 ,, 郭佳, 王三, 陈聪萍, 孙昌辉, 邓晓建, 王平荣
,, 郭佳, 王三, 陈聪萍, 孙昌辉, 邓晓建, 王平荣 ,四川农业大学水稻研究所,成都611130
,四川农业大学水稻研究所,成都611130Gene Mapping and Candidate Gene Analysis of Grain Width Mutant gw87 in Rice
ZHANG XiangYu ,, GUO Jia, WANG San, CHEN CongPing, SUN ChangHui, DENG XiaoJian, WANG PingRong
,, GUO Jia, WANG San, CHEN CongPing, SUN ChangHui, DENG XiaoJian, WANG PingRong ,Rice Research Institute, Sichuan Agricultural University, Chengdu 611130
,Rice Research Institute, Sichuan Agricultural University, Chengdu 611130通讯作者:
责任编辑: 李莉
收稿日期:2020-11-18接受日期:2021-01-15网络出版日期:2021-06-16
| 基金资助: |
Received:2020-11-18Accepted:2021-01-15Online:2021-06-16
作者简介 About authors
张翔宇,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (3182KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
张翔宇, 郭佳, 王三, 陈聪萍, 孙昌辉, 邓晓建, 王平荣. 水稻粒宽突变体gw87的基因定位及候选基因分析[J]. 中国农业科学, 2021, 54(12): 2487-2498 doi:10.3864/j.issn.0578-1752.2021.12.001
ZHANG XiangYu, GUO Jia, WANG San, CHEN CongPing, SUN ChangHui, DENG XiaoJian, WANG PingRong.
开放科学(资源服务)标识码(OSID):

0 引言
【研究意义】籽粒大小是影响水稻产量的一个重要因素,由粒长、粒宽、粒厚等性状共同决定。因此,发掘与籽粒大小相关的突变体,并开展基因克隆和功能分析,有助于阐明水稻籽粒发育的分子机制,促进水稻高产分子育种。【前人研究进展】水稻产量由大量的数量性状基因(quantitative trait locus,QTL)控制[1,2]。迄今,很多与籽粒大小相关的QTL被定位,且不少主效基因/QTL实现了克隆并探明生物学功能。其中,与籽粒宽度有关的主效基因包括GW2、GW5/qSW5、GS5、OsSPL16/GW8等[3,4,5,6,7,8,9]。GW2调控粒宽和粒重,该基因编码一个具有E3泛素连接酶活性的RING蛋白,通过将其底物锚定到蛋白酶体进行降解,从而负调控细胞的分裂[3]。GW5/qSW5通过增加水稻小花外颖的细胞数目,增加水稻颖壳的容量,进而增加粒宽;该基因编码一个由144个氨基酸组成的核蛋白,可能通过泛素-蛋白酶体途径在种子发育过程中调节细胞的分裂[4,5]。GS5编码一个丝氨酸羧肽酶,正向调控水稻籽粒大小;GS5启动子区域的2个关键的多态性单核苷酸影响了水稻幼穗中GS5的表达,GS5高表达具有更大的籽粒[6,7]。OsSPL16/GW8编码一个包含SBP结构域的转录因子,调控水稻粒宽,能够直接与GW7启动子结合并抑制它的表达[8,9]。油菜素内酯(brassinosteroid,BR)是一类多羟基类固醇化合物,在植物的生长、发育和繁殖过程中起着重要作用,在水稻中还可调节株型和籽粒大小等性状[10,11]。例如,水稻BR合成途径中的OsDWARF4、D2、D11、BRD1和BRD2等基因突变,会出现植株矮化、茎秆变短、叶片直立或卷曲、穗变小、籽粒小而圆等表型[12,13,14,15,16]。在水稻BR信号转导途径中,OsBRI1/d61编码BR信号受体蛋白OsBRI1,与拟南芥BRI1同源[17,18],d61突变体植株变矮、穗颈节增长、叶片直立,对外源BL不敏感[17]。OsGSK2与拟南芥BIN2同源,在BR信号途径中起关键作用,能直接与OsGRF4相互作用并抑制OsGRF4转录激活活性,介导BR对水稻籽粒长度的特异性调控[19]。DLT/GS6编码了植物特有的GRAS家族蛋白,正向调节BR应答,是GSK2的直接下游靶标,GSK2可以直接作用于DLT并磷酸化DLT[20];dlt和gs6突变体均表现分蘖减少、植株半矮等表型,gs6还表现籽粒宽度和粒重显著提高[20,21]。RLA1/SMOS1作为BR信号通路的正向调控因子发挥功能,与GSK2互作;GSK2可以磷酸化RLA1/SMOS1,使RLA1/SMOS1稳定性降低[22];rla1突变体表现植株半矮化,叶片直立,smos1突变体表现各种器官变小[22,23]。同时,RLA1/ SMOS1能与GS6/DLT互作,形成水稻生长素-油菜素甾醇信号互作中的关键复合体,影响水稻的生长发育[24]。【本研究切入点】利用化学诱变获得一份籼稻粒宽突变体gw87(grain width87),它是smos1、shb、rla1和ngr5的新等位突变体,但与这些突变体表型不同,gw87的粒宽和千粒重显著增加,对该突变体进行研究有助于增加对GW87调控水稻籽粒发育的了解。【拟解决的关键问题】本研究通过对gw87突变体的表型观察和主要农艺性状调查,外源BL敏感性、叶绿素含量及光合速率的测定,遗传分析、基因定位和候选基因遴选,以及DNA和cDNA测序验证等研究,为探明该基因对水稻籽粒大小的调控机制及应用潜力奠定基础。1 材料与方法
1.1 供试材料
粒宽突变体gw87(grain width87)是四川农业大学水稻研究所品质改良研究室利用化学诱变剂甲基磺酸乙酯(ethyl methanesulfonate,EMS)对籼稻恢复系材料676R进行诱变获得,经连续多代自交和田间观察,突变性状能够稳定遗传。1.2 主要农艺性状分析
野生型亲本676R和突变体gw87田间种植设3次重复,每次重复的每个小区栽种4行,每行10株,株行距16.7 cm×25 cm。在水稻的各个生育期中,观察分析并记录野生型和突变体的形态特征及农艺性状。成熟时,每个小区随机选择中间的10株进行田间农艺性状调查和室内考种,主要农艺性状包括株高、单株有效穗、节间长、主穗穗长和主穗着粒数、结实率、粒长、粒宽和粒重等。1.3 BR敏感性检测
参照TONG等[25]方法,用不同浓度的外源油菜素内酯BL处理野生型676R和突变体gw87的幼苗,测量并记录植株的叶夹角、胚芽鞘和根长,绘制3个性状在不同浓度BL处理下的柱形图或曲线图。1.4 叶绿素含量及光合速率的测定
在水稻抽穗期,分别取676R和gw87剑叶相同部位的新鲜叶片,去除主叶脉,在避光条件下剪碎,放入80%丙酮溶液于4℃浸提48 h,用UV-1700分光光度计在663、646和470 nm波长测出各个提取液的吸光值,参照LICHTENTHALER等[26]方法计算光合色素的含量。在抽穗期,分别选取676R和gw87长势良好且一致的剑叶各3片,于晴朗无云的上午9:00—11:30用便携式光合仪LI-6400(Li-COR Inc., USA)测定光合参数。1.5 遗传分析和高通量测序
用突变体gw87与野生型676R杂交得到F1,并自交得到F 2分离群体。在水稻各生育期,观察gw87与676R的表型特征以及相应F1的表型和F2群体的分离情况,统计、分析其遗传分离方式。从该F2群体中选取具有典型突变体表型的植株30株,将叶片叠放在一起,剪取相同部位叶片约0.5 g,混匀后,送至诺禾致源公司进行高通量测序,并对测序结果进行MutMap分析,获得与突变表型连锁的染色体区段和可能的突变位点,遴选候选基因。1.6 定位群体构建及分子标记分析
用突变体gw87与粳稻品种日本晴杂交,获得F2定位群体,从中选择突变体表型的植株,分单株取叶片提取总DNA。参照MCCOUCH等[27]方法,用候选基因所在染色体区域的SSR标记进行初步定位验证。然后,设计新的InDel(插入/缺失)标记进行PCR扩增和电泳检测,验证MutMap分析所得的候选基因。最后,对突变体gw87及其亲本676R中该候选基因的DNA和cDNA进行测序,进一步验证突变位点。1.7 总RNA提取及实时荧光定量PCR分析
取野生型676R和突变体gw87苗期的叶片,于-80℃保存;将野生型和突变体在28℃培养箱中黑暗培养10 d,剪下第1叶叶环连接部位约0.5 cm叶片和叶鞘,放入100 nmol·L-1 BL溶液中黑暗培养3 h后提取RNA[22]。用RNA isolater Kit试剂盒(Vazyme)提取样品中的RNA,电泳检测所提RNA的浓度和质量,并吸取2—3 μL的RNA进行反转录得到cDNA(Vazyme)。设计OsDWARF4、D11和D2的定量PCR引物(表1),加入0.1 μmol·L-1的正、反向引物、1×SYBR green PCR master mix(Vazyme),以及适宜浓度的cDNA,每个qRT-PCR反应总体积10 μL;仪器为CFX96 real-time PCR system(Bio-Rad);反应条件为96℃ 3 min;96℃ 10 s,55℃ 30 s,40个循环。每个试验3次生物学重复。Table 1
表1
表1qRT-PCR分析的相关引物
Table 1
| 基因 Gene | 正向引物 Forward primers (5′-3′) | 反向引物 Reverse primers (5′-3′) |
|---|---|---|
| OsDWARF4 | TCGTCGGCGAGACGTTCG | GGTAGCTGCACTCGAACA |
| D11 | TTGGTGAGACGCTGAGGT | CACAGGACACTATGGTGGG |
| D2 | GGAATTTATTGTCGGCCTCA | CTCGCCATCTTCTTCTTGG |
| Actin1 | CCCCCATGCTATCCTTCGT | GGCCGTTGTGGTGAATGACT |
新窗口打开|下载CSV
2 结果
2.1 gw87突变体的表型和农艺性状分析
与野生型676R比较,突变体gw87在苗期生长较缓慢,表现出植株半矮,叶片宽大且短;从分蘖期开始,gw87植株呈直立型,叶夹角变小,节间缩短、粗壮,叶片仍宽大且短,叶色深绿(图1)。在成熟期,突变体gw87株高、单株有效穗、主穗穗长、主穗二次枝梗数和结实率都比野生型亲本显著降低,降幅分别为32.0%、15.5%、32.4%、28.2%和16.6%;但gw87的主穗一次枝梗数和千粒重都明显增加,增幅分别为12.8%和12.0%;而gw87主穗着粒数与野生型差异不明显(表2)。图1
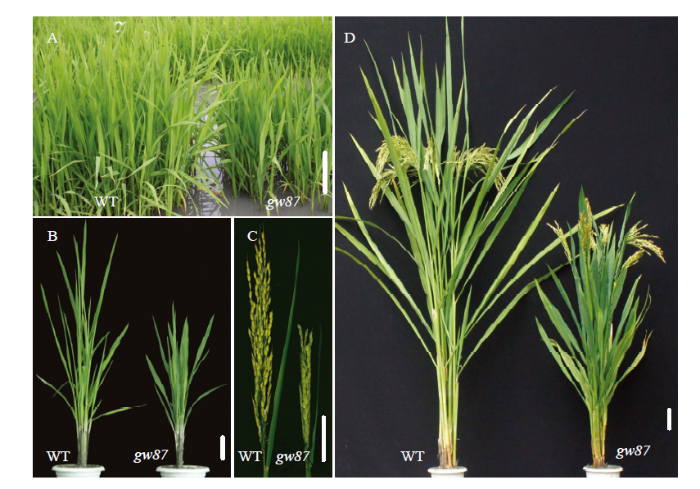 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1gw87突变体和野生型676R(WT)的植株表型
A、B、D:分别在苗期、分蘖期和灌浆结实期的植株;C:在灌浆结实期的叶夹角。Bar=5 cm
Fig. 1Plant phenotypes of gw87 mutant and its wild type (WT)
A, B and D: Plants at seedling stage, tillering stage and grain-filling stage, respectively; C: Leaf angle at grain-filling stage. Bar=5 cm
Table 2
表2
表2gw87与其野生型亲本676R主要农艺性状的比较
Table 2
| 性状Trait | 676R (WT) | gw87 | 比WT增减 Compared with CK (%) |
|---|---|---|---|
| 株高 Plant height (cm) | 118.0±1.9 | 80.2±1.7 | -32.0** |
| 单株有效穗 No. of productive panicles per plant | 5.8±0.4 | 4.9±0.2 | -15.5* |
| 主穗穗长 Length of main panicle (cm) | 27.5±1.0 | 18.6±1.2 | -32.4** |
| 主穗一次枝梗数 No. of primary branches per main panicle | 14.8±0.5 | 16.7±1.0 | 12.8* |
| 主穗二次枝梗数 No. of secondary branches per main panicle | 42.5±1.1 | 30.5±1.0 | -28.2** |
| 主穗着粒数 Total No. of grains per main panicle | 214.6±2.5 | 216.8±5.1 | 1.0 |
| 结实率Seed setting rate (%) | 83.9±0.4 | 67.3±2.0 | -16.6** |
| 千粒重1000-grain weight (g) | 37.4±0.2 | 41.9±0.2 | 12.0** |
新窗口打开|下载CSV
在成熟期,调查各节间的形态和长度,结果表明,与野生型相比,gw87的倒一节间呈现扭曲,且长度缩短最明显;gw87突变体的主穗穗长和其他节间长度也均有缩短(图2-A和图2-B)。在农艺性状调查中发现gw87的千粒重明显增加,特别是gw87籽粒宽度增加尤为显著,因此,对gw87与676R的籽粒大小进一步比较,结果显示gw87的粒长比野生型减少了4.4%,但粒宽则增加了21.1%(图2-C—图2-F),从而导致了突变体gw87的千粒重增加12.0%。
图2
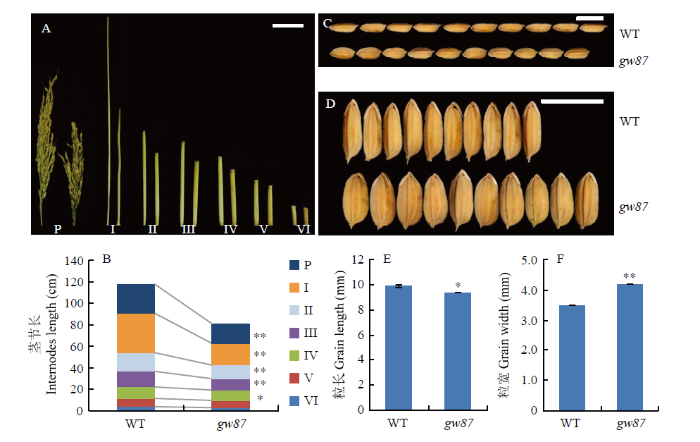 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2gw87突变体和野生型676R(WT)的穗子、茎秆和籽粒表型
A:野生型(左)和gw87(右)的稻穗和茎秆(Ⅰ—Ⅵ依次为倒1—倒6节间),Bar=5 cm;B:穗长、倒1—倒6节间长度统计;C、D:籽粒长度和宽度比较,Bar=1 cm;E、F:籽粒长度和宽度统计;*、**分别表示在P<0.05和P<0.01水平上差异显著。下同
Fig. 2Panicles, stems and grains of gw87 mutant and its wild type (WT)
A: Panicles and internodes of WT (left) and gw87 (right) (P indicates panicle, Ⅰ-Ⅵ indicate the first to the sixth internodes from the top), Bar=5 cm; B: Length of panicles and internode; C and D: Grains of WT and gw87, Bar=1 cm; E and F: Length and width of grains, respectively; * and ** indicate significant difference at P<0.05 and P<0.01, respectively. The same as below
此外,与野生型676R相比,突变体gw87的叶片在整个生育期均表现出长度减少,宽度增加(图3)。在苗期和抽穗期,gw87叶片长度分别降低了10.9%和42.5%,而其叶片宽度分别增加了12.5%和26.1%。同时,在抽穗期,突变体gw87植株的叶片颜色更为深绿,倒一叶和倒二叶的表面有明显皱褶、甚至扭曲。
图3
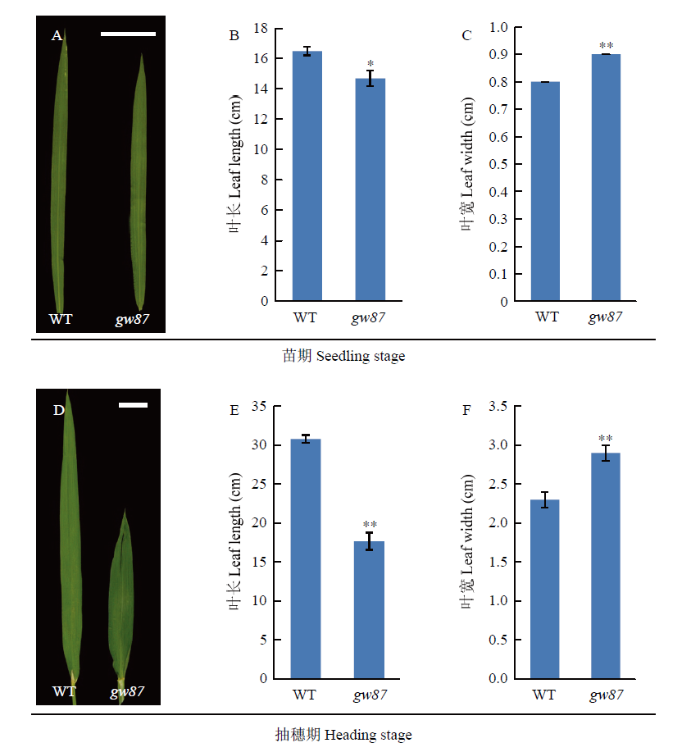 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3gw87突变体和野生型676R(WT)的叶片比较
A、B、C:在苗期第3片完全展开叶的形态以及长度和宽度统计;D、E和F:在抽穗期剑叶的形态以及长度和宽度统计。Bar=3 cm
Fig. 3Leaf comparison of gw87 mutant and its wild type (WT)
A, B and C: Morphology, length and width of the third fully expanding leaf at seeding stage; D, E and F: Morphology, length and width of the flag leaf at heading stage. Bar=3 cm
2.2 gw87突变体幼苗对油菜素内酯的响应
与野生型676R比较,突变体gw87表现出半矮、叶片直立、叶色深并有褶皱等表型,该表型类似于油菜素内酯(BR)突变体。为了检测gw87是否与BR途径相关,用外源BL对676R和gw87的幼苗进行处理,对根、胚芽鞘和叶夹角进行测定。结果显示,与未加BL处理的对照相比,BL处理的野生型676R根伸长明显受到抑制,胚芽鞘伸长增加,叶夹角明显增大(图4-A、图4-C、图4-E和图4-G—图4-I);而BL处理的gw87的根和胚芽鞘的伸长与未加BL的gw87相同,叶夹角随BR浓度增加也有所增大,但增大的幅度明显低于野生型(图4-B、图4-D和图4-F—图4-I)。以上结果表明,gw87幼苗对外源BL的敏感性低于676R幼苗,突变体gw87与BR途径有关。图4
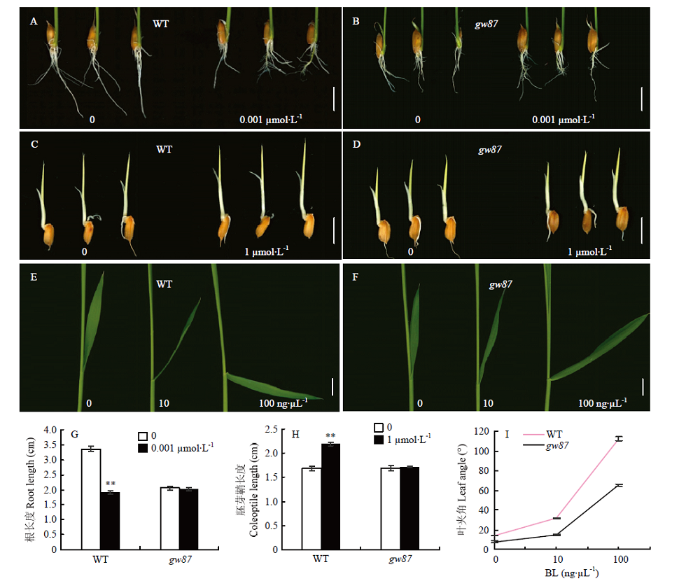 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4gw87突变体和野生型676R(WT)对外源BL的响应
A、B、G:光照条件下施用外源BL后根长度的变化;C、D、H:黑暗条件下施用外源BL后胚芽鞘长度的变化;E、F、I:施用不同浓度外源BL后叶夹角的变化。Bar=1 cm
Fig. 4Responses of gw87 mutant and its wild type (WT) to exogenous BL
A, B and G: Change of root length under presence or absence of 0.001 μmol·L-1 exogenous BL in the dark; C, D and H: Change of coleoptile length under presence or absence of 1 μmol·L-1 exogenous BL under light; E, F and I: Change of leaf angle under different concentrations of exogenous BL. Bar=1 cm
2.3 gw87突变体抽穗期的叶绿素含量及光合速率
与野生型亲本676R相比,突变体gw87叶片颜色更为浓绿。为了探究gw87基因突变是否会影响植株的光合特性,在抽穗期对676R和gw87的叶绿素含量和相关光合参数进行了测定。结果显示,突变体gw87的叶绿素a、叶绿素b和总叶绿素的含量都比野生型显著增加,增幅分别为16.5%、24.5%和18.4%,同时,gw87的类胡萝卜素含量也增加了10.9%(表3),表明gw87叶色浓绿是由于叶绿素含量的增加造成的。同时,对该时期的光合参数进行测定,结果显示突变体gw87的净光合速率和蒸腾速率分别比野生型提高了7.0%和7.1%,气孔导度和胞间CO2浓度分别降低了10.3%和11.8%(表4),说明gw87的光合作用有所增强,但未达到统计上的显著差异。Table 3
表3
表3突变体gw87与野生型676R叶片的光合色素含量
Table 3
| 材料 Material | 叶绿素a Chl a | 叶绿素b Chl b | 总叶绿素 Total Chl | Chla/Chlb | 类胡萝卜素 Caro |
|---|---|---|---|---|---|
| 676R(WT) | 2.18±0.15 | 0.49±0.04 | 2.67±0.19 | 4.38±0.05 | 0.46±0.04 |
| gw87 | 2.54±0.15 | 0.61±0.05 | 3.16±0.19 | 4.18±0.12 | 0.51±0.03 |
| 与WT相比Compared to WT (%) | 16.5* | 24.5* | 18.4* | -4.6 | 10.9 |
新窗口打开|下载CSV
Table 4
表4
表4突变体gw87与野生型676R叶片的光合参数测定
Table 4
| 材料 Material | 净光合速率Pn Net photosynthetic rate (μmol·m-2.s-1) | 蒸腾速率Tr Transpiration rate (μmol·m-2.s-1) | 气孔导度Gs Stomatal conductance to water vapor (μmol·m-2.s-1) | 胞间CO2浓度Ci Intercellular CO2 concentration (μmol·m-2.s-1) |
|---|---|---|---|---|
| 676R(WT) | 21.65±2.91 | 4.52±0.36 | 0.29±0.04 | 225.04±29.92 |
| gw87 | 23.16±2.32 | 4.84±0.22 | 0.26±0.02 | 198.56±26.79 |
| 与WT相比Compared with WT (%) | 7.0 | 7.1 | -10.3 | -11.8 |
新窗口打开|下载CSV
2.4 gw87突变体的遗传分析
将突变体gw87与野生型亲本676R杂交,所得F1植株的表型均与亲本676R相同;F1自交得到F2群体,分离出籽粒大小和株高都正常、籽粒变宽且植株矮化2种类型,统计结果表明这两种类型植株的分离比符合3﹕1(χ2=3.08<3.84(χ2(0.05,1)),表5),表明gw87的突变性状是由1对隐性核基因控制。Table 5
表5
表5gw87突变体与野生型亲本杂交F2代的遗传分离
Table 5
| F2组合 Combination | 总株数 Total No. of plants | 正常植株 No. of normal plants | 矮秆宽粒植株 No. of plants with dwarf and wide grain | 理论比 Expected ratio | χ2 |
|---|---|---|---|---|---|
| gw87×676R | 800 | 622 | 178 | 3﹕1 | 3.08 |
新窗口打开|下载CSV
2.5 gw87突变基因定位及候选基因分析
从突变体gw87与野生型亲本676R杂交F2群体中,选择具有典型突变表型的植株30株,每株取等量叶片混合,提取基因组DNA进行高通量测序,并以四川农业大学水稻研究所品质改良研究室测序676R和网上公开籼稻498R的基因组序列为参考基因组,对测序数据进行MutMap分析,结果显示,gw87突变基因位点与第5染色体中部的单核苷酸多态性(single-nucleotide polymorphism,SNP)连锁。在该染色体区域中,有12个位点发生碱基突变,其中5个位点位于基因上游,4个位点位于基因下游,1个位点位于2个基因之间,2个位点位于基因编码区。位于基因编码区的2个突变位点,其中1个位点突变不引起编码氨基酸变化,另外1个位点突变会引起该基因发生可变剪接,该基因为LOC_Os05g32270(表6)。因此,推测LOC_Os05g32270可能是突变体gw87的候选基因。Table 6
表6
表6gw87突变基因的MutMap分析结果以及用于精细定位的InDel和SSR标记
Table 6
| 标记名称 SNP and marker name | 物理位置 Physical location (bp) | SNP指数 SNP Index | 注释基因及碱基突变位点,或分子标记引物序列 Annotated gene and its nucleotide mutation site, or marker primers |
|---|---|---|---|
| SNP01 | 9834287 | 0.956 | LOC_Os05g17170 downstream, G2464A |
| SNP02 | 10490240 | 0.920 | LOC_Os05g18240 downstream, G1713A |
| SNP03 | 11888797 | 0.909 | LOC_Os05g20290 upstream, -2662G>A |
| SNP04 | 12878107 | 1 | LOC_Os05g22680 downstream,79G>A |
| SNP05 | 13399064 | 0.962 | Intergenic_region, LOC_Os05g23430-LOC_Os05g23440,13399064C>T |
| SNP06 | 13926842 | 0.960 | LOC_Os05g24140 upstream,-1434C>T |
| SNP07 | 13966225 | 0.958 | LOC_Os05g24190 upstream,-3398C>T |
| SNP08 | 14365938 | 0.906 | LOC_Os05g24790 upstream,-2425C>T |
| SNP09 | 14647328 | 0.913 | LOC_Os05g25240,synonymous,93C>T|p.Cys31Cys |
| SNP10 | 14908086 | 0.960 | LOC_Os05g25640 upstream_gene, -3616G>A |
| X1 | 18307365 | F: TATAGCCAGGATGAGAAG, R: CTGCTGTGGATCATCTTG | |
| X2 | 18753900 | F: GTACCCATCATAGCAAGA, R: AATAGGGTTGGAAGAATG | |
| SNP11 | 18813290 | 1 | LOC_Os05g32270, splice_donor_variant&intron_variant,1040+1G>A |
| X3 | 18854840 | F: CTATTTGCTATCGGACAC, R: ATTACTCTTTGGGTTTGG | |
| SNP12 | 18932427 | 1 | LOC_Os05g32430 downstream,117G>A |
| X4 | 18932825 | F: CGGCAACAAGAGGAGGAT, R: ACAGCAATGTCCGCAACGGTTTACA | |
| RM163 | 19251955 | F: ATCCATGTGCGCCTTTATGAGGA, R: CGCTACCTCCTTCACTTACTAGT |
新窗口打开|下载CSV
随后,从gw87与粳稻品种日本晴杂交F2群体中选择与gw87表型相似的突变植株142株进行分子标记连锁分析和验证。用第5染色体中部区域的SSR引物,以及在LOC_Os05g32270两侧的染色体区域设计InDel(插入/缺失)引物进行PCR扩增和电泳分析,结果与MutMap分析一致,gw87突变基因位于InDel标记X2和X3之间,与这两个标记的遗传距离分别为0.35和0.70 cM(表6、图5-A和图5-B);X2和X3之间的染色体区域长约101 kb,内含15个注释基因,LOC_Os05g32270也在其中(图5-C)。
图5
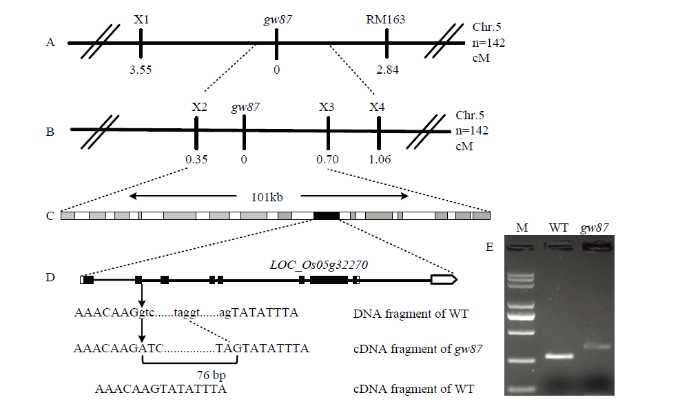 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5gw87突变体的基因定位及候选基因分析
A:突变基因定位于SSR标记RM163和InDel标记X1之间;B:突变基因定位于InDel标记X2和X3之间101 kb染色体区域;C:该101 kb区域含有15个注释基因;D:突变体中候选基因LOC_Os05g32270的第1 041位碱基G突变为A,导致该位点相邻的76 bp内含子序列被剪接为外显子,外显子和内含子序列分别用大写和小写字母表示,箭头表示突变位点;E:突变体LOC_Os05g32270的cDNA中含76 bp内含子特异序列的扩增;M:DL5000 DNA Marker
Fig. 5Gene mapping and candidate gene analysis of gw87 mutant
A: The gw87 locus was mapped to a region between SSR marker RM163 and InDel marker X1 on chromosome 5; B: The locus was narrowed down to a 101-kb genomic region between InDel markers X2 and X3; C: The 101 kb region contains 15 putative genes; D: A single nucleotide G-to-A substitution occurred at position 1041 in candidate gene LOC_Os05g32270 in the gw87 mutant, which was indicated with an arrow. Due to the point mutation, 76 bp of intron sequences were spliced into exons in the cDNA sequence of LOC_Os05g32270 in the mutant. The sequences of extron and intron are shown in upper and lower case, respectively; E: Amplification of LOC_Os05g3227 cDNA containing the specific sequence of 76 bp intron in gw87. M: DL5000 DNA Marker
接下来,对突变体gw87和野生型亲本676R中该候选基因DNA和cDNA进行测序验证,结果显示,突变体LOC_Os05g32270中DNA序列第1 041位的碱基G突变为A,该位点位于基因第2个外显子和第2个内含子交界处的内含子第1个碱基,造成该突变位点相邻的76 bp内含子序列被剪接成外显子,从而引起基因阅读框移位,蛋白翻译提前终止(图5-D)。同时,在该突变位点附近设计特异引物(F:5′-CATCT ATAGGCATAGGTG-3′和R:5′-TCATTGCCAAGCA AGTGT-3′)扩增候选基因cDNA片段,野生型片段长度应为368 bp,突变体gw87片段长度应为444 bp,琼脂糖凝胶电泳检测结果显示突变体和野生型的扩增条带大小分别与其对应的测序结果一致(图5-E),从而佐证了突变体cDNA确有76 bp内含子序列被剪接成外显子。因此,gw87突变基因是smos1、shb、rla1和ngr5的一个新等位基因。
2.6 gw87影响BR途径相关基因的表达
SMOS1/RLA1参与了BL信号途径,为了进一步验证gw87突变体中BR信号是否发生改变,对参与BR生物合成途径的OsDWARF4、D11和D2表达进行实时荧光定量PCR分析,其中,OsDWARF4编码的CYP90B1和D11编码的CYP724B1是BR合成途径限速酶,催化C-22羟基化;D2编码CYP90D2,催化6-脱氧茶甾酮或茶甾酮分别形成3-脱氢6-脱氧茶甾酮或3-脱氢茶甾酮。结果显示,与野生型相比,gw87突变体BR合成途径中的这三个基因表达量显著增加(图6-A)。同时,用BL处理突变体和野生型,检测这三个基因的表达,结果显示,与没有BL处理的野生型相比,BL处理后这三个基因在野生型中的表达量显著下降,在突变体中D11表达量也显著下降,但OsDWARF4和D2表达量下降很少甚至没有显著差异(图6-B)。以上结果表明gw87突变体中BR信号减弱。图6
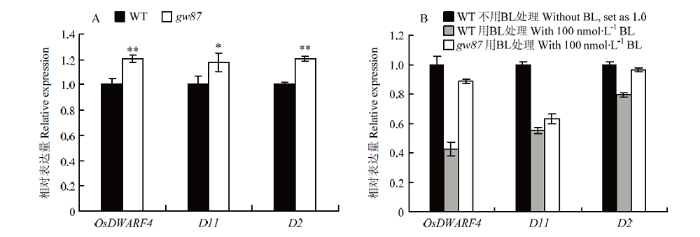 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6BR生物合成途径基因在突变体gw87和野生型676R(WT)中的表达分析
A:苗期突变体和野生型中OsDWARF4、D11和D2的表达水平。在每个基因在野生型中表达量设为1.0,以此计算出突变体基因的表达量。B:用BL处理突变体和野生型幼苗后OsDWARF4、D11和D2的表达水平。幼苗在黑暗中水培10 d,剪取第1叶叶环连接部位约0.5 cm叶片和叶鞘,用100 nmol·L-1 BL处理3 h,提取RNA进行检测
Fig. 6Expression analysis of the genes for BR biosynthesis in gw87 mutant and its wild type (WT)
A: The relative transcript levels of OsDWARF4, D11 and D2 in gw87 and its wild type at seedling stage. The transcript level of each gene in wild type was defined as 1, and that in gw87 mutant was calculated accordingly. B: The relative transcript levels of OsDWARF4, D11 and D2 in gw87 and its wild type without or with 100 nmol·L-1 BL treatment for 3 h. Total RNAs were extracted from 0.5 cm of the lamina joints of the first intact leaf
3 讨论
本研究通过EMS化学诱变获得了一份籽粒宽度明显增大的突变体gw87,并通过遗传分析、高通量测序、MutMap分析及分子标记验证,确定了候选基因LOC_Os05g32270,该基因编码一个含AP2/EREBP DNA绑定结构域的转录因子,gw87是smos1、shb、rla1和ngr5的新等位突变基因[22-23, 28-29]。在这些突变体中,smos1-1、smos1-2和smos1-3突变体具有相似的表型,即细胞变小、数目增加和微管方向异常,各种器官均变小;smos1-1和smos1-3中突变位点位于基因LOC_Os05g32270第7个外显子中单个核苷酸突变,而smos1-2中第6个外显子内插入单核苷酸,均引起翻译提前终止;SMOS1的表达受外源生长素(auxin)诱导,SMOS1启动子上的顺式生长素响应元件能够与生长素响应因子互作[23]。shb突变体是赤霉素(GA)缺陷突变体,表型为主根较短,植株矮化;突变位点是在基因第5内含子中插入T-DNA,显著降低了该基因的表达水平;SHB能够直接激活GA合成基因KS1的转录,因而水稻根分生组织大小受到SHB介导的GA合成的调控,以发育特异阶段的形式调控分生组织细胞的伸长和增殖[28]。rla1突变体则表现出半矮化、节间变短、叶片直立等典型的BR功能缺失表型,且对油菜素甾酮(Castasterone,CS)的敏感性降低;突变位点是在基因起始密码子ATG之前插入了8 bp序列,导致该基因表达量显著降低。RLA1/SMOS1可以和BR信号因子OsBZR1互作从而增强OsBZR1的转录活性;BR信号的负调节子GSK2能与RLA1/SMOS1互作,并对RLA1/SMOS1进行磷酸化,进而降低它的稳定性,对水稻生长和BR信号转导起重要作用[22]。最近报道的ngr5突变体显示分蘖数降低,且对氮素响应不敏感,该突变位于基因第7外显子上,NGR5是GA信号传导途径的一个新的关键元件,GA通过促进NGR5蛋白降解,导致表观遗传修饰降低,进而增强靶基因的转录活性,实现GA抑制植物分枝生长发育[29]。本研究中,gw87的突变发生在基因第2外显子和第2内含子的交界处,导致mRNA编辑时将突变位点相邻的76 bp内含子序列剪接为外显子,引起阅读框移码,翻译提前终止;突变体gw87植株表现出半矮化,叶片直立、变宽、变短,叶色深绿等表型,农艺性状调查显示gw87突变体粒宽增加了21.1%,导致gw87的千粒重增加了12.0%。gw87突变体对BL敏感性降低,该突变体中BR合成途径基因OsDWARF4、D11和D2的表达量升高,说明该突变体中BR信号减弱。虽然以上突变体均等位,但不同突变体之间表型有较大差异,如gw87突变体的粒宽显著增加,导致千粒重增大。根据突变位点的在LOC_Os05g32270中的位置(图7),rla1突变体可以翻译出完整的蛋白质,smos1、sbh和ngr5突变体能翻译出部分蛋白质,而gw87仅能翻译出该蛋白质N端的139个氨基酸(主要是AP2结构域中的部分氨基酸),说明gw87蛋白质功能损伤最严重;另一方面,gw87突变基因对水稻籽粒宽度和粒重产生重要影响,这与水稻BR信号通路中DLT/GS6的突变体gs6籽粒宽度和粒重显著提高的表型相似[21]。因此,gw87与其等位突变体smos1、shb、rla1和ngr5的表型差异,可能是该基因内的突变位点不同造成的,此外,不同突变体的野生型亲本的遗传背景差异也可能对突变体表型产生了影响。图7
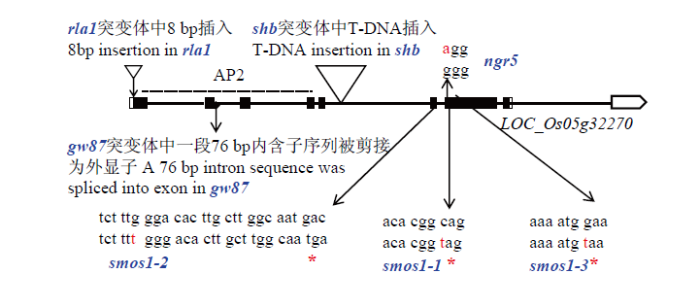 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7突变体smos1、shb、rla1、ngr5与gw87中的突变位点比较
Fig. 7Comparison of mutation sites among smos1, shb, rla1, ngr5 and gw87 mutants
对smos1、shb、rla1、ngr5等突变体研究表明,LOC_Os05g32270在植物体内具有多种生物学功能,在生长素、赤霉素、油菜素内酯等多种激素的信号途径中起了重要作用[22-23, 28-29]。gw87突变体籽粒宽度增大、千粒重增加,对该突变体及基因的进一步研究,将丰富对该基因调控籽粒生长发育机理的认识,也有助探明该基因在水稻分子育种上的应用潜力。
4 结论
通过EMS诱变籼稻恢复系材料676R,获得了一份籽粒宽度增大突变体gw87,其植株表现出半矮化,叶片直立、变宽、变短,叶色颜色更为深绿等表型,同时,该突变体籽粒宽度显著增大,千粒重增加。gw87的突变性状受1对隐性核基因控制,其候选基因为LOC_Os05g32270,突变体中该基因的第2外显子和第2个内含子的交界处碱基由G突变为A,导致突变位点相邻的76 bp内含子序列被剪接成外显子,引起阅读框移码,翻译提前终止。gw87突变体的籽粒宽度显著增大,这与以前报道的等位突变体smos1、shb、rla1和ngr5的表型明显不同,可能是该基因内的突变位点不同,导致其编码蛋白的功能活性受损程度不同所造成的。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1146/annurev.ge.27.120193.001225URL [本文引用: 1]
DOI:10.1016/S1369-5266(00)00148-5URL [本文引用: 1]
DOI:10.1038/ng2014URL [本文引用: 2]
DOI:10.1038/ng.169URL [本文引用: 2]
DOI:10.1038/cr.2008.307URL [本文引用: 2]
DOI:10.1038/ng.977URL [本文引用: 2]
DOI:10.1093/jxb/erv058URL [本文引用: 2]
DOI:10.1038/ng.2327URL [本文引用: 2]
DOI:10.1038/ng.3352URL [本文引用: 2]
DOI:10.1016/j.tplants.2018.08.007URL [本文引用: 1]
[本文引用: 1]
DOI:10.1038/nbt1173URL [本文引用: 1]
DOI:10.1105/tpc.014712URL [本文引用: 1]
DOI:10.1105/tpc.104.024950URL [本文引用: 1]
DOI:10.1046/j.1365-313X.2002.01438.xURL [本文引用: 1]
DOI:10.1007/s00299-015-1889-3URL [本文引用: 1]
DOI:10.1105/tpc.12.9.1591URL [本文引用: 2]
DOI:10.1104/pp.106.077081URL [本文引用: 1]
DOI:10.1038/nplants.2015.195URL [本文引用: 1]
DOI:10.1105/tpc.112.097394URL [本文引用: 2]
DOI:10.1111/jipb.12062URL [本文引用: 2]
DOI:10.1105/tpc.16.00611URL [本文引用: 6]
DOI:10.1093/pcp/pcu023URL [本文引用: 4]
DOI:10.1016/j.molp.2016.12.013URL [本文引用: 1]
[本文引用: 1]
DOI:10.1042/bst0110591URL [本文引用: 1]
[本文引用: 1]
DOI:10.1371/journal.pgen.1005464URL [本文引用: 3]
[本文引用: 3]
