 ,, 吴飞, 陈学秋, 黄艳, 时恒枝, 杜爱芳
,, 吴飞, 陈学秋, 黄艳, 时恒枝, 杜爱芳 ,, 杨怡
,, 杨怡 ,浙江大学动物科学学院/浙江省动物预防医学重点实验室,杭州 310058
,浙江大学动物科学学院/浙江省动物预防医学重点实验室,杭州 310058Hc-hrg-2 of Haemonchus Contortus Rescues the Growth of Heme Deficient Yeast Strain
ZHOU JingRu ,, WU Fei, CHEN XueQiu, HUANG Yan, SHI HengZhi, DU AiFang
,, WU Fei, CHEN XueQiu, HUANG Yan, SHI HengZhi, DU AiFang ,, YANG Yi
,, YANG Yi ,College of Animal Science, Zhejiang University/Key Laboratory of Animal Preventive Medicine of Zhejiang Province, Hangzhou 310058
,College of Animal Science, Zhejiang University/Key Laboratory of Animal Preventive Medicine of Zhejiang Province, Hangzhou 310058通讯作者:
责任编辑: 林鉴非
收稿日期:2020-05-6接受日期:2020-10-15网络出版日期:2021-04-16
| 基金资助: |
Received:2020-05-6Accepted:2020-10-15Online:2021-04-16
作者简介 About authors
周静茹,Tel:18868105390;E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (2902KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
周静茹, 吴飞, 陈学秋, 黄艳, 时恒枝, 杜爱芳, 杨怡. 捻转血矛线虫Hc-hrg-2拯救血红素缺陷型酵母生长表型[J]. 中国农业科学, 2021, 54(8): 1795-1804 doi:10.3864/j.issn.0578-1752.2021.08.018
ZHOU JingRu, WU Fei, CHEN XueQiu, HUANG Yan, SHI HengZhi, DU AiFang, YANG Yi.
开放科学(资源服务)标识码(OSID):

0 引言
【研究意义】捻转血矛线虫(Haemonchus contortus)是世界分布的具有重大经济意义的反刍动物寄生性线虫[1],严重地区感染率可达100%[2]。该线虫寄生宿主皱胃以吸食血液为生[3,4],可引起宿主消化不良,贫血及贫血综合征,严重影响宿主的健康[5,6]。目前捻转血矛线虫病的防控主要以药物使用为主,随着耐药问题日益严重,迫切需要新型抗线虫药物缓解现状[7,8,9]。血红素作为多种蛋白质的辅助因子,在能量代谢、氧气运输和存储、细胞色素依赖性的电子转运等生物学过程中发挥重要作用[10]。然而,包括捻转血矛线虫在内的绝大多数线虫(营自由生活和寄生)都缺乏完整的功能性血红素生物合成途径,需要摄取和利用源自于宿主的血红素[11]。因此,合理调控血红素的摄取及转运对寄生虫的生存和繁殖至关重要,探明该机制可能为寻找药物潜在靶标提供依据。【前人研究进展】作为一种自由生活的血红素缺陷型线虫,秀丽隐杆线虫(Caenorhabditis elegans)是研究血红素依赖性信号通路的最佳模式生物,在血红素转运机制研究上取得了很多进展。通过转录组分析、RNAi筛选和表型分析,目前已经鉴定了几种介导肠细胞血红素摄取以及胞内胞间血红素转运的蛋白质如Ce-HRG-1、HRG-2、HRG-3、HRG-4、MRP-5、HRG-7等[12],为寄生性线虫的相关研究奠定了重要基础。介于生活史的复杂性和研究手段的局限性,寄生性线虫血红素摄取和转运机制的研究相对滞缓。目前研究明确了巴西日圆线虫(Nippostrongylus brasiliensis)和马来丝虫(Brugia malayi)对宿主血红素的摄取和利用,并且对马来丝虫中Ce-HRGs的同源物BmHRG-1、BmHRG-2和BmMRP-5进行了功能探究 [13,14]。同时已有研究通过高通量液相质谱-串联质谱技术(High throughput LC-MS/MS)对捻转血矛线虫排泄分泌蛋白质组进行分析,推测其体内存在由天冬氨酸蛋白酶、半胱氨酸蛋白酶、金属蛋白酶以及外肽酶构成的蛋白酶水解级联体系,该体系能参与血红蛋白的降解[15]。血红素作为血红蛋白的重要组成部分,血红蛋白的降解必然伴随着血红素的产生[16]。但是目前捻转血矛线虫血红素转运相关的研究甚少。本研究团队前期对捻转血矛线虫中Ce-HRG-2的同源物Hc-HRG-2进行了功能探究,结果表明Hc-HRG-2作为内质网膜蛋白,能够在体外与血红素结合并抑制蛋白的GST酶活性[17]。在多细胞生物中对单个基因进行功能研究时往往会受到其旁系同源物的干扰,因此在血红素合成缺陷型酿酒酵母(Saccharomyces cerevisiae)hem1基因敲除株中对寄生虫血红素转运相关基因进行功能验证得到了广泛运用,目前已经成功运用于秀丽隐杆线虫、利什曼原虫(Leishmania amazonensis)、克鲁氏锥虫(Trypanosoma cruzi)、曼氏血吸虫(Schistosoma mansoni)等 [18,19,20,21]。【本研究切入点】前期研究表明,Hc-hrg-2属于血红素应答基因,其蛋白具有血红素结合特性。本研究拟通过构建血红素合成缺陷型酿酒酵母hem1基因敲除株,验证捻转血矛线虫Hc-hrg-2参与细胞内血红素转运的生物学功能。【拟解决的关键问题】构建血红素缺陷型酿酒酵母Δhem1敲除株,异源表达Hc-hrg-2进行酵母生长拯救试验,并探明其发挥作用的关键功能域,以期为进一步研究Hc-hrg-2参与调控捻转血矛线虫血红素稳态的相关机制奠定基础。1 材料与方法
试验于2019 年 4月至 2020 年 1月在浙江大学预防兽医研究所寄生虫病理生物学研究室进行。1.1 菌株与质粒
酿酒酵母菌株BY4741购自德国EUROSCARF,大肠杆菌(E. coli)菌株TOP10由浙江省动物预防医学重点实验室制备并保存;质粒pYES2-CT(携带URA3筛选基因)和pESC-LEU (携带LEU筛选基因和GAL1启动子)购自淼灵质粒平台,质粒 pMD19-T vector 购自宝生物工程(大连)有限公司。1.2 试剂与试剂盒
LA Taq DNA 聚合酶、T4 DNA连接酶、DNA Marker、各种限制性内切酶购自宝生物工程(大连)有限公司。高保真性KOD-FX PCR酶购自东洋纺(上海)生物科技有限公司。NovoRec Plus PCR一步定向克隆试剂盒购自上海近岸科技有限公司。质粒抽提试剂盒、DNA 凝胶回收试剂盒均购自浙江易思得生物科技有限公司。酵母YPD培养基、葡萄糖、琼脂粉购自生工生物工程(上海)股份有限公司;筛选培养基SD/- URA和酵母转化试剂盒购自宝生物工程(大连)有限公司;酵母氮源基础培养基(YNB)、Minimal SD Base Gal/Raf培养基以及缺陷型氨基酸DO Supplement -Leu/-Ura购自北京酷来博科技有限公司;酵母基因组提取试剂盒购自北京天根生化科技有限公司。
5-氨基乙酰丙酸(ALA)、血红素和抗Flag标签鼠源单克隆抗体购自Sigma公司,DNA提取酚试剂购自索莱宝试剂平台,苯甲基磺酰氟(PMSF)、二硫苏糖醇(DTT)和HRP标记的山羊抗鼠IgG购自杭州弗德生物科技有限公司。
1.3 引物设计与合成
根据酿酒酵母基因组数据库(Saccharomyces genome database)中hem1(SGD:S000002640)及其5' 和3' 侧翼区序列设计敲除引物;根据图1,设计鉴定酵母敲除株的引物。以pESC-LEU为载体,设计构建表达质粒的引物。所有引物的合成和测序均有浙江易思得生物科技有限公司完成,引物序列见表1。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1引物设计的原则
A和B位于hem15'和3'侧翼区(FR)的两侧;C、D、E、F位于ORF上;G、H、I、J位于筛选标记URA3上
Fig. 1Principles for primer design
A and B are located outside the 5 'and 3' flanking regions (FR) of the hem1 ; C, D, E, and F are on the ORF; G, H, I, and J are on the selection marker URA3
Table 1
表1
表1本试验所用的引物序列
Table 1
| 引物名称 Primer name | 上下游引物(5'-3')Primer |
|---|---|
| 5' FR-F | CACGGTTTCCTTTGCCAATT |
| 5' FR-R | TATGCTATACGAAGTTATACTGAAAAAAAAACCTAAGTACTGTTATG |
| 3' FR-F | TACATTATACGAAGTTATAACAACCAATATATGCATGGGCTGA |
| 3' FR-R | CTAAAGAATGTCCAAATATCGCCGG |
| URA3-F | ATAACTTCGTATAGCATACATTATACGAAGTTATTTCAATTCATCATTTTTT |
| URA3-R | ATAACTTCGTATAATGTATGCTATACGAAGTTATTTAGTTTTGCTGGCCGCA |
| A | CATAGGAAAACGGTTAAAAGGCCCTGCTTCTACC |
| B | GCTATTATGGAGGAACCCTGTTCAAACCGG |
| C | ATGCAACGCTCCATTTTTGC |
| D | TTACTGCTTGATACCACTAGAAACCTC |
| E | GCTGCTGCATGTGTTGATGACGCTG |
| F | CTTGAACCCTAATGTTAGAGACCCC |
| G | GAGAAGATGCGGCCAGCAAAACTAA |
| H | CTAAAGAATGTCCAAATATCGCCGGC |
| I | TCTGTGCTCCTTCCTTCGTTCTTCC |
| J | GAGAAGATGCGGCCAGCAAAACTAA |
| Hc-hrg-2-F | aattcaaccctcactaaagg ATGATTCTCTTGGTTTCTGTTGCTG |
| Hc-hrg-2-R | tcatccttgtaatccatcgaTTCTTCAGCAAACTCTTTTCCAAAAACCGTA |
| Ce-hrg-4-F | aattcaaccctcactaaaggATGACTGCTGAAAATCGAGGATTCT |
| Ce-hrg-4-R | tcatccttgtaatccatcgaACTTTTAATGACTTCAACATCGTCATC |
| pESC-F | TGTCAACAACGTATCTACCA |
| pESC-R | GGCTCTTTACATTTCCACAA |
新窗口打开|下载CSV
1.4 酵母hem1敲除组件的构建
以BY4741基因组为模板,采用引物5' FR-F/R、3' FR-F /R(5' FR-R和3' FR-F含18 bp loxp序列)分别进行PCR扩增,获得hem1的上下游同源臂序列。以质粒pYES2-CT为模板,用引物URA3-F/R(上下游引物含方向相同的loxp序列)进行PCR扩增,获得筛选基因URA3序列。分别利用引物5' FR-F /URA3-R和5' FR-F / 3' FR-R,通过两次重叠PCR技术依次将上游同源臂、URA3、下游同源臂序列串联成基因敲除组件,总长度为3 088 bp。普通PCR扩增的条件为:95 ℃ 5 min;95 ℃ 30 s,55 ℃ 30 s,72 ℃ 1 min,30个循环;72 ℃ 10 min。第一次重叠PCR扩增的条件为:95 ℃ 5 min;95 ℃ 30 s,55 ℃ 30 s,72 ℃ 2 min,25个循环;72 ℃ 10 min。第二次重叠PCR扩增的条件为:95 ℃ 5 min;95 ℃ 30 s,55 ℃ 30 s,72 ℃ 3 min,25个循环;72 ℃ 10 min。PCR 产物经1%琼脂糖凝胶电泳分离回收目的片段,连接至 pMD19-T载体,再将连接产物转入E. coli TOP10 感受态细胞中,菌液 PCR 鉴定阳性菌落,将阳性菌液送至浙江易思得生物科技有限公司测序分析。以测序正确的pMD19-T-基因组敲除组件质粒为模板,用高保真KOD-FX酶进行PCR扩增(100 μL体系),PCR条件:94 ℃ 2 min;98 ℃ 10 s,55 ℃ 30 s,68 ℃ 3 min,30个循环。按照PCR产物﹕DNA提取酚试剂﹕氯仿 = 2﹕1﹕1的比例(体积比),剧烈混匀,12 000×g离心10 min,取最上层上清。按照上述步骤重复一次。上清用1/10 体积的3 mol·L-1 醋酸钾(pH 5.5)溶液和2.5倍体积的无水乙醇在-20℃条件下沉淀1 h,12 000×g离心10 min,弃上清,沉淀用70%乙醇洗涤一次,开盖风干,用20 μL去离子水溶解备用。
1.5 酵母hem1敲除株的筛选与鉴定
制备酿酒酵母BY4741感受态细胞,利用醋酸锂转化法转化1 μg敲除组件片段到50 μL BY4741感受态细胞中[22]。转化后的菌液涂在含有250 μmol·L-1 ALA的SD/-URA平板上,28℃培养2—3 d,将单菌落在含有250 μmol·L-1 ALA的SD/-URA平板上划线,28℃培养2—3 d,挑选单克隆接种含有250 μmol·L-1 ALA的SD/-URA液体培养基中,30℃,200 r/min,摇床培养2 d,收集菌体。利用基因组提取试剂盒提取菌体DNA基因组作为模板,用引物A-B、C-D、A-E、F-B、G-H、A-I、J-B进行PCR验证,PCR条件:95℃ 5 min;95℃ 30 s,55℃ 30 s,72℃ 4 min,30个循环;72℃ 10 min。扩增产物进行琼脂糖凝胶电泳分析并将条带回收送至浙江易思得生物科技有限公司测序。1.6 酵母表达载体构建和表达株的表型鉴定
以含有捻转血矛线虫ZJ株 Hc-hrg-2 基因序列(GenBank number: MK371241)及其功能域缺失序列Hc-hrg-2(Δgst-n)和Hc-hrg-2(Δgst-c)的质粒为模板[17],分别以Hc-hrg-2-F/R为引物,用KOD-FX酶进行PCR扩增;同样地,以秀丽隐杆线虫的cDNA为模板,用Ce-hrg-4-F/R引物进行PCR扩增获得参与肠道血红素摄入的Ce-hrg-4序列(WBGene00009493),上述PCR条件:94 ℃ 2 min;98 ℃ 10 s,55 ℃ 30 s,68 ℃ 3 min,30个循环。PCR 产物经1%琼脂糖凝胶电泳分离回收目的片段。酵母表达载体pESC-LEU经限制性内切酶Not I单酶切后经1%琼脂糖凝胶电泳分离回收载体片段。目的片段和载体片段用无缝克隆试剂盒连接,转入 E. coli TOP10 感受态细胞中,菌液 PCR 鉴定阳性菌落,将阳性菌液送至浙江易思得生物科技有限公司测序分析。用质粒回收试剂盒提取测序正确的阳性菌质粒,制备Δhem1酵母感受态,利用醋酸锂转化法转化1 μg质粒到50 μL Δhem1感受态细胞中, 转化后的菌液涂在含有250 μmol L-1 ALA的SD/-URA/-LEU平板上,28 ℃培养2—3 d,挑选单克隆直接用通用引物pESC-F/R进行PCR鉴定。PCR条件:95 ℃ 15 min;95 ℃ 30 s,55 ℃ 30 s,72 ℃ 1 min,37个循环;72 ℃ 10 min。扩增产物进行琼脂糖凝胶电泳分析,有目的带的均为含有表达质粒的阳性克隆。
将鉴定成功的转化子用牙签蘸取少许分别接种到3 mL含有或不含有250 μmol L-1 ALA的SD/-URA/ -LEU液体培养基中,30 ℃,200 r/min,摇床培养2 d,拍照记录转化子在两种培养基中的生长情况。
1.7 斑点生长试验
参照YUAN等的方法[23],对该试验进行了优化,具体步骤如下:将含有表达质粒的阳性克隆接种到5 mL含有250 μmol·L-1 ALA的SD/-URA/-LEU液体培养基中,30 ℃,200 r/min,摇床培养2 d,收集菌体,接种部分菌体到5 mL含有250 μmol·L-1 ALA的SD(Gal/Raf)/-URA/-LEU液体培养基(不含葡萄糖)中,使得OD600 = 0.4,30℃,200 r/min,摇床培养24 h,诱导酵母表达目的蛋白。收集菌液,用5 mL SD (Gal/Raf)/-URA/-LEU液体培养基(不含葡萄糖)重悬,30 ℃,200 r/min,摇床培养18 h(耗尽酵母本身含有的血红素)。菌体用去离子水重悬至OD600 = 0.2,用去离子水进行5倍比稀释,取4 μL稀释后的菌液点到含有250 μmol·L-1 ALA或者不同浓度血红素 (0、0.04、0.1、1、10 μmol·L-1) 的SD(Gal/Raf)/-URA/ -LEU固体平板上,28 ℃培养2—3 d后,拍照记录酵母生长状况,根据斑点的数目比较生长状况,斑点数相差N个,则生长倍数相差5N倍。1.8 诱导表达目的蛋白的鉴定
斑点生长试验多余的菌体用1 mL PBS缓冲液重悬,加入蛋白酶抑制剂PMSF(1 mmol·L-1) 和二硫苏糖醇DTT(1 mmol·L-1),用超裂仪超裂破碎,45%功率,工作2 s / 间隔1.5 s,4 ℃超裂40 min,12 000× g离心10 min,收集溶解于PBS中的蛋白;沉淀用8 mol·L-1尿素溶解,12 000× g离心10 min,收集溶解于尿素中的蛋白。将上述两种蛋白混合后,进行SDS-PAGE电泳,转印到NC膜上,以mouse anti-flag tag(1﹕1 000)为一抗,山羊抗鼠(1﹕5 000)为二抗,进行Western Blot鉴定。2 结果
2.1 酵母hem1敲除组件的构建
以BY4741基因组为模板,用设计的引物分别进行hem1上下游同源臂序列的扩增,PCR结果可见约1 kb的条带(图2)。以质粒pYES2-CT为模板,用设计的引物进行筛选基因URA3的扩增,PCR结果可见约1 kb的条带(图2)。通过两次重叠PCR技术依次将测序正确的上游同源臂、URA3、下游同源臂序列串联成基因敲除组件,PCR结果可见约3 kb的条带(图2),回收目的片段,连接至 pMD19-T 载体,转化E. coli TOP10 感受态细胞,阳性菌落送测序。以测序正确的pMD19-T-基因组敲除组件质粒为模板,用高保真KOD-FX酶进行PCR扩增,将PCR产物进行醇沉,获得高浓度的敲除组件片段。图2
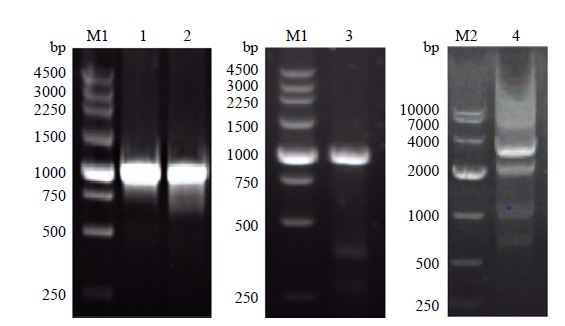 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2hem1敲除组件的构建
M1:DL250 DNA marker; M2:DL10000 DNA marker; 1-3:依次为上游同源臂、URA3、下游同源臂的PCR产物;4:敲除组件的两次重叠PCR的终产物
Fig. 2The construction of hem1 knockout component
M1: DL250 DNA marker; M2: DL10000 DNA marker; 1 - 3: PCR products of upstream homology sequence, URA3, and downstream homology sequence; 4: Final product of two overlapping PCRs of knockout components
2.2 酵母hem1敲除株的筛选与鉴定
采用醋酸锂转化法将敲除组件转化至酿酒酵母BY4741中,其两端与酵母基因组同源的序列进行同源重组,最终以loxP-URA3-loxP取代基因组中的hem1,使得转化子能在SD/-URA平板上(含250 μmol·L-1 ALA)存活,转化结果见图3。为避免假阳性,对转化子进行进一步验证。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3Δhem1敲除株的筛选
Fig. 3Screening of Δhem1 knockout strain
提取转化子Δhem1的基因组,用引物A-B、C-D、A-E、F-B、G-H、A-I、J-B进行PCR验证。正确重组了敲除组件的转化子用C-D、A-E、F-B 3对引物扩增没有条带,而用G-H、A-I、J-B 3对引物扩增目的带大小分别为1 357、1 251和1 088 bp, 用引物A-B扩增目的带为3 495 bp。以野生株BY4741基因组为对照用引物C-D、A-E、F-B 3对引物扩增目的带大小分别为1 358、1 246和1 647 bp,而用G-H、A-I、J-B 3对引物扩增没有条带,用引物A-B扩增目的带为4 054 bp。PCR扩增结果见图4,测序结果与预期一致,说明成功获得了hem1基因敲除株。
图4
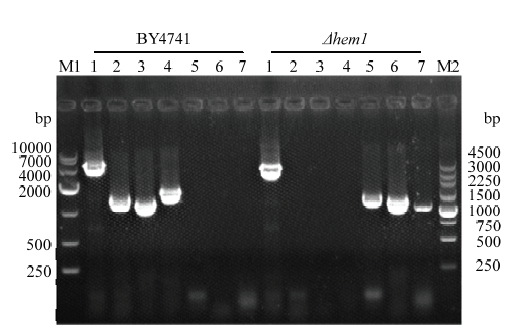 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4Δhem1敲除株的PCR鉴定
M1:DL10000 DNA marker; M2:DL250 DNA marker; 1-7:依次为以A-B、C-D、A-E、F-B、G-H、A-I、J-B为引物时的PCR扩增产物
Fig. 4PCR identification of Δhem1 knockout strain
M1: DL10000 DNA marker; M2: DL250 DNA marker; 1-7: PCR amplification products when A-B, C-D, A-E, F-B, G-H, A-I, J-B are used as primers
2.3 Δhem1表达载体构建与异源表达株的表型分析
已有研究表明,秀丽隐杆线虫Ce-hrg-4基因参与线虫肠道血红素的摄取,在低血红素浓度下(<1 μmol·L-1)可以拯救酵母Δhem1缺失株的生长[23,24],因此本研究将Ce-hrg-4作为酵母斑点生长试验的阳性对照组。将成功连接到pESC-LEU的Hc-hrg-2、Hc-hrg-2(Δgst-n)、Hc-hrg-2 (Δgst-c)、Ce-hrg-4的阳性质粒及空载转化到Δhem1敲除株中,挑选单克隆直接进行PCR鉴定,结果表明(图5),目的条带符合预期大小,5种表达载体已成功转化到敲除株内。图5
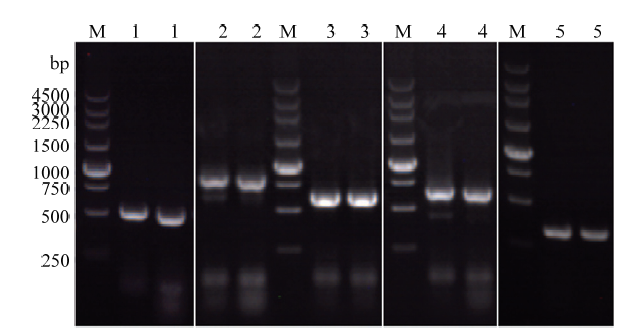 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5Δhem1异源表达株的PCR鉴定
Fig. 5PCR identification of Δhem1 exogenous expression strain
M: DL250 DNA marker; 1: pESC-LEU-Ce-hrg-4; 2: pESC-LEU-Hc-hrg-2; 3: pESC-LEU-Hc-hrg-2(Δgst-n); 4: pESC-LEU-Hc-hrg-2 (Δgst-c); 5: pESC-LEU
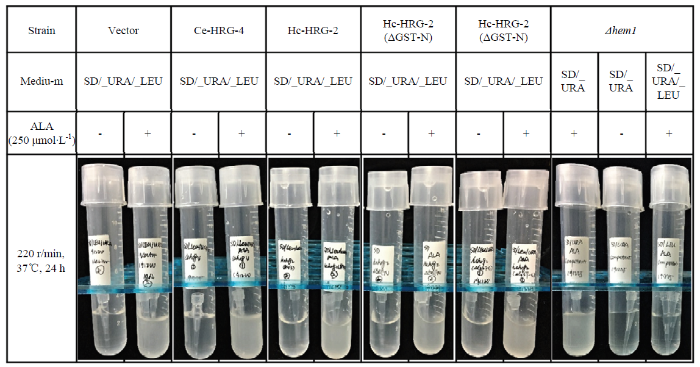
为排除载体对酵母缺失株的影响,对转化成功的异源表达株进行表型验证,结果表明(图6),和添加250 μmol·L-1 ALA的培养基相比,在不添加ALA的培养基中,Δhem1敲除株以及异源表达株的生长受到明显抑制,证明Δhem1确实是血红素合成缺陷株且单纯转化5种表达载体对缺失株的表型没有拯救作用。
图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6Δhem1敲除株及其异源表达株的表型验证
Fig. 6Phenotypic verification of Δhem1 knockout strain and its exogenous expression strain
2.4 目的蛋白的鉴定与斑点生长试验
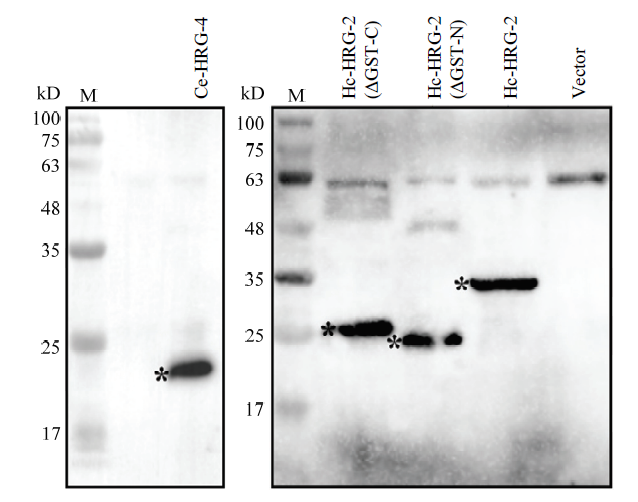
预测各蛋白大小,Hc-HRG-2约为33.6 kD,Ce-HRG-4约为20.3 kD,Hc-HRG-2 (ΔGST-N) 约为25 kD,Hc-HRG-2 (ΔGST-C) 约为25.7 kD,利用Western Blot对含有阳性表达质粒敲除株的目的蛋白进行鉴定。结果表明(图7),除了空载组没有目的带,其余4种目的片段都在酵母中得到了表达,蛋白大小与预期一致。图7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7目的蛋白的Western Blot鉴定
M:蛋白marker; *表示目的条带
Fig. 7Western Blot identification of target proteins
M: Protein marker; Asterisks indicate the target bands
将剩余菌液5倍比稀释后点至含有250 μmol·L-1 ALA或者不同浓度血红素的平板上,生长情况见图8。在0.04 μmol·L-1血红素浓度下,与空载组相比,表达Hc-HRG-2的酵母生长提高了5倍,表达Ce-HGR-4的酵母生长提高了25倍,表达功能域缺失蛋白的酵母的生长没有明显提高。在0.1 μmol·L-1血红素浓度下,与空载组相比,表达Hc-HRG-2的酵母的生长提高了25倍,表达Ce-HGR-4的酵母生长提高了625倍;表达Hc-HRG-2 (ΔGST-N) 的酵母生长提高了25倍,但是生长状况与Hc-HRG-2组相比稍差一些;表达Hc-HRG-2 (ΔGST-C) 的酵母生长仅提高了5倍。随着血红素浓度的增加,试验组和空载组之间的酵母生长差异会逐渐减少。上述结果表明,表达Hc-hrg-2能够在低血红素浓度下(≤1 μmol·L-1)促进酵母对血红素的摄取,拯救敲除株的生长缺陷;Hc-hrg-2的硫氧还蛋白样结构域(GST-N)和谷胱甘肽S-转移酶C末端结构域(GST-C)在敲除株的表型拯救中发挥重要作用, 谷胱甘肽S-转移酶C末端结构域相对更重要一些。
图8
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8Δhem1敲除株及其各异源表达株在不同血红素浓度的平板上的生长情况
Fig. 8Growth of Δhem1 knockout strain and its exogenous expression strains on plates with different heme concentrations
3 讨论
捻转血矛线虫是一种以吸食宿主血液为生的寄生性线虫,探明其对血液中血红素的利用及调控机制可以为药靶的筛找以及疫苗的开发提供新的思路。由于捻转血矛线虫寄生阶段的离体培养比较复杂[25],借助结构相对简单且研究技术成熟的真核模式生物进行功能研究成为了普遍的技术手段[26,27,28]。酿酒酵母 hem1编码5-氨基乙酰丙酸合成酶是血红素合成途径中的第一个酶,在血红素合成过程中发挥着重要作用[29]。因此hem1缺失菌株需要在生长培养基中添加5-氨基乙酰丙酸或者血红素(≥10 μmol·L-1)才能生长[30]。根据上述特性,已有团队成功在血红素缺陷型酿酒酵母中对血液寄生原虫以及秀丽隐杆线虫等进行了建模,对血红素应答相关基因进行了功能验证[18, 20-21],但是对捻转血矛线虫的建模尚未见报道。以酿酒酵母中缺失Hc-hrg-2的同源物为前提,本研究首次成功在血红素缺陷型酿酒酵母中对捻转血矛线虫进行建模,并初步探究了捻转血矛线虫血红素转运相关基因的功能。
前期试验表明,Hc-hrg-2是血红素应答基因,高浓度血红素的刺激可导致该基因的转录水平上调,其蛋白能够在体外和血红素结合并抑制蛋白的GST酶活性[17]。为了进一步验证Hc-hrg-2是否参与血红素的转运,本试验在真核模式生物酿酒酵母中进行了相关功能的验证。以BY4741为模板,通过同源重组技术成功获得hem1敲除株Δhem1。与野生株BY4741相比,Δhem1只有在培养基中添加5-氨基乙酰丙酸时才能生长,说明该敲除株的血红素合成通路的确存在缺陷,可以用于建模。利用无缝克隆技术成功构建Hc-hrg-2及其功能域缺失序列的酵母表达载体,对转化成功的异源表达株进行表型验证,结果表明,和添加250 μmol·L-1 ALA的培养基相比,在不添加ALA的培养基中,Δhem1敲除株以及异源表达株的生长受到明显抑制。理论上,Δhem1敲除株以及异源表达株在不添加ALA的培养基中不能生长,由于在本试验中酵母是从含有ALA的培养基中直接挑选单克隆至不添加ALA的液体培养基中培养,酵母内源ALA可以维持酵母进行有限的扩增,所以结果中可以看到不添加ALA的培养基组酵母存在一定程度的生长。Western Blot鉴定结果表明C端标记有FLAG表位的Hc-HRG-2系列蛋白能够在酵母敲除株内被半乳糖诱导表达,且其分子量的大小与预测一致。在酵母斑点生长试验中,在≤0.1μmol·L-1 血红素浓度下表达Ce-HRG-4对酵母生长有显著的促进作用,与前人的研究结果相一致[23],说明本试验条件下完全能够实现在血红素缺陷型酿酒酵母中对秀丽隐杆线虫的建模。以此为前提,在≤1μmol·L-1 血红素浓度下表达Hc-HRG-2对酵母的生长有一定的促进作用,表明Hc-hrg-2确实能拯救敲除株的生长缺陷,促进酵母细胞摄入外源血红素,利用血红素缺陷型酿酒酵母对捻转血矛线虫的建模确实可行。研究中还发现Hc-hrg-2的两个关键功能域硫氧还蛋白样结构域和谷胱甘肽S-转移酶C末端结构域在敲除株的表型拯救中都发挥重要作用, 且在≤1μmol·L-1 血红素浓度下可以观察到谷胱甘肽S-转移酶C末端结构域的缺失会导致拯救程度降低,表明该功能域相对更关键。值得注意的是,在低血红素浓度下Hc-hrg-2对敲除株的拯救程度明显弱于Ce-hrg-4,该结果与CHEN等在Δhem1敲除株中对秀丽隐杆线虫Ce-HRG-2进行建模时的结果类似[31],表明促进细胞对血红素的摄取可能不是Hc-hrg-2的主要功能。参考Ce-HRG-2能够介导参与隔离和再分配胞内血红素的功能[31],作为同源物的Hc-hrg-2在血红素调控中扮演的角色还需要进一步探究。
4 结论
本试验首次成功在血红素缺陷型酿酒酵母中对捻转血矛线虫进行建模,证明了血红素应答基因Hc-hrg-2能促进细胞对血红素的利用,并且其两个谷胱甘肽S-转移酶相关功能域发挥重要作用。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1016/bs.apar.2016.02.015URLPMID:27238012 [本文引用: 1]

Parasitic roundworms (nematodes) cause substantial mortality and morbidity in animals globally. The barber's pole worm, Haemonchus contortus, is one of the most economically significant parasitic nematodes of small ruminants worldwide. Although this and related nematodes can be controlled relatively well using anthelmintics, resistance against most drugs in common use has become a major problem. Until recently, almost nothing was known about the molecular biology of H. contortus on a global scale. This chapter gives a brief background on H. contortus and haemonchosis, immune responses, vaccine research, chemotherapeutics and current problems associated with drug resistance. It also describes progress in transcriptomics before the availability of H. contortus genomes and the challenges associated with such work. It then reviews major progress on the two draft genomes and developmental transcriptomes of H. contortus, and summarizes their implications for the molecular biology of this worm in both the free-living and the parasitic stages of its life cycle. The chapter concludes by considering how genomics and transcriptomics can accelerate research on Haemonchus and related parasites, and can enable the development of new interventions against haemonchosis.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1371/journal.ppat.1006931URLPMID:29566094 [本文引用: 1]

As part of on-going efforts to control hookworm infection, the
[本文引用: 1]
URLPMID:31071474 [本文引用: 1]
URLPMID:21087517 [本文引用: 1]
DOI:10.1186/s13071-020-3911-zURLPMID:31996262 [本文引用: 3]

BACKGROUND: Haemonchus contortus, a blood-feeding parasite, is constantly surrounded by large quantities of heme released from the catabolism of host red blood cells. To cope with the toxicity of free heme, H. contortus needs to uptake and detoxify the heme, a process believed to be paramount for parasite survival. METHODS: A heme-responsive gene Hc-hrg-2 was identified which is the homologue of Ce-hrg-2. The transcriptional levels in all developmental stages and heme-responsive ability of Hc-hrg-2 were analyzed by qRT-PCR. Immunofluorescence analysis and cell transfections were performed to analyze the expression pattern of Hc-HGR-2. Statistical analyses were performed with GraghPad Prism 6.0 using Student's t-test. RESULTS: To investigate the heme homeostasis of H. contortus, we first identified a heme-responsive gene Hc-hrg-2, a homolog of Ce-hrg-2 that is involved in heme transport in the hypodermis of Caenorhabditis elegans. Using qRT-PCR, we showed that Hc-hrg-2 mRNA was expressed throughout all life-cycle stages of H. contortus with the highest level in the third-stage larvae (L3s). Notably, transcription of Hc-hrg-2 in the exsheathed L3s was significantly upregulated in the presence of high concentration of heme. We found that Hc-HRG-2 protein was mainly located in the hypodermal tissues of adult H. contortus in vivo and the endoplasmic reticulum in the transfected mammalian cells. Our in vitro assay demonstrated that Hc-HRG-2 is a heme-binding protein with glutathione S-transferase activity and heme had a significant effect on its enzymatic activity when a model substrate 1-chloro-2, 4-dinitrobenzene (CDNB) was used. CONCLUSIONS: Hc-hrg-2 is a heme-responsive gene and engaged in heme homeostasis regulation in hypodermal tissues during the free-living stages of H. contortus.
[本文引用: 2]
DOI:10.1111/febs.13368URLPMID:26153121 [本文引用: 1]

Schistosomes ingest host erythrocytes, liberating large quantities of haem. Despite its toxicity, haem is an essential factor for numerous biological reactions, and may be an important iron source for these helminths. We used a fluorescence haem analogue, palladium mesoporphyrin, to investigate pathways of haem acquisition, and showed that palladium mesoporphyrin accumulates in the vitellaria (eggshell precursor glands) and ovary of female Schistosoma mansoni. Furthermore, incubation of adult females in 10-100 mum cyclosporin A (IC50 = 2.3 mum) inhibits the uptake of palladium mesoporphyrin to these tissues, with tenfold reductions in fluorescence intensity of the ovary. In vitro exposure to cyclosporin A resulted in significant perturbation of egg production, reducing egg output from 34 eggs per female to 5.7 eggs per female over the incubation period, and retardation of egg development. We characterized a S. mansoni homologue of the haem-responsive genes of Caenorhabditis elegans. The gene (Smhrg-1) encodes a protein with a molecular weight of approximately 17 kDa. SmHRG-1 was able to rescue growth in haem transport-deficient HEM1Delta yeast. Transcriptional suppression of Smhrg-1 in adult S. mansoni worms resulted in significant delay in egg maturation, with 47% of eggs from transcriptionally suppressed worms being identified as immature compared with only 27% of eggs laid by control worms treated with firefly luciferase. Our findings indicate the presence of transmembrane haem transporters in schistosomes, with a high abundance of these molecules being present in tissues involved in oogenesis.
[本文引用: 2]
DOI:10.1016/j.cmet.2014.03.030URLPMID:24836561 [本文引用: 2]

Several lines of evidence predict that specific pathways must exist in metazoans for the escorted movement of heme, an essential but cytotoxic iron-containing organic ring, within and between cells and tissues, but these pathways remain obscure. In Caenorhabditis elegans, embryonic development is inextricably dependent on both maternally derived heme and environmentally acquired heme. Here, we show that the multidrug resistance protein MRP-5/ABCC5 likely acts as a heme exporter, and targeted depletion of mrp-5 in the intestine causes embryonic lethality. Transient knockdown of mrp5 in zebrafish leads to morphological defects and failure to hemoglobinize red blood cells. MRP5 resides on the plasma membrane and endosomal compartments and regulates export of cytosolic heme. Together, our genetic studies in worms, yeast, zebrafish, and mammalian cells identify a conserved, physiological role for a multidrug resistance protein in regulating systemic heme homeostasis. We envision other MRP family members may play similar unanticipated physiological roles in animal development.
[本文引用: 1]
URLPMID:22174408 [本文引用: 3]
DOI:10.1038/nature06934URLPMID:18418376 [本文引用: 1]

Haems are metalloporphyrins that serve as prosthetic groups for various biological processes including respiration, gas sensing, xenobiotic detoxification, cell differentiation, circadian clock control, metabolic reprogramming and microRNA processing. With a few exceptions, haem is synthesized by a multistep biosynthetic pathway comprising defined intermediates that are highly conserved throughout evolution. Despite our extensive knowledge of haem biosynthesis and degradation, the cellular pathways and molecules that mediate intracellular haem trafficking are unknown. The experimental setback in identifying haem trafficking pathways has been the inability to dissociate the highly regulated cellular synthesis and degradation of haem from intracellular trafficking events. Caenorhabditis elegans and related helminths are natural haem auxotrophs that acquire environmental haem for incorporation into haemoproteins, which have vertebrate orthologues. Here we show, by exploiting this auxotrophy to identify HRG-1 proteins in C. elegans, that these proteins are essential for haem homeostasis and normal development in worms and vertebrates. Depletion of hrg-1, or its paralogue hrg-4, in worms results in the disruption of organismal haem sensing and an abnormal response to haem analogues. HRG-1 and HRG-4 are previously unknown transmembrane proteins, which reside in distinct intracellular compartments. Transient knockdown of hrg-1 in zebrafish leads to hydrocephalus, yolk tube malformations and, most strikingly, profound defects in erythropoiesis-phenotypes that are fully rescued by worm HRG-1. Human and worm proteins localize together, and bind and transport haem, thus establishing an evolutionarily conserved function for HRG-1. These findings reveal conserved pathways for cellular haem trafficking in animals that define the model for eukaryotic haem transport. Thus, uncovering the mechanisms of haem transport in C. elegans may provide insights into human disorders of haem metabolism and reveal new drug targets for developing anthelminthics to combat worm infestations.
[本文引用: 1]
[本文引用: 1]
URLPMID:29167662 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1096/fj.201900888RRURLPMID:31907982 [本文引用: 1]

In the free-living nematode Caenorhabditis elegans, the serine/threonine-specific protein kinase, AKT, is known to play a key role in dauer formation, life-span, and stress-resistance through the insulin-like signaling pathway. Although the structure and function of AKT-coding genes of C. elegans are understood, this is not the case for homologous genes in parasitic nematodes. In the present study, we explored a C. elegans akt-1 gene homolog in the parasitic nematode Haemonchus contortus, investigated its transcript isoforms (Hc-akt-1a and Hc-akt-1b), and studied expression and function using both homologous and heterologous functional genomic tools. In C. elegans, we showed that the predicted promoter of Hc-akt-1 drives substantial expression in ASJ neurons of the N2 (wild-type) strain. In H. contortus (Haecon-5 stain), RNAi (soaking) led to a significantly decreased transcript abundance for both Hc-akt-1a and Hc-akt-1b, and reduced larval development in larval stages in vitro. Chemical inhibition was also shown to block larval development. Taken together, the evidence from this study points to a key functional role for Hc-akt-1 in H. contortus.
[本文引用: 1]
[本文引用: 1]
DOI:10.1074/jbc.M111.307694URLPMID:22303006 [本文引用: 2]

The roundworm Caenorhabditis elegans is a heme auxotroph that requires the coordinated actions of HRG-1 heme permeases to transport environmental heme into the intestine and HRG-3, a secreted protein, to deliver intestinal heme to other tissues including the embryo. Here we show that heme homeostasis in the extraintestinal hypodermal tissue was facilitated by the transmembrane protein HRG-2. Systemic heme deficiency up-regulated hrg-2 mRNA expression over 200-fold in the main body hypodermal syncytium, hyp 7. HRG-2 is a type I membrane protein that binds heme and localizes to the endoplasmic reticulum and apical plasma membrane. Cytochrome heme profiles are aberrant in HRG-2-deficient worms, a phenotype that was partially suppressed by heme supplementation. A heme-deficient yeast strain, ectopically expressing worm HRG-2, revealed significantly improved growth at submicromolar concentrations of exogenous heme. Taken together, our results implicate HRG-2 as a facilitator of heme utilization in the Caenorhabditis elegans hypodermis and provide a mechanism for the regulation of heme homeostasis in an extraintestinal tissue.
