 ,1,2, 平俊爱
,1,2, 平俊爱 ,1,2, 王玉斌1,2, 张福耀1,2, 吕鑫1,2, 李慧明1,2, 楚建强1,2
,1,2, 王玉斌1,2, 张福耀1,2, 吕鑫1,2, 李慧明1,2, 楚建强1,2Molecular Aided Breeding System of Photosensitive Forage Sorghum Based on SSR
NIU Hao ,1,2, PING JunAi
,1,2, PING JunAi ,1,2, WANG YuBin1,2, ZHANG FuYao1,2, Lü Xin1,2, LI HuiMing1,2, CHU JianQiang1,2
,1,2, WANG YuBin1,2, ZHANG FuYao1,2, Lü Xin1,2, LI HuiMing1,2, CHU JianQiang1,2通讯作者:
责任编辑: 李莉
收稿日期:2019-06-6接受日期:2019-08-8网络出版日期:2020-07-16
| 基金资助: |
Received:2019-06-6Accepted:2019-08-8Online:2020-07-16
作者简介 About authors
牛皓,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (3578KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
牛皓, 平俊爱, 王玉斌, 张福耀, 吕鑫, 李慧明, 楚建强. 基于SSR的光敏型饲草高粱分子辅助育种体系[J]. 中国农业科学, 2020, 53(14): 2795-2803 doi:10.3864/j.issn.0578-1752.2020.14.004
NIU Hao, PING JunAi, WANG YuBin, ZHANG FuYao, Lü Xin, LI HuiMing, CHU JianQiang.
0 引言
【研究意义】饲草高粱是中国主要青贮饲料,由于其再生能力强、产量高、蛋白质丰富、易消化等特点,被广泛应用于农牧饲喂及渔业养殖领域[1,2]。光敏感特性是影响饲草高粱产量和品质的主要因素[3,4]。在北方夏季长日照地区,当光照时长超过12.3 h时,具有光敏感特性的饲草高粱不抽穗结实,只进行营养生长,其高度可超过4 m,部分材料在新疆地区可长至6 m,因而产量、蛋白质含量比普通饲草高粱高,若部分替代玉米作为哺乳期奶牛饲料,在增加牛奶乳脂肪含量上有显著效果[5,6,7,8,9]。然而,在光敏型饲草高粱品种选育的过程中,稳定后代是否具有光敏特性,必须将其与普通的不育系测交,第二年种植于大田中,秋天观察抽穗性状才能判断,选育过程中物力、人力、地力投入较多,育种成本非常高,因此,利用分子标记辅助育种技术对传统育种方法进行改良,提高育种效率显得尤为重要。【前人研究进展】生物质产量是将木质素转化为生物燃料或生物能源的生物质或生物能源作物最重要的属性之一,生长期是生物质产量的首要决定因素。因此,假设环境条件允许产量潜力得以发挥,光敏型高粱积累最多的生物质,可达到普通高粱的2倍以上[10]。目前,已知与高粱光敏性相关的基因共有6个,为Ma1—Ma6[10,11]。早在1945年,美国****QUINBY等[12]认为有3个基因影响高粱的花期和成熟期,分别为Ma1、Ma2和Ma3。Ma2、Ma3在没有Ma1的情况下不会表达,Ma3在显性Ma2存在的情况下也不会表达。同时Ma1的作用受光周期的影响,开花时间控制着叶片的数量、生长时间和植株的最终大小。1965年,QUINBY[13,14]又发现了第4个与高粱成熟期相关的基因,并将它命名为Ma4,认为这4个基因的表达受环境的影响,特别是受光周期和温度的影响,且花期开始的早晚取决于品种的成熟基因型。20世纪90年代,MORGAN等[11]、MAJOR等[15]从环境、温度等因素对这一概念又进行了阐述,之后有关高粱光敏基因的研究丰富起来。CHILDS等[16]认为Ma3是第一个被克隆的与高粱成熟期相关的基因,并且有3个等位基因分别为Ma3、ma3、ma3R。ROONEY等[17,18]和MORGAN等[19]将高粱恢复系EBA-3去雄后与其他非光敏(photo-insensitive)恢复系杂交,发现8个F2群体中PS/PI分离比率为9﹕7,表明2个独立的基因位点在互补显性上位中相互作用,而这两个与光周期敏感度相关的基因与先前的Ma1、Ma2、Ma3和Ma4不同,从而定名为Ma5和Ma6,并发明了基于Ma5/Ma6系统的生物质能源高粱的选育方法。MURPHY等[20]认为蛋白PRR37控制高粱的开花时间。YANG等[21,22]认为高粱CONSTANS是一种开花的激活剂,在开花抑制剂PRR37的长时间内在转录后受到抑制,有助于光敏感型短日照高粱的开花。WANG等[23]对Ma3的进化做了分析。另外,针对饲草高粱生物产量与光敏感特性的研究,WOLABU等[24,25]认为不同类型的高粱在温带地区的应用主要取决于其开花时间的控制,光周期不敏感的高粱用于粮食生产,而光周期敏感基因型则用于饲料和生物量原料生产。关于光敏感基因定位的研究,MURPHY等[20]将Ma1定位在高粱第6染色体上的标记Xtxi62和Xtxi58之间约86 kb的区段内。MULLET等[26]将Ma2定位在高粱第2染色体67 923 811—68 393 290 bp,认为该基因可能通过抑制由光敏色素B和C介导的调节高粱光照依赖性产物来调控高粱的开花时间。Ma3是第一个被成功克隆的调控高粱感光性的基因,被定位于高粱第1染色体长臂的末端,在60 910 479—60 917 763 bp。Ma3至少有3种等位基因,分别为显性Ma3、隐性Ma3和一种特殊的隐性基因Ma3[27,28,29]。Ma4被定位在高粱第10染色体,与RFLP标记txs1163相邻[30,31]。Ma5被定位到高粱第1染色体6 762 743—6 767 650 bp,在遗传图谱上的位置为23—26 cM,对应的基因编码光敏色素C[26]。Ma6被定位在高粱第6染色体39 379 760—42 610 705 bp,它和Ma1均受Ma3、Ma5的遗传上位性影响,通过抑制一系列成花素基因的表达,使高粱在长日照条件下开花时间延迟。Ma6是调控高粱籽粒数量、株高和抽穗期的基因——SbGHD7[26]。牛皓等[32]对144对SSR引物(包括PRR37(Ma1)、GHD7(Ma6)、PHYB(Ma3)、PHYC(Ma5)等基因对应的引物)进行筛选,筛出4对疑似光敏感引物,但经过与F1杂交种扩大群体验证,发现它们并不是特异性引物,不能将光敏和非光敏的材料区分开,因而不具备生产使用价值。【本研究切入点】高粱光敏感基因的理论研究丰富,但利用已有成果形成育种体系应用于田间育种的研究鲜有报道。【拟解决的关键问题】本研究以具有光敏感特性(photosensitive)的饲草高粱恢复系晋光1R和普通非光敏(photo-insensitive)饲草高粱恢复系BMRC-3-2为研究对象,通过杂交构建F2群体,利用微卫星分子标记技术,定位光敏基因,筛选特异性引物,为今后改良高粱品种传统育种方式,提高育种效率,降低育种成本,进而形成新的育种体系奠定理论基础。1 材料与方法
1.1 试验材料
亲本材料:具有光敏基因的饲草高粱恢复系晋光1R,普通饲草高粱恢复系BMRC-3-2。F2群体:2017年,在山西榆次以光敏恢复系晋光1R为母本,非光敏恢复系BMRC-3-2为父本,经人工去雄有性杂交得到F0种子;2017年冬,在海南三亚种植F0种子,并自交,得到F1种子,2018年,获得晋光1R/BMRC-3-2的F2群体。
F1杂交种:以晋光1R为亲本选育的高代稳定恢复系作为父本,与其他不育系测配得到的F1杂交种。
部分SSR标记序列来源于NCBI网站,部分由上海派森诺生物科技有限公司依据候选基因序列设计合成。
材料种子由山西省农业科学院高粱研究所提供。所有试验材料均种植于山西省农业科学院高粱研究所东白试验基地。田间管理与其他普通高粱一致,2018年8月底调查抽穗情况。
1.2 表型鉴定及遗传分析
取样前,对亲本及群体单株进行光敏和非光敏性状调查统计。根据表型鉴定结果计算分离比例,并进行卡方检测,确定光敏性状在F2群体中的分离情况,用于遗传分析。1.3 DNA提取及近等基因池的构建
为确保取样的准确性,在8月底高粱抽穗尾期,按照光敏(不抽穗)与非光敏(抽穗)将F2分两类,每类随机各选30株,分别挂牌标记,取顶部新鲜幼叶放入2 mL离心管中,液氮速冻,由上海派森诺生物科技有限公司提DNA及进行后续相关试验。同时,将1.1内容中F1杂交种,也按照光敏与非光敏分两类,每类随机各取50株顶端新鲜幼叶放入2 mL离心管中,液氮速冻,由上海派森诺生物科技有限公司进行微卫星标记验证试验。1.4 BSA试验
构建基于Illumina HiSeq测序平台,利用第二代测序技术(next-generation sequencing,NGS),对插入片段为400的文库进行双末端(paired-end,PE)测序。通过对目标性状区域分析,确定基因位置。1.5 微卫星标记验证试验
将F1杂交种DNA进行毛细管电泳检测,验证特异性引物。2 结果
2.1 表型鉴定和遗传分析
晋光1R与BMRC-3-2杂交,对F2进行性状调查,其中,251株不抽穗,169株抽穗,经χ2检验光敏与非光敏(PS/PI)的分离比例符合9﹕7(表1)。Table1
表1
表1F2光敏性状的遗传模式
Table1
| 性状Traits | 实际值Actual value | 理论值Theoretical value | 分离比Separation ratio | χ2 |
|---|---|---|---|---|
| 不抽穗Photosensitive | 251 | 236 | 9﹕7 | 1.1 |
| 抽穗Photo-insensitive | 169 | 184 |
新窗口打开|下载CSV
2.2 基因初步定位及功能注释
经过测序,选取99%阈值线最高点前后约250 kb的区域作为性状相关的候选区域,区域总长度为500 kb,这个范围内满足条件的SNP位点共400个,对这些SNP位点注释,其中,非同义突变或者stop-gain或者stop-loss的SNP位点有6个,分布在4个基因。关联区域位于第7染色体810 000—1 310 000 bp(图1)。图1
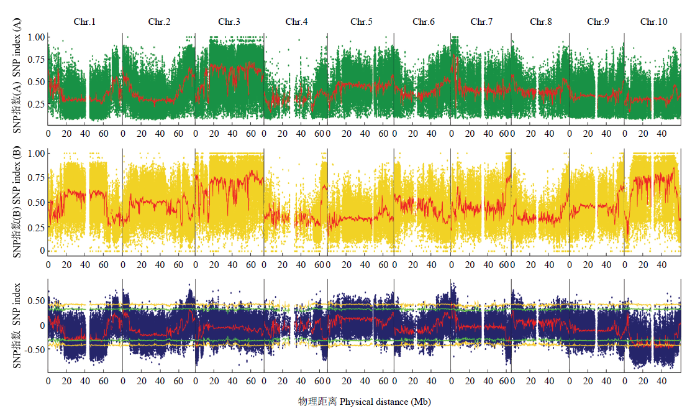 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1子代混池 SNP index和△(SNP index)在染色体上的分布
Fig. 1Distribution of SNP index and△(SNP index) on chromosomes in offspring mixed pools
4个候选基因分别为LOC8068537、LOC110437009、LOC8068548和LOC8066082。通过与GO、KEFG、Swiss Prot、NR等数据库比对注释,其中LOC8068537和LOC8068548都是剪切因子基因,因而,认为它们可能就是与饲草高粱光敏感相关的基因。
2.3 特异性SSR引物的获得及分子辅助育种体系的建立
在F1材料的DNA中选取光敏与非光敏单株各2株,根据候选基因序列设计合成的引物共51对,进行荧光定量PCR,每组引物前2条带为非光敏F1,后2条带为光敏F1(图2)。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2F1样品的PCR检测
Fig. 2PCR detection of F1 generation samples
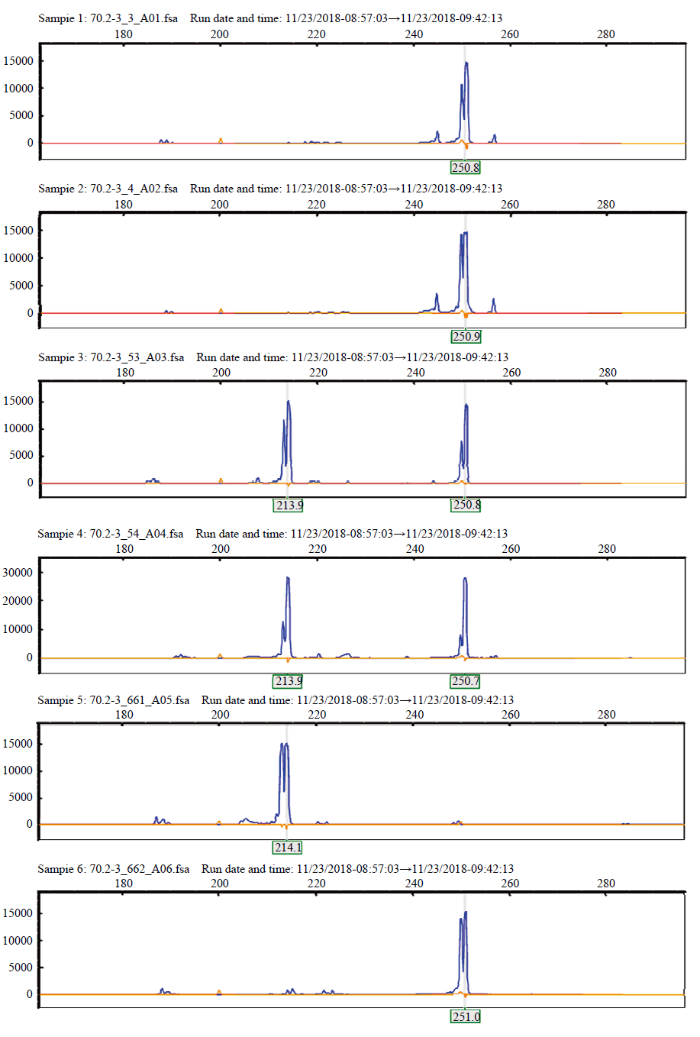
根据图2结果,将个别差异极不明显的引物排除,其余引物扩大样本至100个F1材料进行毛细管电泳验证。结果表明,仅有引物70.2-3可以区分光敏与非光敏F1材料(图3)。非光敏型F1杂交种在251 bp
图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3引物70.2-3对亲本和F1植株的毛细管电泳图
Sample1和Sample2为非光敏型F1杂交种,Sample3和Sample4为光敏型F1杂交种,Sample5为光敏亲本晋光1R,Sample6为非光敏亲本BMRC-3-2
Fig. 3Capillary electrophoresis of primer 70.2-3 pairs of parents and F1 plants
Sample1 and Sample2 are photo-insensitive F1 hybrids, Sample3 and Sample4 are photosensitive F1 hybrids, Sample5 is the photosensitive parent Jinguang1R, Sample6 is the photo-insensitive parent BMRC-3-2
附近出现“单峰”;而光敏型则在214和251 bp两处附近出现“双峰”;光敏亲本和非光敏亲本则分别在214和251 bp附近出现“单峰”。以251 bp处出现“单峰”为判断依据,引物70.2-3对50个非光敏型F1杂交种的鉴选准确率达到100%,所有材料均在251 bp附近出现峰值。以214和251 bp两处附近出现“双峰”为判断依据,该引物对另外50个光敏型F1杂交种鉴选的准确率达到90%,其中F1-69、F1-70、F1-71这三个材料在214和262 bp附近出现“双峰”;F1-81仅在251 bp处有“单峰”而F1-86则在251和232 bp两处附近出现“双峰”。据此,认为所选引物70.2-3为特异性引物,可以区分光敏和非光敏杂交种,可以作为田间生产指导。其序列为FORWARD PRIMER:5′- CCTCCTCTTCCTCGGATAGC-3′和REVERSEPRIMER:5′-ATGATCGGTGGT TG GAAGAG-3′。
综上所述,对传统的光敏感饲草高粱选育体系进行改良,利用实验室筛选代替田间测交,形成新的分子辅助育种体系(图4)。
图4
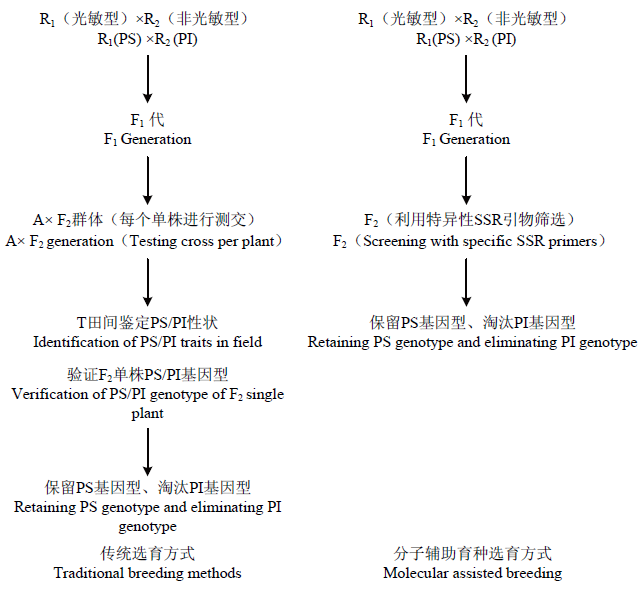 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4新旧育种体系对比示意图
Fig. 4Comparison of old and new breeding systems
3 讨论
3.1 传统选育方式
光敏型饲草高粱恢复系的选育,以传统高粱恢复系选育方法为基础,选择具有光敏特性的饲草高粱恢复系与普通非光敏但具有其他优良性状的饲草高粱恢复系人工去雄有性杂交,获得F0种子,通过南繁加代也可下一年种植大田得到F1,F1套袋自交后第二年得到F2群体。从这一步开始传统的育种方法需另选不育系A与选出的F2群体单株一一测交,得到杂交种T,翌年田间种植后再观察抽穗情况,从而判定上年测交F2群体中哪株为光敏型。3.2 分子辅助育种选育方式
分子辅助育种选育方式与传统育种方式在获得F2群体的方法上保持一致,但将工作量最繁琐的田间测交验证这一步用实验室荧光定量PCR方法取代,利用筛选的特异性SSR引物70.2-3将所有F2群体中的个体进行毛细管电泳检测,观察214和251 bp处的“峰值”情况,两处均出现“峰”则判定为光敏型,非光敏型则淘汰。结果表明,该引物筛选光敏材料准确度非常高,但仍有极少部分材料出现峰值位移,这与部分“中间型”材料取样分类误差有关。同时,候选基因LOC8068537和LOC8068548定位在第7染色体,与目前所有已知的与高粱光敏感性相关的基因位置都不同,是否是新的光敏基因,其调控机理如何还需进一步研究确定,但F2群体中光敏与非光敏(PS/PI)分离比例为9﹕7,这说明饲草高粱光敏感特性是由2个独立的互补显性基因控制,这与ROONEY[17]研究结果相同。3.3 分子辅助育种体系优点
本研究创造的光敏型饲草高粱分子辅助育种体系是对传统育种体系的改良,优点主要体现在:1)节约育种时间;2)节省育种成本。通过对比可发现,传统的育种体系比分子辅助育种体系的育种年限要多1年,而多出来的这一年必然会引起育种成本的增加。假设F2群体共200株,每株均与不育系A测交,收获测交种子200份,第二年以每15 m2小区种6行,每2行种1个材料来计算,大约需要67个小区0.1 hm2,以2019年每公顷地育种成本来计算,需约6 000元,而实验室荧光定量PCR耗材费用不超过2 000元。如果F2群体数量庞大,后者将节省更多的育种成本。综上所述,调控高粱抽穗期性状的基因很多,而且等位基因也很丰富,并且影响不同杂交组合产生后代的抽穗期性状的QTL位点各不相同,调控高粱抽穗期性状的基因之间互作方式也是多种多样。因此,尽可能多的发掘调控高粱抽穗期性状的基因位点,明确基因功能及互作模式,对于揭示高粱光敏感特性的机理、提高育种效率、节约育种成本有着重要的意义。
4 结论
发现控制高粱抽穗期的候选基因LOC8068537和LOC8068548,位于高粱第7染色体810 000— 1 310 000 bp。同时特异性SSR引物70.2-3的获得,在饲草高粱光敏性鉴定上有极高的准确率,基于该引物构建的光敏型饲草高粱选育体系,利用实验室验证替代田间测交观察,节省育种成本,提高育种效率。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:22394582 [本文引用: 1]
DOI:10.1371/journal.pone.0212154URLPMID:30969968 [本文引用: 1]

Sorghum bicolor is a drought-resilient facultative short-day C4 grass that is grown for grain, forage, and biomass. Adaptation of sorghum for grain production in temperate regions resulted in the selection of mutations in Maturity loci (Ma1 -Ma6) that reduced photoperiod sensitivity and resulted in earlier flowering in long days. Prior studies identified the genes associated with Ma1 (PRR37), Ma3 (PHYB), Ma5 (PHYC) and Ma6 (GHD7) and characterized their role in the flowering time regulatory pathway. The current study focused on understanding the function and identity of Ma2. Ma2 delayed flowering in long days by selectively enhancing the expression of SbPRR37 (Ma1) and SbCO, genes that co-repress the expression of SbCN12, a source of florigen. Genetic analysis identified epistatic interactions between Ma2 and Ma4 and located QTL corresponding to Ma2 on SBI02 and Ma4 on SBI10. Positional cloning and whole genome sequencing identified a candidate gene for Ma2, Sobic.002G302700, which encodes a SET and MYND (SYMD) domain lysine methyltransferase. Eight sorghum genotypes previously identified as recessive for Ma2 contained the mutated version of Sobic.002G302700 present in 80M (ma2) and one additional putative recessive ma2 allele was identified in diverse sorghum accessions.
[本文引用: 1]
[本文引用: 1]
URLPMID:30223789 [本文引用: 1]
[本文引用: 1]
DOI:10.3168/jds.2017-12552URLPMID:28527803 [本文引用: 1]

Double cropping and increasing crop diversity could improve dairy farm economic and environmental sustainability. In this experiment, corn silage was partially replaced with 2 alternative forages, brown midrib-6 brachytic dwarf forage sorghum (Sorghum bicolor) or fall-grown oat (Avena sativa) silage, in the diet of lactating dairy cows. We investigated the effect on dry matter (DM) intake, milk yield (MY), milk components and fatty acid profile, apparent total-tract nutrient digestibility, N utilization, enteric methane emissions, and income over feed cost. We analyzed the in situ DM and neutral detergent fiber disappearance of the alternative forages versus corn silage and alfalfa haylage. Sorghum was grown in the summer and harvested in the milk stage. Oats were grown in the fall and harvested in the boot stage. Compared with corn silage, neutral detergent fiber and acid detergent fiber concentrations were higher in the alternative forages. Lignin content was highest for sorghum silage and similar for corn silage and oat silage. The alternative forages had less than 1% starch compared with the approximately 35% starch in the corn silage. Ruminal in situ DM effective degradability was similar, although statistically different, for corn silage and oat silage, but lower for sorghum silage. Diets with the alternative forages were fed in a replicated 3 x 3 Latin square design experiment with three 28-d periods and 12 Holstein cows. The control diet contained 44% (DM basis) corn silage. In the other 2 diets, sorghum or oat silages were included at 10% of dietary DM, replacing corn silage. Sorghum silage inclusion decreased DM intake, MY, and milk protein content but increased milk fat and maintained energy-corrected MY similar to the control. Oat silage had no effect on DM intake, MY, or milk components compared to the control. The oat silage diet increased apparent total-tract digestibility of dietary nutrients, except starch, whereas the sorghum diet slightly decreased DM, organic matter, crude protein, and starch digestibility. Cows consuming the oat silage diet had higher milk urea N and urinary urea N concentrations. Milk N efficiency was decreased by the sorghum diet. Diet did not affect enteric methane or carbon dioxide emissions. This study shows that oat silage can partially replace corn silage at 10% of the diet DM with no effect on MY. Brown midrib sorghum silage harvested at the milk stage with <1% starch may decrease DM intake and MY in dairy cows.
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:12228479 [本文引用: 1]
[本文引用: 2]
[本文引用: 1]
DOI:10.2135/cropsci2002.1791URL [本文引用: 1]
DOI:10.1073/pnas.1106212108URLPMID:21930910 [本文引用: 2]

Optimal flowering time is critical to the success of modern agriculture. Sorghum is a short-day tropical species that exhibits substantial photoperiod sensitivity and delayed flowering in long days. Genotypes with reduced photoperiod sensitivity enabled sorghum's utilization as a grain crop in temperate zones worldwide. In the present study, Ma(1), the major repressor of sorghum flowering in long days, was identified as the pseudoresponse regulator protein 37 (PRR37) through positional cloning and analysis of SbPRR37 alleles that modulate flowering time in grain and energy sorghum. Several allelic variants of SbPRR37 were identified in early flowering grain sorghum germplasm that contain unique loss-of-function mutations. We show that in long days SbPRR37 activates expression of the floral inhibitor CONSTANS and represses expression of the floral activators Early Heading Date 1, FLOWERING LOCUS T, Zea mays CENTRORADIALIS 8, and floral induction. Expression of SbPRR37 is light dependent and regulated by the circadian clock, with peaks of RNA abundance in the morning and evening in long days. In short days, the evening-phase expression of SbPRR37 does not occur due to darkness, allowing sorghum to flower in this photoperiod. This study provides insight into an external coincidence mechanism of photoperiodic regulation of flowering time mediated by PRR37 in the short-day grass sorghum and identifies important alleles of SbPRR37 that are critical for the utilization of this tropical grass in temperate zone grain and bioenergy production.
[本文引用: 1]
DOI:10.1371/journal.pone.0105352URLPMID:25122453 [本文引用: 1]

Light signaling by phytochrome B in long days inhibits flowering in sorghum by increasing expression of the long day floral repressors PSEUDORESPONSE REGULATOR PROTEIN (SbPRR37, Ma1) and GRAIN NUMBER, PLANT HEIGHT AND HEADING DATE 7 (SbGHD7, Ma6). SbPRR37 and SbGHD7 RNA abundance peaks in the morning and in the evening of long days through coordinate regulation by light and output from the circadian clock. 58 M, a phytochrome B deficient (phyB-1, ma3R) genotype, flowered approximately 60 days earlier than 100 M (PHYB, Ma3) in long days and approximately 11 days earlier in short days. Populations derived from 58 M (Ma1, ma3R, Ma5, ma6) and R.07007 (Ma1, Ma3, ma5, Ma6) varied in flowering time due to QTL aligned to PHYB/phyB-1 (Ma3), Ma5, and GHD7/ghd7-1 (Ma6). PHYC was proposed as a candidate gene for Ma5 based on alignment and allelic variation. PHYB and Ma5 (PHYC) were epistatic to Ma1 and Ma6 and progeny recessive for either gene flowered early in long days. Light signaling mediated by PhyB was required for high expression of the floral repressors SbPRR37 and SbGHD7 during the evening of long days. In 100 M (PHYB) the floral activators SbEHD1, SbCN8 and SbCN12 were repressed in long days and de-repressed in short days. In 58 M (phyB-1) these genes were highly expressed in long and short days. Furthermore, SbCN15, the ortholog of rice Hd3a (FT), is expressed at low levels in 100 M but at high levels in 58 M (phyB-1) regardless of day length, indicating that PhyB regulation of SbCN15 expression may modify flowering time in a photoperiod-insensitive manner.
URLPMID:25961888 [本文引用: 1]
DOI:10.1080/15592324.2016.1261232URLPMID:27854155 [本文引用: 1]

Sorghum is a short day plant with strong photoperiod response and its cultivation for grain in temperate regions necessitated the development of photoperiod insensitive mutants that can flower rapidly in the long days of summer. Wild type genotypes grow vegetatively in summer accumulating significant biomass before floral transition ensues during the shorter days of fall. Thus, photoperiod insensitive mutants are grown for grain production while photoperiod sensitive wild type genotypes are grown for forage and biomass feedstock production in the United States. However, the molecular mechanism of photoperiod response and floral transition is poorly understood in sorghum. We have previously reported 3 FLOWERING LOCUS T homologues (SbFT1, SbFT8 and SbFT10) that serve as the ultimate mediators of photoperiod response and floral transition, but more work remains to be done to clearly define the molecular function of the upstream regulatory factors. One of the major QTL that accounts for 85% of the flowering time variation, which was reported to be encoding the PRR37 protein is now debated to be encoding the SbFT12 protein, raising further questions as to how SbFT12 may regulate sorghum florigens. Further molecular analyses will uncover the true nature of the day length sensors in sorghum and the mechanisms of their interactions with florigens to modulate photoperiod dependent vegetative growth and floral transition.
URLPMID:26765652 [本文引用: 1]
[本文引用: 3]
DOI:10.1104/pp.113.2.611URL [本文引用: 1]
URLPMID:16668953 [本文引用: 1]
DOI:10.1104/pp.97.2.714URLPMID:16668457 [本文引用: 1]

Physiological processes controlled by phytochrome were examined in three near-isogenic genotypes of Sorghum bicolor, differing at the allele of the third maturity gene locus. Seedlings of 58M (ma(3) (R)ma(3) (R)) did not show phytochrome control of anthocyanin synthesis. In contrast, seedlings of 90M (ma(3)ma(3)) and 100M (Ma(3)Ma(3)) demonstrated reduced anthocyanin synthesis after treatment with far red and reversal of the far red effect by red. De-etiolation of 48-hour-old 90M and 100M dark-grown seedlings occurred with 48 hours of continuous red. Dark-grown 58M seedlings did not de-etiolate with continuous red treatment. Treatment of seedlings with gibberellic acid or tetcyclacis, a gibberellin synthesis inhibitor, did not alter anthocyanin synthesis. Levels of chlorophyll and anthocyanin were lower in light-grown 58M seedlings than in 90M and 100M. Etiolated seedlings of all three genotypes have similar amounts of photoreversible phytochrome. Crude protein extracts from etiolated seedlings were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose. Phytochrome was visualized with Pea-25, a monoclonal antibody directed to phytochrome from etiolated peas. The samples from all three genotypes contained approximately equivalent amounts of a prominent, immunostaining band at 126 kD. However, the sample from 58M did not show a fainter, secondary band at 123 kD that was present in 90M and 100M. The identity and importance of this secondary band at 123 kD is unknown. We propose that 58M is a phytochrome-related mutant that contains normal amounts of photoreversible phytochrome and normal phytochrome protein when grown in the dark.
[本文引用: 1]
DOI:10.1023/A:1017513608309URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
