 ,, 郝志云, 沈继源, 柯娜, 黄兆春, 梁维炜, 罗玉柱, 胡江, 刘秀, 李少斌甘肃农业大学动物科学技术学院/甘肃省草食动物生物技术重点实验室/甘肃省牛羊基因改良工程实验室,兰州 730070
,, 郝志云, 沈继源, 柯娜, 黄兆春, 梁维炜, 罗玉柱, 胡江, 刘秀, 李少斌甘肃农业大学动物科学技术学院/甘肃省草食动物生物技术重点实验室/甘肃省牛羊基因改良工程实验室,兰州 730070Screening, Identification and Functional Analysis of Important LncRNAs for Lactation Traits in Small-Tailed Han Sheep
WANG JiQing ,, HAO ZhiYun, SHEN JiYuan, KE Na, HUANG ZhaoChun, LIANG WeiWei, LUO YuZhu, HU Jiang, LIU Xiu, LI ShaoBinCollege of Animal Science and Technology/Gansu Key Laboratory of Herbivorous Animal Biotechnology/Gansu Engineering Lab of Genetic Improvement in Ruminants, Gansu Agricultural University, Lanzhou 730070
,, HAO ZhiYun, SHEN JiYuan, KE Na, HUANG ZhaoChun, LIANG WeiWei, LUO YuZhu, HU Jiang, LIU Xiu, LI ShaoBinCollege of Animal Science and Technology/Gansu Key Laboratory of Herbivorous Animal Biotechnology/Gansu Engineering Lab of Genetic Improvement in Ruminants, Gansu Agricultural University, Lanzhou 730070责任编辑: 林鉴非
收稿日期:2020-11-13接受日期:2020-02-4
| 基金资助: |
Received:2020-11-13Accepted:2020-02-4
作者简介 About authors
王继卿,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (1128KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
王继卿, 郝志云, 沈继源, 柯娜, 黄兆春, 梁维炜, 罗玉柱, 胡江, 刘秀, 李少斌. 小尾寒羊泌乳性状重要lncRNAs的筛选、鉴定及功能分析[J]. 中国农业科学, 2021, 54(14): 3113-3123 doi:10.3864/j.issn.0578-1752.2021.14.016
WANG JiQing, HAO ZhiYun, SHEN JiYuan, KE Na, HUANG ZhaoChun, LIANG WeiWei, LUO YuZhu, HU Jiang, LIU Xiu, LI ShaoBin.
开放科学(资源服务)标识码(OSID):

0 引言
【研究意义】小尾寒羊是我国为数不多的高繁殖力绵羊品种之一(平均产羔率为270%),通过多年引种,已广泛分布于我国北方和中部农区。但小尾寒羊也存在饲料转化率不高、肉用性能不足、肉质一般等缺点[1]。因此,多羔成活率成为决定小尾寒羊养殖效益的主要因素之一。研究发现,母羊泌乳量主要决定了多羔的成活率[2],也显著影响了羔羊的哺乳期生长速度和发育。母羊的产奶量和乳成分受到乳腺发育程度的直接调控[3],若能解析调控乳腺发育的分子机理,则能为泌乳性状的遗传改良提供理论指导。【前人研究进展】长链非编码RNA(long noncoding RNAs, LncRNAs)的长度大于200 nt,在细胞内的表达量通常低于线性mRNA,具有组织特异性和时空特异性的表达特点[4]。虽然哺乳动物基因组转录产生了较少的lncRNAs分子(仅占4%—9%)[5],但它们却在细胞增殖、分化、凋亡、衰老等活动中发挥了重要作用,还参与了表观遗传调控、剂量补偿效应、基因组印迹、X染色体失活、生长发育和疾病等生物学过程[6,7],因此lncRNAs已成为解析动物复杂性状遗传机理的热点方向之一[8]。LncRNAs可通过多种途径调控功能基因的表达。首先,lncRNAs通过顺式作用调控了邻近功能基因的表达量[9]。其次,一些lncRNAs可以作为“海绵体”,与小RNAs(microRNAs, miRNAs)结合,从而减轻miRNAs对功能基因的抑制作用,最终提高功能基因的表达量[9]。最后,lncRNAs也可以与核糖核酸蛋白结合形成lncRNAs核糖核酸蛋白复合物,从而调控靶基因的表达[10]。乳腺是母畜特有的外分泌腺,具有分泌乳汁的功能,一生经历了多次周期性的增殖、分化和退化等过程。研究已证实,lncRNAs在哺乳动物的乳腺发育和乳汁生成过程中发挥了重要作用[11,12,13]。例如,敲除lncRNA Neat1后,造成小鼠乳腺异常发育,导致泌乳能力显著下降[11]。小鼠敲除LncRNA PINC1.0后,乳腺上皮细胞凋亡数量显著增加。相反,lncRNA PINC1.6的敲除,诱导了乳腺上皮细胞的增殖[12]。在转基因小鼠的乳腺上皮细胞中过表达lncRNA SRA后,显著增强了乳腺上皮细胞的增殖和分化能力,增大了乳腺的侧向分支,这表明该lncRNA对小鼠乳腺分化有正向调控作用[13]。随着RNA-Seq技术在动物遗传育种领域的广泛应用,人们已在动物乳腺中鉴定出越来越多的lncRNAs,并研究了它们的生物学功能。例如,YANG[14]以泌乳期和干乳期中国荷斯坦奶牛为研究对象,在乳腺中发现了23 495个lncRNAs,其中3 746个lncRNAs在两个时期差异表达,这些差异表达lncRNAs的靶基因主要富集在细胞循环、JAK-STAT等与泌乳相关的信号通路上。ZHENG[15]在泌乳高峰期和泌乳后期荷斯坦奶牛的乳腺组织中发现了117个差异表达lncRNAs,其中72个差异表达lncRNAs与蛋白编码基因共表达,靶基因显著富集在PPAR、AMPK等脂类和葡萄糖代谢有关的信号通路上。【本研究切入点】lncRNAs广泛参与了奶牛[14,15]、奶山羊[16,17]和母猪[18]的乳腺发育和泌乳过程调控。然而,目前绵羊乳腺组织中的lncRNAs功能研究甚少,只有CHEN[19]和HAO[20]分别研究了不同发育时期和不同品种间绵羊乳腺的lncRNAs表达特征。【拟解决的关键问题】本研究选择3只高泌乳性能小尾寒羊和3只低泌乳性能小尾寒羊,采集泌乳期乳腺组织,采用RNA-Seq技术,研究lncRNAs表达谱,筛选出两组小尾寒羊中的差异表达lncRNAs,分析靶基因的富集通路,旨在为解析绵羊泌乳性能的分子机理提供理论基础。1 材料与方法
1.1 试验羊和乳腺组织样品采集
2019年3—6月,在甘肃省天祝县金子河绵羊繁育公司相同的饲养管理条件下,应用羔羊自由哺乳前后体重差法[21],测定小尾寒羊母羊的泌乳量,并采集乳汁样本,测定乳脂率等乳成分。根据测定的表型数据,选择高泌乳性能(高泌乳量、高乳脂率)和低泌乳量性能(低泌乳量、低乳脂率)小尾寒羊各3只,要求所有羊只健康、3岁、产双羔、第4胎。这些高泌乳性能和低泌乳性能母羊产后30d的平均泌乳量为1 456和830 g·d-1,它们的乳脂率分别为7.36%和5.72%。参照韦科龙等[22]描述的方法,活体采集相同部位的乳腺实质部分后,迅速放入液氮中储存。1.2 绵羊乳腺组织总RNA提取及RNA文库构建
用Trizol试剂(Invitrogen, CA, USA)提取乳腺组织中的总RNA,分别用Bioanalyzer 2100和Nanodrop 2000仪器测定RNA的完整性(RNA integrity number, RIN)和纯度。挑选3.0 μg、RIN>7的RNA样本,用Epicentre Ribo-zero™ rRNA去除里面的核糖体RNA,然后用NEB Next® Ultra™ Directional RNA Library Prep Kit RNA构建6个样品的RNA文库。1.3 RNA测序、lncRNAs鉴定和表达量检测
用Illumina® HiSeqPE Cluster Kit在cBot簇生成系统上进行样品聚类,随后在上海派森诺生物科技有限公司的Illumina Hiseq 3000平台上进行2×150 bp双末端测序。RNA-Seq获得的原始数据经FastQC v0.10.1质控后,过滤去除质量分数低于Q20的序列和接头序列,得到高质量的Clean reads。用Bowite v2、HISAT v2和Stringtie v1.2.4软件,建立参考基因组索引,将Clean Reads比对到绵羊参考基因组Oar_rambouillet_v1.0上,鉴定出已注释的绵羊lncRNAs。最后,按照HAO等[20]描述的方法,识别绵羊基因组中未注释的lncRNAs。用Stringtie v1.2.4统计每千个碱基的转录每百万映射读取的片段(Fragments per kilobase of transcript Per million reads mapped, FPKM),对表达量进行均一化后获得lncRNAs的表达量。在每组3个样本中,当某一lncRNA在其中2个样本中的FPKM值大于0.01时,定义该lncRNA是表达的。
1.4 差异表达lncRNAs筛选及其靶基因的GO和KEGG分析
根据|fold change|>2且P-value<0.05这一条件,用DESeq v1.18.0软件筛选两组小尾寒羊中的差异表达lncRNAs。根据lncRNAs靶基因的预测规则,本研究搜寻了差异表达lncRNA上下游100 kb范围以内的结构基因,将其作为lncRNA的顺式调控靶基因[4]。用基因本体论(gene ontology, GO)和KEGG(kyoto encyclopedia of genes and genomes, KEGG)数据库,分别分析靶基因的生物学功能和参与的生物学通路。1.5 LncRNAs的靶向miRNAs预测
用miRnada预测lncRNAs的靶向miRNA海绵体,然后用Cytoscape 3.0进行可视化处理,最后用Starbase v2.0构建lncRNA-miRNA调控网络。1.6 差异表达lncRNAs的RT-qPCR验证
在高泌乳性能小尾寒羊中,挑选8个上调lncRNAs和8个下调lncRNAs,把GAPDH作为管家基因[23],进行实时荧光定量PCR(reverse transcription-quantitative PCR, RT-qPCR),验证RNA-Seq测序数据的质量。所选lncRNAs和内参基因的引物信息见表1。Table 1
表1
表1RT-qPCR引物信息
Table 1
| 引物名称 Name | 正向序列(5'→3') Forward sequence (5'→3') | 反向序列(5'→3') Reverse sequence (5'→3') |
|---|---|---|
| MSTRG.59580.8 | ACCAGGTTGCCTAAGGAGGC | TGGAGTACAGTGGCTATTCACAA |
| MSTRG.59580.9 | ACCAGGTTGCCTAAGGAGG | TGGAGTACAGTGGCTATTCACA |
| MSTRG.59580.10 | TCGGGTGTCCGCACTAAG | GTTGCCCAGGCTGGAGTA |
| MSTRG.38680.1 | GGTCTGCCTTCTTGGGTTC | TGGAGATGGAGCAGGGATC |
| MSTRG.47064.1 | GCCGACTAAGGTCCATCT | GGCACTCAGCCTTCTTCA |
| MSTRG.137650.1 | GTGCCGAAGAATAGATGC | TTCCTCCAAAGAAATCCC |
| MSTRG.59580.12 | ACCAGGTTGCCTAAGGAGG | TGGAGTACAGTGGCTATTCACA |
| MSTRG.80056.21 | CACTTCACTTAGCACGAT | CAACAGATAAATGGACGA |
| MSTRG.125242.6 | GCACGAACAGCGACATCAG | CAGGGTCTCAGCAAGTTAGGAG |
| MSTRG.119809.14 | TGTAGTCCTTTCCCAGTT | TGAATACCAGACCACCTTA |
| MSTRG.59580.14 | GGCTGGAGGATCGCTTGA | TTGACCTGCTCCGTTTCC |
| MSTRG.59580.11 | CAGCCTGGGCAACATAGC | CCGAACTTAGTGCGGACAC |
| MSTRG.114625.4 | TGATCGCCAGGGTTGATT | GGATGGTCGTCCTCTTCG |
| MSTRG.59580.3 | ACCAGGTTGCCTAAGGAGG | TGGAGTACAGTGGCTATTCACA |
| MSTRG.106261.2 | TGTCACCCTCCACCAATG | CAGGCTGAAGTCCCAAAA |
| rna-XR_003586216.1 | TACACGGTATTTCCTCCAA | CCAATTCTAAGATGCGATT |
| GAPDH | ATCTCGCTCCTGGAAGATG | TCGGAGTGAACGGATTCG |
新窗口打开|下载CSV
将原始RNA样本进行反转录之后得到cDNA,用SYBR Green I染料法进行RT-qPCR,然后用2-ΔΔCt法计算lncRNAs在乳腺组织中的相对表达量,最后用Log2FoldChange进行标准化处理。
2 结果
2.1 绵羊乳腺组织RNA-Seq
在高泌乳性能和低泌乳性能小尾寒羊乳腺组织中分别得到19.48G和19.71G的原始测序数据,两组测序数据的平均Q30值分别为90.0%%和90.9%。进一步对原始测序数据进行质控后,在两组中分别得到16.5G和16.9G的Clean data,其中90.8%和91.2%的Clean data能够比对到绵羊参考基因组Oar_ rambouillet_v1.0的唯一位置上。2.2 绵羊乳腺组织lncRNAs表达特征
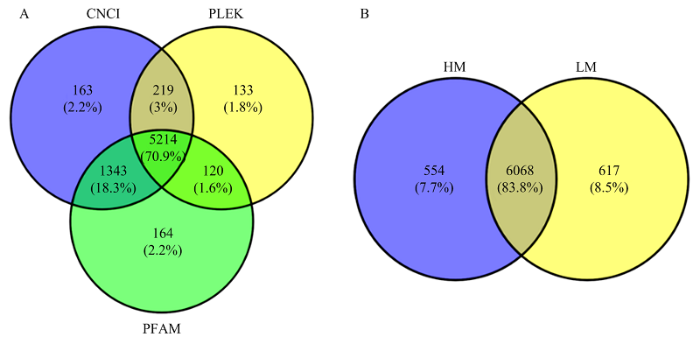
在乳腺组织中共检测到75 303个转录本,经与绵羊参考基因组中的已知lncRNAs库比对,发现了3 943个已知的lncRNAs。经CPC、CNCI和PFAM 3种软件同时预测,发现了5 214个新的lncRNAs(图1-A)。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1小尾寒羊乳腺组织中新预测的lncRNAs(A)和lncRNAs表达Venn图(B)
HM:高泌乳性能小尾寒羊乳腺组织;LM:低泌乳性能小尾寒羊乳腺组织。下同
Fig. 1The predicted novel lncRNAs in the mammary gland tissues of Small-Tailed Han sheep (A) and the venn diagram of lncRNAs expression in the two groups of Small-Tailed Han ewe (B)
‘HM’ and ‘LM’ represent the mammary gland tissues from high-lactating performance and low-lactating performance Small-tailed Han sheep, respectively. The same as below
根据lncRNAs的表达定义,在绵羊乳腺组织中发现了7 239个可表达的lncRNAs,其中包括2 262个已知lncRNAs和4 977个新lncRNAs。在所有表达lncRNAs中,6 068个lncRNAs在两组中共表达,554个lncRNAs仅在高泌乳性能小尾寒羊乳腺组织中特异性表达,617个lncRNAs仅在低泌乳性能小尾寒羊乳腺组织中特异性表达(图1-B)。
2.3 差异表达lncRNAs筛选
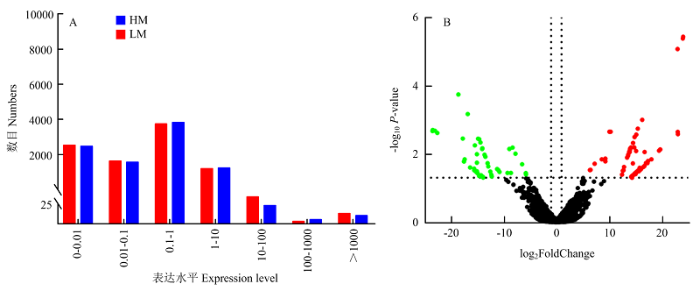
对所有lncRNAs的FPKM值统计后发现,在高、低泌乳性能组中,分别有82.2%和81.9%的lncRNAs的FPKM值低于1(图2-A),说明lncRNAs在绵羊乳腺组织中呈中低丰度表达。在所有鉴定到的lncRNAs中,MSTRG.114625.1的表达量最高。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2小尾寒羊乳腺组织中lncRNAs表达量分布统计(A)和差异表达lncRNAs的火山图(B)
图B中,红色表示在高泌乳性能小尾寒羊乳腺组织中上调表达的lncRNAs,绿色表示下调表达的lncRNAs,黑色表示两组中表达量没有差异的lncRNAs (P>0.05)
Fig. 2The statistical distribution of expression levels of lncRNAs in the mammary gland of Small-Tailed Han sheep (A) and volcano plot of the differentially expressed lncRNAs (B)
The red and green dots indicate upregulated and downregulated lncRNAs in high-lactating performance Small-Tailed Han sheep, respectively. The black dots indicate the lncRNAs that had no significantly difference in the two groups (P>0.05)
在两组小尾寒羊中共筛选出120个差异表达lncRNAs,其中68个lncRNAs的表达量在高泌乳性能小尾寒羊中显著上调,最显著上调的3个lncRNAs分别是MSTRG.59580.8、MSTRG.38680.1和MSTRG. 124434.2;52个lncRNAs的表达量显著下调(图2-B),最显著下调的3个lncRNAs是MSTRG.125242.6、MSTRG.119809.14和MSTRG.59580.14。
2.4 差异表达lncRNAs靶基因的GO和KEGG功能富集分析
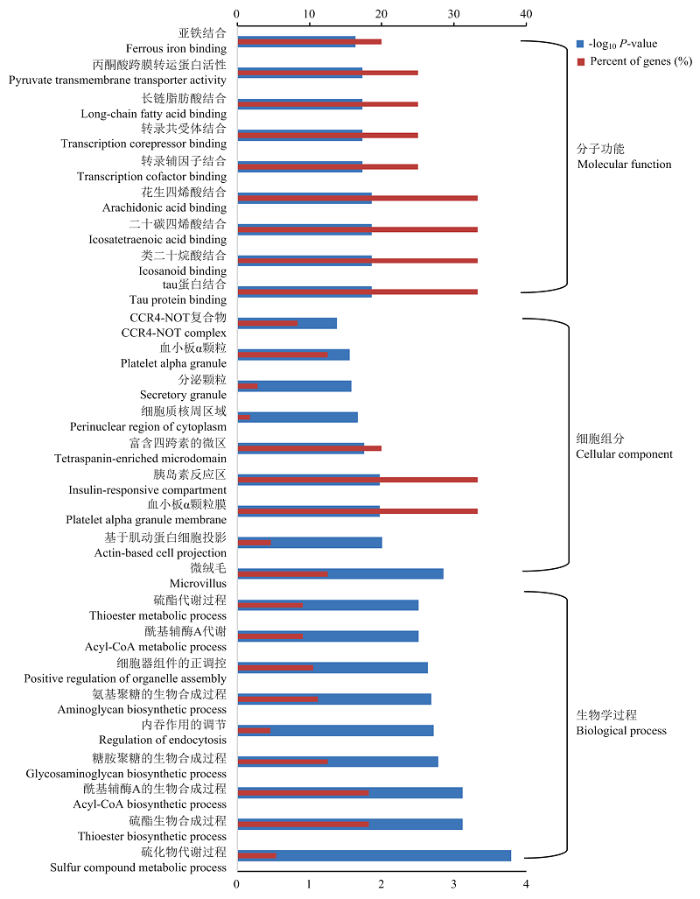
GO数据库注释发现,在生物学过程中,差异表达lncRNAs的靶基因主要富集到硫化物代谢过程、硫酯生物合成过程和酰基辅酶A的生物合成过程等条目上;在细胞组分中,靶基因主要富集在微绒毛、基于肌动蛋白细胞投影和血小板α颗粒膜等条目上;在分子功能中,靶基因组主要富集在tau蛋白结合、类二十烷酸结合和二十碳四烯酸结合等条目上(图3)。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3差异表达lncRNAs靶基因的GO功能富集注释
Fig. 3The GO enrichment results of the target genes of differentially expressed lncRNAs
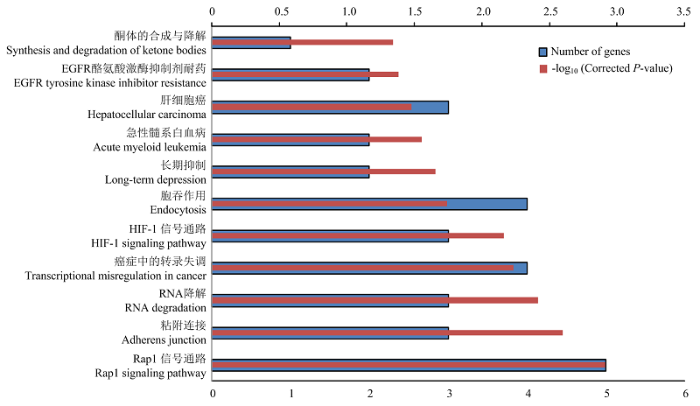
KEGG数据库注释发现,靶基因显著富集到Rap1信号通路(P=0.001)、粘附连接(P=0.002)、RNA降解(P=0.003)等信号通路中(图4)。
图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4差异表达lncRNAs靶基因的KEGG通路注释
Fig. 4The KEGG analysis of the target genes of differentially expressed lncRNAs
2.5 lncRNA-miRNA网络分析
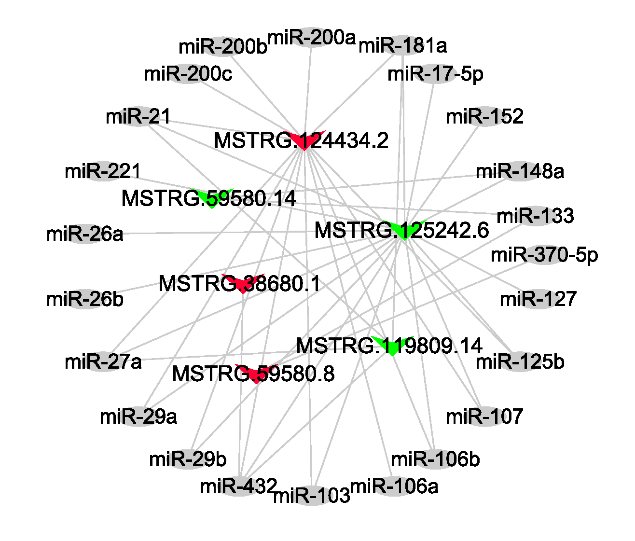
选择在高泌乳性能小尾寒羊乳腺组织中最显著上调的3个lncRNAs(MSTRG.59580.8、MSTRG.38680.1和MSTRG.124434.2)和最显著下调的3个lncRNAs(MSTRG.125242.6、MSTRG.119809.14和MSTRG. 59580.14),用miRnada预测了它们的靶基因,从中选择分值较高的miRNAs,构建了lncRNA-miRNA互作网络图。结果表明,这些lncRNAs的部分靶向miRNAs在动物乳腺发育及泌乳过程中发挥了重要作用,例如miR-148a、miR-181a和miR-221等(图5)。图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5lncRNA-miRNA网络调控图
红色和绿色三角分别表示在高泌乳性能小尾寒羊中上调和下调表达的lncRNAs
Fig. 5The network of lncRNA-miRNA
The red and green triangles indicate up-regulated and down-regulated lncRNAs in mammary gland tissues of high-lactating performance Small-Tailed Han sheep, respectively
2.6 RT-qPCR验证差异表达lncRNAs
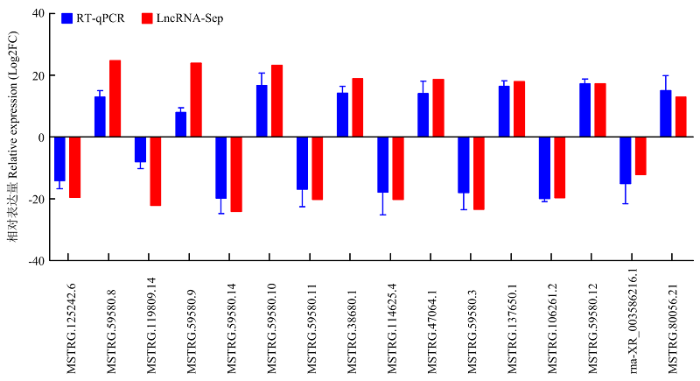
RT-qPCR结果表明,lncRNAs MSTRG.59580.8、MSTRG.59580.9、MSTRG.59580.10、MSTRG.38680.1、MSTRG.47064.1、MSTRG.137650.1、MSTRG.59580.12和MSTRG.80056.21在高泌乳性能组小尾寒羊中的表达量高于低泌乳性能组,而lncRNAs MSTRG.125242.6、MSTRG.119809.14、MSTRG.59580.14、MSTRG.59580. 11、MSTRG.114625.4、MSTRG.59580.3、MSTRG. 106261.2和rna-XR_003586216.1在高泌乳性能组中的表达量低于低泌乳性能组( 图6)。这表明16个lncRNAs的RT-qPCR结果与RNA-Seq测序结果完全一致,进一步证实了RNA-Seq测序结果的可靠性。图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图616个差异表达lncRNAs的RT-qPCR验证
Fig. 6The RT-qPCR verification of 16 differentially expressed lncRNAs
3 讨论
泌乳性状是受微效多基因控制的重要经济性状[24]。除了编码蛋白质的功能基因以外,它还受到非编码RNA的调控。现已发现,lncRNAs在奶牛、奶山羊和小鼠的乳腺发育、乳汁合成以及乳品质调控方面发挥了重要作用,但是它在绵羊乳腺中的生物学作用鲜有报道。本研究以高泌乳性能和低泌乳性能小尾寒羊为研究对象,用RNA-Seq技术发现,554个lncRNAs仅在高泌乳性能小尾寒羊乳腺组织中表达,617个lncRNAs仅在低泌乳性能乳腺组织中表达,表明这些特异性表达的lncRNAs在调控小尾寒羊泌乳量和乳脂率方面发挥了重要作用。本研究中,80%以上lncRNAs的FPKM值小于1(图2-A),说明lncRNAs在绵羊乳腺组织中呈中低丰度表达,这与前人研究结果相一致。IBEAGHA- AWEMU等[7]在奶牛乳腺组织中鉴定出4 955个lncRNAs,其中表达量最高的lncRNA的FPKM值仅为11.93,绝大部分lncRNAs呈低丰度表达。
本研究得到的大多数lncRNAs呈低丰度表达,它是通过何种途径调控绵羊的乳腺发育和泌乳性能呢?一种解释是,这些lncRNAs能与对乳腺发育和泌乳性能有重要作用的功能基因发挥交互作用,从而间接影响上述性能。在非编码的环状RNA中已发现,低表达量的环状RNA可以完全实现对靶基因的顺式调控[25]。在本研究鉴定的lncRNAs中,MSTRG.114625.1的表达量最高。该lncRNA位于绵羊第20号染色体上,经靶向关系预测,它能调控肠细胞激酶(intestinal cell kinase, ICK)、F盒蛋白9(F-box protein 9, FBXO9)、谷胱甘肽S-转移酶α1(glutathione S-transferase Alpha 1, GSTA1)和胶质细胞缺失转录因子1(glial cells missing transcription factor 1, GCM1)基因的表达。研究发现,ICK参与了细胞周期转换过程中催化结构域和相关激酶活性的调控,进而影响了哺乳动物的细胞周期和细胞凋亡[26]。VERARDO等[27]采用GWAS方法在345头母猪中发现,与乳头数量相关的19个SNPs显著富集到13个功能基因上,其中就包括ICK,该基因受到核受体亚家族3 C组成员1(nuclear receptor subfamily 3 group C member 1, NR3C1)和核因子白介素3(nuclear factor interleukin 3, NFIL3)这两个转录因子的共同调控。研究发现,NR3C1和NFIL3调控了小鼠乳腺上皮细胞的增殖和凋亡[28,29]。GSTA1对清除奶牛乳腺上皮细胞中的代谢毒素有重要作用[30]。此外,降低GSTA1的表达量将提高乳腺癌的患病风险[31]。因此,表达量最高的MSTRG.114625.1可能通过调控它的靶基因的表达,从而参与绵羊乳腺发育和泌乳过程。
本研究在两组小尾寒羊乳腺组织中鉴定出120个差异表达lncRNAs,其中在高泌乳性能小尾寒羊中上调表达的有68个,下调表达的有52个。MSTRG. 59580.8是最显著上调表达的lncRNA(P<0.01)。该lncRNA位于绵羊第7号染色体上,调控了出核调节因子(nuclear export mediator factor, NEMF)基因的表达。研究发现,NEMF不仅调控了蛋白酶体的降解过程[32],它还在细胞核内物质的出入中发挥了重要作用[33]。另外,MSTRG.59580.8可以作为miR-133和miR-370- 5p的海绵体(图5)。虽然目前尚未研究miR-133对乳腺上皮细胞的作用,但它可以靶向LIM和SH3蛋白1(LIM and SH3 protein 1, LASP1)的编码基因,来促进肾小球系膜细胞的凋亡[34]。根据海绵体作用原理,MSTRG.59580.8能抑制miR-133对肾小球系膜细胞凋亡的促进作用,从而起到抗细胞凋亡作用。因此推测,MSTRG.59580.8也能抑制绵羊乳腺上皮细胞的凋亡,从而提高母羊的泌乳量[24]。然而,这还需要进一步研究加以证实。
在高泌乳性能小尾寒羊中,MSTRG.125242.6的表达量最显著下调(P<0.01)。该lncRNA来自于钙电压门控通道亚基α H(calcium voltage-gated channel subunit Alpha1 H, CACNA1H)基因的编码区域。靶基因预测发现,它能顺式调控CACNA1H的表达。研究已表明,CACNA1H的碱基突变改变了细胞膜通透性,进而增加了Ca2+的活性[35]。Ca2+对于细胞稳态具有重要调控作用,它能维持乳腺中酪蛋白的合成[36]。因此,MSTRG.125242.6通过调控CACNA1H的表达量,进而影响羊奶中酪蛋白的合成量和泌乳量。MSTRG.125242.6还能作为miR-148a的海绵体(图5),来调控绵羊乳腺发育过程和泌乳性能。研究发现,在奶山羊乳腺上皮细胞中过表达miR-148a后,可以下调PPARGC1A的表达量,从而促进甘油三酯的合成[37]。这表明MSTRG.125242.6的表达量与乳汁中的乳脂含量呈负相关,即:MSTRG.125242.6通过吸附miR-148a的含量,抑制了甘油三酯的合成,最终降低了绵羊乳汁中的乳脂含量和比例。这与MSTRG.125242.6在乳脂含量较低的低泌乳性能小尾寒羊中呈上调表达的事实相一致。
差异表达lncRNAs的靶基因显著富集到硫化物代谢过程、硫酯生物合成过程和酰基辅酶A的生物合成等GO条目中。硫酯是ATP合成和分解过程中的中间代谢产物,它能为乳汁合成提供能量[38]。KEGG注释结果表明,差异表达lncRNAs的靶基因也显著富集到Rap1信号通路、粘附连接、RNA降解等信号通路中(图4)。HOU等[39]在奶牛中发现,向乳腺上皮细胞中添加蛋氨酸,能显著促进乳汁中酪蛋白的合成量,此过程中的功能基因显著富集到Rap1信号通路上,这表明Rap1信号通路与乳蛋白合成相关。细胞粘附连接能促进TGF-β3信号通路下游基因的吞噬作用,从而清除了小鼠乳腺上皮细胞中的凋亡细胞,最终提高了小鼠的泌乳量[40]。
相比于同为非编码RNA的miRNAs,lncRNA研究起步较晚,研究相对滞后,对其发挥生物学作用的途径还未完全研究清楚。目前,人们的研究更多的局限于用RNA-Seq技术鉴定特定组织中的lncRNAs,研究它们在不同时间或不同遗传背景中的差异表达情况,进而分析差异表达lncRNAs靶基因的GO和KEGG通路,来最终解析lncRNAs的生物学功能。本研究也遵循了这一经典方法,在筛选出的120个差异表达lncRNAs中,挑选了16个差异表达lncRNAs,用RT-qPCR方法证实了RNA-Seq结果的准确性和可靠性。但这并不能完全排除假阳性的概率,将来需要对更多的差异表达lncRNAs进行RT-qPCR验证。同时,对于本研究筛选出的最显著差异表达lncRNAs,以及仅在一组中特异性表达的lncRNAs,需要在绵羊乳腺上皮细胞中,通过过表达和沉默等方法,来进一步阐释它们的生物学功能和发挥功能的途径。
4 结论
本研究在小尾寒羊乳腺组织中发现了7 239个lncRNAs,其中68个lncRNAs在高泌乳性能小尾寒羊乳腺组织中上调表达,而52个lncRNAs呈下调表达。差异表达lncRNAs的靶基因主要参与了Rap1信号通路、粘附连接、RNA降解等与乳腺发育和泌乳相关的生物学过程。该结果为解析lncRNAs在绵羊乳腺发育和泌乳过程中的生物学功能提供了理论基础。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
DOI:10.1080/00288233.1970.10430507URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1038/nature10887URL [本文引用: 2]
[本文引用: 1]
[本文引用: 1]
DOI:10.1101/gad.1800909URL [本文引用: 1]
DOI:10.3390/ijms19113610URL [本文引用: 2]
DOI:10.1016/j.biochi.2011.07.031URL [本文引用: 1]
DOI:10.1016/j.cell.2011.09.028URL [本文引用: 2]
[本文引用: 1]
DOI:10.1261/rna.047332.114URL [本文引用: 2]
[本文引用: 2]
DOI:10.1128/MCB.23.20.7163-7176.2003URL [本文引用: 2]
DOI:10.1186/s12864-018-4974-5URL [本文引用: 2]
DOI:10.3168/jds.2018-14900URL [本文引用: 2]
DOI:10.18632/oncotarget.v8i58URL [本文引用: 1]
DOI:10.1186/s12864-020-6656-3URL [本文引用: 1]
DOI:10.1016/j.ygeno.2020.04.021URL [本文引用: 1]
DOI:10.3389/fgene.2020.00946URL [本文引用: 1]
DOI:10.3390/ani10091565URL [本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
DOI:10.3168/jds.2017-12983URL [本文引用: 2]
DOI:10.1038/nsmb.2959URL [本文引用: 1]
DOI:10.1016/j.ajhg.2008.12.017URL [本文引用: 1]
DOI:10.1007/s13353-014-0240-yURL [本文引用: 1]
DOI:10.1210/me.2004-0068URL [本文引用: 1]
PMID:12386933 [本文引用: 1]

E4BP4, a mammalian basic leucine zipper (bZIP) transcription factor, was first identified through its ability to bind and repress viral promoter sequences. Subsequently, E4BP4 and homologues in other species have been implicated in a diverse range of processes including commitment to cell survival versus apoptosis, the anti-inflammatory response and, most recently, in the mammalian circadian oscillatory mechanism. In some of these cases at least, E4BP4 appears to act antagonistically with members of the related PAR family of transcription factors with which it shares DNA-binding specificity. This diversity of function is mirrored by the regulatory pathways impinging on E4BP4, which include regulation by ras via the lymphokine IL-3 in murine B-cells, by thyroid hormone during Xenopus tail resorption, by glucocorticoids in murine fibroblasts and by calcium in rat smooth muscle cells. This article will cover the unfolding role/s of and regulation of E4BP4, E4BP4-like proteins and PAR factors in species as diverse as mouse and C. elegans. Copyright 2002 Wiley-Periodicals, Inc.
DOI:10.1016/j.tiv.2019.03.002URL [本文引用: 1]
DOI:10.1093/carcin/bgl038URL [本文引用: 1]
DOI:10.1016/j.molcel.2014.12.015URL [本文引用: 1]
DOI:10.1038/sj.onc.1208962URL [本文引用: 1]
DOI:10.1016/j.yexmp.2020.104384URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1080/15476286.2016.1276149URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1017/S0022029920000199URL [本文引用: 1]
DOI:10.1038/cdd.2015.82URL [本文引用: 1]
