 ,信阳农林学院牧医工程学院,河南信阳 464000
,信阳农林学院牧医工程学院,河南信阳 464000Lentivirus Mediated Interference Silencing MAT2A and MAT2B Inhibited Differentiation of Porcine Intramuscular Preadipocytes
ZHAO CunZhen, YI BenChi, CHEN PeiRong, LI JianZhu, ZHAO YunHuan, ZHU ZhongKe ,College of Animal Science and Veterinary Medicine, Xinyang College of Agriculture and Forestry, Xinyang 464000, Henan
,College of Animal Science and Veterinary Medicine, Xinyang College of Agriculture and Forestry, Xinyang 464000, Henan通讯作者:
收稿日期:2018-06-14接受日期:2019-07-3网络出版日期:2019-08-01
| 基金资助: |
Received:2018-06-14Accepted:2019-07-3Online:2019-08-01

摘要
关键词:
Abstract
Keywords:
PDF (2480KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
赵存真, 易本驰, 陈培荣, 李建柱, 赵云焕, 朱忠珂. 慢病毒介导shRNA干扰MAT2A与MAT2B基因抑制猪肌内脂肪细胞分化[J]. 中国农业科学, 2019, 52(15): 2706-2715 doi:10.3864/j.issn.0578-1752.2019.15.014
ZHAO CunZhen, YI BenChi, CHEN PeiRong, LI JianZhu, ZHAO YunHuan, ZHU ZhongKe.
0 引言
【研究意义】慢病毒载体作为一个高效实用的基因转移工具,其在基础和应用研究领域已得到广泛认可[1,2]。RNAi是可以抑制正常生物体内特定基因表达的一种工具,在进化过程中高度保守,由双链RNA (double-stranded RNA,dsRNA)诱发目的mRNA高效特异性降解的现象,其利用RNA介导,可特异性地阻断和降低目的基因 [3]。慢病毒载体作为一类来源于逆转录病毒的载体,可感染分裂期和非分裂期细胞,其感染效率高[3]。利用RNA介导,可特异性地阻断和降低目的基因的表达,同时载体感染细胞的范围扩大,外源的short-hairpin RNAs (shRNAs)既可用于细胞特定基因功能的研究,还可用于基因治疗[4]。【前人研究进展】哺乳动物有两种形式的腺苷甲硫氨酸转移酶,一种是肝脏特异性的MAT1A,编码MAT I/III ;另外一种是非肝脏特异性的MAT2A,编码MATII[5]。另外一个基因——MAT2B,编码β调控亚基,与MAT2A 编码的α2催化亚基一起调控MATII的酶活性[6]。MATI/III主要在肝脏细胞中表达并维持着这些细胞的分化状态;MATII在所有的肝外细胞中表达,在正常的肝细胞中检测不到其表达,但是在肝癌细胞的激活或去分化状态时能够被激活[7]。在肝癌细胞中,c-Myb、Sp1、NF-κB 和 AP-1等转录因子能够促进MAT2A启动子的转录,同时MAT2A的表达能够响应于TNF-α的处理[8,9]。MAT2A作为MafK的一个转录抑制子与染色质调控基因一起为SAMe提供甲基转移酶[10]。除此之外,在人类的结肠癌和肝癌细胞中,MATα2泛素化可以正向的调控Bal-2的表达[11]。在大鼠的肝癌细胞中, PPARγ负调控处于静息状态肝癌细胞时的MAT2A基因表达,但是在由静息状态转向激活状态时,则可以上调MAT2A基因的表达[12]。同时,MAT2A能特定地与 H3K9 甲基转移酶SETDB1互作以及结合MAT 2B促进 COX-2基因位点的H3K9三甲基化而抑制COX-2基因表达[13]。【本研究切入点】先前研究采用基因芯片技术发现MAT2B与猪肌内脂肪含量密切相关,其在脂肪型猪和瘦肉型猪的脂肪和肌肉组织中存在着显著的差异表达,推测可能影响猪肌内脂肪沉积[14,15]。目前关于MAT2A和MAT2B基因的研究层面主要集中在癌细胞等方面,在脂肪细胞分化及其调控机制方面并未有相关报道。【拟解决的关键问题】本研究通过构建MAT2A和MAT2B基因的慢病毒干扰载体,进一步探讨其对脂肪细胞分化的作用及分子机理。1 材料与方法
试验于2016年10月至2017年11月在信阳农林学院牧医工程学院和信阳师范学院生命科学学院实验室进行。1.1 材料
3—7日龄健康黑猪由河南正阳种猪场提供。慢病毒载体系统pLenti Hl-shRNA,CMV-△8.9,CMV- VSVG及 E. coli 菌株和HEK293T细胞系由信阳农林学院牧医工程学院和信阳师范学院生命科学学院实验室保存。质粒小提试剂盒和凝胶回收试剂盒(Omega公司,美国);PCR反应试剂,限制性内切酶(BamH I 和Xho I),T4 DNA连接酶试剂盒及反转录试剂盒购于日本TaKaRa公司;实时定量试剂盒(Vazyme公司,美国);DMEM/F12、I型胶原酶、Opti-MEM、LipofectamineTM3000购于Invitrogen公司;胎牛血清(Science Cell,美国);MAT2A,MAT2B抗体购于Abcam公司;其他试剂均为国产或进口分析纯。1.2 方法
1.2.1 猪肌内脂肪细胞分离培养 3—7日龄健康小猪,取样前用0.5%新洁尔灭清洗,75%酒精擦拭3遍,电击处死。无菌条件下分离背最长肌组织。用含双倍双抗(200X)的PBS缓冲液清洗3次,去除肉眼可见结缔组织或血管后,剪成1—2 mm3组织块。每克组织加入12 500 U的I胶原酶进行消化,置37℃振荡摇床上消化1—2 h。用等体积的10%FBS的DMEM/F12培养基中和,70和200目不锈钢筛网过滤。所收集滤液1 500×g离心10 min,弃上清;加入无血清DMEM/F12培养基重悬清洗,1 500×g离心2次。细胞计数仪计数后,以2.5×105活细胞密度接种于100 mm(Corning公司,美国)细胞培养皿中,置37℃,5% CO2培养箱中培养2 h后,细胞贴壁后,用PBS缓冲液清洗3次,并更换新鲜培养基;换液后,细胞置于37℃,5% CO2培养箱中继续培养。待其长至80%汇合度时传代,以6×104/cm2活细胞密度接种于12孔细胞培养板中(Corning公司,美国),用于后续诱导分化试验。1.2.2 猪pLentiH1-MAT2A/2B shRNA慢病毒重组质粒构建与鉴定 根据GenBank中猪MAT2A基因序列(Accession No.NM_001167650.1)和MAT2B基因序列(Accession No. NM_001142832.1),利用Invitrogen 公司在线软件BLOCK-iTTM RNAi Designer分别筛选3条shRNA靶序列,合成针对靶序列的相应寡核苷酸序列(表1)。将合成的单链寡核苷酸退火形成双链,与经过BamH I和Xho I(TaKaRa)双酶切后的pLenti-Hl载体连接并转化后,挑选阳性克隆提取质粒,经过酶切鉴定正确后送北京华大公司测序。
Table 1
表1
表1猪MAT2A和MAT2B的shRNA序列参数
Table 1
| RNAi | 序列 Primer sequence (5′ to 3′) |
|---|---|
| sh-MAT2A-1 sh-MAT2A-2 sh-MAT2A-3 sh-MAT2B-1 sh-MAT2B-2 sh-MAT2B-3 sh-scramble | 5′-GATCCGCAGCAGTCACCAGATATTGCctcgagGCAATATCTGGTGACTGCTGCTTTTTC-3′ 5′-TCGAGAAAAAGCAGCAGTCACCAGATATTGCctcgagGCAATATCTGGTGACTGCTGCG-3′ 5′-GATCCGCACACAAGCTCAATGCCAAActcgagTTTGGCATTGAGCTTGTGTGCTTTTTC-3′ 5′-TCGAGAAAAAGCACACAAGCTCAATGCCAAActcgagTTTGGCATTGAGCTTGTGTGCG-3′ 5′-TCGAGAAAAAGCCAAGTGGCAGATTTGTTATctcgagATAACAAATCTGCCACTTGGCG-3′ 5′-GATCCGGAGAGTGCCGTGACTGTTATctcgagATAACAGTCACGGCACTCTCCTTTTTC-3′ 5′-GATCCGCACAGCGACGAGTACAAGActcgagTCTTGTACTCGTCGCTGTGCTTTTTC-3′ 5′-TCGAGAAAAAGCACAGCGACGAGTACAAGActcgagTCTTGTACTCGTCGCTGTGCG-3′ 5′-GATCCGGAGAGTGCCGTGACTGTTATctcgagATAACAGTCACGGCACTCTCCTTTTTC-3′ 5′-TCGAGAAAAAGGAGAGTGCCGTGACTGTTATctcgagATAACAGTCACGGCACTCTCCG -3′ 5′-GATCCGCGAACACCATTTCGAATTGGctcgagCCAATTCGAAATGGTGTTCGCTTTTTC-3′ 5′-TCGAGAAAAAGCGAACACCATTTCGAATTGGctcgagCCAATTCGAAATGGTGTTCGCG-3′ 5′-GATCCGACACCTACGCAAAACCCTctcgagGACACCTACGCAAAACCCTTTTTTC-3′ 5′-TCGAGAAAAAGACACCTACGCAAAACCCTctcgagGACACCTACGCAAAACCCTG-3′ |
新窗口打开|下载CSV
1.2.3 重组慢病毒pLentiH1-MAT2A/2B shRNA包装、浓缩及滴度测定 293T细胞密度达90%左右时进行转染,转染前2—4 h换成新鲜的培养基,使细胞适应。转染时,需要配置两种液体:A液,溶解有质粒的溶液: 10 μg转移载体+7.5 μg Δ8.9+5 μg VSVG溶解在1.5 mL Opti-DMEM培养基中,混匀;B液,溶解有脂质体的溶液:35 μL X-tremeGENE HP DNA溶解在1.5 mL Opti-MEM,混匀;A液和B液,室温静置5 min;将两种液体混合,混匀,室温静置20 min,均匀滴加到培养皿中,轻微震荡,放培养箱培养。加入质粒后24 h观察荧光蛋白表达情况,48 h和72 h后分别收集上清。将收集的病毒上清于2 000 r/min离心10 min去除细胞碎片保存备用。0.45 μm滤膜过滤上清液,采用100 000 r/min离心30 min得到浓缩的病毒颗粒。按照10倍比例稀释病毒,以10-2—10-6浓度梯度分别感染293T细胞,48 h统计绿色荧光表达情况,按公式:病毒滴度(pfu·mL-1)= GFP阳性细胞数×病毒稀释倍数÷0.01 mL,计算病毒滴度。
1.2.4 油红O染色与定量分析 病毒感染后,诱导分化,取分化第8天的细胞,弃去培养基,PBS缓冲液清洗;采用4%多聚甲醛室温下固定30 min,PBS清洗;60%油红O工作液浸染细胞30 min,PBS清洗3次;显微镜下观察照相。定量分析时,100%异丙醇萃取与脂滴结合的油红O,分别等量收集到比色皿中,在波长为510 nm的分光光度计下测定其吸光值(A)。
1.2.5 RNA提取和Real-time PCR定量分析 根据NCBI 中GenBank 数据库中猪MAT2A和MAT2B以及成脂标志基因序列,采用Primer Primer 5. 0设计qPCR定量引物,引物序列见表2。按照TRIzol试剂盒提取不同时间点收集的猪肌内脂肪细胞的RNA。按照Vazyme反转录试剂盒操作说明进行反转录,合成cDNA第一链;然后以此作为模板,采用IQ5 (Bio-Rad公司)实时定量PCR系统检测基因表达情况。荧光定量反应体系为20 μL:SYBR Mix为10 μL,上下游引物各0.8 μL(10 μmol·L-1),cDNA模板为2 μL,用ddH2O补至20 μL。采用的扩增程序为95℃预变性10 min,95℃变性15 s,60℃退火20 s,共40个循环。基因相对表达量采用2-ΔΔCt法计算,以β-actin作为内参基因。每组试验至少进行重复3次。
Table 2
表2
表2实时定量PCR扩增所用的引物序列
Table 2
| Gene | 序列号Accession No. | 序列Primer sequence (5′ to 3′) |
|---|---|---|
| MAT2A | NM_001167650.1 | S: GTGGTTCGTGAAACCATTAAG A: ATCAGTGGCATAACCAAACAT |
| MAT2B | NM_001142832.1 | S: TAGGAGCTGCTGTTTTGAGA A: CACACGCCATTTCATACTTG |
| PPARγ | NM_214379.1 | S:AGGACTACCAAAGTGCCATCAAA A:GAGGCTTTATCCCCACAGACAC |
| aP2 | HM_453202 | S:GAGCACCATAACCTTAGATGGA |
| A:AAATTCTGGTAGCCGTGACA | ||
| CEBP/α | AF_103944.1 | S: GCACTTGCAGTTCCAGATCG A:ACCCGGTACTCGTTGCTGTT |
| β-actin | NM_007393 | S: GGACTTCGAGCAGGAGATGG A: AGGAAGGAGGGCTGGAAGAG |
新窗口打开|下载CSV
1.2.6 蛋白提取及Western blot印迹分析 处理后的猪肌内脂肪细胞,PBS清洗3次,加入适量的RIPA蛋白裂解液(含10 g·L-1 PMSF), 采用BCA法测定蛋白浓度,加入5×蛋白上样缓冲液并确保蛋白浓度一致,煮沸10 min后,12% SDS-PAGE凝胶电泳,电泳结束后转移至PVDF膜上,采用5%脱脂奶粉常温封闭2 h。一抗和二抗4℃孵育过夜,蛋白清洗液清洗4次后进行蛋白曝光。
1.3 统计分析
采用SPSS13.0统计分析软件One-way ANOVA进行方差分析试验数据,以平均值±标准误(mean±S.E.)表示,用t 检验(t-test)对不同试验处理之间的差异进行显著性分析。2 结果
2.1 MAT2A/2B重组慢病毒干扰载体的构建与鉴定
利用Invitrogen在线软件BLOCK-iTTM RNAi Designer(https://www.rnaid esigner. invitrogen.com/ rnaiexpress/)设计猪MAT2A和MAT2B基因各3条shRNA,并与无关序列的单链寡核苷酸序列一起在上海生工合成(表1)。经退火后形成双链DNA(图1-A),与经过BamH I和Xho I双酶切的LentiH1载体连接(图1-B),转化提取质粒后,将酶切鉴定成功的质粒(图1-C)送公司测序,确保插入载体的序列与设计合成的靶基因链完全一致。经鉴定,MAT2A与MAT2B基因的shRNA与无关序列的寡核苷酸均无突变,插入正确。图1
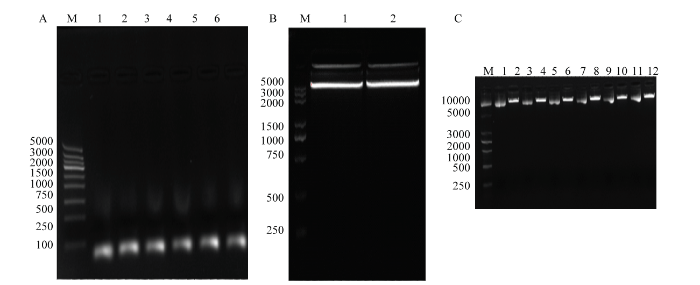 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1重组慢病毒干扰载体的构建与鉴定
(A):琼脂糖凝胶电泳检测合成的shRNA退火所形成双链。1-3:3条sh-MAT2A形成的双链;4-6:3条sh-MAT2B形成的双链。(B) :LentiH1 载体的Xho I和BamH I双酶切检测。(C):重组质粒的琼脂糖凝胶电泳酶切鉴定。1,3,5,7,9,11经酶切的质粒鉴定,2, 4, 6, 8, 10, 12未经过酶切的质粒鉴定
Fig. 1Construction and identification of recombinant plasmid LentiH1-shRNA
(A): Produce shRNA interference fragments sense and antisense oligo nucleotides were annealed to form complementary double strands DNA. M: DNA marker (DL 5000); 1-3: sh-MAT2A RNA; 4-6: sh-MAT2B RNA. (B): LentiH1 shuttle plasmid was double digested by Xho I and BamH I. 1-2: the product of LentiH1 shuttle plasmid was double digested; all released two fragments (7612 bp and 59 bp). M: DNA marker (DL 5000). (C): recombinant plasmids were identified by digesting. M: DNA marker (DL 5000). 1, 3, 5, 7, 9, 11: digested plasmid (as a control); 2, 4, 6, 8, 10, 12: undigested plasmid (2-6: sh-MAT2A; 8-12: sh-MAT2B)
2.2 pLentiHl-MAT2A/2B慢病毒干扰载体的包装和滴度测定
293T细胞生长密度为90%左右,并且状态良好时,采用X-tremeGENE-HP DNA转染试剂与重组质粒以及包装质粒(CMV-Δ8.9和CMV-VSVG)一起转染293T细胞,48 h后观察绿色荧光蛋白(GFP)的表达情况。如图2所示,细胞状态良好,绿色荧光强度高, 90%以上细胞表达绿色荧光蛋白;继续培养,分别于转染质粒48 h和72 h后收集病毒上清,并进行离心浓缩。采用病毒梯度稀释法测定病毒滴度。病毒滴度结果分别为7.31×107、6.70×107和7.0×107 pfu/mL,满足侵染原代脂肪细胞的试验要求。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2pLentiH1-MAT2A/2B shRNA慢病毒干扰载体的包装
A-C:分别是无关序列,sh-MAT2A及sh-MAT2B包装48 h的绿色荧光蛋白的表达情况
Fig. 2The packaging of pLentiH1-MAT2A/2B shRNA vector
A-C: The GFP expression of sh-scramble and sh-MAT2A and sh-MAT2B that separately infected for 48 h
2.3 pLentiHl-MAT2A/2B shRNA侵染猪肌内前体脂肪细胞效果
为检测所包装的慢病毒载体对猪原代脂肪细胞内MAT2A和MAT2B基因的干扰效率。试验将病毒载体sh-scaramble (设为对照),sh-MAT2A和 sh-MAT2B侵染猪肌内脂肪细胞,24 h观察到绿色荧光蛋白的表达,但是侵染效率较低;72 h后肌内脂肪细胞出现大面积的绿色荧光蛋白(GFP),如图3-A、B和C所示。分别收集RNA采用Real-time qPCR检测sh-MAT2A及sh-MAT2B的干扰情况,与sh-scramble对照相比,sh-MAT2A-3和sh-MAT2B-2干扰效果最佳,其干扰效率分别在70%和60%以上,达到预期效果。为了进一步证明sh-MAT2A和 sh-MAT2B是否对MAT2A和MAT2B蛋白表达水平产生影响,采用Western blot试验及蛋白分析验证。结果如图4所示,干扰MAT2A基因,MAT2A蛋白表达水平降低40%左右,达到极显著水平(图4-A和C);同样干扰MAT2B基因后,也显著抑制了MAT2B蛋白水平表达(图4-B和D)。这些结果表明MAT2A及MAT2B慢病毒干扰载体构建成功,可以进行后续试验。图3
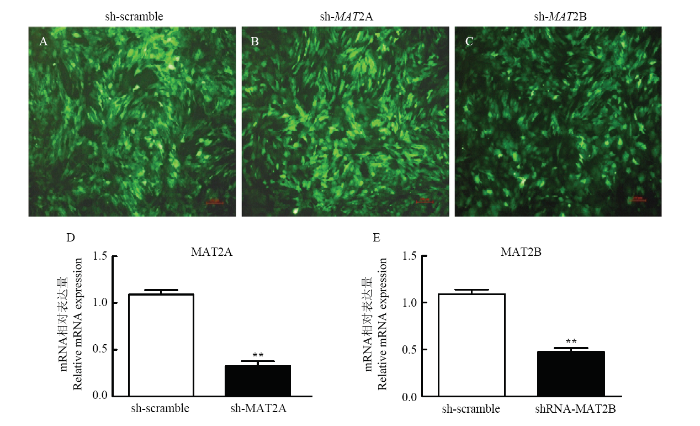 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3sh-MAT2A/2B重组慢病毒感染猪前体脂肪细胞的效率
A-C: sh-scaramble, sh-MAT2A 和 sh-MAT2B侵染猪肌内脂肪细胞72 h的荧光表达。D-E: Real-time qPCR 检测MAT2A和MAT2B基因的干扰效率
Fig. 3The efficiency of cultured preadipocytes that were infected with recombinant lentivirus
A-C Porcine preadipocytes were separately infected by sh-scaramble (as a control), sh-MAT2A and sh-MAT2B for 72 h. D-E: Real-time qPCR to analyze the relative expression of MAT2A and MAT2B (Data are presented as means ± SEM, n=3. *P<0.05, **P<0.01)
图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4Western blot检测MAT2A 和MAT2B蛋白干扰效率
A-B:Western blot 检测MAT2A和MAT2B蛋白的干扰效率。C-D:蛋白定量分析MAT2A和MAT2B基因的干扰效率
Fig. 4The detection of MAT2A and MAT2B interference efficiency
A-B: The Interference efficiency of MAT2A and MAT2B was detected using Western blot. C-D: Quantification to analysis the protein level of MAT2A and MAT2B (Data are presented as means ± SEM, n=3. *P<0.05, **P<0.01)
2.4 干扰MAT2A和MAT2B基因抑制猪肌内脂肪细胞分化
猪肌内前体脂肪细胞侵染慢病毒携带的干扰片段sh-MAT2A 48 h后(设转染无义序列sh-scramble为对照组),MDI培养基诱导分化至成熟。结果显示,与sh-scramble对照组相比,脂滴聚积能力在sh-MAT2A处理组中显著降低(图5-A);干扰MAT2B同样也显著抑制了脂滴的积累(图5-A)。吸光度定量分析结果显示,在MAT2A及MAT2B基因缺失的猪肌内脂肪细胞中,脂滴聚积的能力显著低于对照组(图5-B)。RT-PCR检测干扰MAT2A及MAT2B基因后,成脂标志基因的表达情况。结果如下图所示(图5-C),降低MAT2A和MAT2B基因的表达后,显著抑制成脂标志基因PPARγ,aP2及CEBP/α的表达。这些结果表明慢病毒介导的MAT2A和MAT2B基因干扰显著抑制了猪肌内脂肪细胞分化。图5
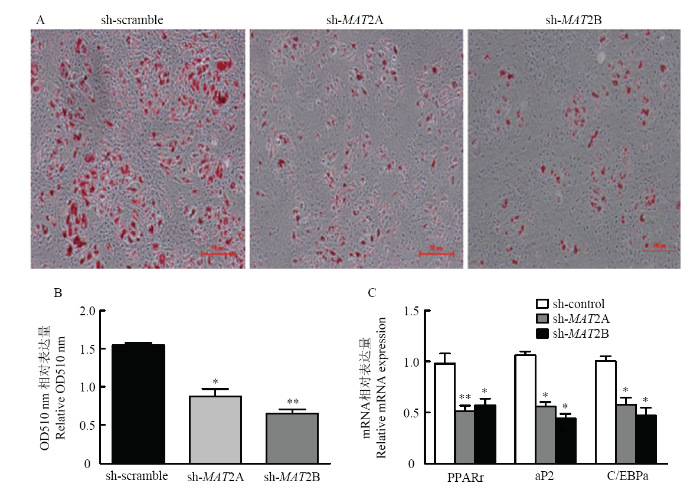 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5干扰MAT2A/2B抑制猪肌内脂肪细胞分化
A-B:猪肌内脂肪细胞内干扰MAT2A和MAT2B基因的油红O染色及定量。C:实时定量检测干扰MAT2A和MAT2B对PPARγ,aP2及CEBP/α的影响
Fig. 5Silencing MAT2A and MAT2B inhibited differentiation of porcine intramuscular adipocytes
A-B: Cells were stained with Oil red O and quantification to assess lipid accumulation. C: The relative mRNA expression of MAT2A and MAT2B detected by RT-qPCR. (Data are presented as means ± SEM; n=3; *P<0.05, **P<0.01)
3 讨论
通过构建慢病毒干扰载体可成功的敲低MAT2A和MAT2B在猪肌内脂肪细胞中的表达(干扰效率可达到70%左右),构建干扰载体抑制基因表达是研究一个基因的功能作用必不可少的一方面。通过化学合成的siRNA在转染试剂的介导下也可达到抑制基因表达的目的,但存在转染试剂介导RNAi转染率低、基因抑制表达效率低、持续时间短、使用成本大且不适合活体实验等缺点[16]; 但慢病毒介导的shRNA作用持久,同时载体感染细胞的范围较广,既可用于细胞特定基因功能的研究,还可用于基因治疗,将两种技术结合起来,已成为肿瘤性疾病治疗中的一种有效方法。通过慢病毒将肿瘤的治疗基因安全地转移到靶细胞内,在各种恶性肿瘤的基础研究中有广泛的应用。例如,PFEIFER等将慢病毒干扰系统shRNA导入小鼠受精卵中,培育后得到特定基因表达沉默的小鼠个体,用于特定基因缺失后对细胞功能的影响研究及有关疾病机制的基础研究等领域[17]。POESCHLA等通过构建慢病毒载体转染于患有帕金森病的大鼠,发现可起到减轻PD神经症状的效果[18]。本试验室采用慢病毒干扰系统在原代脂肪细胞中可成功地实现将实验目的基因敲除[19,20],通过慢病毒介导的干扰载体注射小鼠或其他模式动物的研究,从而研究目的基因对脂肪组织影响的试验,目前正在开展。肌内脂肪含量与猪肉品质密切相关,原代肌内脂肪细胞是研究脂肪沉积及其调控机制必不可少的实验工具[21]。先前研究发现MAT2B在脂肪型猪和瘦肉型猪的背最长肌中存在差异表达[15],但是其是否在小猪和大猪中存在差异表达以及是否在猪肌内脂肪细胞中存在作用是未知的。我们先前的研究结果表明MAT2B在3日龄的皮下脂肪组织表达显著低于180日龄大猪,但是在3日龄的小猪的背最长肌中却显著高于180日龄大猪。进一步的试验证明MAT2B在成熟脂肪组织中要高于前体脂肪细胞,且MAT2B随着分化时间的增加呈现出逐渐上升的趋势。这些结果均说明MAT2B在脂肪细胞分化过程中可能存在一定的作用[22]。本次试验通过干扰掉MAT2B后,脂滴积累明显减少,与先前的研究结果一致[23]。作为与MAT2B一起调控MATII活性的MAT2A ,先前的研究主要集中与肝癌细胞的增殖与凋亡[24,25,26,27]。在本研究中,我们首次发现干扰MAT2A后,猪肌内脂肪细胞中脂滴的积累显著减少,与MAT2B的作用功能一致。MAT催化生物体内重要的甲基供体—S-腺苷甲硫氨酸(SAM)的生成[6]。SAM水平的变化,必将影响DNA,RNA,组蛋白等方面的甲基化水平的变化[28,29]。例如,SAMe可以调控神经髓鞘施旺细胞全基因组DNA甲基化水平,从而影响周围神经髓鞘形成[30]。但是MAT2A和MAT2B如何来调控猪肌内脂肪细胞分化的呢,以及MAT催化的MATII 酶其酶活是否影响了脂肪细胞的分化,还有待进一步的验证。总体来看,本试验通过构建慢病毒介导的MAT2A/2B干扰载体,克隆包装等获得猪MAT2A和MAT2B基因慢病毒,实现猪肌内脂肪细胞内MAT2A/2B基因的干扰, 此为深入研究MAT2A和MAT2B调控猪脂肪细胞分化的作用及分子机制奠定基础。
4 结论
成功地构建了猪MAT2A和MAT2B基因慢病毒干扰载体,分别命名为sh-MAT2A和sh-MAT2B。成功获得重组慢病毒,滴度达到6.7×107和7×107 pfu/mL。感染细胞后,能有效降低猪肌内前体脂肪细胞内MAT2A和MAT2B基因的表达;进一步验证干扰MAT2A及MAT2B基因,可抑制猪肌内脂肪细胞分化。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
