 ,, 严常燕, 杨伟聪, 张云晓, 于洋, 黄显会
,, 严常燕, 杨伟聪, 张云晓, 于洋, 黄显会 ,华南农业大学兽医学院,广州 510642
,华南农业大学兽医学院,广州 510642Pharmacokinetics of Chlortetracycline Microspheres in Pigs
XU Ying ,, YAN ChangYan, YANG WeiCong, ZHANG YunXiao, YU Yang, HUANG XianHui
,, YAN ChangYan, YANG WeiCong, ZHANG YunXiao, YU Yang, HUANG XianHui ,College of Veterinary Medicine, South China Agricultural University, Guangzhou 510642
,College of Veterinary Medicine, South China Agricultural University, Guangzhou 510642通讯作者:
责任编辑: 林鉴非
收稿日期:2019-10-30接受日期:2020-06-30网络出版日期:2020-10-01
| 基金资助: |
Received:2019-10-30Accepted:2020-06-30Online:2020-10-01
作者简介 About authors
许颖,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (637KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
许颖, 严常燕, 杨伟聪, 张云晓, 于洋, 黄显会. 金霉素微囊颗粒在猪体内的比较药动学研究[J]. 中国农业科学, 2020, 53(19): 4083-4091 doi:10.3864/j.issn.0578-1752.2020.19.020
XU Ying, YAN ChangYan, YANG WeiCong, ZHANG YunXiao, YU Yang, HUANG XianHui.
0 引言
【研究意义】金霉素(chlortetracycline,CTC),又称氯西霉素,是由美国氰胺公司于1949年开发的四环素类广谱抗生素,它通过与细菌核糖体30s亚基不可逆的结合抑制细菌蛋白质的合成,对革兰阳性菌、阴性菌、支原体和立克次体等有广泛的抑制作用,自发现以来被广泛用于畜牧养殖业[1,2,3,4]。【前人研究进展】金霉素被FDA批准用于鸡、猪、牛、羊等动物多种疾病的预防与治疗,国内主要用于治疗断奶仔猪腹泻、猪气喘病、增生性肠炎等。对于增生性肠炎、猪痢疾等仔猪腹泻性疾病,金霉素有很好的治疗效果,它可以影响肠道炎症因子的表达,改善断奶仔猪肠道黏膜的形态和结构,降低腹泻率,相比于泰妙菌素和林可霉素,金霉素的使用成本更低[5,6]。金霉素能够有效减轻猪肺炎支原体感染引起的损伤,治疗支原体肺炎[7,8],改善呼吸道症状,降低病变[9],在饲料中定期添加金霉素还可以控制衣原体和钩端螺旋体感染,改善母猪的健康状况,提高种猪的繁殖性能[10,11,12]。金霉素虽在猪、鸡、牛、羊等动物上已有相关药动学报道[13,14,15,16,17,18,19,20],但剂型多为盐酸金霉素和普通的金霉素预混剂。研究表明,盐酸金霉素或普通金霉素预混剂给药后吸收迅速,达峰时间短,分布广泛而且消除缓慢,生物利用度低,但在不同动物种属之间有较大的差异。目前,随着兽药制剂技术的发展,微囊化制剂由于掩味、能够减少胃酸对药物的破坏、肠溶缓释等优点[21,22],在兽医临床使用越来越普遍。金霉素微囊化后,能够减少药物与饲料中其他物质的接触、减少在胃液中崩解、释放,还能够调节在肠道内的释药速度,但国内尚未见金霉素微囊颗粒在猪体内的药动学研究报道。【本研究切入点】本研究探讨了金霉素微囊颗粒在猪体内的药动学过程,比较了禁食与非禁食对金霉素生物利用度的影响,为金霉素微囊颗粒临床使用制定合理的给药方案提供依据。【拟解决的关键问题】本研究旨在通过静脉注射和灌胃口服的给药方式,探讨金霉素微囊颗粒在猪体内的比较药动学,通过相关药动学参数的比较,为金霉素的进一步开发和临床应用提供科学依据。1 材料与方法
1.1 实验材料
1.1.1 药品与试剂 盐酸金霉素对照品,含量92.1%,批号K0011209,由中国兽医药品监察所提供;盐酸金霉素原料药,含量93.2%,批号H20130112,10%金霉素微囊颗粒,规格100 g:10 g,批号WN20130702,15%金霉素微囊颗粒:规格100 g:15 g,批号WN20130703,均由金河生物科技股份有限公司提供。乙腈、甲醇、甲酸为HPLC级,德国CNW公司;氢氧化钠、盐酸、十二水磷酸氢二钠、一水合柠檬酸、EDTA二钠均为国产分析纯;水为符合GB/T6682规定的二级水。1.1.2 仪器与设备 高效液相色谱仪:Agilent 1200型液相色谱仪,美国安捷伦公司,配备四元泵,自动进样器,柱温箱等;电喷雾-串联四级杆质谱仪:API 4000 MS/MS,美国应用生物系统公司,配Analyst4.1.5软件;色谱柱:CNW Athena C18 WP(150 mm×2.1 mm,5 μm),上海安谱公司;Mach 1.6 R冷冻型离心机:美国Thermo Fisher Scientific公司;Milli Q 去离子水发生器:美国Millipore 公司;轨道式摇床:HS250,马来西亚IKA公司;pH 计:pHs-25 型,上海精科雷磁仪器厂;电子分析天平:AE 160型,瑞士Mettler公司;移液器:Eppendorf Research型,德国EPPENDORF公司;氮吹仪:D10-24型,杭州奥盛仪器有限公司;固相萃取小柱:Poly-Sery HLB SPE,60 mg/3mL,德国CNW科技公司;有机针式尼龙微孔滤膜,13 mm×0.22 μm,上海安谱科学仪器有限公司。
1.1.3 试液配制 0.1%甲酸水溶液:取1.0 mL甲酸,加水稀释至1 000 mL,混匀,超声脱气;
1 mol·L-1氢氧化钠溶液:称取氢氧化钠4 g,加水溶解并稀释至100 mL;
McIlvaine缓冲液:将0.1 mol·L-1柠檬酸溶液1 000 mL与0.2 mol·L-1磷酸氢二钠溶液652 mL 混合,摇匀即可。必要时用氢氧化钠溶液或盐酸溶液调pH至4.0±0.05;
0.1 mol·L-1 Na2EDTA-McIlvaine缓冲液:称取60.5 g乙二胺四乙酸二钠倒入1 625 mL McIlvaine缓冲溶液中,超声使其溶解,摇匀。
1.1.4 试验动物 本试验于2013年6—12月于华南农业大学兽医学院进行,试验选取16头健康的杜洛克×长白×约克夏杂交猪,公母各半,7周龄左右,(20±2.5)kg,按常规饲养,自由饮水和采食,饲料为全价日粮,不含抗菌药物。试验前观察1周,临床表现健康。
1.2 试验方法
1.2.1 给药 16头健康猪随机分为2组,A组为非禁食组,B组为禁食组,每组8头。临床观察健康后,采用自身对照随机三药剂、三周期、三交叉试验设计方法,将每组的8头猪随机编号,给药方案见表1。A组在给药0.5 h前正常饲喂,B组试验前16 h起及给药后3 h期间禁食,仅自由饮水。一个药动采血周期结束,经过7 d的清洗期后,给药制剂互换。试验猪只采用单剂量给药,静脉注射和灌胃口服给药剂量分别为10 mg·kg-1 b.w.和40 mg·kg-1 b.w.(以金霉素计)。Table 1
表1
表1金霉素制剂给药方案
Table 1
| 组别Group | A组 (非禁食组)) A group (feeding condition) | B组(禁食组) B group (fasted condition) | ||||
|---|---|---|---|---|---|---|
| 1、2、3 | 4、5、6 | 7、8 | 10、11、12 | 13、14、15 | 16、17 | |
| 周期1 Period 1 | 药剂1 Drug1 | 药剂2 Drug2 | 药剂3 Drug3 | 药剂1 Drug1 | 药剂2 Drug2 | 药剂3 Drug3 |
| 周期2 Period 2 | 药剂3 Drug3 | 药剂1 Drug1 | 药剂2 Drug2 | 药剂3 Drug3 | 药剂1 Drug1 | 药剂2 Drug2 |
| 周期3 Period 3 | 药剂2 Drug2 | 药剂3 Drug3 | 药剂1 Drug1 | 药剂2 Drug2 | 药剂3 Drug3 | 药剂1 Drug1 |
新窗口打开|下载CSV
1.2.2 样品采集 猪只仰卧保定,从前腔静脉采血。给药前采一次空白血。给药后分别于5、10、15、20、30、45 min及1、1.5、2、3、4、6、8、12、16、24、36、48、72 h采集血样。每次采血3 mL左右,置于含肝素的离心管中,4 000 r/min离心10 min,分离血浆,-20℃冰箱保存,待测。
1.2.3 血浆中金霉素测定
(1)样品前处理 血浆样品自然解冻后摇匀,准确吸取0.5 mL血浆于15 mL塑料离心管中,加入5 mL EDTA-McIlvaine缓冲液,旋涡混匀1 min,水浴超声10 min,摇床振荡10 min后,于4℃下8 000 r/min离心10 min,上清液待净化。
分别用3 mL甲醇和纯水活化HLB SPE小柱,上清液过柱,自然流速。上清液流出后,3 mL纯水淋洗,负压抽干后用(2+2)mL甲醇洗脱,收集洗脱液,于35℃水浴下氮气吹干,残渣用1 mL甲醇溶解,转至2 mL离心管,于4℃下12 000 r/min离心10 min,上清过0.22 μm滤膜,供LC-MS/MS测定。
(2)液相色谱条件
色谱柱:CNW Athena C18 WP(150 mm×2.1 mm,5 μm),上海安谱公司;
流动相:A相为0.1%甲酸水溶液,B相为乙腈;梯度洗脱程序见表2:
Table 2
表2
表2液相色谱梯度洗脱条件
Table 2
| 时间 Time (min) | 流速 Flow (mL·min-1) | A (%) | B (%) |
|---|---|---|---|
| 0.0 | 0.25 | 90.0 | 10.0 |
| 1.0 | 0.25 | 30.0 | 70.0 |
| 4.0 | 0.25 | 30.0 | 70.0 |
| 4.5 | 0.25 | 90.0 | 10.0 |
| 13.0 | 0.25 | 90.0 | 10.0 |
新窗口打开|下载CSV
流速:0.25 mL·min-1;
柱温:35℃;
进样量:5 μL。
(3)质谱条件 采用多反应监测(MRM)扫描模式;电喷雾离子源(ESI):正离子扫描;喷雾电压(ionspray voltage,IS):4 000.0 V;离子源温度:700.0℃;气帘气压力(curtain gas,CUR)15 psi;雾化气压力(nebulizer gas,GS1)55 psi;辅助气流速(ion source gas2,GS2)35 L·min-1;碰撞室压力(CAD)6 psi;定量离子m/z= 444.1,辅助定性离子m/z= 154.2。
1.2.4 检测限与定量限 以最低检出浓度计算,信噪比S/N≥3 为检测限(LOD),S/N≥10为定量限(LOQ)。
1.2.5 标准曲线与线性范围 空白猪血浆按照1.2.3中“(1)样品前处理”方法进行处理。洗脱液在氮吹浓缩后,用1.00 mL相应浓度的标准工作液复溶,其中第一管加入1.00 mL色谱甲醇做空白对照,其余各管基质加标样品浓度分别为5、10、20、50、150、500 ng·mL-1,进行LC-MS/MS测定,将金霉素的药物浓度(C)对色谱峰面积(A)作线性回归,求得回归方程和相关系数(r)。
1.2.6 回收率与精密度 准确移取0.49 mL空白猪血浆,分别加入低、中、高不同浓度的标准工作液0.01 mL,制得10 ng·mL-1、50 ng·mL-1、100 ng·mL-1 3个浓度水平的空白加标样品,按1.2.3中“(1)样品前处理”方法处理。每个浓度做5个重复,共做4个批次,不同批次隔一天或者数天。同时,取空白血浆0.5 mL按1.2.3中“(1)样品前处理”方法处理,洗脱液经氮气吹干后,残渣加入1.00 mL低、中、高3个浓度的金霉素标准工作液,使得基质加标样品的浓度与空白加标样品的上机浓度一致。将处理后的样品进行LC-MS/MS测定,以测得的药物峰面积计算回收率和批内、批间的平均值及标准差和变异系数。
1.2.7 血药样品浓度测定 给药后不同时间采集的血浆,按1.2.3中“(1) 样品前处理”方法处理样品后进行LC-MS/MS测定,以标准曲线回归方程计算血浆中金霉素的浓度。如样品血药浓度超出线性范围,需将样品用空白基质稀释至线性范围内,然后再进行血药浓度测定。
1.2.8 数据处理与分析 使用Analyst1.5软件处理实测血浆中金霉素浓度-时间数据,采用美国Pharsight公司药动学软件 Winnonlin5.2.1 的非房室模型来处理药动数据,拟合时选择消除相后4—5个点,计算出每头试验猪的药动学参数,求出关键药动学参数(F、AUC、Tmax、Cmax等),然后计算平均值($\bar{X}$)及标准差(SD),同时以血药浓度平均值对时间作药-时曲线。
2 结果
2.1 方法学验证
按照1.2.3中“(1)样品前处理”方法处理样品,再按(2)、(3)色谱工作条件进行LC-MS/MS分析测定,测得猪血浆中金霉素的检测限(LOD)为5 ng·mL-1,定量限(LOQ)为10 ng·mL-1,在5—500 ng·mL-1范围内,猪血浆中添加金霉素的线性关系良好,相关系数均在0.9990以上,金霉素在10、50、100 ng·mL-1 3个浓度水平的平均回收率为76.90%—89.25%,批内变异系数为2.97%—9.45%,批间变异系数为6.16%—13.39%,均满足兽药临床药代动力学研究要求。2.2 金霉素微囊颗粒在猪体内的药动学特征
本试验的动物分为两组,禁食组(试验前禁食16 h以上,给药后禁食3 h)和非禁食组(给药前0.5 h饲喂,给药后自由饮食),每组通过自身对照随机三药剂、三周期、三交叉方法设计,采用非房室模型分析给药后的药动学数据,探索金霉素微囊颗粒在猪体内的吸收、分布和消除规律,通过药动学主要参数的比较分析,从而得知金霉素微囊颗粒在猪体内的动力学特征。禁食组和非禁食组猪的药时曲线分别见图1、图2,血药浓度-时间数据见表3,药动学参数见表4。图1
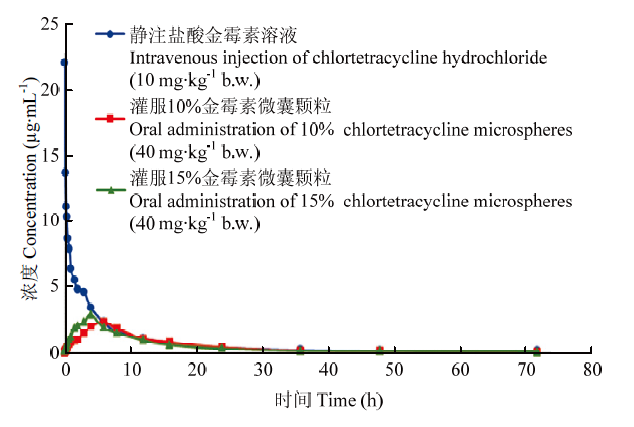 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1金霉素对禁食组猪的血浆药时曲线
静注盐酸金霉素(10 mg·kg-1 b.w.)、灌服10%和15%金霉素微囊颗粒(40 mg·kg-1 b.w.)
Fig. 1Plasma concentration-time curves of pigs in the fasting group with chlortetracycline
Intravenous injection of chlortetracycline hydrochloride (10 mg·kg-1 b.w.) and oral administration of 10% and 15% chlortetracycline micropheres (40 mg·kg-1 b.w.)
图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2金霉素对非禁食组猪的血浆药时曲线
静注盐酸金霉素(10 mg·kg-1 b.w.)、灌服10%和15%金霉素微囊颗粒(40 mg·kg-1 b.w.)
Fig. 2Plasma concentration-time curves of pigs in the feeding group with chlortetracycline
Intravenous injection of chlortetracycline hydrochloride (10 mg·kg-1 b.w.) and oral administration of 10% and 15% chlortetracycline microspheres (40 mg·kg-1 b.w.)
Table 3
表3
表3禁食组、非禁食组猪单剂量静注盐酸金霉素溶液(10.0 mg·kg-1 b.w.)与灌服金霉素微囊颗粒(40.0 mg·kg-1 b.w.)后平均血药浓度(mg·mL-1, $\bar{X}$±SD, n=8)
Table 3
| 采血时间Time(h) | 禁食组 Fasting group | 非禁食组 Feeding group | ||||
|---|---|---|---|---|---|---|
| 静注盐酸 金霉素溶液 CTC, i.v. | 灌服10%金霉素 微囊颗粒 10% CTC Micropheres, p.o. | 灌服15%金霉素 微囊颗粒 15% CTC Micropheres, p.o. | 静注盐酸 金霉素溶液 CTC, i.v. | 灌服10%金霉素 微囊颗粒 10% CTC Micropheres, p.o. | 灌服15%金霉素 微囊颗粒 15% CTC Micropheres, p.o. | |
| 0.083 | 21.96±10.25 | 0.05±0.03 | 0.08±0.06 | 15.07±9.43 | 0.06±0.03 | 0.06±0.04 |
| 0.167 | 13.60±6.41 | 0.09±0.04 | 0.22±0.16 | 9.12±5.88 | 0.08±0.06 | 0.09±0.07 |
| 0.25 | 11.03±5.02 | 0.15±0.09 | 0.34±0.27 | 6.72±3.65 | 0.12±0.07 | 0.12±0.09 |
| 0.333 | 10.23±4.42 | 0.28±0.21 | 0.44±0.41 | 5.78±2.91 | 0.16±0.10 | 0.17±0.17 |
| 0.5 | 8.62±3.67 | 0.40±0.32 | 0.73±0.56 | 4.68±2.22 | 0.24±0.12 | 0.23±0.20 |
| 0.75 | 7.84±3.60 | 0.64±0.44 | 0.94±0.62 | 4.23±1.82 | 0.38±0.19 | 0.34±0.31 |
| 1 | 6.28±2.82 | 0.75±0.48 | 1.16±0.89 | 3.70±1.83 | 0.53±0.23 | 0.42±0.37 |
| 1.5 | 5.42±2.80 | 0.92±0.56 | 1.83±0.85 | 3.30±1.57 | 0.53±0.17 | 0.48±0.30 |
| 2 | 4.71±2.99 | 0.99±0.71 | 2.04±1.09 | 2.95±1.47 | 0.63±0.21 | 0.58±0.32 |
| 3 | 4.55±2.72 | 1.44±0.91 | 2.27±1.04 | 2.32±0.90 | 0.83±0.28 | 0.72±0.33 |
| 4 | 3.33±1.01 | 1.96±0.85 | 2.80±1.93 | 2.09±1.07 | 0.88±0.34 | 0.82±0.37 |
| 6 | 2.26±0.93 | 2.32±1.07 | 1.87±0.75 | 1.35±0.76 | 0.84±0.46 | 0.73±0.36 |
| 8 | 1.58±0.75 | 1.81±0.77 | 1.46±0.56 | 1.00±0.69 | 0.84±0.49 | 0.78±0.31 |
| 12 | 1.02±0.63 | 1.00±0.36 | 0.91±0.27 | 0.56±0.29 | 0.53±0.26 | 0.55±0.22 |
| 16 | 0.73±0.44 | 0.78±0.25 | 0.56±0.17 | 0.39±0.23 | 0.46±0.21 | 0.52±0.27 |
| 24 | 0.35±0.23 | 0.36±0.08 | 0.29±0.07 | 0.20±0.20 | 0.30±0.15 | 0.31±0.12 |
| 36 | 0.18±0.13 | 0.14±0.01 | 0.12±0.04 | 0.10±0.07 | 0.12±0.05 | 0.12±0.07 |
| 48 | 0.12±0.06 | 0.10±0.03 | 0.09±0.04 | 0.07±0.03 | 0.12±0.08 | 0.08±0.05 |
| 72 | 0.08±0.04 | 0.07±0.03 | 0.06±0.04 | 0.10±0.10 | 0.05±0.03 | 0.04±0.02 |
新窗口打开|下载CSV
Table 4
表4
表4禁食组、非禁食组猪单剂量静注盐酸金霉素溶液(10.0 mg·kg-1 b.w.)与灌服金霉素微囊颗粒(40.0 mg·kg-1 b.w.)后的主要药动学参数($\bar{X}$±SD, n=8)
Table 4
| 药动参数 Parameter | 禁食组 Fasting Group | 非禁食组 Feeding Group | ||||
|---|---|---|---|---|---|---|
| 静注盐酸 金霉素溶液 CTC, i.v. | 灌服10%金霉素 微囊颗粒 10%CTC Micropheres, p.o. | 灌服15%金霉素 微囊颗粒 15%CTC Micropheres, p.o. | 静注盐酸 金霉素溶液 CTC, i.v. | 灌服10%金霉素 微囊颗粒 10%CTC Micropheres, p.o. | 灌服15%金霉素 微囊颗粒 15%CTC Micropheres, p.o. | |
| Kel (1/h) | 0.04±0.01 | 0.04±0.01 | 0.04±0.01 | 0.03±0.01 | 0.04±0.01 | 0.05±0.01 |
| t1/2 (h) | 19.93±5.26 | 16.87±3.49 | 17.13±3.58 | 27.79±12.82 | 18.57±10.67 | 16.64±5.21 |
| Tmax (h) | — | 4.88±1.25 | 3.13±1.55 | — | 6.38±4.44 | 8.00±5.24 |
| Cmax (mg·mL-1) | 21.96±10.25 | 2.48±1.05 | 2.97±1.88 | 15.07±9.43 | 1.02±0.38 | 0.95±0.32 |
| AUC0–∞ (h·mg·mL-1) | 57.42±23.53 | 34.46±10.28 | 33.15±12.76 | 37.58±21.30 | 20.81±7.46 | 19.72±5.69 |
| V/F (L·kg-1) | 5.67±2.12 | 31.53±15.98 | 32.30±9.69 | 12.59±6.43 | 52.40±22.90 | 52.47±19.69 |
| MRT (h) | 13.87±2.00 | 19.93±3.83 | 17.41±1.80 | 22.17±14.47 | 24.67±9.52 | 23.37±4.21 |
| F (%) | — | 17.03±0.08 | 15.82±5.16 | — | 16.07±6.78 | 15.26±5.26 |
新窗口打开|下载CSV
禁食组猪静注盐酸金霉素溶液(10.0 mg·kg-1)、灌服10%金霉素微囊颗粒(40 mg·kg-1)和15%金霉素微囊颗粒(40 mg·kg-1)后,金霉素静注给药后表观分布容积(V/F)为(5.67±2.12) L·kg-1,消除半衰期(t1/2)为(19.93±5.26) h;灌服10%金霉素微囊颗粒和15%金霉素微囊颗粒后表观分布容积(V/F)分别为(31.53± 15.98)和(32.30±9.69) L·kg-1,消除半衰期(t1/2)分别为(16.87±3.49)和(17.13±3.58)h,达峰浓度(Cmax)分别为(2.48±1.05)和(2.97±1.88)mg·mL-1,达峰时间(Tmax)分别为(4.88±1.25)和(3.13±1.55)h,生物利用度(F)分别为(17.03±0.08)%和(15.82±5.16)%。
非禁食组猪静注盐酸金霉素溶液(10.0 mg·kg-1)及灌服10%金霉素微囊颗粒(40 mg·kg-1)和15%金霉素微囊颗粒(40 mg·kg-1)后,金霉素静注给药后表观分布容积(V/F)为(12.59±6.43) L·kg-1,消除半衰期(t1/2)为(27.79±12.82)h;灌服10%金霉素微囊颗粒和15%金霉素微囊颗粒后表观分布容积(V/F)分别为(52.40±22.90)和(52.47±19.69)L·kg-1,消除半衰期(t1/2)分别为(18.57±10.67)和(16.64±5.12)h,达峰浓度(Cmax)分别为(1.02±0.38)和(0.95±0.32)mg·mL-1,达峰时间(Tmax)分别为(6.38±4.44)和(8.00±5.24)h,生物利用度(F)分别为(16.07±6.78)%和(15.26±5.26)%。禁食组与非禁食组中10%和15%金霉素微囊颗粒吸收、消除均较慢,表观分布容积较高,在两组中10%金霉素微囊颗粒在猪体内均表现出更高的生物利用度,但无统计学差异(P>0.05)。与禁食组相比,非禁食组10%和15%金霉素微囊颗粒均表现出达峰时间更慢,峰浓度更低,但表观分布容积更大,生物利用度稍低,但无统计学差异(P>0.05)。
3 讨论
在禁食和非禁食的情况下,V/F结果表明:金霉素分布广泛,与猪禁食和非禁食情况下静注盐酸金霉素(11 mg·kg-1 )后V/F分别为0.97和1.39 L·kg-1 比较[16],显示出相似的分布特征。在其他动物亦可见相似的特征,但存在一定的种属差异,如荷斯坦牛、育肥鸡的V/F分别为40.9、1.63 L·kg-1 [15, 23]。t1/2结果表明:金霉素在猪体内消除慢,高于方炳虎等报道的金霉素在鸡体内的消除半衰期[17],也显著高于金霉素在育肥鸡(静注7.96 h,口服13.15 h)[14]的半衰期,与REINBOLD报道的荷斯坦牛(灌服16.2 h)[15]的消除半衰期近似。金霉素通过胆汁排泄,部分可以在肠内被重新吸收[24],这可能是导致金霉素在动物体内半衰期较长的原因。禁食组和非禁食组猪灌服药物后,禁食组达峰浓度和达峰时间与NIELSEN等报道的(2.4±1.1)mg·mL-1、(3.7±1.9)h相似,非禁食组达峰浓度与报道的0.8 mg·mL-1 相似,但达峰时间比3.2 h明显延迟[13]。试验中金霉素在禁食组和非禁食组猪中的生物利用度均较低,金霉素通过肠道少部分吸收,禁食组生物利用度稍高于非禁食组,但两者无统计学差异,与KILROY等报道盐酸金霉素在禁食组和非禁食组猪中的F[(19.12±8.3)%、(17.88±5.32)%]相似[16],但高于NIELSEN等报道的猪灌服盐酸金霉素后的F [(11±5)%、(6±2)%)][13]。JEROME 通过对金霉素在猪体内的药动学数据进行Mantel Haenszel分析发现猪对金霉素的生物利用度随饲料中钙含量的增高而降低,由17%降至9%[25]。而本研究Cmax和F说明饲料虽然可以影响金霉素的吸收速率,但对金霉素在猪胃肠道中的吸收量未表现显著影响,这可能与金霉素微囊化后,药物以分子状态在肠道缓慢释放、被吸收,从而减少饲料对药物的影响有关。金霉素抗菌作用较广,对革兰氏阳性菌(链球菌、炭疽杆菌、葡萄球菌、破伤风杆菌)、革兰氏阴性菌(巴氏杆菌、沙门氏菌、嗜血杆菌)及一些衣原体、支原体、立克次体、螺旋体具有一定的抗菌活性。非禁食组中,40 mg·kg-1 b.w.单剂量给猪灌服15%微囊金霉素后,约8 h后达到最高血药浓度0.95 mg·mL-1 ,至12、24 h血浆中药物浓度仍可维持0.55、0.31 mg·mL-1 。同样单剂量灌服10%金霉素微囊颗粒后,约6 h后达到最高血药浓度1.02 mg·mL-1 、至12、24 h血浆中药物浓度仍可维持0.53、0.30 mg·mL-1 。金霉素对大肠杆菌、金黄色葡萄球菌、猪霍乱沙门氏菌和铜绿假单胞菌等临床分离菌的MIC分别为4、0.0625、4和2 mg·mL-1 [26],对猪肺炎支原体的MIC为1.25 mg·mL-1 [27],HAYER评估美国2006至2016年副猪嗜血杆菌对13种抗菌素的耐药率变化,其中金霉素对副猪嗜血杆菌的MIC90为1 mg·mL-1 [28]。无论禁食组还是非禁食组,金霉素在体内的表观分布容积远远大于1 L·kg-1,有报道金霉素在胆汁中的浓度是在血清中观测到的浓度的8至16倍[29],药物的组织浓度要高于血浆浓度,药物在体内分布广泛[30]。此外,日粮中添加75和150 mg·kg-1 的金霉素,腹泻率下降22.1%和52.23%[6]。对此,按40 mg·kg-1添加金霉素微囊颗粒,可用于预防敏感菌导致的猪肠道和呼吸道疾病。
4 结论
猪灌服金霉素微囊颗粒后,药物在体内吸收缓慢、分布广、消除较慢、生物利用度较低,与禁食组相比,非禁食组生物利用度偏低,但无统计学差异。表明饲料不影响金霉素微囊颗粒在猪胃肠道中的吸收,但会改变金霉素进入体内的药代动力学过程。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1128/MMBR.65.2.232-260.2001URLPMID:11381101 [本文引用: 1]

Tetracyclines were discovered in the 1940s and exhibited activity against a wide range of microorganisms including gram-positive and gram-negative bacteria, chlamydiae, mycoplasmas, rickettsiae, and protozoan parasites. They are inexpensive antibiotics, which have been used extensively in the prophlylaxis and therapy of human and animal infections and also at subtherapeutic levels in animal feed as growth promoters. The first tetracycline-resistant bacterium, Shigella dysenteriae, was isolated in 1953. Tetracycline resistance now occurs in an increasing number of pathogenic, opportunistic, and commensal bacteria. The presence of tetracycline-resistant pathogens limits the use of these agents in treatment of disease. Tetracycline resistance is often due to the acquisition of new genes, which code for energy-dependent efflux of tetracyclines or for a protein that protects bacterial ribosomes from the action of tetracyclines. Many of these genes are associated with mobile plasmids or transposons and can be distinguished from each other using molecular methods including DNA-DNA hybridization with oligonucleotide probes and DNA sequencing. A limited number of bacteria acquire resistance by mutations, which alter the permeability of the outer membrane porins and/or lipopolysaccharides in the outer membrane, change the regulation of innate efflux systems, or alter the 16S rRNA. New tetracycline derivatives are being examined, although their role in treatment is not clear. Changing the use of tetracyclines in human and animal health as well as in food production is needed if we are to continue to use this class of broad-spectrum antimicrobials through the present century.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 2]

Seventy-two piglets (Duroc×Large White×Landrace) weaned at 21 days weighting (5.06±0.49)kg were used to study the effects of chlortetracycline on the activity of intestinal nuclear factor NF-κB for a period of 15 days. The piglets were randomly allotted to three groups, each of which was replicated three times with eight pigs per replicate. The piglets were reared on a conventional corn-based diet supplemented with 0(control group), 75mg/kg(test 1) and 150mg/kg chlortetracycline(test 2). All piglets were given ad libitum access to both feed and water. The results showed that supplementation of chlortetracycline 75mg/kg and 150mg/kg significantly increased AFI by 3.62% and 8.35%(P<0.05) and decreased diarrhea rate by 22.10% (P<0.05) and 52.23%(P<0.01)respectively over the control group. The lengths of the villus within the jejunal and ileal sections of the small intestine were increased significantly (P<0.05) and the crypt depth were decreased significantly (P<001) relative to control group. The activity of NF-κB in jejunum of weaning piglets were decreased 41.57%(P<0.05)and 91.01%(P<0.01) and IκK-β mRNA expression decreased 56.16%(P<0.05)and 89.72%(P<0.01)in test 1 and test 2 group respectively. Nuclear factor NF-κB and IκB kinase(IκK-β) may play an important role in the modulation of macrophage and functions of digestive tract in weaning piglets
URL [本文引用: 2]

Seventy-two piglets (Duroc×Large White×Landrace) weaned at 21 days weighting (5.06±0.49)kg were used to study the effects of chlortetracycline on the activity of intestinal nuclear factor NF-κB for a period of 15 days. The piglets were randomly allotted to three groups, each of which was replicated three times with eight pigs per replicate. The piglets were reared on a conventional corn-based diet supplemented with 0(control group), 75mg/kg(test 1) and 150mg/kg chlortetracycline(test 2). All piglets were given ad libitum access to both feed and water. The results showed that supplementation of chlortetracycline 75mg/kg and 150mg/kg significantly increased AFI by 3.62% and 8.35%(P<0.05) and decreased diarrhea rate by 22.10% (P<0.05) and 52.23%(P<0.01)respectively over the control group. The lengths of the villus within the jejunal and ileal sections of the small intestine were increased significantly (P<0.05) and the crypt depth were decreased significantly (P<001) relative to control group. The activity of NF-κB in jejunum of weaning piglets were decreased 41.57%(P<0.05)and 91.01%(P<0.01) and IκK-β mRNA expression decreased 56.16%(P<0.05)and 89.72%(P<0.01)in test 1 and test 2 group respectively. Nuclear factor NF-κB and IκB kinase(IκK-β) may play an important role in the modulation of macrophage and functions of digestive tract in weaning piglets
DOI:10.1136/vr.100976URLPMID:23136309 [本文引用: 1]

The efficacy of chlortetracycline (CTC) in-feed medication to treat pigs with clinical respiratory disease was investigated in a farrow-to-finish pig herd infected with Mycoplasma hyopneumoniae, and with clinical respiratory disease in growing pigs. In total, 533 pigs were included. The animals were vaccinated against M hyopneumoniae and porcine circovirus type 2 at weaning. At onset of clinical respiratory disease, they were randomly allocated to one of the following treatment groups: chlortetracycline 1 (CTC1) (two consecutive weeks, 500 ppm), chlortetracycline 2 (CTC2) (two non-consecutive weeks, with a non-medicated week interval in between, 500 ppm) or tylosin (T) (three consecutive weeks, 100 ppm). Performance (daily weight gain, feed conversion ratio), pneumonia lesions at slaughter and clinical parameters (respiratory disease score) were assessed. Only numeric differences in favour of the CTC2 group were obtained for the performance and the clinical parameters. The prevalence of pneumonia lesions was 20.5, 13.1 and 23.0 per cent (P<0.05) for the CTC1, CTC2 and T groups, respectively. The study demonstrated that CTC, when administered at onset of clinical respiratory disease via the feed at a dose of 500 ppm during two alternative weeks, was able to decrease the prevalence of pneumonia lesions, and numerically reduce performance losses and clinical signs.
[本文引用: 1]
DOI:10.1136/vr.100976URLPMID:23136309 [本文引用: 1]

The efficacy of chlortetracycline (CTC) in-feed medication to treat pigs with clinical respiratory disease was investigated in a farrow-to-finish pig herd infected with Mycoplasma hyopneumoniae, and with clinical respiratory disease in growing pigs. In total, 533 pigs were included. The animals were vaccinated against M hyopneumoniae and porcine circovirus type 2 at weaning. At onset of clinical respiratory disease, they were randomly allocated to one of the following treatment groups: chlortetracycline 1 (CTC1) (two consecutive weeks, 500 ppm), chlortetracycline 2 (CTC2) (two non-consecutive weeks, with a non-medicated week interval in between, 500 ppm) or tylosin (T) (three consecutive weeks, 100 ppm). Performance (daily weight gain, feed conversion ratio), pneumonia lesions at slaughter and clinical parameters (respiratory disease score) were assessed. Only numeric differences in favour of the CTC2 group were obtained for the performance and the clinical parameters. The prevalence of pneumonia lesions was 20.5, 13.1 and 23.0 per cent (P<0.05) for the CTC1, CTC2 and T groups, respectively. The study demonstrated that CTC, when administered at onset of clinical respiratory disease via the feed at a dose of 500 ppm during two alternative weeks, was able to decrease the prevalence of pneumonia lesions, and numerically reduce performance losses and clinical signs.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:12753551 [本文引用: 1]
DOI:10.1111/j.1365-2885.1996.tb00054.xURLPMID:8866460 [本文引用: 3]

The disposition of oxytetracycline (OTC), tetracycline (TC) and chlortetracycline (CTC) was measured after intravenous and oral administration to pigs. Eighteen healthy pigs (six for each compound) weighing 22-43 kg received a dose of 10 mg/kg intravenously, and 45 mg/kg (OTC and TC) or 40 mg/kg (CTC) orally in both a fasted and a fed condition in a three-way crossover design. The three tetracyclines were present in plasma up to 30 hours after intravenous and after oral administration to fasted as well as fed pigs. The volume of distribution was 1.4, 1.2 and 0.7 L/kg body weight for OTC, TC and CTC respectively. The bioavailability was in general low for all the three tetracyclines. The presence of food did not affect the bioavailability of OTC, which was only 3% in both fasted and fed pigs. For TC there was a significantly higher bioavailability in fasted (18%) than in fed (5%) pigs, whereas for CTC the difference was not significant, being 11% in fasted vs. 6% in fed pigs. Even though the presence of food affected the bioavailability only for TC, it prolonged the absorption phase for all three tetracyclines. Based on the bioavailability and the resulting plasma concentrations, it is concluded that it is not possible to obtain a therapeutically active concentration in plasma or tissues after oral administration of any of the three tetracyclines to fed or fasted pigs.
DOI:10.1016/j.fct.2012.05.007URLPMID:22595330 [本文引用: 2]

Chickens were used to investigate plasma disposition of chlortetracycline after single IV (15 mg/kg) and multiple oral administration (60 mg/kg, 5 days) and residue depletion of chlortetracycline after multiple oral doses (60 mg/kg, 5 days). Plasma and tissue samples were analyzed by HPLC. Mean elimination half-lives in plasma were 7.96 and 13.15 h after IV and multiple oral administration. Maximum plasma concentration was 4.33 mug/ml and the interval from oral administration until maximal concentration was 1.79 h. Oral bioavailability was 17.76%. After multiple oral dose, mean kidney, liver and muscle tissue concentrations of chlortetracycline+4-epi-chlortetracycline of 835.3, 192.7, and 126.3 mug/kg, respectively, were measured 1 day after administration of the final dose of chlortetracycline. Chlortetracycline residues were detected in kidney and liver (205.4 and 81.7 mug/kg, respectively), but not in muscle, 3 days after the end of chlortetracycline treatment. The mean chlortetracycline+4-epi-chlortetracycline concentrations were below LOQ at 3 and 5 days after cessation of medication in muscle and liver, respectively. A withdrawal time of 3 days was necessary to ensure that the chlortetracycline residues were less than the maximal residue limits (MRLs) established by the European Union (100, 300, and 600 mug/kg in muscle, liver, and kidney, respectively).
DOI:10.1111/j.1365-2885.2009.1116.xURLPMID:20444029 [本文引用: 3]

Chlortetracycline HCl (CTC) has impacted profitable livestock production since 1945. However, pharmacokinetic parameters for CTC in ruminating cattle are unavailable in peer-reviewed literature. A total of 18 steers were randomized to 4.4, 11, or 22 mg/kg/day p.o. CTC treatment groups (n = 6). Chlortetracycline treatment was offered as one-half of the daily dose b.i.d. (160 total doses/group) for 80 days. Blood samples were collected at selected time points throughout an 83-day study and analyzed with a solid phase extraction technique and novel ultrahigh performance liquid chromatography-mass spectroscopy/mass spectroscopy analytical method. Noncompartmental analysis (NCA) determined individual pharmacokinetic parameters by treatment group with coefficient of variation (CV %) estimates. A one-compartment open model with first order absorption and elimination, where absorption rate constant was equal to elimination rate constant, was fitted using nonlinear mixed effects modeling (NLMEM). NLMEM determined the primary pharmacokinetic parameters: volume of distribution (V/F, 40.9 L/kg) and rate constant (k, 0.0478 h(-1)), and the secondary parameters: dose-normalized area under the curve (AUC/D, 0.29 h x microg/L), clearance (Cl/F, 1.8 L/kg/h), elimination half-life (t(1/2), 16.2 h), C(max/Dose) (4.5 ng/mL), and time of C(max) (T(max), 23.3 h) with improved CV estimates over NCA. Dose linearity was confirmed by anova of parameters derived from NCA by treatment group. Further studies are necessary for determining absolute bioavailability and pharmacokinetic-pharmacodynamic relationships of CTC in group fed, ruminating cattle.
DOI:10.1111/j.1365-2885.1990.tb00747.xURLPMID:2319636 [本文引用: 3]

Chlortetracycline hydrochloride was administered intra-arterially (11 mg/kg) and as an oral drench (33 mg/kg) to ten 21.0-31.5-kg pigs. Five of the pigs were fasted 18 h prior to dosing and five of the pigs were fed ad libitum prior to dosing. The mean volume of distribution determined by area-under-the-curve calculations for the fasted pigs (0.967 +/- 0.210 l/kg) was significantly less (P less than 0.05) than the mean volume of distribution for the fed pigs (1.39 +/- 0.31 l/kg). Mean total body clearance of the drug was also significantly less (P less than 0.05) in the fasted pigs (0.165 +/- 0.055 l/kg/h) as compared to the fed pigs (0.307 +/- 0.053 l/kg/h). The elimination constants (beta) were not found to be statistically different (P less than 0.05): 0.1811 +/- 0.0057 for the fasted pigs; 0.2260 +/- 0.0461 for the fed pigs. The bioavailability for both groups was similar; 19.12 +/- 8.3% for the fasted pigs and 17.88 +/- 5.3% for the fed pigs. In a second experiment three groups of six pigs which weighed 34.5-44.1 kg were fed a corn-soy diet ad libitum. The rations were fortified with chlortetracycline at 100, 400 or 1000 mg chlortetracycline hydrochloride/kg feed. Chlortetracycline concentrations were determined in plasma samples collected over a 6-day period. Plasma chlortetracycline concentrations reach a plateau within 24 h after initial access to the trial diets and were highly correlated with the dose of the drug consumed (r2 = 0.97).
URL [本文引用: 2]
URL [本文引用: 2]
DOI:10.1111/jvp.12144URLPMID:25131164 [本文引用: 1]

The objectives of this study were to determine plasma concentrations and pharmacokinetic parameters of feed-grade chlortetracycline (CTC) in sheep after oral administration of 80 or 500 mg/head daily, divided into two equal doses given at 12-h intervals for 8 days. These are the approved, and commonly used but unapproved, feed additive doses, respectively, in the United States for the prevention of ovine infectious abortion. Blood samples were collected just prior to dosing at 0, 12, 24, 72, 96, and 192 h, as well as 4, 8, 12, 24, and 36 h after the last dose, and noncompartmental pharmacokinetic analysis was performed to estimate elimination half-life and area under the plasma concentration-time curve (AUC). Mean observed maximum CTC concentrations (Cmax ) were 20.0 ng/mL (80 mg dose) and 101 ng/mL (500 mg dose). Mean apparent elimination half-life was 18 h (80 mg dose) and 20 h (500 mg dose). Although published data do not exist to estimate plasma CTC concentrations necessary for the prevention of ovine infectious abortion, concentrations reached in our study suggest that either the FDA-approved and FDA-unapproved dosages are not high enough or that the pharmacodynamic parameter relating preventive dose to pathogen minimum inhibitory concentrations is yet to be determined.
URLPMID:3354964 [本文引用: 1]

The pharmacokinetics of chlortetracycline (CTC) in plasma and bile after intravenous administration in turkeys was ascertained. After a dose of CTC (0.9 mg/kg) was administered IV, 8.5% of the dose appeared in the bile in 4 hours. The peak bile/plasma concentration ratio for CTC was 254 at 2 hours. The bile/plasma concentration ratio was greater than 1 from 10 to 240 minutes after CTC administration.
URLPMID:4087164 [本文引用: 1]
URL [本文引用: 1]

微囊是利用天然或合成高分子作外膜,将极其微小的内芯物质(液体、气体或固体等)包覆成半透性或密闭性的微小囊状物.第一个微囊产品是1936 年大西洋海岸渔业公司在液体石蜡中制备的鱼肝油明胶微囊.1949年,Wurster 发明了微囊的空气悬浮法,改进了药物的总包衣过程.1954 年,Green 成功的将微囊技术应用于无碳复写纸的生产,从而开始了微囊技术的工业化.
URL [本文引用: 1]

微囊是利用天然或合成高分子作外膜,将极其微小的内芯物质(液体、气体或固体等)包覆成半透性或密闭性的微小囊状物.第一个微囊产品是1936 年大西洋海岸渔业公司在液体石蜡中制备的鱼肝油明胶微囊.1949年,Wurster 发明了微囊的空气悬浮法,改进了药物的总包衣过程.1954 年,Green 成功的将微囊技术应用于无碳复写纸的生产,从而开始了微囊技术的工业化.
URL [本文引用: 1]

中药制荆现代化是中药现代化的重要组成部分,将现代药物制剂新技术与新方法引入传统中药制剂中,优化传统中药的使用方式,提高中药在临床的利用率,促进中医药的发展.
URL [本文引用: 1]

中药制荆现代化是中药现代化的重要组成部分,将现代药物制剂新技术与新方法引入传统中药制剂中,优化传统中药的使用方式,提高中药在临床的利用率,促进中医药的发展.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.rvsc.2019.11.010URLPMID:31785428 [本文引用: 1]

Swine respiratory disease complex (SRDC) causes massive economic losses to the swine industry and is a major animal welfare concern. Antimicrobials are mainstay in treatment and control of SRDC. However, there is a lack of data on the prevalence and trends in resistance to antimicrobials in bacterial pathogens associated with SRDC. The objective of this study was to estimate the prevalence and changes in resistance to 13 antimicrobials in swine bacterial pathogens (Streptococcus suis, Pasteurella multocida, Actinobacillus suis and Haemophilus parasuis) in the U.S.A using data collected at University of Minnesota Veterinary Diagnostic Laboratory between 2006 and 2016. For antimicrobials for which breakpoints were available, prevalence of resistance remained below 10% except for tetracycline in S. suis and P. multocida isolates, and these prevalence estimates remained consistently low over the years despite statistical significance (p < .05) in trend analysis. For antimicrobial-bacterial combinations without available breakpoints, the odds of isolates being resistant increased by >10% annually for 7 and 1 antimicrobials in H. parasuis and S. suis isolates respectively, and decreased >10% annually for 4 and 1 antimicrobials in A. suis and H. parasuis isolates, respectively, according to the ordinal regression models. Clinical implications of changes in AMR for A. suis and H. parasuis should be interpreted cautiously due to the lack of interpretive criteria and challenges in antimicrobial susceptibility tests in the case of H. parasuis. Future studies should focus on surveillance of antimicrobial resistance and establishment of standardized susceptibility testing methodologies and interpretive criteria for these animal pathogens of critical importance.
DOI:10.1002/cpt19612151URLPMID:13755136 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
