 ,, 李强
,, 李强 ,西南大学/中国农业科学院柑桔研究所,重庆400712
,西南大学/中国农业科学院柑桔研究所,重庆400712Cloning and Expression Analysis of the Citrus Bacterial Canker-Related Gene CsPGIP in Citrus
HU AnHua, QI JingJing, ZHANG QingWen, CHEN ShanChun, ZOU XiuPing, XU LanZhen, PENG AiHong, LEI TianGang, YAO LiXiao, LONG Qin, HE YongRui ,, LI Qiang
,, LI Qiang ,Citrus Research Institute, Southwest University/Chinese Academy of Agricultural Sciences, Chongqing 400712
,Citrus Research Institute, Southwest University/Chinese Academy of Agricultural Sciences, Chongqing 400712通讯作者:
收稿日期:2018-10-13接受日期:2018-11-26网络出版日期:2019-02-16
| 基金资助: |
Received:2018-10-13Accepted:2018-11-26Online:2019-02-16
作者简介 About authors
胡安华,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (5361KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
胡安华, 祁静静, 张庆雯, 陈善春, 邹修平, 许兰珍, 彭爱红, 雷天刚, 姚利晓, 龙琴, 何永睿, 李强. 柑橘溃疡病相关基因CsPGIP的克隆与表达[J]. 中国农业科学, 2019, 52(4): 639-650 doi:10.3864/j.issn.0578-1752.2019.04.006
HU AnHua, QI JingJing, ZHANG QingWen, CHEN ShanChun, ZOU XiuPing, XU LanZhen, PENG AiHong, LEI TianGang, YAO LiXiao, LONG Qin, HE YongRui, LI Qiang.
0 引言
【研究意义】柑橘是我国南方最重要的果树作物,其中柑橘溃疡病(citrus bacterial canker,CBC)是影响柑橘产业发展最为严重的病害之一。柑橘溃疡病是由地毯黄单胞柑橘致病变种(Xanthomonas citri subsp. citri,Xcc)引起的世界性检疫病害[1,2,3]。目前为控制柑橘溃疡病危害通常采取化学防治为主,生物防治为辅的综合防治策略[4]。由于以上防治措施对环境不友好,需要投入大量的人力、物力,因此培育抗病新品种是减少柑橘溃疡病危害的根本途径。近年来日趋成熟的分子育种技术具有效率高、周期短、可对性状进行定向改良等优点,愈来愈受到关注。通过分子育种挖掘溃疡病相关的候选基因对于柑橘产业的发展具有重要意义。【前人研究进展】多聚半乳糖醛酸酶抑制蛋白(polygalacturonase inhibitor protein,PGIP)基因是一个常用的抗病基因,陈波等通过挖掘、分析柑橘中的PGIP(登录号:BAA31841.1)编码蛋白质序列,证明PGIP是一个编码327个氨基酸并包含两个富含亮氨酸重复序列(leucine-rich repeat,LRR)LRR-2、LRR-1的基因[5]。LRR结构域在植物生长发育和抗病反应等方面发挥着重要作用[6],与识别病原体的特异性有一定关系,且决定与配体结合的专一性[7],PGIP通过抑制病原菌多聚半乳糖醛酸酶(polygalacturonase,PGs)的活性防止病原菌侵染植物组织[8,9,10,11,12,13,14,15]。大量的研究证明PGIP可提高植物对真菌病害的抗性,例如棉花[16]、烟草[9,17-18]、小麦[19]、拟南芥[8,20]、谷子[21,22]等。但是越来越多的研究发现PGIP在抗细菌病方面也发挥重要的作用。组成型表达梨PcPGIP后发现PcPGIP对细菌叶缘焦枯菌(Xylella fastidiosa)有明显的抗性[23];HWANG等在烟草和结球甘蓝中转入芜菁的PGIP2后,发现该基因增强了对细菌性病害软腐病菌(Pectobacterium carotovorum)的抗性[14];青枯菌(Ralstonia solanacearum)中PGs的活性可被番茄茎中提取的PGIP强烈抑制[24];纹枯病菌(Rhizoctonia solani)中PGs活性可以被水稻OsPGIPI的原核表达产物抑制[25];FENG等[26]的研究则发现超表达OsPGIP4可增强水稻对条斑病菌(Xanthomonas oryzae)的抗性,而抑制表达OsPGIP4使水稻对条斑病更加敏感。【本研究切入点】前期转录组研究发现,溃疡病高感品种晚锦橙(Citrus sinensis)和高抗品种四季橘(Citrus mitis)在感染溃疡病菌前后CsPGIP表达差异显著,推测CsPGIP可能与柑橘溃疡病的抗性相关。【拟解决的关键问题】以柑橘溃疡病抗性品种四季橘和感性品种晚锦橙为材料,通过生物信息学分析、亚细胞定位、表达分析和转基因功能验证等研究,探索CsPGIP与柑橘溃疡病抗、感性的关系,为柑橘抗溃疡病分子育种提供理论依据。1 材料与方法
试验于2016年12月至2018年8月在西南大学/中国农业科学院柑桔研究所国家柑桔工程技术研究中心完成。1.1 植物材料与病原菌
选取4年生晚锦橙和四季橘叶片(完全展开的3个月叶龄的春稍叶片)、2年生资阳香橙(Citrus junos)砧木为供试材料。材料取自西南大学温网室和国家柑桔品种改良中心育种圃(19° 51′ N,106° 37′ E)。晚锦橙种子取自成熟果实,消毒后无菌条件下播种于MS培养基,3周后取上胚轴切成1 cm茎段作为转化外植体;溃疡病菌是由西南大学柑桔研究所保存的亚洲种A株系。1.2 CsPGIP的克隆与分析
晚锦橙和四季橘RNA提取采用RNA快速提取试剂盒(Aidlab),并反转录为cDNA(TaKaRa);根据Phytozome甜橙基因组[27]中CsPGIP基因序列(ID: orange1.1g020203m)设计特异引物OE-CsPGIP-f/r(表1)并分别以晚锦橙和四季橘cDNA为模板PCR扩增CsPGIP;PCR产物连接pGEM-T easy载体(Promega)并转化E. coli感受态菌株DH5α(TaKaRa),阳性克隆委托擎科生物有限公司测序;利用MEGA6[28]进行氨基酸多序列比对分析并绘制NJ系统发育树。Table 1
表1
表1本研究使用的引物
Table 1
| 引物名称Primer name | 引物序列Primer sequence (5′-3′) | 酶切位点Enzyme site |
|---|---|---|
| OE-CsPGIP-f | CGGGATCCATGAGCAACACGTCACTGTTGTCT | BamHI |
| OE-CsPGIP-r | CGGAATTCTCACTTGCAGCTTTCGAGGGGCGC | EcoRI |
| SCL-CsPGIP-f | GGGGTACCATGAGCAACACGT CACTGTTGT | KpnI |
| SCL-CsPGIP-r | TCCCCCGGGCTTGCAGCTTTCG AGGGGCGCG | SmaI |
| qPCR-CsPGIP-f | AGAAGCTTGGCGCTCTTCAT | N/A |
| qPCR-CsPGIP-r | TCGCCTTCAAGCTTGTTCCT | N/A |
| qPCR-Actin-f | CATCCCTCAGCACCTTCC | N/A |
| qPCR-Actin-r | CCAACCTTAGCACTTCTCC | N/A |
| OE-f (35S) | AGTAAGGATCGAT CCCACAAAGT | N/A |
| OE-r (CsPGIP) | TTTTTGAAGAGTAGTGAAGCTGCA | N/A |
新窗口打开|下载CSV
1.3 CsPGIP的亚细胞定位
利用BaCelLo[29]和SignalP4.0[30]进行CsPGIP的亚细胞定位和信号肽预测;根据CsPGIP序列设计不含终止密码子的特异引物SCL-CsPGIP-f/r(表1)并以晚锦橙cDNA为模板进行PCR扩增,产物与pSAT6- mGFP-N1载体连接,构建CsPGIP::mGFP融合基因,再将融合基因连接到pLGN-2x35s载体,最终得到pLGN::CsPGIP::mGFP载体;将含有pLGN::CsPGIP:: mGFP载体和pLGN::mGFP载体的EHA105农杆菌(OD=0.1)注射洋葱下表皮,28℃暗培养36 h,制片并用荧光显微镜(OLYMPUS:BX51)观察明、暗视野下的表达情况。1.4 柑橘溃疡病菌对CsPGIP的诱导表达分析
将高感品种晚锦橙和高抗品种四季橘叶片用自来水清洗干净并用75%的乙醇擦拭消毒,无菌水冲净后置于无菌培养皿。将OD600=0.5的溃疡病菌菌悬液注射到晚锦橙和四季橘叶片下表皮,对照组注射无菌LB液体培养基,于28℃光照培养。分别于0、12、24、36、48 h取样,切取叶片的接种部位提取总RNA并反转录。根据CsPGIP基因特异性区域和柑橘内参基因Actin设计定量PCR引物qPCR-CsPGIP-f/r and qPCR-Actin-f/r(表1)。利用实时荧光定量PCR(quantitative real-time PCR,qRT-PCR)分析CsPGIP的相对表达量。每个处理进行3次生物学重复和3次技术重复。1.5 CsPGIP超表达载体构建与转化
将克隆的具有BamHI和EcoRI酶切位点的片段和pLGNe-2×35S-MCS-nos超表达载体用BamHI和EcoRI双酶切,酶切后的基因片段和载体片段连接构建pLGNe-CsPGIP-2×35S-MCS-nos超表达载体并转化农杆菌(EHA105)。柑橘转化参照PENG等[31]的方法。含有重组质粒的农杆菌于LB液体培养基28℃培养至OD600=0.5,侵染晚锦橙外植体(1 cm上胚轴茎段)15 min,外植体用灭菌滤纸擦干后均匀摆放到共培养基(含2 mg·L-1 IP、1 mg·L-1 IAA和2,4-D、0.1 mg·L-1 AS的MS培养基)于28℃暗培养,72 h后转移到筛选培养基(含2 mg·L-1 BA、1 mg·L-1 IAA、50 mg·L-1 Kana的MS培养基)于28℃暗培养,7 d后转移到28℃光照培养。1.6 转基因株系鉴定
光照培养约50 d后,待不定芽生长到1 cm左右,切取少量芽进行GUS染色,显色为蓝色的芽初步认定为拟转化芽。初筛得到的拟转化芽嫁接到砧木,待长大后取其叶片提取基因组DNA,以此为模板用特异CsPGIP基因验证引物OE-f(35S)/OE-r(CsPGIP)(表1)进行PCR鉴定。阳性转基因株系提取RNA并反转录为cDNA,利用qRT-PCR分析各株系中CsPGIP的表达量。1.7 转基因株系的抗性评价
转基因株系的抗病性评价参照PENG等[32]的方法进行。选取完全展开的3个月叶龄转基因株系及野生型晚锦橙叶片,进行离体抗性评价。用接种针在每片叶片的背面刺4—6组孔,每组6个,每个针孔接种溃疡病菌1 μL(OD600=0.5),同时对照组接种无菌LB培养基。28℃光照培养10 d,拍照记录病斑。用软件Image J V1.47(National Institutes of Health,Bethesda,MD)统计病斑面积(lesion area,LA,mm2)。按照病斑面积大小将病情分为8个级别,用字母LA表示病斑面积,0级(LA≤0.5 mm2),1级(0.5 mm2<LA≤1.0 mm2),2级(1.0 mm2<LA≤1.5 mm2),3级(1.5 mm2<LA≤2.0 mm2),4级(2.0 mm2<LA≤2.5 mm2),5级(2.5 mm2<LA≤3.0 mm2),6级(3.0 mm2<LA≤3.5 mm2),7级(LA>3.5 mm2)。根据以下公式计算病情指数(disease index,DI):DI=100×Σ(各级病斑数×相应级数值)/(病斑总数×最大级数)。1.8 qRT-PCR与统计分析
相对表达量采用2-ΔΔCt法(ΔCt = CtCsPGIP-CtActin)计算,使用Excel进行数据统计分析并绘图。P<0.05表示差异显著,P<0.01表示差异极显著。2 结果
2.1 CsPGIP生物信息学分析
晚锦橙和四季橘的CsPGIP编码的CsPGIP均含有328个氨基酸,与已报道柑橘PGIP(BAA31841.1)[5]同源性为99.39%,3个基因编码的PGIP均含有PGIP关键结构域LRR_1和LRR_2,属于同源基因(图1)。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1柑橘CsPGIP序列比对
深蓝色为相同氨基酸序列,浅蓝色为不同的氨基酸序列,LRR_1和LRR_2为LRR结构域
Fig. 1Sequence alignment of the CsPGIP in three Citrus spp.
Dark blue and light blue represent the same and different amino acid sequences, respectively. LRR_1 and LRR_2 are LRR structural domains in PGIP
通过对CsPGIP与其他8个物种(拟南芥、高粱、水稻、亚麻属、苜蓿、杨树、谷子和葡萄)共38条PGIP序列进行系统发育分析,结果显示不同物种间PGIP序列具有很强的保守性,相同物种具有较高的相似度;单子叶和双子叶植物单独聚在一起,分成两个大组;柑橘CsPGIP与葡萄PGIP(GSVIVT01033370001)遗传距离最近,相似度达到62.97%(图2)。
图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2不同物种PGIP的系统发育树
以上基因均来自Phytozome(
Fig. 2The phylogenetic tree of PGIPs from different species
Genes in this study are all from Phytozome (
2.2 CsPGIP亚细胞定位
利用BaCelLo进行CsPGIP亚细胞定位预测,定位于细胞膜上的预测分值(2.272)显著高于其他部位(≤1.494),CsPGIP可能定位在细胞膜上。信号肽预测结果显示其N端有含23个氨基酸的信号肽:MSNTSLLSLFFFLCLCISPSLSD,表明CsPGIP为分泌蛋白。为验证亚细胞定位和信号肽的预测,以柑橘CsPGIP与GFP构建融合表达载体,通过洋葱表皮瞬时表达进行亚细胞定位,显微观察显示融合蛋白定位在细胞膜和细胞壁结合部(图3 A1-A3),进一步进行质壁分离后观察显示融合蛋白在细胞膜和细胞壁中都有积累(图3 B1-B3),而对照组定位在整个细胞中(图3 C1-C3、D1-D3)。CsPGIP定位在细胞膜和细胞壁中的观察结果与预测一致。图3
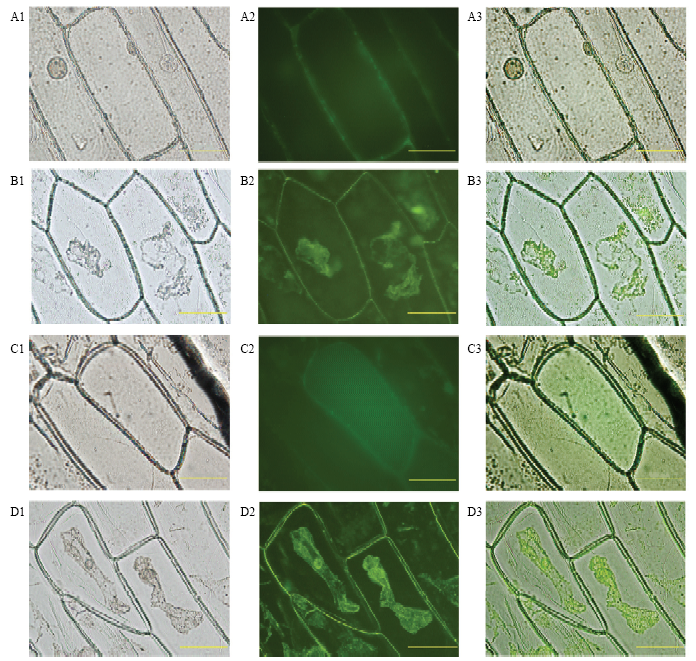 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3CsPGIP的亚细胞定位
A1:明视野观察CsPGIP-GFP融合蛋白Image of CsPGIP-GFP under bright field;A2:暗视野观察CsPGIP-GFP融合蛋白Image of CsPGIP-GFP under dark field;A3:A1、A2视野叠加Overlap of A1 and A2;B1:明视野观察CsPGIP-GFP融合蛋白质壁分离Image of CsPGIP-GFP under bright field (plasmolysis);B2:暗视野观察CsPGIP-GFP融合蛋白质壁分离Image of CsPGIP-GFP under dark field (plasmolysis);B3:B1、B2视野叠加Overlap of B1 and B2;C1:明视野观察GFP表达Image of GFP under bright field;C2:暗视野观察GFP表达Image of GFP under dark field;C3:C1、C2视野叠加Overlap of C1 and C2;D1:明视野观察GFP质壁分离GFP of plasmolysis under bright field;D2:暗视野观察GFP质壁分离GFP of plasmolysis under dark field;D3:D1、D2视野叠加Overlap of D1 and D2;标尺Scale:100 μm
Fig. 3The subcellular localization of CsPGIP
2.3 柑橘溃疡病菌对CsPGIP的诱导表达分析
实时荧光定量PCR结果分析显示柑橘CsPGIP在5个时间点(0、12、24、36和48 h)表达水平存在不同程度的差异(图4),其中高感品种晚锦橙在接种溃疡病菌12 h后CsPGIP的表达出现显著下调并维持在较低的水平。而高抗品种四季橘在接种溃疡病菌后CsPGIP表达出现不同程度上调,在12 h时表达量最高,为0 h的2.91倍,12 h后仍维持在较高水平。结果表明CsPGIP表达与溃疡病菌的侵染具有密切关系,经溃疡病菌诱导而显著上调可能是四季橘抗溃疡病的原因之一。
图4
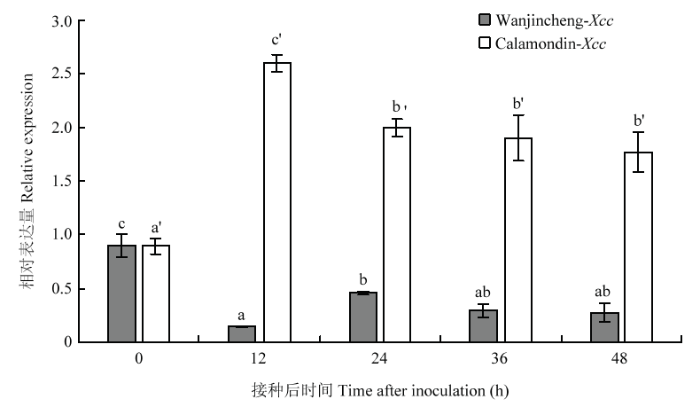 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4柑橘溃疡病菌对CsPGIP的诱导表达
不同小写字母表示差异显著(P<0.05)
Fig. 4The expression of CsPGIP induced by Xcc
Different lowercases indicate significant difference (P<0.05)
2.4 转基因株系的鉴定及CsPGIP表达分析
经GUS染色初筛(结果未显示)结合PCR鉴定,共获得9个转基因株系,分别为OE1、OE3、OE4、OE5、OE6、OE9、OE10、OE12和OE14(图5-A)。以转基因株系和野生型对照同期叶片提取RNA,qRT-PCR进行CsPGIP表达量测定,相对于野生型对照,以上9个株系CsPGIP表达量均出现不同程度的上调表达,其中OE10上调表达最高(图5-B)。图5
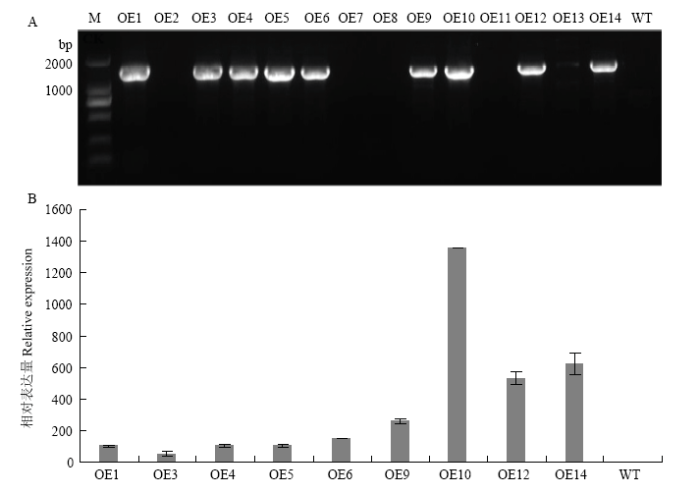 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5转基因株系的鉴定及CsPGIP表达分析
A:CsPGIP转基因株系的PCR鉴定PCR amplification of CsPGIP in over-expression transgenic lines;B:转基因株系中CsPGIP的相对表达量检测The relative expression level of CsPGIP in over-expression transgenic lines。 M:分子量标准Marker;OE1—OE14:GUS初筛的转基因材料Lines verified from GUS staining;WT:野生型Wild-type;阳性株系特异扩增条带为1 530 bp PCR product size of positive lines is 1 530 bp
Fig. 5Identification of transgenic lines and CsPGIP expression profiles
2.5 转基因株系的表型分析
观察分析9株转基因株系表型,3个树龄一年的株系OE1、OE3和OE4中,OE3与野生型对照差异明显,植株较矮小(图6-A、6-D)。7个树龄6个月的株系OE5、OE6、OE9、OE10、OE12和OE14与野生型比较,OE14株系出现了异常,植株矮小(图6-B、6-E)、叶片卷曲、增厚(图6-C)。图6
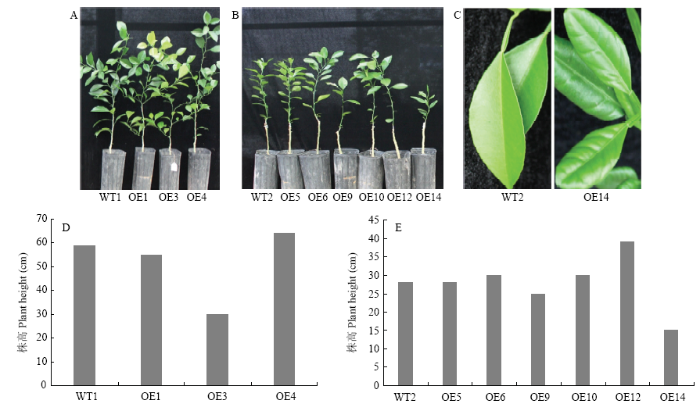 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6转基因株系的表型分析
A:树龄1年的转基因株系(OE1、OE3、OE4)和野生型对照(WT1)植株The phenotype of 1-year-old transgenic lines (OE1, OE3, OE4) and the wild-type control (WT1);B:树龄6个月的转基因株系(OE5、OE6、OE9、OE10、OE12、OE14)和野生型对照(WT2)植株Phenotype of 6-month-old transgenic lines (OE5, OE6, OE9, OE10, OE12, OE14) and the wild-type control (WT2);C:野生型对照WT2和转基因株系OE14的叶片Leaves of WT2 and OE14;D:树龄1年的转基因株系(OE1、OE3、OE4)和野生型对照(WT1)的株高(测量方法:从嫁接口到顶梢的距离)Height of 1-year-old transgenic lines (OE1, OE3, OE4) and the wild-type control (measurement method: distance from the graft to the top tip);E:树龄6个月的转基因株系(OE5、OE6、OE9、OE10、OE12、OE14)和野生型对照(WT2)的株高Height of 6-month-old transgenic lines (OE5, OE6, OE9, OE10, OE12, OE14) and the wild-type control
Fig. 6The phenotype analysis of over-expressed transgenic lines
2.6 转基因株系的溃疡病抗性评价
对8个转基因株系(OE1、OE3、OE4、OE5、OE6、OE9、OE10和OE12)进行了抗病性评价。采用针刺法离体接种溃疡病菌,以接种LB培养基的叶片作为对照。10 d后,接种LB培养基的叶片均未发病(图7-A),而接种溃疡病菌的叶片均不同程度发病,病斑大小存在一定的差异(图7-B);经过统计分析,转基因株系病斑面积显著小于野生型对照(图7-C),仅为野生型对照病斑面积的24.11%—83.88%;转基因株系病情指数仅为野生型对照的23.12%—86.52%(图7-D)。从转基因株系接种溃疡病菌抗性评价结果得出株系OE1、OE4、OE5、OE6、OE9、OE10和OE12可显著减小叶片溃疡病病情指数,其中OE1株系对柑橘溃疡病抗性得到极显著提高。图7
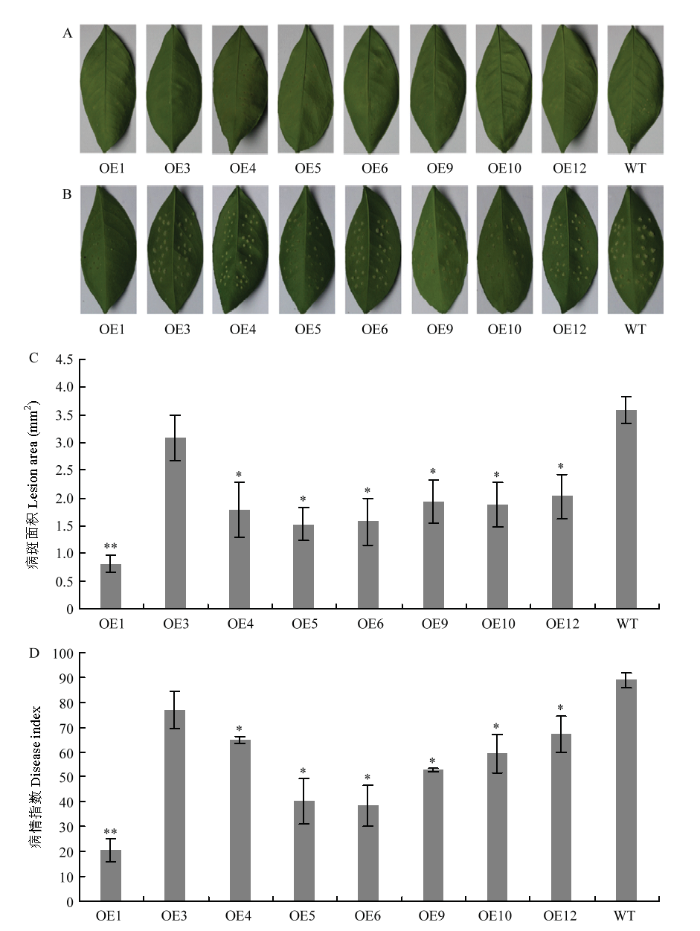 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7转基因株系的抗性评价
A:接种LB培养基的转基因株系和野生型对照叶片Disease spots of transgenic lines and the wild-type inoculated with LB;B:接种溃疡病菌的转基因株系和野生型对照叶片Disease spots of transgenic lines and the wild-type inoculated with Xcc;C:接种溃疡病菌的转基因株系和野生型对照病斑面积Lesion area of transgenic lines and the wild-type inoculated with Xcc;D:接种溃疡病菌的转基因株系和野生型对照病情指数Disease index of transgenic lines and the wild-type inoculated with Xcc。WT:野生型对照Wild-type control;OE1—OE12:转基因株系Transgenic lines。*表示差异显著(P<0.05),**表示差异极显著(P<0.01)* represents significant difference (P<0.05), ** represents extremely significant difference (P<0.01)
Fig. 7The Xcc resistance of over-expressed transgenic lines
3 讨论
植物细胞壁是抵御病菌入侵的第一道防线,病原细菌和真菌必须通过植物细胞壁在植物体内建立生物营养感染的定殖位点后进行扩大感染[33]。PGIP是植物细胞壁产生的LRR类防御蛋白,能特异性的抑制病原菌分泌的PGs,从而抑制病原菌对植株的侵染。LRR基序是参与蛋白质之间互作的结构域[34],PGIP通过LRR基序抑制PGs的活性[35]。有研究表明PGIP在多种物种中对提高病害的抗性有显著作用,棉花GhPGIP1可增强植株对黄萎病和镰孢菌枯萎病的抗性[16];过表达PGIP2增强了结球甘蓝对细菌性软腐病的抗性[14];CaPGIPs在植物的抗病方面起着重要作用[36];葡萄VvPGIP1可以降低转基因烟草对灰霉病菌的敏感性,并对病原菌的PGs有不同程度的抑制作用[17]。本研究结果表明,晚锦橙中的CsPGIP超量表达可增强柑橘对溃疡病菌的抗性。在溃疡病菌的诱导下,CsPGIP在高感品种晚锦橙中下调表达而在高抗品种四季橘中显著上调表达。晚锦橙和四季橘中的CsPGIP蛋白仅存在3个氨基酸的差异,但它们具有相同的LRR类防御蛋白特有的结构域LRR_1和LRR_2(图1),因而这两种蛋白本身对病原菌的抵抗能力可能差异不大。导致CsPGIP在不同溃疡病抗性的柑橘品种中差异表达的原因可能是调控机制的差异。柑橘抵抗溃疡病菌的入侵是一个复杂的调控网络。溃疡病病原菌主要的效应因子pthA4通过III型分泌系统进行柑橘基因组后,与柑橘体内的溃疡病感病基因CsLOB1结合[37]。研究表明,不同溃疡病抗性的柑橘品种中都含有CsLOB1,但CsLOB1在不同抗性的柑橘品种中存在启动子序列的差异[31],这种启动子序列的差异可能会引起基因表达的差异。在不同的抗、感溃疡病柑橘品种中,CsPGIP虽然相同,但其转录后可能存在转录后修饰现象,转录呈现多态性,这种转录后的修饰也会导致在不同抗性的柑橘品种中CsPGIP表达的差异。因而进一步克隆晚锦橙和四季橘中CsPGIP的启动子,分析启动子序列差异;同时对不同溃疡病抗性的柑橘品种中CsPGIP转录的结构多态性进行研究,有望阐明CsPGIP在不同溃疡病抗性的柑橘品种中差异表达的原因。
本研究对9个转基因株系进行表型分析,发现仅有两个转基因株系出现了植株矮小的现象,其中一个株系(OE14)的叶片卷曲增厚。多个物种的PGIP已在不同的植株中进行超表达,但转基因并未引起植株表型的差异[16,17,18,19,20,21,22,23,24,25,26]。本研究中转基因植株表型变化可能是CsPGIP随机整合到柑橘基因组中时引起某些基因或调控序列失活造成的。由于仅有两个株系出现了表型变化且其中一株过于矮小无法进行表型相关研究,后期将会对溃疡病抗性评价、插入位点、基因表达和细胞组织结构进行综合研究,以探究CsPGIP对植物生长发育的影响。
溃疡病抗性评价结果显示不同的转基因株系可不同程度显著减小叶片病情指数,其中OE1株系对柑橘溃疡病抗性极显著提高且表型正常。目前CsPGIP对柑橘溃疡病抗性机理尚未清楚,因而筛选出的抗溃疡病的转基因柑橘可以作为材料进一步研究CsPGIP的作用机理。
4 结论
CsPGIP为定位于细胞壁和细胞膜的蛋白,受溃疡病菌诱导表达。CsPGIP的表达特性表明该基因是柑橘响应溃疡病侵染的重要基因,超表达该基因可以提高柑橘对溃疡病的抗性,该基因在柑橘抗溃疡病机理研究方面具有较大的应用价值,可作为柑橘抗溃疡病分子育种的一个候选基因。(责任编辑 岳梅)
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1038/hortres.2015.42URLPMID:4595992 [本文引用: 1]

Abstract Citrus canker, caused by the bacterial pathogen Xanthomonas citri ssp. citri (Xcc), has been attributed to millions of dollars in loss or damage to commercial citrus crops in subtropical production areas of the world. Since identification of resistant plants is one of the most effective methods of disease management, the ability to screen for resistant seedlings plays a key role in the production of a long-term solution to canker. Here, an inverse correlation between reactive oxygen species (ROS) production by the plant and the ability of Xcc to grow and form lesions on infected plants is reported. Based on this information, a novel screening method that can rapidly identify citrus seedlings that are less susceptible to early infection by Xcc was devised by measuring ROS accumulation triggered by a 22-amino acid sequence of the conserved N-terminal part of flagellin (flg22) from X. citri ssp. citri (Xcc-flg22). In addition to limiting disease symptoms, ROS production was also correlated with the expression of basal defense-related genes such as the pattern recognition receptors LRR8 and FLS2, the leucine-rich repeat receptor-like protein RLP12, and the defense-related gene PR1, indicating an important role for pathogen-associated molecular pattern-triggered immunity (PTI) in determining resistance to citrus canker. Moreover, the differential expression patterns observed amongst the citrus seedlings demonstrated the existence of genetic variations in the PTI response among citrus species/varieties.
DOI:10.3864/j.issn.0578-1752.2017.13.008URL [本文引用: 1]

[目的]分析柑橘BZIP转录因子家族并克隆柑橘溃疡病菌相关的转录因子CsBZIP40,对其进行亚细胞定位并研究外源激素及机械损伤对该基因的诱导表达,确定其诱导表达模式,分析其与溃疡病菌侵染的关系.[方法]基于全基因组公共数据库,对柑橘BZIP转录因子进行综合专业注释以获得柑橘中BZIP家族的所有成员信息,并根据染色体定位对其进行命名;利用MEME分析其结构域;利用MEGA 6.0分析柑橘BZIP与拟南芥BZIP的系统发育关系,并根据系统发育关系对该基因家族进行分类,确定本研究关注的CsBZIP40所属的类型;结合染色体定位和系统发育树确定该家族的基因复制情况;利用实时荧光定量PCR(qRT-PCR)方法验证感染溃疡病菌前后由柑橘转录组筛选出的CsBZIP40表达模式;分析CsBZIP40、启动子元件(plantCARE)和核定位信号(cNLSmapper),构建GFP融合载体,用洋葱表皮进行亚细胞定位分析,来对预测的核定位信息进行验证;利用qRT-PCR技术分析水杨酸(SA)、茉莉酸甲酯(MeJA)、乙烯利(ET)以及机械损伤(wounding)对该基因的诱导表达模式,揭示该转录因子与激素代谢途径的关系.[结果]从柑橘(甜橙)全基因组数据库中共注释到47个BZIP,这些BZIP基因位于9号染色体之外的所有染色体上,其中3号染色体基因密度最大为4.5× 10-2个/Mb,2号染色体上的BZIP基因密度最小,仅占所有BZIP基因的2%;柑橘BZIP基因家族含有较少的基因复制事件,所以相对其他已测序物种其BZIP家族较小;CsBZIP40基因全长5 756 bp,开放阅读框1 530 bp,编码509个氨基酸;经BZIP家族染色体定位、结构域和系统发育分析结果显示CsBZIP40基因序列特异性较好,且CsBZIP40与拟南芥中AT1g08320属于同源基因,属于柑橘1 0个亚家族中参与病菌防御的D亚类;上游启动子元件含多个与植物逆境或激素应答相关的顺式作用元件,例如Box-W1、HSE、ERE等;此基因含有核定位信号,亚细胞定位表明此蛋白定位于细胞核,具备转录因子发挥作用的前提;外源水杨酸并不会使四季橘和纽荷尔脐橙中CsBZIP40的表达水平显著上调,纽荷尔脐橙在茉莉酸甲酯诱导后有明显的差异表达,但乙烯利可以使CsBZIP40在四季橘和纽荷尔脐橙中均有较明显的差异表达;柑橘溃疡病菌侵染可诱导抗病品种四季橘中此基因上调表达,而在感病品种纽荷尔脐橙中,该基因对柑橘溃疡病菌侵染没有响应.[结论]CsBZIP40是响应溃疡病菌侵染的一个转录因子,该基因可作为柑橘抗溃疡病菌分子育种的一个候选基因来进行进一步功能性验证,具有潜在的分子育种价值.
DOI:10.3864/j.issn.0578-1752.2017.13.008URL [本文引用: 1]

[目的]分析柑橘BZIP转录因子家族并克隆柑橘溃疡病菌相关的转录因子CsBZIP40,对其进行亚细胞定位并研究外源激素及机械损伤对该基因的诱导表达,确定其诱导表达模式,分析其与溃疡病菌侵染的关系.[方法]基于全基因组公共数据库,对柑橘BZIP转录因子进行综合专业注释以获得柑橘中BZIP家族的所有成员信息,并根据染色体定位对其进行命名;利用MEME分析其结构域;利用MEGA 6.0分析柑橘BZIP与拟南芥BZIP的系统发育关系,并根据系统发育关系对该基因家族进行分类,确定本研究关注的CsBZIP40所属的类型;结合染色体定位和系统发育树确定该家族的基因复制情况;利用实时荧光定量PCR(qRT-PCR)方法验证感染溃疡病菌前后由柑橘转录组筛选出的CsBZIP40表达模式;分析CsBZIP40、启动子元件(plantCARE)和核定位信号(cNLSmapper),构建GFP融合载体,用洋葱表皮进行亚细胞定位分析,来对预测的核定位信息进行验证;利用qRT-PCR技术分析水杨酸(SA)、茉莉酸甲酯(MeJA)、乙烯利(ET)以及机械损伤(wounding)对该基因的诱导表达模式,揭示该转录因子与激素代谢途径的关系.[结果]从柑橘(甜橙)全基因组数据库中共注释到47个BZIP,这些BZIP基因位于9号染色体之外的所有染色体上,其中3号染色体基因密度最大为4.5× 10-2个/Mb,2号染色体上的BZIP基因密度最小,仅占所有BZIP基因的2%;柑橘BZIP基因家族含有较少的基因复制事件,所以相对其他已测序物种其BZIP家族较小;CsBZIP40基因全长5 756 bp,开放阅读框1 530 bp,编码509个氨基酸;经BZIP家族染色体定位、结构域和系统发育分析结果显示CsBZIP40基因序列特异性较好,且CsBZIP40与拟南芥中AT1g08320属于同源基因,属于柑橘1 0个亚家族中参与病菌防御的D亚类;上游启动子元件含多个与植物逆境或激素应答相关的顺式作用元件,例如Box-W1、HSE、ERE等;此基因含有核定位信号,亚细胞定位表明此蛋白定位于细胞核,具备转录因子发挥作用的前提;外源水杨酸并不会使四季橘和纽荷尔脐橙中CsBZIP40的表达水平显著上调,纽荷尔脐橙在茉莉酸甲酯诱导后有明显的差异表达,但乙烯利可以使CsBZIP40在四季橘和纽荷尔脐橙中均有较明显的差异表达;柑橘溃疡病菌侵染可诱导抗病品种四季橘中此基因上调表达,而在感病品种纽荷尔脐橙中,该基因对柑橘溃疡病菌侵染没有响应.[结论]CsBZIP40是响应溃疡病菌侵染的一个转录因子,该基因可作为柑橘抗溃疡病菌分子育种的一个候选基因来进行进一步功能性验证,具有潜在的分子育种价值.
URL [本文引用: 1]

【目的】溃疡病是严重危害柑橘的一种细菌性病害,通常在高温下容易发生。论文旨在阐明高温下柑橘易发生溃疡病的机制,揭示其代谢变化,为利用药剂防治溃疡病提供重要的理论指导。【方法】以感病的甜橙(Citrus sinensis)为研究对象,在21℃和30℃下预培养3 d,然后均接种同样浓度(108 cfu/m L)的柑橘溃疡病菌(Xanthomonas citri subsp.citri,Xcc)10μL,比较两组植株的发病率,采用半定量RT-PCR分析两种温度下4个抗病基因AOS、CHI、GPX和PR4A的表达量,利用HPLC测定预培养3 d后叶片内源多胺(腐胺、亚精胺和精胺)的含量。在此基础上,利用外源亚精胺(0.4 mmol·L-1)处理甜橙植株(以清水处理为对照),比较亚精胺和清水处理的植株接种溃疡病菌后的发病率和病情指数,分析亚精胺处理对内源多胺含量和抗病基因AOS、CHI、GPX和PR4A表达的影响。【结果】溃疡病菌接种后观察发现,21℃培养植株溃疡病的发病率在前期低于30℃培养的植株,至第10天时,两个处理组植株的发病率接近;同时HPLC测定发现,21℃培养植株叶片3种自由态多胺(腐胺、亚精胺和精胺)含量高于30℃培养植株;RT-PCR分析表明,CHI、GPX和PR4A这3个抗病基因的表达量在21℃培养植株中高于30℃培养植株,而AOS表达水平在两组材料中差异不明显。外源亚精胺处理显著增加了内源腐胺和亚精胺的含量,降低了所处理植株接种溃疡病菌后的发病率和病情指数,接种后14 d发病率比对照降低45%,病情指数比对照降低4.8,而由表型可见对照发病程度重于亚精胺处理材料。此外,亚精胺处理能够增强AOS、CHI、GPX和PR4A 4个抗病基因的表达量。【结论】高温下甜橙更易发生溃疡病的可能机制是高温抑制抗病基因的表达和多胺合成。外源多胺处理能够降低甜橙发生溃疡病,可能机制是多胺处理后增强了抗病基因的表达,诱发植株的抗病反应最终表现出抗病。因此,高温是影响溃疡病发生的一个关键环境因子,多胺有助于提高对柑橘溃疡病的抗性。
URL [本文引用: 1]

【目的】溃疡病是严重危害柑橘的一种细菌性病害,通常在高温下容易发生。论文旨在阐明高温下柑橘易发生溃疡病的机制,揭示其代谢变化,为利用药剂防治溃疡病提供重要的理论指导。【方法】以感病的甜橙(Citrus sinensis)为研究对象,在21℃和30℃下预培养3 d,然后均接种同样浓度(108 cfu/m L)的柑橘溃疡病菌(Xanthomonas citri subsp.citri,Xcc)10μL,比较两组植株的发病率,采用半定量RT-PCR分析两种温度下4个抗病基因AOS、CHI、GPX和PR4A的表达量,利用HPLC测定预培养3 d后叶片内源多胺(腐胺、亚精胺和精胺)的含量。在此基础上,利用外源亚精胺(0.4 mmol·L-1)处理甜橙植株(以清水处理为对照),比较亚精胺和清水处理的植株接种溃疡病菌后的发病率和病情指数,分析亚精胺处理对内源多胺含量和抗病基因AOS、CHI、GPX和PR4A表达的影响。【结果】溃疡病菌接种后观察发现,21℃培养植株溃疡病的发病率在前期低于30℃培养的植株,至第10天时,两个处理组植株的发病率接近;同时HPLC测定发现,21℃培养植株叶片3种自由态多胺(腐胺、亚精胺和精胺)含量高于30℃培养植株;RT-PCR分析表明,CHI、GPX和PR4A这3个抗病基因的表达量在21℃培养植株中高于30℃培养植株,而AOS表达水平在两组材料中差异不明显。外源亚精胺处理显著增加了内源腐胺和亚精胺的含量,降低了所处理植株接种溃疡病菌后的发病率和病情指数,接种后14 d发病率比对照降低45%,病情指数比对照降低4.8,而由表型可见对照发病程度重于亚精胺处理材料。此外,亚精胺处理能够增强AOS、CHI、GPX和PR4A 4个抗病基因的表达量。【结论】高温下甜橙更易发生溃疡病的可能机制是高温抑制抗病基因的表达和多胺合成。外源多胺处理能够降低甜橙发生溃疡病,可能机制是多胺处理后增强了抗病基因的表达,诱发植株的抗病反应最终表现出抗病。因此,高温是影响溃疡病发生的一个关键环境因子,多胺有助于提高对柑橘溃疡病的抗性。
URLMagsci [本文引用: 1]

<FONT face=Verdana>【目的】筛选鉴定柑橘溃疡病菌拮抗菌,研究其培养特性及拮抗物质的初步性质,为柑橘溃疡病的生物制剂研制奠定基础。【方法】利用对峙培养法筛选对柑橘溃疡病菌具有良好抑制作用的生防菌,并通过对菌株形态、生理生化特征以及16S rDNA序列分析鉴定其分类地位;以单因素试验和正交设计方法对影响CQBS03菌株抑菌活性物质产生的各种培养条件进行优化;利用硫酸铵沉淀获得抑菌物质粗提物并测定其对温度、蛋白酶及氯仿的敏感性。【结果】经鉴定CQBS03为枯草芽孢杆菌Bacillus subtilis。CQBS03抑菌活性成分主要存在于培养液中,能被80%饱和度硫酸铵沉淀,最高可耐受的温度范围为60~70℃;活性成分在280 nm 处有最大吸收峰,分子量大于10 kD,对蛋白酶K和胰蛋白酶稳定,而对氯仿部分敏感。培养特性研究表明,CQBS03最适培养基为YPG液体培养基,抑菌物质产生的最适培养条件为:pH 8.0左右,28℃培养72 h。【结论】枯草芽孢杆菌CQBS03所产生的抑菌物质主要成分是蛋白质,且属于外泌型蛋白;该抑菌蛋白对多种植物病原菌有抑菌作用,尤其对柑橘溃疡病菌抑菌活性高,稳定性好,是一株极具开发潜力的生防菌株。</FONT>
URLMagsci [本文引用: 1]

<FONT face=Verdana>【目的】筛选鉴定柑橘溃疡病菌拮抗菌,研究其培养特性及拮抗物质的初步性质,为柑橘溃疡病的生物制剂研制奠定基础。【方法】利用对峙培养法筛选对柑橘溃疡病菌具有良好抑制作用的生防菌,并通过对菌株形态、生理生化特征以及16S rDNA序列分析鉴定其分类地位;以单因素试验和正交设计方法对影响CQBS03菌株抑菌活性物质产生的各种培养条件进行优化;利用硫酸铵沉淀获得抑菌物质粗提物并测定其对温度、蛋白酶及氯仿的敏感性。【结果】经鉴定CQBS03为枯草芽孢杆菌Bacillus subtilis。CQBS03抑菌活性成分主要存在于培养液中,能被80%饱和度硫酸铵沉淀,最高可耐受的温度范围为60~70℃;活性成分在280 nm 处有最大吸收峰,分子量大于10 kD,对蛋白酶K和胰蛋白酶稳定,而对氯仿部分敏感。培养特性研究表明,CQBS03最适培养基为YPG液体培养基,抑菌物质产生的最适培养条件为:pH 8.0左右,28℃培养72 h。【结论】枯草芽孢杆菌CQBS03所产生的抑菌物质主要成分是蛋白质,且属于外泌型蛋白;该抑菌蛋白对多种植物病原菌有抑菌作用,尤其对柑橘溃疡病菌抑菌活性高,稳定性好,是一株极具开发潜力的生防菌株。</FONT>
URL [本文引用: 2]

柑橘是重要的经济园艺植物,但是部分人群在食用柑橘后会造成过敏现象,其中多聚半乳糖醛酸酶抑制蛋白(PGIP)是柑橘中重要的过敏原。本文利用Jameson-Wolf法、Kyte-Doolittle法、Emini法和Karplus-Schulz法对柑橘过敏原蛋白PGIP的抗原指数、亲水性、蛋白表面可及性及柔韧性进行分析,通过构建PET-28 a-PGIP载体转移到大肠杆菌(Escherichia coli)中进行原核表达。结果表明:当PGIP二级结构是β-转角和无规则卷曲,同时氨基酸序列的抗原指数>0、柔韧性>0、亲水性>0和蛋白表面可及性>1时的氨基酸时,过敏原蛋白PGIP的B细胞表位结合氨基酸的序列区域是在19~22、37~41、49~52、68~69、117~120、163~164、173~177、221~226、236~240。通过分析氨基酸的区域及原核表达,为寻找柑橘过敏原蛋白PGIP的B细胞抗原表位的最佳优势区域提供支持,同时也为消除柑橘过敏原蛋白PGIP对人体的影响的进一步研究提供理论基础。
URL [本文引用: 2]

柑橘是重要的经济园艺植物,但是部分人群在食用柑橘后会造成过敏现象,其中多聚半乳糖醛酸酶抑制蛋白(PGIP)是柑橘中重要的过敏原。本文利用Jameson-Wolf法、Kyte-Doolittle法、Emini法和Karplus-Schulz法对柑橘过敏原蛋白PGIP的抗原指数、亲水性、蛋白表面可及性及柔韧性进行分析,通过构建PET-28 a-PGIP载体转移到大肠杆菌(Escherichia coli)中进行原核表达。结果表明:当PGIP二级结构是β-转角和无规则卷曲,同时氨基酸序列的抗原指数>0、柔韧性>0、亲水性>0和蛋白表面可及性>1时的氨基酸时,过敏原蛋白PGIP的B细胞表位结合氨基酸的序列区域是在19~22、37~41、49~52、68~69、117~120、163~164、173~177、221~226、236~240。通过分析氨基酸的区域及原核表达,为寻找柑橘过敏原蛋白PGIP的B细胞抗原表位的最佳优势区域提供支持,同时也为消除柑橘过敏原蛋白PGIP对人体的影响的进一步研究提供理论基础。
[本文引用: 1]
URLPMID:12441626 [本文引用: 1]

Abstract This article reviews recent advances that shed light on plant disease resistance genes, beginning with a brief overview of their structure, followed by their genomic organization and evolution. Plant disease resistance genes have been exhaustively investigated in terms of their structural organization, sequence evolution and genome distribution. There are probably hundreds of NBS-LRR sequences and other types of R-gene-like sequences within a typical plant genome. Recent studies revealed positive selection and selective maintenance of variation in plant resistance and defence-related genes. Plant resistance genes are highly polymorphic and have diverse recognition specificities. R-genes occur as members of clustered gene families that have evolved through duplication and diversification. These genes appear to evolve more rapidly than other regions of the genome, and domains such as the leucine-rich repeat, are subject to adaptive selection
[本文引用: 2]
[本文引用: 2]
DOI:10.1016/S1673-8527(08)60084-3URLPMID:18937920 [本文引用: 1]

Polygalacturonase-inhibiting proteins (PGIPs) are extracellular proteins that belong to the leucine-rich repeat (LRR) protein superfamily. PGIPs inhibit fungal polygalacturonases (PGs) and promote accumulation of oligogalacturonides, which activate plant defense responses. PGIPs play important roles in resistance to infection of pathogens. In this study, reverse transcriptase-polymerase chain reaction (RT-PCR) and RNA ligase-mediated rapid amplification of cDNA ends (RLM-RACE) were used to isolate the full-length PGIP cDNA from Populus deltoides (GenBank accession no. of PdPGIP2 and PdPGIP4: EF684913 and EF684912). Domain analysis revealed that the deduced amino acid sequences of PdPGIP2 and PdPGIP4 had a typical PGIP topology. Phylogenetic analysis of known PGIPs indicated that the two PdPGIPs were clustered to the defense-related PGIP clade. Using real-time RT-PCR, the expression patterns of the two PdPGIPs following treatment with a fungal pathogen and defense-related signaling molecules were studied. The expression levels of PdPGIP2 and PdPGIP4 were both up-regulated when inoculated with the phytopathogenic fungus Marssonina brunnea. Therefore, it was proposed that the two PGIPs might be involved in the resistance to Marssonina brunnea in P. deltoides.
[本文引用: 1]
DOI:10.1094/MPMI-21-2-0171URLPMID:18184061 [本文引用: 1]

Abstract A possible strategy to control plant pathogens is the improvement of natural plant defense mechanisms against the tools that pathogens commonly use to penetrate and colonize the host tissue. One of these mechanisms is represented by the host plant's ability to inhibit the pathogen's capacity to degrade plant cell wall polysaccharides. Polygalacturonase-inhibiting proteins (PGIP) are plant defense cell wall glycoproteins that inhibit the activity of fungal endopolygalacturonases (endo-PGs). To assess the effectiveness of these proteins in protecting wheat from fungal pathogens, we produced a number of transgenic wheat lines expressing a bean PGIP (PvPGIP2) having a wide spectrum of specificities against fungal PGs. Three independent transgenic lines were characterized in detail, including determination of the levels of PvPGIP2 accumulation and its subcellular localization and inhibitory activity. Results show that the transgene-encoded protein is correctly secreted into the apoplast, maintains its characteristic recognition specificities, and endows the transgenic wheat with new PG recognition capabilities. As a consequence, transgenic wheat tissue showed increased resistance to digestion by the PG of Fusarium moniliforme. These new properties also were confirmed at the plant level during interactions with the fungal pathogen Bipolaris sorokiniana. All three lines showed significant reductions in symptom progression (46 to 50%) through the leaves following infection with this pathogen. Our results illustrate the feasibility of improving wheat's defenses against pathogens by expression of proteins with new capabilities to counteract those produced by the pathogens.
DOI:10.1007/s00425-009-1039-7URLPMID:19885675 [本文引用: 1]

Polygalacturonase-inhibiting proteins (PGIPs) are plant defense proteins. To date, no spatial distribution of PGIPs and interaction between PGIPs and nitric oxide (NO) in plant were described. Here, we first reported the full-length cDNA sequence of PGIP of Chorispora bungeana (CbPGIP1). Notably, immunofluorescence localization showed that the CbPGIP was evenly distributed in leaves but it was mainly localized in epidermis and vascular bundle in stems and roots. Further studies indicated that CbPGIP had higher abundance in roots than in stems and leaves. Conversely, the bulk PGIP of C. bungeana showed a higher activity in leaves than in stems and roots. In addition, quantitative real-time polymerase chain reaction demonstrated that CbPGIP1 expression was induced by Stemphylium solani, salicylic acid (SA), 4, -4掳C and NO. This is a first report attempting to predict if NO can induce the PGIP expression. Taken together, these findings showed that the gene was spatially regulated and NO and SA might take part in CbPGIP1 expression induced by biotic and abiotic stresses. This study highlighted the potential importance of CbPGIP1 and NO in plant resistance.
[本文引用: 3]
DOI:10.1104/pp.104.044644URL [本文引用: 1]
DOI:10.1038/srep39840URLPMID:5228132 [本文引用: 3]

Polygalacturonase-inhibiting protein (PGIP), belonging to a group of plant defence proteins, specifically inhibits endopolygalacturonases secreted by pathogens. Herein, we showed that purified GhPGIP1 is a functional inhibitor ofVerticillium dahliaeandFusarium oxysporumf. sp.vasinfectum, the two fungal pathogens causing cotton wilt. Transcription ofGhPGIP1was increased in cotton upon infection, wounding, and treatment with defence hormone and H2O2. Resistance by GhPGIP1 was examined by its virus-induced gene silencing in cotton and overexpression inArabidopsis. GhPGIP1-silenced cotton was highly susceptible to the infections. GhPGIP1 overexpression in transgenicArabidopsisconferred resistance to the infection, accompanied by enhanced expression of pathogenesis-related proteins (PRs), isochorismate synthase 1 (ICS1), enhanced disease susceptibility 1 (EDS1), and phytoalexin-deficient 4 (PAD4) genes. Transmission electron microscopy revealed cell wall alteration and cell disintegration in plants inoculated with polygalacturonase (PGs), implying its role in damaging the cell wall. Docking studies showed that GhPGIP1 interacted strongly with C-terminal ofV. dahliaePG1 (VdPG1) beyond the active site but weakly interacted with C-terminal ofF. oxysporumf. sp.vasinfectum(FovPG1). These findings will contribute towards the understanding of the roles of PGIPs and in screening potential combat proteins with novel recognition specificities against evolving pathogenic factors for countering pathogen invasion.
[本文引用: 3]
DOI:10.3389/fpls.2012.00268URLPMID:23264779 [本文引用: 2]

We have tested whether a gene encoding a polygalacturonase-inhibiting protein (PGIP) protects tobacco against a fungal pathogen (Rhizoctonia solani) and two oomycetes (Phytophthora parasiticavar.nicotianaeandPeronospora hyoscyamif. sp.tabacina). The trials were performed in greenhouse conditions forR. solaniandP. parasiticaand in the field forP. hyoscyami. Our results show that expression of PGIP is a powerful way of engineering a broad-spectrum disease resistance.
DOI:10.1007/s10142-014-0428-6URLPMID:25487419 [本文引用: 2]

Take-all (caused by the fungal pathogen Gaeumannomyces graminis var. tritici, Ggt) and common root rot (caused by Bipolaris sorokiniana) are devastating root diseases of wheat ( Triticum aestivum...
[本文引用: 2]
DOI:10.1007/s11033-015-3850-5URLPMID:255967221 [本文引用: 2]

Polygalacturonase-inhibitor proteins (PGIPs) are important plant defense proteins which modulate the activity of microbial polygalacturonases聽(PGs) leading to elicitor accumulation. Very few studies have been carried out towards understanding the role of PGIPs in monocot host defense. Hence, present study was taken up to characterize a native PGIP from pearl millet and understand its role in resistance against downy mildew. A native glycosylated PGIP ( Pgl PGIP1) of ~43聽kDa and pI 5.9 was immunopurified from pearl millet. Comparative inhibition studies involving Pgl PGIP1 and its non-glycosylated form (r Pgl PGIP1; recombinant pearl millet PGIP produced in Escherichia coli ) against two PGs, PG-II isoform from Aspergillus niger ( An PGII) and PG-III isoform from Fusarium moniliforme , showed both PGIPs to inhibit only An PGII. The protein glycosylation was found to impact only the pH and temperature stability of PGIP, with the native form showing relatively higher stability to pH and temperature changes. Temporal accumulation of both Pgl PGIP1 protein (western blot and ELISA) and transcripts (real time PCR) in resistant and susceptible pearl millet cultivars showed significant Sclerospora graminicola -induced accumulation only in the incompatible interaction. Further, confocal PGIP immunolocalization results showed a very intense immuno-decoration with highest fluorescent intensities observed at the outer epidermal layer and vascular bundles in resistant cultivar only. This is the first native PGIP isolated from millets and the results indicate a role for Pgl PGIP1 in host defense. This could further be exploited in devising pearl millet cultivars with better pathogen resistance.
DOI:10.1093/abbs/gms015URLPMID:22411686 [本文引用: 2]
[本文引用: 2]
DOI:10.1016/j.plaphy.2011.02.001URLPMID:21367611 [本文引用: 2]

Polygalacturonases (PGs) of wild-type and non-virulent phenotype conversion mutant (PC) strains of Highlights? A plant PGIP activity against a bacterial pathogen is reported for the first time. ? PC mutant strain reveals 4-35 times higher PG activity. ? Mainly endo-PG activity of R. solanacearum PC mutant is triggered by media supplements. ? The interaction between PGs and PGIP leads to generation or avoidance of elicitor-active oligomers. ? Presence of absence of these oligomers may contribute to the compatible or incompatible interaction.
DOI:10.1007/s11103-014-0269-7URLPMID:25488398 [本文引用: 2]

As one of the most devastating diseases of rice, sheath blight causes severe rice yield loss. However, little progress has been made in rice breeding for sheath blight resistance. It has been reported that polygalacturonase inhibiting proteins can inhibit the degradation of the plant cell wall by polygalacturonases from pathogens. Here, we prokaryotically expressed and purified OsPGIP1 protein, which was verified by Western blot analysis. Activity assay confirmed the inhibitory activity of OsPGIP1 against the PGase from Rhizoctonia solani. In addition, the location of OsPGIP1 was determined by subcellular localization. Subsequently, we overexpressed OsPGIP1 in Zhonghua 11 ( Oryza sativa L. ssp. japonica), and applied PCR and Southern blot analysis to identify the positive T transgenic plants with single-copy insertions. Germination assay of the seeds from T transgenic plants was carried out to select homozygous OsPGIP1 transgenic lines, and the expression levels of OsPGIP1 in these lines were analyzed by quantitative real-time PCR. Field testing of R. solani inoculation showed that the sheath blight resistance of the transgenic rice was significantly improved. Furthermore, the levels of sheath blight resistance were in accordance with the expression levels of OsPGIP1 in the transgenic lines. Our results reveal the functions of OsPGIP1 and its resistance mechanism to rice sheath blight, which will facilitate rice breeding for sheath blight resistance.
DOI:10.1007/s00425-016-2480-zURLPMID:26945855 [本文引用: 2]

ABSTRACT Main conclusion: OsPGIP4 overexpression enhances resistance to bacterial leaf streak in rice. Polygalacturonase-inhibiting proteins are thought to play important roles in the innate immunity of rice against fungi. Here, we show that the chromosomal location of OsPGIP4 coincides with the major bacterial leaf streak resistance quantitative trait locus qBlsr5a on the short arm of chromosome 5. OsPGIP4 expression was up-regulated upon inoculation with the pathogen Xanthomonas oryzae pv. oryzicola strain RS105. OsPGIP4 overexpression enhanced the resistance of the susceptible rice variety Zhonghua 11 to RS105. In contrast, repressing OsPGIP4 expression resulted in an increase in disease lesions caused by RS105 in Zhonghua 11 and in Acc8558, a qBlsr5a resistance donor. More interestingly, upon inoculation, the activated expression of pathogenesis-related genes was attenuated for those genes involved in the salicylic acid pathway, while the activated expression of jasmonic acid pathway markers was increased in the overexpression lines. Our results not only provide the first report that rice PGIP could enhance resistant against a bacterial pathogen but also indicate that OsPGIP4 is a potential component of the qBlsr5a locus for bacterial leaf streak in rice.
DOI:10.1093/nar/gkr944URLPMID:22110026 [本文引用: 1]

http://nar.oxfordjournals.org/lookup/doi/10.1093/nar/gkr944
DOI:10.1093/molbev/mst197URLPMID:24132122 [本文引用: 1]

We announce the release of an advanced version of the Molecular Evolutionary Genetics Analysis (MEGA) software, which currently contains facilities for building sequence alignments, inferring phylogenetic histories, and conducting molecular evolutionary analysis. In version 6.0, MEGA now enables the inference of timetrees, as it implements the RelTime method for estimating divergence times for all branching points in a phylogeny. A new Timetree Wizard in MEGA6 facilitates this timetree inference by providing a graphical user interface (GUI) to specify the phylogeny and calibration constraints step-by-step. This version also contains enhanced algorithms to search for the optimal trees under evolutionary criteria and implements a more advanced memory management that can double the size of sequence data sets to which MEGA can be applied. Both GUI and command-line versions of MEGA6 can be downloaded from www.megasoftware.net free of charge.
DOI:10.1093/bioinformatics/btl222URL [本文引用: 1]
DOI:10.1038/nmeth.1701URLPMID:21959131 [本文引用: 1]

Nat Methods. 2011 Sep 29;8(10):785-6. doi: 10.1038/nmeth.1701. Letter
[本文引用: 2]
DOI:10.1111/pbi.12733URLPMID:5698050 [本文引用: 1]

Abstract Citrus canker, caused by Xanthomonas citri subsp. citri (Xcc), is severely damaging to the global citrus industry. Targeted editing of host disease-susceptibility genes represents an interesting and potentially durable alternative in plant breeding for resistance. Here, we report improvement of citrus canker resistance through CRISPR/Cas9-targeted modification of the susceptibility gene CsLOB1 promoter in citrus. Wanjincheng orange (Citrus sinensis Osbeck) harbours at least three copies of the CsLOB1 G allele and one copy of the CsLOB1 - allele. The promoter of both alleles contains the effector binding element (EBE P thA4 ), which is recognized by the main effector PthA4 of Xcc to activate CsLOB1 expression to promote citrus canker development. Five pCas9/CsLOB1sgRNA constructs were designed to modify the EBE P thA4 of the CsLOB1 promoter in Wanjincheng orange. Among these constructs, mutation rates were 11.5%-64.7%. Homozygous mutants were generated directly from citrus explants. Sixteen lines that harboured EBE P thA4 modifications were identified from 38 mutant plants. Four mutation lines (S2-5, S2-6, S2-12 and S5-13), in which promoter editing disrupted CsLOB1 induction in response to Xcc infection, showed enhanced resistance to citrus canker compared with the wild type. No canker symptoms were observed in the S2-6 and S5-13 lines. Promoter editing of CsLOB1 G alone was sufficient to enhance citrus canker resistance in Wanjincheng orange. Deletion of the entire EBE P thA4 sequence from both CsLOB1 alleles conferred a high degree of resistance to citrus canker. The results demonstrate that CRISPR/Cas9-mediated promoter editing of CsLOB1 is an efficient strategy for generation of canker-resistant citrus cultivars. 2017 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
DOI:10.1094/MPMI.2000.13.9.942URLPMID:10975651 [本文引用: 1]

Transgenic tomato plants expressing the pear fruit polygalacturonase inhibitor protein (pPGIP) were used to demonstrate that this inhibitor of fungal pathogen endo-polygalacturonases (endo-PGs) influences disease development. Transgenic expression of pPGIP resulted in abundant accumulation of the heterologous protein in all tissues and did not alter the expression of an endogenous tomato fruit PGIP (tPGIP). The pPGIP protein was detected, as expected, in the cell wall protein fraction in all transgenic tissues. Despite differential glycosylation in vegetative and Fruit tissues, the expressed pPGIP was active in both tissues as an inhibitor of endo-PGs from Botrytis cinerea. The growth of B. cinerea on ripe tomato fruit expressing pPGIP was reduced, and tissue breakdown was diminished by as much as 15%, compared with nontransgenic fruit. In transgenic leaves, the expression of pPGIP reduced lesions of macerated tissue approximately 25%, a reduction of symptoms of fungal growth similar to that observed with a B. cinerea strain in which a single endo-PG gene, Bcpg1, had been deleted (A. ten Have, W, Mulder, J. Visser, and J A L van Kan, Mel. Plant-Microbe Interact. 11:1009-1016, 1998), Heterologous expression of pPGIP has demonstrated that PGTP inhibition of fungal PGs slows the expansion of disease lesions and the associated tissue maceration.
DOI:10.1146/annurev.phyto.39.1.313URL [本文引用: 1]
DOI:10.1016/S0959-440X(01)00266-4URLPMID:11751054 [本文引用: 1]

Leucine-rich repeats (LRRs) are 20–29-residue sequence motifs present in a number of proteins with diverse functions. The primary function of these motifs appears to be to provide a versatile structural framework for the formation of protein–protein interactions. The past two years have seen an explosion of new structural information on proteins with LRRs. The new structures represent different LRR subfamilies and proteins with diverse functions, including GTPase-activating protein rna1p from the ribonuclease-inhibitor-like subfamily; spliceosomal protein U2A′, Rab geranylgeranyltransferase, internalin B, dynein light chain 1 and nuclear export protein TAP from the SDS22-like subfamily; Skp2 from the cysteine-containing subfamily; and YopM from the bacterial subfamily. The new structural information has increased our understanding of the structural determinants of LRR proteins and our ability to model such proteins with unknown structures, and has shed new light on how these proteins participate in protein–protein interactions.
DOI:10.1007/s11103-013-0007-6URLPMID:23334855 [本文引用: 1]

Polygalacturonase-inhibiting proteins (PGIPs) are plant cell wall glycoproteins that can inhibit fungal endopolygalacturonases (PGs). The PGIPs directly reduce the aggressive potential of PGs. Here, we isolated and functionally characterized three members of the pepper (Capsicum annuum) PGIP gene family. Each was up-regulated at a different time following stimulation of the pepper leaves by Phytophthora capcisi and abiotic stresses including salicylic acid, methyl jasmonate, abscisic acid, wounding and cold treatment. Purified recombinant proteins individually inhibited activity of PGs produced by Alternaria alternata and Colletotrichum nicotianae, respectively, and virus-induced gene silencing in pepper conferred enhanced susceptibility to P. capsici. Because three PGIP genes acted similarily in conferring resistance to infection by P. capsici, and because individually purified proteins showed consistent inhibition against PG activity of both pathogens, CaPGIP1 was selected for manipulating transgenic tobacco. The crude proteins from transgenic tobacco exhibited distinct enhanced resistance to PG activity of both fungi. Moreover, the transgenic tobacco showed effective resistance to infection and a significant reduction in the number of infection sites, number of lesions and average size of lesions in the leaves. All results suggest that CaPGIPs may be involved in plant defense response and play an important role in a plant's resistance to disease.
[本文引用: 1]
