phases of the cycle (growth, regression, resting, shedding, then growth again), the follicle demonstrates the unusual ability to completely regenerate itself. The basis for this regeneration rests in the unique follicular epithelial and mesenchymal components and their interactions. Recently, some of the molecular signals making up these interactions have been defined. They involve gene families also found in other regenerating systems such as fibroblast growth factor, transforming growth factor-beta, Wnt pathway, Sonic hedgehog, neurotrophins, and homeobox. For the immediate future, our challenge is to define the molecular basis for hair follicle growth control, to regenerate a mature hair follicle in vitro from defined populations, and to offer real solutions to our patients' problems.
URLPMID:10441606 [本文引用: 1]

Author information: (1)Department of Dermatology, University Hospital Eppendorf, University of Hamburg, Germany. paus@uke.uni-hamburg.de
URLPMID:11181640 [本文引用: 2]

Abstract The murine hair follicle undergoes pronounced cyclic expansion and regression, leading to rapidly changing demands for its vascular support. Our study aimed to quantify the cyclic changes of perifollicular vascularization and to characterize the biological role of VEGF for hair growth, angiogenesis, and follicle cycling. We found a significant increase in perifollicular vascularization during the growth phase (anagen) of the hair cycle, followed by regression of angiogenic blood vessels during the involution (catagen) and the resting (telogen) phase. Perifollicular angiogenesis was temporally and spatially correlated with upregulation of VEGF mRNA expression by follicular keratinocytes of the outer root sheath, but not by dermal papilla cells. Transgenic overexpression of VEGF in outer root sheath keratinocytes of hair follicles strongly induced perifollicular vascularization, resulting in accelerated hair regrowth after depilation and in increased size of hair follicles and hair shafts. Conversely, systemic treatment with a neutralizing anti-VEGF antibody led to hair growth retardation and reduced hair follicle size. No effects of VEGF treatment or VEGF blockade were observed in mouse vibrissa organ cultures, which lack a functional vascular system. These results identify VEGF as a major mediator of hair follicle growth and cycling and provide the first direct evidence that improved follicle vascularization promotes hair growth and increases hair follicle and hair size.
URLPMID:10771470 [本文引用: 1]

After the completion of skin development, angiogenesis, i.e., the growth of new capillaries from pre-existing blood vessels, is held to occur in the skin only under pathologic conditions. It has long been noted, however, that hair follicle cycling is associated with prominent changes in skin perfusion, that the epithelial hair bulbs of anagen follicles display angiogenic properties, and that the follicular dermal papilla can produce angiogenic factors. Despite these suggestive observations, no formal proof is as yet available for the concept that angiogenesis is a physiologic event that occurs all over the mature mammalian integument whenever hair follicles switch from resting (telogen) to active growth (anagen). This study uses quantitative histomorphometry and double-immunohistologic detection techniques for the demarcation of proliferating endothelial cells, to show that synchronized hair follicle cycling in adolescent C57BL/6 mice is associated with substantial angiogenesis, and that inhibiting angiogenesis in vivo by the intraperitoneal application of a fumagillin derivative retards experimentally induced anagen development in these mice. Thus, angiogenesis is a physiologic event in normal postnatal murine skin, apparently is dictated by the hair follicle, and appears to be required for normal anagen development. Anagen-associated angiogenesis offers an attractive model for identifying the physiologic controls of cutaneous angiogenesis, and an interesting system for screening the effects of potential antiangiogenic drugs in vivo.
URLPMID:8602241 [本文引用: 2]

The endothelial cell-specific vascular endothelial growth factor (VEGF) and its cellular receptors Flt-1 and Flk-1 have been implicated in the formation of the embryonic vasculature. This is suggested by their colocalized expression during embryogenesis and the impaired vessel formation in Flk-1 and Flt-1 deficient embryos. However, because Flt-1 also binds placental growth factor, a VEGF homologue, the precise role of VEGF was unknown. Here we report that formation of blood vessels was abnormal, but not abolished, in heterozygous VEGF-deficient (VEGF+/-) embryos, generated by aggregation of embryonic stem (ES) cells with tetraploid embryos (T-ES) and even more impaired in homozygous VEGF-deficient (VEGF-/-) T-ES embryos, resulting in death at mid-gestation. Similar phenotypes were observed in F1-VEGF+/- embryos, generated by germline transmission. We believe that this heterozygous lethal phenotype, which differs from the homozygous lethality in VEGF-receptor-deficient embryos, is unprecedented for a targeted autosomal gene inactivation, and is indicative of a tight dose-dependent regulation of embryonic vessel development by VEGF.
URLPMID:8602242 [本文引用: 1]

Angiogenesis is required for a wide variety of physiological and pathological processes. The endothelial cell-specific mitogen vascular endothelial growth factor (VEGF) is a major mediator of pathological angiogenesis. Also, the expression of VEGF and its two receptors, Flt-1 and Flk-1/KDR, is related to the formation of blood vessels in mouse and rat embryos. Mice homozygous for mutations that inactivate either receptor die in utero between days 8.5 and 9.5. However, ligand(s) other than VEGF might activate such receptors. To assess the role of VEGF directly, we disrupted the VEGF gene in embryonic stem cells. Here we report the unexpected finding that loss of a single VEGF allele is lethal in the mouse embryo between days 11 and 12. Angiogenesis and blood-island formation were impaired, resulting in several developmental anomalies. Furthermore, VEGF-null embryonic stem cells exhibit a dramatically reduced ability to form tumours in nude mice.
URLPMID:16633338 [本文引用: 1]

Vascular endothelial growth-factor receptors (VEGFRs) regulate the cardiovascular system. VEGFR1 is required for the recruitment of haematopoietic precursors and migration of monocytes and macrophages, whereas VEGFR2 and VEGFR3 are essential for the functions of vascular endothelial and lymphendothelial cells, respectively. Recent insights have shed light onto VEGFR signal transduction and the interplay between different VEGFRs and VEGF co-receptors in development, adult physiology and disease.
URLPMID:17658244 [本文引用: 1]

Vascular endothelial growth factors (VEGFs) regulate vascular development, angiogenesis and lymphangiogenesis by binding to a number of receptors. VEGFR-1 is required for the recruitment of haematopoietic stem cells and the migration of monocytes and macrophages, VEGFR-2 regulates vascular endothelial function and VEGFR-3 regulates lymphatic endothelial cell function. Over the last decade, considerable progress has been made in delineating the VEGFR-2 specific intracellular signalling cascades leading to proliferation, migration, survival and increased permeability, each of which contributes to the angiogenic response. Furthermore, therapeutic inhibition of VEGFR-2 action is now having an impact in the clinic for the treatment of a number of diseases.
URLPMID:19309374 [本文引用: 1]

Background. Vascular endothelial growth factor (VEGF) promotes angiogenesis and plays important roles in neovascularization and development of tissues. VEGF receptors (VEGFRs) are high-affinity receptors for VEGF and are originally considered specific to endothelial cells. We have previously shown that keratinocytes from human normal skin express VEGFRs. This poses the question of whether these receptors are also expressed by epidermal appendages, as epidermal appendages are lined with epithelial cells. Objective. To investigate the expression of VEGFR-2 compare with VEGF in epidermal appendages, including hair follicles, eccrine sweat glands and sebaceous glands. Methods. Monoclonal antibodies to VEGF and VEGFR-2 were used for immunohistochemical examination of cryostat-cut sections of normal human skin specimens from 11 donors undergoing cosmetic surgery. Results. Immunoreactivities for VEGF and VEGFR-2 principally showed parallel intense expression in anagen hair follicle (including outer root sheat, inner root sheath, dermal papillae epidermal matrix), sebaceous glands (ductal and secretory portions) and eccrine sweat glands (ductal and secretory portions), respectively. In particular, abundant expression of VEGF was found in the follicular basement membrane zone surrounding the bulb matrix and in the ductal and secretory portions of eccrine sweat glands. Conclusion. A potential VEGF/VEGFR-2 autocrine pathway may be defined by the coexpression of VEGF and VEGFR-2 in human skin epidermal appendages.
URLPMID:22707147 [本文引用: 1]

Abstract stimulated proliferation of ORS cells and upregulated expression of VEGFR-2 in a dose-dependent manner. Moreover, VEGF induced phosphorylation of VEGFR-2, PLC-γ1, PKC-α, MEK, and p44/42 MAPK (ERK1/2) in a time-dependent manner. Taken together, human ORS cells express functional VEGF receptor-2 and exogenous VEGF upregulates expression of VEGFR-2 and stimulates proliferation of ORS cells via VEGFR-2 mediated ERK signaling pathway.
URLPMID:22659165 [本文引用: 2]

78 We examine the expression of VEGFR-2 on cultured human dermal papilla (DP) cells. 78 VEGF165 stimulated proliferation of human DP cells in a dose-dependent manner. 78 This stimulation was through VEGFR-2-mediated activation of ERK.
URLPMID:11240268 [本文引用: 1]

The effect of cultured normal human dermal papilla cells (DPCs) and conditioned medium prepared with cultured DPCs on chemotactic migration of human hair outer root sheath cells (ORSCs) was examined quantitatively. ORSCs showed significantly increased migration toward both cultured DPCs and the conditioned medium suggesting that DPCs produce and secrete a paracrine factor(s), which attracts hair follicle epithelial cells. Some soluble factors, which are reportedly produced by DPCs, such as insulin-like growth factor-I (IGF-I), hepatocyte growth factor (HGF), vascular endothelial cell growth factor (VEGF), and transforming growth factor-β1 (TGF-β1), were also examined. ORSCs showed dramatically increased migration toward IGF-I and HGF at concentrations of 1–10 ng/ml. On the other hand, neither VEGF nor TGF-β1 showed any effect on the chemotaxis of ORSCs. It is interesting that all factors involving mitogenic activity did not always have chemotactic activity for ORSCs. This is the first report to establish that IGF-I and HGF have not only a growth stimulatory but also a chemotactic effect on ORSCs. In addition, the method presented here may help to simplify chemotaxis assays of any type of epithelial keratinocytes with poor mobility.
URL [本文引用: 1]
[本文引用: 2]
URLPMID:9665379 [本文引用: 1]

Vascular endothelial growth factor (VEGF) has been implicated in the pathologic angiogenesis observed in psoriasis and other chronic inflammatory skin diseases that are characterized by enhanced expression of VEGF by epidermal keratinocytes and of VEGF receptors by tortuous microvessels in the upper dermis. To investigate the functional importance of chronic VEGF overexpression in vivo, we used a keratin 14 promoter expression cassette containing the gene for murine VEGF164 to selectively target VEGF expression to basal epidermal keratinocytes in transgenic mice. These mice demonstrated an increased density of tortuous cutaneous blood capillaries with elevated expression levels of the high affinity VEGF receptors, VEGFR-1 and VEGFR-2, most prominently during the neonatal period. In contrast, no abnormalities of lymphatic vessels were detected. In addition, the number of mast cells in the upper dermis was significantly increased in transgenic skin. Intravital fluorescence microscopy revealed highly increased leukocyte rolling and adhesion in postcapillary skin venules that were both inhibited after injection of blocking antibodies against E- and P-selectin. Combined blocking antibodies against intercellular adhesion molecule-1 and lymphocyte function-associated antigen-1 were without effect, whereas an anti-vascular cell adhesion molecule-1/VLA-4 antibody combination almost completely normalized the enhanced leukocyte adhesion in transgenic mice. This study reveals VEGF as a growth factor specific for blood vessels, but not lymphatic vessels, and demonstrates that chronic orthotopic overexpression of VEGF in the epidermis is sufficient to induce cardinal features of chronic skin inflammation, providing a molecular link between angiogenesis, mast cell accumulation, and leukocyte recruitment to sites of inflammation.
URL [本文引用: 2]

旨在克隆内蒙古白绒山羊血管内皮生长因子(vascular endothelial growth factor,VEGF164)基因并分析其基本表达模式。采用RT-PCR技术克隆基因,将得到的基因cDNA序列及其编码的氨基酸序列进行生物信息学分析。利用半定量RT-PCR方法进行组织表达检测。获得了内蒙古白绒山羊VEGF164基因编码区cDNA全长序列,扩增片段全长573 bp,包含了完整的ORF,编码190个氨基酸残基。核苷酸序列与绵羊的VEGF164(EU857623.1)基因同源性为99%,相应的氨基酸序列同源性为99%。SMART程序分析表明,ORF编码的蛋白质具有信号肽序列及血小板衍生和血管内皮生长因子家族(PDGF,VEGF)结构域。Psite程序分析表明,有1个蛋白激酶C磷酸化位点,4个酪蛋白激酶磷酸化位点。ProtComp Version 9.0程序分析将其定位于细胞外。RT-PCR检测表明,VEGF164基因在绒山羊脑、心脏、睾丸、胰腺、脾、肾和肺组织中均有表达。
URL [本文引用: 2]

旨在克隆内蒙古白绒山羊血管内皮生长因子(vascular endothelial growth factor,VEGF164)基因并分析其基本表达模式。采用RT-PCR技术克隆基因,将得到的基因cDNA序列及其编码的氨基酸序列进行生物信息学分析。利用半定量RT-PCR方法进行组织表达检测。获得了内蒙古白绒山羊VEGF164基因编码区cDNA全长序列,扩增片段全长573 bp,包含了完整的ORF,编码190个氨基酸残基。核苷酸序列与绵羊的VEGF164(EU857623.1)基因同源性为99%,相应的氨基酸序列同源性为99%。SMART程序分析表明,ORF编码的蛋白质具有信号肽序列及血小板衍生和血管内皮生长因子家族(PDGF,VEGF)结构域。Psite程序分析表明,有1个蛋白激酶C磷酸化位点,4个酪蛋白激酶磷酸化位点。ProtComp Version 9.0程序分析将其定位于细胞外。RT-PCR检测表明,VEGF164基因在绒山羊脑、心脏、睾丸、胰腺、脾、肾和肺组织中均有表达。
URLPMID:24296159 [本文引用: 1]

The functional state of vasculature is tightly controlled by vascular endothelial growth factor receptor-2 (VEGFR-2). Recent studies revealed that VEGFR-2 is expressed on hair follicle keratinocytes. We proposed to investigate its effect on proliferation, adhesion and migration of cultured human outer root sheath cells from central hair follicle epithelium. These studies were undertaken in vitro using human outer root sheath cells from central hair follicle epithelium, immunohistochemistry analysis, immunofluorescence microscopy, western blot analysis, MTT, trans well analysis, and RT-PCR. Our results show that VEGFR-2 is expressed in these cells in vivo and in vitro. Furthermore, proliferation and migration of cultured human outer root sheath cells from central hair follicle epithelium is increased by VEGF165, while homotypic adhesion is decreased but heterotypic adhesion is increased. VEGF165 upregulates integrin 1 but dowregulates lgr6 expression. In addition, phosphorylation of VEGFR-2, Erk1/2, c-Jun and p38, are increased following VEGF165 treatment and these effects are reversed by a VEGFR-2 neutralizing antibody. Our results suggest a role of VEGF/VEGFR-2 beyond angiogenesis in hair follicle regulation.
URLPMID:24796185 [本文引用: 1]

Objective To obtain rat hair follicle stem cells(rHFSCs) which can constantly and highly express vascular endothelial growth factor 165(VEGF165), and to observe the expression of VEGF165 gene in rat HFSCs. Methods The cirri skin of 1-week-old Sprague Dawley rat was harvested and digested by using combination of Dispase and type IV collagenases. The bulge was isolated under microscope. The rHFSCs were cultured by tissue block method. After purified by rapid adhering on collagen type IV, the growth curve of different generations rHFSCs was drawn. The cells were identified by immunofluorescence staining and real time quantitative PCR(RT-qPCR) analysis that tested the expression level of correlated genes. Lentivirus of pLV-internal ribosome entry site(IRES)-VEGF165-enhanced green fluorescent protein(EGFP)(experimental group) and pLVIRESEGFP empty vector(control group) was packaged by calcium transfected method and the rHFSCs were transfected. The green fluorescent protein expression was observed by inverted fluorescence microscope, and VEGF165 mRNA and protein expressions were detected using RT-PCR and Western blot. Results The rHFSCs which were isolated, cultured, and purified were like the "slabstone", and had strong adhesion ability and colony formation ability. The purified cells were in latent growth phase at 2-3 days; they were in exponential growth phase at 5-6 days. The expressions of cytokeration 15(CK15), integrin 6, and integrin 1(markers of HFSCs) were positive by immunocytochemistry. The RT-qPCR analysis showed that CK15, CK19, integrin 6, and integrin 1 expressed highly, but CD34(a marker of epidermal stem cells) and CK10(a marker of keratinocyte) expressed lowly. After 14 days, the transfection efficiency was up to 85.76% 1.91%. RT-PCR analysis and Western blot showed that VEGF165 mRNA and protein expressions were positive in experimental group, and were negative in controlgroup. Conclusion The rHFSCs with high purity and strong proliferation ability can be obtained by using microscope combined with tissue cultivation and rapid cell adhesion on collagen type IV. The rHFSCs with high expression of VEGF165 can be successfully obtained by lentiviral transfection. This method provides good seeding cells for tissue engineering to construct artificial hair follicles, blood vessels, and skins.
PMID:28244687 [本文引用: 1]

Within the vascular endothelial growth factor (VEGF) family of five subtypes, VEGF165 secreted by endothelial cells has been identified to be the most active and widely distributed factor that plays a vital role in courses of angiogenesis, vascularization and mesenchymal cell differentiation. Hair follicle stem cells (HFSCs) can be harvested from the bulge region of the outer root sheath of the hair follicle and are adult stem cells that have multi-directional differentiation potential. Although the research on differentiation of stem cells (such as fat stem cells and bone marrow mesenchymal stem cells) to the endothelial cells has been extensive, but the various mechanisms and functional forms are unclear. In particular, study on HFSCs' directional differentiation into vascular endothelial cells using VEGF165 has not been reported. In this study, VEGF165 was used as induction factor to induce the differentiation from HFSCs into vascular endothelial cells, and the results showed that Notch signalling pathway might affect the differentiation efficiency of vascular endothelial cells. In addition, the in vivo transplantation experiment provided that HFSCs could promote angiogenesis, and the main function is to accelerate host-derived neovascularization. Therefore, HFSCs could be considered as an ideal cell source for vascular tissue engineering and cell transplantation in the treatment of ischaemic diseases.
URL [本文引用: 1]
URLPMID:21385837 [本文引用: 1]

The ventricular conduction system represents the electrical wiring responsible for the co-ordination of cardiac contraction. Defects in the circuit produce a delay or conduction block and induce cardiac arrhythmias. Understanding how this circuit forms and identification of the factors important for its development thus provide insights into the origin of cardiac arrhythmias. Recent advances, using genetically modified mouse models, have contributed to our understanding of how the ventricular conduction system is established during heart development. Transgenic mice carrying different reporter genes have highlighted the conservation of the anatomy and development of the ventricular conduction system between mice and humans. Lineage tracing and retrospective clonal analysis have established the myogenic origin of the ventricular conduction system and determined properties of conductive progenitor cells. Finally, gene knock-out models reproducing human cardiac defects have led to the identification of transcription factors important for the development of the ventricular conduction system. These transcription factors operate at the levels of both conduction system morphogenesis and differentiation by controlling the expression of genes responsible for the electrical activity of the heart. In summary, defects in the ventricular conduction system are a major cause of arrhythmias, and deciphering the molecular pathways responsible for conduction system morphogenesis and the differentiation of conductive myocytes furthers our understanding of the mechanisms underlying heart disease.
[本文引用: 1]
URLPMID:14987374 [本文引用: 1]

Cystic fibrosis (CF) is a common and fatal recessive disease, which is caused by dysfunction of a chloride ion channel, termed the CF transmembrane conductance regulator (CFTR). The CF gene was cloned in 1989; subsequently, several mouse models have been created using gene targeting within embryonic stem cells. This report describes how such animal models provide the opportunity to elucidate disease pathogenesis, correlate genotype with phenotype and develop novel therapies. The current models encompass mice with a complete knockout of CFTR function, with residual CFTR function, and with precise mutations corresponding to those in humans that precipitate CF. All the CF mice demonstrate the characteristic CF ion-transport defect and show some evidence of intestinal disease, but they have a variable level of survival. Genetic background has also been shown to affect the intestinal phenotype of CF mice and this has allowed identification of a genetic modifier locus of CF in humans. Lung disease in human CF is the major cause of death in early adulthood. This is not entirely reproduced in CF mice, but repeat exposure of the lung to clinical pathogens does reveal a significantly abnormal pathogen-related response in the residual-function mice. CF mice have been successfully used to investigate the safety and efficacy of various pharmacological and gene-therapy protocols. As new cloning techniques become available, the models can be refined to ensure that in vivo models continue to be an essential tool for studying CF.
URLPMID:9879835 [本文引用: 1]

Abstract Hair follicle vascularization appears to be closely related to the processes involved in hair cycle regulation, in which growth factors, cytokines and other bioactive molecules are involved. In particular, vascular endothelial growth factor (VEGF), essential for angiogenesis and vascular permeability, may be responsible for maintaining proper vasculature around the hair follicle during the anagen growth phase. The aim of our study was to compare the in vitro angiogenic capacity, i.e. the steady-state expression of the VEGF gene, of different cultured cell types derived from normal human hair follicles, corresponding to different follicular compartments. Human dermal papilla cells (DPC), fibrous sheath fibroblasts, dermal fibroblasts, and follicular and interfollicular keratinocytes were cultured and studied in vitro for VEGF expression at the mRNA level using RT-PCR, and for VEGF protein synthesis by radioimmunoprecipitation and immunocytochemistry. In vivo examination for VEGF expression in human terminal hair follicles was performed using immunohistochemical methods. In the present report the expression of four different VEGF molecular isoforms, differing in their angiogenic capacity, are described in different cultured follicular cell types for the first time. Cultured follicular cells strongly expressed mRNA of four VEGF molecular species identified as the 121-, 145-, 165- and 189-amino acid splice variants, the most prominent being the 121-amino acid molecule. DPC, and also other mesenchymal cells such as fibrous sheath fibroblasts and dermal fibroblasts, in vivo and in vitro strongly expressed VEGF mRNA and synthesized a 46-kDa VEGF protein, whereas follicular and interfollicular keratinocytes in vitro expressed lower levels of VEGF mRNA and proteins than mesenchymal cells. As the highest expression of VEGF was found in DPC, we suggest that DPC are mainly responsible for angiogenic processes possibly related to the human hair cycle.
URLPMID:25178380 [本文引用: 1]

To detect goat vascular endothelial growth factor (VEGF)-mediated regrowth of hair, full-length VEGF164 cDNA was cloned from Inner Mongolia cashmere goat () into the pET-his prokaryotic expression vector, and the recombinant plasmid was transferred into BL21 cells. The expression of recombinant 6×his-gVEGF164 protein was induced by 0.5 mM isopropyl thio-β-D-galactoside at 32°C. Recombinant goat VEGF164 (rgVEGF164) was purified and identi ed by western blot using monoclonal anti-his and anti-VEGF antibodies. The rgVEGF164 was smeared onto the dorsal area of a shaved mouse, and we noted that hair regrowth in this area was faster than in the control group. Thus, rgVEGF164 increases hair growth in mice.
URLPMID:12699676 [本文引用: 1]

This study is an investigation to evaluate how the controlled release of different growth factors modifies the hair follicle growth of mice. For the controlled release, basic fibroblast growth factor or hepatocyte growth factor was incorporated into a biodegradable gelatin hydrogel, while vascular endothelial growth factor was incorporated into a biodegradable collagen hydrogel. After subcutaneous implantation of the two different hydrogels incorporating growth factors into the backs of mice, hair follicle growth was evaluated photometrically and histologically 10 days later. The darkness of the reverse side of skin implanted with every hydrogel incorporating growth factor was significantly higher than that of skin injected with the corresponding growth factor in the solution. Implantation of the hydrogel incorporating growth factor increased the area of the hair follicles to a significantly greater extent than other control groups, whereas no effect on the skin thickness was observed. The length of hair shaft elongated was significantly high by the hydrogel incorporating every growth factor. Neither empty gelatin nor collagen hydrogels affected hair follicle growth. These findings indicate that the controlled release enabled the growth factor to positively act on the hair growth cycle of mice.
URLPMID:12778165 [本文引用: 1]

Vascular endothelial growth factor (VEGF) is a key regulator of physiological angiogenesis during embryogenesis, skeletal growth and reproductive functions. VEGF has also been implicated in pathological angiogenesis associated with tumors, intraocular neovascular disorders and other conditions. The biological effects of VEGF are mediated by two receptor tyrosine kinases (RTKs), VEGFR-1 and VEGFR-2, which differ considerably in signaling properties. Non-signaling co-receptors also modulate VEGF RTK signaling. Currently, several VEGF inhibitors are undergoing clinical testing in several malignancies. VEGF inhibition is also being tested as a strategy for the prevention of angiogenesis, vascular leakage and visual loss in age-related macular degeneration.
URLPMID:10327068 [本文引用: 1]

KDR/FIk-1 tyrosine kinase, one of the two VEGF receptors induces mitogenesis and differentiation of vascular endothelial cells. We have previously reported that a major target molecule of KDR/Flk-1 kinase is PLC-gamma, and that VEGF induces activation of MAP kinase, mainly mediated by protein kinase C (PKC) in the NIH3T3 cells overexpressing KDR/FIk-1 (Takahashi and Shibuya, 1997). However, the signal transduction initiated from VEGF in endothelial cells remains to be elucidated. In primary sinusoidal endothelial cells which showed strictly VEGF-dependent growth, we found that VEGF stimulated the activation of Raf-1-MEK-MAP kinase cascade. To our surprise, an important regulator, Ras was not efficiently activated to a significant level in response to VEGF. Consistent with this, dominant-negative Ras did not block the VEGF-induced phosphorylation of MAP kinase. On the other hand, PKC-specific inhibitors severely reduced VEGF-dependent phosphorylation of MEK, activation of MAP kinase and subsequent DNA synthesis. A potent PI3 kinase inhibitor, Wortmannin, could not inhibit either of them. These results suggest that in primary endothelial cells, VEGF-induced activation of Raf-MEK-MAP kinase and DNA synthesis are mainly mediated by PKC-dependent pathway, much more than by Ras-dependent or PI3 kinase-dependent pathway.
URLPMID:3624707 [本文引用: 1]

Regulation of endothelial cell apoptosis is a critical modulator of normal and pathological angiogenesis. In this study, we examined the role of the protein kinase Akt/PKB in endothelial cell survival in response to growth factor and matrix attachment signals. Vascular endothelial growth factor(VEGF)-induced cytoprotection of endothelial cell monolayers correlated with the wortmannin-sensitive induction of Akt activity. Transfection of an adenovirus expressing a dominant-negative Akt mutant decreased endothelial cell viability in the presence of VEGF. Conversely, adenoviral transduction of wild-type Akt facilitated the cell survival effects of VEGF, whereas transduction of constitutively active Akt conferred endothelial cell survival in the absence of VEGF. Constitutively active Akt also conferred survival to endothelial cells in suspension culture, whereas stimulation with VEGF did not. In suspension cultures, VEGF stimulation was unable to activate Akt, and Akt protein levels were repressed in cells undergoing anoikis. These data suggest that cross-talk between growth factor- and anchorage-dependent signaling pathways are essential for Akt activation and endothelial cell survival.
URLPMID:18684741 [本文引用: 1]

Dlx homeobox transcription factors regulate epidermal, neural andosteogenic cellular differentiation. Here, we demonstrate the central role ofDlx3 as a crucial transcriptional regulator of hair formation andregeneration. The selective ablation of Dlx3 in the epidermis results incomplete alopecia owing to failure of the hair shaft and inner root sheath toform, which is caused by the abnormal differentiation of the cortex.Significantly, we elucidate the regulatory cascade that positions Dlx3downstream of Wnt signaling and as an upstream regulator of othertranscription factors that regulate hair follicle differentiation, such asHoxc13 and Gata3. Colocalization of phospho-Smad1/5/8 and Dlx3 is consistentwith a regulatory role for BMP signaling to Dlx3 during hair morphogenesis.Importantly, mutant catagen follicles undergo delayed regression and displaypersistent proliferation. Moreover, ablation of Dlx3 expression in the telogenbulge stem cells is associated with a loss of BMP signaling, precludingre-initiation of the hair follicle growth cycle. Taken together with hairfollicle abnormalities in humans with Tricho-Dento-Osseous (TDO) syndrome, anautosomal dominant ectodermal dysplasia linked to mutations in theDLX3 gene, our results establish that Dlx3 is essential for hairmorphogenesis, differentiation and cycling programs.
URLPMID:21935929 [本文引用: 1]

Thymosin β4 (Tβ4) has been suggested to regulate multiple cell signal pathways and a variety of cellular functions such as cell migration, proliferation, survival, and angiogenesis. Here, we investigated the effect of Tβ4 on endothelial progenitor cells (EPCs) apoptosis induced by serum deprivation and the corresponding signal transduction pathways involved in this process. Circulating EPCs, isolated from healthy volunteers, were cultured in the absence or presence of Tβ4 and various signal cascade inhibitors. Apoptosis was evaluated with Annexin V immunostaining and cytosolic cytochrome c expression. Incubation of EPCs with Tβ4 caused a concentration dependent increase in cell viability and proliferation activity. It also caused an inhibitory effect on EPCs apoptosis, which was abolished by PI3K inhibitors (either LY294002 or Wortmannin) or JNK MAPK inhibitor SP600125. In addition, the expression and activity of caspase-3 and -9 were decreased by Tβ4, which markedly increased the Bcl-2/Bax ratio within EPCs. Furthermore, Tβ4 was immunoprecipitated with integrin-linked kinase (ILK), accompanied by augmentation of ILK activity. Transfection of EPCs with ILK-siRNA resulted in abolishment of the activation of ILK-Akt and the ameliorative effect on apoptosis by Tβ4. Together, Tβ4 mediated inhibitory effect on EPCs apoptosis under serum deprivation can be attributed, at least in part, to ILK-Akt activation. The activation of JNK MAPK might also be involved in this process. J. Cell. Physiol. 226: 2798–2806, 2011. 08 2011 Wiley-Liss, Inc.
URL [本文引用: 1]

http://www.genesdev.org/cgi/doi/10.1101/gad.11.24.3286
