 ,, 王艳秋, 李金红, 朱凯
,, 王艳秋, 李金红, 朱凯 ,辽宁省农业科学院高粱研究所,沈阳 110161
,辽宁省农业科学院高粱研究所,沈阳 110161Dwarfing Effect and Molecular Mechanism of An Elite Sorghum Male Sterile Line 01-26A in Its Hybrids
ZOU JianQiu ,, WANG YanQiu, LI JinHong, ZHU Kai
,, WANG YanQiu, LI JinHong, ZHU Kai ,Sorghum Research Institute, Liaoning Academy of Agricultural Sciences, Shenyang 110161
,Sorghum Research Institute, Liaoning Academy of Agricultural Sciences, Shenyang 110161通讯作者:
责任编辑: 李莉
收稿日期:2019-07-31接受日期:2019-09-22网络出版日期:2020-07-16
| 基金资助: |
Received:2019-07-31Accepted:2019-09-22Online:2020-07-16
作者简介 About authors
邹剑秋,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (4233KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
邹剑秋, 王艳秋, 李金红, 朱凯. 优异高粱雄性不育系01-26A的组配降秆效应及其分子机理[J]. 中国农业科学, 2020, 53(14): 2814-2827 doi:10.3864/j.issn.0578-1752.2020.14.006
ZOU JianQiu, WANG YanQiu, LI JinHong, ZHU Kai.
0 引言
【研究意义】高粱是中国重要的粮食、饲料作物和酿酒原料,是世界上仅次于小麦、玉米、水稻、大麦的第五大作物,并具有抗旱、耐涝、耐盐碱、耐瘠薄等多重抗性和强大的杂种优势[1,2]。随着生产水平的不断提高,高粱机械化生产成为未来高粱产业持续健康发展的必然方向,而现阶段中国适宜机械化栽培高粱资源较少,很多优良的高粱资源因组配的杂交种株高较高,影响了其利用效率和利用潜力,导致机械化专用品种选育相对落后,限制了高粱产业的进一步提升[3,4,5]。因此,开展高粱株高矮化遗传效应及调控基因位点研究,解析高粱株高的遗传机理,以便更加有针对性地应用高粱资源,对高粱株高进行科学合理地调控,促进高粱产业向机械化和规模化发展尤为重要。【前人研究进展】关于高粱株高的调控效应,****们开展了大量研究,曾有报道气候条件、种植密度和理化调控等手段会对株高造成较大影响[6];而更多人从株高遗传方面开展了研究,有研究认为株高受茎秆节间长度、节数、穗柄长和穗长等因素影响[7,8,9];也有报道认为高粱的株高基因是所有已知的最有用的遗传因子之一,可使粒用高粱植株变矮,但对甜高粱株高的矮化效应并不明晰[10];ROSS等[11]研究表明高粱株高的调控基因主要有4个,即Dw1—Dw4。另外,卢庆善等[12]提出通过选择具有株高矮化基因的高粱资源与其他资源组配,可显著降低杂种F1的株高,并且确定了4对非连锁矮化基因控制高粱株高的遗传,即高秆与矮秆品种杂交,在杂种一代高秆对矮秆表现为部分显性,同时将株高分为5个等级:0-矮级、1-矮级、2-矮级、3-矮级、4-矮级。此外,****在调控高粱株高的Dw1—Dw4 4个基因作用效应方面开展了研究,指出Dw1和Dw2在调控茎秆节间长度方面效应明显,而Dw3作为在高粱上第一个被克隆的矮化基因,除对茎秆节间长度存在调控外,还对分蘖数、抗倒伏和产量性状具有显著影响[9]。Dw4至今还没有被克隆,但曾在帚高粱中被检测到[13],虽没有深度报道,但分析可能对穗柄长具有影响,进而调控株高。同时,大量研究表明,株高矮化调控同时受一个或多个基因共同作用,高粱在株高调控中往往是由Dw1—Dw4协同完成[14]。【本研究切入点】迄今为止,在已收集到的全世界高粱资源中,虽然资源非常丰富,株高的幅度在55—655 cm,但在资源利用方面还存在很多问题,且先前的研究表明,从株高遗传研究角度而言,大多数高粱资源依然是1-矮基因型和2-矮基因型材料[15],3-矮基因型和4-矮基因型材料相对较少。另外,虽然前人在高粱株高调控方面开展了许多研究,但仍然缺少株高组配降秆效应的系统研究。【拟解决的关键问题】本研究利用育种过程中发现的具有和现有粒用高粱恢复系组配F1都能降低株高的矮秆雄性不育系01-26A为株型调控材料,并且选择不具矮化株高效应的不育系7050A作为对照,系统开展株高矮化遗传效应和调控基因位点研究。旨在探明01-26A矮化基因关键调控位点(包括Dw1、Dw2和Dw3,而Dw4至今还未被克隆),解析高粱株高的遗传机理,从遗传角度解决高粱株高矮化调控问题,促进高粱向机械化、规模化发展。1 材料与方法
1.1 试验材料
1.1.1 2个高粱雄性不育系和7个高粱恢复系 2个高粱雄性不育系,包括具有矮化株高效应的01-26A和不具矮化株高效应的7050A,其中,01-26A具有一般配合力好、特殊配合力高、高抗丝黑穗病、抗蚜虫、抗叶斑病、抗旱、抗涝、耐瘠薄、活秆成熟等特点,最重要的是其具有和所有粒用高粱恢复系组配F1都能降低株高的特性。7个高粱恢复系,包括粒用高粱恢复系6个(LNR-4、NK1、0-01、3535、3550和BR92)和甜高粱恢复系1个(LTR168)。以上9个材料的系谱见表1。Table 1
表1
表12个高粱雄性不育系和7个高粱恢复系的系谱
Table 1
| 材料Material | 系谱 Pedigree |
|---|---|
| 01-26A | 以7050B×P932065B为母本,以天变624B×296B×428B为父本去雄杂交,育成01-26B并经过多代回交转育而成 Take 7050B×P932065B as female parent, and Tianbian 624B×296B×428B was used as male parent to cross, the developed 01-26B was transformed into 01-26A by multiple backcross |
| 7050A | 以421B(SPL–132B)为母本,以TAM428B为父本去雄杂交,育成7050B并经过多代回交转育而成 The 421B (SPL-132B) was used as the female parent, and the TAM428B was used as the male parent to cross, the developed 7050B was transformed into by multiple backcross |
| LNR-4 | 以9544为母本,7037为父本杂交 Take 9544 as the female parent and 7037 as the male parent to cross |
| NK1 | 以LR9198为母本,0-01为父本杂交 Take LR9198 as the female parent and 0-01 as the male parent to cross |
| 0-01 | 以4003×忻粱52为母本,116为父本杂交 Take 4003×Xinliang 52 as the female parent and 116 as the male parent to cross |
| 3535 | 以T719为母本,0-01为父本杂交 Take T719 as the female parent and 0-01 as the male parent to cross |
| 3550 | 以矮大穗为母本,LR625为父本杂交 Take short stigma as the female parent and LR625 as the male parent to cross |
| BR92 | 以F15为母本,LR9198为父本杂交 Take F15 as the female parent and LR9198 as the male parent to cross |
| LTR168 | 以辽宁地方农家品种为母本,ICSV111为父本杂交 Take Liaoning local variety as the female parent and ICSV111 as the male parent to cross |
新窗口打开|下载CSV
1.1.2 84个杂交种 由2个雄性不育系和7个恢复系不完全双列杂交的14个杂交种F1;粒用杂交组合(F1)57个,其中,01-26A与恢复系杂交组合33个,7050A与与恢复系杂交组合24个;甜高粱F1杂交组合13个,其中,与01-26A组配7个,与7050A组配6个。
1.2 试验设计
试验于2016—2018年连续3年在辽宁省农业科学院研究基地进行,对2个不育系、7个恢复系及其不完全双列杂交形成的14个杂交组合进行种植。随机区组设计,6行区,行长3 m,行宽0.6 m,小区面积10.8 m2,3次重复。完全抽穗后测定株高和株高参数,其中株高参数包括穗柄下茎秆高、穗柄长、穗长、茎秆节数和节间长。2019年对以上参试材料在辽宁省农业科学院人工气候室(温度:25℃,湿度:45%,光照12 h/黑暗12 h)进行幼苗培养,进行矮化基因提取与分析。
此外,2018年对01-26A与粒用恢复系杂交33个组合、7050A与粒用恢复系杂交24个组合、01-26A及7050A与甜高粱恢复系杂交13个组合的株高进行了测定,进一步验证01-26A的株高矮化效应。
1.3 测定项目与方法
1.3.1 株高及其参数测定 待高粱穗完全抽出后,采用伸缩性直尺测定株高,株高测定时去掉边行,每个高粱品种(品系)测定3株,求其平均值。同时,对2个不育系、7个恢复系(包括粒用高粱和甜高粱)及其不完全双列杂交形成的14个杂交组合进行株高参数测定,将每小区测定株高的3个植株去掉叶和叶鞘,分离穗、穗柄和穗柄下茎秆,分别用直尺测定其长度并求平均值,之后将3年结果求平均值。1.3.2 株高基因Dw1、Dw2和Dw3的检测 在人工气候室进行幼苗培养,六叶期取叶片进行矮化基因提取与分析。利用改良CTAB法提取三叶期高粱幼苗DNA,根据https://phytozome.jgi.doe.gov/pz/portal.html#! search?show=KEYWORD&method=Org_Sbicolor网站 公布的调控株高的基因序列设计引物(表2),进行Dw1、Dw2和Dw3的扩增,扩增体系为Mix 10 μL primer(100 μmol·L-1)0.5 μL、DNA 0.5 μL和ddH2O 8.25 μL。扩增条件为94℃ 2 min;94℃ 30 s,55℃ 30 s,72℃ 45 (120) s,32个循环;72℃ 10 min。Dw1和Dw2的PCR产物送至大连TaKaRa公司测序,然后用DNAman软件进行序列分析。Dw3的PCR产物进行琼脂糖检测。
Table 2
表2
表2PCR引物扩增序列
Table 2
| 引物名称 Primer name | 引物序列 Primer sequence (5′-3′) |
|---|---|
| Dw1-F | TGGCGGTCCAACGTCTAAT |
| Dw1-R | CCTGAAGTATGGCGTGTCT |
| Dw2-F | CAGTTCAAATCAACGAGGAG |
| Dw2-R | TCCGTCGTGAAATGAGAATA |
| Dw3-F | CGTCATCGTCCAGAACTCGG |
| Dw3-R | GACCCTTGCTCCACCACCTT |
新窗口打开|下载CSV
1.4 数据分析
采用DPS7.05和Excel 2007软件对亲本和F1各参数进行统计分析,计算变异系数(coefficient of variation,CV)。变异系数CV(%)=σ/X,σ为杂交F1数据的标准差,X为杂交F1数据的平均值。2 结果
2.1 株高及其参数遗传分析
2.1.1 株高遗传分析 参试的01-26A和7050A 2个高粱雄性不育系与6个粒用高粱恢复系组配的F1代杂交种株高存在显著差异(表3)。值得注意的是,01-26A与6个粒用高粱恢复系组配杂交种株高变幅为141.3—159.7 cm,变异系数(CV)为13.6%—22.9%,而7050A组配的杂交种株高变幅为159.6—185.7 cm,CV为10.9%—23.7%,01-26A组配的粒用高粱F1杂交种株高的平均值比7050A组配的粒用高粱F1杂交种降低了15.8%。说明01-26A对杂种F1具有显著的矮化效应。F1株高与亲本关联分析发现,01-26A组配的粒用高粱F1杂交种株高基本在双亲之间或略高于双亲最高值,而7050A组配的粒用高粱F1杂交种则均表现为高于双亲最高值。Table 3
表3
表3不育系、恢复系及其F1株高遗传效应
Table 3
| 品种类型 Variety type | 组合(母本×父本) Cross (♀×♂) | 株高Plant height (cm) | F1株高 平均值 F1X (cm) | F1株高变 异系数 CV (%) | F1株高与亲本关联 F1 PH associated with the parent | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ♀ | ♂ | 平均值 Average | 小于低亲 PH<PH (Min) | 双亲间 Between parents | 大于高亲 PH> PH(Max) | |||||
| 01-26A/LNR-4 | 103.5 | 145.3 | 124.4 | 141.3 | 13.6 | — | + | — | ||
| 粒用 Grain sorghum | 01-26A/3550 | 103.5 | 164.8 | 134.2 | 152.5 | 17.4 | — | + | — | |
| 01-26A/0-01 | 103.5 | 139.2 | 121.4 | 141.9 | 18.7 | — | — | + | ||
| 01-26A/BR92 | 103.5 | 162.4 | 133.0 | 146.6 | 15.3 | — | + | — | ||
| 01-26A/NK1 | 103.5 | 139.1 | 121.3 | 159.7 | 22.9 | — | — | + | ||
| 01-26A/3535 | 103.5 | 145.5 | 124.5 | 144.8 | 10.3 | — | + | — | ||
| 均值Average | 103.5 | 149.4 | 126.5 | 147.8 | 16.4 | |||||
| 7050A/LNR-4 | 136.7 | 145.3 | 141.0 | 173.1 | 18.4 | — | — | + | ||
| 7050A/3550 | 136.7 | 164.8 | 150.8 | 185.7 | 23.7 | — | — | + | ||
| 7050A/0-01 | 136.7 | 139.2 | 138.0 | 178.4 | 15.7 | — | — | + | ||
| 7050A/BR92 | 136.7 | 162.4 | 149.6 | 179.2 | 16.3 | — | — | + | ||
| 7050A/NK1 | 136.7 | 139.1 | 137.9 | 177.6 | 14.9 | — | — | + | ||
| 7050A/3535 | 136.7 | 145.5 | 141.1 | 159.6 | 10.9 | — | — | + | ||
| 均值Average | 136.7 | 149.4 | 143.1 | 175.6 | 16.7 | |||||
| T检验T test | * | ns | ** | ns | ||||||
| 甜高粱 Sweet sorghum | 01-26A/LTR168 | 103.5 | 247.3 | 175.4 | 341.7 | 21.4 | — | — | + | |
| 7050A/LTR168 | 136.7 | 247.3 | 192.0 | 355.3 | 20.8 | — | — | + | ||
| T检验 T test | * | ns | * | ns | ||||||
新窗口打开|下载CSV
与甜高粱恢复系组成的杂交F1中,01-26A和7050A与甜高粱恢复系LTR168的杂种F1株高增加明显,均明显高于双亲最高值,但二者差异很小。说明01-26A与甜高粱杂交株高遗传中,没有明显的株高矮化遗传效应。2.1.2 穗柄下茎秆高度遗传分析 01-26A和7050A与粒用高粱恢复系组配的F1穗柄下茎秆高度存在显著差异(表4)。雄性不育系01-26A比7050A穗柄下茎秆高度低35.9%,它们分别与6个粒用高粱恢复系组配的杂交种F1穗柄下茎秆高度变平均值01-26A比7050A降低19.9%,差异达极显著水平,介于双亲之间。说明穗柄下茎秆高度在01-26A矮化株高效应中发挥着重要作用。在01-26A为母本时,除01-26A/NK1外,其他5个粒用高粱组合F1杂交组合穗柄下茎秆高度均介于双亲之间并且高粱两亲本的平均值;7050A与所有粒用组合杂种F1穗柄下茎秆高度均高于亲本最高值,与株高遗传趋势相一致。在与甜高粱杂交F1中,01-26A和7050A与甜高粱恢复系杂种F1穗柄下茎秆高度差异不显著,其变化趋势与株高遗传基本一致,进一步说明了穗柄下茎秆高度对株高变化的重要作用。
Table 4
表4
表4不育系、恢复系及其F1穗柄下茎秆高遗传效应
Table 4
| 品种类型 Variety type | 组合(母本×父本) Cross (♀×♂) | 穗柄下茎秆高度 Stalk height under peduncle(cm) | F1穗柄下 茎秆高度 平均值 F1X (cm) | F1穗柄下茎秆高度变异系数 CV(%) | F1穗柄下茎秆高度与亲本关联 F1 SP associated with the parent | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ♀ | ♂ | 平均值Average | 小于低亲 SP<SP(Min) | 双亲间 Between parents | 大于高亲 SP> SP(Max) | |||||
| 01-26A/LNR-4 | 47.6 | 82.2 | 64.9 | 75.3 | 14.7 | — | + | — | ||
| 粒用 Grain sorghum | 01-26A/3550 | 47.6 | 97.4 | 72.5 | 81.6 | 16.9 | — | + | — | |
| 01-26A/0-01 | 47.6 | 83.3 | 65.5 | 78.1 | 22.1 | — | + | — | ||
| 01-26A/BR92 | 47.6 | 105.6 | 76.6 | 80.6 | 12.9 | — | + | — | ||
| 01-26A/NK1 | 47.6 | 86.9 | 67.3 | 92.7 | 17.4 | — | — | + | ||
| 01-26A/3535 | 47.6 | 86.5 | 67.1 | 80.4 | 16.5 | — | + | — | ||
| 均值 Average | 47.6 | 90.3 | 69.0 | 81.5 | 16.8 | |||||
| 7050A/LNR-4 | 74.3 | 82.2 | 78.3 | 92.6 | 13.3 | — | — | + | ||
| 7050A/3550 | 74.3 | 97.4 | 85.9 | 115.3 | 21.6 | — | — | + | ||
| 7050A/0-01 | 74.3 | 83.3 | 78.8 | 107.7 | 19.5 | — | — | + | ||
| 7050A/BR92 | 74.3 | 105.6 | 90.0 | 103.4 | 28.7 | — | — | + | ||
| 7050A/NK1 | 74.3 | 86.9 | 80.6 | 101.8 | 23.1 | — | — | + | ||
| 7050A/3535 | 74.3 | 86.5 | 80.4 | 89.7 | 15.4 | — | — | + | ||
| 均值 Average | 74.3 | 90.3 | 82.3 | 101.8 | 20.3 | |||||
| T检验 T test | * | ns | ** | ns | ||||||
| 甜高粱 Sweet sorghum | 01-26A/LTR168 | 47.6 | 183.4 | 115.5 | 264.6 | 25.2 | — | — | + | |
| 7050A/LTR168 | 74.3 | 183.4 | 128.9 | 277.2 | 25.6 | — | — | + | ||
| T检验 T test | * | ns | * | ns | ||||||
新窗口打开|下载CSV
2.1.3 穗柄长遗传分析 穗柄长作为株高的重要组成部分,在01-26A和7050A与粒用高粱恢复系组配的F1杂交种中变化活跃,01-26A作母本组配的F1穗柄长显著低于7050A(表5)。01-26A和7050A 2个母本差异不显著,而在与相同父本杂交F1中,01-26A作母本与粒用高粱组配的F1穗柄长比7050A的F1降低10.7%,且变异系数较小。说明穗柄长在01-26A矮化株高效应中发挥着重要作用,且效应值与株高相近。此外,在01-26A为母本时,与6个粒用高粱恢复系杂种F1组合穗柄下茎秆高度均高于亲本最高值,其遗传变化大于株高;7050A与所有粒用组合杂种F1穗柄下茎秆高度均高于亲本最高值,与株高遗传趋势相一致。另外,01-26A和7050A与甜高粱杂交F1中穗柄长差异不显著,可能是因为这两个不育系存在相同或相似的调控穗柄长度的基因。
Table 5
表5
表5不育系、恢复系及其F1穗柄长遗传效应
Table 5
| 品种类型 Variety type | 组合(母本×父本) Cross(♀×♂) | 穗柄长 Peduncle length (cm) | F1穗柄长 平均值 F1X(cm) | F1穗柄长 变异系数 CV (%) | F1穗柄长与亲本关联 F1 PL associated with the parent | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ♀ | ♂ | 平均值Average | 小于低亲 PL<PL(Min) | 双亲间 Between parents | 大于高亲 PL> PL(Max) | |||||
| 01-26A/LNR-4 | 29.7 | 35.5 | 32.6 | 38.1 | 6.5 | — | — | + | ||
| 粒用 Grain sorghum | 01-26A/3550 | 29.7 | 39.1 | 34.4 | 41.5 | 12.3 | — | — | + | |
| 01-26A/0-01 | 29.7 | 29.2 | 29.5 | 33.7 | 11.1 | — | — | + | ||
| 01-26A/BR92 | 29.7 | 32.8 | 31.3 | 38.2 | 7.6 | — | — | + | ||
| 01-26A/NK1 | 29.7 | 27.4 | 28.6 | 40.9 | 4.8 | — | — | + | ||
| 01-26A/3535 | 29.7 | 31.6 | 30.7 | 36.5 | 8.5 | — | — | + | ||
| 均值 Average | 29.7 | 32.6 | 31.2 | 38.2 | 8.5 | |||||
| 7050A/LNR-4 | 33.1 | 35.5 | 34.3 | 49.7 | 6.5 | — | — | + | ||
| 7050A/3550 | 33.1 | 39.1 | 36.1 | 40.3 | 8.7 | — | — | + | ||
| 7050A/0-01 | 33.1 | 29.2 | 31.2 | 36.9 | 11.3 | — | — | + | ||
| 7050A/BR92 | 33.1 | 32.8 | 33.0 | 46.5 | 7.2 | — | — | + | ||
| 7050A/NK1 | 33.1 | 27.4 | 30.3 | 40.6 | 6.2 | — | — | + | ||
| 7050A/3535 | 33.1 | 31.6 | 32.4 | 42.8 | 8.7 | — | — | + | ||
| 均值 Average | 33.1 | 32.6 | 32.9 | 42.8 | 8.1 | |||||
| T检验 T test | ns | ns | * | ns | ||||||
| 甜高粱 Sweet sorghum | 01-26A/LTR168 | 29.7 | 37.3 | 33.5 | 49.9 | 8.1 | — | — | + | |
| 7050A/LTR168 | 33.1 | 37.3 | 35.2 | 52.1 | 6.5 | — | — | + | ||
| T检验 T test | ns | ns | ns | ns | ||||||
新窗口打开|下载CSV
2.1.4 穗长遗传分析 01-26A和7050A与粒用高粱组配的F1穗长差异不显著(表6)。2个母本01-26A和7050A穗长差异不显著,父本相同,01-26A和7050A分别与6个粒用高粱恢复系组配杂交种F1中,01-26A作母本F1的穗长变幅为28.7—33.4 cm,平均变异系数(CV)仅为3.2%,而7050A作母本的F1穗长变幅为26.7—32.2 cm,平均变异系数(CV)为4.5%,说明01-26A作母本组配的杂种F1穗长较7050A相对更加整齐,但穗长的变化较穗柄下茎秆高度和穗柄长对杂种F1株高影响相对较小。
Table 6
表6
表6不育系、恢复系及其F1穗长遗传效应
Table 6
| 品种类型 Variety type | 组合(母本×父本) Cross (♀×♂) | 穗长 Head length (cm) | F1代穗长 平均值 F1X (cm) | F1代穗长 变异系数 CV (%) | F1穗长与亲本关联 F1 HL associated with the parent | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ♀ | ♂ | 平均值Average | 小于低亲 HL<HL(Min) | 双亲间 Between parents | 大于高亲 HL> HL(Max) | |||||
| 01-26A/LNR-4 | 26.2 | 27.6 | 26.9 | 27.9 | 2.1 | — | — | + | ||
| 粒用 Grain sorghum | 01-26A/3550 | 26.2 | 28.3 | 27.3 | 29.4 | 1.4 | — | + | — | |
| 01-26A/0-01 | 26.2 | 26.7 | 26.4 | 30.1 | 4.5 | — | — | + | ||
| 01-26A/BR92 | 26.2 | 24.0 | 25.1 | 27.8 | 5.3 | — | — | + | ||
| 01-26A/NK1 | 26.2 | 24.8 | 25.4 | 26.1 | 2.9 | — | — | + | ||
| 01-26A/3535 | 26.2 | 27.4 | 26.7 | 27.9 | 3.2 | — | — | + | ||
| 均值 Average | 26.2 | 26.5 | 26.3 | 28.2 | 3.2 | |||||
| 7050A/LNR-4 | 29.3 | 27.6 | 28.5 | 30.8 | 4.2 | — | — | + | ||
| 7050A/3550 | 29.3 | 28.3 | 28.8 | 30.1 | 2.6 | + | — | — | ||
| 7050A/0-01 | 29.3 | 26.7 | 28.0 | 33.8 | 3.9 | — | + | — | ||
| 7050A/BR92 | 29.3 | 24.0 | 26.7 | 29.3 | 7.8 | — | — | + | ||
| 7050A/NK1 | 29.3 | 24.8 | 27.1 | 35.2 | 5.2 | — | + | — | ||
| 7050A/3535 | 29.3 | 27.4 | 28.4 | 27.1 | 3.1 | + | — | — | ||
| 均值 Average | 29.3 | 26.5 | 27.9 | 31.1 | 4.5 | — | + | — | ||
| T检验 T test | ns | ns | ns | * | ||||||
| 甜高粱Sweet sorghum | 01-26A/LTR168 | 26.2 | 26.6 | 26.4 | 27.2 | 3.7 | + | — | — | |
| 7050A/LTR168 | 29.3 | 26.6 | 27.9 | 26.0 | 2.9 | + | — | — | ||
| T检验 T test | ns | ns | ns | * | ||||||
新窗口打开|下载CSV
2.1.5 茎节参数对株高遗传的影响 不育系01-26A和7050A与粒用高粱组配的F1的节间长、茎节数与株高存在相关(表7)。分析发现,01-26A组配的多数F1中下层和中层节间长度与株高变化相关性较大,而与7050A组配的的F1则表现为中上层和上层节间长度与株高关联密切。所以,分析认为01-26A杂交组配的F1株高的矮化效应很可能是由于茎秆中下层和中层节间长度变短所致;而对于甜高粱,01-26A和7050A组配的F1均表现为中层和中上层节间长度与株高相关性较大,也和上述结果相吻合。此外,2个母本无论是与粒用高粱还是甜高粱恢复系组配的杂种F1均表现出节数与株高关联密切。
Table 7
表7
表7茎节相关参数对杂种F1株高遗传的影响
Table 7
| 品种类型 Variety type | 组合(母本×父本) Cross (♀×♂) | 与株高相关系数R Correlation coefficient with plant height R (%) | |||||
|---|---|---|---|---|---|---|---|
| 节间长Internode length | 节数 Internode number | ||||||
| 下层Lower | 中下层Lower middle | 中层Middle | 中上层Up middle | 上层Upper | |||
| 粒用 Grain sorghum | 01-26A/LNR-4 | 15.3 | 65.2* | 52.6* | 18.7 | 22.3 | 75.3* |
| 01-26A/3550 | -3.6 | 42.6 | 63.2* | 55.6* | 45.2* | 64.2* | |
| 01-26A/0-01 | 34.5 | 51.8* | 71.8** | 23.4 | 12.7 | 26.9 | |
| 01-26A/BR92 | -12.4 | 63.2* | 51.4* | 42.3* | 26.9 | 65.8* | |
| 01-26A/NK1 | 12.3 | 68.2* | 76.3** | 9.8 | 23.7 | 69.7* | |
| 01-26A/3535 | 23.5 | 42.3 | 55.2* | 48.5 | 14.3 | 54.8* | |
| 7050A/LNR-4 | 5.3 | 29.7 | 38.5 | 68.9* | 24.1 | 76.7* | |
| 7050A/3550 | -2.9 | 15.4 | 25.6 | 45.6* | 82.5** | 72.3* | |
| 7050A/0-01 | 14.3 | 42.4 | 42.1 | 68.7** | 43.6 | 71.9* | |
| 7050A/BR92 | 21.6 | 28.5 | 23.6 | 76.3* | 67.1* | 52.3* | |
| 7050A/NK1 | 12.3 | 9.7 | -9.7 | 77.9** | 69.9** | 88.9** | |
| 7050A/3535 | 18.5 | 41.2 | 45.3 | 56.2* | 48.7 | 65.3* | |
| 甜高粱 Sweet sorghum | 01-26A/LTR168 | 8.4 | 31.4 | 55.9* | 75.3** | 42.1 | 61.5* |
| 7050A/LTR168 | 9.7 | 15.6 | 58.4* | 65.3* | 28.6 | 65.3* | |
新窗口打开|下载CSV
2.2 组配F1杂交种株高矮化效应验证
为进一步验证01-26A的株高矮化效应,对01-26A与33个恢复系、7050A与24个恢复系的杂种F1亲本的株高进行了测定与分析(图1)。通过F1株高分类和出现频次分析发现,01-26A组配的杂种F1株高在100—160 cm,且多数品种株高在140—160 cm;而与7050A组配的杂种F1株高在140—190 cm,多数品种株高集中在160—190 cm。对这两个不育系与13个甜高粱恢复系组配分析发现01-26A与甜高粱组配的杂种F1株高主要都集中在340—380 cm,7050A与甜高粱组配的杂种F1代株高主要都集中在360—400 cm,与先前(2.1.1)的研究结果相吻合。通过验证进一步说明01-26A可显著降低杂种F1粒用高粱的株高,但对甜高粱没有矮化株高的效应。图1
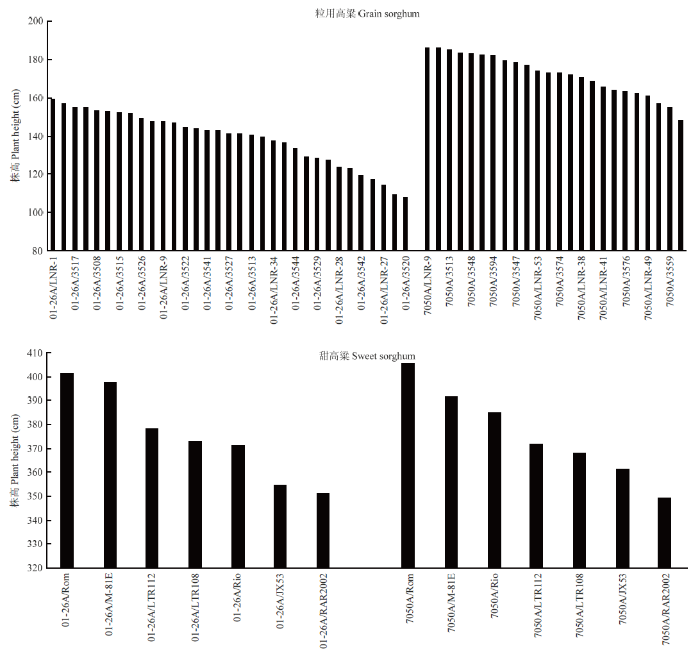 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1组配F1杂交种株高矮化效应验证
Fig. 1Verification of the dwarf effect of F1 hybrids
2.3 株高矮化基因位点研究
2.3.1 高粱Dw1序列分析 为进一步分析01-26A对粒用高粱杂种F1株高的矮化效应和对甜高粱杂种F1株高无矮化效应的作用机制,对试验材料进行了矮化基因检测。分析发现雄性不育系01-26A和7050A、7个恢复系(6个粒用、1个甜高粱)以及它们组配的F1杂交种在Dw1位点上存在差异(图2)。01-26A作为母本,在Dw1基因组序列中第1 350位上碱基由A突变为T,造成Dw1发生突变,即01-26A具有矮化基因dw1dw1,而与之组配的6个粒用高粱恢复系(LNR-4、NK1、0-01、3550、3535、BR92)除3550基因型发生突变(由A到T)为dw1外(01-26A与3550杂种F1基因型dw1dw1,表型矮秆),其他5个品系基因型均为Dw1Dw1,与之组配的杂交种基因型均为杂合,即Dw1dw1,由于在杂种F1高粱高秆对矮秆表现为部分显性[12],因此,01-26A对F1的株高矮化起到了一定的作用,此结果也与株高表型(2.1.1和2.2)的研究结果相呼应。01-26A与甜高粱恢复系杂交F1基因型为Dw1dw1,株高表现为小幅下降。7050A作为母本,在基因组第1 350位上碱基未发生突变,所以其基因型为Dw1Dw1,不具矮化基因,除与3535杂种F1基因型Dw1dw1外,与其他恢复系(包括粒用和甜高粱)杂交F1的基因型均为Dw1Dw1,所组配组合表现为株高普遍高于01-26A组配的杂交组合,与表型结果相吻合。图2
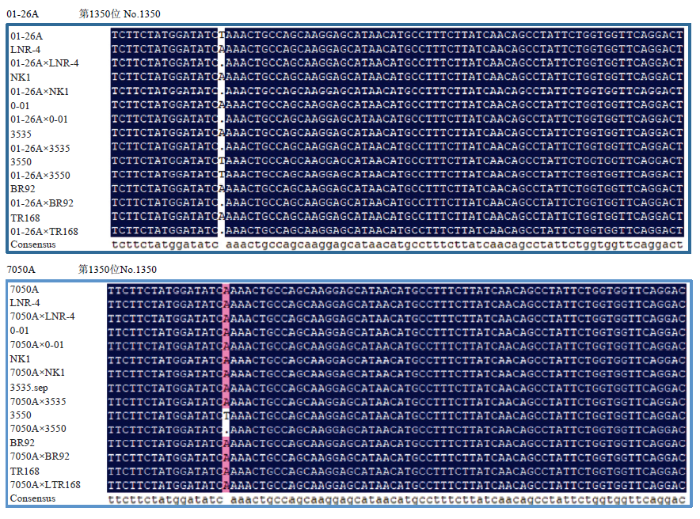 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2高粱Dw1位点分析
Fig. 2Analysis of Dw1 in sorghum
2.3.2 高粱Dw2序列分析 由图3可以看出,在Dw2基因组第549位上,01-26A碱基序列GA并未缺失(GA缺失为dw2突变的特征位点),说明01-26A基因型为Dw2Dw2,而其他所有参试恢复系组配F1杂交种(粒用和甜高粱)除恢复系0-01发生基因缺失杂合(导致杂种F1 01-26A/0-01为Dw2dw2外,其他F1杂交种基因型均为Dw2Dw2。7050A在Dw2位点的基因型与01-26A相同(Dw2Dw2),其组配的F1杂交种与01-26A一致,所以Dw2在这两个不育系杂交F1组合株高矮化调控中没有起到明显作用。
图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3高粱Dw2基因位点分析
**:基因缺失;··:杂合
Fig. 3Analysis of Dw2 gene locus in sorghum
**: Gene deletion; ··: Heterozygosity
2.3.3 高粱Dw3位点分析 矮化基因Dw3在第五外显子插入882 bp的重复序列,使其变成dw3,基因片段大小由1 450 bp变为2 330 bp。由图4、表8可以看出,01-26A(第17泳道)和7050A(第8泳道)在Dw3位点基因型均为dw3dw3,而参试恢复系LNR-4、3550和BR92为野生型(Dw3Dw3),其他恢复系均为突变型(dw3dw3)。从Dw3位点角度分析发现01-26A与粒用杂交组合01-26A/3550、01-26A/BR92、01-26A/LNR-4及甜高粱杂交组合01-26A/LTR168基因型均为Dw3dw3,此研究结果与先前(2.1.1和2.2)株高表型的研究结果基本吻合;而7050A与粒用杂交组合7050A/NK1、7050A/3535和7050A/3550基因型均为dw3dw3,其他组合基因型为Dw3dw3,也与基因株高表型变化(2.1.1和2.2)基本一致。综上所述,01-26A的dw3dw3在对杂种F1株高矮化调控中发挥了重要作用。而01-26A和7050A与甜高粱恢复系杂交组配均为Dw3dw3,也进一步解释了2个母本组配的甜高粱杂交种均表现为高秆的内在原因。
图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4高粱Dw3基因位点分析
1、2、3、7、9、10、12、13、14、20、21和23泳道片段大小为1263 bp,其他片段大小为2145 bp
Fig. 4Analysis of Dw3 in sorghum
1, 2, 3, 7, 9, 10, 12, 13, 14, 20, 21 and 23 are 1263 bp in size and the other fragments are 2145 bp in size
Table 8
表8
表8Dw3检测序号及对应品系/组合
Table 8
| 序号 Serial number | 品系或组合 Line or combination | 基因型 Genotype | 序号 Serial number | 品系或组合 Line or combination | 基因型 Genotype | |
|---|---|---|---|---|---|---|
| 1 | LNR-4 | Dw3Dw3 | 13 | 7050A/LNR-4 | Dw3dw3 | |
| 2 | 3550 | Dw3Dw3 | 14 | 7050A/BR92 | Dw3dw3 | |
| 3 | BR92 | Dw3Dw3 | 15 | 7050A/NK1 | dw3dw3 | |
| 4 | 3535 | dw3dw3 | 16 | 7050A/3535 | dw3dw3 | |
| 5 | 0-01 | dw3dw3 | 17 | 01-26A | dw3dw3 | |
| 6 | NK1 | dw3dw3 | 18 | 01-26A/0-01 | dw3dw3 | |
| 7 | LTR168 | Dw3Dw3 | 19 | 01-26A/NK1 | dw3dw3 | |
| 8 | 7050A | dw3dw3 | 20 | 01-26A/3550 | Dw3dw3 | |
| 9 | 7050A/LTR168 | Dw3dw3 | 21 | 01-26A/BR92 | Dw3Dw3 | |
| 10 | 01-26A/LTR168 | Dw3dw3 | 22 | 01-26A/3535 | dw3dw3 | |
| 11 | 7050A/0-01 | dw3dw3 | 23 | 01-26A/LNR-4 | Dw3dw3 | |
| 12 | 7050A/3550 | Dw3dw3 |
新窗口打开|下载CSV
2.3.4 矮化基因型及平均株高 对参试的2个雄性不育系、7个恢复系和其组配形成的14个杂交种的基因型和平均株高进行分析(表9)。发现01-26A和7050A的Dw2基因型相同,均为Dw2Dw2;01-26A的Dw1和Dw3矮化基因型分别为dw1dw1和dw3dw3。而7050A的Dw1基因型为Dw1Dw1,Dw3基因型为dw3dw3,其组配的粒用杂交组合的平均株高总体高于01-26A组配的杂交种。此外,Dw4尚未被克隆,没有检测,但其基因型很可能也与F1株高密切相关。
Table 9
表9
表9矮化基因型及其平均株高
Table 9
| 品系或组合Line or combination | 基因型Genotype | 平均株高Average plant height (cm) |
|---|---|---|
| LNR-4 | Dw1Dw1Dw2Dw2Dw3Dw3 | 145.3 |
| 3550 | dw1dw1Dw2Dw2Dw3Dw3 | 164.8 |
| BR92 | Dw1Dw1Dw2Dw2Dw3Dw3 | 162.4 |
| 3535 | Dw1Dw1Dw2Dw2dw3dw3 | 145.5 |
| 0-01 | Dw1Dw1dw2dw2dw3dw3 | 139.2 |
| NK1 | Dw1Dw1Dw2Dw2dw3dw3 | 139.1 |
| LTR168 | Dw1Dw1Dw2Dw2Dw3Dw3 | 247.3 |
| 7050A | Dw1Dw1Dw2Dw2dw3dw3 | 136.7 |
| 7050A/LTR168 | Dw1Dw1Dw2Dw2Dw3dw3 | 355.3 |
| 01-26A/LTR168 | Dw1dw1Dw2Dw2Dw3dw3 | 341.7 |
| 7050A/0-01 | Dw1Dw1Dw2dw2dw3dw3 | 178.4 |
| 7050A/3550 | Dw1dw1Dw2Dw2Dw3dw3 | 185.7 |
| 7050A/LNR-4 | Dw1Dw1Dw2Dw2Dw3dw3 | 173.1 |
| 7050A/BR92 | Dw1Dw1Dw2Dw2Dw3dw3 | 179.2 |
| 7050A/NK1 | Dw1Dw1Dw2Dw2dw3dw3 | 177.6 |
| 7050A/3535 | Dw1Dw1Dw2Dw2dw3dw3 | 159.6 |
| 01-26A | dw1dw1Dw2Dw2dw3dw3 | 103.5 |
| 01-26A/0-01 | Dw1dw1Dw2dw2dw3dw3 | 141.9 |
| 01-26A/NK1 | Dw1dw1Dw2Dw2dw3dw3 | 159.7 |
| 01-26A/3550 | dw1dw1Dw2Dw2Dw3dw3 | 152.5 |
| 01-26A/BR92 | Dw1dw1Dw2Dw2Dw3Dw3 | 146.6 |
| 01-26A/3535 | Dw1dw1Dw2Dw2dw3dw3 | 144.8 |
| 01-26A/LNR-4 | Dw1dw1Dw2Dw2Dw3dw3 | 141.3 |
新窗口打开|下载CSV
3 讨论
株高作为高粱群体构成和机械化生产的重要指标备受人们的关注,而通过遗传手段矮化株高是调控株高最有效、最稳定的手段之一[16]。本研究通过将01-26A与粒用高粱和甜高粱恢复系杂交,对亲本和组配F1杂交种的株高进行分析,发现该材料可显著降低粒用杂种F1的株高,而对甜高粱杂种F1株高没有显著的矮化效应。此研究结果与管延安等[17]对普通高粱与甜高粱杂交组合株高主基因多基因模型遗传效应研究得出的株高遗传变化趋势基本一致,李延玲等[18]对高粱株型性状数量遗传分析也得出类似的结论。此外,OLSON等[19]研究认为,株高是高粱株型调节的关键农艺性状,通过遗传改良株高是高粱矮化育种的必然方向,与本研究结果相吻合。本研究通过遗传分析认为01-26A矮化株高遗传效应主要表现在杂种F1代穗柄下茎秆高度明显降低,同时穗柄长降低也是造成株高变矮的重要原因,穗长对株高变化的影响很小。此研究结果与MULTANI[20]对高粱株高调控的研究结果相一致;ORDONIO等[21]以粒用高粱对高粱叶夹角、株高、穗长、平均茎节长度遗传分析也得出类似的结论。有研究认为,小麦株高与其构成因素呈极显著遗传正相关,株高构成因素对株高的作用大小依次为倒一节>倒二节>倒三节>倒五节>倒四节>穗的结论[22],此结果与本研究结果基本吻合,但影响的节间位置略有差异,可能是由于作物不同,植株高度差异和分蘖数不同所致。
前人已经有过报道,高粱株高的调控基因主要有Dw1、Dw2、Dw3和Dw4,株高矮化是这4个基因以及其他未知基因共同作用的结果[23,24,25]。虽然本研究中通过对01-26A矮化基因型的检测确定其基因型为dw1Dw2dw3,对dw4没有检测,但鉴于其与30余个恢复系杂交F1均为株高降低的表型数据分析,尤其是穗柄长度的分析(较7050A组配的杂交组合显著降低),可推断01-26A dw4位点很可能为dw4,即01-26A的基因型为dw1dw1Dw2Dw2dw3dw3dw4dw4,即3-矮高粱雄性不育系。而7050A很可能是Dw1Dw1Dw2Dw2dw3dw3dw4dw4的2-矮不育系。此推断与前人通过隐性等位基因在Milo系中鉴定出Dw1和Dw2,而在Kafir背景中鉴定了Dw3的隐性等位基因的论断基本一致[26,27,28]。本研究还发现,01-26A的dw1和dw3在其杂种F1株高矮化调控方面发挥了重要作用,可促使茎秆中下部节间变短从而降低株高。此结果与JIA等[29]研究并提出的Dw1和Dw2具有降低株高的效应,但Dw1作用效应值更大的结果相吻合;但与THURBER等[30]研究并指出的Dw3在高粱株高调控中起着关键作用,中部茎秆影响程度最大,茎秆受影响程度较大的结果略有差异,可能是选用矮化材料差异所致,也可能是几个基因互作差异所致。截至目前,虽然Dw4还没有被克隆,报道也很少,但普遍认为Dw4影响高粱穗柄长[31]。
控制高粱株高的基因除Dw1—Dw4外,还可能受其他基因或其他内在因素的调控,尚需进一步研究;同时本研究采用的高粱恢复系类型有限,通过01-26A与更多矮化基因型和更多种类恢复系杂交组配进行株高矮化效应分析还有待于进一步研究。
4 结论
高粱雄性不育系01-26A确定含有dw1dw1Dw2Dw2dw3dw3 3对基因,同时通过其大量株高矮化效应验证,推断其很可能具有dw4,即为基因型dw1dw1Dw2Dw2dw3dw3dw4dw4的三矮高粱不育系。01-26A具有和大多数粒用高粱恢复系组配都能矮化杂种F1株高的遗传效应,可主要通过降低杂种F1中下部节间长度和穗柄长度降低F1株高,实现对高粱株高的矮化调控,但其与甜高粱恢复系杂交未发现其具有明显矮化效应。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1073/pnas.1215985110URLPMID:23267105 [本文引用: 1]

Accelerating crop improvement in sorghum, a staple food for people in semiarid regions across the developing world, is key to ensuring global food security in the context of climate change. To facilitate gene discovery and molecular breeding in sorghum, we have characterized ~265,000 single nucleotide polymorphisms (SNPs) in 971 worldwide accessions that have adapted to diverse agroclimatic conditions. Using this genome-wide SNP map, we have characterized population structure with respect to geographic origin and morphological type and identified patterns of ancient crop diffusion to diverse agroclimatic regions across Africa and Asia. To better understand the genomic patterns of diversification in sorghum, we quantified variation in nucleotide diversity, linkage disequilibrium, and recombination rates across the genome. Analyzing nucleotide diversity in landraces, we find evidence of selective sweeps around starch metabolism genes, whereas in landrace-derived introgression lines, we find introgressions around known height and maturity loci. To identify additional loci underlying variation in major agroclimatic traits, we performed genome-wide association studies (GWAS) on plant height components and inflorescence architecture. GWAS maps several classical loci for plant height, candidate genes for inflorescence architecture. Finally, we trace the independent spread of multiple haplotypes carrying alleles for short stature or long inflorescence branches. This genome-wide map of SNP variation in sorghum provides a basis for crop improvement through marker-assisted breeding and genomic selection.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
URLPMID:21930910 [本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
DOI:10.1038/srep28366URL [本文引用: 1]
URLPMID:26351684 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 1]

对普通高粱与甜高粱杂交组合(石红137×L-甜)的株高与糖度进行主基因多基因遗传模型分析,以期研究株高、糖度的遗传效应。获得了2个性状的最适遗传模型,株高的最适遗传模型为2对完全显性主基因+加性-显性多基因混合遗传模型,主基因遗传率为74.4%,多基因遗传率为22.1%;糖锤度的最适遗传模型为1对加性-显性主基因+加性-显性-上位性多基因混合遗传模型,主基因遗传率为65.72%,多基因遗传率为20.43%。主基因个数和基因效应的预测与分子检测的主效QTL个数和基因效应基本相符。
URL [本文引用: 1]

对普通高粱与甜高粱杂交组合(石红137×L-甜)的株高与糖度进行主基因多基因遗传模型分析,以期研究株高、糖度的遗传效应。获得了2个性状的最适遗传模型,株高的最适遗传模型为2对完全显性主基因+加性-显性多基因混合遗传模型,主基因遗传率为74.4%,多基因遗传率为22.1%;糖锤度的最适遗传模型为1对加性-显性主基因+加性-显性-上位性多基因混合遗传模型,主基因遗传率为65.72%,多基因遗传率为20.43%。主基因个数和基因效应的预测与分子检测的主效QTL个数和基因效应基本相符。
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1126/science.1086072URLPMID:14526073 [本文引用: 1]

Agriculturally advantageous reduction in plant height is usually achieved by blocking the action or production of gibberellins. Here, we describe a different dwarfing mechanism found in maize brachytic2 (br2) mutants characterized by compact lower stalk internodes. The height reduction in these plants results from the loss of a P-glycoprotein that modulates polar auxin transport in the maize stalk. The sorghum ortholog of br2 is dwarf3 (dw3), an unstable mutant of long-standing commercial interest and concern. A direct duplication within the dw3 gene is responsible for its mutant nature and also for its instability, because it facilitates unequal crossing-over at the locus.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:26316679 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1186/1471-2229-14-148URL [本文引用: 1]
