 ,山东农业大学动物科技学院,山东泰安 271018
,山东农业大学动物科技学院,山东泰安 271018Recombinant Expression and Antimicrobial Activity of Apidaecin in Apis cerana cerana
CHEN WenFeng, WANG HongFang, LIU ZhenGuo, ZHANG WeiXing, CHI XuePeng, XU BaoHua ,College of Animal Science and Technology, Shandong Agricultural University, Taian 271018, Shandong
,College of Animal Science and Technology, Shandong Agricultural University, Taian 271018, Shandong通讯作者:
收稿日期:2018-09-4接受日期:2018-10-9网络出版日期:2019-02-16
| 基金资助: |
Received:2018-09-4Accepted:2018-10-9Online:2019-02-16
作者简介 About authors
陈文凤,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (1010KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
陈文凤, 王红芳, 刘振国, 张卫星, 郗学鹏, 胥保华. 中华蜜蜂Apidaecin的重组表达及其抗菌活性[J]. 中国农业科学, 2019, 52(4): 767-776 doi:10.3864/j.issn.0578-1752.2019.04.016
CHEN WenFeng, WANG HongFang, LIU ZhenGuo, ZHANG WeiXing, CHI XuePeng, XU BaoHua.
0 引言
【研究意义】抗生素对于人类的健康和社会的发展具有重要作用,但目前抗生素滥用现象已引起国内外的密切关注,并且抗菌药物的耐药性已被视为全球最危害公众健康的问题之一。为了应对耐药性菌株感染,研究者一方面在对传统抗生素进行结构改造降低病原菌耐药性,提高药物疗效,另一方面也在不断研究和开发新型抗菌药物。自第一个昆虫源的抗菌肽报道以来,抗菌肽作为一种潜在的替代药物立即引起了人们的高度关注。蜜蜂是各种作物的有效传粉者,对农业和生态环境中都具有至关重要的作用,同时蜜蜂也经常暴露于各种病原体的胁迫环境中。在长期与外来病原物作斗争的过程中,蜜蜂形成了与之相对应的分子进化策略,发展出反应灵敏且高表达的Apidaecin抗菌肽基因家族以及其他抗菌肽家族,由于其具有天然的抗菌活性,有望成为抗生素的替代品。从天然组织中分离抗菌肽工作量大,难度高,且获得的量很少;化学合成虽然能提供大量的抗菌肽,但价格昂贵,无法满足研究和潜在应用的需求。随着现代基因工程技术的不断发展,使大规模生产抗菌肽并降低生产成本具备了可行性,因此探索适合的基因表达系统表达出大量具有活性的抗菌肽具有重要意义。【前人研究进展】在过去几十年的研究中,人们对大肠杆菌(Escherichia coli)系统的遗传学、生物化学和分子生物学方面有了充分的认识,使之成为许多外源蛋白的首选表达系统。大肠杆菌遗传图谱明确,易培养、周期短、费用低[1],对许多蛋白质有较强的耐受能力,能较高水平地表达多种蛋白[2],所以成为应用于DNA重组技术的主要宿主细菌。然而抗菌肽的抗菌特性使得它们对宿主菌具有潜在的致命毒性,这成为抗菌肽在大肠杆菌中重组表达面临的巨大挑战。酵母具有比大肠杆菌更完备的基因表达调控机制和对表达产物的加工修饰和分泌能力,并且不会产生内毒素,是基因工程中良好的真核基因受体菌[3],但发酵周期相对较长。枯草芽孢杆菌(Bacillus subtilis)属于芽孢杆菌属,是一类好氧型、内生抗逆性孢子的革兰氏阳性菌。对于饲用抗菌制剂的开发,枯草芽孢杆菌表达系统拥有独特的优点。与大肠杆菌表达系统相比较,枯草芽孢杆菌细胞壁不含内毒素,是被公认的安全微生物,可直接添加于饲料中;与酵母表达系统相比较,枯草芽孢杆菌发酵周期短,操作简单,没有明显的密码子偏好性;枯草芽孢杆菌对培养基的要求远低于酵母,生产成本低[4,5,6]。此外,枯草芽孢杆菌是猪等动物肠道中的一种益生菌,对维持动物肠道健康具有重要作用。同时蛋白酶缺陷型菌株的构建提高了目的蛋白的表达水平,新型载体的设计提高了质粒的稳定性等[7]。因此,枯草芽孢杆菌表达系统适于大多数饲用抗菌肽的重组表达。蜜蜂抗菌肽是蜜蜂在受到病原物感染后,其脂肪体迅速合成的一些具有抗菌活性的肽类,然后释放到血淋巴中发挥抑制细菌、真菌、病毒的作用[8]。蜜蜂抗菌肽属于先天免疫,在体液内有4个基因家族:Apidaecin[9]、Abaecin[10]、Hymenoptaecin[11]、Defensin[12]。其中,Apidaecin是蜜蜂体液中最早被研究的一种抗菌肽,主要对革兰氏阴性菌起作用。另外,独特的前体结构使Apidaecin受到极少量的病原物刺激后即迅速表达[9]。并且自然界中的野生中华蜜蜂(Apis cerana cerana)种群由于一直受到相对较强的环境选择压力,从而保持了中华蜜蜂原有的抗菌肽基因的多样性,长期的家养驯化使得意大利蜜蜂(Apis mellifera ligustica)抗菌肽基因退化。【本研究切入点】抗菌肽作为昆虫体液免疫系统的重要成分,为昆虫抵御病原物的侵袭建立了坚固的屏障。但目前有关蜜蜂抗菌肽的研究报道几乎都集中于意大利蜜蜂,中华蜜蜂的相关报道很少,其中对于中华蜜蜂重组抗菌肽抗菌活性的研究更少。【拟解决的关键问题】通过基因工程技术,利用枯草芽孢杆菌表达系统表达中华蜜蜂抗菌肽Apidaecin,并对其进行体内和体外抗菌活性的验证,为开发新型、安全具有抗菌和免疫调节功能的抗菌肽制剂提供理论依据。1 材料与方法
试验于2016—2017年在山东农业大学动物科技学院完成。1.1 材料
供试昆虫:中华蜜蜂取自山东农业大学试验蜂群。供试小鼠为屏障环境SPF级KM小鼠。主要试剂:总RNA提取试剂盒(Trizol)、大肠杆菌感受态DH5α购自北京全式金公司;原核表达载体Pet-30a(+)由笔者实验室提供;DNA分子质量标准(DNA marker DL2000)、M-MLV反转录酶及SYBR Green Ⅱ定量试剂均购自日本TaKaRa公司;异丙基硫代半乳糖苷(IPTG)、dNTP、LA-Taq DNA聚合酶及凝胶回收试剂盒均购自美国PUEX公司;枯草芽孢杆菌WB800N以及枯草芽孢杆菌表达载体his-pHT43购自于淼灵质粒平台(http://www.miaolingbio.com/);血清免疫球蛋白试剂盒以及肠道分泌型免疫球蛋白IgA试剂盒购自于科诺迪生物;His标签蛋白纯化试剂盒(可溶性蛋白)购自于康为世纪生物科技有限公司;Easy II Protein Quantitative Kit (BCA)试剂盒购自北京全式金公司。
1.2 方法
1.2.1 总RNA提取及cDNA的合成 Trizol法提取蜜蜂以及小鼠肠道样品的总RNA,紫外分光光度计测定总RNA浓度和纯度。用反转录试剂盒对总RNA进行反转录,反转录反应条件:体系混匀后,42℃反应40 min,75℃灭活5 min。反转录产物保存于-20℃。1.2.2 中华蜜蜂Apidaecin引物设计、PCR扩增、克隆、测序以及诱导表达 引物设计:由于中华蜜蜂和意大利蜜蜂为两个亲缘关系最近的物种,同一基因的序列在这两个物种间变异很小,因此,根据GenBank中意大利蜜蜂Apidaecin基因序列(GenBank登录号LOC406115),利用Primer 5.0软件,设计基因序列引物,引物序列见表1,引物由上海生工生物工程技术有限公司(简称上海生工)合成。
Table 1
表1
表1基因引物序列
Table 1
| 基因名称 Gene name | 引物序列 Primer sequence (5′-3′) | 引物用途 Description | 登录号 GenBank accession number |
|---|---|---|---|
| API-F | TGTGGGTTGAATAACTATTGATAA | cDNA sequence primer, forward | NM_001011613.1 |
| API-R | GTTATTTCACGTGCTTCATATTC | cDNA sequence primer, reverse | |
| GAPDH-F | GGTTGTCTCCTGCGACTTCA | Standard control primer, forward | NM_001289726.1 |
| GAPDH-R | TGGTCCAGGGTTTCTTACTCC | Standard control primer, reverse | |
| IL10-F | ACTGCTATGCTGCCTGCTCT | Real-time PCR primer, forward | NM_010548.2 |
| IL10-R | GACTGGGAAGTGGGTGCAGT | Real-time PCR primer, reverse | |
| TNF-α-F | CCAGCCGATGGGTTGTACCT | Real-time PCR primer, forward | NM_001278601.1 |
| TNF-α-R | CAAATCGGCTGACGGTGTGG | Real-time PCR primer, reverse | |
| IL6-F | TGGGACTGATGCTGGTGACA | Real-time PCR primer, forward | NM_031168.2 |
| IL6-R | ACAGGTCTGTTGGGAGTGGT | Real-time PCR primer, reverse | |
| ZO-2-F | CCACCTCGCACGCATCACAG | Real-time PCR primer, forward | XM_017318137.1 |
| ZO-2-R | TGGTCCTTCACCTCTGAGCACTAC | Real-time PCR primer, reverse | |
| Claudin-1-F | TCGGCTCCATCGTCAGCACTG | Real-time PCR primer, forward | NM_016674.4 |
| Claudin-1-R | AGATGGCCTGAGCGGTCACG | Real-time PCR primer, reverse | |
| IFN-γ-F | GGCTCTGGAGGCTGGAGGAAG | Real-time PCR primer, forward | NM_008337.4 |
| IFN-γ-R | TGATAGGCGGTGAGGCTACAAGG | Real-time PCR primer, reverse |
新窗口打开|下载CSV
在PCR扩增中,反应体系(25 μL):5 μL cDNA、2.5 μL 10×PCR缓冲液、1 μL dNTP、1 μL模板,上下游引物各1 μL、0.25 μL LA-Taq DNA聚合酶,18.25 μL双蒸水。扩增程序:94℃预变性4 min;94℃变性40 s,50℃退火30 s,72℃延伸30 s,35个循环;72℃延伸10 min;4℃保存待测。将PCR产物经1%琼脂糖凝胶电泳并割胶纯化,克隆于T1载体内,最后将鉴定的阳性克隆菌送至上海生工进行测序。
根据测序结果和比对结果,选择比对后的特异性序列,然后将含特异性序列的菌液送至上海生工并由其克隆至载体pHT43。
1.2.3 菌株转化、蛋白诱导表达纯化以及蛋白浓度测定 枯草芽孢杆菌感受态细胞的制备和转化以及诱导表达参见MoBiTec所提供的制备方法(
1.2.4 实时荧光定量PCR(qRT-PCR) 取1 μL cDNA加入到20 μL荧光定量体系中,按照荧光定量试剂盒(TaKaRa)操作指南,用7500 real-time PCR仪(ABI 7500,USA)检测目的基因相对表达量。反应程序:预变性95℃,10 s;变性95℃,5 s;退火60℃ 40 s,40个循环,熔解曲线添加,1个循环。目的基因引物设计参考序列来自于NCBI数据库,以β-actin为内参,采用Primer 5.0进行引物设计,委托上海生工合成引物,引物序列如表1所示。
1.2.5 小鼠分组与处理 18—20 g体重的雄性小鼠60只,随机分成6组,每组10只,购自济南朋悦实验动物繁育有限公司;小鼠日粮为小鼠SPF级大小鼠维持饲料,购自华阜康生物有限公司;饲养环境:屏障系统,温度控制在22—25℃,相对湿度40%—60%,黑暗12 h,光照12 h。6组不同处理参考小鼠试验分组(表2),接菌后,取肠道相关样品进行试验。
Table 2
表2
表2重组抗菌肽Apidaecin对小鼠感染大肠杆菌K88保护作用试验分组
Table 2
| 分组 Group | 步骤1 Step 1 (第1—6天腹腔注射 Intraperitoneal injection at 1st-6th days) | 步骤2 Step 2 (第6天腹腔注射后1 h再注射 Repeated intraperitoneal injection was performed 1 h after the first injection at 6th day) |
|---|---|---|
| A:正常对照组 Normal control group | 0.5 mL生理盐水0.5 mL Saline | 0.5 mL生理盐水0.5 mL Saline |
| B:pHT43空载体 pHT43 Empty carrier | 0.5 mL pHT43 | 0.5 mL生理盐水 0.5 mL Saline |
| C:染菌对照组Infection control group | 0.5 mL生理盐水0.5 mL Saline | 0.5 mL菌悬液0.5 mL Bacteria suspension |
| D:pHT43空载体染菌组 pHT43 Empty carrier infection group | 0.5 mL pHT43 | 0.5 mL菌悬液0.5 mL Bacteria suspension |
| E:20 mg·L-1重组Apidaecin染菌组 20 mg·L-1 Recombinant Apidaecin infection group | 0.5 mL重组Apidaecin 0.5 mL Recombinant Apidaecin | 0.5mL菌悬液0.5 mL Bacteria suspension |
| F:150 mg·L-1头孢噻肟染菌组 150 mg·L-1 Cefotaxime infection group | 0.5 mL头孢噻肟溶液 0.5 mL Cefotaxime solution | 0.5 mL菌悬液0.5 mL Bacteria suspension |
新窗口打开|下载CSV
1.2.6 抑菌圈检测重组抗菌肽的抗菌活性 环划线接种大肠杆菌于琼脂平板,37℃生化培养箱过夜培养至长出单菌落。挑取单菌落接种于5 mL新鲜肉汤培养基中,于37℃摇床中恒温振荡培养过夜;将上述过夜培养菌悬液50 μL转接至5 mL新鲜肉汤培养基中,于37℃恒温振荡培养2—3 h至吸光值OD600=0.5左右。取100 μL菌悬液均匀涂布于固体培养基上,将纸片轻轻放置于涂布好菌液的培养基上,滴加3—4 μL相应的液体,设置阴性(灭菌水)和阳性(头孢霉素)对照,按照同样的方法和步骤,设置4个重复的固体培养基,倒置于37℃恒温培养箱中过夜,观察并拍照记录结果。
1.2.7 最小抑菌浓度法(MIC)检测重组抗菌肽的抗菌活性 将同1.2.6方法培养的OD600=0.5的菌悬液10 μL转接至10 mL新鲜肉汤培养基中,涡旋振荡混匀;此时细菌密度在1×105 cfu/mL,用于MIC的测定。将抗菌肽与抗生素进行梯度稀释,至终浓度为2 560、1 280、640、320、160、40、20、10、5和2.5 μg·mL-1系列浓度稀释液;向无菌96孔圆底板中加入90 μL制备好的菌悬液,再逐一加入10 μL相应浓度的抗菌肽稀释液或抗生素稀释液,待测终浓度分别为256、128、64、32、16、8、4、2、1、0.5、0.25 μg·mL-1;设菌悬液为阳性对照孔,肉汤培养基为阴性对照,重组抗菌肽和抗生素(头孢菌素)均设3次重复,将96孔板置于37℃生化培养箱中保湿静置培养18—24 h,培养后观察各孔底部是否有细菌沉淀产生,无肉眼可见细菌沉淀的最小浓度可判定为该抗菌物质的MIC。
1.3 数据分析
数据采用SAS 9.2软件进行单因素方差分析(one- way ANOVA)和Turkey检验进行比较分析,结果表示为平均值±标准差。P<0.05表示差异显著。2 结果
2.1 中华蜜蜂Apidaecin cDNA的克隆以及序列分析
以中华蜜蜂总RNA反转录的cDNA为模板,利用引物API-F/ API-R进行PCR扩增,获得一条183 bp特异性片段(图1),通过ExPASy网站(http://www. expasy.cn)进行分析获得中华蜜蜂Apidaecin,含有183 bp的碱基,编码60个氨基酸,编码蛋白质命名为AccApidaecin,其分子量为15.6 kD,等电点为5.33。其氨基酸序列分析如图2所示。图1
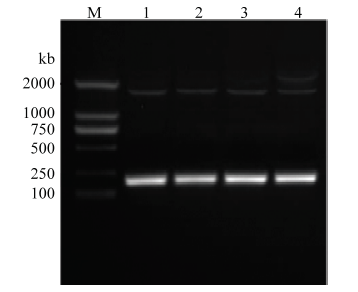 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1AccApidaecin克隆
M:DL 2000 DNA Marker;1—4:基因扩增产物Product of genome walking
Fig. 1Gene cloning of AccApidaecin
图2
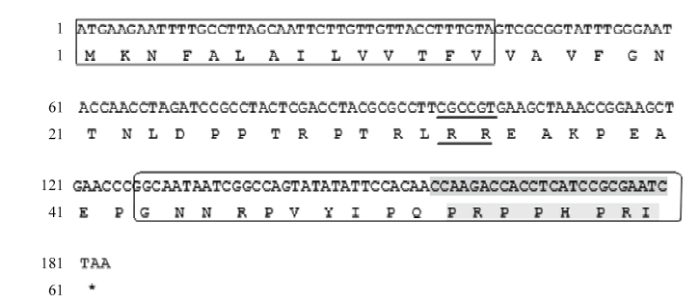 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2AccApidaecin cDNA分析
方框表示AccApidaecin的信号肽序列;下划线部分为一个基础的RR二肽;椭圆形边框表示抗菌肽AccApidaecin氨基酸序列,阴影部分为高度保守的8个氨基酸序列
Fig. 2The cDNA analysis of AccApidaecin
The box indicates signal peptide sequence of AccApidaecin; The underlined part represents a basic RR dipeptide; The oval border represents the amino acid sequence of AccApidaecin, and the shaded part is a highly conservative sequence of 8 amino acids
2.2 AccApidaecin蛋白的纯化与分离
在枯草芽孢杆菌中重组表达了具有活性的AccApidaecin。在IPTG终浓度为3 mmol·L-1,诱导温度为30℃,诱导表达12 h后进行分离纯化。抗菌肽主要分布在胞外,分泌效率高。通过His标签蛋白纯化试剂盒(可溶性蛋白)从1 L表达上清中纯化得到了约20 mg抗菌肽,图3为SDS-PAGE电泳结果。图3
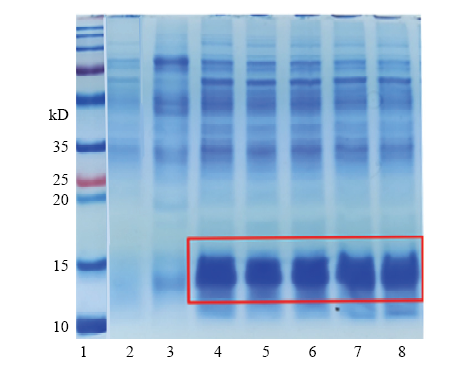 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3重组抗菌肽AccApidaecin的SDS-PAGE分析
1:蛋白Marker Protein marker;2:诱导表达的pHT43空载体Induced overexpression of pHT43;3:未诱导表达的pHT43(+)-AccApidaecin Non-induced overexpression of pHT43(+)-AccApidaecin;4—8:诱导表达的pHT43(+)-AccApidaecin Induced overexpression of pHT43(+)- AccApidaecin
Fig. 3SDS-PAGE analysis of recombinant antimicrobial peptide AccApidaecin
2.3 抑菌圈检测重组抗菌肽的抗菌活性
将OD600=0.5的E. coli K88均匀涂布于固体培养基上,5个0.5 cm的纸片上分别滴加3 μL的灭菌双蒸水、10 mg·L-1重组AccApidaecin、20 mg·L-1的重组AccApidaecin、10 mg·L-1的头孢霉素和100 mg·L-1的头孢霉素,涂好的培养基放于37℃培养箱中培养12 h。结果如图4所示,与阴性对照1号相比,其余均有明显抑菌圈出现,表明重组抗菌肽在体外具有良好的抗菌活性。图4
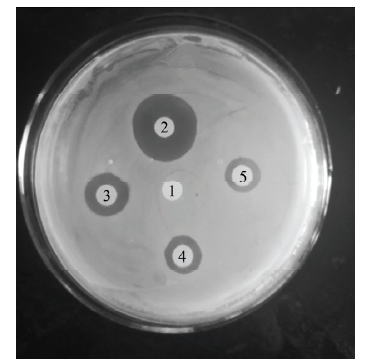 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4重组抗菌肽AccApidaecin的抗菌分析
1:灭菌双蒸水Sterilized double distilled water;2:100 mg·L-1的头孢霉素Cafotaxime (100 mg·L-1);3:20 mg·L-1的重组Apidaecin Recombinant Apidaecin (20 mg·L-1);4:10 mg·L-1的头孢噻肟Cafotaxime (10 mg·L-1);5:10 mg·L-1重组AccApidaecin Recombinant AccApidaecin (10 mg·L-1)
Fig. 4Bacteriostatic analysis of recombinant antimicrobial peptide AccApidaecin
2.4 最小浓度抑菌法检测重组抗菌肽的抗菌活性
重组抗菌肽AccApidaecin对E. coli K88的最小抑菌浓度为10 mg·L-1,头孢噻肟(Cafotaxime Na salt)对E. coli K88的最小抑菌浓度为5 mg·L-1。2.5 重组抗菌肽对染菌小鼠血清免疫球蛋白含量与肠道sIgA水平的影响
如图5所示,染菌对照组免疫球蛋白含量与肠道sIgA水平最高,并与其他各组差异显著。血清IgA、IgG和IgM水平变化与sIgA—致,表明重组抗菌肽AccApidaecin显著缓解由于染菌导致的血清和肠道免疫球蛋白含量的升高。图5
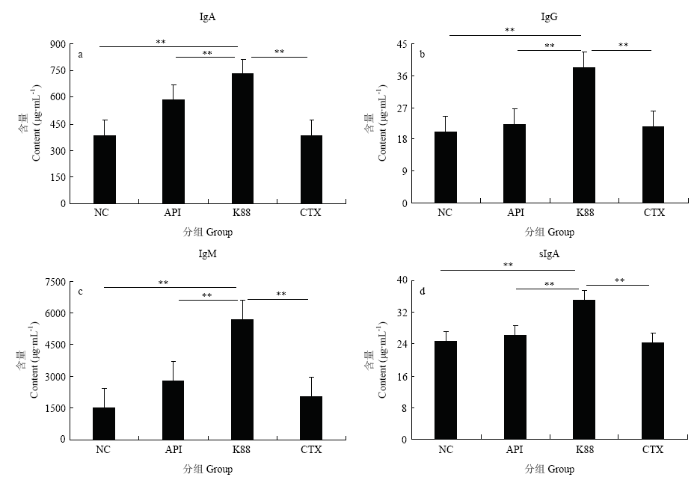 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5重组抗菌肽对染菌小鼠免疫球蛋白与肠道sIgA水平的影响
NC:正常对照组 Normal control group;API:20 mg·L-1重组Apidaecin染菌组 20 mg·L-1 Recombinant Apidaecin infection group;K88:染菌对照组 Infection control group;CTX:150 mg·L-1头孢噻肟染菌组150 mg·L-1 Cefotaxime infection group。
Fig. 5Effect of recombinant antimicrobial peptide on immunoglobulin and sIgA levels in mice infected with E. coli K88
2.6 重组抗菌肽对染菌小鼠肠道相关蛋白基因表达的影响
采用qRT-PCR法对小鼠肠道紧密连接蛋白基因(claudin-1、ZO-2)以及小鼠肠道细胞因子(促炎因子TNF-α、IFN-γ、IL6和抑炎因子IL10)的表达水平进行了测定,结果表明染菌后TNF-α、IL6和IL10表达水平显著升高(P<0.05),而claudin-1、ZO-2和IFN-γ表达水平显著降低(P<0.05)(图6-a、6-b);然而染菌后,注射了重组抗菌肽和头孢霉素的试验组,都显著抑制TNF-α、IL6和IL10表达水平的升高以及claudin-1、ZO-2和IFN-γ表达水平的降低(P<0.05)(图6-c、6-d、6-e、6-f)。综合来看,重组抗菌肽Apidaecin能够有效保护大肠杆菌K88侵染后的小鼠肠道。图6
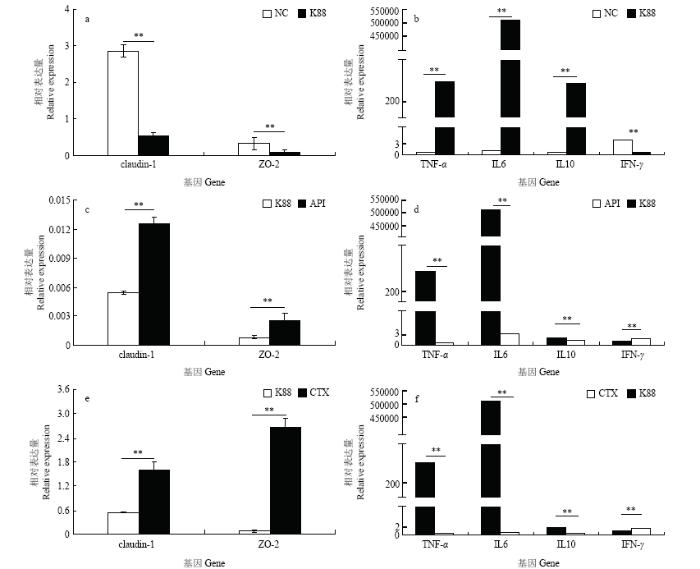 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6重组抗菌肽对染菌小鼠肠道相关蛋白基因表达的影响
Fig. 6Effect of recombinant antimicrobial peptide on related intestinal protein genes mRNA expression in mice infected with E. coli K88
3 讨论
CASTEELS-JOSSON等[14]分析发现一个Apidaecin前体蛋白可加工生产最多可达12个Apidaecin的单元,即有重复单元的现象,导致的直接结果是可以迅速地增强昆虫本身的免疫应答,产生超量的蜜蜂肽。具有生物活性的Apidaecin是由两个加工序列EAEPEAEP(变异体)和RR连接在一起的。如图2所示,对克隆得到的序列进行分析,GNNRPVYIPQPRPPHPRI是Apidaecin的18个氨基酸序列,Apidaecin是富含脯氨酸的短肽,在C端具有高度保守的8个氨基酸(PRPPHPRL/I),而它们的N-末端是可变区,与其抗菌活性息息相关。目前,抗菌肽已在很多表达系统运用各种表达策略成功表达,例如大肠杆菌中的融合表达[15,16,17]和杂合表达[18,19,20]、毕赤酵母菌表达系统[21]、乳酸乳球菌表达系统[22]等,但对于重组抗菌肽的抗菌活性并没有进一步的研究与验证。Apidaecin对革兰氏阴性菌效果显著,与细菌表面结合,然后转运到细胞质中,最后与靶蛋白结合(主要是热休克蛋白DnaK)。由于这种靶向特异性,Apidaecin对哺乳动物细胞是无毒的和非溶血的,因此被认为是新的抗生素候选药物[23]。本研究以大肠杆菌K88作为致病原对重组抗菌肽体外抗菌活性以及小鼠的体内抗菌活性进行验证,抑菌圈试验结果表明重组抗菌肽Apidaecin在体外具有良好的抗菌活性,重组抗菌肽Apidaecin对大肠杆菌K88的最小抑菌浓度为10 mg·L-1。
重组抗菌肽Apidaecin在体外具有良好的抗菌活性,但体内环境远比体外环境复杂。体内pH、离子强度以及宿主的蛋白酶等都会对抗菌肽造成影响。因此,本试验在获得重组抗菌肽的基础上,以小鼠为试验动物模型,从肠道屏障和免疫功能两个方面探讨重组抗菌肽Apidaecin对感染大肠杆菌K88小鼠的保护作用。紧密连接是肠黏膜机械屏障的重要组成部分,主要由跨膜蛋白和胞质蛋白组成,其中跨膜蛋白包括occludin、claudin-1、连接黏附分子等;胞质蛋白主要为ZO家族,与紧密连接结构的其他蛋白以及细胞骨架相连。紧密连接具有物质大小和电荷选择性,是决定肠道通透性的关键因素,维持紧密连接形态和功能的完整性对保护肠黏膜屏障、防止细菌移位有着重要意义[24]。小鼠肠道细胞因子(促炎因子和抑炎因子)平衡失调被认为是肠道炎症性疾病的一个重要发病机制,细胞因子异常表达与结肠炎的发生和发展密切相关[25]。本试验采用qRT-PCR法对小鼠肠道紧密连接蛋白基因(claudin-1、ZO-2)以及小鼠肠道细胞因子(促炎因子TNF-α、IFN-γ、IL6和抑炎因子IL10)的表达水平进行了测定,结果发现大肠杆菌K88侵染小鼠后,导致claudin-1、ZO-2和IFN-γ表达水平显著低于正常对照组,而TNF-α、IL6和IL10表达水平显著升高,与齐珂珂等[26]的研究相一致。而腹腔注射重组抗菌肽Apidaecin以及头孢霉素皆可以有效抵御大肠杆菌K88侵染小鼠带来的一系列炎症反应,表明重组抗菌肽Apidaecin 能够有效保护大肠杆菌K88侵染后的小鼠肠道。
本试验还检测了血清中免疫球蛋白(IgA、IgG、IgM)以及肠道中sIgA的含量,结果显示染菌组显著高于正常对照组,与之前余树培[27]报道的大肠杆菌K88可导致小鼠腹泻相符,表明大肠杆菌K88可导致小鼠染病并激起机体的免疫应答。空载体正常组与染菌组相比差异不明显,而重组抗菌肽Apidaecin以及头孢霉素的正常组与染菌组相比差异显著,这几种免疫球蛋白或细胞因子的水平在一定程度上反应了免疫功能[27,28,29],表明腹腔注射的重组抗菌肽Apidaecin能提高小鼠的免疫机能,从而抵抗大肠杆菌K88对小鼠的侵染。
目前,关于抗菌肽的抗菌机制有众多的猜想,但是抗菌肽抗菌机制研究只针对了个别几种,所以还没有一个能够涵盖所有种类抗菌肽作用机理的假说,而且也不确定哪种假说更接近真实情况[30]。常见的抗菌肽作用机制有桶板模型[31]、毯式模型[32]、环形孔模型[33]、凝聚模型以及其他作用机制[34]。对于重组抗菌肽Apidaecin的抗菌机制,还需要进一步深入研究。
4 结论
在枯草芽孢杆菌中成功重组表达得到了中华蜜蜂抗菌肽Apidaecin,其在体内/体外均对大肠杆菌K88有良好的抗菌效果,并且能够提高小鼠的免疫机能,抵抗大肠杆菌K88对小鼠的侵染。(责任编辑 岳梅)
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1016/j.peptides.2012.09.020URL [本文引用: 1]
DOI:10.1016/j.jbiotec.2004.08.004URLPMID:15607230 [本文引用: 1]

Preparations enriched by a specific protein are rarely easily obtained from natural host cells. Hence, recombinant protein production is frequently the sole applicable procedure. The ribosomal machinery, located in the cytoplasm is an outstanding catalyst of recombinant protein biosynthesis. Escherichia coli facilitates protein expression by its relative simplicity, its inexpensive and fast high-density cultivation, the well-known genetics and the large number of compatible tools available for biotechnology. Especially the variety of available plasmids, recombinant fusion partners and mutant strains have advanced the possibilities with E. coli. Although often simple for soluble proteins, major obstacles are encountered in the expression of many heterologous proteins and proteins lacking relevant interaction partners in the E. coli cytoplasm. Here we review the current most important strategies for recombinant expression in E. coli. Issues addressed include expression systems in general, selection of host strain, mRNA stability, codon bias, inclusion body formation and prevention, fusion protein technology and site-specific proteolysis, compartment directed secretion and finally co-overexpression technology. The macromolecular background for a variety of obstacles and genetic state-of-the-art solutions are presented.
[本文引用: 1]
DOI:10.1016/j.resmic.2004.05.002URLPMID:15380546 [本文引用: 1]

Bacillus subtilis is an alternative host for expression and secretion of heterologous proteins. However, low yields of protein production limit its use on a wide scale. The secretory pathway of proteins can be divided into three functional stages: the early stage, involving the synthesis of secretory pre-proteins, their interaction with chaperones and binding to the secretory translocase; the second stage, translocation across the cytoplasmic membrane; and the last stage, including removal of the signal peptide, protein refolding and passage through the cell wall. Five bottlenecks for expression and secretion of heterologous proteins are described in this review: transcription, protein folding, translocation, signal peptide processing and proteolysis.
URL [本文引用: 1]
DOI:10.1016/j.bbamcr.2004.02.011URLPMID:15546673 [本文引用: 1]

Bacillus subtilis is a rod-shaped, Gram-positive soil bacterium that secretes numerous enzymes to degrade a variety of substrates, enabling the bacterium to survive in a continuously changing environment. These enzymes are produced commercially and this production represents about 60% of the industrial-enzyme market. Unfortunately, the secretion of heterologous proteins, originating from Gram-negative bacteria or from eukaryotes, is often severely hampered. Several bottlenecks in the B. subtilis secretion pathway, such as poor targeting to the translocase, degradation of the secretory protein, and incorrect folding, have been revealed. Nevertheless, research into the mechanisms and control of the secretion pathways will lead to improved Bacillus protein secretion systems and broaden the applications as industrial production host. This review focuses on studies that aimed at optimizing B. subtilis as cell factory for commercially interesting heterologous proteins.
[本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
DOI:10.1002/j.1460-2075.1989.tb08368.xURLPMID:2676519 [本文引用: 2]

Although insects lack the basic entities of the vertebrate immune system, such as lymphocytes and immunoglobulins, they have developed alternative defence mechanisms against infections. Different types of peptide factors, exhibiting bactericidal activity, have been detected in some insect species. These humoral factors are induced upon infection. The present report describes the discovery of the apidaecins, isolated from lymph fluid of the honeybee (Apis mellifera). The apidaecins represent a new family of inducible peptide antibiotics with the following basic structure: GNNRP(V/I)YIPQPRPPHPR(L/I). These heat-stable, non-helical peptides are active against a wide range of plant-associated bacteria and some human pathogens, through a bacteriostatic rather than a lytic process. Chemically synthesized apidaecins display the same bactericidal activity as their natural counterparts. While only active antibacterial peptides are detectable in adult honeybee lymph, bee larvae contain considerable amounts of inactive precursor molecules.
DOI:10.1111/j.1432-1033.1990.tb15315.xURLPMID:2298215 [本文引用: 1]

Honeybees ( Apis mellifera ) are frequently exposed to and likely to be infected by plant-associated bacteria. We mimicked this process by injecting bees with live bacteria and isolated five induced antibacterial substances by comparative liquid chromatographic mapping of the hemolymph. Three of these antibiotics belong to a unique family of small (18 amino acids) peptides: the apidaecins [Casteels et al. (1989) EMBO J. 8 , 2387芒聙聯2391]. We have now characterized a fourth bee immune response peptide. The complete sequence was established by Edman degradation of the peptide and fragments thereof. It is 34 amino acids long and contains 10 proline residues. The amino-terminal half is related to the apidaecins; similar proline motifs are also present in the aminoterminal quarter of the much longer fly diptericins. The newly identified peptide's broad spectrum, lower specific activities against Gram-negative plant pathogens and its inability to inhibit bacterial growth at medium ionic strength are different from the apidaecins. Moreover, the highest observed specific activity was against an apidaecin-resistant Xanthomonas strain. In contrast to the immediate action of apidaecins, bactericidal activity is delayed. We propose the name 'abaecin' for this new antibacterial response peptide.
[本文引用: 1]
DOI:10.1006/bbrc.1994.1234URLPMID:8123032 [本文引用: 1]

Insect resistance to bacterial infections is dependent on the production of specialized defense peptides. We report here that lethal activities of apidaecin, a small peptide from honeybees, cannot possibly be the result of a conventional 'lytic' mechanism. Evidence includes the complete lack of membrane permeabilization, at concentrations that exceed lethal doses by four orders of magnitude, and undiminished sensitivity of apidaecin-resistant mutants to 'poreforming' peptides. In addition, the D-enantiomer of apidaecin is completely devoid of antibacterial activities. We propose therefore, that the antagonistic effects of apidaecin involve stereoselective recognition of a chiral cellular target, establishing this peptide as functionally unique among insect antibacterials. Identification of the apidaecin target may provide the scientific basis for rational drug design.
[本文引用: 1]
DOI:10.1002/j.1460-2075.1993.tb05801.xURLPMID:8467807 [本文引用: 1]

Abstract Apidaecins are the most prominent components of the honeybee humoral defense against microbial invasion. Our analysis of cDNA clones indicated that up to 12 of these short peptides (2 kDa) can be generated by processing of single precursor proteins; different isoforms are hereby linked in one promolecule. Assembly of the multipeptide precursors and the putative three-step maturation are strongly reminiscent of yeast alpha-mating factor. Bioactive apidaecins are flanked by the two 'processing' sequences, EAEPEAEP (or variants) and RR; joined together, they form a single unit that is repeated numerous times. The number of such repeats is variable and was reflected in the observed diversity of transcript lengths. Each such transcript is likely to be encoded by a different gene, forming a tight gene cluster. While transcriptional activation upon bacterial challenge is not exceptionally fast, the multigene and multipeptide precursor nature of the apidaecin genetic information allows for amplification of the response, resulting in a real overproduction of peptide antibiotic. Enhanced efficiency of the 'immune' response to bacterial infection through such a mechanism is, to our knowledge, unique among insects.
DOI:10.1016/0378-1119(94)90205-4URLPMID:8026760 [本文引用: 1]

A set of vectors has been constructed to facilitate the fusion of heterologous sequences to the C terminus of the maltose-binding protein (MBP) of Escherichia coli. The plasmids carry a cloning region comprising two blunt cloning sites, a BamHI site and multiple stop codons, and this has been placed in each reading frame so that translational fusions to MBP can be generated and manipulated with ease. To demonstrate the utility of this system, recombinant proteins have been engineered in which staphylococcal enterotoxin A has been fused to MBP.
DOI:10.1128/AAC.46.5.1503-1509.2002URLPMID:15104145 [本文引用: 1]

Human Csk Homologous Kinase (CHK), a protein of 527 amino acid residues, is involved in suppression of breast tumors. The kinase domain of CHK ( amino acid residues 228 to 485) expressed with C-terminal 6HIS fusion in Pichia pastoris is heavily glycosylated. Expression of the C-terminal 6HIS fused kinase domain of CHK, with an N-terminal glutathione S-transferase fusion, in Pichia pastoris alleviated the hyperglycosylation. The expressed protein was purified by affinity chromatography to 1 mg l(-1) culture and remained active. A simple plate assay to identify colonies of P. pastoris expressing the recombinant protein is also presented.
DOI:10.1016/j.jbiotec.2004.03.013URLPMID:15196769 [本文引用: 1]

An expression/purification system was developed using artificial oil bodies (AOB) as carriers for producing recombinant proteins. A target protein, green fluorescent protein (GFP), was firstly expressed in Escherichia coli as an insoluble recombinant protein fused to oleosin, a unique structural protein of seed oil bodies, by a linker sequence susceptible to factor Xa cleavage. Artificial oil bodies were constituted with triacylglycerol, phospholipid, and the insoluble recombinant protein, oleosin–Xa–GFP. After centrifugation, the oleosin-fused GFP was exclusively found on the surface of artificial oil bodies presumably with correct folding to emit fluorescence under excitation. Proteolytic cleavage with factor Xa separated soluble GFP from oleosin embedded in the artificial oil bodies; thus after re-centrifugation, GFP of high yield and purity was harvested simply by concentrating the ultimate supernatant.
DOI:10.1016/j.pep.2007.04.018URLPMID:17572103 [本文引用: 1]

The hybrid antibacterial peptide CA–MA [cecropinA(1-8)–magainin2(1-12)] with potent antimicrobial properties but no hemolytic activity is a potential alternative antibiotic. To explore a new approach for high-level expression of the hybrid peptide CA–MA in -UBI-CA–MA. The fusion protein was expressed in soluble form under the optimized conditions at high level (more than 36% of the total proteins). With (His)-tag, the fusion protein was easily purified by Ni–NTA chromatography and 3602mg of fusion protein was purified from 102L of culture medium. The fusion protein was efficiently cleaved by ubiquitin C-terminal hydrolase (UCH), yielding recombinant CA–MA with high antimicrobial activity. After removing the contaminants by Ni–NTA chromatography, recombinant CA–MA was purified to homogeneity by reversed-phase HPLC and 6.802mg of pure active CA–MA was obtained from 102L culture medium. Analysis of recombinant CA–MA by MALDI-TOF-MS showed that the molecular weight of the purified recombinant CA–MA was 255902Da, which perfectly matches the mass (255902Da) calculated from the amino acid sequence. Analysis of CA–MA by circular dichroism (CD) revealed that the secondary structures of CA–MA in water solution were 17.4% α-helix and 82.6% random coil but no β-sheet. Our results demonstrated that functional CA–MA can be produced in sufficient quantities using the ubiquitin fusion technique. This is the first report on the heterologous expression of a hybrid antibacterial peptide fused to ubiquitin in E. coli.
[本文引用: 1]
DOI:10.1007/s00253-011-3816-zURLPMID:22189867 [本文引用: 1]

Abstract65=6516–6402μg/ml) against given strains and did not show hemolytic activity for human erythrocytes. The results indicated that the hybrid peptide LFT33 could serve as a promising candidate for pharmaceutical agents.
DOI:10.13701/j.cnki.kqyxyj.2017.05.002URL [本文引用: 1]

目的;利用基因工程的方法,在毕赤酵母菌(Pichia patoris,P.pastoris)中成功表达Apidaecin型抗菌肽。方法:利用PCR技术在Apidaecin基因N端插入EcoR I酶切位点、GST标签(并连接DDDDK肠激酶位点),且在C端插入终止密码子和Not I酶切位点,后将上述片段扩增后与表达载体pPICZαA连接,构建重组表达载体pPICZαA-GST-Apidaecin,并测序鉴定;将pPICZαA-GST-Apidaecin重组质粒电转至P.pastoris X33中并进行发酵表达;表达产物经GST亲和层析纯化后进行SDS-PAGE凝胶电泳鉴定,EK肠激酶切除GST标签后进行抑菌实验。结果:SDS-PAGE凝胶电泳结果显示GST-Apidaecin融合蛋白表达的产物条带位于分子量约为28KD处,与理论分子量一致;抑菌实验表明,用EK肠激酶切除GST融合标签后,Apidaecin型抗菌肽对大肠杆菌有明显的抑菌活性。结论:Apidaecin型抗菌肽在P.pastoris菌中得到成功表达并具有抑菌活性。
DOI:10.13701/j.cnki.kqyxyj.2017.05.002URL [本文引用: 1]

目的;利用基因工程的方法,在毕赤酵母菌(Pichia patoris,P.pastoris)中成功表达Apidaecin型抗菌肽。方法:利用PCR技术在Apidaecin基因N端插入EcoR I酶切位点、GST标签(并连接DDDDK肠激酶位点),且在C端插入终止密码子和Not I酶切位点,后将上述片段扩增后与表达载体pPICZαA连接,构建重组表达载体pPICZαA-GST-Apidaecin,并测序鉴定;将pPICZαA-GST-Apidaecin重组质粒电转至P.pastoris X33中并进行发酵表达;表达产物经GST亲和层析纯化后进行SDS-PAGE凝胶电泳鉴定,EK肠激酶切除GST标签后进行抑菌实验。结果:SDS-PAGE凝胶电泳结果显示GST-Apidaecin融合蛋白表达的产物条带位于分子量约为28KD处,与理论分子量一致;抑菌实验表明,用EK肠激酶切除GST融合标签后,Apidaecin型抗菌肽对大肠杆菌有明显的抑菌活性。结论:Apidaecin型抗菌肽在P.pastoris菌中得到成功表达并具有抑菌活性。
[D].
[本文引用: 1]
[D].
[本文引用: 1]
DOI:10.1016/j.peptides.2006.03.016URLPMID:16675061 [本文引用: 1]

Apidaecins (apidaecin-type peptides) refer to a series of small, proline-rich (Pro-rich), 18- to 20-residue peptides produced by insects. They are the largest group of Pro-rich antimicrobial peptides (AMPs) known to date. Structurally, apidaecins consist of two regions, the conserved (constant) region, responsible for the general antibacterial capacity, and the variable region, responsible for the antibacterial spectrum. The small, gene-encoded and unmodified apidaecins are predominantly active against many Gram-negative bacteria by special antibacterial mechanisms. The mechanism of action by which apidaecins kill bacteria involves an initial non-specific binding of the peptides to an outer membrane (OM) component. This binding is followed by invasion of the periplasmic space, and by a specific and essentially irreversible combination with a receptor/docking molecule that may be a component of a permease-type transporter system on inner membrane (IM). In the final step, the peptide is translocated into the interior of the cell where it meets its ultimate target. Evidence that apidaecins are non-toxic for human and animal cells is a prerequisite for using them as novel antibiotic drugs. This review presents the biodiversity, structure unction relationships, and mechanism of action of apidaecins.
DOI:10.3969/j.issn.1007-6948.2015.01.037URL [本文引用: 1]

临床上许多疾病的发生发展都与肠黏膜屏障功能有关,紧密连接是肠黏膜上皮细胞之间的主要连接方式,对维持肠黏膜屏障机械结构完整和正常功能发挥起重要作用。Claudin-1属紧密连接蛋白claudins家族,是构成紧密连接的主要骨架蛋白之一。作为紧密连接蛋白原纤维形成的主要成分,Claudin-1基因表达具有组织特异性,在正常肠黏膜上皮细胞间高表达,参与维持上皮细胞极性和调节肠屏障的通透性,对于维持肠上皮结构和功能,保护肠道屏障完整性,防止毒性大分子物质进入体内等方面起着重要作用。
DOI:10.3969/j.issn.1007-6948.2015.01.037URL [本文引用: 1]

临床上许多疾病的发生发展都与肠黏膜屏障功能有关,紧密连接是肠黏膜上皮细胞之间的主要连接方式,对维持肠黏膜屏障机械结构完整和正常功能发挥起重要作用。Claudin-1属紧密连接蛋白claudins家族,是构成紧密连接的主要骨架蛋白之一。作为紧密连接蛋白原纤维形成的主要成分,Claudin-1基因表达具有组织特异性,在正常肠黏膜上皮细胞间高表达,参与维持上皮细胞极性和调节肠屏障的通透性,对于维持肠上皮结构和功能,保护肠道屏障完整性,防止毒性大分子物质进入体内等方面起着重要作用。
DOI:10.3748/wjg.14.1972URLPMID:18395894 [本文引用: 1]

Although the aetiology of inflammatory bowel disease (IBD) remains unknown, the pathogenesis is gradually being unravelled, seeming to be the result of a combination of environmental, genetic, and immunological factors in which an uncontrolled immune response within the intestinal lumen leads to inflammation in genetically predisposed individuals. Multifactorial evidence suggests that a defect of innate immune response to microbial agents is involved in IBD. This editorial outlines the immunopathogenesis of IBD and their current and future therapy. We present IBD as a result of dysregulated mucosal response in the intestinal wall facilitated by defects in epithelial barrier function and the mucosal immune system with excessive production of cytokines growth factors, adhesion molecules, and reactive oxygen metabolites, resulting in tissue injury. Established and evolving therapies are discussed in the second part of this editorial and at the end of this section we review new therapies to modulate the immune system in patients with IBD.
DOI:10.3969/j.issn.1006-267x.2014.09.037URLMagsci [本文引用: 1]

本试验旨在研究聚乙二醇(PEG)修饰猪胰高血糖素样肽-2(pGLP-2)对结肠炎小鼠肠道紧密连接蛋白和炎性因子基因表达的影响。试验选取24只BALB/C小鼠,随机分为4组,葡聚糖硫酸钠(DSS)组小鼠饮用3% DSS建立小鼠结肠炎模型,DSS+pGLP-2组和DSS+PEG-pGLP-2组饮用3% DSS且在试验第8天腹腔分别注射pGLP-2和PEG-pGLP-2,饮水组小鼠正常饮水。试验期10 d。结果表明:与饮水组相比,DSS组小鼠结肠紧密连接闭锁小带基因(<em>ZO</em>-1)的mRNA相对表达量极显著降低(<em>P</em><0.01);与DSS组相比,注射pGLP-2对<em>ZO</em>-1的mRNA相对表达量没有改善(<em>P</em>>0.05),而注射PEG-pGLP-2可极显著增加<em>ZO</em>-1的mRNA相对表达量(<em>P</em><0.01)。与饮水组相比,DSS组小鼠结肠白细胞介素-6(<em>IL</em>-6)、白细胞介素-10(<em>IL</em>-10)和干扰素-γ基因(<em>INF</em>-<em>γ</em>)的mRNA相对表达量极显著增加(<em>P</em><0.01);与DSS组相比,注射pGLP-2和PEG-pGLP-2可极显著降低<em>IL</em>-6、<em>IL</em>-10和<em>IFN</em>-<em>γ</em>的mRNA相对表达量(<em>P</em><0.01)。结果提示,PEG-pGLP-2通过增加肠道紧密连接蛋白的表达、降低结肠炎小鼠炎性细胞因子的表达抑制其炎性病变,且作用效果优于pGLP-2。
DOI:10.3969/j.issn.1006-267x.2014.09.037URLMagsci [本文引用: 1]

本试验旨在研究聚乙二醇(PEG)修饰猪胰高血糖素样肽-2(pGLP-2)对结肠炎小鼠肠道紧密连接蛋白和炎性因子基因表达的影响。试验选取24只BALB/C小鼠,随机分为4组,葡聚糖硫酸钠(DSS)组小鼠饮用3% DSS建立小鼠结肠炎模型,DSS+pGLP-2组和DSS+PEG-pGLP-2组饮用3% DSS且在试验第8天腹腔分别注射pGLP-2和PEG-pGLP-2,饮水组小鼠正常饮水。试验期10 d。结果表明:与饮水组相比,DSS组小鼠结肠紧密连接闭锁小带基因(<em>ZO</em>-1)的mRNA相对表达量极显著降低(<em>P</em><0.01);与DSS组相比,注射pGLP-2对<em>ZO</em>-1的mRNA相对表达量没有改善(<em>P</em>>0.05),而注射PEG-pGLP-2可极显著增加<em>ZO</em>-1的mRNA相对表达量(<em>P</em><0.01)。与饮水组相比,DSS组小鼠结肠白细胞介素-6(<em>IL</em>-6)、白细胞介素-10(<em>IL</em>-10)和干扰素-γ基因(<em>INF</em>-<em>γ</em>)的mRNA相对表达量极显著增加(<em>P</em><0.01);与DSS组相比,注射pGLP-2和PEG-pGLP-2可极显著降低<em>IL</em>-6、<em>IL</em>-10和<em>IFN</em>-<em>γ</em>的mRNA相对表达量(<em>P</em><0.01)。结果提示,PEG-pGLP-2通过增加肠道紧密连接蛋白的表达、降低结肠炎小鼠炎性细胞因子的表达抑制其炎性病变,且作用效果优于pGLP-2。
[D].
[本文引用: 2]
[D].
[本文引用: 2]
DOI:10.3969/j.issn.1001-991X.2005.02.002URL [本文引用: 1]

乳铁蛋白(LF)是一种多功能 的糖蛋白,乳铁蛋白活性多肽(Lfcin)是从LF上被胃蛋白酶水解下来的25个氨基酸残基的小肽。文中阐述了乳铁蛋白及其活性多肽的结构,介绍了乳铁蛋 白及其活性多肽的主要生理作用:抗菌、抑菌、抗病毒、抗氧化,调节机体的免疫和提高肠道对铁离子的吸收等作用。根据乳铁蛋白制备的研究进展,讨论了乳铁蛋 白及其活性多肽在乳、食品和动物生产中作为添加剂的应用前景。
DOI:10.3969/j.issn.1001-991X.2005.02.002URL [本文引用: 1]

乳铁蛋白(LF)是一种多功能 的糖蛋白,乳铁蛋白活性多肽(Lfcin)是从LF上被胃蛋白酶水解下来的25个氨基酸残基的小肽。文中阐述了乳铁蛋白及其活性多肽的结构,介绍了乳铁蛋 白及其活性多肽的主要生理作用:抗菌、抑菌、抗病毒、抗氧化,调节机体的免疫和提高肠道对铁离子的吸收等作用。根据乳铁蛋白制备的研究进展,讨论了乳铁蛋 白及其活性多肽在乳、食品和动物生产中作为添加剂的应用前景。
DOI:10.3969/j.issn.1002-1280.2005.10.002URL [本文引用: 1]

通过两个试验探讨了重组猪乳铁蛋白(rPLF)对断奶仔猪血清免疫因子IL-1和IL-2水 平的影响.两试验都按体重将90头28日龄断奶仔猪分成3个处理组:对照组(基础日粮)、抗生素组(基础日粮+20 mg/kg黄霉素+110 mg/kg金霉素)和rPLF组(基础日粮+730 mg/kgrPLF),试验期30 d.试验1采用杜长嘉断奶仔猪;试验2采用杜长大断奶仔猪,分两期试验(断奶后1~15 d和16~30 d).试验结束后,各处理组分别取3头体重相近的小母猪屠宰,测定血清中IL-1和IL-2的浓度.结果表明,与对照组相比,rPLF能显著提高杜长嘉仔 猪血清中IL-2的水平,达46.64%(P<0.01);显著提高杜长大仔猪断奶前期和后期血清中IL-2水平(P<0.05);IL-1也有提高趋 势.两试验结果揭示,rPLF能通过提高断奶仔猪血清中IL-1和IL-2的水平而增强断奶仔猪的免疫力.
DOI:10.3969/j.issn.1002-1280.2005.10.002URL [本文引用: 1]

通过两个试验探讨了重组猪乳铁蛋白(rPLF)对断奶仔猪血清免疫因子IL-1和IL-2水 平的影响.两试验都按体重将90头28日龄断奶仔猪分成3个处理组:对照组(基础日粮)、抗生素组(基础日粮+20 mg/kg黄霉素+110 mg/kg金霉素)和rPLF组(基础日粮+730 mg/kgrPLF),试验期30 d.试验1采用杜长嘉断奶仔猪;试验2采用杜长大断奶仔猪,分两期试验(断奶后1~15 d和16~30 d).试验结束后,各处理组分别取3头体重相近的小母猪屠宰,测定血清中IL-1和IL-2的浓度.结果表明,与对照组相比,rPLF能显著提高杜长嘉仔 猪血清中IL-2的水平,达46.64%(P<0.01);显著提高杜长大仔猪断奶前期和后期血清中IL-2水平(P<0.05);IL-1也有提高趋 势.两试验结果揭示,rPLF能通过提高断奶仔猪血清中IL-1和IL-2的水平而增强断奶仔猪的免疫力.
DOI:10.1016/j.vaccine.2005.01.118URLPMID:16823923 [本文引用: 1]

The recent discovery of pathogen-associated molecular patterns (PAMPs) as potential ligands for the evolutionary conserved innate immune receptors termed Toll-like receptors has enabled a modern era of immunotherapy using synthetic mimics of pathogen molecules. Among the PAMPs, bacterial DNA or synthetic oligodeoxynucleotides (ODNs) that contain unmethylated CpG motif shows promising effect on activation of systemic innate immune response. We could document that CpG ODN is capable of mobilizing a potent innate immunity in the mucosal tissues. Thus, intravaginal, intrarrectal, or intragastric delivery of mice with CpG ODN elicits potent innate chemokine responses in the respective mucosal tissues. Interestingly, we could show that the immunostimulatory effect of CpG DNA is much improved when chemically conjugated to the non-toxic B subunit of cholera toxin.
DOI:10.1016/j.tim.2015.11.004URLPMID:4733415 [本文引用: 1]

Single-cell, real-time observations provide a remarkably detailed picture of the timing, sequence, and subcellular location of specific events during the attack of antimicrobial peptides (AMPs) on live bacteria. In addition to permeabilizing membranes, AMPs induce a variety of ‘downstream effects’. Specific peptides may interfere with cell wall synthesis; induce osmotic shock; disrupt synthesis of DNA, RNA, or proteins; destroy the proton-motive force; or induce oxidative stress. Environmental factors can modulate potency by enabling specific bacteriostatic mechanisms or by altering the AMP structure. LL-37 is more effective againstEscherichia coliin aerobic metabolism than in anaerobic conditions. In 2011, Schroeder and coworkers found that the reduced, unfolded form of human β-defensin-1 is much more potent against some intestinal bacteria that live in a naturally reducing environment.
DOI:10.1016/j.tibtech.2011.05.001URLPMID:21680034 [本文引用: 1]

Antimicrobial peptides (AMPs) are an integral part of the innate immune system that protect a host from invading pathogenic bacteria. To help overcome the problem of antimicrobial resistance, cationic AMPs are currently being considered as potential alternatives for antibiotics. Although extremely variable in length, amino acid composition and secondary structure, all peptides can adopt a distinct membrane-bound amphipathic conformation. Recent studies demonstrate that they achieve their antimicrobial activity by disrupting various key cellular processes. Some peptides can even use multiple mechanisms. Moreover, several intact proteins or protein fragments are now being shown to have inherent antimicrobial activity. A better understanding of the structure ctivity relationships of AMPs is required to facilitate the rational design of novel antimicrobial agents.
DOI:10.1016/j.physbeh.2009.07.008URLPMID:127115 [本文引用: 1]

The 13-residue dermaseptin S4 derivative K(4)S4(1-13)a (P) was previously shown to kill intraerythrocytic malaria parasites through the lysis of the host cells. In this study, we have sought peptides that will kill the parasite without lysing the erythrocyte. To produce such peptides, 26 compounds of variable structure and size were attached to the N terminus of P and screened for antiplasmodium and hemolytic activities in cultures of Plasmodium falciparum. Results from this screen indicated that increased hydrophobicity results in amplified antiplasmodium effect, irrespective of the linearity or bulkiness of the additive. However, increased hydrophobicity also was generally associated with increased hemolysis, with the exception of two derivatives: propionyl-P (C3-P) and isobutyryl-P (iC4-P). Both acyl-peptides were more effective than P, with 50% growth inhibition at 3.8, 4.3, and 7.7 microM, respectively. The antiparasitic effect was time dependent and totally irreversible, implying a cytotoxic effect. The peptides were also investigated in parallel for their ability to inhibit parasite growth and to induce hemolysis in infected and uninfected erythrocytes. Whereas the dose dependence of growth inhibition and hemolysis of infected cells overlapped when cells were treated with P, the acyl-peptides exerted 50% growth inhibition at concentrations that did not cause hemolysis. Noticeably, the acyl derivatives, but not P, were able to dissipate the parasite plasma membrane potential and cause depletion of intraparasite potassium under nonhemolytic conditions. These results clearly demonstrate that the acyl-peptides can affect parasite viability in a manner that is dissociated from lysis of the host cell. Overall, the data indicate the potential usefulness of this strategy for development of selective peptides as investigative tools and eventually as antimalarial agents.
DOI:10.1128/AAC.00209-09URLPMID:19470516 [本文引用: 1]

Abstract Lacticin Q is a pore-forming bacteriocin produced by Lactococcus lactis QU 5, and its antimicrobial activity is in the nanomolar range. Lacticin Q induced calcein leakage from negatively charged liposomes. However, no morphological changes in the liposomes were observed by light scattering. Concomitantly with the calcein leakage, lacticin Q was found to translocate from the outer to the inner leaflet of the liposomes, after it initially bound to the membrane within 2 s. Lacticin Q also induced lipid flip-flop. These results reveal that the antimicrobial mechanism of lacticin Q can be described by the toroidal pore model. This is the first report of a bacteriocin of gram-positive bacteria that forms a toroidal pore. From liposomes, lacticin Q leaked fluorescence-labeled dextran with a diameter of 4.6 nm. In addition, lacticin Q caused the leakage of small proteins, such as the green fluorescent protein, from live bacterial cells. There are no other reports of antimicrobial peptides that exhibit protein leakage properties. The proposed pore formation model of lacticin Q is as follows: (i) quick binding to outer membrane leaflets; (ii) the formation of at least 4.6-nm pores, causing protein leakage with lipid flip-flop; and (iii) the migration of lacticin Q molecules from the outer to the inner membrane leaflets. Consequently, we termed the novel pore model in the antimicrobial mechanism of lacticin Q a "huge toroidal pore."
