 ,1, 高玉林2, 张方梅2,3, 杨超霞1,2, 蒋健2, 朱勋2, 张云慧
,1, 高玉林2, 张方梅2,3, 杨超霞1,2, 蒋健2, 朱勋2, 张云慧 ,2, 李祥瑞
,2, 李祥瑞 ,2
,2Cloning of Heat Shock Protein Gene Ld-hsp70 in Leptinotarsa decemlineata and Its Expression Characteristics under Temperature Stress
ZHENG HaiXia ,1, GAO YuLin2, ZHANG FangMei2,3, YANG ChaoXia1,2, JIANG Jian2, ZHU Xun2, ZHANG YunHui
,1, GAO YuLin2, ZHANG FangMei2,3, YANG ChaoXia1,2, JIANG Jian2, ZHU Xun2, ZHANG YunHui ,2, LI XiangRui
,2, LI XiangRui ,2
,2通讯作者:
责任编辑: 岳梅
收稿日期:2020-05-28接受日期:2020-06-29网络出版日期:2021-03-16
| 基金资助: |
Received:2020-05-28Accepted:2020-06-29Online:2021-03-16
作者简介 About authors
郑海霞,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (1212KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
郑海霞, 高玉林, 张方梅, 杨超霞, 蒋健, 朱勋, 张云慧, 李祥瑞. 马铃薯甲虫热激蛋白基因Ld-hsp70的克隆及温度胁迫下的表达特性[J]. 中国农业科学, 2021, 54(6): 1163-1175 doi:10.3864/j.issn.0578-1752.2021.06.008
ZHENG HaiXia, GAO YuLin, ZHANG FangMei, YANG ChaoXia, JIANG Jian, ZHU Xun, ZHANG YunHui, LI XiangRui.
开放科学(资源服务)标识码(OSID):

0 引言
【研究意义】马铃薯甲虫(Leptinotarsa decemlineata)是鞘翅目(Coleoptera)叶甲科(Chrysomelidae)的一种世界公认的毁灭性害虫,是我国重大检疫对象之一。该虫以成虫、幼虫危害马铃薯(Solanum tuberosum)、茄子(Solanum melongena)和番茄(Solanum lycopersicum)等多种茄科植物的茎叶、花蕾等部位[1,2],同时传播马铃薯褐斑病菌和环腐病菌等,造成马铃薯产量的重大损失[3,4]。马铃薯甲虫成虫不仅具有自主扩散能力[5,6],还对温度[7]、杀虫剂[1]等有着极强的抵抗力,成为了亚洲、欧洲、北美等地茄科植物的重要害虫[1]。温度是影响物种种群扩散和分布的重要因素[8],大量研究也证实马铃薯甲虫具有很强的耐寒性和耐高温能力,与其发育起点温度、有效积温、发育历期、繁殖力及扩散等都有着直接的关系[9,10,11,12]。因此,研究马铃薯甲虫对温度胁迫的响应机制对其科学防控具有重要意义。【前人研究进展】热激蛋白(heat shock protein,HSP)是一种在生物体内广泛存在的抗逆蛋白,在进化上高度保守。当生物体遭受不利环境条件胁迫时,其迅速产生可保证生物体的正常生理活动[13]。由于热激蛋白分子量大小不同,一般将其分为4个家族,分别为HSP90、HSP70、HSP60和小分子HSP家族[14,15]。其中HSP70家族是最为保守的一类,存在于原核生物和真核生物体的所有细胞区室和器官中。一般情况下,HSP70以稳定态存在于细胞浆内,在应激环境下,会迅速转移到细胞核内,表达也迅速增加,仅有少量存在于细胞浆内;当应激刺激消失,细胞恢复正常状态,核内的HSP70会迅速消失,而细胞浆内仍有低水平的表达[16]。HSP70家族包括两种蛋白质:组成型热激蛋白70(HSC70)和诱导型热激蛋白70(HSP70),组成型热激蛋白在正常环境下表达,不受外界因素的胁迫;而诱导型热激蛋白在温度等胁迫因子处理后,表达量急剧上升[17]。HSP70作为热激蛋白家族中研究最广泛、深入的一类,在生物体高、低温胁迫中起着至关重要的作用[18,19,20]。国内外对多种昆虫的HSP70基因序列及其在温度胁迫下的表达做了大量的研究。例如,高温处理南美斑潜蝇(Liriomyza huidobrensis)[21]、梨小食心虫(Grapholitha molesta)[22]和空心莲子草叶甲(Agasicles hygrophila)[23]等均可显著提高HSP70基因的表达及昆虫的耐热性。不同龄期的赤拟谷盗(Tribolium castaneum)在40℃高温处理1 h后,HSP70基因表达比对照增加了1.1—2.0倍[24]。在低温胁迫下,HSP70在昆虫耐受性中同样挥发作用。例如,黑腹果蝇(Drosophila melanogaster)在-7℃处理1 h后,HSP70基因的相对表达量显著上升[25]。褐飞虱(Nilaparvata lugens)在高温胁迫下HSP70基因的表达量未发生改变,而低温胁迫下其表达量明显下降[26]。此外,HSP70不同亚族基因在相同温度胁迫下表达模式也存在差异。比如三叶斑潜蝇(Liriomyza trifolii)的Lthsp701在高、低温胁迫下表达量均能显著增高,而Lthsp70和Lthsp702对温度的胁迫不敏感,在受到温度胁迫后其表达量无显著变化[27]。褐飞虱HSP70家族的不同基因对高、低温胁迫也表现出不同的响应模式[28]。【本研究切入点】本团队前期已研究了马铃薯甲虫HSP90和HSP60在高温和低温胁迫下的表达特点,表明二者可能在马铃薯甲虫高温胁迫下发挥作用[29,30]。但是不同的HSP基因在抵抗温度胁迫时作用机制不同,有关马铃薯甲虫HSP70基因(Ld-hsp70)的温度胁迫作用还有待探究。【拟解决的关键问题】利用生物信息学分析方法、RT-PCR及RACE技术,对Ld-hsp70的全长基因进行克隆、分析,同时结合实时荧光定量PCR(qRT-PCR)技术检测马铃薯甲虫雌、雄成虫在高、低温胁迫条件下的表达情况,探讨Ld-hsp70在温度胁迫中的作用,为明确马铃薯甲虫适应温度响应的机制及其科学防控提供理论依据。1 材料与方法
试验昆虫的采集分别于2012年6月和2018年6月在新疆农业科学院植物保护研究所安宁渠试验基地马铃薯试验田完成;试验昆虫的处理及解剖于2018年6月在新疆农业科学院植物保护研究所完成;基因克隆试验于2012年、其余所有试验于2018—2019年在中国农业科学院植物保护研究所完成。1.1 试验昆虫
马铃薯甲虫试验种群采自于新疆农业科学院植物保护研究所安宁渠试验基地马铃薯试验田(87°28′28°°E,43°57′7°°N)。采回当地实验室后,在室温(25℃)条件下,置于圆柱形养虫盒内(直径12 cm,高12 cm)饲养,养虫盒内放入新鲜的马铃薯叶片,利用纱布网封口保证透气。温度处理时,将马铃薯甲虫雌、雄成虫分别取出,放入铺有马铃薯叶片的培养皿(直径9 cm)中,每皿10只,置于不同设置温度的培养箱中进行处理,设定温度分别为-10、0、38和44℃,处理时间为1和4 h,每个处理重复3次,以室温(25℃)作为对照。处理完成后,将马铃薯甲虫迅速取出,用解剖刀切成厚度不超过0.5 cm的组织块,放入含有RNAlater的1.5 mL离心管中,于4℃冰箱中过夜,保证RNAlater全部浸透组织,最后置于-80℃冰箱保存备用。1.2 试剂及仪器
主要试剂:TRIzol试剂盒和M-MLV反转录酶购于美国Invitrogen公司;3° Full RACE Core Set Ver. 2.0试剂盒、dNTP、Taq酶和SYBR Premix Ex TaqTM购于大连TaKaRa公司;胶回收试剂盒和质粒提取试剂盒购于德国OMEGA公司;克隆载体pEASY-T1、感受态细胞Trans1-T1和DNA marker购于法国Transgene公司。引物合成与测序由北京三博远志生物技术有限责任公司完成。主要仪器:ND-2000微量分光光度仪(美国Thermo Scienfic公司);PCR仪536-TouchGene? Gradient(英国EDVOTEK公司);实时荧光定量PCR仪7500 Fast(美国Applied Biosystems公司)。
1.3 试验方法
1.3.1 马铃薯甲虫总RNA的提取及一链cDNA的合成 用Trizol法抽提马铃薯甲虫的总RNA,用微量分光光度仪检测总RNA的浓度,2%的琼脂糖凝胶电泳进一步检测其质量。最后,根据M-MLV反转录试剂盒的操作说明书合成cDNA第一链。1.3.2 马铃薯甲虫HSP70基因片段的筛选及克隆 在NCBI库中检索筛选马铃薯甲虫HSP70的cDNA序列,共检索到3类,分别命名为HSP70a、HSP70b和HSP70c。利用Primer 5.0软件设计特异性引物,引物序列及对应的PCR退火温度见表1。通过多轮PCR扩增获得马铃薯甲虫HSP70的序列片段。具体PCR 反应条件为95℃预变性5 min;95℃变性30 s,55℃退火30 s,72℃延伸30 s,共35个循环;72℃充分延伸10 min。利用胶回收试剂盒对目的片段进行回收纯化,再连接到pEASY-T1载体中,转化至Trans1-T1感受态细胞,最后进行菌落PCR阳性克隆鉴定,送至北京三博远志生物技术有限责任公司测序验证序列的准确性。
Table1
表1
表1试验所用引物
Table1
| 引物名称 Primer name | 引物序列 Primer sequence (5°-3°) | 引物功能 Function of primers | 退火温度 Annealing temperature (℃) |
|---|---|---|---|
| hsp70aF1 | GCCATGAACCCCAATAACAC | hsp70a基因片段扩增 Amplification of hsp70a gene fragment | 56.0 |
| hsp70aR1 | AGATGGTACCGGCATCTTTG | ||
| hsp70aF2 | AGGAAGGACTTGACGAGCAA | 56.0 | |
| hsp70aR2 | TTTCTGCACCTTGGGGATAC | ||
| hsp70aF3 | CAAGATCACCATCACCAACG | 56.0 | |
| hsp70aR3 | GATGGGGTTGCAGATGTTCT | ||
| hsp70aF4 | CATACCTGGGCAAGACCG | 56.0 | |
| hsp70aR4 | CCAAAGACAGTGGCGTGA | ||
| hsp70bF1 | GATGTTGGCACAGTCATTGG | hsp70b基因片段扩增 Amplification of hsp70b gene fragment | 56.0 |
| hsp70bR1 | TGCTGAACAGTGGAGTCTGTG | ||
| hsp70bF2 | AGCCAAGAGAGCCTTGTCTG | 55.0 | |
| hsp70bR2 | CAACGAGGACGATTTCATCA | ||
| hsp70cF1 | GGCATTGATCTTGGAACCAC | hsp70c基因片段扩增 Amplification of hsp70c gene fragment | 56.0 |
| hsp70cR1 | CTTCCTGGATCTTGGGATCA | ||
| hsp70aF1 | CAAGATCACCATCACCAACG | hsp70a 3° RACE扩增 3° RACE of hsp70a | 56.0 |
| hsp70bF1 | TGATGAAATCGTCCTCGTT | hsp70b 3° RACE扩增 3° RACE of hsp70b | 52.0 |
| hsp70bF2 | CAGCTCCCAGAGGTGTTC | ||
| hsp70cF1 | GCCAGGTAGCCATGAATC | hsp70c 3° RACE扩增 3° RACE of hsp70c | 52.0 |
| hsp70aFQ | ACGGTGACAAGTCTGAGGAGGT | 实时荧光定量PCR qRT-PCR | 60.0 |
| hsp70aRQ | TGGTGGTGTTACGCTTGATGAG | ||
| hsp70bFQ | AGCAGCTATTGCCTATGGTTTG | 60.0 | |
| hsp70bRQ | TTCCTGATGTCTTTGCCCTTC | ||
| hsp70cFQ | AACCAGTGGAGAAGGCTCTAACA | 60.0 | |
| hsp70cRQ | TGTACTGCCGCACCATAAGC | ||
| GADPH-F | GATTCCACTCACGGACGATT | 内参基因扩增 Amplification of the reference gene | 60.0 |
| GADPH-R | CATATTTGCCCCAAGGAATG | ||
| TUB-F | CTGCCTCTTTGAGGTTCGAC | 60.0 | |
| TUB-R | TTACTGGGGCGTAAGTGACC |
新窗口打开|下载CSV
1.3.3 马铃薯甲虫HSP70的全长序列扩增 根据1.3.2验证的马铃薯甲虫HSP70的cDNA片段,设计3个Ld-hsp70的特异性引物(表1),进行3′ RACE扩增获得HSP70的全长序列,具体按照试剂盒说明书中的操作完成。
1.3.4 Ld-hsp70在温度胁迫下的表达 提取不同温度处理下的马铃薯甲虫雌、雄成虫的总RNA,取1 μg进行反转录合成cDNA第一链。将反转录的cDNA稀释10倍作为模板,用于qRT-PCR反应。以室温(25℃)作为对照,每个处理重复3次,每次取3头整虫提取总RNA混样,每个样品重复3次。以甘油醛-3-磷酸脱氢酶GADPH(glyceraldehyde-3- phosphate dehydrogenase)和α-微管蛋白TUB(alpha-tubulin)基因作为内参基因[28],用于qRT-PCR的引物和内参特异性引物见表1。具体qRT-PCR反应条件为95℃预变性30 s;95℃变性3 s,60℃退火与延伸30 s,40个循环;最后在60℃收集荧光信号。采用2-ΔΔCt法计算Ld-hsp70的相对表达量。
1.4 序列和数据分析
利用DNAMAN软件对马铃薯甲虫HSP70的全长序列进行拼接,然后输入NCBI数据库进行Blast比对分析。利用Biology WorkBench(利用SAS9.2(SAS Insitute Inc.,Cary,NC,美国)软件,采用单因素方差(One-way,ANOVA,LSD分析法)分析Ld-hsp70相对表达量在不同温度胁迫下的差异显著性;并采用独立样本t检验(t-test)分析Ld-hsp70相对表达量在某一胁迫温度下不同处理时间之间的差异显著性。
2 结果
2.1 Ld-hsp70的克隆
通过在NCBI数据库中检索比对,获取3个马铃薯甲虫的HSP70a片段,分别设计特异性引物对hsp70aF1和hsp70aR1,hsp70aF2和hsp70aR2,hsp70aF3和hsp70aR3,得到了长度分别为316、297和322 bp的3个片段。进而设计特异性引物hsp70aF4和hsp70aR4,以合成的cDNA为模板,进行第2轮PCR扩增,获得了长度为807 bp的片段。以hsp70aF2和hsp70aR3配对,进行第3轮PCR扩增,获得了一段长1 071 bp的片段,确定为HSP70a的5°端全长序列(图1-A)。接着,以3° RACE的cDNA为模板,以hsp70aF3和3° RACE Inner Primer配对,进行3° RACE扩增,获得一段长为611 bp的3°端全长序列(图1-B)。最后设计特异性引物扩增,得到编码ORF区域的全长序列为1 947 bp(图1-C)。该全长序列的5′ UTR长92 bp,3′ UTR长160 bp,将其命名为Ld-hsp70a,提交NCBI数据库,登录号为KC544268。马铃薯甲虫HSP70b全长cDNA序列的克隆过程同上(图1-D—F)。最后获得长度为2 397 bp的HSP70b全长cDNA序列,其ORF长1 974 bp,5′ UTR长132 bp,3′ UTR长291 bp,将其命名为Ld-hsp70b,NCBI登录号为KC544269。马铃薯甲虫HSP70c全长cDNA序列的克隆过程同上(图1-G—I)。最后经DNAMAN软件拼接后得到了长度为2 482 bp的全长cDNA序列,其ORF长1 890 bp,5′ UTR长289 bp,3′ UTR长303 bp,将其命名为Ld-hsp70c,NCBI登录号为KC544270。图1
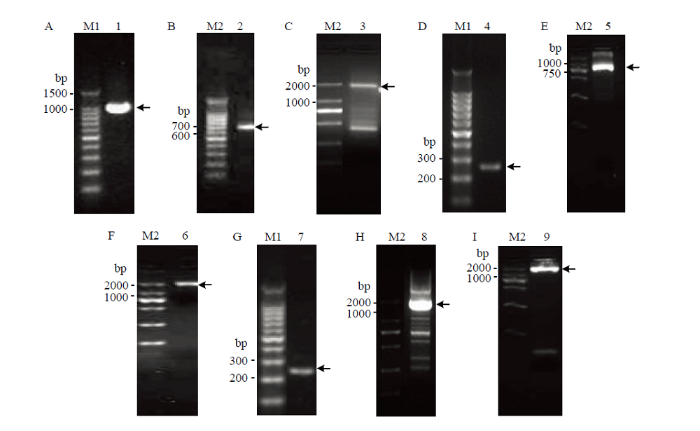 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1Ld-hsp70 RT-PCR和3′ RACE产物
A:Ld-HSP70a筛选片段的PCR产物PCR product of Ld-HSP70a fragment;B:3′ RACE产物PCR product of 3′ RACE;C:全长ORF的PCR产物PCR product of full-length ORF;D:Ld-HSP70b筛选片段的PCR产物PCR product of Ld-HSP70b fragment;E:3′ RACE产物PCR product of 3′ RACE;F:全长ORF的PCR产物PCR product of full-length ORF;G:Ld-HSP70c筛选片段的PCR产物PCR product of Ld-HSP70c fragment;H:3′ RACE产物PCR product of 3′ RACE;I:全长ORF的PCR产物PCR product of full-length ORF;M1:100 bp ladder DNA maker;M2:BM2000 DNA maker;1—9(箭头Arrow): PCR 产物 PCR products
Fig. 1The RT-PCR and 3′ RACE products of Ld-hsp70s
2.2 Ld-hsp70序列分析
Ld-hsp70a、Ld-hsp70b和Ld-hsp70c的开放阅读框分别编码648、657和629个氨基酸,3′端非翻译区含有PolyA尾及多聚腺苷酸信号序列AATAAA。预测蛋白相对分子量分别为71.07、72.86和68.72 kD,等电点分别为5.33、5.06和5.58。3个HSP70序列中均具有HSP70家族3段完整的签名序列,分别为GIDLGTTYSCV、FDLGGGTFDVS、VLVGGSTRIPK,说明此3种蛋白属于HSP70家族。Ld-hsp70a和Ld-hsp70c的氨基酸C末端序列为EEVD,表明该蛋白质具有胞质型热激蛋白氨基酸特征;Ld-hsp70b的氨基酸C末端序列为KDEL,表明该蛋白质具有内质网型热激蛋白氨基酸特征,属于内质网型热激蛋白。此外,Ld-hsp70a的C末端具有两个GGXP四肽序列,而Ld-hsp70b和Ld-hsp70c C末端均无该序列(图2)。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2马铃薯甲虫5种Ld-hsp70的氨基酸序列比对
HSP70蛋白来源及GenBank登录号 Origin of HSP70 protein and their GenBank accession numbers:马铃薯甲虫Leptinotarsa decemlineata Ld-hsp70a(KC544268);Ld-hsp70b(KC544269);Ld-hsp70c(KC544270);LdHSP70A(AF288978);LdHSP70B(AF322911)。下划线:3段保守的HSP70家族签名序列Underlined: three conserved motifs of the HSP70 family;方框:胞质型标志性基序(EEVD)和内质网型HSPC末端标志性基序(KDEL)Box: the cytoplasmic HSP70 carboxyl terminal region (EEVD) and the endoplasmic reticulum HSP70 carboxyl terminal region (KDEL);箭头:C末端具有两个GGXP四肽序列Arrows: two GGXP quaternary peptide sequences at the C-terminal;黑色区域:高度保守的氨基酸Black blocks: highly conserved amino acids
Fig. 2Alignment of five heat shock protein sequences of Ld-hsp70s in L. decemlineata
序列比对发现,Ld-hsp70a、Ld-hsp70b和Ld-hsp70c三者之间的氨基酸序列相似性为71.50%。Ld-hsp70a与Ld-hsp70c的氨基酸序列相似性为71.85%,而Ld-hsp70a与Ld-hsp70b、Ld-hsp70b与Ld-hsp70c的序列相似性分别为59.26%和56.50%。将3个HSP70的氨基酸序列与已报道的两个马铃薯甲虫的HSP70(GenBank登录号分别为AF288978和AF322911)氨基酸序列进行比对,其序列相似性为70.94%(图2)。
利用SWISS-MODEL工具建模,以家牛(Bos taurus)的HSC70为模板(PDB登录号:3c7n),预测Ld-hsp70a和Ld-hsp70c编码蛋白的三维结构(图3-A、3-C),以家牛的HSC70为模板(PDB登录号: 1yuw),预测Ld-hsp70b编码蛋白的三维结构(图3-B)。结果表明,马铃薯甲虫3个HSP70的三维结构与已知其他物种的HSP70相似度很高,3个HSP70蛋白N端44 kD的片段内含有4个域形成两瓣,两瓣中间形成一个深槽,该区域包含高度保守的ATPase功能域(ATP binding domain);靠近C端18 kD保守的肽结合域(peptide binding domain),由两个四联反向平行的β-折叠和一个α-螺旋组成。
图3
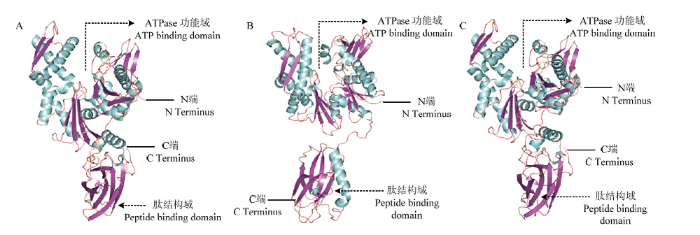 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3Ld-hsp70蛋白的三维结构预测
A: Ld-hsp70a; B: Ld-hsp70b; C: Ld-hsp70c
Fig. 3Deduced three-dimensional structure of Ld-hsp70s
2.3 系统发育树分析
选取来自于膜翅目、双翅目、鳞翅目、鞘翅目等昆虫的38个HSP70基因和马铃薯甲虫的5个HSP70基因构建系统系发育树(图4)。结果表明,马铃薯甲虫5个HSP70分别聚类到三大支,Ld-hsp70a和Ld-hsp70b与其他物种的HSP70分别独聚为一支,Ld-hsp70c与已报道的马铃薯甲虫两个HSP70聚为一支,进化距离较近,与Ld-hsp70a和Ld-hsp70b的进化距离较远。图4
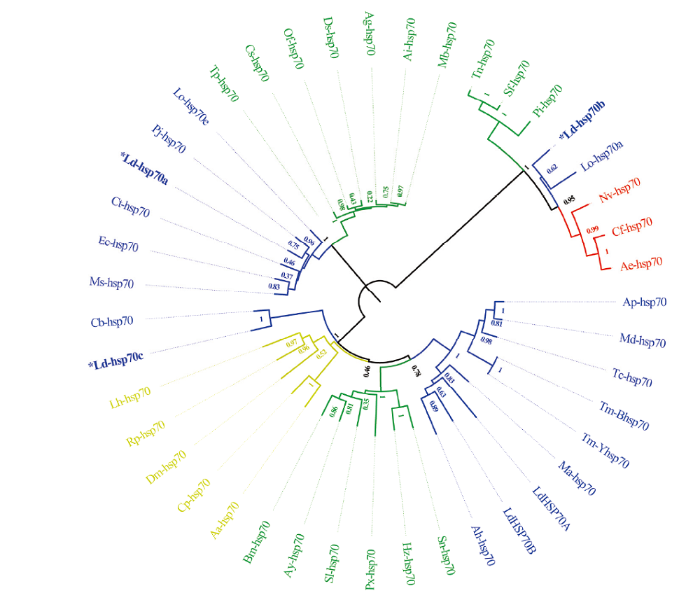 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4昆虫HSP70氨基酸序列系统进化树
蓝色Blue:鞘翅目Coleoptera;绿色Green:鳞翅目Lepidoptera;黄色Yellow:双翅目Diptera;红色Red:膜翅目Hymenoptera。星号*标注为本文中的3个HSP70 Asterisks* are three HSP70s in this study。HSP70s选取的物种来源及其在GenBank中的登录号 Origin species of HSP70s and their accession numbers in GenBank:鳞翅目Lepidoptera:天蚕Antheraea yamamai(BAD18974),家蚕Bombyx mori(BAF69068),细纹夜蛾Spodoptera litura(ADV03160),粉茎螟Sesamia nonagrioides(ABZ10939),玉米夜蛾Helicoverpa zea(ACV32640),小菜蛾Plutella xylostella(ADK94697),蒲氏钩蝠蛾Thitarodes pui(ADA61012),二化螟Chilo suppressalis(ADE05296),玉米螟Ostrinia furnacalis(ADR00357),落叶松毛虫Dendrolimus superans(ABM90551),黎豆夜蛾Anticarsia gemmatalis(ADO32621),球菜夜蛾Agrotis ipsilon(AEG78288),甘蓝夜蛾Mamestra brassicae(BAF03556),印度谷螟Plodia interpunctella(ABM88156),粉纹夜蛾Trichoplusia ni(ABH09735),草地贪夜蛾Spodoptera frugiperda(AAN86047);鞘翅目Coleoptera:空心莲子草叶甲Agasicles hygrophila(KF792067),黄粉虫Tenebrio molitor(JQ219848,JQ219849),谢氏宽漠王Mantichorula semenowi(GU289400),光滑鳖甲Anatolica polita borealis(EF569673),稻水象甲Lissorhoptrus oryzophilus(KC620437,KC620428),龟纹瓢虫Propylea japonica(KJ483960),大猿叶甲Colaphellus bowringi(KF214746),赤拟谷盗Tribolium castaneum(MK000439),中华豆芫菁Epicauta chinensis(KR181952),准噶尔小胸鳖甲Microdera dzhungarica punctipennis(JF421286),三开蜣螂Copris tripartitus(EF208962),松墨天牛Monochamus alternatus(AF288978),马铃薯甲虫Leptinotarsa decemlineata(AF288978,AF322911,KC544268,KC544269,KC544270);双翅目Diptera:埃及伊蚊Aedes aegypti(ACJ64193),尖音库蚊Culex pipiens(AAX84696),果蝇Drosophila melanogaster(AAG26887),苹果实蝇Rhagoletis pomonella(ABL06948),南美斑潜蝇Liriomyza huidobrensis(AAW32098);膜翅目Hymenoptera:金小蜂Nasonia vitripennis(XP_001606463),佛罗里达弓背蚁Camponotus floridanus(EFN61604),叶切蚁Acromyrmex echinatior(EGI70210)
Fig. 4Phylogenetic tree of amino acid sequences of HSP70s in insects
与其他物种比较,Ld-hsp70a与龟纹瓢虫(Propylaea japonica)的Pj-hsp70聚为一支,同源性最高,Ld-hsp70b与稻水象甲(Lissorhoptrus oryzophilus)的Lo-hsp70同源性最高,Ld-HSP70c与大猿叶甲(Colaphellus bowringi)的Cb-hsp70同源性最高。膜翅目、双翅目、鳞翅目、鞘翅目也都能各自聚类。该聚类结果能将昆虫归类到各自传统的分类地位中,说明HSP70可以作为系统进化分析的参照基因。
2.4 温度胁迫下Ld-hsp70的表达
不同的温度和时间处理均能诱导马铃薯甲虫雌、雄成虫Ld-hsp70一定程度的表达。不同温度胁迫1 h后,马铃薯甲虫雌、雄成虫体内3个Ld-hsp70的相对表达量虽有一定程度的升高或降低,但与对照组(25℃)相比均无显著差异;不同温度胁迫处理4 h后,雌、雄成虫Ld-hsp70a的相对表达量均有提高,其Ld-hsp70a的相对表达量与对照相比分别在低温-10℃和高温44℃时显著上调至2.24和2.41倍(图5-A、5-B)。图5
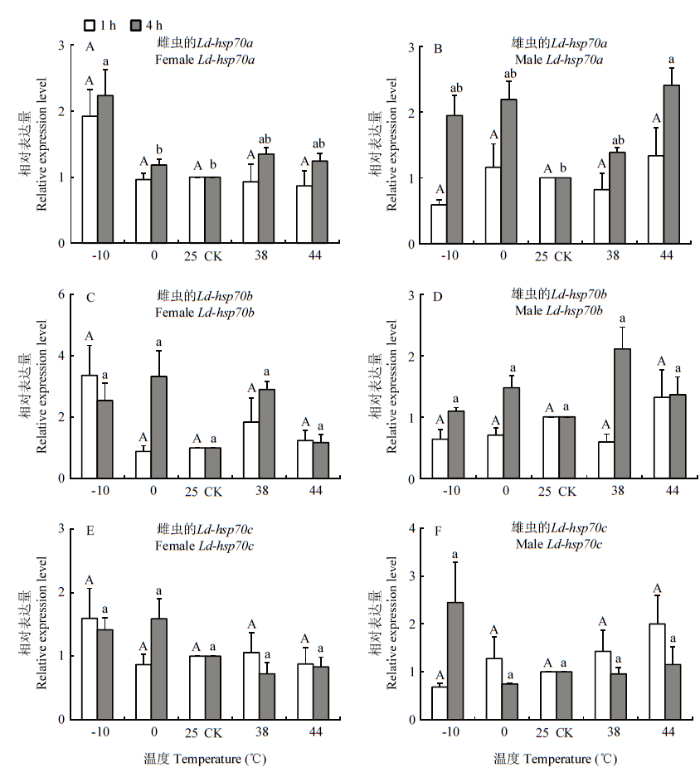 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5不同温度和不同时间处理后马铃薯甲虫雌(A、C、E)和雄(B、D、F)成虫中Ld-hsp70的相对表达量
图中数据为平均值±标准误;柱上不同大、小写字母分别表示处理1 h和4 h时不同温度处理间差异显著(P<0.05)(LSD法)
Fig. 5Relative expression levels of Ld-hsp70s in female (A, C, E) and male (B, D, F) adults of L. decemlineata after exposure to different temperatures at different durations
Data in the figure are mean±SE and different letters above the bars indicate significant difference among different treatment temperatures for 1 h and 4 h, respectively (P<0.05) (LSD method)
Ld-hsp70b和Ld-hsp70c在高低温胁迫处理4 h后,相对表达量与对照相比均无显著差异(图5-C—F);另外,无论是雌虫还是雄虫,3个Ld-hsp70的相对表达量在同一温度不同的胁迫处理时间下均无显著差异。
3 讨论
3.1 Ld-hsp70序列分析
本研究成功克隆了3个马铃薯甲虫Ld-hsp70的全长序列,并且它们编码的氨基酸序列均包含了完整保守结构域和3段保守的HSP70家族的典型基序,表明这3个Ld-hsp70属于典型的HSP家族。这些基因在氨基酸C末端均具有保守的亚细胞定位基序,Ld-hsp70b的C末端具有内质网保守基序KDEL,Ld-hsp70a和Ld-hsp70c的C末端具有细胞质保守基序EEVD,表明Ld-hsp70b蛋白存在于内质网中,而Ld-hsp70a和Ld-hsp70c蛋白存在于细胞质中[31,32],这种相似的保守基序也存在于HSP90家族中(细胞质定位基序MEEVD;内质网定位基序KDEL或HDEL)[33]。马铃薯甲虫HSP70家族的3个成员位于不同的细胞器中,在其他昆虫中也有相同的发现,如珍珠边豹纹蝶(Melitaea cinxia)有7个HSP70基因,1个位于内质网,6个位于细胞质[34];褐飞虱HSP70的15个家族成员,2个位于内质网,3个位于线粒体,其余位于细胞质或细胞核[28]。序列比对发现,Ld-HSP70a与Ld-HSP70c的氨基酸序列相似性为71.85%,而Ld-HSP70b与Ld-HSP70a、Ld-HSP70c的序列相似性分别只有59.26%和56.50%,表明在相同物种中,同一细胞区室内的HSP70之间相似度更高。根据HSP70在靠近C端是否具有简并重复的四肽序列GGXP,可将HSP70分为诱导型和组成型[35,36]。本研究中Ld-hsp70a的编码蛋白有两个短肽GGXP序列出现在C端,可以推断Ld-hsp70a属于诱导型HSP70,该类型只有受到外界因素诱导后才会大量增加,且这些重复序列可提供带负电且柔和的疏水性界面,促进蛋白合成中间体的排列折叠[37];而其他两个Ld-hsp70在C端无GGXP序列,属于组成型HSP70,一般在无外界诱导时便有一定量的表达[38]。
系统进化树分析显示,3个Ld-hsp70分别聚类到3个分支上,表明其可能在马铃薯甲虫抵御不良的环境胁迫中发挥的作用不同。研究表明,HSP70基因可以作为系统进化研究的标志基因[39]。在马铃薯甲虫的HSP70系统进化树中,不同昆虫HSP70按照不同目单独聚类,亲缘关系较近的昆虫在系统进化树上HSP70位置较近,该结果与烟蚜(Myzus persicae)[40]、三叶斑潜蝇[27]等昆虫的HSP70聚类结果一致。这一研究结果也与马铃薯甲虫HSP90进化分析结果一致,均可作为生物系统进化分析的候选基因[29]。
马铃薯甲虫HSP70蛋白的三维结构分析表明,3个HSP70蛋白N端均包含ATPase功能域,对ATP具有极高的亲和能力,ATPase功能域参与热激蛋白构象的转换、形成HSP70的分子伴侣复合物,并且在底物蛋白折叠的过程中至关重要[18,41]。马铃薯甲虫HSP70蛋白的C端是多肽结合区,研究发现其可以通过蛋白结构上的一段铰链区连接到N端核酸部位,引导多肽的折叠,起到分子伴侣作用[42]。与马铃薯甲虫热激蛋白60、90相比,这3类蛋白在结构上存在较大差异,如HSP90具有3个结构域:LM结构域(large middle domain)、SM结构域(small middle domain)和C结构域(C-terminal domain),LM结构域包含与分子伴侣、ATP和有害化学物质结合的位点,SM结构域主要参与分子伴侣和下游蛋白结合,C结构域参与二聚化,且含有与有害化学物质结合的位点[29];HSP60也具有3个结构域:顶端域、赤道域和中间域,顶端域是底物结合部位,赤道域是ATP结合部位,中间域连接顶端域和赤道域[30]。这些高度保守的三维结构与其功能密不可分,表明马铃薯甲虫的这3类热激蛋白在其应激刺激中可能发挥不同的作用。
3.2 Ld-hsp70在温度胁迫下的功能分析
HSP70是与生物体温度耐受性关系最为密切的一类蛋白质,许多报道已证实高温和低温均能诱导生物体内hsp70的表达[22-23,26-27,43]。本研究同样发现马铃薯甲虫的3个Ld-hsp70在不同温度和时间胁迫下均能诱导表达,但是3个Ld-hsp70具有不同的诱导表达特异性,这一结果与三叶斑潜蝇的3个hsp70表达结果相似[26]。Ld-hsp70a在马铃薯甲虫雌、雄成虫中的最佳诱导温度不同。在雌虫中,低温-10℃胁迫处理4 h后,Ld-hsp70a的相对表达量升至对照的2.24倍,表明在低温胁迫后马铃薯甲虫通过提高体内Ld-hsp70a的表达量,来帮助新生肽链的正确折叠,提高马铃薯甲虫对低温胁迫的适应力。而雄虫在高温44℃胁迫处理4 h后相对表达量显著上升,说明雌、雄两性成虫Ld-hsp70a可能对温度的敏感性存在差异。此种现象在其他昆虫中也有类似发现,如CHEN等[22]发现梨小食心虫在极端温度胁迫下雌虫HSP70基因的表达相比雄虫更敏感。杨苑钊[44]发现西藏飞蝗(Locusta migratoria tibetensis)雄虫应对环境胁迫时HSP70基因诱导表达更为敏感。Ld-hsp70a的相对表达量随着温度升高和胁迫时间的延长而增加,表明该基因属于诱导型蛋白,序列分析结果也证实了这一点。因此,笔者推测Ld-hsp70a在马铃薯甲虫热耐受性过程中具有重要作用。本研究发现Ld-hsp70b和Ld-hsp70c在不同温度和不同时间胁迫下相对表达量与对照相比无显著差异,推测这两个Ld-hsp70的作用并不能提高马铃薯甲虫的温度胁迫耐受性,而是受温度以外的其他因子调控。有研究发现,HSP70蛋白在机体细胞内的合成有一定的阈值,一旦超过这个阈值,HSP70蛋白的合成将出现下降[45,46]。因此,Ld-hsp70b和Ld-hsp70c的相对表达量出现下降现象,也可能是长时间温度胁迫损伤了马铃薯甲虫的部分机体,造成的损伤超出了Ld-hsp70对机体的保护能力,从而导致马铃薯甲虫热激蛋白表达途径的关闭。在三叶斑潜蝇[45]、美洲斑潜蝇、南美斑潜蝇[21]、褐飞虱[46]等昆虫中均有类似的报道。另外,本研究还发现马铃薯甲虫成虫体内的3个Ld-hsp70相对表达量在同一温度、不同胁迫处理时间下均无显著差异,表明Ld-hsp70在本试验设定的同一温度下的相对表达量与处理时间之前没有显现出相关性。
同一物种不同组织间HSP70的同源性低于不同物种相同组织[28]。位于内质网和线粒体的HSP70与一般HSP70的亲缘关系较远,遗传距离大于不同物种间相同组织来源的HSP70。且位于不同组织的HSP70在减少昆虫所受胁迫伤害中具有一定的协同作用,如位于内质网和细胞质的HSP70可通过协同作用提高昆虫机体对高温的适应性[47]。本研究对3个Ld-hsp70的氨基酸序列分析表明,Ld-hsp70b属于内质网型热激蛋白,Ld-hsp70a和Ld-hsp70c属于胞质型热激蛋白,Ld-hsp70a表达量变化相对显著,而Ld-hsp70b和Ld-hsp70c却与对照相比无显著差异,3个Ld-hsp70的表达情况也进一步验证了这一观点。在应对温度胁迫时,马铃薯甲虫体内并非某一热激蛋白起作用,而是由不同热激蛋白共同协作抵抗逆境胁迫。而马铃薯甲虫体内这些不同Ld-hsp70是如何协作来抵御环境胁迫还需要进一步探讨。
4 结论
马铃薯甲虫Ld-hsp70a在雌虫遭受低温胁迫时显著升高,在雄虫遭受高温胁迫时显著升高,其对高、低温均敏感,可能在马铃薯甲虫抵御极端温度胁迫中发挥作用。而Ld-hsp70b和Ld-hsp70c对高、低温均不敏感。推测马铃薯甲虫同一家族的3个Ld-hsp70在相同刺激下可能具有不同功能。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 3]
URLPMID:29365170 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 1]
URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 1]
DOI:10.1093/jisesa/ieu015URLPMID:25347841 [本文引用: 1]

A chironomid midge, Cricotopus lebetis (Sublette) (Diptera: Chironomidae), was discovered attacking the apical meristems of Hydrilla verticillata (L.f. Royle) in Crystal River, Citrus Co., Florida in 1992. The larvae mine the stems of H. verticillata and cause basal branching and stunting of the plant. Temperature-dependent development, cold tolerance, and the potential distribution of the midge were investigated. The results of the temperature-dependent development study showed that optimal temperatures for larval development were between 20 and 30 degrees C, and these data were used to construct a map of the potential number of generations per year of C. lebetis in Florida. Data from the cold tolerance study, in conjunction with historical weather data, were used to generate a predicted distribution of C. lebetis in the United States. A distribution was also predicted using an ecological niche modeling approach by characterizing the climate at locations where C. lebetis is known to occur and then finding other locations with similar climate. The distributions predicted using the two modeling approaches were not significantly different and suggested that much of the southeastern United States was climatically suitable for C. lebetis.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 1]
URL [本文引用: 1]
URL [本文引用: 1]
URL [本文引用: 1]
[本文引用: 1]
URLPMID:8451637 [本文引用: 1]
URL [本文引用: 1]
URL [本文引用: 1]
DOI:10.1016/0092-8674(84)90345-3URLPMID:6421488 [本文引用: 1]

The intracellular distribution of the major Drosophila heat-shock protein hsp70 was determined by indirect immunofluorescence with monoclonal antibodies. During heat shock the protein concentrates strongly in nuclei while a small quantity remains cytoplasmic. During recovery hsp70 leaves the nuclei and becomes distributed throughout the cytoplasm. With a second heat shock it is rapidly transported back into the nucleus. Nuclear translocation depends not on the temperature per se, but on the physiological state of the cell since it also occurs after exposure to an anoxic atmosphere at normal temperatures. We also provide evidence that hsps protect cells from the toxic effects of anoxia, as well as heat, and conclude that nuclear translocation of hsp70 is related to its function in protecting the organism from both forms of environmental stress.
DOI:10.1016/s0163-7258(98)00028-xURLPMID:9839771 [本文引用: 1]

Heat shock proteins (HSPs) are detected in all cells, prokaryotic and eukaryotic. In vivo and in vitro studies have shown that various stressors transiently increase production of HSPs as protection against harmful insults. Increased levels of HSPs occur after environmental stresses, infection, normal physiological processes, and gene transfer. Although the mechanisms by which HSPs protect cells are not clearly understood, their expression can be modulated by cell signal transducers, such as changes in intracellular pH, cyclic AMP, Ca2+, Na+, inositol trisphosphate, protein kinase C, and protein phosphatases. Most of the HSPs interact with other proteins in cells and alter their function. These and other protein-protein interactions may mediate the little understood effects of HSPs on various cell functions. In this review, we focus on the structure of the HSP-70 family (HSP-70s), regulation of HSP-70 gene expression, their cytoprotective effects, and the possibility of regulating HSP-70 expression through modulation of signal transduction pathways. The clinical importance and therapeutic potential of HSPs are discussed.
DOI:10.1007/s00018-004-4464-6URLPMID:15770419 [本文引用: 2]

Hsp70 proteins are central components of the cellular network of molecular chaperones and folding catalysts. They assist a large variety of protein folding processes in the cell by transient association of their substrate binding domain with short hydrophobic peptide segments within their substrate proteins. The substrate binding and release cycle is driven by the switching of Hsp70 between the low-affinity ATP bound state and the high-affinity ADP bound state. Thus, ATP binding and hydrolysis are essential in vitro and in vivo for the chaperone activity of Hsp70 proteins. This ATPase cycle is controlled by co-chaperones of the family of J-domain proteins, which target Hsp70s to their substrates, and by nucleotide exchange factors, which determine the lifetime of the Hsp70-substrate complex. Additional co-chaperones fine-tune this chaperone cycle. For specific tasks the Hsp70 cycle is coupled to the action of other chaperones, such as Hsp90 and Hsp100.
[本文引用: 1]
URLPMID:21717055 [本文引用: 1]
DOI:10.1111/j.1365-2583.2007.00744.xURLPMID:17651238 [本文引用: 2]

Studies have demonstrated differences in temperature tolerance between two Liriomyza species, L. huidobrensis and L. sativae. To investigate whether the heat shock proteins (Hsps) in the two species have different expression profiles during temperature stress, we cloned hsp90, 70, 60, 40 and 20, and analysed their expression profiles across temperature gradients by real-time quantitative PCR and Western blotting. The results revealed that the number of TATA-box-like elements and A/T-rich insertion/deletions within the 5' UTRs of the hsps are different in the two species. The temperatures for onset (T(on)) or maximal (T(max)) induction of hsp expression in L. huidobrensis were generally 2.5-10 degrees C lower than those in L. sativae, and the T(on) were highly consistent with the temperature limits of the northern boundary of the range of these two leafminer species. These studies confirmed, in terms of gene expression levels, that L. huidobrensis is more cold tolerant than L. sativae, which is more heat tolerant, and suggest that the T(on) (or T(max)) of hsps can represent the differences in temperature tolerance of these two leafminer species, and may be used to determine their natural geographical distribution limits.
URLPMID:24006328 [本文引用: 3]
DOI:10.3389/fphys.2019.01593URLPMID:31992993 [本文引用: 2]

Thermal adaptation plays a fundamental role in the expansion and distribution of insects, and heat shock proteins (Hsps) play important roles in the temperature adaptation of various organisms. To determine the roles of Hsp genes (Hsp70, Hsp21, and sHsp21) on the high temperature tolerance of Agasicles hygrophila, we obtained complete cDNA (complementary DNA) sequences for Hsp70, Hsp21, and sHsp21 by rapid amplification of cDNA ends (RACE), analyzed their expression profiles under different high temperature treatments by quantitative reverse transcription polymerase chain reaction (RT-qPCR), and performed functional verification by RNA interference (RNAi). The open reading frames of Hsp70, Hsp21, and sHsp21 were 1940, 543, and 567 bp, encoding 650, 180, and 188 amino acids, respectively. Their molecular weights (MWs) were 71.757, 20.879, and 21.510 kDa, and the isoelectric points were 5.63, 6.45, and 6.24, respectively. Phylogenetic tree analysis showed that the Hsp70, Hsp21, and sHsp21 genes of A. hygrophila were relatively conserved in evolution. The Hsp70 and Hsp21 genes in A. hygrophila were homologous to those in Leptinotarsa decemlineata (87 and 79% similarity, respectively), and the sHsp21 gene in A. hygrophila was homologous to that in Lissorhoptrus oryzophilus (74% similarity). The amino acid polypeptide chain had highly conserved sequences of DLGGGTFD, VLVGGSTR, and GPTIEEVD. The sequence of EEVD was the characteristic motif of cytoplasmic Hsp70, and the highly conserved sequences of MALFR and MSLLP were characteristic sequences of Hsp2 and sHsp21, respectively. Relative quantitative real time PCR showed that the three Hsps could be induced by 4-h treatment at high temperatures. Significant upregulation of these Hsps was observed when the temperature was further increased. The RNAi results showed that the injection of the three Hsps' dsRNA could suppress the expression at the gene level significantly. Compared with the control group, high temperature heat shock reduced the fecundity of A. hygrophila significantly, and the fecundity decreased with the increase in temperature. Our results suggest that Hsp70, Hsp21, and sHsp21 might play key roles in high temperature adaptation of A. hygrophila and help improve our understanding of their mechanism of thermotolerance.
[本文引用: 1]
DOI:10.1016/j.jinsphys.2010.08.008URLPMID:20713057 [本文引用: 1]

In this study, we investigated the physiological mechanisms underlying temperature tolerance using Drosophila melanogaster lines with rapid, intermediate, or slow recovery from heat or chill coma that were established by artificial selection or by free recombination without selection. Specifically, we focused on the relationships among their recovery from heat or chill coma, survival after severe heat or cold, and survival enhanced by rapid cold hardening (RCH) or heat hardening. The recovery time from heat coma was not related to the survival rate after severe heat. The line with rapid recovery from chill coma showed a higher survival rate after severe cold exposure, and therefore the same mechanisms are likely to underlie these phenotypes. The recovery time from chill coma and survival rate after severe cold were unrelated to RCH-enhanced survival. We also examined the expression of two genes, Heat-shock protein 70 (Hsp70) and Frost, in these lines to understand the contribution of these stress-inducible genes to intraspecific variation in recovery from temperature coma. The line showing rapid recovery from heat coma did not exhibit higher expression of Hsp70 and Frost. In addition, Hsp70 and Frost transcription levels were not correlated with the recovery time from chill coma. Thus, Hsp70 and Frost transcriptional regulation was not involved in the intraspecific variation in recovery from temperature coma.
DOI:10.1016/j.pestbp.2017.01.011URLPMID:29107232 [本文引用: 3]

The brown planthopper, Nilaparvata lugens, possesses a strong adaptability to extreme temperature and insecticide stresses. Heat shock proteins (Hsps) are highly conserved molecular chaperones and play a pivotal role in response to various environmental stresses in insects. However, little is known about the response of Hsps to stresses in N. lugens. In the present study, an inducible Hsp70 (NlHsp70) was isolated from this insect and transcriptional expression patterns of NlHsp70 under temperature and insecticide stresses were analyzed. The full-length of NlHsp70 was 2805bp with an open reading frame (ORF) of 1896bp, showing high homology to its counterparts in other species. Expression of NlHsp70 was not altered by heat shock for 1h, nor following recovery from thermal stress. Conversely, decreased expression of NlHsp70 was observed in response to cold shock. In addition, the expression of NlHsp70 increased after imidacloprid exposure. RNA interference experiment combined with insecticide injury assay also demonstrated that NlHsp70 was essential for resistance against insecticide exposure. These observations indicated that NlHsp70 was an important gene involved in the resistance or tolerance to environmental stresses in N. lugens. Interestingly, weak changes in mRNA expression levels of two thermal-inducible Hsp genes, NlHsp90 and NlHsc70 were observed in imidacloprid-exposed N. lugens adults, suggesting that different Hsps may respond differential to the extreme temperature and insecticide stresses.
DOI:10.1017/S0007485318000354URLPMID:29743123 [本文引用: 3]

Heat shock proteins (HSPs) participate in diverse physiological processes in insects, and HSP70 is one of the most highly conserved proteins in the HSP family. In this study, full-length cDNAs of three HSP70 genes (Lthsc70, Lthsp701, and Lthsp702) were cloned and characterized from Liriomyza trifolii, an important invasive pest of vegetable crops and horticultural crops worldwide. These three HSP70s exhibited signature sequences and motifs that are typical of the HSP70 family. The expression patterns of the three Lthsp70s during temperature stress and in different insect development stages were studied by real-time quantitative PCR. Lthsp701 was strongly induced by high- and low-temperature stress, but Lthsc70 and Lthsp702 were not very sensitive to temperature changes. All three Lthsp70s were expressed during insect development stages, but the expression patterns were quite different. The expression of Lthsc70 and Lthsp702 showed significant differences in expression during leafminer development; Lthsc70 was most highly expressed in female adults, whereas Lthsp702 was abundantly expressed in larvae and prepupae. Lthsp701 expression was not significantly different among leafminer stages. These results suggest that functional differentiation within the LtHSP70 subfamily has occurred in response to thermal stress and insect development.
URL [本文引用: 4]
[本文引用: 4]
URL [本文引用: 3]
URL [本文引用: 3]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
URLPMID:8151709 [本文引用: 1]
DOI:10.1093/oxfordjournals.molbev.a040281URLPMID:8524040 [本文引用: 1]

The heat shock protein (Hsp) sequences, because of their ubiquity and high degree of conservation, provide useful models for phylogenetic analysis. In this paper I have carried out a global alignment of all available sequences (a total of 31) for the 90-kD heat shock protein (Hsp90) family. The minimum amino acid identity that is seen between presently known Hsp90 homologs is about 40% over the entire length, indicating that it is a highly conserved protein. Based on the alignment, a number of signature sequences that either are distinctive of the Hsp90 family or that distinguish between the cytosolic and the endoplasmic reticular forms of Hsp90 have been identified. Detailed phylogenetic analyses based on Hsp90 sequences reported here strongly indicate that the cytosolic and the endoplasmic reticulum (ER) resident forms of Hsp90 constitute paralogous gene families which arose by a gene duplication event that took place very early in the evolution of eukaryotic cells. A minimum of two additional gene duplication events, which took place at a later time, are required to explain the presence of two different forms of Hsp90 that are found in fungi and vertebrate species. In a consensus neighbor-joining bootstrap tree based on Hsp90 sequences, plants and animals species grouped together 989 times of 1,000 (a highly significant score), indicating a closer relationship between them as compared to fungi. A closer affiliation of plant and animal species was also observed in the maximum-parsimony tree, although the relationship was not significantly supported by this method. A survey of the recent literature on this subject indicates that depending on the protein sequence and the methods of phylogenetic analysis, the animal species are indicated as closer relatives to either plants or fungi with significant statistical support for both topologies. Thus the relationship among the animal, plant, and fungi kingdoms remains an unresolved issue at the present time.
DOI:10.1016/j.gene.2014.11.043URLPMID:25433328 [本文引用: 1]

Temperature variation in the environment is a great challenge to organisms. Induction of heat shock protein 70 (HSP70) is a common genetic mechanism to cope with thermal stress. The Glanville fritillary butterfly (Melitaea cinxia) is a model species in population and evolutionary biology, and its behavior and life history are greatly influenced by ambient temperature. We cloned and sequenced the full coding sequences of seven hsp70 genes from the Glanville fritillary. Of those genes, McHsc70-1 and McHsc70-2 were identified as heat shock cognate 70 (hsc70), of which the latter located in endoplasmic reticulum. We analyzed the expression patterns of different hsp70s under various thermal stresses using quantitative PCR. Heat shock at 40 degrees C for 2h induced high expression of McHsp70-1, McHsp70-2 and McHsc70-2. Only McHsc70-2 had a small increase after cold shock at 0 degrees C for 2h. Acclimation at 35 degrees C for three days before heat shock reduced expression of McHsp70 after heat shock. The maximum mRNA level of McHsp70s was reached in the first 2h after the heat shock. This study uncovers the complexity of the hsp70 system, and provides the valuable information for further temperature-related research in the Glanville fritillary butterfly.
URLPMID:8310296 [本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.cell.2006.04.027URLPMID:16751100 [本文引用: 1]

GroEL and GroES form a chaperonin nano-cage for proteins up to approximately 60 kDa to fold in isolation. Here we explored the structural features of the chaperonin cage critical for rapid folding of encapsulated substrates. Modulating the volume of the GroEL central cavity affected folding speed in accordance with confinement theory. Small proteins (approximately 30 kDa) folded more rapidly as the size of the cage was gradually reduced to a point where restriction in space slowed folding dramatically. For larger proteins (approximately 40-50 kDa), either expanding or reducing cage volume decelerated folding. Additionally, interactions with the C-terminal, mildly hydrophobic Gly-Gly-Met repeat sequences of GroEL protruding into the cavity, and repulsion effects from the negatively charged cavity wall were required for rapid folding of some proteins. We suggest that by combining these features, the chaperonin cage provides a physical environment optimized to catalyze the structural annealing of proteins with kinetically complex folding pathways.
URLPMID:2427013 [本文引用: 1]
DOI:10.1016/j.jtherbio.2016.03.005URLPMID:27033046 [本文引用: 1]

The western flower thrips, Frankliniella occidentalis, is an important invasive pest with a strong tolerance for extreme temperatures; however, the molecular mechanisms that regulate thermotolerance in this insect remain unclear. In this study, four heat shock protein genes were cloned from F. occidentalis and named Fohsp90, Fohsc701, Fohsc702 and Fohsp60. These four Hsps exhibited typical characteristics of heat shock proteins. Subcellular localization signals and phylogenetic analysis indicated that FoHsp90 and FoHsc701 localize to the cytosol, whereas FoHsc702 and FoHsp60 were located in the endoplasmic reticulum and mitochondria, respectively. Analysis of genomic sequences revealed the presence of introns in the four genes (three, four, seven, and five introns for Fohsp90, Fohsc701, Fohsc702 and Fohsp60, respectively). Both the number and position of introns in these four genes were quite different from analogous genes in other species. qRT-PCR indicated that the four Fohsps were detected in second-stage larvae, one-day-old pupae, and one-day-old adults, and mRNA expression levels were lowest in larvae and highest in pupae. Fohsc701 and Fohsc702 possessed similar expression patterns and were not induced by cold or heat stress. Expression of Fohsp60 was significantly elevated by heat, and Fohsp90 was rapidly up-regulated after exposure to both cold and heat stress. Exposure to -8 degrees C had no effect on expression of the four Fohsps; however, expression of Fohsp90 and Fohsp60 was highest after a 2-h incubation at 39 degrees C. Furthermore, cold and heat hardening led to significant up-regulation of the four Fohsps compared to their respective controls. Collectively, our results indicate that the four FoHsps contribute to insect development and also function in rapid cold or heat hardening; furthermore, FoHsp90 and FoHsp60 contribute to thermotolerance in F. occidentalis.
[本文引用: 1]
[本文引用: 1]
URLPMID:16678092 [本文引用: 1]
URLPMID:8658133 [本文引用: 1]
DOI:10.1371/journal.pone.0031446URLPMID:22319631 [本文引用: 1]

Individuals of widely spread species are expected to show local adaption in temperature tolerance as they encounter a range of thermal conditions. We tracked thermal adaptations of the Colorado potato beetle (Leptinotarsa decemlineata) that invaded Europe within the last 100 years. It has occupied various conditions although, like the majority of invasive species, it lost a measurable amount of neutral genetic variation due to bottleneck effect when it invaded Europe. We exposed diapausing beetles originated from three different latitudes (54 degrees N, 59 degrees N, 60 degrees N) to cold shock (-5 degrees C, 1.5 hrs) in order to test if beetles from the northern populations express differential levels of cold-induced and constitutive Hsp70 compared to the beetles from milder temperature regime. The level of cold-induced Hsp70 was lowest in the northernmost beetle populations while the level of constitutive Hsp70 did not differ with the population. Moreover, the southernmost beetles were more plastic in their response to cold shock than the northernmost beetles. These results suggest that physiological adaptation, like the synthesis of Hsp70, can evolve very quickly.
[D].
[本文引用: 1]
[D].
[本文引用: 1]
URL [本文引用: 2]
URL [本文引用: 2]
[本文引用: 2]
[本文引用: 2]
URLPMID:21876766 [本文引用: 1]
