 ,1, 孟春春2
,1, 孟春春2Construction of Chicken TIGAR Gene Eukaryotic Expression Plasmid and Evaluation of Its Anti-Apoptotic Function
LI YongHua1,2, CHE LuPing2, QIU XuSheng2, TAN Lei2, SUN YingJie2, LIU WeiWei2, SONG CuiPing2, LIAO Ying2, DING Chan2, WANG JinQuan ,1, MENG ChunChun2
,1, MENG ChunChun2通讯作者:
责任编辑: 林鉴非
收稿日期:2018-08-20接受日期:2018-12-13网络出版日期:2019-03-16
| 基金资助: |
Received:2018-08-20Accepted:2018-12-13Online:2019-03-16
作者简介 About authors
栗永华,E-mail: 1345346792@qq.com。

摘要
关键词:
Abstract
Keywords:
PDF (1446KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
栗永华, 车路平, 仇旭升, 谭磊, 孙英杰, 刘炜炜, 宋翠萍, 廖瑛, 丁铲, 王金泉, 孟春春. 鸡TIGAR基因真核表达质粒的构建及其抗凋亡功能评价[J]. 中国农业科学, 2019, 52(6): 1102-1109 doi:10.3864/j.issn.0578-1752.2019.06.013
LI YongHua, CHE LuPing, QIU XuSheng, TAN Lei, SUN YingJie, LIU WeiWei, SONG CuiPing, LIAO Ying, DING Chan, WANG JinQuan, MENG ChunChun.
0 引言
【研究意义】新城疫(newcastle disease,ND)是危害我国养禽业严重疫病之一,研究表明新城疫病毒(NDV)可诱导受感染细胞中caspase依赖的内源性和外源性凋亡途径[1],从而产生病变效应[2]。细胞凋亡的特征是细胞形态和生化变化,包括细胞膜泡、caspase活化和DNA断裂[3]。P53抑癌蛋白在细胞应激反应和抑制恶性发展中起着重要作用,P53抑癌蛋白通过协调DNA修复、诱导细胞周期阻滞、凋亡、衰老和维持基因组稳定性等多种机制调节肿瘤细胞的增殖和侵袭力[4],防止肿瘤的形成[5,6,7,8]。【前人研究进展】BENSAAD等提出p53在细胞代谢中起着直接作用,TP53诱导的糖酵解和凋亡调节因子(TP53-induced glycolysis and apoptosis regulator,TIGAR)是p53下游的靶基因,具有调节糖酵解和抗氧化作用的蛋白[9]。在细胞中TIGAR表达降低了果糖-2,6-二磷酸的水平来抑制磷酸果糖激酶(PFK),进而有利于果糖-6-磷酸(fructose-6-phosphate,F-6-P)和葡萄糖-6-磷酸(glucose-6-phosphatase,G-6-P)的形成,从而抑制了糖酵解[5]。TIGAR可通过增加磷酸戊糖途径(pentose phosphate pathway, PPP)[10,11,12],促进还原型烟酰胺腺嘌呤二核苷酸磷酸(nicotinamide adenine dinucleotide phosphate,NADPH)和谷胱甘肽(glutathione,GSH)的产生,从而去除活性氧(Reactive oxygen species,ROS)[13],研究证实 ROS 在 p53 介导的细胞凋亡中有着重要调节作用,可以由多种途径抑制细胞生长、诱发细胞凋亡[14,15]、自噬[16,17,18]。YE等用RNA干扰沉默HepG 2细胞中的TIGAR mRNA后,主要通过细胞凋亡和自噬作用抑制细胞生长[19]。细胞内ROS水平的降低而导致细胞对p53以及其他与ROS相关凋亡信号的敏感性[11, 20-21]。TIGAR可以在缺氧条件下与线粒体的己糖激酶2形成复合体,从而提高己糖激酶2的活性,降低线粒体膜电位,减少ROS产生,保护细胞免受ROS的损伤并保持线粒体的完整性[22,23,24]。KO等在研究中表明TIGAR在乳腺癌细胞中的表达促进了肿瘤的代谢分化和线粒体的乳酸和谷氨酰胺分解代谢,是一种很有潜力的乳腺癌治疗方法[25]。【本研究切入点】前人已扩增出人、鼠、牛等动物的TIGAR基因,并研究TIGAR的抗凋亡及在肿瘤的发生、增殖和转移中发挥的重要作用。本研究首次从SPF鸡的脾脏中扩增出鸡的TIGAR基因,并研究了其抗凋亡的作用。【拟解决的关键问题】验证本研究扩增出的TIGAR是否具有抗凋亡功能,过表达TIGAR基因后再感染病毒是否可以增加病毒滴度,并有利于病毒感染细胞的存活;为后续构建过表达TIGAR的细胞系和筛选适合新城疫病毒繁殖的高产细胞株提供前期基础。1 材料与方法
试验于2018年在中国农业科学院上海兽医研究所完成。1.1 试验材料
限制性内切酶(EcoR I、BamH I)、Trizol、Alexa FlourTM 488 Annexin V/Dead Cell Apoptosis Kit为Thermo公司产品,Taq聚合酶、dNTP、T4DNA连接酶为诺唯赞公司产品,M-MLV、RNasin、FuGen为Promega公司产品,DH5α感受态细胞为天根公司产品,ECL发光液为圣尔公司产品。1.2 试验方法
1.2.1 RT-PCR扩增TIGAR全长 取SPF鸡的脾脏,加PBS反复研磨3次,7 000 r/min离心10 min,取上清。采用Trizol裂解细胞提取RNA,加入随机引物6nt和Olig18t各1 μL,70℃10 min,冰浴5 min,按照Promega公司试剂盒的操作方法逆转录为cDNA,反转录的温度为37℃ 2 h、42℃ 2 h,75℃ 5 min灭活。根据GenBank(GenBank登录号:XM_417232.6)上预测的原鸡TIGAR序列设计并合成上游引物(5′-CCGGAATTCATGGTTCGCTTCGGG CTGACC-3′,限制性内切酶为EcoR I)和下游引物(5′-CGCG GATCCGAACATTTTCGGAGCTACACA TTCAGCACC-3′,限制性内切酶为BamH I),以cDNA为模板利用PCR扩增出鸡TIGAR的CDS区(扩增程序:95℃ 预变性30 s,95℃变性10 s,62℃退火30 s,72℃延伸1 min进行30个循环,72℃延伸10 min)。PCR结束后,产物经1%琼脂糖凝胶电泳并在紫外灯下观察,用胶回收试剂盒收集PCR产物,并送至上海生物工程公司测序。1.2.2 绘制哺乳动物、水生动物的TIGAR基因进化树 TIGAR基因位于12号染色体短臂1区3带3亚带,含6个可能编码外显子的区域和2个P53结合位点[9, 26-27],BENSAAD发现在脊椎动物中(从鱼到人)高度保守[9]。利用GenBank上找到脊椎动物的TIGAR基因以及本研究扩增的TIGAR基因构建进化树。
1.2.3 质粒转染DF1细胞 在200 μL的Opti-MEM转染试剂中加入6 μL FuGen,室温静置5 min,再加入Flag-TIGAR或Flag-CMV14 2 μg,轻轻混匀,室温静置20 min后加入6孔板中,于37℃培养4—6 h,更换成10%DMEM继续培养。
1.2.4 TIGAR蛋白表达检测和PARP检测 将细胞接种至6孔板(细胞密度为1×105),待细胞长至70%时转染2 μg重组质粒(Flag-TIGAR)和空载体(Flag-CMV14),转染24 h后感染Herts33(1MOI),在感染后18、24、30、36 h收集样品,每孔加入200 μL 2×Loading Buffer裂解细胞,收集入离心管,100℃ 煮样10 min,12 000 r/min离心3 min。取等量样品上样于10%聚丙烯酰胺凝胶进行电泳,电转移至硝酸纤维素膜上,加入5%脱脂乳室温封闭2 h;按照1:1 000稀释一抗(Flag、NP、PARP)4℃封闭过夜,用TBST洗膜3次,每次10 min;按照1:5 000稀释二抗室温封闭1 h,TBST洗膜3次,每次10 min。用化学发光法显色。
1.2.5 流式检测细胞凋亡 将细胞接种至6孔板(细胞密度为1×105),待细胞长至70%时分别转染重组质粒(Flag-TIGAR)和空载体(Flag-CMV14),转染后24、48 h收集细胞,用PBS洗后,加500 μL不含EDTA的胰酶消化2 min,用PBS吹下细胞并收集入离心管中,4℃1 000 r/min离心5 min,弃上清,用预冷的PBS洗3次,加入500 μL 1×Binding Buffer重悬细胞,分别加入5 μL PI(用1×Binding Buffer做10倍稀释)、25 μL Annexin V-FITC,室温避光孵育15 min,用流式仪检测细胞凋亡情况。
2 结果
2.1 TIGAR的扩增及遗传进化分析
扩增的全长TIGAR基因经1%琼脂糖凝胶电泳,结果显示扩增片段大小为843 bp,与预期结果相符(图1)。图1
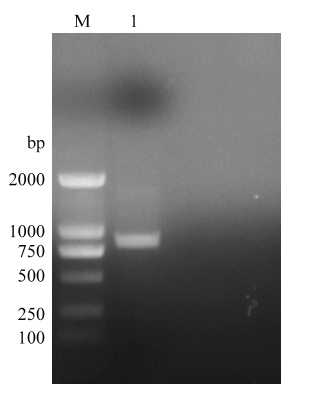 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1RT-PCR扩增鸡TIGAR基因
M:DL2000 Marker;1:RT-PCR扩增TIGAR基因的产物
Fig. 1Amplification chicken TIGAR gene with RT-PCR
1:The production of amplification chicken TIGAR gene by RT- PCR
本研究扩增的TIGAR基因全长3 206 bp,CDS区843 bp编码281个氨基酸,已上传至GenBank并获得登录号(MH511993.1),将扩增的鸡TIGAR基因的序列与其他脊椎动物的TIGAR基因的序列进行比对并构建进化树(图2),发现TIGAR基因在脊椎动物中高度保守。
图2
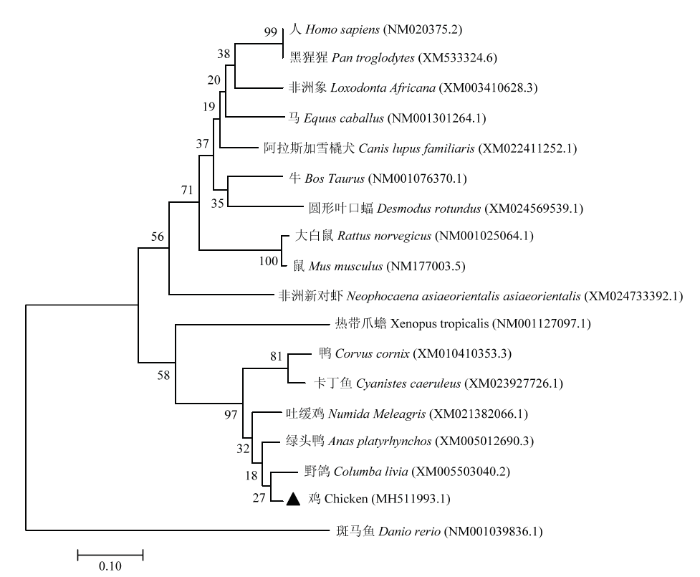 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2哺乳动物、水生动物的TIGAR基因的进化树
Fig. 2Phylogenetic tree of TIGAR gene in mammals and aquatic animals
2.2 Flag-TIGAR真核质粒的鉴定
将Flag-TIGAR重组质粒和空载转染至DF1细胞系中,转染后24、36、48 h的样品利用Western Blot检测蛋白表达情况,结果显示转染重组质粒的样品在30 kD的位置出现了条带,大小与预期结果一致;转染空载体(Flag-CMV14)和未转染细胞的样品未出现条带,表明Flag-TIGAR重组质粒构建成功(图3)。图3
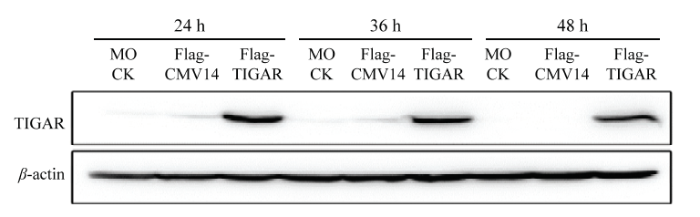 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3Flag-TIGAR真核质粒表达(Flag抗体)
Fig. 3Expression of Flag-TIGAR with Western Blot(Flag antibody)
2.3 Western Blot检测PARP
将Flag-TIGAR重组质粒和空载转染至DF1细胞系中,转染后24 h感染Herts/33(1MOI),Western Blot检测NP、FLAG、PARP的表达情况,结果显示转染重组质粒的样品均表达TIGAR;感染组均表达NP;在30 h、36 h的样品中PARP均有裂解条带且转染重组质粒(Flag-TIGAR)的样品裂解出PARP的表达量明显低于未转染质粒(MOCK)组的样品,且差异极显著(P<0.01)(图4)。图4
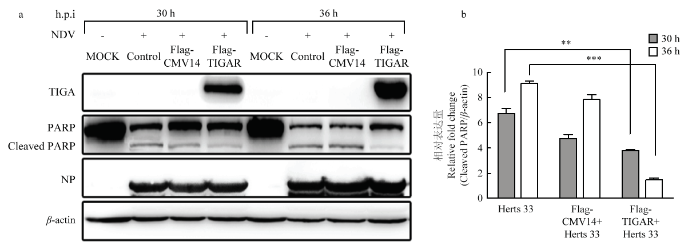 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4Western Blot检测PARP表达
** P<0.01;*** P<0.001。a:Western Blot检测PARP、NP、TIGAR的表达情况;b:Cleaved PARP/β-actin的相对表达情况
Fig. 4Detection PARP expression with Western Blot
a:The expression of PARP, NP, TIGAR was detection by Western Blot;b:Relative expression of Cleaved PARP/β-actin
2.4 FCM检测转染TIGAR对细胞凋亡的影响
将Flag-TIGAR和Flag-CMV14转染入DF1细胞,在转染后24、48 h收集细胞用流式细胞仪检测细胞凋亡情况(收集样品前2 h用药物Staurosporine诱导细胞凋亡,按照1:1 000的比例使用)。结果显示,24 h转染Flag-CMV14后细胞总凋亡率为11%(早期凋亡7.8%,晚期凋亡3.2%),而转染Flag-TIGAR后细胞总凋亡率为4%(早期凋亡3.7%,晚期凋亡0.3%),转染Flag-CMV14的早期凋亡率和晚期凋亡率均高于转染Flag-TIGAR组,且差异显著(P<0.05);48 h转染Flag-CMV14后细胞总凋亡率为20.3%(早期凋亡14.3%,晚期凋亡6.0%),而转染Flag-TIGAR后细胞总凋亡率为6.4%(早期凋亡4.8%,晚期凋亡1.6%),转染Flag-CMV14的早期凋亡率和晚期凋亡率均高于转染Flag-TIGAR组,且差异极显著(P<0.01)(图5)。图5
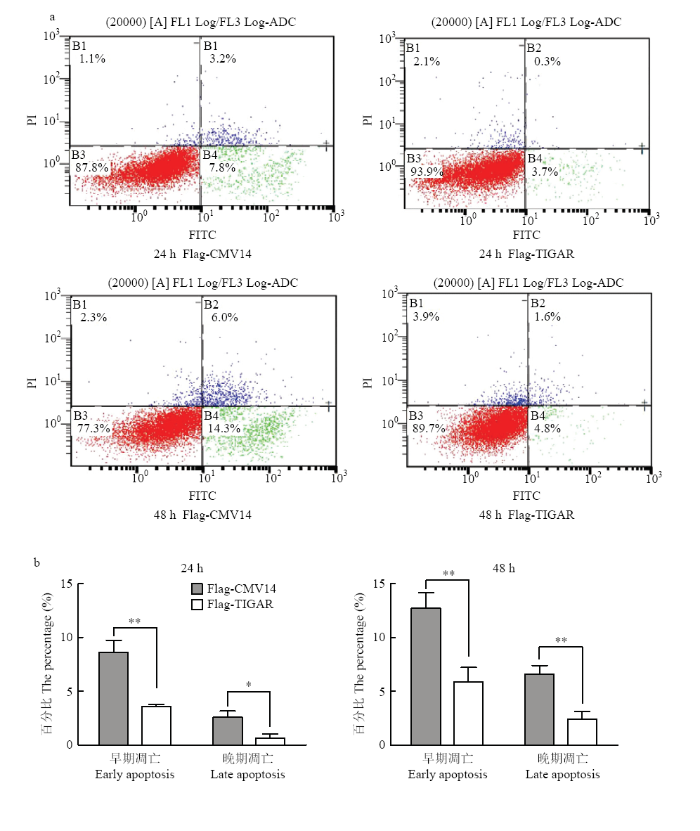 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5FCM检测细胞凋亡率
* P<0.05;** P<0.01。a:流式细胞仪检测细胞凋亡;b:统计分析细胞凋亡率
Fig. 5Detection the rate of apoptosis with FCM
a: Detection the apoptosis of cells by flow cytometry;b: Statistical analysis of apoptosis rate
3 讨论
TIGAR是具有调节糖酵解和抗氧化作用的蛋白,是存在于机体内的p53基因诱导的糖酵解和凋亡调节因子,通过抑制糖酵解途径,增加磷酸戊糖途径(PPP),可产生大量的5-磷酸核糖,是DNA修复和合成的重要原料[26];还可以通过维持还原型谷胱甘肽所需的NADPH水平来调节ROS水平,保护细胞免受ROS的损伤并保持线粒体的完整性,细胞内NADPH升高,活性氧(ROS)和自噬活性降低,因此TIGAR既可以抑制细胞凋亡又抑制自噬[21, 28]。本试验利用鸡脾脏组织cDNA为模板扩增出鸡的TIGAR基因,经测序分析后,并将TIGAR基因序列上传至GenBank。本研究通过检测凋亡蛋白(PARP)以及流式检测,结果表明扩增的鸡TIGAR基因具有抗凋亡的功能。
研究表明TIGAR在抗细胞凋亡及在肿瘤的发生、增殖和转移中发挥的重要作用。周骏浩通过试验预测TIGAR在脑预适应中的作用可能是中风预防和治疗[29]。细胞自噬是真核细胞内维持细胞自稳态的正常的分解代谢活动, 通过溶酶体将长寿蛋白、损伤的细胞器以及外源病原微生物吞噬、降解的过程, 是机体内一种重要的保护和防御机制。冯婧等干扰TIGAR蛋白后,细胞色素c在胞质内含量明显增加而在线粒体内含量减少,且干扰组细胞凋亡率明显升高,表明细胞凋亡增加;并检测细胞中自噬标志蛋白LC3-II/LC3-I、Beclin1表达显著上升,同时P62表达下降,表明细胞自噬增强[30]。细胞发生凋亡后不利于病毒在细胞内的复制[31,32,33],所以本研究为建立一株抑制病毒凋亡和自噬,利于病毒复制的细胞系奠定了前期基础。
4 结论
首次扩增出鸡的TIGAR基因,并对其功能进行研究,确认其具有抗凋亡的作用。为进一步研究鸡TIGAR基因在减少细胞凋亡,促进细胞增殖能力方面的研究提供了借鉴。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1016/j.virusres.2009.05.012URLPMID:19501124 [本文引用: 1]

Newcastle disease virus (NDV) causes economically significant Newcastle disease (ND) in almost all birds worldwide. Previous studies have shown that NDV induces caspase dependent apoptotic pathways in infected cells. In the present study, time course induction of apoptotic pathways in Vero cells is described. In NDV-infected cells, caspase-8 activity, percentage of cells showing TRAIL expression was higher at 24 h p.i. (post-infection) compared to 48 h p.i. In contrast, caspase-9 activity, efflux of cytochrome c, loss of mitochondrial membrane potential was higher at 48 h compared to 24 h p.i. The caspase-3 activity was high both times. Based on these results, it was concluded that at 24 h p.i., NDV induces apoptosis through extrinsic apoptotic pathway while at 48 h p.i. predominantly through intrinsic apoptotic pathway.
DOI:10.1016/j.virusres.2008.12.008URLPMID:19152817 [本文引用: 1]

The velogenic Newcastle disease virus (NDV) causes highly infectious and economically significant Newcastle disease (ND) in birds of various species. In cell culture NDV induces cytopathic effect (CPE) characterized by rounding, vacuolation, syncytia formation and cell death. Aside from cell to cell fusion caused by the F and HN glycoprotein of the virus molecular events leading to cell death are not known. In the current study, NDV-infected Vero cells, at 48 h p.i., showed nuclear condensation, cytoplasm blebbing, DNA fragmentation, and phosphatidylserine translocation to the cell surface. In addition, virus-infected cells demonstrated decreased DNA content and an increased Bax to Bcl-2 ratio, p53 level and caspase 3, 8, 9 expression compared to mock-infected cells. Based on these results, it was concluded that CPE in NDV-infected cells was caused by to the induction of apoptosis with the involvement of p53 and the Bax, dependent apoptotic pathways.
DOI:10.1038/bjc.1972.33URLPMID:4561027 [本文引用: 1]

The BJC is owned by Cancer Research UK, a charity dedicated to understanding the causes, prevention and treatment of cancer and to making sure that the best new treatments reach patients in the clinic as quickly as possible. The journal reflects these aims. It was founded more than fifty years ago and, from the start, its far-sighted mission was to encourage communication of the very best cancer research from laboratories and clinics in all countries. The breadth of its coverage, its editorial independence and it consistent high standards, have made BJC one of the world's premier general cancer journals. Its increasing popularity is reflected by a steadily rising impact factor.
DOI:10.3969/j.issn.1673-4130.2016.06.014URL [本文引用: 1]

目的探讨P53下游基因TIGAR在肺癌细胞A549增殖、迁移及侵袭中的作用。方法采用siRNA技术在A549细胞中干扰TIGAR的表达,细胞计数试剂盒(CCK-8)检测细胞增殖,小室法检测细胞迁移,肿瘤细胞侵袭实验检测细胞侵袭,免疫印迹杂交检测相关蛋白水平变化。结果在A549细胞中成功干扰TIGAR后,细胞增殖显著降低(P0.05),细胞迁移和侵袭能力显著减弱,侵袭相关蛋白基质金属蛋白酶2(MMP-2)和基质金属蛋白酶9(MMP-9)的表达量均下调。结论 TIGAR促进肺癌细胞A549的增殖,并促进细胞的迁移和侵袭能力。
.
DOI:10.3969/j.issn.1673-4130.2016.06.014URL [本文引用: 1]

目的探讨P53下游基因TIGAR在肺癌细胞A549增殖、迁移及侵袭中的作用。方法采用siRNA技术在A549细胞中干扰TIGAR的表达,细胞计数试剂盒(CCK-8)检测细胞增殖,小室法检测细胞迁移,肿瘤细胞侵袭实验检测细胞侵袭,免疫印迹杂交检测相关蛋白水平变化。结果在A549细胞中成功干扰TIGAR后,细胞增殖显著降低(P0.05),细胞迁移和侵袭能力显著减弱,侵袭相关蛋白基质金属蛋白酶2(MMP-2)和基质金属蛋白酶9(MMP-9)的表达量均下调。结论 TIGAR促进肺癌细胞A549的增殖,并促进细胞的迁移和侵袭能力。
.
DOI:10.1016/j.cell.2006.06.032PMID:16839873 [本文引用: 2]

The p53 tumor suppressor pathway coordinates DNA repair, cell-cycle arrest, apoptosis, and senescence to preserve genomic stability and prevent tumor formation. The discovery of three new target genes for p53 reveals unexpected functions for this tumor suppressor in the regulation of glucose metabolism and autophagy.
DOI:10.1038/35042675 [本文引用: 1]
DOI:10.1186/s12943-018-0839-4URLPMID:29753331 [本文引用: 1]

TIGAR is a p53 target gene that is known to protect cells from ROS-induced apoptosis by promoting the pentose phosphate pathway. The role of TIGAR in tumor cell invasion and metastasis remains...
[本文引用: 1]
DOI:10.1016/j.cell.2006.05.036URLPMID:16839880 [本文引用: 3]

The p53 tumor-suppressor protein prevents cancer development through various mechanisms, including the induction of cell-cycle arrest, apoptosis, and the maintenance of genome stability. We have identified a p53-inducible gene named TIGAR ( TP53- induced glycolysis and apoptosis regulator). TIGAR expression lowered fructose-2,6-bisphosphate levels in cells, resulting in an inhibition of glycolysis and an overall decrease in intracellular reactive oxygen species (ROS) levels. These functions of TIGAR correlated with an ability to protect cells from ROS-associated apoptosis, and consequently, knockdown of endogenous TIGAR expression sensitized cells to p53-induced death. Expression of TIGAR may therefore modulate the apoptotic response to p53, allowing survival in the face of mild or transient stress signals that may be reversed or repaired. The decrease of intracellular ROS levels in response to TIGAR may also play a role in the ability of p53 to protect from the accumulation of genomic damage.
[本文引用: 1]
DOI:10.1038/srep27096URLPMID:27256465 [本文引用: 2]

Previous study showed that TIGAR (TP53-induced glycolysis and apoptosis regulator) protected ischemic brain injury via enhancing pentose phosphate pathway (PPP) flux and preserving mitochondria function. This study was aimed to study the role of TIGAR in cerebral preconditioning. The ischemic preconditioning (IPC) and isoflurane preconditioning (ISO) models were established in primary cultured cortical neurons and in mice. Both IPC and ISO increased TIGAR expression in cortical neurons. Preconditioning might upregulate TIGAR through SP1 transcription factor. Lentivirus mediated knockdown of TIGAR significantly abolished the ischemic tolerance induced by IPC and ISO. ISO also increased TIGAR in mouse cortex and hippocampus and alleviated subsequent brain ischemia-reperfusion injury, while the ischemic tolerance induced by ISO was eliminated with TIGAR knockdown in mouse brain. ISO increased the production of NADPH and glutathione (GSH), and scavenged reactive oxygen species (ROS), while TIGAR knockdown decreased GSH and NADPH production and increased the level of ROS. Supplementation of ROS scavenger NAC and PPP product NADPH effectively rescue the neuronal injury caused by TIGAR deficiency. Notably, TIGAR knockdown inhibited ISO-induced anti-apoptotic effects in cortical neurons. These results suggest that TIGAR participates in the cerebral preconditioning through reduction of ROS and subsequent cell apoptosis.
DOI:10.1016/j.joca.2017.10.007URLPMID:29061494 [本文引用: 1]

Objective Hypoxia has been shown to inhibit reactive oxygen species(ROS)production in nucleus pulposus(NP)cells.The TP53-induced glycolysis and apoptosis regulator(TIGAR)has been reported to suppress oxidative stress.We sought to explore the role of TIGAR in the effect of hypoxia on ROS production and apoptosis.Methods An intervertebral disc degeneration(IDD)model of Sprague-Dawley(SD)rat caudal spine was established by stabbing their intervertebral discs.TIGAR expression was detected by immunohistochemistry in human and SD rat NP tissues of degenerated discs.Rat primary NP cells treated with hypoxia and cobalt chloride(CoCl_2)were analyzed by western blotting for TIGAR expression.After TIGAR inhibition with TIGAR siRNA transfection,apoptosis percentage,mitochondrial and total intracellular ROS levels were measured.H_2O_2 was used to further check the effects of TIGAR on oxidative stress.Finally,NADPH/NADP~+and GSH/GSSH ratio were examined after TIGAR silencing under hypoxic conditions and after H_2O_2 treatment.Results degree-dependent increase in TIGAR expression was observed in human and rat degenerated NP tissues.Hypoxia and hypoxia-inducer CoCl_2enhanced TIGAR and P53 expressions in rat NP cells.TIGAR inhibition reversed the inhibitory effects of hypoxia on intracellular and mitochondrial ROS production,as well as apoptosis percentage.However,TIGAR inhibition aggravated H_2O_2-induced ROS production.In addition,TIGAR increased NADPH/NADP~+and GSH/GSSH ratio in NP cells.Conclusions hese results suggested that TIGAR appears to mediate the protective role of hypoxia on ROS production and apoptosis percentage by enhancing NADPH/NADP+and GSH/GSSH ratio.
DOI:10.1152/ajprenal.00459.2014URLPMID:25503731 [本文引用: 1]

Tp53-induced glycolysis and apoptosis regulator (TIGAR) activation blocks glycolytic ATP synthesis by inhibiting phosphofructokinase-1 activity. Our data indicate that TIGAR is selectively induced and activated in renal outermedullary proximal straight tubules (PSTs) after ischemia-reperfusion injury in a p53-dependent manner. Under severe ischemic conditions, TIGAR expression persisted through 48 h postinjury and induced loss of renal function and histological damage. Furthermore, TIGAR upregulation inhibited phosphofructokinase-1 activity, glucose 6-phosphate dehydrogenase (G6PD) activity, and induced ATP depletion, oxidative stress, autophagy, and apoptosis. Small interfering RNA-mediated TIGAR inhibition prevented the aforementioned malevolent effects and protected the kidneys from functional and histological damage. After mild ischemia, but not severe ischemia, G6PD activity and NADPH levels were restored, suggesting that TIGAR activation may redirect the glycolytic pathway into gluconeogenesis or the pentose phosphate pathway to produce NADPH. The increased level of NADPH maintained the level of GSH to scavenge ROS, resulting in a lower sensitivity of PST cells to injury. Under severe ischemia, G6PD activity and NADPH levels were reduced during reperfusion; however, blockade of TIGAR enhanced their levels and reduced oxidative stress and apoptosis. Collectively, these results demonstrate that inhibition of TIGAR may protect PST cells from energy depletion and apoptotic cell death in the setting of severe ischemia-reperfusion injury. However, under low ischemic burden, TIGAR activation induces the pentose phosphate pathway and autophagy as a protective mechanism.
[D].
[本文引用: 1]
[D].
[本文引用: 1]
DOI:10.1002/bit.21589URLPMID:17680685 [本文引用: 1]

During Chinese hamster ovary (CHO) cell culture for foreign protein production, cells are subjected to programmed cell death (PCD). A rapid death at the end of batch culture is accelerated by nutrient starvation. In this study, type II PCD, autophagy, as well as type I PCD, apoptosis, was found to take place in two antibody-producing CHO cell lines, Ab1 and Ab2, toward the end of batch culture when glucose and glutamine were limiting. The evidence of autophagy was observed from the accumulation of a common autophagic marker, a 16 kDa form of LC3-II during batch culture. Moreover, a significant percentage of the total cells (80% of Ab1 cells and 86% of Ab2 cells) showed autophagic vacuoles containing cytoplasmic material by transmission electron microscopy. An increased level of PARP cleavage and chromosomal DNA fragmentation supported that starvation-induced apoptosis also occurred simultaneously with autophagy. Biotechnol. Bioeng. 2008;99: 678-685. 2007 Wiley Periodicals, Inc.
DOI:10.1074/mcp.RA118.000620URLPMID:29776966 [本文引用: 1]

Maternal obesity has been reported to impair oocyte quality in mice, however, the underlying mechanisms remain unclear. In the present study, by conducting a comparative proteomic analysis, we identified a reduced expression of TIGAR (TP53-induced glycolysis and apoptosis regulator) protein in ovulated oocytes from high-fat diet (HFD)-fed mice. Specific depletion of TIGAR in mouse oocytes results in the marked elevation of reactive oxygen species (ROS) levels and the failure of meiotic apparatus assembly. Importantly, forced expression of TIGAR in HFD oocytes not only attenuates ROS production, but also partly prevents spindle disorganization and chromosome misalignment during meiosis. Meantime, we noted that TIGAR knockdown in oocytes induces a strong activation of autophagy, whereas overexpression of TIGAR significantly reduces the LC3 accumulation in HFD oocytes. By anti-oxidant treatment, we further demonstrated that such an autophagic response is dependent on the TIGAR-controlled ROS production. In summary, our data indicate a role for TIGAR in modulating redox homeostasis during oocyte maturation, and uncover that loss of TIGAR is a critical pathway mediating the effects of maternal obesity on oocyte quality.
DOI:10.1038/s41401-018-0001-2URLPMID:29769743 [本文引用: 1]

Abstract Our previous study showed that TP53-induced glycolysis and apoptosis regulator (TIGAR) regulated ROS, autophagy, and apoptosis in response to hypoxia and chemotherapeutic drugs. Aescin, a triterpene saponin, exerts anticancer effects and increases ROS levels. The ROS is a key upstream signaling to activate autophagy. Whether there is a crosstalk between TIGAR and aescin in regulating ROS, autophagy, and apoptosis is unknown. In this study, we found that aescin inhibited cell viability and colony formation, and induced DNA damage, cell cycle arrest, and apoptosis in cancer cell lines HCT-116 and HCT-8 cells. Concurrently, aescin increased the expression of TIGAR, ROS levels, and autophagy activation. Knockdown of TIGAR enhanced the anticancer effects of aescin in vitro and in vivo, whereas overexpression of TIGAR or replenishing TIGAR downstream products, NADPH and ribose, attenuated aescin-induced apoptosis. Furthermore, aescin-induced ROS elevation and autophagy activation were further strengthened by TIGAR knockdown in HCT-116 cells. However, autophagy inhibition by knockdown of autophagy-related gene ATG5 or 3-methyladenine (3-MA) exaggerated aescin-induced apoptosis when TIGAR was knocked down. In conclusion, TIGAR plays a dual role in determining cancer cell fate via inhibiting both apoptosis and autophagy in response to aescin, which indicated that inhibition of TIGAR and/or autophagy may be a junctional therapeutic target in treatment of cancers with aescin.
DOI:10.1016/j.biochi.2015.07.016URLPMID:26212201 [本文引用: 1]

61Resveratrol reduces TIGAR protein to promote ROS induced apoptosis & autophagy.61TIGAR overexpression inhibits apoptosis and autophagy induced by resveratrol.61TIGAR knock down promotes apoptosis and autophagy induced by resveratrol.61Autophagy inhibition by Chloroquine promotes resveratrol induced cell death.
DOI:10.1016/j.bbrc.2013.06.072URLPMID:23817040 [本文引用: 1]

Apoptosis and autophagy are crucial mechanisms regulating cell death, and the relationship between apoptosis and autophagy in the liver has yet to be thoroughly explored. TIGAR (TP53-induced glycolysis and apoptosis regulator), which is a p53-inducible gene, functions in the suppression of ROS (reactive oxygen species) and protects U2OS cells from undergoing cell death. In this study, silencing TIGAR by RNAi (RNA interference) in HepG2 cells down-regulated both TIGAR mRNA (similar to 75%) and protein levels (similar to 80%) and led to the inhibition of cell growth (P < 0.01) by apoptosis (P < 0.001) and autophagy. We demonstrated that TIGAR can increase ROS levels in HepG2 cells. The down-regulation of TIGAR led to the induction of LC-3 II (specific autophagic marker), the formation of the autophagosome, and increased Beclin-1 expression. 3-MA (3-Methyladenine), an inhibitor of autophagic sequestration blocker, inhibited TIGAR siRNA-enhanced autophagy, as indicated by the decrease in LC-3 II levels. Consequently, these data provide the first evidence that targeted silencing of TIGAR induces apoptotic and autophagic cell death in HepG2 cells, and our data raise hope for the future successful application of TIGAR siRNA in patients with hepatocellular carcinoma (HCC). (C) 2013 The Authors. Published by Elsevier Inc. All rights reserved.
DOI:10.1101/gad.271130.115URLPMID:26679840 [本文引用: 1]

Abstract Reactive oxygen species (ROS) participate in numerous cell responses, including proliferation, DNA damage, and cell death. Based on these disparate activities, both promotion and inhibition of ROS have been proposed for cancer therapy. However, how the ROS response is determined is not clear. We examined the activities of ROS in a model of Apc deletion, where loss of the Wnt target gene Myc both rescues APC loss and prevents ROS accumulation. Following APC loss, Myc has been shown to up-regulate RAC1 to promote proliferative ROS through NADPH oxidase (NOX). However, APC loss also increased the expression of TIGAR, which functions to limit ROS. To explore this paradox, we used three-dimensional (3D) cultures and in vivo models to show that deletion of TIGAR increased ROS damage and inhibited proliferation. These responses were suppressed by limiting damaging ROS but enhanced by lowering proproliferative NOX-derived ROS. Despite having opposing effects on ROS levels, loss of TIGAR and RAC1 cooperated to suppress intestinal proliferation following APC loss. Our results indicate that the pro- and anti-proliferative effects of ROS can be independently modulated in the same cell, with two key targets in the Wnt pathway functioning to integrate the different ROS signals for optimal cell proliferation. 2016 Cheung et al.; Published by Cold Spring Harbor Laboratory Press.
DOI:10.1158/0008-5472.CAN-13-3517URLPMID:25085248 [本文引用: 2]

The p53-induced glycolysis and apoptosis regulator (TIGAR) inhibits glycolysis, resulting in higher intracellular NADPH, lower reactive oxygen species (ROS) and autophagy activity. In this study, we investigated whether TIGAR might exert dual impacts on cancer cell survival based on its ability to inhibit both apoptosis and autophagy. In liver or lung cancer cells treated with the anticancer drug epirubicin, TIGAR levels increased in a dose- and time-dependent manner. TIGAR silencing enhanced epirubicin-induced elevations in ROS levels and apoptosis rates, in a manner that was blocked by ectopic addition of NADPH or N-acetyl cysteine. These findings were correlated with reduced tumorigenicity and increased chemosensitivity in mouse xenograft tumor assays. In parallel, TIGAR silencing also enhanced the epirubicin-induced activation of autophagy, in a manner that was also blocked by ectopic addition of NADPH. Notably, TIGAR silencing also licensed epirubicin-mediated inactivation of the mTOR pathway, suggesting TIGAR also exerted a negative impact on autophagy. However, genetic or pharmacologic inhibition of autophagy increased epirubicin-induced apoptosis in TIGAR-silenced cells. Overall, our results revealed that TIGAR inhibits both apoptosis and autophagy, resulting in a dual impact on tumor cell survival in response to tumor chemotherapy. Cancer Res; 74(18); 5127-38. 2014 AACR.
DOI:10.1016/j.neuropharm.2018.01.012URLPMID:29331305 [本文引用: 1]

正Background:The inflammatoryresponse of glial cells contributes to neuronal damage caused by brainischemia/reperfusion insult.We previously demonstrated a protective role ofTP53-induced glycolysis and apoptosis regulator(TIGAR)in ischemic neuronal injurythrough increasing the flow of pentose phosphate pathway(PPP).The presentstudy investigated the possible role of TIGAR in ischemia/reperfusion-induced inflammatoryresponse of
DOI:10.1073/pnas.1206530109URLPMID:23185017 [本文引用: 1]

The p53-inducible protein TIGAR (Tp53-induced Glycolysis and Apoptosis Regulator) functions as a fructose-2,6-bisphosphatase (Fru-2,6-BPase), and through promotion of the pentose phosphate pathway, increases NADPH production to help limit reactive oxygen species (ROS). Here, we show that under hypoxia, a fraction of TIGAR protein relocalized to mitochondria and formed a complex with hexokinase 2 (HK2), resulting in an increase in HK2 activity. Mitochondrial localization of TIGAR depended on mitochondrial HK2 and hypoxia-inducible factor 1 (HIF1) activity. The ability of TIGAR to function as a Fru-2,6-BPase was independent of HK2 binding and mitochondrial localization, although both of these activities can contribute to the full activity of TIGAR in limiting mitochondrial ROS levels and protecting from cell death.
DOI:10.3892/ol.2017.7303URLPMID:5766069 [本文引用: 1]

Mitochondria have been described as ‘the powerhouse of the cell’ as the organelle generates the majority of adenosine triphosphate (ATP) in cells to support life. Mitochondria can be damaged due to stress, for example by reactive oxygen species (ROS). TP53-induced glycolysis and apoptosis regulator (TIGAR) serves a role in suppressing ROS damage and may protect mitochondria integrity. In the present study, the localization of TIGAR on mitochondria in 5–8F cells was demonstrated. Furthermore, it was indicated that the knockdown of TIGAR using lentivirus-short hairpin RNA induces the loss of mitochondrial membrane potential and cytochrome c leakage. However, these damaged mitochondria were not degraded in cells, but exhibited an abnormal appearance as indicated by mitochondrial swelling, crista collapse and vacuolization, with physiological dysfunction marked by reduced ATP production. Therefore, TIGAR maybe an indispensable protein for mitochondrial protection and degradation following cellular damage.
DOI:10.1074/jbc.M116.740209URLPMID:27803158 [本文引用: 1]

A subgroup of breast cancers has several metabolic compartments. The mechanisms by which metabolic compartmentalization develop in tumors are poorly characterized. TP53 inducible glycolysis and apoptosis regulator (TIGAR) is a bisphosphatase that reduces glycolysis and is highly expressed in carcinoma cells in the majority of human breast cancers. Hence we set out to determine the effects of TIGAR expression on breast carcinoma and fibroblast glycolytic phenotype and tumor growth. The overexpression of this bisphosphatase in carcinoma cells induces expression of enzymes and transporters involved in the catabolism of lactate and glutamine. Carcinoma cells overexpressing TIGAR have higher oxygen consumption rates and ATP levels when exposed to glutamine, lactate, or the combination of glutamine and lactate. Coculture of TIGAR overexpressing carcinoma cells and fibroblasts compared with control cocultures induce more pronounced glycolytic differences between carcinoma and fibroblast cells. Carcinoma cells overexpressing TIGAR have reduced glucose uptake and lactate production. Conversely, fibroblasts in coculture with TIGAR overexpressing carcinoma cells induce HIF (hypoxia-inducible factor) activation with increased glucose uptake, increased 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3), and lactate dehydrogenase-A expression. We also studied the effect of this enzyme on tumor growth. TIGAR overexpression in carcinoma cells increases tumor growth in vivo with increased proliferation rates. However, a catalytically inactive variant of TIGAR did not induce tumor growth. Therefore, TIGAR expression in breast carcinoma cells promotes metabolic compartmentalization and tumor growth with a mitochondrial metabolic phenotype with lactate and glutamine catabolism. Targeting TIGAR warrants consideration as a potential therapy for breast cancer.
URL [本文引用: 2]

TP53 诱导的糖酵解和凋亡调节因子(TP53-induced glycolysis andapoptosis regulator,TIGAR)是p53 下游的直接靶基因,其编码蛋白能够降解2,6-二磷酸果糖,后者是肿瘤细胞糖酵解途径中关键酶6-磷酸果糖激酶1(6-phosphofructokinase 1,PFK1)的最强激活剂,从而对糖酵解产生抑制作用。TIGAR 可促使糖代谢更多地流向磷酸戊糖途径(pentosephosphate pathway,PPP),促进烟酰胺腺嘌呤二核苷酸磷酸(nicotinamideadenine dinucleotide phosphate,NADPH)和谷胱甘肽(glutathione,GSH)的产生,降低细胞内活性氧(reactive oxygen species,ROS)水平。因此,TIGAR 是维持肿瘤细胞氧化还原状态平衡的重要因子,对细胞凋亡起到重要的调节作用。最新研究表明,TIGAR 在肿瘤发生、进展及转移过程中发挥重要作用,而且干扰TIGAR 表达可以提高多种肿瘤细胞对放化疗的敏感性,提示TIGAR 可能成为一个极具潜力的肿瘤治疗靶点。本文对近年来TIGAR 在肿瘤发生、进展和转移中的作用以及其靶向治疗的研究进展进行简要综述。
.
URL [本文引用: 2]

TP53 诱导的糖酵解和凋亡调节因子(TP53-induced glycolysis andapoptosis regulator,TIGAR)是p53 下游的直接靶基因,其编码蛋白能够降解2,6-二磷酸果糖,后者是肿瘤细胞糖酵解途径中关键酶6-磷酸果糖激酶1(6-phosphofructokinase 1,PFK1)的最强激活剂,从而对糖酵解产生抑制作用。TIGAR 可促使糖代谢更多地流向磷酸戊糖途径(pentosephosphate pathway,PPP),促进烟酰胺腺嘌呤二核苷酸磷酸(nicotinamideadenine dinucleotide phosphate,NADPH)和谷胱甘肽(glutathione,GSH)的产生,降低细胞内活性氧(reactive oxygen species,ROS)水平。因此,TIGAR 是维持肿瘤细胞氧化还原状态平衡的重要因子,对细胞凋亡起到重要的调节作用。最新研究表明,TIGAR 在肿瘤发生、进展及转移过程中发挥重要作用,而且干扰TIGAR 表达可以提高多种肿瘤细胞对放化疗的敏感性,提示TIGAR 可能成为一个极具潜力的肿瘤治疗靶点。本文对近年来TIGAR 在肿瘤发生、进展和转移中的作用以及其靶向治疗的研究进展进行简要综述。
URL [本文引用: 1]

目的:构建TP53诱导的糖酵解和凋亡调控子(T1GAR)全长cDNA的真核表达载体 pEGFP—TIGAR,观察TIGAR存人肝细胞HepG2中的作用:方法:RT-PCR技术扩增获得TIGAR全长cDNA,将扩增的产物克隆,经酶 切、DNA测序鉴定正确.构成pEGFP—TIGAR的真核表达重组质粒:利用脂质体将重组质粒转染入HepG2细胞,流式细胞仪和TUNEL法检测细胞 凋亡、流式细胞仪检测细胞周期、MIB-1(Ki-67抗原测定)法检测细胞增殖、实时荧光PCR检测mRNA表达和Westem-blot检测蛋白质的 表达。结果:构建完成的真核表达重组质粒pEGFP-TIGAR,经DNA测序与GenBank中的人TIGARcDNA序列一致。荧光显做镜观察到转染 重组质粒的细胞中有绿色荧光蛋白的表达。检测结果显示,转染pEGFP-TIGAR质粒的HepG2晚期凋亡细胞明显减少(P〈0.05);S期细胞百分 数则明显增加(P〈0.05).增殖能力明显增高(P〈0.05):结论:本实验成功构建了pEGFP-TIGAR真核表达质粒,过表达的TIGAR可降 低HeDG2细胞发生晚期凋亡和促进细胞增殖,为进一步研究TIGAR基因在肿瘤细胞中作用提供了基础.
.
URL [本文引用: 1]

目的:构建TP53诱导的糖酵解和凋亡调控子(T1GAR)全长cDNA的真核表达载体 pEGFP—TIGAR,观察TIGAR存人肝细胞HepG2中的作用:方法:RT-PCR技术扩增获得TIGAR全长cDNA,将扩增的产物克隆,经酶 切、DNA测序鉴定正确.构成pEGFP—TIGAR的真核表达重组质粒:利用脂质体将重组质粒转染入HepG2细胞,流式细胞仪和TUNEL法检测细胞 凋亡、流式细胞仪检测细胞周期、MIB-1(Ki-67抗原测定)法检测细胞增殖、实时荧光PCR检测mRNA表达和Westem-blot检测蛋白质的 表达。结果:构建完成的真核表达重组质粒pEGFP-TIGAR,经DNA测序与GenBank中的人TIGARcDNA序列一致。荧光显做镜观察到转染 重组质粒的细胞中有绿色荧光蛋白的表达。检测结果显示,转染pEGFP-TIGAR质粒的HepG2晚期凋亡细胞明显减少(P〈0.05);S期细胞百分 数则明显增加(P〈0.05).增殖能力明显增高(P〈0.05):结论:本实验成功构建了pEGFP-TIGAR真核表达质粒,过表达的TIGAR可降 低HeDG2细胞发生晚期凋亡和促进细胞增殖,为进一步研究TIGAR基因在肿瘤细胞中作用提供了基础.
DOI:10.1016/j.bcp.2017.07.018URLPMID:28774732 [本文引用: 1]

Physapubenolide (PB) is a cytotoxic withanolide isolated from Physalis angulata that was used as a traditional Chinese medicine. In this study, we investigated the role of TIGAR and ROS in PB-induced apoptosis and autophagosome formation in human breast carcinoma MDA-MB-231 and MCF-7 cells. PB induced apoptosis by decreasing mitochondrial membrane potential and elevating the Bax/Bcl-2 protein expression ratio in MDA-MB-231 and MCF-7 cells. Caspase inhibitor Z-VAD-FMK treatment partly blocked PB induced cytotoxicity, suggesting that apoptosis serves as an important role in the anti-proliferative effect of PB. Meanwhile, PB induced autophagosome formation, as characterized by increased acridine orange-stained positive cells, accumulation of punctate LC3B fluorescence and a greater number of autophagic vacuoles under electron microscopy. Furthermore, PB inhibited autophagic flux as reflected by the overlapping of mCherry and GFP fluorescence when MDA-MB-231 cells were transfected with GFP-mCherry-LC3 plasmid. Depletion of LC3B, ATG5 or ATG7 reduced PB-induced cytotoxicity, indicating that autophagosome associated cell death participated in the anti-cancer effect of PB. Moreover, PB-induced apoptosis and autophagosome formation were linked to the generation of intracellular ROS, and pre-treatment with the antioxidant NAC obviously mitigated the effects. Interestingly, PB treatment slightly increased TIGAR expression at low concentrations but decreased TIGAR expression drastically at high concentrations. Downregulation of TIGAR by small interfering RNA augmented low concentrations of PB-induced apoptosis and autophagosome formation, which contributed to the observed anti-cancer effect of PB and were reversed by NAC pre-treatment. Consistently, in MDA-MB-231 or MCF-7 xenograft mouse model, PB suppressed tumor growth through ROS induced apoptosis and autophagosome associated cell death accompanied with the downregulation of TIGAR. Taken together, these results indicate that downregulation of TIGAR increased PB-induced apoptosis and autophagosomes associated cell death through promoting ROS generation in MDA-MB-231 and MCF-7 cells.
[D].
[本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
DOI:10.3870/j.issn.1672-0741.2018.01.025URL [本文引用: 1]

细胞自噬是广泛存在于真核细胞内的一种溶酶体依赖性降解途径,涉及细胞内成分如长寿蛋白和受损伤细胞器以及外源病原微生物的吞噬、降解,是机体内一种重要的保护和防御机制[1].自噬起源于希腊文"eating of self(自食)",最早由比利时科学家Christian de Duve在1963年溶酶体国际会议中提出,他基于在灌注胰高血糖素的大鼠肝脏里,发现能降解线粒体和其他细胞内结构的溶酶体而提出自噬的概念[2].
DOI:10.3870/j.issn.1672-0741.2018.01.025URL [本文引用: 1]

细胞自噬是广泛存在于真核细胞内的一种溶酶体依赖性降解途径,涉及细胞内成分如长寿蛋白和受损伤细胞器以及外源病原微生物的吞噬、降解,是机体内一种重要的保护和防御机制[1].自噬起源于希腊文"eating of self(自食)",最早由比利时科学家Christian de Duve在1963年溶酶体国际会议中提出,他基于在灌注胰高血糖素的大鼠肝脏里,发现能降解线粒体和其他细胞内结构的溶酶体而提出自噬的概念[2].
[D].
[本文引用: 1]
[D].
[本文引用: 1]
URL [本文引用: 1]
URL [本文引用: 1]
