 ,中国农业科学院农业资源与农业区划研究所/农业部农业微生物资源收集与保藏重点实验室,北京 100081
,中国农业科学院农业资源与农业区划研究所/农业部农业微生物资源收集与保藏重点实验室,北京 100081Effects of pH and Buffering on the Growth of Lentinula edodes Mycelium
DUAN YingCe, HU ZiYi, YANG Fan, LI JinTao, WU XiangLi, ZHANG RuiYing ,Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences / Key Laboratory of Agricultural Microbial Resources Collection and Preservation, Ministry of Agriculture, Beijing 100081
,Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences / Key Laboratory of Agricultural Microbial Resources Collection and Preservation, Ministry of Agriculture, Beijing 100081通讯作者:
责任编辑: 赵伶俐
收稿日期:2020-04-6接受日期:2020-09-4网络出版日期:2020-11-16
| 基金资助: |
Received:2020-04-6Accepted:2020-09-4Online:2020-11-16
作者简介 About authors
段应策,Tel:18210633620;E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (3599KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
段应策, 胡姿仪, 杨帆, 李金涛, 邬向丽, 张瑞颖. pH和缓冲作用对香菇菌丝生长的影响[J]. 中国农业科学, 2020, 53(22): 4683-4690 doi:10.3864/j.issn.0578-1752.2020.22.014
DUAN YingCe, HU ZiYi, YANG Fan, LI JinTao, WU XiangLi, ZHANG RuiYing.
开放科学(资源服务)标识码(OSID):

0 引言
【研究意义】香菇(Lentinula edodes)是中国食用菌中重要的大宗栽培种类,不仅味道鲜美,还有非常高的营养价值和药用价值,深受市场欢迎。近年来香菇生产规模不断扩大,栽培技术也发生了巨大的变化,工厂化制棒和液体菌种技术开始广泛应用。工厂化制棒对质量控制提出了更高的要求,pH是衡量培养料质量和培养条件的一个重要指标[1],在香菇菌棒工厂中,大量木屑通常是露天堆放,被雨淋湿后,易发酵变酸[2]。生产中通常添加石灰将木屑pH调至6.0—7.0,但仍然经常出现菌丝生长缓慢甚至不萌发的现象。在液体菌种发酵过程中,pH不断下降,后期有时能降至pH 2.5以下,对菌种质量的影响尚不清楚。因此,亟需深入研究pH对香菇菌丝生长的影响机制,为香菇生产和新技术的开发应用提供理论依据。【前人研究进展】pH是影响食用菌生长发育的一个重要因子。不同食用菌对pH的要求不同,平菇(Pleurotus ostreatus)能在pH 4.0—7.0的范围内生长,但pH 6.0时生长速度最高[3],金针菇(Flammulina velutipes)液体发酵的最适pH为5.5—7.4[4],黑木耳(Auricularia heimuer)菌丝生长的最适pH为7.0[5]。有些食用菌生长发育过程中基质pH是动态变化的,香菇菌棒在培养过程中,pH不断下降,在出菇前达到pH 4.0,有报道建议将pH 4.0作为衡量香菇菌棒生理成熟的一个指标[6]。大多数真菌通过合成草酸调控基质pH[7],草酰乙酸水解酶和乙醛酸脱氢酶是真菌合成草酸的主要酶[8,9,10],香菇中草酰乙酸水解酶基因已经被成功克隆[11]。【本研究切入点】香菇菌丝能够在pH 3.0—7.0的范围内生长,传统上认为香菇对基质pH的适应性较强,缺乏精确和系统的研究。【拟解决的关键问题】本研究为了探索pH对香菇菌丝生长的影响,首先利用HCL和NaOH调节PDA培养基pH,检测菌丝生长的最适pH;然后分析木屑中尤其是酸化木屑中的有机酸,利用有机酸、钙盐和缓冲液调节培养基pH,检测其对香菇菌丝生长的影响,揭示pH和缓冲作用对香菇菌丝生长的影响机制。1 材料与方法
试验于2017年在中国农业科学院农业资源与农业区划研究所进行。1.1 菌株
香菇菌株由国家食用菌标准菌株库(China center for mushroom spawn standards and control,CCMSSC)提供,编号为CCMSSC 04311,品种名为L808,是国内常用的一个主栽品种。1.2 培养基制备
为了检测有机酸和钙盐对香菇菌丝生长的影响,在PDA培养基(DifcoTM)中分别添加有机酸和钙盐,有机酸为柠檬酸和乳酸;钙盐为柠檬酸钙、乳酸钙、硫酸钙和碳酸钙。每种物质设置3个浓度,分别为5、25和50 mmol?L-1。未添加有机酸和钙盐的PDA培养基作为对照(CK)。为了检测pH对香菇菌丝生长的影响,设计5个pH梯度,分别为pH 3.0、pH 4.0、pH 5.0、pH 6.0和pH 7.0。利用3种方法分别调节PDA培养基的pH,第一种是用盐酸和氢氧化钠溶液调节;第二种是利用25和50 mmol?L-1的柠檬酸-柠檬酸钠缓冲液(CBS)调节;第三种是利用25和50 mmol?L-1磷酸-磷酸氢二钠-磷酸二氢钠缓冲液(PBS)调节。利用平面样品pH电极(梅特勒)测定PDA培养基的pH。
1.3 木屑中有机酸的提取
从香菇菌棒厂分别取酸化木屑和正常木屑,用塑料袋封装带回实验室。称取10 g木屑样品,加20 mL水,匀浆;超声波处理30 min;50℃水浴30 min;10 000 r/min离心10 min,取10 mL上清液,用0.22 μm过滤器过滤,用pH电极(梅特勒)测定pH。1.4 GC-MS分析
利用GC-MS进行有机酸的定性分析。GC-MS分析之前,利用硅烷化方法对样品进行衍生化处理[12,13]。将过滤液冷冻干燥;加100 μL甲氧胺的吡啶溶液(20 mg?mL-1),38℃反应90 min;加入100 μL的N-甲基三甲基硅基三氟乙酰胺(N-methyl-N- trimethylsilytrifluoroacetamide,MSTFA)反应30 min。有机酸样品衍生化之后直接进行GC-MS分析[14],色谱柱为DB-5MS(50 m×0.25 mm,0.25 μm);程序升温条件为70℃保持2 min,4℃?min-1升至300℃,保持10 min;质谱扫描范围为33—500 m/z;进样量1 μL;内标为己二酸。
1.5 HPLC分析
利用HPLC进行有机酸的定量分析[15,16],仪器为Agilent1100,有机酸分析柱SB-Aq,4.6 mm×150 mm,5 μm;流动相为硫酸溶液(pH 1.96)和甲醇(色谱级),配比为9﹕1;柱温为30℃;流速0.3 mL?min-1;检测波长为210 nm。根据标准品制作的标准曲线,计算样品中有机酸的含量。1.6 生长速度测定
每个培养皿装20 mL培养基,接种后25℃避光培养,利用游标卡尺测量菌落直径,计算菌丝生长速度和抑制率[17]。菌丝生长速度(mm?d-1)=菌落直径/培养天数;相对生长速度(%)=处理的生长速度/CK的生长速度。1.7 统计分析
每组试验重复3—5次,利用Prism(version 8.0)进行数据统计分析。2 结果
2.1 香菇菌丝生长的最适pH
利用浓度为1 mol?L-1 HCL或NaOH将PDA培养基的pH调节到3.0、4.0、5.0、6.0和7.0,分析香菇菌丝生长的最适pH,结果如图1所示,香菇菌丝在pH 3.0—7.0的范围内都能生长,其中pH 4.0时生长速度最快;当pH>4.0时,pH越高,菌丝生长速度越慢;CK不含HCL或NaOH,pH为5.5,菌丝生长速度介于pH 5.0和pH 6.0之间。因此,在PDA培养基上,香菇菌丝生长的最适pH为4.0。图1
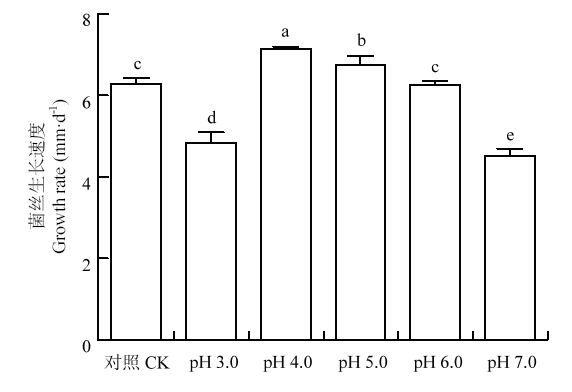 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1不同初始pH对香菇菌丝生长的影响
不同字母表示差异显著(Dunnett检验,P<0.05)。下同
Fig. 1Effects of different initial pH on mycelial growth rate of L. edodes on PDA media
Different letters indicate a statistically significant difference (Dunnett’s test, P<0.05).
2.2 酸化木屑中主要有机酸的种类及含量
利用GC-MS对酸化木屑和正常木屑中有机酸进行鉴定,样品中共发现9种主要有机酸,其中草酸、苯甲酸和香草酸可能主要是木屑的成分。乙酸、丙二酸、乳酸、琥珀酸、α-酮戊二酸和柠檬酸可能不仅是木屑成分,也可能是微生物发酵代谢的产物[18,19],针对这6种有机酸,利用HPLC检测正常木屑和酸化木屑中的含量,结果表明乙酸、丙二酸、琥珀酸和α-酮戊二酸4种有机酸在两种木屑中的含量都非常低,无明显差异;乳酸和柠檬酸在两种木屑中的差异非常大(图2),正常木屑中几乎检测不到乳酸和柠檬酸,酸化木屑中乳酸和柠檬酸的含量分别达到(4.6±0.22)mg?g-1和(23±3.35)mg?g-1(按干重计算),推测酸化木屑中的柠檬酸和乳酸主要为堆放过程中微生物发酵代谢产生。正常木屑样品和酸化木屑样品的pH分别为6.0和3.0。正常木屑的pH主要与树种有关,大多数阔叶树木材的pH为5.0—7.0[20,21,22]。木屑露天堆放过程中,尤其是碳源充足时,发酵易产生有机酸[2],本研究样品中柠檬酸含量达到了23 mg?g-1,足以使pH降至3.0,由此推测酸化木屑pH的降低主要是微生物发酵代谢产生柠檬酸的结果。
图2
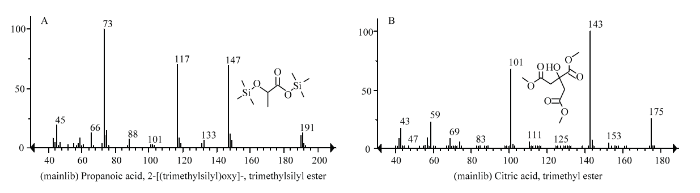 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2酸化木屑中主要有机酸的种类
A:乳酸 Lactic acid;B:柠檬酸 Citric acid
Fig. 2Types of main organic acids in acidified wood chips
2.3 有机酸和钙盐在PDA培养基上对香菇菌丝生长的影响
5 mmol?L-1乳酸对香菇菌丝生长具有促进作用,25和50 mmol?L-1时具有不同程度抑制作用;而5、25和50 mmol?L-1的柠檬酸对香菇菌丝生长均有不同程度的抑制作用(图3)。图3
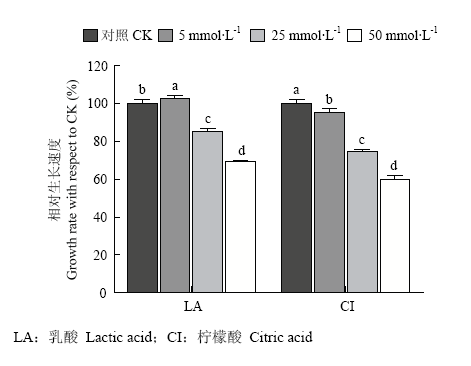 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3不同浓度有机酸对香菇菌丝生长的影响
LA:乳酸 Lactic acid;CI:柠檬酸 Citric acid
Fig. 3Effects of different concentration of organic acids on mycelial growth of L. edodes on PDA media
碳酸钙和柠檬酸钙对香菇菌丝的抑制作用最强,并且浓度越高,抑制越强;乳酸钙在5 mmol?L-1时无显著影响,浓度为25和50 mmol?L-1时对香菇菌丝具有一定的抑制作用;硫酸钙对香菇菌丝无显著影响(图4)。
有机酸和钙盐对香菇菌丝的抑制作用非常复杂,可能与钙离子、pH、缓冲作用和酸根离子等多种因素有关。硫酸钙对菌丝生长无显著影响,说明本研究中钙离子不是影响菌丝生长的主要原因。有机酸使培养基pH下降,如添加50 mmol?L-1柠檬酸或50 mmol?L-1乳酸的PDA培养基pH分别为2.9和3.2,远低于最适pH 4.0,菌丝的相对生长速度分别为69.1%和60.1%;柠檬酸钙和碳酸钙等碱性钙盐使培养基pH升高,如添加50 mmol?L-1柠檬酸钙和碳酸钙的培养基pH都为7.0,菌丝的相对生长速度分别为49.5%和31.7%(图4),说明pH是有机酸和碱性钙盐抑制香菇菌丝的一个主要原因。pH 7.0时,碳酸钙和柠檬酸钙对菌丝的抑制作用远高于NaOH,由于碳酸钙和柠檬酸钙在pH 6.4左右具有很强的缓冲作用,推测钙盐的抑制作用可能还与缓冲作用有关。
图4
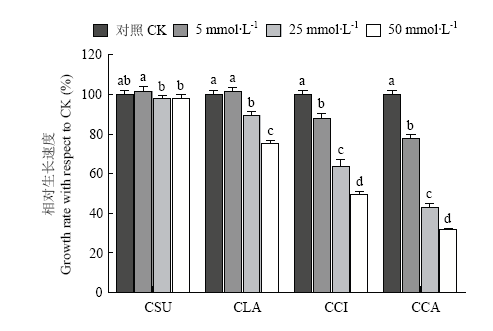 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4不同浓度钙盐对香菇菌丝生长的影响
CSU:硫酸钙 Calcium sulfate;CLA:乳酸钙 Calcium lactate;CCI:柠檬酸钙 Calcium citrate;CCA:碳酸钙 Calcium carbonate
Fig. 4Effects of different concentrations of calcium salt on mycelia growth of L. edodes on PDA media
2.4 PDA培养基的缓冲作用对香菇菌丝生长的影响
柠檬酸钙和碳酸钙在PDA培养基内有沉淀,为了更准确的检测缓冲作用对香菇菌丝生长的影响,分别用CBS和PBS调节培养基pH。两种缓冲液的最适pH都是4.0;pH 4.0时,缓冲液浓度不影响菌丝生长;pH>4.0时,缓冲液浓度越高,抑制作用越强。当缓冲能力达到一定的阈值,能导致菌种完全不萌发。CBS为25 mmol?L-1时,菌丝在pH 6.0和pH 7.0的PDA培养基上不能萌发;50 mmol?L-1时,菌丝在pH 5.0的PDA培养基上也不能萌发(图5)。缓冲液浓度越高,缓冲能力越大,由此可见,当偏离最适pH时,培养基的缓冲作用也是影响菌丝生长的一个重要因素。图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5缓冲液对香菇菌丝生长的影响
CBS:柠檬酸-柠檬酸钠缓冲液 Citric acid sodium citrate buffer;PBS:磷酸-磷酸氢二钠-磷酸二氢钠缓冲液 Phosphoric acid disodium hydrogen phosphate sodium dihydrogen phosphate buffer solution
Fig. 5Effect of buffer on mycelial growth of L. edodes on PDA media
2.5 香菇菌丝生长对PDA培养基pH的影响
在菌丝生长过程中,定期检测PDA培养基pH的变化,结果如图6所示,添加HCL或NaOH的处理,接种前pH>4.0,接种后pH下降,最终降至pH 4.0左右。添加有机酸的处理,接种前PDA培养基的pH<4.0,接种后pH升高;添加钙盐的培养基接种前pH>4.0,接种后pH下降,最终均调节至pH 4.0左右。由此说明,香菇菌丝对环境pH具有一定的调节能力。当培养基pH>4.0时,香菇菌丝通过代谢调节基质pH,最终将基质pH变为4.0,以利于菌丝的生长。如果培养基中含有缓冲系统,阻碍pH的变化,则抑制菌丝的正常生长。由此可以解释缓冲液和钙盐对香菇菌丝生长的影响机制。图6
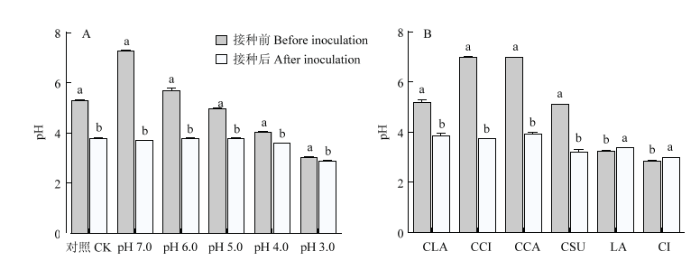 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6接种前后PDA培养基pH变化
A:PDA培养基含不同浓度的HCL或NaOH;B:PDA培养基中分别含50 mmol?L-1的乳酸钙(CLA)、柠檬酸钙(CCI)、碳酸钙(CCA)、硫酸钙(CSU)、乳酸(LA)和柠檬酸(CI)
Fig. 6Changes in pH of PDA media before and after inoculation
A: PDA media were adjusted with HCL or NaOH; B: PDA media were supplemented with 50 mmol?L-1 calcium lactate (CLA), calcium carbonate (CCA), calcium citrate (CCI), calcium sulfate (CSU), lactic acid (LA) and citric acid (CI), respectively
3 讨论
不同的研究中最适pH并不完全一致,如MARKOVIC等[23]测定的最适pH为3.0—3.63;AMINUDDIN等[24]测定的最适pH为6.0;万洪善[25]测定的最适pH为5.6。造成这种差异的原因除了所用的菌株和方法不同之外,可能还与香菇调控环境pH的能力有关。本研究表明当环境pH>4.0时,香菇菌丝通过分泌草酸降低pH[11,15],最终将培养基pH调节至4.0,以满足生长需求。当环境pH<4.0时,香菇对pH的影响较小,而污叉丝孔菌(Dichomitus squalens)在低pH条件下,草酸脱羧酶和甲酸脱氢酶等基因的表达上调,通过降解基质中的草酸,提高pH[26,27]。香菇菌棒中pH的变化与本研究在PDA培养基的检测结果一致[6]。不论用HCL-NaOH还是缓冲液调节PDA培养基的pH,初始pH 4.0时,菌丝生长速度最快;并且当pH>4.0时,随着菌丝的生长,最终pH均为4.0,由此说明香菇菌丝生长的最适pH为4.0。目前关于缓冲作用对香菇菌丝生长影响的研究报道相对较少,pH>4.0时,香菇菌丝产草酸调节培养基pH,缓冲作用阻碍了培养基pH的变化,从而增加了pH对香菇菌丝生长的影响。本研究中,当缓冲作用达到一定的阈值,pH 5.0—7.0的范围内,缓冲液可能完全抑制香菇菌丝的生长,导致菌种不萌发。生产中,木屑发酵产生有机酸,用石灰调节pH时形成有机酸钙,如果钙盐在pH 5.0—7.0范围内具有缓冲作用,则可能抑制香菇菌丝的生长。柠檬酸钙和碳酸钙在pH 6.4左右具有较强的缓冲作用,在PDA培养基上能显著抑制香菇菌丝的生长;乳酸的pKa=3.86,乳酸钙在pH 5.0—7.0范围内的缓冲作用较弱,其在PDA培养基上的抑制作用也相对较弱。由此可见,生产中调节培养料的pH时,不仅要考虑pH,还要考虑培养料内各种成分的缓冲作用。
有机酸对食用菌菌丝生长具有一定的影响。柠檬酸能促进杏鲍菇(P. eryngii)、金针菇、双孢蘑菇(Agaricus bisporus)和黑柄炭角菌(Xylaria nigripes)的菌丝生长[28,29,30,31];乳酸能抑制杏鲍菇和平菇菌丝生长[17]。本研究中,除5 mmol?L-1的乳酸轻微促进香菇菌丝生长外,其他浓度的乳酸和柠檬酸均抑制香菇菌丝生长。有机酸的影响机制可能与pH和有机酸根离子两个方面有关,柠檬酸和柠檬酸钙对香菇菌丝都有抑制作用,pH 4.0时,50 mmol?L-1的柠檬酸缓冲液不影响菌丝生长,说明本研究中柠檬酸根离子不是影响香菇菌丝生长的主要原因,柠檬酸对香菇菌丝的抑制作用主要与pH有关。
4 结论
香菇菌丝能够在pH 3.0—7.0的范围内生长,最适pH为4.0。当pH>4.0时,香菇菌丝生长过程中通过代谢降低环境pH,如果培养基中含有阻碍pH调节的缓冲系统,则抑制香菇菌丝的生长;当缓冲能力达到一定的阈值,可导致菌种完全不萌发。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
DOI:10.15376/bioresURL [本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/BF01177917URL [本文引用: 2]
DOI:10.1007/BF00166839URL [本文引用: 1]
DOI:10.1073/pnas.191389598URL [本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.aspen.2017.12.003URL [本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
DOI:10.1038/nprot.2010.108URLPMID:20885382 [本文引用: 1]

This protocol describes an analytical platform for the analysis of intra- and extracellular metabolites of microbial cells (yeast, filamentous fungi and bacteria) using gas chromatography-mass spectrometry (GC-MS). The protocol is subdivided into sampling, sample preparation, chemical derivatization of metabolites, GC-MS analysis and data processing and analysis. This protocol uses two robust quenching methods for microbial cultures, the first of which, cold glycerol-saline quenching, causes reduced leakage of intracellular metabolites, thus allowing a more reliable separation of intra- and extracellular metabolites with simultaneous stopping of cell metabolism. The second, fast filtration, is specifically designed for quenching filamentous micro-organisms. These sampling techniques are combined with an easy sample-preparation procedure and a fast chemical derivatization reaction using methyl chloroformate. This reaction takes place at room temperature, in aqueous medium, and is less prone to matrix effect compared with other derivatizations. This protocol takes an average of 10 d to complete and enables the simultaneous analysis of hundreds of metabolites from the central carbon metabolism (amino and nonamino organic acids, phosphorylated organic acids and fatty acid intermediates) using an in-house MS library and a data analysis pipeline consisting of two free software programs (Automated Mass Deconvolution and Identification System (AMDIS) and R).
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
DOI:10.1016/j.scienta.2015.12.035URL [本文引用: 2]
DOI:10.1016/S0960-8524(99)00161-3URL [本文引用: 1]
DOI:10.1111/1567-1364.12221URL [本文引用: 1]

Weak acids are present in lignocellulosic hydrolysate as potential inhibitors that can hamper the use of this renewable resource for fuel and chemical production. To study the effects of weak acids on yeast growth, physiological investigations were carried out in batch cultures using glucose as carbon source in the presence of acetic, formic, levulinic, and vanillic acid at three different concentrations at pH 5.0. The results showed that acids at moderate concentrations can stimulate the glycolytic flux, while higher levels of acid slow down the glycolytic flux for both aerobically and anaerobically grown yeast cells. In particular, the flux distribution between respiratory and fermentative growth was adjusted to achieve an optimal ATP generation to allow a maintained energy level as high as it is in nonstressed cells grown exponentially on glucose under aerobic conditions. In addition, yeast cells exposed to acids suffered from severe reactive oxygen species stress and depletion of reduced glutathione commensurate with exhaustion of the total glutathione pool. Furthermore, a higher cellular trehalose content was observed as compared to control cultivations, and this trehalose probably acts to enhance a number of stress tolerances of the yeast.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.5897/SREURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1099/mic.0.028860-0URL [本文引用: 1]
DOI:10.1371/journal.pone.0087959URLPMID:24505339 [本文引用: 1]

Oxalic acid is a prevalent fungal metabolite with versatile roles in growth and nutrition, including degradation of plant biomass. However, the toxicity of oxalic acid makes regulation of its intra- and extracellular concentration crucial. To increase the knowledge of fungal oxalate metabolism, a transcriptional level study on oxalate-catabolising genes was performed with an effective lignin-degrading white-rot fungus Dichomitus squalens, which has demonstrated particular abilities in production and degradation of oxalic acid. The expression of oxalic-acid decomposing oxalate decarboxylase (ODC) and formic-acid decomposing formate dehydrogenase (FDH) encoding genes was followed during the growth of D. squalens on its natural spruce wood substrate. The effect of high proton concentration on the regulation of the oxalate-catabolising genes was determined after addition of organic acid (oxalic acid) and inorganic acid (hydrochloric acid) to the liquid cultures of D. squalens. In order to evaluate the co-expression of oxalate-catabolising and manganese peroxidase (MnP) encoding genes, the expression of one MnP encoding gene, mnp1, of D. squalens was also surveyed in the solid state and liquid cultures. Sequential action of ODC and FDH encoding genes was detected in the studied cultivations. The odc1, fdh2 and fdh3 genes of D. squalens showed constitutive expression, whereas ODC2 and FHD1 most likely are the main responsible enzymes for detoxification of high concentrations of oxalic and formic acids. The results also confirmed the central role of ODC1 when D. squalens grows on coniferous wood. Phylogenetic analysis revealed that fungal ODCs have evolved from at least two gene copies whereas FDHs have a single ancestral gene. As a conclusion, the multiplicity of oxalate-catabolising genes and their differential regulation on wood and in acid-amended cultures of D. squalens point to divergent physiological roles for the corresponding enzymes.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/s00253-020-10718-5URLPMID:32533305 [本文引用: 1]

High temperature is a major threat to Pleurotus ostreatus cultivation. In this study, a potential mechanism by which P. ostreatus mycelia growth is inhibited under heat stress was explored. Lactate, as a microbial fermentation product, was found unexpectedly in the mycelia of P. ostreatus under heat stress, and the time-dependent accumulation and corresponding inhibitory effect of lactate on mycelial growth was further confirmed. The addition of a glycolysis inhibitor, 2-deoxy-D-glucose (2DG), reduced the lactate content in mycelia and slightly restored mycelial growth under high-temperature conditions, which indicated the accumulation of lactate can be inhibited by glycolysis inhibition. Further data revealed mitochondrial dysfunction under high-temperature conditions, with evidence of decreased oxygen consumption and adenosine triphosphate (ATP) synthesis and increased reactive oxygen species (ROS). The removal of ROS with ascorbic acid decreased the lactate content, and mycelial growth recovered to a certain extent, indicating lactate accumulation could be affected by the mitochondrial ROS. Moreover, metabolic data showed that glycolysis and the tricarboxylic acid cycle were enhanced. This study reported the accumulation of lactate in P. ostreatus mycelia under heat stress and the inhibitory effect of lactate on the growth of mycelia, which might provide further insights into the stress response mechanism of edible fungi. Key Points * Lactate can accumulate in Pleurotus ostreatus mycelia under heat stress and inhibit its growth. * The accumulation of lactate may be due to the acceleration of glycolysis and the dysfunction of mitochondria of P. ostreatus mycelia under high-temperature stress. * The glycolysis and tricarboxylic acid cycle of P. ostreatus mycelia were accelerated under high-temperature stress.
