 ,2,*
,2,*Heterologous Overexpression of Desiccation-tolerance Moss ScABI3 Gene Changes Stomatal Phenotype and Improves Drought Resistance in Transgenic Arabidopsis
Yigong Zhang1, Yi Zhang1, Ayibaiheremu Mutailifu1, Daoyuan Zhang ,2,*
,2,*通讯作者: *E-mail:zhangdy@ms.xjb.ac.cn
责任编辑: 朱亚娜
收稿日期:2020-12-26接受日期:2021-05-8
| 基金资助: |
Corresponding authors: *E-mail:zhangdy@ms.xjb.ac.cn
Received:2020-12-26Accepted:2021-05-8

摘要
ABI3是ABA信号通路中关键的转录调控因子, 参与种子休眠、质体发育及苔藓耐干等重要生理过程, 在植物抗逆中发挥关键作用。以荒漠耐干苔藓——齿肋赤藓(Syntrichia caninervis)为材料, 克隆了抗逆基因ScABI3并获得3个独立的pCAMBIA1301-ScABI3转基因拟南芥(Arabidopsis thaliana)纯合株系。结果表明, 转基因拟南芥叶片气孔孔径增大, 单位面积气孔数量减少, 植株水分利用效率提高; 在干旱处理14天后转基因拟南芥植株存活率显著高于野生型, 离体叶片失水率显著低于野生型。进一步研究发现, ScABI3转基因拟南芥通过提高自身活性氧(ROS)清除能力增强植株抗旱性。研究结果可为开发利用荒漠植物基因资源培育抗逆作物品种奠定基础。
关键词:
Abstract
ABI3 is a key transcriptional factor of the ABA signaling pathway, which is involved in seed dormancy, plastid development, and desiccation tolerance of bryophytes, and plays an important role in the stress tolerance of plant. This study obtained three independent pCAMBIA1301-ScABI3 transgenic Arabidopsis homozygous lines. The results showed that transgenic plants increased leaf stomata diameter, reduced the number of stomata in unit area, and improved plant water use efficiency; transgenic plants survival rate was significantly higher than wild type (WT) after 14 days of drought treatment; the water loss rate of transgenic leaves was significantly lower than that of WT. Further research has found that ScABI3 transgenic plants had higher drought resistance characteristics by improving reactive oxygen species (ROS) scavenging ability. These results may be contributed to the development and utilization of genetic resources of desert plants, also provide the theoretical and practical base for molecular breeding.
Keywords:
PDF (5474KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
引用本文
张一弓, 张怡, 阿依白合热木·木台力甫, 张道远. 异源过表达齿肋赤藓ScABI3基因改变拟南芥气孔表型并提高抗旱性. 植物学报, 2021, 56(4): 414-421 doi:10.11983/CBB20212
Zhang Yigong, Zhang Yi, Mutailifu Ayibaiheremu, Zhang Daoyuan.

脱落酸(abscisic acid, ABA)是植物在水分胁迫下产生的响应逆境反应及信号转导的一种关键植物激素, 调控植物的蒸腾、耐干、种子成熟以及休眠等过程, 抑制侧根和花序形成(Chen et al., 2020)。相比于维管植物, 苔藓植物中ABA的研究开展较晚, 同时, 由于苔藓在植物由水生到陆生的进化中处于关键位置, 因此认为苔藓植物中ABA在植株的脱水和干燥过程中起重要作用(Xiao et al., 2018)。
齿肋赤藓(Syntrichia caninervis)是一种荒漠苔藓, 广泛分布于新疆古尔班通古特沙漠, 它能适应沙漠中水分的迅速变化, 是沙漠生物结皮的优势种(Pan et al., 2016)。齿肋赤藓具有极强的耐干能力、迅速的再水化过程和光合恢复速率, 以及迅速的蛋白合成能力(Duff et al., 1999; Zhang et al., 2011)。因此, 齿肋赤藓被认为是荒漠地区特殊生境植物研究的优良材料, 其迅速的复水过程、耐干机理以及丰富的抗逆基因资源已成为新的研究热点。
ABI3 (ABSCISIC ACID INSENSITIVE 3)是ABA信号转导过程中的关键调控因子, 是植物特有的B3结构域转录因子。ABI3参与调控植物的质体发育、花芽休眠、开花时间以及侧根发育等生物过程(Rohde et al., 2002; Brady et al., 2003)。在棉花(Gossypium spp.)中过量表达拟南芥AtABI3基因会增强干旱耐受能力, 同时提高光合效率(Mittal et al., 2014)。在杨树(Populus)中过量表达PtABI3基因会影响杨树胚芽叶的生长和分化(Rohde et al., 2002)。小立碗藓(Physcomitrella patens) abi3突变体在添加ABA的培养基上不能存活, 突变体中与ABA相关的基因不表达或者表达量显著降低, 表明ABI3在苔藓获得耐干性中也发挥作用(Khandelwal et al., 2010)。对小立碗藓abi3突变体进行生物信息学分析, 推测ABI3通过调控miRNAs和ta-siRNAs的表达而获得耐干性(Xia et al., 2016)。利用同位素标记相对和绝对定量技术(iTRAQ), 发现小立碗藓abi3突变体中LEA蛋白、短链脱氢酶以及干燥相关蛋白均发生明显变化, 证实植物中ABI3诱导获得耐干性可能具有相同的遗传背景(Yotsui et al., 2016)。苔藓植物ABI3在植株获得耐干性方面发挥重要作用。研究表明, 从小立碗藓中克隆到3个ABI3基因, 过量表达和突变敲除实验显示, PpABI3是ABA诱导下游基因表达和获得耐干性的关键基因(Marella et al., 2006; Khandelwal et al., 2010)。本研究以齿肋赤藓为材料, 克隆了ScABI3基因并构建pCAMBIA1301-ScABI3表达载体, 获得相应的转基因拟南芥株系。结果表明, 异源过表达ScABI3基因能够改变转基因拟南芥气孔表型, 并增强植株的抗旱性。
1 材料与方法
1.1 植物材料及培养条件
拟南芥(Arabidopsis thaliana L.)采用Columbia生态型。培养条件: 22°C, 光照强度为100 μmol·m-2·s-1, 光周期为16小时光照/8小时黑暗, 相对湿度为60%。1.2 植物表达载体构建及拟南芥遗传转化
根据齿肋赤藓(Syntrichia caninervis Mitt.)转录组信息(NCBI, SRA数据库, accession number: SRP 150575)设计引物, 分别在上、下游引物中加入SalI和KpnI酶切位点。以齿肋赤藓干旱处理后复水24小时的cDNA为模板, 进行Reverse Transcription PCR, 克隆得到ScABI3基因的CDS序列(全长1 695 bp)。通过SalI和KpnI双酶切插入到表达载体pCAMBIA1301, 完成pCAMBIA1301-ScABI3过表达载体构建。采用液氮冻融转化法将重组质粒转入农杆菌(Agrobacterium tumefaciens) EHA105。采用浸花法进行野生拟南芥遗传转化。将ScABI3转基因拟南芥T0代种子在含有50 mg·L-1潮霉素的筛选培养基上筛选, 直至获得T3代纯合株系, 进行后续实验。1.3 转基因拟南芥鉴定
选取潮霉素筛选培养基中的6个阳性植株进行移苗, 生长至4周龄后提取叶片DNA, 用特异引物进行PCR检测, 确认ScABI3基因已成功转入拟南芥中。然后随机选取3个阳性植株, 命名为Line 1、Line 2和Line 3, 采用SYBR Premix Ex Taq II 荧光定量试剂盒(TaKaRa, Cat No.RR820), 以α-tublin为内参基因, 用qRT-PCR方法检测转基因拟南芥中ScABI3基因的表达量。1.4 转基因拟南芥气孔表型与水分利用率测定
采用300 mmol·L-1甘露醇(mannitol)渗透处理3天以模拟干旱胁迫。选取干旱胁迫下的转基因和野生型(WT)拟南芥叶片, 切取样块后进行固定、冻干和喷金等处理。通过扫描电镜观察样品, 每个视野中随机选取10个气孔, 用Image J软件测量气孔的长度与宽度(气孔器保卫细胞的长轴长度和短轴长度)。选取正常条件下生长至4周龄的拟南芥植株, 在上午(10:00-11:00), 自然光照, 25°C下, 使用便携式光合作用测定仪(LI-6400), 测定转基因和WT成熟叶片净光合速率(Pn)和蒸腾速率(Tr), 重复5次, 计算水分利用率(WUE)=Pn/Tr。
1.5 转基因拟南芥ABA含量测定
转基因拟南芥种子萌发后, 移入营养土生长至4周龄。用300 mmol·L-1甘露醇处理3天后取样, 参照文献(Shinde et al., 2012)报道方法, 采用超高效液相色谱-质谱法(Ultra performance liquid chromatography- mass spectrometry, UPLC-MS)测定内源ABA含量。1.6 转基因拟南芥耐干表型及失水率测定
将正常条件下生长至4周龄的拟南芥停止浇水, 自然干旱处理14天后观察植株的长势, 统计存活率。再从正常生长至4周龄的拟南芥上剪取大小相同的叶片0.5 g左右, 立即称重, 室温放置, 自然干燥, 相对湿度保持在60%。选择8个时间点(0.5、1、2、3、4、5、6和8小时), 分别称重, 计算每个时间点的失水率。失水率=(最初重量-对应时间点重量)/最初重量。每个株系10次重复。1.7 转基因拟南芥非生物胁迫下的生理指标测定
选取正常条件下生长至4周龄的拟南芥, 用300 mmol·L-1甘露醇处理3天。分别对处理植株叶片进行取样, 参照南京建成生物工程研究所生产的试剂盒, 采用紫外分光光度计法测定丙二醛(Cat No. A003-3)和H2O2 (Cat No.A064-1)含量以及超氧化物歧化酶(SOD) (Cat No.A001-1)和过氧化物酶(POD) (Cat No.A084-3)活性。1.8 统计分析
使用SPSS18.0软件对数据进行单因素方差分析及差异显著性检验(One-way ANOVA, *表示差异显著(P<0.05); **表示差异极显著(P<0.01))。2 结果与讨论
2.1 ScABI3转基因拟南芥过表达株系鉴定
PCR结果显示, 有5个转基因拟南芥株系(泳道1、3、4、5和6)检测为阳性, 与阳性对照pCAMBIA-ScABI3质粒条带(泳道7)大小相同, 而WT植株未检测出条带, 表明ScABI3基因已成功转入拟南芥(图1)。随机选取3株转基因阳性苗, 以4周龄叶片为材料, 提取总RNA后反转录为cDNA, 利用qRT-PCR进一步确定ScABI3在转基因拟南芥中的表达水平(图2)。图1
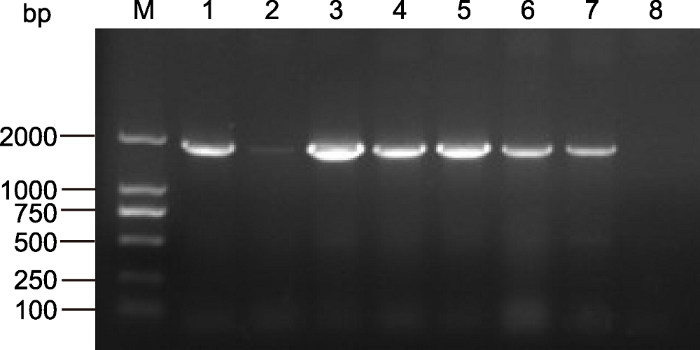 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1转基因拟南芥植株PCR检测
M: DL2000分子量标准品; 1-6: 转基因株系; 7: 阳性对照; 8:野生型
Figure 1PCR test of ScABI3 overexpression transgenic Arabidopsis
M: DL2000 marker; 1-6: Transgenic lines; 7: Positive control; 8: Wild type
图2
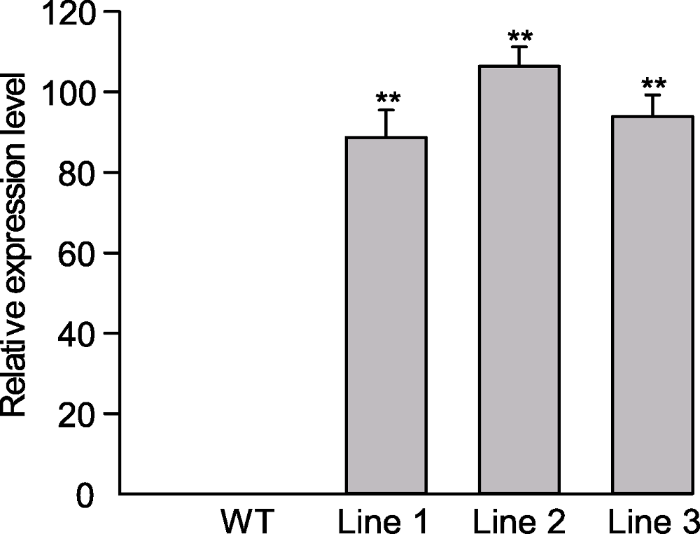 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2转基因拟南芥叶片ScABI3基因表达水平
** 表示差异极显著(P<0.01)。
Figure 2Expression level of ScABI3 in leave of Arabidopsis transgenic lines
** indicate extremely significant differences at P<0.01.
2.2 转基因拟南芥气孔表型及水分利用率
转基因拟南芥和WT在对照和甘露醇处理下的气孔表型如图3所示。在正常条件下, 转基因拟南芥和WT气孔保卫细胞形态相似; 在甘露醇处理下, WT植株气孔保卫细胞明显下陷, 而转基因植株气孔表型无变化或变化幅度较小(图3)。对气孔长度和宽度进行测量, 发现在正常条件和甘露醇处理下, 转基因植株(除Line 3的气孔长度外)均显著高于WT (图4A, B)。正常条件下, 转基因植株单位面积(mm2)气孔数量显著低于WT (图5A, B); 转基因和WT株系的WUE无显著差异, 干旱处理14天后, 转基因植株WUE显著高于WT, 约为WT的2倍(图5C), 这可能与干旱条件下转基因植株具有更高的净光合速率有关。上述研究结果表明, ScABI3转基因植株叶片气孔孔径增大, 单位面积气孔数量减少, 植株水分利用效率提高。图3
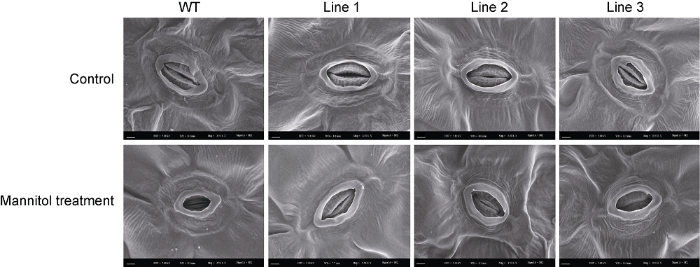 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3转基因及野生型(WT)拟南芥在干旱处理下的气孔表型
Bars=2 μm
Figure 3Stomata phenotype of the transgenic Arabidopsis and wild type (WT) under mannitol treatment
Bars=2 μm
图4
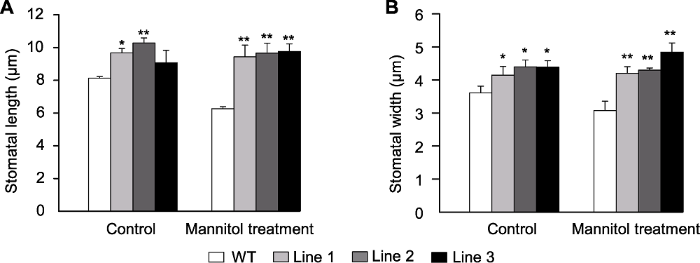 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4甘露醇处理下转基因和野生型(WT)拟南芥气孔长度(A)和宽度(B)
* 表示差异显著(P<0.05), ** 表示差异极显著(P<0.01)。
Figure 4Stomata length (A) and width (B) of the transgenic Arabidopsis and wild type (WT) under mannitol treatment
* indicate significant differences at P<0.05, ** indicate extremely significant differences at P<0.01.
图5
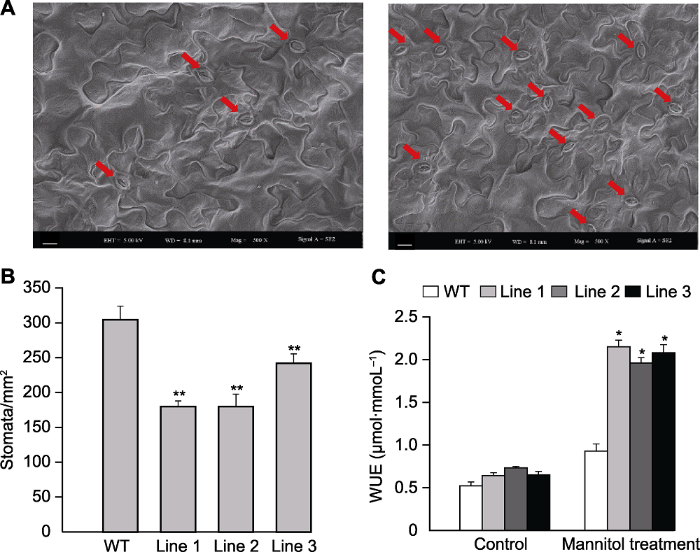 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5转基因和野生型(WT)拟南芥气孔密度和水分利用率(WUE)
(A) 转基因(左)和野生型(右)拟南芥单位面积上的气孔数目; (B) 转基因和野生型拟南芥单位面积气孔数目统计; (C) 甘露醇处理下转基因和野生型拟南芥水分利用率。红色箭头指示气孔; * 表示差异显著(P<0.05), ** 表示差异极显著(P<0.01)。(A) Bars=10 μm
Figure 5The stomatal density and water use efficiency (WUE) in the transgenic Arabidopsis and wild type (WT)
(A) Stomatal number of transgenic (left) Arabidopsis and WT (right); (B) Stomatal number statistics of the transgenic Arabidopsis and WT; (C) WUE of transgenic Arabidopsis and WT under mannitol treatment. The red arrows indicate stomatas; * indicate significant differences at P<0.05, ** indicate extremely significant differences at P<0.01. (A) Bars=10 μm
2.3 转基因拟南芥内源ABA含量分析
在正常条件下, ScABI3转基因植株和WT内源ABA含量无显著差异。在甘露醇处理下, ScABI3基因过表达会引起植株内源ABA含量明显升高(图6)。图6
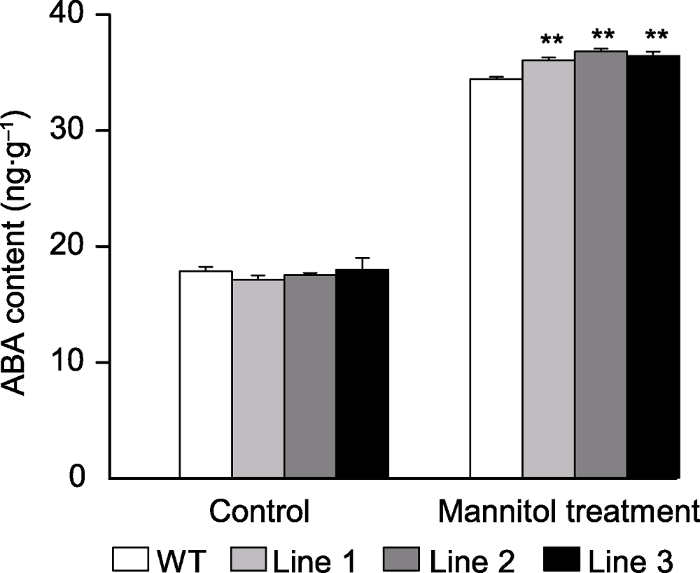 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6甘露醇处理下转基因和野生型(WT)拟南芥ABA含量
** 表示差异极显著(P<0.01)。
Figure 6The content of ABA in the transgenic Arabidopsis and wild type (WT) under mannitol treatment
** indicate extremely significant differences at P<0.01.
2.4 转基因拟南芥耐干性分析
在正常条件下, 转基因拟南芥和WT长势基本一致(图7A); 干旱处理14天后, ScABI3基因过表达株系存活率显著高于WT (图7B)。转基因株系和WT离体叶片失水率测定结果(图7C)显示, 干燥0.5小时失水率无显著差异; 干燥1小时, 二者仍无显著差异; 干燥2-5小时, 3个过表达植株失水率均显著低于WT (图7C, D)。推测ScABI3基因可能通过降低失水率的方式来提高抗旱性。图7
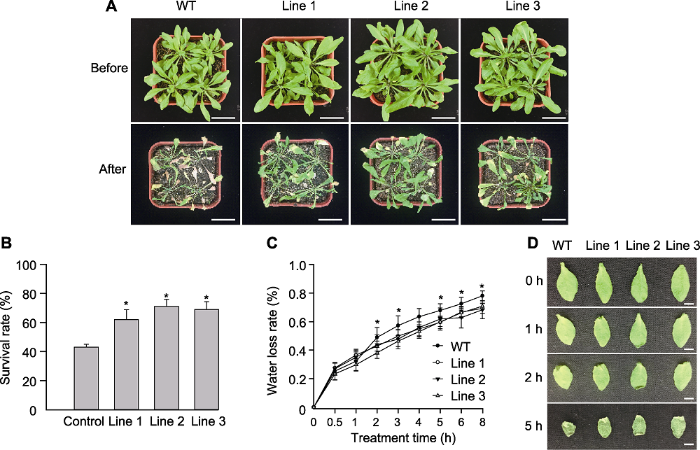 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7干旱条件下转基因和野生型(WT)拟南芥表型比较
自然干旱下转基因和野生型拟南芥表型(A)、存活率(B)、失水率(C)以及离体叶片失水表型(D)。* 表示差异显著(P<0.05)。(A) Bars=2 cm; (D) Bars=10 mm
Figure 7Comparisons of the transgenic Arabidopsis and wild type (WT) under drought stress
Phenotype (A), survival rate (B), water loss rate (C) and the phenotype of water loss to detached leaves (D) of the transgenic plant and WT under drought stress. * indicate significant differences at P<0.05. (A) Bars=2 cm; (D) Bars=10 mm
2.5 转基因拟南芥干旱相关生理指标
正常条件下, 转基因拟南芥和WT的SOD和POD酶活性以及MDA和H2O2含量无显著差异; 在甘露醇处理下, 转基因植株SOD和POD的活性显著升高, MDA和H2O2含量则显著降低(图8A-D)。表明ScABI3基因过表达可改变转基因拟南芥的耐旱生理指标。图8
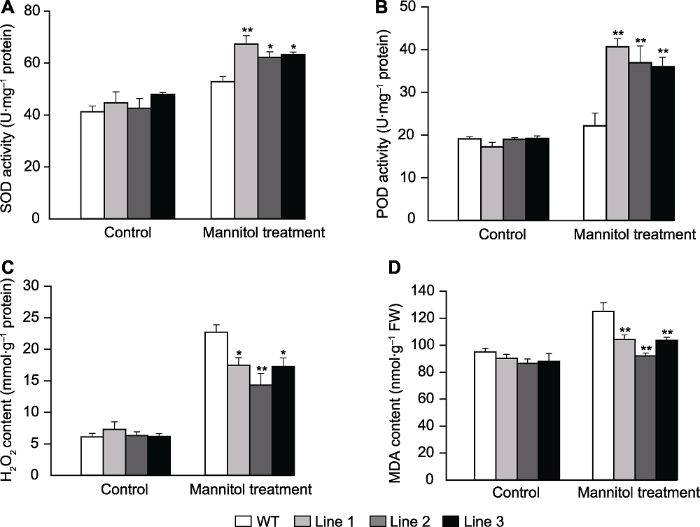 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8甘露醇处理下转基因和野生型拟南芥生理指标
(A) 超氧化物歧化酶(SOD)活性; (B) 过氧化物酶(POD)活性; (C) H2O2含量; (D) 丙二醛(MDA)含量。* 表示差异显著(P<0.05), ** 表示差异极显著(P<0.01)。
Figure 8Physiological indicators of the transgenic Arabidopsis and wild type (WT) under mannitol stress
(A) Superoxide dismutase (SOD) activity; (B) Peroxidase (POD) activity; (C) H2O2 contents; (D) Malondialdehyde (MDA) contents. * indicate significant differences at P<0.05, ** indicate extremely significant differences at P<0.01.
2.6 讨论
苔藓代表植物从水生到陆生进化的关键节点(Oliver et al., 2004)。齿肋赤藓作为荒漠耐干苔藓, 近年来被认为是研究植物耐干生理和分子机制的理想模式物种(Wood and Oliver, 2004; Wu et al., 2012)。ABA是一种通用的植物激素, 普遍存在于高等植物中, 但针对耐旱苔藓中ABA的功能研究才刚开始。ABI3是ABA信号通路中的重要转录因子, 在种子成熟和获得耐干性方面发挥重要作用(Mönke et al., 2012)。在植物干燥脱水时ABI3基因显著表达, 在复水时表达量成倍增高(Bedi et al., 2016)。异源表达ABI3基因在外源ABA处理下会导致组织中种子特异性转录物质的积累, 还会增强组织抗冻性和耐低温能力(Tamminen et al., 2001)。气孔是植物与外界进行气体和水分交换的重要通道, 植物可通过调控气孔运动调节光合与蒸腾作用, 多种非生物逆境以及外源ABA处理均可引起植物内源ABA水平升高(Yu et al., 2008)。ABA诱导气孔发育的信号途径是细胞信号转导领域研究较为深入的热点问题, 越来越多的信使物质及调节因子被成功鉴定(王雅静等, 2019)。ABA功能缺陷突变体aba2-2子叶小, 能持续形成拟分生组织细胞和保卫细胞母细胞, 气孔数目增加, 表皮细胞扩增变小(王宏亮等, 2018)。在ABA过度积累的cyp707a1/cyp707a3双突变体或者外源施加ABA可使气孔细胞数目变少, 并且导致表皮细胞扩增加大, 表明ABA作为激素不仅诱导气孔关闭而且抑制气孔发育(Tanaka et al., 2013)。本研究发现, 在拟南芥中过量表达ScABI3基因会改变植株的气孔发育特征, 变为气孔下陷、气孔长度和宽度增大、单位面积气孔数量减少以及植株水分利用率提高, 从而增强植株的耐干性(图3-5)。同时, 作为ABA信号途径的重要基因, 在拟南芥中过表达ScABI3, 正常生长条件下并不会引起内源ABA含量的显著变化, 但当受到干旱处理后, ScABI3转基因植株中ABA含量极显著高于WT (图6), 说明过表达ABI3基因可能涉及ABA合成或代谢过程, 由此推测ScABI3转录因子在调控脱落酸信号与合成过程中可能存在“交叉对话”。
对转基因拟南芥耐干性进行分析, 我们发现在成苗干旱处理和离体叶片脱水实验中, 转基因拟南芥都表现出更高的存活率和含水量(图7), 表明ScABI3基因可能通过降低失水率的方式提高抗旱性。进一步分析转基因植株干旱处理下的相关生理指标, 结果显示, 甘露醇处理下转基因拟南芥SOD和POD的活性更高, MDA和H2O2的含量更低, 表现出更高的活性氧(ROS)清除能力(图8), 推测过量表达ScABI3基因导致ROS关键基因转录活性或者酶活性增强, ROS清除能力增强, 从而间接提高植株的抗旱性。
综上所述, 与被子植物相比, 苔藓的水分吸收和干燥耐受性具有不同的策略, 这些策略是其适应环境的关键机制。齿肋赤藓是一种变水性耐干苔藓, 能适应陆地环境, 表现出全面的胁迫耐受性。本研究初步阐明ScABI3基因参与调节植物气孔表型和水分利用机制, 通过提高ROS清除能力使植株具有更高的耐旱性。研究结果可为深入揭示ABA在苔藓植物耐干过程中的分子机理和信号转导机制奠定基础。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
DOI:10.11983/CBB18115 [本文引用: 1]

磷脂酸(PA)是应答多种生理过程的第二信使, 其作为一个脂质信号快速积累从而响应多种环境。PA主要通过磷脂酶D (PLD)和磷脂酶C/甘油二酯激酶(PLC/DGK)途径产生。基于PLDs的生化特性、激活机制以及在不同类型胁迫下被激活的特定同种型的差异, 不同类型胁迫下会产生特定分子种组成的PA。PA在多种环境下起信号转导作用, 在调节气孔运动中, PA的作用模式主要是通过与多种蛋白结合, 激活或抑制这些蛋白的活性, 进而执行其信使功能。该文主要综述PA的生化特性以及信号途径中PA互作蛋白的研究进展, 并提出PA研究中亟待解决的问题及今后的重点研究方向。
DOI:10.1016/j.plantsci.2016.06.006URL [本文引用: 1]
DOI:10.1046/j.1365-313X.2003.01707.xURL [本文引用: 1]
DOI:10.1111/jipb.v62.1URL [本文引用: 1]
DOI:10.2307/3244230URL [本文引用: 1]
DOI:10.1126/science.1183672PMID:20110497 [本文引用: 2]

We show in bryophytes that abscisic acid (ABA) pretreatment of moss (Physcomitrella patens) cells confers desiccation tolerance. In angiosperms, both ABA and the transcriptional regulator ABSCISIC ACID INSENSITIVE 3 (ABI3) are required to protect the seed during desiccation. ABA was not able to protect moss cells in stable deletion lines of ABI3 (DeltaPpabi3). Hence, moss has the same functional link between ABA, ABI3, and the desiccation tolerance phenotype that is found in angiosperms. Furthermore, we identified 22 genes that were induced during ABA pretreatment in wild-type lines. When their expression was compared with that of DeltaPpabi3 during ABA pretreatment and immediately after desiccation, a new target of ABI3 action appears to be in the recovery period.
DOI:10.1111/tpj.2006.46.issue-6URL [本文引用: 1]
DOI:10.1111/pbi.12162URL [本文引用: 1]
DOI:10.1093/nar/gks594URL [本文引用: 1]
DOI:10.1186/1471-2164-5-89URL [本文引用: 1]
DOI:10.1038/nplants.2016.76URL [本文引用: 1]
DOI:10.1105/tpc.003186URL [本文引用: 2]
DOI:10.1111/j.1469-8137.2012.04193.xPMID:22591374 [本文引用: 1]

• Physcomitrella patens is a bryophyte belonging to early diverging lineages of land plants following colonization of land in the Ordovician period. Mosses are typically found in refugial habitats and can experience rapidly fluctuating environmental conditions. The acquisition of dehydration tolerance by bryophytes is of fundamental importance as they lack water-conducting tissues and are generally one cell layer thick. • Here, we show that dehydration induced oscillations in the steady-state transcript abundances of two group 3 late embryogenesis abundant (LEA) protein genes in P. patens protonemata, and that the amplitudes of these oscillations are reflective of the severity of dehydration stress. • Dehydration stress also induced elevations in the concentrations of abscisic acid (ABA), and ABA alone can also induce dosage-dependent oscillatory increases in the steady-state abundance of LEA protein transcripts. Additionally, removal of ABA resulted in rapid attenuation of these oscillatory increases. • Our data demonstrate that dehydration stress-regulated expression of LEA protein genes is temporally dynamic and highlight the importance of oscillations as a robust mechanism for optimal responses. Our results suggest that dehydration stress-induced oscillations in the steady-state abundance of LEA protein transcripts may constitute an important cellular strategy for adaptation to life in a constantly changing environment.© 2012 The Authors. New Phytologist © 2012 New Phytologist Trust.
PMID:11169177 [本文引用: 1]

The plant hormone abscisic acid (ABA) regulates several physiological and developmental processes in plants, including stress adaptation and seed maturation. ABA-mediated processes appear to be central in plant cold acclimation and expression of cold acclimation-related genes. Ectopic expression of ABI3 encoding a seed-specific transcriptional activator confers on Arabidopsis vegetative tissues the ability to accumulate seed-specific transcripts in response to ABA, and also influences some ABA-mediated vegetative responses. In the present study we characterized the effect of ectopic expression of ABI3 on cold acclimation and development of freezing tolerance in Arabidopsis. We first determined the effect of ABI3 on ABA-induced expression of cold acclimation-related genes. Expression of ABI3 increased the ABA-induced accumulation of transcripts for several ABA/cold/drought-responsive genes such as RAB18 and LTI78. Enhanced expression of these genes was evident even after transient application of ABA, and the enhanced expression was correlated with increased freezing tolerance in ABI3 transgenic plants. Ectopic expression of ABI3 also appeared to modulate low temperature-induced freezing tolerance. The ABI3 transgenic plants acclimated faster than the wild-type plants, and the maximum tolerance obtained was significantly higher. These data showed that lower levels of ABA were needed to trigger the expression of the genes and to maintain the freezing-tolerant state in the ABI3 transgenic plants, and indicate that ectopic expression of ABI3 leads to enhanced responsiveness to ABA. The ectopic expression of ABI3 could provide a new strategy for engineering plant stress tolerance.
DOI:10.1111/tpj.2013.74.issue-3URL [本文引用: 1]
[本文引用: 1]
DOI:10.1179/1743282011Y.0000000043URL [本文引用: 1]
DOI:10.1038/srep30118URL [本文引用: 1]
DOI:10.1111/pce.v41.1URL [本文引用: 1]
DOI:10.1016/j.bbrc.2016.02.024URL [本文引用: 1]
DOI:10.1105/tpc.108.058263PMID:18451323 [本文引用: 1]

Drought is one of the most important environmental constraints limiting plant growth and agricultural productivity. To understand the underlying mechanism of drought tolerance and to identify genes for improving this important trait, we conducted a gain-of-function genetic screen for improved drought tolerance in Arabidopsis thaliana. One mutant with improved drought tolerance was isolated and designated as enhanced drought tolerance1. The mutant has a more extensive root system than the wild type, with deeper roots and more lateral roots, and shows a reduced leaf stomatal density. The mutant had higher levels of abscisic acid and Pro than the wild type and demonstrated an increased resistance to oxidative stress and high levels of superoxide dismutase. Molecular genetic analysis and recapitulation experiments showed that the enhanced drought tolerance is caused by the activated expression of a T-DNA tagged gene that encodes a putative homeodomain-START transcription factor. Moreover, overexpressing the cDNA of the transcription factor in transgenic tobacco also conferred drought tolerance associated with improved root architecture and reduced leaf stomatal density. Therefore, we have revealed functions of the homeodomain-START factor that were gained upon altering its expression pattern by activation tagging and provide a key regulator that may be used to improve drought tolerance in plants.
DOI:10.1007/s11099-011-0002-6URL [本文引用: 1]
植物气孔发育机制研究进展
1
2018
... 气孔是植物与外界进行气体和水分交换的重要通道, 植物可通过调控气孔运动调节光合与蒸腾作用, 多种非生物逆境以及外源ABA处理均可引起植物内源ABA水平升高(
植物磷脂酸的特性及其在ABA诱导气孔运动中的作用
1
2019
... 气孔是植物与外界进行气体和水分交换的重要通道, 植物可通过调控气孔运动调节光合与蒸腾作用, 多种非生物逆境以及外源ABA处理均可引起植物内源ABA水平升高(
ABI3 mediates dehydration stress recovery response in Arabidopsis thaliana by regulating expression of downstream genes
1
2016
... 苔藓代表植物从水生到陆生进化的关键节点(
The ABSCISIC ACID INSENSITIVE 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis
1
2003
... ABI3 (ABSCISIC ACID INSENSITIVE 3)是ABA信号转导过程中的关键调控因子, 是植物特有的B3结构域转录因子.ABI3参与调控植物的质体发育、花芽休眠、开花时间以及侧根发育等生物过程(
Abscisic acid dynamics, signaling, and functions in plants
1
2020
... 脱落酸(abscisic acid, ABA)是植物在水分胁迫下产生的响应逆境反应及信号转导的一种关键植物激素, 调控植物的蒸腾、耐干、种子成熟以及休眠等过程, 抑制侧根和花序形成(
A Tortula ruralis cDNA encoding small-subunit ribosomal protein S3a: polysomal retention of transcript in response to desiccation and rehydration
1
1999
... 齿肋赤藓(Syntrichia caninervis)是一种荒漠苔藓, 广泛分布于新疆古尔班通古特沙漠, 它能适应沙漠中水分的迅速变化, 是沙漠生物结皮的优势种(
Role of ABA and ABI3 in desiccation tolerance
2
2010
... ABI3 (ABSCISIC ACID INSENSITIVE 3)是ABA信号转导过程中的关键调控因子, 是植物特有的B3结构域转录因子.ABI3参与调控植物的质体发育、花芽休眠、开花时间以及侧根发育等生物过程(
... ;
Characterization and functional analysis of ABSCISIC ACID INSENSITIVE3-like genes from Physcomitrella patens
1
2006
... ABI3 (ABSCISIC ACID INSENSITIVE 3)是ABA信号转导过程中的关键调控因子, 是植物特有的B3结构域转录因子.ABI3参与调控植物的质体发育、花芽休眠、开花时间以及侧根发育等生物过程(
Related to ABA-insensitive3 (ABI3)/Viviparous1 and AtABI5 transcription factor coexpression in cotton enhances drought stress adaptation
1
2014
... ABI3 (ABSCISIC ACID INSENSITIVE 3)是ABA信号转导过程中的关键调控因子, 是植物特有的B3结构域转录因子.ABI3参与调控植物的质体发育、花芽休眠、开花时间以及侧根发育等生物过程(
Toward the identification and regulation of the Arabidopsis thaliana ABI3 regulon
1
2012
... 苔藓代表植物从水生到陆生进化的关键节点(
The rehydration transcriptome of the desiccation-tolerant bryophyte Tortula ruralis: transcript classification and analysis
1
2004
... 苔藓代表植物从水生到陆生进化的关键节点(
The upside-down water collection system of Syntrichia caninervis
1
2016
... 齿肋赤藓(Syntrichia caninervis)是一种荒漠苔藓, 广泛分布于新疆古尔班通古特沙漠, 它能适应沙漠中水分的迅速变化, 是沙漠生物结皮的优势种(
PtABI3 impinges on the growth and differentiation of embryonic leaves during bud set in poplar
2
2002
... ABI3 (ABSCISIC ACID INSENSITIVE 3)是ABA信号转导过程中的关键调控因子, 是植物特有的B3结构域转录因子.ABI3参与调控植物的质体发育、花芽休眠、开花时间以及侧根发育等生物过程(
... 基因会影响杨树胚芽叶的生长和分化(
Dehydration stress-induced oscillations in LEA protein transcripts involves abscisic acid in the moss, Physcomitrella patens
1
2012
... 转基因拟南芥种子萌发后, 移入营养土生长至4周龄.用300 mmol·L-1甘露醇处理3天后取样, 参照文献(
Ectopic expression of ABI3 gene enhances freezing tolerance in response to abscisic acid and low temperature in Arabidopsis thaliana
1
2001
... 苔藓代表植物从水生到陆生进化的关键节点(
ABA inhibits entry into stomatal-lineage development in Arabidopsis leaves
1
2013
... 气孔是植物与外界进行气体和水分交换的重要通道, 植物可通过调控气孔运动调节光合与蒸腾作用, 多种非生物逆境以及外源ABA处理均可引起植物内源ABA水平升高(
Molecular biology and genomics of the desiccation tolerant moss Tortula ruralis
1
... 苔藓代表植物从水生到陆生进化的关键节点(
Membrane stability of the desert moss Syntrichia caninervis Mitt. during desiccation and rehydration
1
2012
... 苔藓代表植物从水生到陆生进化的关键节点(
Endogenous small-noncoding RNAs and potential functions in desiccation tolerance in Physcomitrella patens
1
2016
... ABI3 (ABSCISIC ACID INSENSITIVE 3)是ABA信号转导过程中的关键调控因子, 是植物特有的B3结构域转录因子.ABI3参与调控植物的质体发育、花芽休眠、开花时间以及侧根发育等生物过程(
Desiccation tolerance in Physcomitrella patens: rate of dehydration and the involvement of endogenous abscisic acid (ABA)
1
2018
... 脱落酸(abscisic acid, ABA)是植物在水分胁迫下产生的响应逆境反应及信号转导的一种关键植物激素, 调控植物的蒸腾、耐干、种子成熟以及休眠等过程, 抑制侧根和花序形成(
Large-scale proteome analysis of abscisic acid and ABSCISIC ACID INSENSITIVE3-dependent proteins related to desiccation tolerance in Physcomitrella patens
1
2016
... ABI3 (ABSCISIC ACID INSENSITIVE 3)是ABA信号转导过程中的关键调控因子, 是植物特有的B3结构域转录因子.ABI3参与调控植物的质体发育、花芽休眠、开花时间以及侧根发育等生物过程(
Activated expression of an Arabidopsis HD-START protein confers drought tolerance with improved root system and reduced stomatal density
1
2008
... 气孔是植物与外界进行气体和水分交换的重要通道, 植物可通过调控气孔运动调节光合与蒸腾作用, 多种非生物逆境以及外源ABA处理均可引起植物内源ABA水平升高(
Photosynthetic and cytological recovery on remoistening Syntrichia caninervis Mitt., a desiccation-tolerant moss from Northwestern China
1
2011
... 齿肋赤藓(Syntrichia caninervis)是一种荒漠苔藓, 广泛分布于新疆古尔班通古特沙漠, 它能适应沙漠中水分的迅速变化, 是沙漠生物结皮的优势种(
