 ,1, 董育红1, 姜菊玲1, 钱建宁1, 魏文涛1, 宋国亮2, 焦金波2, 关新新2, 姬郭彪2, 张业炘1
,1, 董育红1, 姜菊玲1, 钱建宁1, 魏文涛1, 宋国亮2, 焦金波2, 关新新2, 姬郭彪2, 张业炘1Based on PK15 Cell Line for PCV2 Fully Suspension Culture Process
WANG JiaQi ,1, DONG YuHong1, JIANG JuLing1, QIAN JianNing1, WEI WenTao1, SONG GuoLiang2, JIAO JinBo2, GUAN XinXin2, JI GuoBiao2, ZHANG YeXin1
,1, DONG YuHong1, JIANG JuLing1, QIAN JianNing1, WEI WenTao1, SONG GuoLiang2, JIAO JinBo2, GUAN XinXin2, JI GuoBiao2, ZHANG YeXin1责任编辑: 林鉴非
收稿日期:2020-03-20接受日期:2020-06-30网络出版日期:2021-03-16
| 基金资助: |
Received:2020-03-20Accepted:2020-06-30Online:2021-03-16
作者简介 About authors
王嘉琪,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (1051KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
王嘉琪, 董育红, 姜菊玲, 钱建宁, 魏文涛, 宋国亮, 焦金波, 关新新, 姬郭彪, 张业炘. 基于PK15细胞的猪圆环病毒2型全悬浮培养工艺[J]. 中国农业科学, 2021, 54(6): 1280-1287 doi:10.3864/j.issn.0578-1752.2021.06.017
WANG JiaQi, DONG YuHong, JIANG JuLing, QIAN JianNing, WEI WenTao, SONG GuoLiang, JIAO JinBo, GUAN XinXin, JI GuoBiao, ZHANG YeXin.
开放科学(资源服务)标识码(OSID):

0 引言
【研究意义】猪圆环病毒病(porcine circovirus diseases, PCVDs)是影响养猪业的重要传染病,其病原为猪圆环2型(porcine circovirus type 2, PCV2)。PCV2可引起断奶仔猪多系统衰竭综合症,还与皮炎肾病综合症、增生性坏死性肺炎、猪繁殖与呼吸综合征、仔猪传染性先天性震颤等多种疾病相关,但临床上以多系统衰竭综合症最为常见[1,2,3,4,5,6],是严重危害养猪业健康发展的疫情之一[7,8]。猪肾细胞(PK15)是目前用于PCV2病毒培养的主要细胞系,用于增殖PCV2和制备猪疫苗。但由于PCV2病毒的编码区非常有限,对宿主细胞具有高度的依赖性[9],且在PK15细胞系不同的细胞株上增殖的病毒含量不同, 难以达到制备疫苗的要求[10-12] ,是制约PCV2全病毒灭活苗的技术瓶颈之一。同时PK15细胞贴壁培养时存在需要血清、放大困难和成本高等问题,需筛选出一株对PCV2敏感,且易于放大和产业化生产的病毒培养工艺。【前人研究进展】PK15细胞现已广泛应用于猪圆环病毒,但由于PCV2毒力弱,且不产生细胞病变,获得高病毒含量PCV2难度较大,加之PK15细胞贴壁培养时存在血清,而且放大困难,成本高等问题,因此PCV2的培养病毒含量高低已成为制约现有疫苗质量的关键瓶颈之一[13,14]。刘俊斌等[15,16,17,18]报道了使用片状载体和微载体在生物反应器中培养PK15贴壁细胞并感染PCV2的工艺研究,通过优化微载体使用浓度、细胞接种密度、细胞初始搅拌方式、生长阶段搅拌速度、培养基补给方式、接毒剂量和收获时间等关键技术参数,提高PCV2产量,确定了反应器生产PCV2的最佳工艺;彭伍平等[19,20,21]报道了通过筛选贴壁单克隆,找到一株对PCV2敏感的克隆株,从而提高了PCV2病毒含量;刘天伦等 [22,23]报道了经贴壁降血清、低血清悬浮优化传代、无血清悬浮传代培养,对一株贴壁PK15细胞进行了无血清全悬浮驯化,并可稳定传代,以期为传代细胞系低血清驯化培养及圆环病毒在低血清培养细胞上的增殖研究奠定基础;李雨慈[24]报道使用昆虫细胞基因表达技术,首次使用悬浮培养生产的猪圆环病毒病疫苗;郭玲花等[25,26,27,28]报道了猪圆环病毒2型悬浮培养工艺的研究进展,对目前国内外市场上PCV2疫苗的制备工艺及其优缺点进行对比分析,可利用全悬浮培养工艺培养PK-15细胞来增殖PCV2;此外,未见全悬浮培养工艺中以悬浮细胞制备种毒的研究报道。【本研究切入点】筛选出1株PCV2高产克隆细胞株,并建立起无血清全悬浮病毒培养工艺。【拟解决的关键问题】通过全悬浮细胞培养技术,制备出病毒含量高和适合猪圆环病毒病灭活疫苗规模化生产工艺。1 材料与方法
1.1 材料
1.1.1 试验时间和地点 试验于2018年12月至2019年12月在甘肃健顺生物科技有限公司实验室进行。1.1.2 细胞株 试验所用细胞株为PK15(猪圆环病毒1型及2型阴性)[PK15, PK-15]ATCC? CCL-33?,甘肃健顺生物科技有限公司保存。
1.1.3 病毒株 PCV2(猪圆环2型病毒分离株,PK15贴壁获得P14病毒,病毒含量106.4TCID50/mL,支原体检验、BVDV、CSFV、致细胞病变检查及红细胞吸附性外源病毒检验均为阴性)由洛阳惠中生物技术有限公司提供。
1.1.4 主要试剂和耗材 DMEM高糖、CD PK15 259(PK15悬浮细胞生长培养基)、PK15接毒培养基、0.25%胰蛋白酶-EDTA、接毒培养基A、接毒培养基B(以上试剂均由甘肃健顺生物科技有限公司提供),FBS(兰州荣晔生物科技有限公司),PCV2一抗、羊抗鼠二抗(南京华恩生物科技有限公司),荧光显微镜(OLYMPUS),Vi-Cell细胞计数仪。
1.2 方法
1.2.1 贴壁PK15细胞克隆 采用有限稀释法,将细胞铺于96孔培养板,于37℃、5%CO2培养箱中培养,第4天于显微镜下初步挑选单克隆,每两天显微镜下观察,保证孔中细胞为单克隆团,将克隆细胞逐步放大培养。1.2.2 克隆细胞株悬浮驯化 待细胞扩大至T225瓶时,消化,计数,离心,采用PK15悬浮生长培养基CD PK15 259按1.0×106个细胞/mL密度重悬细胞,并于37℃、5%CO2摇床培养箱中培养,至细胞适应悬浮培养基可正常生长。
1.2.3 PCV2高产细胞筛选 待驯化悬浮的PK15-2F11、1E5、1C8细胞生长至第3天时,分别使用PK15接毒培养基A和PK15接毒培养基B将细胞稀释至1.0×106个细胞/mL,工作体积30mL分别接于125mL摇瓶中,按体积比5%加入PCV2病毒,37℃、5%CO2于摇床培养箱中培养,每天取样计数,72h后收毒,采用测定病毒含量。并将收获病毒液,对应细胞第二次感染各克隆细胞。
1.2.4 接毒工艺优化 接毒培养基A将培养至第3天的PK15-1C8细胞稀释至1.0×106个细胞/mL,按0.1、0.2、0.5MOI(PCV2来源于贴壁细胞毒)接毒,试验重复3次,或按0.1、0.2、0.5MOI(PCV2来源于悬浮细胞毒)接毒,试验重复5次,不同时间段收获病毒液,测定病毒含量(表1)。
Table 1
表1
表1接毒工艺优化试验参数
Table 1
| 试验 Experiment | 感染培养基 Infection medium | 感染时细胞密度 Infection cell density(cells/mL) | 感染量 MOI | 收获时间 Harvest time (h) | 重复次数 Repeat times | |
|---|---|---|---|---|---|---|
| 贴壁种毒 Virus from adherent cell | 感染MOI摸索 Infection MOI explore | 接毒培养基A Infection medium A | 1.0×106 | 0.1、0.2、0.5 | 72 | 3 |
| 收获时间摸索 Harvest time explore | 1.0×106 | 0.1 | 48、72、96、120 | 3 | ||
| 悬浮种毒 Virus from suspension cell | 感染MOI摸索 Infection MOI explore | 接毒培养基A Infection medium A | 1.0×106 | 0.1、0.2、0.5 | 72 | 5 |
| 收获时间摸索 Harvest time explore | 1.0×106 | 0.2 | 48、72、96 | 5 | ||
新窗口打开|下载CSV
1.2.5 病毒含量测定 用含2%血清的DMEM培养基将所取病毒液作10倍系列稀释,取10-4、10-5、10-6、10-7 4个稀释度,分别接种在单层PK15细胞的96孔细胞培养板中,每个稀释度接种6孔,100μL /孔,同时设正常细胞对照组,采用Reed-Müench法计算TCID50。
2 结果
2.1 PK15单克隆细胞驯化悬浮
将挑出来的4个单克隆贴壁细胞及PK15原细胞株驯化悬浮,成功驯化3株克隆和PK15细胞,细胞生长曲线如图1所示,PK15、PK15-2F11和1C8 3株细胞均1代便从贴壁细胞驯化为悬浮细胞,且细胞生长稳定,PK15-1E5前5代生长较慢,6代后细胞生长变快渐渐生长稳定。4株细胞生长稳定之后,3d生长情况相似,密度几乎都为10×106个细胞/mL,PK15、PK15-2F11、1E5、1C8细胞倍增时间为分别为21、21、20和21 h。图1
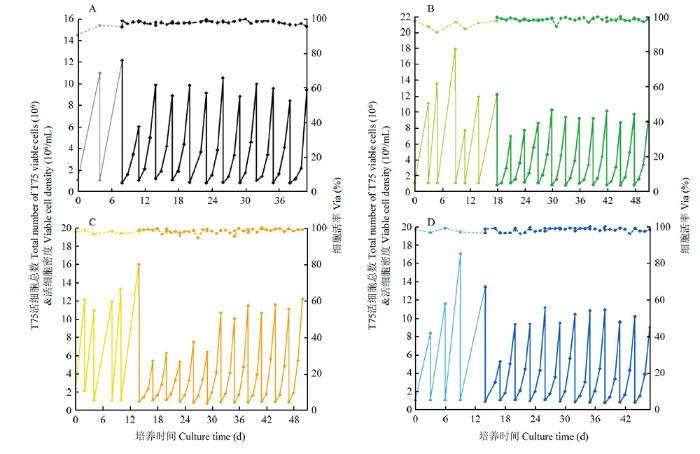 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图14株单克隆细胞驯化悬浮生长曲线
A.PK15细胞驯化生长曲线。B.PK15-2F11细胞驯化生长曲线。C.PK15-1E5细胞驯化生长曲线。D. PK15-1C8细胞驯化生长曲线。其中,实线为细胞生长密度曲线,虚线为细胞活率曲线。浅色线为3种单克隆和PK15贴壁细胞生长曲线,深色线条为3种单克隆和PK15细胞驯化悬浮细胞生长曲线。
Fig. 1Adaption suspension growth curves of four monoclonal cells
A. PK15 cells adapted growth curve. B. Acclimation growth curve of PK15-2F11 cells. C. Acclimation growth curve of PK15-1E5 cells. D. Acclimation growth curve of PK15-1C8 cells. Among them, the solid line is the cell growth density curve, and the dashed line is the cell viability curve. The light-colored line is the growth curve of 3 kinds of monoclonal and PK15 adherent cells, and the dark line is the growth curve of the acclimated suspension cells of 3 kinds of monoclonal and PK15 cells.
2.2 PCV2敏感细胞株筛选
将PK15细胞及PK15-2F11、PK15-1E5、PK15-1C8 4株细胞感染PCV2(种毒由悬浮细胞获得),TCID50结果如图2-A所示,3株克隆细胞毒价均高于原始细胞株,PK15-1C8试验组较其他2株克隆细胞毒价更高,病毒含量为106.0TCID50/mL,使用接毒培养基A接毒组(PK15-1C8-C1)与使用接毒培养基B接毒组(PK15-1C8-C2)病毒含量无差异。为了检测第一次试验结果的可靠性,将第一次所收获病毒液,对应细胞株进行第二次接毒(图2-B),3株克隆细胞感染PCV2后仍然是PK15-1C8更高,是2F11、1E5的10倍,病毒含量维持在106.0TCID50/mL。图2
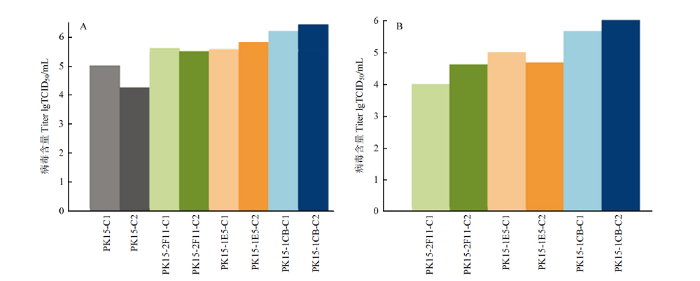 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2PK15细胞感染PCV2后TCID50
A.PK15、PK15-2F11、PK15-1E5、PK15-1C8四株细胞分别感染PCV2后TCID50。B.PK15-2F11、PK15-1E5、PK15-1C8三株细胞分别对应感染图A收获PCV2病毒。其中,C1为使用接毒培养基A接毒实验组,C2为使用接毒培养基B接毒实验组。
Fig. 2TCID50 after PCV2 infection in PK15 cells
A. TCID50 of PK15, PK15-2F11, PK15-1E5, PK15-1C8 cells infected with PCV2. B. PK15-2F11, PK15-1E5, and PK15-1C8 were infected with PCV2 virus harvest from Figure A. Among them, C1 is the experimental group infected with inoculation medium A, and C2 is the experimental group infected with inoculation medium B.
2.3 接毒工艺优化
2.3.1 贴壁细胞收获病毒为感染种毒工艺优化 如表1所示进行试验,不同MOI感染细胞重复3次试验后,3种不同MOI感染的细胞毒价差异不大均在106.5TCID50/mL左右(表2),因此选取病毒添加较少的0.1MOI接毒。Table 2
表2
表2不同MOI、不同时间的PCV2感染PK15-1C8病毒含量
Table 2
| 参数 Parameter | 条件 Condition | 实验中病毒含量(lgTCID50/mL) Virus content(lgTCID50/mL) | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| 感染病毒量 MOI | 0.1 | 6.5 | 6.6 | 6.0 |
| 0.2 | 6.7 | 6.4 | 6.2 | |
| 0.5 | 6.3 | 6.5 | 6.2 | |
| 取样时间/h Sample Time/h | 48 | 6.4 | 5.8 | 6.4 |
| 72 | 6.5 | 6.3 | 6.3 | |
| 96 | 6.7 | 6.4 | 5.6 | |
| 120 | 6.7 | 6.7 | 5.6 | |
新窗口打开|下载CSV
选取接毒计量(MOI)为0.1的感染条件,于培养48、72、96、120 h取样,检测病毒含量,综合3次试验结果72 h收获病毒含量lgTCID50/mL较高且更稳定。
2.3.2 悬浮细胞收获病毒为感染种毒来源相关工艺确认 接毒计量及收毒时间摸索, 如表3所示,分别按0.1、0.2、0.5MOI将由悬浮PK15细胞所获PCV2病毒感染PK15-1C8细胞,0.2MOI感染后,病毒含量更稳定。因此当种毒来源于悬浮细胞时,0.2MOI接毒更佳。
Table 3
表3
表3不同MOI、不同时间的PCV2感染PK15-1C8病毒含量
Table 3
| 参数 Parameter | 条件 Condition | 实验中病毒含量(lgTCID50/mL) Virus content(lgTCID50/mL) | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 感染病毒量 MOI | 0.1 | 6.5 | 6.1 | 6.2 | 6.0 | 6.0 |
| 0.2 | 7.0 | 6.7 | 6.5 | 6.3 | 6.3 | |
| 0.5 | 6.8 | 6.7 | 6.3 | 5.5 | 5.7 | |
| 取样时间 Sample time (h) | 48 | 6.5 | 6.2 | 6.4 | 6.3 | 6.0 |
| 72 | 7.2 | 7.3 | 6.9 | 6.5 | 6.3 | |
| 96 | 6.7 | 7.2 | 6.4 | 6.5 | 6.3 | |
新窗口打开|下载CSV
以0.2MOI的接毒量,相同试验方法接毒,检测 48、72、96 h病毒含量。48 h病毒含量低于72与96 h,但72与96 h病毒含量差异不大,从病毒含量和生产的时间效率考虑,以悬浮细胞制备的病毒为种毒,接毒量为0.2MOI 72h收获更佳。
3 讨论
PK15细胞作为PCV2主要感染细胞,广泛应用于PCV2疫苗的生产,但由于PCV2 DNA的合成依赖于细胞周期S期表达的酶,随宿主细胞的复制而复制,因此病毒的复制周期更长,使得PCV2体外增值能力较差[29,30,31]。彭伍平等[19,20,21]通过对PK15贴壁细胞进行有限稀释后,挑选出的单克隆细胞表现出了对PCV2的高敏感性,提示我们可以通过该方法提高病毒产量,但贴壁细胞生产放大采用的细胞工厂或转瓶工艺,劳动强度大,可使用的细胞密度低[18],而微载体技术虽然可以有效提高细胞密度,但仍存在生产成本高等弊端,且血清的使用对产品的可控性、安全性、批间差产生一定影响[22,23]。而悬浮培养细胞,可以在较小的空间内达到较高的细胞密度,同时无血清培养基的使用,使得细胞培养成本低、产品可控、重复性高。因此本文采用有限稀释法挑选出单克隆细胞,并将其驯化悬浮,筛选出对PCV2高敏感的PK15悬浮细胞,以期解决PCV2在生产中病毒含量低的问题。同时通过查阅文献发现,病毒感染时补料的添加、不同感染MOI及收获时间也是影响病毒产量的原因[16,32],因此在挑选出单克隆悬浮细胞的基础上,进一步进行了工艺优化,确定了最佳的病毒感染MOI及收获时间。刘天伦等[22]的研究,通过贴壁细胞逐渐降血清,到悬浮细胞低血清,再到悬浮细胞无血清的过程,经过了23代传代,将PK15贴壁细胞驯化为悬浮细胞,悬浮细胞72h最高生长密度为4.0×106/mL。而本文通过将贴壁PK15使用有限稀释法稀释获得单克隆细胞,并从贴壁细胞含10%血清直接驯化为悬浮无血清,驯化后第二代即可高密度生长,细胞生长稳定,以3d按1﹕10的比例进行扩大,生长密度可达到10× 106/mL,活率均在95%以上。相较刘天伦[22]等的研究,本文细胞驯化时间更短,且驯化悬浮后的细胞生长稳定倍增时间短,可大大缩短生产周期,为工业的放大生产提供有力支持;同时本文对悬浮细胞进行了单克隆筛选,筛选出的克隆细胞PCV2病毒含量较原克隆从105.0TCID50/mL提高至106.5TCID50/mL,可大大提高PCV2病毒疫苗的生产效率,但该悬浮克隆细胞PCV2病毒产量仍未达到理想结果,因此我们进一步进行了工艺优化。
福州大北农与成都天邦开发的DBN-SX07 株灭活疫苗,灭活前半成品病毒含量105.5TCID50/mL以上;武汉中博,科前生物等开发的WH株灭活疫苗,灭活前半成品病毒含量在107.0TCID50/mL以上;江苏南农高科等开发的SH株灭活疫苗,灭活前半成品病毒含量在106.0TCID50/mL以上; 哈维科等开发的LG株灭活疫苗,灭活前半成品病毒含量105.5TCID50/mL以上[33]。为了进一步提高该PCV2敏感克隆悬浮细胞的病毒产量,针对该细胞进行了接毒工艺的摸索,确定了不同种毒(来源于贴壁细胞或者悬浮细胞)来源接毒MOI及收获时间,结果发现,使用悬浮细胞种毒、按照0.2MOI接毒72h收获病毒含量从106.5TCID50/mL提高至107.3TCID50/mL;较传统贴壁工艺和现有的无血清培养基工艺[15-17,23]相比,收获时间缩短,接毒剂量低且首次接种悬浮细胞制备的种毒,病毒含量提高了10倍,可提高生产效率,缩短生产周期,同时整个生产工艺采用无血清培养,降低生产成本和纯化难度,提高产品质量和批间一致性。此工艺经过多次试验验证,确定了工艺的稳定性,为后期的PCV2病毒疫苗的研究和生产奠定了基础。
4 结论
本研究通过细胞克隆、悬浮驯化、高产细胞株筛选及PCV2生产工艺摸索,建立了全悬浮无血清培养的PK15-1C8细胞增殖PCV2工艺,为疫苗企业采用无血清悬浮培养工艺提供了试验依据。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1080/00480169.2007.36792URLPMID:18059655 [本文引用: 1]

CASE HISTORY: Investigations were conducted to determine the cause of an acute, multi-farm outbreak of porcine respiratory disease that included diarrhoea and subsequent loss of body condition in affected pigs. A definition for post-weaning multisystemic wasting syndrome (PMWS) including both clinical and pathological features, previously developed for the pig industry in New Zealand, was applied to the current outbreak. In addition to self-reporting by owners of affected farms, local veterinarians, disease and epidemiology consultants, and animal health officials from the Ministry of Agriculture and Forestry (MAF) were involved in conducting farm visits and submission of diagnostic specimens. CLINICAL FINDINGS AND DIAGNOSIS: Pathogens known to be endemic in the pig industry in New Zealand as well as likely exotic diseases were excluded as causative agents of the outbreak. Clinical signs including dyspnoea, diarrhoea, and rapid loss of body condition were consistent with the New Zealand case definition for PMWS. Interstitial pneumonia, pulmonary oedema, generalised lymph-node enlargement, and presence of porcine circovirus type 2 (PCV2) inclusion bodies were consistently identified in affected pigs. Classical swine fever virus (CSFv), Porcine reproductive and respiratory syndrome virus (PRRSv), and Influenza virus were ruled out, using molecular and traditional virological techniques. Spread of the disease between farms was hypothesised to be facilitated by locally migrating flocks of black-backed seagulls. The original source of the disease incursion was not identified. DIAGNOSIS: Based on the consistent presence of circovirus-associated lesions in lymphoid tissues in combination with generalised enlargement of lymph nodes, histiocytic interstitial pneumonia, clinical wasting, and poor response to antibiotic therapy, a diagnosis of PMWS was made. CLINICAL RELEVANCE: PMWS should be considered in the differential diagnoses of sudden onset of respiratory dyspnoea, diarrhoea, and rapid loss of body condition in young pigs in New Zealand pig herds.
URLPMID:17709139 [本文引用: 1]
URLPMID:17611048 [本文引用: 1]
URLPMID:9187808 [本文引用: 1]
DOI:10.1017/S1466252311000053URLPMID:21676340 [本文引用: 1]

Porcine circovirus type 2 (PCV2) causes great economic losses in growing pigs and there are several reviews on disease manifestations and lesions associated with PCV2 in growing pigs. Reproductive failure in breeding herds, predominately associated with increased numbers of mummies and non-viable piglets at parturition, is one of the disease manifestations of PCV2 infection. Boars shed low amounts of infectious PCV2 in semen for extended time periods, and vertical transmission of PCV2 to fetuses during PCV2 viremia of the dam has been experimentally confirmed. However, intrauterine-infected piglets often are clinically normal. Nevertheless, pigs infected with PCV2 by the intrauterine route can be born viremic, possibly contributing to horizontal spread of PCV2 within the breeding herd and into the nursery. Shedding of PCV2 in semen and prevalence of intrauterine-infected piglets can both be greatly reduced by PCV2 vaccination well ahead of expected PCV2 exposure. This review is a discussion on current knowledge on the effects of PCV2 infection in the dam and in in utero fetuses, including clinical signs, lesions, diagnosis and prevention through vaccination. Infection of boars with PCV2, the potential for PCV2 transmission via semen and prevention of PCV2 shedding are also discussed.
URLPMID:9429277 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:17889922 [本文引用: 1]
DOI:10.1007/s00705-004-0444-2URLPMID:15578238 [本文引用: 1]

In this in vitro study, the replication kinetics of porcine circovirus type 2 (PCV2) in porcine alveolar macrophages (PAM) and fetal cardiomyocytes (FCM), two target cells in vivo, was compared with that in PK-15 cells. Cultures were inoculated with either the postweaning multisystemic wasting syndrome (PMWS)-associated strain Stoon-1010 or the abortion-associated strain 1121. Viral proteins were visualized and virus production was determined. In PK-15 cells, the capsid protein was expressed between 6 and 12 hours post inoculation (hpi), it relocated to the nucleus between 12 and 24 hpi. At that time, Rep protein was also detected in the nucleus. This sequence of events also occurred in FCM and PAM but nuclear localized antigens appeared later (48 hpi) and in a lower percentage of cells. In PAM, clear differences in susceptibility were seen between pigs. In PAM from two out of five tested pigs, nuclear localized antigens were not detected, whereas in PAM from three other pigs they were seen in up to 20% of the antigen-positive cells. Virus production was observed in PK-15 but not in PAM or FCM cultures. In a second study, the replication kinetics of seven different PCV2 strains were compared in PK-15 cells. It was shown that the two abortion-associated strains had a different replication kinetics in comparison with PMWS or porcine dermatitis and nephropathy syndrome associated strains. With the abortion-associated strains, a higher number of infected cells was observed at 24 hpi and the percentage of infected cells with nuclear localised antigens was lower compared to that of other strains.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[D].
[本文引用: 2]
[D].
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
URL [本文引用: 2]
URL [本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 4]
[本文引用: 4]
[本文引用: 3]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.vaccine.2007.12.019URLPMID:18237827 [本文引用: 1]

The efficacy of recently developed porcine circovirus type 2 (PCV2) vaccines has not been tested yet against PCV2 isolates of the two proposed genotypes. In the present work, the efficacy of a subunit vaccine containing PCV2 capsid protein was evaluated by using a challenge model with four different PCV2 isolates of different genotype and geographic origin. The vaccine prevented the development of viremia in all cases as well as significantly decreased nasal and faecal shedding of the virus. Also, the vaccine elicited PCV2-specific neutralizing antibodies to PCV2 even in the presence of maternally derived immunity.
DOI:10.1007/BF01310989URLPMID:3619654 [本文引用: 1]

Multiplication of porcine circovirus (PCV) was found to be inducible by treatment of infected cell cultures with 300 mM glucosamine. One day after glucosamine treatment and after growth in fresh medium, an increase in the number of cells containing virus antigen of up to 50 times as compared to mock-treated cultures was observed. Analysis of this phenomenon revealed that replication of PCV DNA was induced. Only aminohexoses but not hexoses and acetylated aminohexoses were efficacious. The course of PCV replication in synchronized cell cultures infected at different periods of the cell cycle showed that PCV DNA synthesis depends on cellular enzymes expressed during S phase growth of cells. However, whereas in cell cultures treated with glucosamine after infection in G0 or during G1, the start of PCV replication was observed during the first S phase after growth stimulation, the latent period in mock-treated cultures lasted until the second S phase. Also in cell cultures transfected with PCV DNA in G0 or during G1 using DEAE-dextran as mediator, PCV replication started during the first S phase after growth release of the cells. From these findings the conclusion is drawn that glucosamine and DEAE-dextran initiate PCV replication by enabling the PCV genome to get entry to the cell nucleus that normally can be achieved only by inclusion in the daughter nuclei at the end of mitosis.
URLPMID:6295978 [本文引用: 1]
URLPMID:15163723 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
