 ,, 何芳, 邵胜楠, 刘政
,, 何芳, 邵胜楠, 刘政 ,, 黄家风
,, 黄家风 ,石河子大学农学院/新疆绿洲农业病虫害治理与植保资源利用重点实验室,新疆石河子 832003
,石河子大学农学院/新疆绿洲农业病虫害治理与植保资源利用重点实验室,新疆石河子 832003Cloning and Functional Analysis of VdHP1 in Verticillium dahliae from Cotton
SUN Qi ,, HE Fang, SHAO ShengNan, LIU Zheng
,, HE Fang, SHAO ShengNan, LIU Zheng ,, HUANG JiaFeng
,, HUANG JiaFeng ,College of Agriculture/Key Laboratory of Oasis Agricultural Pest Management and Plant Protection Resources Utilization, Xinjiang Uygur Autonomous Region, Shihezi University, Shihezi 832003, Xinjiang
,College of Agriculture/Key Laboratory of Oasis Agricultural Pest Management and Plant Protection Resources Utilization, Xinjiang Uygur Autonomous Region, Shihezi University, Shihezi 832003, Xinjiang通讯作者:
责任编辑: 岳梅
收稿日期:2020-01-10接受日期:2020-02-21网络出版日期:2020-07-16
| 基金资助: |
Received:2020-01-10Accepted:2020-02-21Online:2020-07-16
作者简介 About authors
孙琦,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (3761KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
孙琦, 何芳, 邵胜楠, 刘政, 黄家风. 棉花黄萎病菌VdHP1的克隆及功能分析[J]. 中国农业科学, 2020, 53(14): 2872-2884 doi:10.3864/j.issn.0578-1752.2020.14.011
SUN Qi, HE Fang, SHAO ShengNan, LIU Zheng, HUANG JiaFeng.
0 引言
【研究意义】棉花黄萎病(cotton verticillium wilt)是威胁我国棉花生产的一种重要土传病害,由大丽轮枝菌(Verticillium dahliae)引起[1]。目前有关该病原菌致病机制的解析尚不全面,加之其特殊的存活方式,黑色微菌核可在土壤中存活多年,使得依赖传统方法进行防治难以奏效[2]。因此,通过基因功能鉴定找到调控大丽轮枝菌致病机制的关键因子,对全面解析大丽轮枝菌致病机制,以及利用靶基因进行棉花抗病育种和探索新型防治方法具有重要的理论价值。【前人研究进展】近年来有关大丽轮枝菌致病相关基因及致病机制的研究取得了较大进展,大致分成4种情况:第1类基因编码效应子蛋白,通过干扰或阻止植物免疫过程参与致病,如VdPDA1编码的聚多糖脱乙酰酶,通过对真菌细胞壁组分几丁质寡糖进行脱乙酰作用,阻止几丁质寡糖触发免疫信号传递,进而抑制寄主的免疫反应[3];VdSCP41编码的分泌蛋白直接作用于调节植物免疫的转录因子以阻止植物的免疫反应[4];此类效应子蛋白还有VdNLP1、VdNLP2[5,6]和VdCUT11[7]。第2类基因通过形成侵入结构或分泌结构参与致病,如VdNoxB和VdPls1编码的蛋白复合体定位于附着枝细胞膜,通过促进胞质内活性氧和钙离子的积累激活VdCrz1转录因子及钙离子信号途径,从而调控附着枝分化形成侵染钉,VdNoxB/VdPls1基因敲除导致附着枝不能形成侵染钉,进而使大丽轮枝菌丧失致病力[8];VdSep5蛋白在侵染钉与其侵入寄主后的侵染性菌丝之间形成一种环状结构,即在大丽轮枝菌与寄主互作界面形成菌丝颈环用于分泌蛋白的释放和输出[9]。第3类基因编码转录因子,这类基因通常不仅影响致病力,也参与大丽轮枝菌的产孢、微菌核(或黑色素)形成,如转录因子基因SOM1和VTA3敲除后分别影响大丽轮枝菌的穿透能力和在寄主体内的定殖,并导致大丽轮枝菌产孢量下降、微菌核减少,进一步研究发现,SOM1和VTA3共同调控与产孢、微菌核形成及毒力相关基因的表达[10];Vdpf转录因子通过cAMP依赖信号途径和G蛋白信号途径调控大丽轮枝菌黑色素形成、并且还通过调控与产孢及毒力相关基因的表达影响大丽轮枝菌的致病力和产孢量[11];此类转录因子还有VdSge1、VdCmr1、VdHapX等[12,13,14]。第4类基因通过信号途径参与大丽轮枝菌致病,同时也参与分生孢子、微菌核或黑色素的形成过程,如MAPK信号途径中VdSho1编码的跨膜蛋白与Vst50-Vst7-Vst11蛋白模块共同调控大丽轮枝菌黑色素形成和穿透能力,黑色素含量与大丽轮枝菌的穿透能力密切相关[15];此外,该信号途径中的VMK1、VdMsb、VdMsn2分别敲除均导致大丽轮枝菌致病力下降并伴随产孢量下降和微菌核减少[16,17,18];又如VdPKAC1和VGB分别通过cAMP依赖信号途径和G蛋白信号途径对大丽轮枝菌的致病力、产孢量和微菌核形成产生影响[19,20]。综上所述,参与大丽轮枝菌致病的基因很多,致病机制复杂,各基因参与的途径之间相互作用,共同决定了大丽轮枝菌对寄主的致病作用。【本研究切入点】笔者课题组从前期已构建的大丽轮枝菌T-DNA插入突变体库中筛选到一株致病力显著下降的突变菌株,通过鉴定发现,被插入突变的基因是VDAG_07100基因,其预测编码蛋白(hypothetical protein)没有功能注释,因此将其命名为VdHP1。VdHP1除了影响大丽轮枝菌的致病力外,其他生物学功能尚不明确。【拟解决的关键问题】从大丽轮枝菌强致病力菌株V592中克隆VdHP1,并对VdHP1在棉花根诱导条件下的转录表达及不同组织中的转录表达进行分析;通过获得VdHP1的敲除突变体菌株、互补菌株和过表达菌株,研究该基因对大丽轮枝菌生长发育、致病力及对其他致病相关基因的影响,明确VdHP1的生物学功能。1 材料与方法
试验于2018年6月至2019年8月在新疆石河子大学新疆绿洲农业病虫害治理与植保资源利用重点实验室完成。1.1 材料
1.1.1 菌株和质粒 大丽轮枝菌V592菌株由本实验室分离、鉴定并保存。pMD19-T克隆载体购自TaKaRa公司。敲除载体pGKO-HPT和过表达载体pSUIPH-RG-HPT由中国科学院微生物研究所郭惠珊研究员惠赠。互补载体p1300-Neo-oLiC-Cas9-TtrpC由本实验室构建并保存。1.1.2 植物材料 棉花品种为感病品种军棉1号。
1.2 VdHP1的克隆与测序
参考宋雯等[21]的方法,利用真菌DNA提取试剂盒(BioFlux)及Trizol(QIAGEN)法分别提取V592菌株的DNA和RNA;用反转录试剂盒(TaKaRa)将RNA反转录成cDNA。根据大丽轮枝菌VdLs.17菌株的VDAG_07100基因序列(NCBI),设计引物VdHP1 full-F(5′-ATGCGTTTCTTCGCCTTTTT-3′)和VdHP1 full-R(5′-TAGTCCATTCTGATCCATGT-3′),分别以V592的DNA和cDNA为模板扩增VdHP1的全长和CDS序列并测序。1.3 VdHP1在大丽轮枝菌中的表达
1.3.1 棉花根诱导条件下VdHP1的表达 挑选粒大饱满的军棉1号种子,剥去种皮后置于0.1%的升汞溶液中消毒处理30 min,用清水漂洗3遍,放入装有40 mL MS培养基的三角瓶中,暗培养7 d。待根长至5—10 cm时剪取2 g棉花根段,添加至100 mL Czapek-Dox液体培养基中;再将PDA上培养7 d的V592菌株,取10个直径1 cm的菌饼加入,分别在26℃、200 r/min培养2、4、6、8、10、12、24和48 h;分别以未加棉花根系在同样条件下培养的V592菌株为阴性对照;剔除棉花根段,参照方法1.2,提取各菌株的总RNA并反转录成cDNA;按照SYBR(Life Technologies)试剂盒说明添加样品,通过qPCR反应分析VdHP1受棉花根系诱导后的表达特征。qPCR反应在7500实时PCR系统(Life Technologies)中完成,以β-tubulin作为内参基因对目标基因的转录水平进行标准化,每种菌株设3次生物学重复,每个反应设3次重复,根据2-ΔΔCt方法进行数据处理,再利用SPSS 17.0软件进行统计分析。
1.3.2 VdHP1在大丽轮枝菌不同组织中的表达 参考王春巧等[22]的方法,分别收集V592菌株的菌丝体、分生孢子和微菌核,提取各组织的总RNA并反转录成cDNA,利用上述qPCR方法分析VdHP1在不同组织中的表达情况。
1.4 载体构建及真菌转化
构建针对VdHP1的敲除载体,先用引物VdHP1 up-F和VdHP1 up-R从V592菌株的基因组中扩增VdHP1基因上游780 bp片段,再用引物VdHP1 down-F和VdHP1 down-R从V592基因组DNA中扩增VdHP1基因下游782 bp片段。将获得的2个目标片段与PacⅠ线性化的敲除载体通过多片段重组酶(Vazyme)进行重组构建敲除载体pGKO-HPT::VdHP1,再将其通过ATMT方法转化V592菌株的分生孢子。将获得的转化子参考WANG等[23]的PCR方法进行初步筛选。再分别用HPH的特异引物HygU和HygL,及VdHP1的特异引物VdHP1 full-F和VdHP1 full-R对转化子进行再次筛选。为了获得互补菌株,用引物ECVdHP1-F和ECVdHP1-R从V592菌株的基因组DNA中扩增VdHP1基因编码区全长,将其插入到Xba I/BamH I线性化的互补载体中,通过ATMT转化VdHP1基因敲除突变体,对转化子用抗生素G418和PCR方法进行筛选。为了获得VdHP1基因过表达菌株,用引物对OEVdHP1-F和OEVdHP1-R从V592菌株中扩增VdHP1基因编码区全长,插入到Xba I/Spe I线性化的pSUIPH-RG-HPT载体中。然后通过ATMT方法将该载体转化V592菌株。最后,用引物VdHP1-qPCR-F和VdHP1-qPCR-R通过RT-qPCR对目标基因VdHP1在野生型菌株、敲除突变体菌株、互补菌株和过表达菌株中的转录表达进行确定。载体构建所用引物见表1。Table 1
表1
表1载体构建所用引物
Table 1
| 引物Primer | 引物序列Primer sequence (5′-3′) |
|---|---|
| VdHP1 full-F | ATGCGTTTCTTCGCCTTTTT |
| VdHP1 full-R | TAGTCCATTCTGATCCATGT |
| VdHP1 up-F | CTTGCTGAGGTCTTAATTAAACCTCCAAGGCATCGTTGC |
| VdHP1 up- R | AGTGCTGAGGCATTAATTAACAAACGAGACGCGAATGGTG |
| VdHP1 down-F | CCCGCTGAGGACTTAATTAA GGATCTTGCGTCTCGTAGGT |
| VdHP1 down- R | CTCGCTGAGGGTTTAATTAA AGGCCATTCATTACGATGCC |
| HygU | AACCACGGCCTCCAGAAGAA |
| HygL | AGCCTGACCTATTGCATCTCCC |
| ECVdHP1-F | CGGCCAGTGCCAAGCTT AAATATCGTGTGGTGCGAAA |
| ECVdHP1-R | GCAGCTTCTGCGAATTC TTAGTCCATTCTGATCCATG |
| OEVdHP1-F | AATGAATATAGGCCGTCGACATGCGTTTCTTCGCCTTTTT |
| OEVdHP1-R | CTGCATCCGAATTCACTAGTTTAGTCCATTCTGATCCATGC |
| VdHP1-qPCR-F | AGAGCCAGAGGGTTCGTGGA |
| VdHP1-qPCR-R | ATGCGTTTCTTCGCCTTTTTACAGA |
新窗口打开|下载CSV
1.5 VdHP1基因敲除突变体生物学性状的测定
VdHP1基因敲除突变体的菌落形态、产孢量及微菌核产量参考宋雯等[21]的方法进行测定;菌丝的显微观察:将真菌在PDA平板上划线,再将灭菌的盖玻片斜插到划好的线上,26℃暗培养3 d,取出盖玻片在显微镜下观察菌丝生长情况。玻璃纸穿透试验:参考ZHAO等[8]的方法将灭菌玻璃纸平铺在MM基本培养基上,用牙签挑取菌丝接种至玻璃纸中央,分别培养3、4、5、6和7 d揭去玻璃纸,继续培养7 d观察真菌穿透玻璃纸后的菌落形成情况。每个菌株设3个重复。1.6 致病力测定
将棉苗在MS液体培养基中培养至4—5片真叶展开时[21],参照GAO等[24]的无伤浸根接种法用浓度为1×107 cfu/mL的孢子悬浮液进行接种,每个菌株接种3个营养钵,每个营养钵12棵棉苗。参考ZHANG等[25]的方法进行病情指数统计。1.7 利用qPCR测定感病植株中真菌生物量
对接种28 d的棉花分别提取根、茎、叶的总DNA,通过qPCR测定其中大丽轮枝菌的生物量。qPCR所用引物ITS-F和ITS-R基于核糖体RNA的ITS1和ITS2区设计[25]。每个反应设3个重复。qPCR反应及数据处理与同1.3.1。1.8 其他致病相关基因在VdHP1基因敲除突变体中的表达量测定
通过RT-qPCR对大丽轮枝菌其他致病相关基因在野生型菌株V592、VdHP1敲除突变体和互补菌株中的转录水平进行测定。被检测的致病相关基因及其引物见表2,每个反应设3个重复。RT-qPCR反应及数据处理同1.3.1。Table 2
表2
表2被检测的致病相关基因及其引物
Table 2
| 基因Gene | 引物Primer | 引物序列Primer sequence (5′-3′) |
|---|---|---|
| VdPKS1 (VDAG_00190) | VdPKS1-F | ATGGTCGGCACCATGTCTTTTCTCC |
| VdPKS1-R | GCCTGTTCGAGAAAGGTCTTGGCAA | |
| VMK1 (VDAG_09461.1) | VMK1-F | CGCAGCAACGCCCCTAATC |
| VMK1-R | GGCAGTGGTCATCGGAGAGGT | |
| VdNLP1 (VDAG_04701.1) | VdNLP1-F | TCGGTCTTTGCCCTCGTC |
| VdNLP1-R | GCCTGGTTTGCGTTGTTC | |
| VdNLP2 (VDAG_01995.1) | VdNLP2-F | AAGCCGTACCTCAAGGTGTTCA |
| VdNLP2-R | CCGACCCAAAGTCCGTGTTCT | |
| VdCYC8 (VDAG_07052) | VdCYC8-F | GGATGCCCTCGATGCTTACT |
| VdCYC8-R | CGTCGCTGATCTGGTTGTTG | |
| VGB (JQ665433.1) | VGB-F | GCAATCTCCAAACGACGTGTCG |
| VGB-R | GCGAACTGACGTGTGGTGTCGG | |
| VdSge1 (VDAG_06298.1) | VdSge1-F | CATGGATCCTTCCGAGGCATCTAG |
| VdSge1-R | GATGATGCGGGACGCTTCTGAAC | |
| VdHog1 (VDAG_08982) | VdHog1-F | CTTCCACGTGTCTACTGGCAGG |
| VdHog1-R | TGCTCCTTACCACGACCTTACCGA | |
| VdNoxB (VDAG_09930) | VdNoxB-F | TGCGTGGCAAGCATAAGACATAC |
| VdNoxB-R | GACAGCACGAGTGAAATCACCAAC | |
| VdPls1 (VDAG_01769) | VdPls1-F | ATGGTCAACAAGATCCTCGCGA |
| VdPls1-R | TCCGGCTGCTCAAACATGTTGT | |
| VdSep5 (VDAG_04382) | VdSep5-F | AGCTCGACCTGGACGAGGA |
| VdSep5-R | GAGGCTTCGTTATCAATCTCGTCTC | |
| VdSho1 (VDAG_01836) | VdSho1-F | GAGATAACCCAAAGGGCCATGGG |
| VdSho1-R | GAGAGCGTATCCAATCGCACC | |
| VdCrz1 (VDAG_03208) | VdCrz1-F | ATGGATCAGCAAGCTCAACATCG |
| VdCrz1-R | GATCCAGACCGAGACCGAGAC | |
| VdLAC (VDAG_00189) | VdLAC-F | ATGCTCTTCTCGCGTTTCCTCA |
| VdLAC-R | GCCACTGACCATTGATGCCAAT | |
| VdCmr1 (VDAG_00195) | VdCmr1-F | GCGCCACAAGCTCTGCATCTTC |
| VdCmr1-R | CAGAATCAAGGTGGCGCGATACAC | |
| VdPLP (VDAG_00942) | VdPLP-F | GCTGACCAGTATCTGTCGGAGG |
| VdPLP-R | ATGACGACTGGCTTCTCGGCCT | |
| Vdpf (VDAG_08521.1) | Vdpf-F | ACCATTTTCAACAGTCGGGTACGCG |
| Vdpf-R | GTGTGACGTACCAGCAACCGCTT | |
| β-tubulin (DQ266153) | β-tubulin-F | TCACCAGCCGTGGCAAGGTTG |
| β-tubulin-R | AGCAAAGGGCGGTCTGGACGTTG |
新窗口打开|下载CSV
2 结果
2.1 VdHP1克隆
分别以大丽轮枝菌野生型菌株V592的基因组DNA和cDNA为模板,通过克隆测序得到VdHP1全长为862 bp,预测编码蛋白的cDNA为807 bp,序列分析显示,该基因由2个外显子和1个内含子组成,编码268个氨基酸。VdHP1的预测编码蛋白与大丽轮枝菌VdLs.17同源基因编码的氨基酸序列相似性为98.95%,与非苜蓿轮枝菌(V. nonalfalfae)的D7B24_004502基因编码的氨基酸序列相似性为94.90%,与苜蓿轮枝菌(V. alfalfae)的VDBG_05451基因编码的氨基酸序列相似性为94.48%,而与其他真菌没有任何氨基酸序列相似性;在GenBank中进行检索,VdHP1与已注释的基因没有任何序列相似性。上述结果表明VdHP1是轮枝菌属所特有的基因。2.2 VdHP1在大丽轮枝菌中的表达分析
通过RT-qPCR测定VdHP1受棉花根系诱导后的表达特征,结果显示V592菌株受棉花根系诱导培养6 h后VdHP1的表达水平呈现剧烈上调,10 h达到最大值,12 h后转录表达水平显著下降(图1-A),表明VdHP1可能在大丽轮枝菌侵染早期发挥作用。
对VdHP1在大丽轮枝菌不同组织中的转录表达水平进行测定,结果发现VdHP1在分生孢子中的表达量最高,分别是菌丝和微菌核中的14倍和28倍(图1-B),表明VdHP1在大丽轮枝菌中的表达具有组织差异性。
图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1VdHP1在野生型菌株V592中的转录表达
A:V592菌株被棉花根系诱导后VdHP1的转录表达;B:VdHP1 transcriptional expression in different tissues of V592 strain
不同小写字母表示处理间差异显著(P<0.05)
Fig. 1VdHP1 transcriptional expression in the wild-type strain V592
A:VdHP1 transcriptional expression in V592 strain induced by cotton roots;B:VdHP1 transcriptional expression in different tissues of V592 strain
Different lowercases indicate that the differences are significant at 0.05 level;**:P<0.01。下同 The same as below
2.3 VdHP1基因敲除突变体和互补菌株的筛选
以野生型菌株V592为对照,将PCR初步筛选获得的2个敲除突变体和互补菌株用2对特异引物进行再次确认,结果如图2-B所示,用HPH的特异引物只能从2个敲除突变体(ΔVdHP1-1和ΔVdHP1-2)中扩增出457 bp的HPH基因片段,用VdHP1的特异引物只能从V592菌株和2个互补菌株(ECVdHP1-1和ECVdHP1-2)中扩增出862 bp VdHP1全长。上述结果表明在基因组水平上,敲除突变体中VdHP1已被HPH成功取代,互补菌株中VdHP1得到回补。再对敲除突变体和互补菌株中的VdHP1的转录表达进行测定,结果如图2-C所示,VdHP1在2个敲除突变体ΔVdHP1-1和ΔVdHP1-2中几乎不表达,而在互补菌株ECVdHP1-1和 ECVdHP1-2中的表达恢复至野生型菌株水平,因此从转录水平再次表明,VdHP1在ΔVdHP1-1和ΔVdHP1-2中被成功敲除,在互补菌株ECVdHP1-1和 ECVdHP1-2中得到互补。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2大丽轮枝菌V592菌株VdHP1基因的敲除
A:VdHP1基因敲除载体构建图;B:利用特异引物对目标基因VdHP1进行PCR检测;C:利用RT-qPCR对VdHP1的转录水平进行确定
Fig. 2Knockout of VdHP1 in V. dahliae V592 strain。。。
A:Strategy diagram of VdHP1 knockout vector construction;B:Specific primers were used to detect VdHP1 by PCR;C:Confirmation of transcriptional expression of VdHP1 by RT-qPCR analysis
2.4 VdHP1基因敲除突变体表型特征的观察
在PDA培养基上,VdHP1基因敲除突变体与V592菌株和互补菌株一样形成典型的菌核型菌落,菌落生长速度也无明显差异(图3-A),说明VdHP1基因敲除不影响大丽轮枝菌的菌落生长。将等量分生孢子接种在营养缺乏的培养基上诱导微菌核的形成,接种14 d后,2个VdHP1基因敲除突变体的微菌核干重和湿重与V592菌株和2个互补菌株相比也无明显差异(图3-B),表明VdHP1不参与大丽轮枝菌微菌核的形成。图3
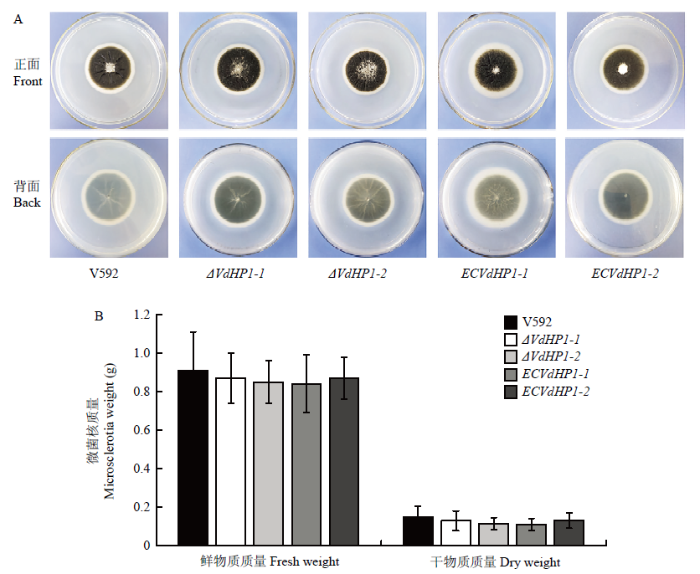 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3VdHP1基因敲除突变体在PDA上的菌落形态及微菌核的质量
A:V592菌株、VdHP1基因敲除突变体和互补菌株在PDA培养基上的培养特征;B:V592菌株、VdHP1敲除突变体和互补菌株的微菌核湿重和干重
Fig. 3Colony morphology on PDA media and microsclerotia weight of VdHP1 gene knockout mutants
A:Colony morphology of V592 strain, VdHP1 gene knockout mutants and complementary strains on PDA media;B:Fresh and dry weights of microsclerotia of V592 strain, VdHP1 gene knockout mutants and complementary strains
2.5 VdHP1基因敲除突变体产孢量的测定
与V592菌株相比,2个敲除突变体ΔVdHP1-1和ΔVdHP1-2的产孢量明显减少(图4),在接种第7 天时,ΔVdHP1-1和ΔVdHP1-2的产孢量分别是V592产孢量的46.62%和45.00%;而2个互补菌株ECVdHP1-1和 ECVdHP1-2的产孢量恢复至野生型菌株的水平,表明VdHP1参与大丽轮枝菌分生孢子的产生。图4
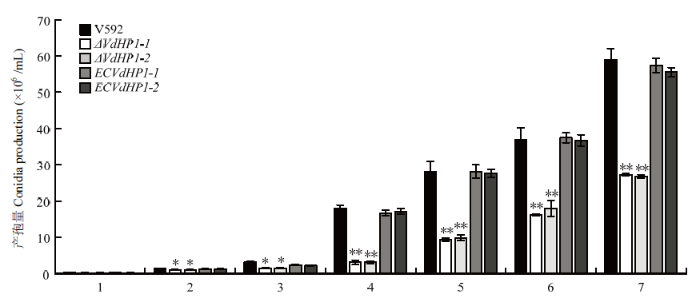 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4VdHP1基因敲除突变体产孢量测定
*:P<0.05。下同
Fig. 4Conidial production of VdHP1 gene knockout mutants
*:P<0.05。The same as below
2.6 VdHP1基因敲除突变体菌丝形态观察
显微观察结果显示,与野生型菌株V592相比,2个VdHP1基因敲除突变体分支菌丝呈现螺旋状,产孢梗减少;而互补菌株ECVdHP1-1和 ECVdHP1-2的菌丝体形态和分生孢子梗恢复至野生型水平(图5)。表明VdHP1与大丽轮枝菌菌丝形态及产孢梗的形成有关,突变体产孢量的减少可能与产孢梗减少有关。图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5显微镜下菌丝的形态特征
Fig. 5Morphology of hyphae under microscope
2.7 VdHP1基因敲除突变体致病力测定
为了明确VdHP1对大丽轮枝菌致病力的影响,以野生型菌株V592为对照,测定了VdHP1基因敲除体对棉花的致病力。结果如图6所示,接种10 d时所有植株开始出现萎蔫症状,随着病情发展,VdHP1基因敲除突变体导致的病害症状与对照相比明显减轻。接种28 d时,V592菌株引起感病植株叶片萎蔫、焦枯及脱落,病情指数达到95.4,而敲除突变体ΔVdHP1-1和ΔVdHP1-2所致病害的病情指数则分别为44.3和43.2;互补菌株ECVdHP1-1和 ECVdHP1-2对棉花的致病力明显得到恢复,病情指数分别为92.2和91.1(图6-A、6-B)。接种30 d时,对感病植株进行剖杆观察,V592菌株及2个互补菌株均导致棉杆维管束明显变褐,而接种2个敲除突变体的棉花,其维管束基本不变色(图6-C)。表明VdHP1基因敲除影响大丽轮枝菌对棉花的致病力。图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6VdHP1基因敲除对大丽轮枝菌致病力的影响
A:感病棉花的症状,照片拍摄于接种后20 d ;B:棉花发病后的病情指数;C:感病棉花维管束变褐现象;D:棉花根、茎、叶中的真菌生物量;E:与侵染钉形成和分泌蛋白释放相关基因在VdHP1基因敲除突变体中的相对表达量;F:玻璃纸穿透试验
Fig. 6Effect of VdHP1 gene knockout on pathogenicity in V. dahliae
A:The symptoms of diseased cotton plants, the photograph were taken at 20βdays post inoculation;B:Disease index of cotton plants;C:Vascular discoloration of diseased cotton plants;D:Relative quantification of fungal biomass in roots, stems and leaves of cotton plants;E: The transcriptional expression of genes involved in penetration peg formation and delivery of secretory protein in VdHP1 gene knockout mutants;F:Cellophane penetration test of VdHP1 gene knockout mutants
为了明确VdHP1是否影响大丽轮枝菌在寄主体内的定殖能力,通过qPCR对棉花中的真菌生物量进行了测定,结果如图6-D显示,VdHP1基因敲除突变体在根组织中的生物量显著高于V592菌株和2个互补菌株,而在茎组织中的生物量又显著低于V592和互补菌株,表明VdHP1基因敲除导致大丽轮枝菌在棉花茎中的扩展受阻。
为了进一步明确致病力下降是否与菌株的穿透能力下降有关,以V592为对照,对VdHP1基因敲除突变体和互补菌株进行了玻璃纸穿透试验,结果如图6-F所示,V592菌株和2个互补菌株均可在接种第3天时穿透玻璃纸在MM培养基上生长,而VdHP1基因敲除突变体则在接种第5天时才能穿透玻璃纸。RT-qPCR结果显示,与野生型V592菌株和互补菌株相比,在VdHP1基因敲除突变体中,与大丽轮枝菌侵染钉形成相关的2个基因VdNoxB和VdPls1以及与分泌结构菌丝颈环形成相关基因VdSep5的表达量均明显下降(图6-E)。表明VdHP1基因敲除导致大丽轮枝菌穿透延迟并且影响侵染钉及菌丝颈环形成相关基因的表达。
2.8 致病相关基因在VdHP1基因敲除突变体中的表达
为了明确VdHP1表达对其他致病相关基因的影响,首先构建了VdHP1基因过表达载体,获得过表达菌株(OEVdHP1-1和OEVdHP1-2),RT-qPCR测定结果显示,VdHP1在过表达菌株中的表达量显著高于野生型菌株V592中的表达量(图7-A)。与野生型菌株V592相比,与黑色素合成相关的基因VdCmr1、VdSho1、VdLAC和VdPKS1在VdHP1基因敲除体中的相对表达量均显著上调,在过表达菌株中除VdCmr1外均显著下调(图7-B);与之相反,与侵染钉形成有关的基因VdCrz1、VdNoxB、VdPls1和分泌蛋白释放有关的基因VdSep5在VdHP1基因敲除体中的相对表达量均显著下调,在过表达菌株中VdNoxB和VdSep5表达量显著上调(图7-C);同样,与分生孢子产生有关的基因Vdpf、VdSge1、VGB、VdPLP、VdCYC8、VdNLP1和VdNLP2在VdHP1基因敲除突变体中的相对表达量显著下调,Vdpf、VGB、VdPLP、VdCYC8、VdNLP1表达量在过表达菌株中显著上调,VdHog1和VMK1的表达则不受VdHP1表达量影响(图7-D)。上述结果表明,VdHP1对与侵染钉形成、分泌蛋白释放及分生孢子产生有关基因的表达具有正调控作用,对黑色素合成有关基因的表达具有负调控作用。图7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7其他致病相关基因在VdHP1基因敲除突变体中的相对表达量
A:VdHP1过表达菌株中的转录水平验证;B:大丽轮枝菌中黑色素合成相关基因的转录表达;C:大丽轮枝菌中与穿透及分泌蛋白释放相关基因的转录表达;D:大丽轮枝菌中产孢相关基因的转录表达
Fig. 7Relative expression of genes involved in pathogenicity in VdHP1 gene knockout mutants
A:Transcriptional expression of VdHP1 in V592 strain and overexpressed strains;B:Transcriptional expression of genes involved in melanin synthesis;C:Transcriptional expression of genes involved in penetration peg and delivery of secretory protein;D:Transcriptional expression of genes involved in conidial production
3 讨论
本研究从大丽轮枝菌中克隆到的VdHP1与GenBank中已有功能注释的基因没有任何序列相似性,并且只与轮枝菌属中的同源基因具有很高的序列相似性,表明该基因是轮枝菌属特有的基因。KLOSTERMAN等通过亚细胞定位和信号肽预测轮枝菌属中大丽轮枝菌(VdLs.17菌株)和黑白轮枝菌(V. albo-atrum)(VaMs.102菌株)分别编码780个和759个分泌蛋白,其中574个基因在2个种间保守[26]。由于许多真菌典型的效应子蛋白是氨基酸数目<400,半胱氨酸数>4的小蛋白[27,28,29],据此轮枝菌属中246个未被注释的基因编码的蛋白为这类典型的效应子蛋白[26]。而本研究克隆到的VdHP1,通过亚细胞定位和信号肽预测编码的蛋白也是分泌蛋白,包含268个氨基酸,但是其半胱氨酸只有2个,因此VdHP1蛋白可能是轮枝菌属中一种非典型效应子蛋白。VdHP1在分生孢子中的表达量是微菌核中的28倍、菌丝的14倍,当VdHP1从大丽轮枝菌中敲除,因其不能在分生孢子和菌丝中表达,导致敲除突变体产孢梗和产孢量明显减少,菌丝形态也发生改变,表明VdHP1参与大丽轮枝菌的产孢过程。已有研究表明,大丽轮枝菌的产孢过程是多基因参与的过程,如Vdpf [11]、VdSge1[12]、VdPKAC1[19]、VGB[20]、VdPLP[30]、VMK1[16]、VdHog1[31]、VdCYC8[32]、VdNLP1[6]和VdNLP2[6]等基因敲除均导致大丽轮枝菌产孢量下降。本研究发现,VdHP1正调控其中一些与产孢相关基因Vdpf、VdSge1、VGB、VdPLP、VdCYC8、VdNLP1、VdNLP2的表达,其中,Vdpf[11]、VdSge1[12]和VdCYC8[32]是参与调控产孢过程的转录因子,VGB是G蛋白信号途径的关键基因[20]。VdHP1基因敲除导致产孢量下降可能与这些产孢相关基因表达量下降有关,同时也表明VdHP1可能通过与其他基因或信号途径互作参与分生孢子的形成过程。
VdHP1敲除突变体在棉花根、茎中的生物量测定结果表明,VdHP1基因敲除导致大丽轮枝菌在棉花茎中的扩展受阻;与之一致的结果是,VdHP1基因敲除体穿透玻璃纸的能力下降,并且与附着枝侵染钉形成相关的基因VdCrz1、VdNoxB、VdPls1在VdHP1基因敲除体中的表达量显著下调[8],表明VdHP1基因敲除导致附着枝形成侵染钉的能力下降,从而导致大丽轮枝菌穿透寄主细胞壁和在寄主体内的扩展能力下降。大丽轮枝菌发挥致病作用的途径之一就是通过分泌各类效应子蛋白与寄主互作,干扰或阻止植物免疫。VdSep5是大丽轮枝菌侵染钉与寄主界面形成菌丝颈环结构所必需的基因,其作用是释放分泌蛋白[9];VdSep5在VdHP1基因敲除体中的表达量显著下调,表明VdHP1基因敲除突变体致病力下降可能与其分泌效应子能力下降有关。此外,Vdpf[11]、VdSge1[12]、VGB[20]、VdPLP[30]、VdCYC8[32]、VdNLP1[6]、VdNLP2[6]不仅是产孢相关基因,也是致病相关基因,它们在VdHP1基因敲除突变体中的表达量显著下调也可能是导致致病力下降的因素之一。
虽然有些致病相关基因的功能鉴定表明,其产孢过程与其微菌核形成过程相互偶联,如Vdpf[11]、VGB[20]和VdCYC8[32]均与分生孢子产生和微菌核的形成有关。本研究结果显示,VdHP1不参与大丽轮枝菌微菌核形成,表明VdHP1参与的产孢和致病过程不与微菌核形成途径偶联。已有研究表明,黑色素含量与大丽轮枝菌的致病力密切相关,VdCmr1[13]、VdSho1[15]、VdPKS1[25]、VdLAC[33]基因敲除均导致大丽轮枝菌致病力显著下降,其中,VdPKS1和VdLAC是真菌DHN黑色素合成途径起始和结束的2个关键基因[13];VdCmr1蛋白是调控黑色素合成途径的转录因子[13];VdSho1编码的跨膜蛋白通过MAPK级联信号途径调控大丽轮枝菌黑色素的合成和穿透寄主的能力[15]。本研究发现,VdHP1对黑色素合成相关基因VdCmr1、VdSho1、VdPKS1、VdLAC的表达具有负调控作用,但是VdHP1与这4个基因所涉及的调控网络是如何相互作用的还有待深入研究。
4 结论
从大丽轮枝菌中克隆到的VdHP1是轮枝菌属特有的新基因。VdHP1基因敲除导致大丽轮枝菌产孢量和产孢梗减少、菌丝形态发生改变、对寄主的致病力下降,表明VdHP1与大丽轮枝菌分生孢子和产孢梗的产生有关,参与大丽轮枝菌致病。其他致病相关基因在VdHP1敲除突变体中的转录分析结果表明,VdHP1对与侵染钉形成、分泌蛋白释放及分生孢子产生相关基因的表达具有正调控作用,对黑色素合成相关基因的表达具有负调控作用。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
URLPMID:11331751 [本文引用: 1]
DOI:10.1038/s41477-019-0527-4URLPMID:31636399 [本文引用: 1]

Soil-borne fungal pathogens that cause crop disease are major threats to agriculture worldwide. Here, we identified a secretory polysaccharide deacetylase (PDA1) from the soil-borne fungus Verticillium dahliae, the most notorious plant pathogen of the Verticillium genus, that facilitates virulence through direct deacetylation of chitin oligomers whose N-acetyl group contributes to host lysine motif (LysM)-containing receptor perception for ligand-triggered immunity. Polysaccharide deacetylases are widely present in fungi, bacteria, insects and marine invertebrates and have been reported to possess diverse functions in developmental processes rather than virulence. A phylogenetics analysis of more than 5,000 fungal proteins with conserved polysaccharide deacetylase domains showed that the V. dahliae PDA1-containing subtree includes a large number of proteins from the Verticillium genus as well as the Fusarium genus, another group of characterized soil-borne fungal pathogens, suggesting that soil-borne fungal pathogens have adopted chitin deacetylation as a major virulence strategy. We showed that a Fusarium PDA1 is required for virulence in cotton plants. This study reveals a substantial virulence function role of polysaccharide deacetylases in pathogenic fungi and demonstrates a subtle mechanism whereby deacetylation of chitin oligomers converts them to ligand-inactive chitosan, representing a common strategy of preventing chitin-triggered host immunity by soil-borne fungal pathogens.
URLPMID:29376824 [本文引用: 1]
DOI:10.1094/MPMI-12-11-0319URLPMID:22414440 [本文引用: 1]

Verticillium dahliae Kleb. is a hemibiotrophic, phytopathogenic fungus that causes wilt disease in a wide range of crops, including cotton. Successful host colonization by hemibiotrophic pathogens requires the induction of plant cell death to provide the saprophytic nutrition for the transition from the biotrophic to the necrotrophic stage. In this study, we identified a necrosis-inducing Phytophthora protein (NPP1) domain-containing protein family containing nine genes in a virulent, defoliating isolate of V. dahliae (V592), named the VdNLP genes. Functional analysis demonstrated that only two of these VdNLP genes, VdNLP1 and VdNLP2, encoded proteins that were capable of inducing necrotic lesions and triggering defense responses in Nicotiana benthamiana, Arabidopsis, and cotton plants. Both VdNLP1 and VdNLP2 induced the wilting of cotton seedling cotyledons. However, gene-deletion mutants targeted by VdNLP1, VdNLP2, or both did not affect the pathogenicity of V. dahliae V592 in cotton infection. Similar expression and induction patterns were found for seven of the nine VdNLP transcripts. Through a comparison of the conserved amino acid residues of VdNLP with different necrosis-inducing activities, combined with mutagenesis-based analyses, we identified several novel conserved amino acid residues, in addition to the known conserved heptapeptide GHRHDWE motif and the cysteine residues of the NPP domain-containing protein, that are indispensable for the necrosis-inducing activity of the VdNLP2 protein.
DOI:10.1094/MPMI-09-12-0222-RURLPMID:23051172 [本文引用: 5]

In this study, we functionally analyzed the gene family encoding necrosis- and ethylene-inducing-like proteins (NLP) of the vascular wilt pathogen Verticillium dahliae. We show that the composition of the NLP gene family varies little among V. dahliae isolates. The cytotoxic activity of NLP family members of a tomato-pathogenic V. dahliae strain was determined, demonstrating that only two of the seven NLP induced plant cell death. The genes encoding these cytotoxic NLP were found to be induced in V. dahliae upon colonization of tomato. Interestingly, targeted deletion of either of the two genes in V. dahliae significantly compromised virulence on tomato as well as on Arabidopsis plants, whereas deletion of only one of the two genes affected virulence on Nicotiana benthamiana. This could be attributed to differential induction of the two NLP genes in V. dahliae upon N. benthamiana colonization, revealing that the in planta induction of NLP genes varies between plant hosts. Intriguingly, one of the NLP genes appears to also affect vegetative growth and conidiospore production, because the corresponding deletion strain produced significantly fewer conidiospores and developed extensive aerial mycelium. In conclusion, we demonstrate that the expanded V. dahliae NLP family shows functional diversification, revealing not only differential cytotoxicity between family members but also that the cytotoxic NLP play a role in vegetative growth and asexual reproduction in addition to their contribution to virulence.
URLPMID:29068240 [本文引用: 1]
URLPMID:27463643 [本文引用: 3]
DOI:10.1371/journal.ppat.1006275URL [本文引用: 2]
URLPMID:30290010 [本文引用: 1]
DOI:10.1111/mpp.12367URLPMID:26857810 [本文引用: 5]

Verticillium dahliae is a soil-borne, hemibiotrophic phytopathogenic fungus that causes wilting in crop plants. Here, we constructed a random insertional mutant library using Agrobacterium tumefaciens-mediated transformation to study the pathogenicity and regulatory mechanisms of V. dahliae. The fungal-specific transcription factor-encoding gene Vdpf was shown to be associated with vegetative growth and virulence, with the highest transcript expression occurring during conidia formation in the V991 strain. The deletion mutants (DeltaVdpf) and insertion mutants (IMDeltaVdpf) produced fewer conidia than did the wild-type (WT) fungi, which contributed to the reduced virulence. Unlike the WT, the complemented strains and IMDeltaVdpf, DeltaVdpf formed swollen, thick-walled and hyaline mycelium rather than melanized microsclerotia. The DeltaVdpf mutants were melanin deficient, with undetectable expression of melanin biosynthesis-related genes (Brn1, Brn2 and Scd1). The melanin deficiency was related to cyclic adenosine monophosphate (cAMP) and the G-protein-coupled signalling pathways in this study. Similar to the WT and complemented strains, the DeltaVdpf and IMDeltaVdpf mutants could also successfully penetrate into cotton and tobacco roots, but displayed reduced virulence because of lower biomass in the plant roots and significantly reduced expression of pathogenicity-related genes in V. dahliae. In conclusion, these results provide insights into the role of Vdpf in melanized microsclerotia formation, conidia production and pathogenicity.
URLPMID:22970788 [本文引用: 4]
URLPMID:29485393 [本文引用: 4]
DOI:10.1128/mSphere.00400-18URLPMID:30185514 [本文引用: 1]

Iron homeostasis is essential for full virulence and viability in many pathogenic fungi. Here, we showed that the bZip transcription factor VdHapX functions as a key regulator of iron homeostasis for adaptation to iron-depleted and iron-excess conditions and is required for full virulence in the vascular wilt fungus, Verticillium dahliae Deletion of VdHapX impaired mycelial growth and conidiation under both iron starvation and iron sufficiency. Furthermore, disruption of VdHapX led to decreased formation of the long-lived survival structures of V. dahliae, known as microsclerotia. Expression of genes involved in iron utilization pathways and siderophore biosynthesis was misregulated in the DeltaVdHapX strain under the iron-depleted condition. Additionally, the DeltaVdHapX strain exhibited increased sensitivity to high iron concentrations and H2O2, indicating that VdHapX also contributes to iron or H2O2 detoxification. The DeltaVdHapX strain showed a strong reduction in virulence on smoke tree seedlings (Cotinus coggygria) and was delayed in its ability to penetrate plant epidermal tissue.IMPORTANCE This study demonstrated that VdHapX is a conserved protein that mediates adaptation to iron starvation and excesses, affects microsclerotium formation, and is crucial for virulence of V. dahliae.
DOI:10.1111/1462-2920.14846URLPMID:31667948 [本文引用: 3]

Verticillium dahliae is a soil-borne fungus that causes vascular wilt on numerous plants worldwide. The fungus survives in the soil for up to 14 years by producing melanized microsclerotia. The protective function of melanin in abiotic stresses is well documented. Here, we found that the V. dahliae tetraspan transmembrane protein VdSho1, a homolog of the Saccharomyces cerevisiae Sho1, acts as an osmosensor, and is required for plant penetration and melanin biosynthesis. The deletion mutant DeltaSho1 was incubated on a cellophane membrane substrate that mimics the plant epidermis, revealing that the penetration of DeltaSho1 strain was reduced compared to the wild-type strain. Furthermore, VdSho1 regulates melanin biosynthesis by a signalling mechanism requiring a kinase-kinase signalling module of Vst50-Vst11-Vst7. Strains, DeltaVst50, DeltaVst7 and DeltaVst11 also displayed defective penetration and melanin production like the DeltaSho1 strain. Defects in penetration and melanin production in DeltaSho1 were restored by overexpression of Vst50, suggesting that Vst50 lies downstream of VdSho1 in the regulatory pathway governing penetration and melanin biosynthesis. Data analyses revealed that the transmembrane portion of VdSho1 was essential for both membrane penetration and melanin production. This study demonstrates that Vst50-Vst11-Vst7 module regulates VdSho1-mediated plant penetration and melanin production in V. dahliae, contributing to virulence.
URLPMID:16003535 [本文引用: 2]
[本文引用: 1]
DOI:10.1016/j.funbio.2017.08.005URLPMID:29122172 [本文引用: 1]

Verticillium dahliae is a notorious pathogen that causes vascular wilt disease in numerous plant species worldwide. The fungus produces melanized microsclerotia, which helps it survive adverse environmental conditions that it may encounter within its hosts and in the soil. Previously, we determined that the high osmolarity glycerol (HOG) pathway is involved in the environmental stress response of V. dahliae. In this study, we investigated the function of VdMsn2, a homologue of the yeast C2H2 transcription factor Msn2, which is predicted to function as a downstream player in the HOG pathway. Disruption of VdMsn2 has a discernible effect on hyphal growth and septation, but not on diverse stresses including hyperosmotic stresses and cell wall inhibitory agents. Furthermore, we show that VdMsn2 deletion mutants produce significantly more microsclerotia than the wild-type and exhibit attenuated virulence to smoke trees because of poor penetration. Taken together, our findings suggest that VdMsn2 controls hyphal growth, microsclerotia formation, and virulence but does not significantly contribute to stress responses in V. dahliae.
DOI:10.1016/j.fgb.2010.01.007URLPMID:20144723 [本文引用: 2]

Verticillium dahliae is a soilborne fungus that causes vascular wilt disease in a broad range of hosts and survives for many years in the soil in the form of microsclerotia. Although the role of cAMP-dependent protein kinase A (PKA) has been extensively studied in foliar pathogens, there is limited information about its role in soilborne fungal pathogens that infect through the root system. Genome database search revealed the presence of two PKA catalytic subunit genes in V. dahliae, named VdPKAC1 and VdPKAC2. A phylogenetic analysis showed that VdPKAC2 groups with fungal PKA catalytic subunits that appear to play a minor role in PKA activity. This gene was expressed considerably lower than that of VdPKAC1. Although disruption of VdPKAC1 did not affect the ability of V. dahliae to infect through the roots of tomato and eggplant, disease severity was significantly reduced. Since pathogen-derived ethylene is presumed to play a major role in symptom induction in vascular wilt diseases, ethylene generation was measured in fungal culture. The mutants defective in VdPKAC1 produced less ethylene than the corresponding wild type strains, suggesting a regulatory role of PKA in ethylene biosynthesis. Growth rates of these mutants were similar to those of wild type strains, while the rate of spore germination was slightly elevated and conidia production was significantly reduced. When grown on minimal media, the mutants showed greater microsclerotia production compared with the wild type strains. These results suggest multiple roles of VdPKAC1, including virulence, conidiation, microsclerotia formation, and ethylene biosynthesis, in the soilborne fungus V. dahliae.
URLPMID:22387367 [本文引用: 5]
[本文引用: 3]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
DOI:10.1094/PHYTO-10-15-0280-RURLPMID:26780432 [本文引用: 1]

The soilborne fungal pathogen Verticillium dahliae infects a broad range of plant species to cause severe diseases. The availability of Verticillium genome sequences has provided opportunities for large-scale investigations of individual gene function in Verticillium strains using Agrobacterium tumefaciens-mediated transformation (ATMT)-based gene-disruption strategies. Traditional ATMT vectors require multiple cloning steps and elaborate characterization procedures to achieve successful gene replacement; thus, these vectors are not suitable for high-throughput ATMT-based gene deletion. Several advancements have been made that either involve simplification of the steps required for gene-deletion vector construction or increase the efficiency of the technique for rapid recombinant characterization. However, an ATMT binary vector that is both simple and efficient is still lacking. Here, we generated a USER-ATMT dual-selection (DS) binary vector, which combines both the advantages of the USER single-step cloning technique and the efficiency of the herpes simplex virus thymidine kinase negative-selection marker. Highly efficient deletion of three different genes in V. dahliae using the USER-ATMT-DS vector enabled verification that this newly-generated vector not only facilitates the cloning process but also simplifies the subsequent identification of fungal homologous recombinants. The results suggest that the USER-ATMT-DS vector is applicable for efficient gene deletion and suitable for large-scale gene deletion in V. dahliae.
DOI:10.1371/journal.pone.0015319URLPMID:21151869 [本文引用: 1]

Verticillium dahliae Kleb. is a phytopathogenic fungus that causes wilt disease in a wide range of crops, including cotton. The life cycle of V. dahliae includes three vegetative phases: parasitic, saprophytic and dormant. The dormant microsclerotia are the primary infectious propagules, which germinate when they are stimulated by root exudates. In this study, we report the first application of Agrobacterium tumefaciens-mediated transformation (ATMT) for construction of insertional mutants from a virulent defoliating isolate of V. dahliae (V592). Changes in morphology, especially a lack of melanized microsclerotia or pigmentation traits, were observed in mutants. Together with the established laboratory unimpaired root dip-inoculation approach, we found insertional mutants to be affected in their pathogenicities in cotton. One of the genes tagged in a pathogenicity mutant encoded a glutamic acid-rich protein (VdGARP1), which shared no significant similarity to any known annotated gene. The vdgarp1 mutant showed vigorous mycelium growth with a significant delay in melanized microsclerotial formation. The expression of VdGARP1 in the wild type V529 was organ-specific and differentially regulated by different stress agencies and conditions, in addition to being stimulated by cotton root extract in liquid culture medium. Under extreme infertile nutrient conditions, VdGARP1 was not necessary for melanized microsclerotial formation. Taken together, our data suggest that VdGARP1 plays an important role in sensing infertile nutrient conditions in infected cells to promote a transfer from saprophytic to dormant microsclerotia for long-term survival. Overall, our findings indicate that insertional mutagenesis by ATMT is a valuable tool for the genome-wide analysis of gene function and identification of pathogenicity genes in this important cotton pathogen.
DOI:10.1007/s11427-017-9075-3URLPMID:28755294 [本文引用: 3]

Verticillium dahliae is a soil-borne phytopathogenic fungus that causes vascular wilt disease in a broad range of hosts. This pathogen survives for many years in soil in the form of melanized microsclerotia. To investigate the melanin synthesis in V. dahliae, we identified a polyketide synthase gene in V. dahliae, namely VdPKS1. PKS1 is known to involve in the dihydroxynaphthalene melanin synthesis pathway in many fungi. We found that VdPKS1 was required for melanin formation but not for microsclerotial production in V. dahliae. The VdPKS1 gene-disruption mutant (vdpks1) formed melanin-deficient albino microsclerotia, which did not affect the fungal colonization in host tissues but significantly reduced the disease severity. Gene transcription analysis in the wild-type and the vdpks1 strains suggested that VdPKS1 gene-disruption influenced the expression of a series of genes involved in ethylene biosynthesis, microsclerotial formation and pathogenesis. Our results suggest that the VdPKS1-mediated melanin synthesis is important for virulence and developmental traits of V. dahliae.
URLPMID:21829347 [本文引用: 2]
URLPMID:18452583 [本文引用: 1]
URLPMID:18660430 [本文引用: 1]
URLPMID:17849712 [本文引用: 1]
[本文引用: 2]
URLPMID:26812120 [本文引用: 1]
DOI:10.1371/journal.pone.0144020URLPMID:26633180 [本文引用: 4]

Verticillium dahliae is the primary causal agent for Verticillium wilt disease on a diverse array of economically important crops, including cotton. In previous research, we obtained the low-pathogenicity mutant T286 from the T-DNA insertional mutant library of the highly virulent isolate Vd080 derived from cotton. In this study, the target disrupted gene VdCYC8 was identified by TAIL-PCR, encoding a homolog of CYC8 proteins involved in glucose repression. The deletion mutant DeltaCYC8 exhibited several developmental deficiencies, including reduced microsclerotia formation, reduced sporulation, and slower growth. Moreover, compared with the wild type strain Vd080, the pathogenicity of strain DeltaCYC8 was significantly decreased on cotton seedlings. However, the complementary mutants DeltaCYC8-C led to restoration of the wild type phenotype or near wild type levels of virulence on cotton. Interestingly, pathogenicity of the strains was correlated with VdCYC8 gene expression levels in complemented mutants. Gene expression analyses in the wild type strain Vd080, the DeltaCYC8-45 strain, and complemented strain DeltaCYC8-C26 indicated that VdCYC8 regulates the transcription levels of several genes in V. dahliae that have roles in melanin and production.
[本文引用: 1]
[本文引用: 1]
