 , 王利
, 王利
Identification and Expression Analysis of Likely Orthologs of Tobacco Salicylic Acid Binding Protein 2 in Common Beans
XUERen-Feng , WANGLi
, WANGLi
通讯作者:
收稿日期:2017-09-10
接受日期:2018-01-8
网络出版日期:2018-01-29
版权声明:2018作物学报编辑部作物学报编辑部
基金资助:
作者简介:
-->
展开
摘要
关键词:
Abstract
Keywords:
-->0
PDF (1852KB)元数据多维度评价相关文章收藏文章
本文引用格式导出EndNoteRisBibtex收藏本文-->
普通菜豆(Phaseolus vulgaris L.)是最重要的食用豆类之一, 是人类摄取蛋白质、维生素和矿质元素的重要来源[1]。普通菜豆多种植于土壤贫瘠区, 因此容易遭受多种病原物危害而减产, 其中以土传病害最为严重。普通菜豆镰孢菌枯萎病(Fusarium wilt)属于普通菜豆维管束类病害的一种, 是由尖镰孢菌菜豆专化型(Fusarium oxysporum f. sp. phaseoli, Fop)引起的典型土传真菌病害, 给普通菜豆生产带来很大危害[2]。
水杨酸(salicylic acid, SA)作为一种介导植物抗病反应重要的信号分子, 在普通菜豆抗镰孢菌枯萎病的防御反应中具有极其重要的作用[3]。植物通过水杨酰甲酯酯酶(methyl salicylate esterase, MES)水解水杨酰甲酯(methyl salicylate, MeSA), 从而释放出具有生物活性的游离SA, 诱导植物细胞内SA相关的抗病防御反应, 提高植物免疫力[4,5]。SA与许多植物镰孢菌枯萎病的抗病性都存在着密切的联系, 受外源SA诱导条件下, 拟南芥[6]、番茄[7]、海枣[8]、鹰嘴豆[9]、普通菜豆[3]等对F. oxysporum的抗性均显著提高。SA是许多种植物防御系统中重要的信号分子, 因此在植物体内直接影响SA水平的调节因子就必然与植物的抗病性存在密切的联系。水杨酸结合蛋白2 (salicylic acid binding protein 2, SABP2)就是这样一类直接参与调控植物体内SA水平的蛋白。SABP2具有MES活性, 在植物体内将MeSA转化成游离SA, 提高植物体内SA水平, 对激发植物系统抗性具有极其关键的作用[10]。相关研究表明, 烟草SABP2基因编码29 kD蛋白质, 该蛋白能够水解MeSA生成SA, 从而诱导寄主产生系统获得性抗性[11,12,13,14]。此外, Chigurupati等[15]研究表明, SABP2是烟草中SA介导的信号传导途径关键因子, 在烟草受非寄主病原诱导发生的抗性反应中发挥重要作用。Vlot等[16]研究表明, 拟南芥中SABP2基因的直系同源基因AtMES基因家族的AtMES-1、AtMES-7和AtMES-9在丁香假单胞菌Pseudomonas syringae诱导条件下表达量显著升高, 功能验证试验结果表明, AtMES-1、AtMES-2、AtMES-7和AtMES-9在诱导拟南芥发生系统获得性抗性反应中具有十分关键的作用。Manosalva等[17]研究表明, 马铃薯中SABP2基因的直系同源基因StMES1在寄主抵御致病疫霉Phytophthora infestans侵染过程中同样也发挥着相似的重要功能。
植物细胞内存在一个SA参与的复杂信号调节网络, 而MES基因作为植物内源SA重要的调控节点蛋白, 目前仅有少数几个基因通过功能验证实验被证明参与了植物的抗病反应, 而且主要集中在模式植物上。因此本研究选择普通菜豆中烟草SABP2基因(NtSABP2)的同源基因作为目的基因, 研究MES基因家族成员在普通菜豆枯萎病抗病反应中的功能, 有望在普通菜豆抗病分子育种和抗病机理的研究中取得重要的突破。
1 材料与方法
1.1 试验材料
普通菜豆品种BRB130 (编号为F0004322)、白刀豆(编号为F0001298)、260205 (编号为F0005035)和黑芸豆(编号为F0004852)由中国农业科学院作物科学研究所提供。将菜豆种子播于灭菌的营养土中, 23~28℃条件下, 每日补充12 h光照, 培养7~10 d, 以备接种。供试菌株为FOP-DM01。病原菌首先接种于PDA (Potato Dextrose Agar)平板上, 25~28℃培养7 d, 然后用于接种。1.2 接种与样品的采集
参照薛仁风等[18]方法接种病原菌; 分别从0、1和3 d接种和未接种的菜豆植株根部取样, 每个处理3次生物学重复; 将样品保存于-80℃, 用于基因表达量、MES活性和SA含量分析。1.3 PvMES家族基因预测与序列分析
利用烟草中SABP2蛋白质序列(登录号为Q6RYA0)搜索普通菜豆基因组数据库(http://www. phytozome.net/), 分析普通菜豆MES家族基因, 获得7个普通菜豆中 NtSABP2基因的直系同源基因序列, 命名为PvMES1~PvMES7。根据推测的PvMES氨基酸序列, 利用ClustalX 1.83和MEGA 4.0软件分别进行氨基酸序列同源性比对和进化树分析。1.4 总RNA的提取与cDNA合成
采用TRIzol试剂(TianGen, China)提取处理和对照各时间点样品的总RNA, 按照 Reverse Transcriptase System试剂盒(Promega, USA)操作说明书合成第1链cDNA, 采用Oligo d(T)15反转录引物。1.5 实时荧光定量PCR分析基因表达量
根据PvMES1~PvMES7和PvPR1的cDNA序列设计定量PCR引物(表1)。以普通菜豆的Actin基因作为内参, 采用SuperRealPreMix (SYBR Green, TianGen, China) 荧光定量PCR试剂和qTOWER 2.2实时定量PCR仪(Analytikjena, Germany), 以各个取样时间点的cDNA为模板分析PvMES家族基因的表达情况。参照试剂说明中的反应体系与程序。反应结束后分析荧光值变化曲线和熔解曲线, 以PCR产物电泳确认扩增产物特异性。采用2-ΔΔCT 法分析数据, 确定基因的相对表达量。设每个取样时间点3个生物学重复, 在同一个批次完成内参基因和目标基因的PCR反应。Table 1
表1
表1实验中所用的引物
Table 1Primers used in the experiment
| 基因名称 Gene name | 引物名称 Primer name | 引物序列 Sequence for qPCR (5°-3°) |
|---|---|---|
| PvMES1 | QMES1F | GCTCTATCAACTTTCCCCTG |
| QMES1R | CATCGTTGGTAATCCAAAGT | |
| PvMES2 | QMES2F | CTATCAACTCTCCCCAACTC |
| QMES2R | CAAAGTAATTGCAAGGTCTAT | |
| PvMES3 | QMES3F | CAGAAAATTGAAGACGTTGG |
| QMES3R | AACATAGGAGGGCTTGCGC | |
| PvMES4 | QMES4F | GAGTACCTTGGGAGAGAAT |
| QMES4R | TAGAGAAGCTCTTCTGTTGG | |
| PvMES5 | QMES5F | GCAATTCCCAGAAAAAATTCTG |
| QMES5R | CTTGGGACCAAAGAACATCAA | |
| PvMES6 | QMES6F | ACCAAGTTTCTGTCCACTGC |
| QMES6R | TACTCTTTTGGAATTGTCAA | |
| PvMES7 | QMES7F | GGAGTTGAATTTTTGAGATCTA |
| QMES7R | CATCAAATTATCTTTTCCAGC | |
| PvPR1 | QPR1F | TGCTAAAGACGCCGATACCA |
| QPR1R | GCAACACCTCCAACTATGCT | |
| Actin | Act-F | GAAGTTCTCTTCCAACCATCC |
| Act-R | TTTCCTTGCTCATTCTGTCCG |
新窗口打开
1.6 MES活性及SA含量的测定
分别取各时间点采集样品, 用液氮研磨成粉末, 溶解于预冷Tris-HCl缓冲液中, 6000×g离心30 min, 收集上清液用于MES活性测定。参照Forouhar等[19]测定方法, 将上清液与MeSA混合, 于37℃温浴30 min, 加入水杨酸甲基转移酶和14C-S-腺苷甲硫氨酸标记水解产生的SA, 生成14C-MeSA, 加入等体积乙酸乙酯, 混匀后离心提取有机相, 通过闪烁计数仪定量分析14C-MeSA含量, 计算上清液MES活性; 另取各时间点采集样品, 用于游离SA含量测定。参照薛仁风等[3]的方法, 取300~1000 mg根组织, 用液氮研磨后溶于3 mL 80%甲醇, 置4℃过夜。将样品 4℃, 9000×g离心10 min, 取上清液, 将沉淀再次悬浮于2 mL甲醇中, 4℃黑暗放置6 h, 4℃条件下10 000×g离心10 min, 取上清液, 将两组上清液混合后取0.5 mL置于40℃条件下真空干燥, 将提取物用0.3 mL 5%三氯乙酸抽提后, 加入2倍体积环戊烷∶乙酸乙酯∶异丙醇(50∶50∶1), 充分混匀后离心, 取有机相真空干燥, 将干燥产物溶解于乙腈, 用高效液相色谱仪(HPLC)测定SA含量。1.7 发病情况调查与评价
采用1~9级的病级评价标准[20], 在接种后每天记录发病植株的病级。共设3个重复, 每个重复10株菜豆幼苗。平均发病级别=Σ(发病级别/调查总株数); 病情指数=Σ(发病级数×相应的发病植株数)/(9×植株总数)×100%。1.8 数据分析
采用SAS统计分析软件中LSD法进行单因素方差分析[21], 采用Microsoft Excel 2010软件绘制图表。2 结果与分析
2.1 PvMES基因序列及编码蛋白质序列分析
根据NtSABP2基因cDNA序列, 利用生物信息学方法在普通菜豆基因组数据库(http://www. phytozome.net/)中搜索NtSABP2基因的同源序列, 共获得7个NtSABP2基因的直系同源基因, 分别命名为PvMES1~PvMES7 (表2)。其中PvMES1~PvMES5位于第3染色体, PvMES6位于第2染色体, PvMES7位于第10染色体。PvMES1~PvMES7基因序列与NtSABP2基因序列一致性各不相同, 其中PvMES3最高, 达到63.8%, PvMES7最低, 仅为45.3%。Table 2
表2
表2PvMES家族基因与烟草水杨酸结合蛋白2核苷酸序列分析
Table 2Nucleotide sequence analysis of PvMES family members and tobacco (Nt) SABP2
| 基因名称 Gene | 基因位点 Genetic locus | 基因序列位置 Sequence location | E值 E-value | 一致性 Identity (%) | 染色体 Chromosome |
|---|---|---|---|---|---|
| PvMES1 | Phvul.003G248200.1 | 48561200-48564505 | 1.7×10-35 | 59.2 | 3 |
| PvMES2 | Phvul.003G248300.1 | 48567955-48569848 | 7.0×10-34 | 57.6 | 3 |
| PvMES3 | Phvul.003G248025.1 | 48530639-48532632 | 1.6×10-29 | 63.8 | 3 |
| PvMES4 | Phvul.003G248050.1 | 48539059-48541466 | 1.9×10-28 | 61.4 | 3 |
| PvMES5 | Phvul.003G248075.1 | 48545538-48547254 | 1.9×10-28 | 63.1 | 3 |
| PvMES6 | Phvul.002G022300.1 | 2389494-2391172 | 3.4×10-25 | 58.4 | 2 |
| PvMES7 | Phvul.010G043800.2 | 6682398-6684451 | 5.1×10-4 | 45.3 | 10 |
新窗口打开
NtSABP2蛋白质序列包括260个氨基酸, 理论蛋白分子质量为29.3 kDa, 等电点为5.39。该蛋白包含3个活性位点(Ser81、Asp210、His238), 共同组成具有水解MeSA活性的结构域。此外, NtSABP2序列中有15个与SA互作的活性位点; PvMES1序列中3个水解活性位点均与NtSABP2相同, SA互作位点有14个与NtSABP2相同; PvMES2序列中有2个水解活性位点和11个SA互作位点与NtSABP2相同; PvMES3~PvMES7序列3个水解活性位点均与NtSABP2相同, 而SA互作位点数则均与NtSABP2不同, 分别为13、11、15、9和6 (图1)。该结果表明, PvMES基因家族不同成员可能具有不同的生化特性和生物学功能。
 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图1PvMES1~PvMES7和NtSABP2氨基酸序列比对分析
相同位点用黑色表示, 相似位点用灰色表示; 水解活性位点用黑色箭头表示。SA结合位点用黑色三角形表示。
-->Fig. 1Multiple sequence alignment of PvMES1-PvMES7 and tobacco SABP2 (NtSABP2)
Identical residues are shaded in black and similar residues in gray; the catalytic triad residues are indicated by arrows, and residues that contact salicylic acid are indicated with black triangles.
-->
PvMES基因家族成员按照与NtSABP2序列的亲缘关系可分为三个亚家族, PvMES1、PvMES2和PvMES5的亲缘关系较近, 组成亚家族I, PvMES3、PvMES4和PvMES6三个成员亲缘关系较近, 组成亚家族II, PvMES7组成亚家族III (图2)。目前在拟南芥、马铃薯等作物中均有MES基因家族的相关研究报道[16,17], 但在普通菜豆中尚无MES基因的研究成果。
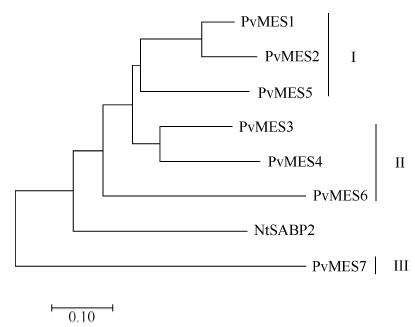 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图2PvMES1~PvMES7和NtSABP2进化树分析
-->Fig. 2Phylogenetic tree of PvMES1-PvMES7 and NtSABP2
-->
2.2 PvMES基因的诱导表达分析
接种FOP-DM01菌株1 d后, 4个菜豆品种根中PvMES1表达量均有所上调, 但不显著, 接种3 d后, 抗病品种黑芸豆和260205根中PvMES1表达量显著高于感病品种BRB130和白刀豆, 与0 d表达量相比, 分别上升至3.9倍和5.6倍; PvMES2基因的表达量在接种病原菌后的4个品种中, 不同时间段并没有发生显著变化; 在接种病原菌1 d后, 抗病品种黑芸豆和260205根中PvMES3表达量显著高于感病品种BRB130和白刀豆, 接种3 d后, 2个抗病品种的表达量继续升高, 显著高于2个感病品种, 与0 d表达量相比, 分别提高至1.9倍和4.3倍; PvMES4表达量在接种病原菌3 d后的4个菜豆品种中均升高, 黑芸豆根中PvMES4表达量升高至2.5倍, 高于其他3个品种; PvMES5表达量在接种病原菌后均显著升高, 接种1 d后, 黑芸豆和260205根中PvMES5表达量显著高于BRB130和白刀豆, 接种3 d后, 黑芸豆和260205根中PvMES5表达量分别升高至0 d表达量的7.6倍和5.4倍; 接种病原菌后, 4个菜豆品种中PvMES6表达量均逐渐升高, 在接种3 d后, 黑芸豆根中PvMES4表达量升高至3.7倍, 显著高于其他3个品种; 而PvMES7基因的表达量在接种病原菌后的4个品种根中没有发生显著变化; 在此互作过程中, 寄主PvPR1基因作为阳性对照, 在接菌1 d后表达量显著升高(图3)。结果表明, PvMES基因家族7个成员中, PvMES1、PvMES3、PvMES4、PvMES5和PvMES6的转录表达受 FOP-DM01菌株诱导均呈现不同程度的升高, 推测这5个MES基因与菜豆镰孢菌枯萎病抗病反应关系密切, 其中黑芸豆PvMES5基因在接种3 d后表达量变化最显著, 升高至7.6倍, 说明该基因可能对抗病品种黑芸豆的抗病性起到关键作用, 而抗病品种260205接种3 d后PvMES1表达量升高最明显, 达5.6倍, 表明其可能对寄主诱导自身抗病反应的发生最重要。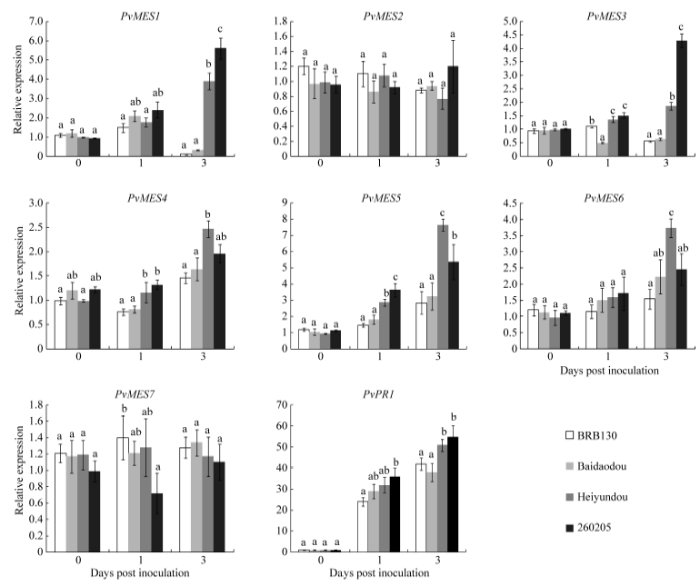 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图3FOP-DM01菌株诱导下普通菜豆根组织PvMES1~PvMES7和PvPR1表达量
相同时间点中标以不同字母的柱值在不同品种间差异显著(P < 0.05)。
-->Fig. 3Expression of PvMES1-PvMES7 and PvPR1 induced by Fusarium oxysporum f. sp. phaseoli DM01 isolate (FOP-DM01)
Bars represented by different letters are significantly different (P < 0.05) among four common bean varieties at the same time point.
-->
2.3 MES活性分析
MES活性与普通菜豆应答枯萎病原菌FOP-DM01菌株侵染密切相关。研究表明, 在接种病原菌1 d后, 4个品种普通菜豆根MES活性均升高, 但并不显著, 接种3 d后, 抗病品种黑芸豆和260205根中MES活性显著升高, 分别为0.043和0.057 μmol min-1 mg-1, 显著高于感病品种BRB130和白刀豆(图4), 表现出对病原菌更强的抗病性。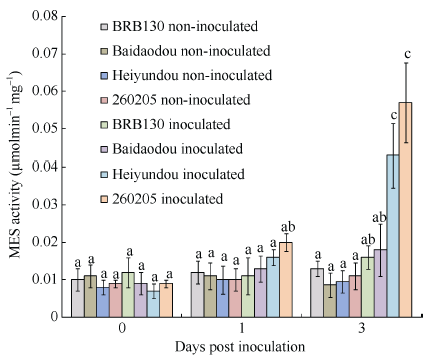 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图4FOP-DM01菌株诱导下普通菜豆根组织MES活性
相同时间点中标以不同字母的柱值在不同品种间差异显著(P < 0.05)。
-->Fig. 4MES activity induced by Fusarium oxysporum f. sp. phaseoli DM01 isolate (FOP-DM01)
Bars represented by different letters are significantly different (P < 0.05) among four common bean varieties at the same time point.
-->
2.4 游离SA含量分析
接种病原菌1 d后, 普通菜豆抗病品种和感病品种根中SA含量略有上升, 但并没有明显差异; 接种病原菌3 d后, 抗病品种黑芸豆和260205根中SA含量显著升高, 分别为264.1和287.0 ng g-1 FW, 均高于感病品种BRB130和白刀豆(图5), MES活性的升高是其更强抗病性的基础。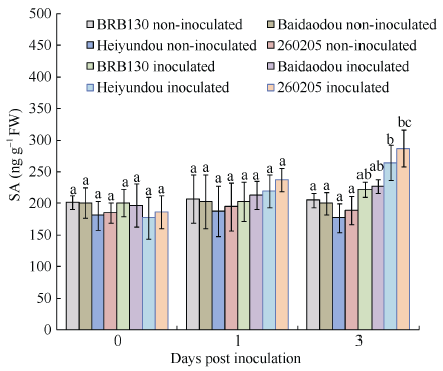 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图5FOP-DM01菌株诱导下普通菜豆根组织SA含量分析
相同时间点中标以不同字母的柱值在不同品种间差异显著(P < 0.05)。
-->Fig. 5SA content induced by Fusarium oxysporum f. sp. phaseoli DM01 isolate (FOP-DM01)
Bars represented by different letters are significantly different (P < 0.05) among four common bean varieties at the same time point.
-->
2.5 不同品种普通菜豆发病级别的调查
接种FOP-DM01菌株3周后, 感病品种BRB130和白刀豆表现出明显的叶片枯萎、褪绿变黄和根系变褐腐烂等发病症状, 而抗病品种黑芸豆和260205仅少数植株表现出轻微的发病症状; BRB130和白刀豆的平均病级数分别达到6.4和7.1, 病情指数分别为74.2和68.3, 而黑芸豆和260205平均病级数仅为2.9和2.7, 病情指数分别为38.1和27.7 (图6)。表型鉴定结果表明, 黑芸豆和260205对FOP-DM01菌株的抗病性要强于BRB130和白刀豆。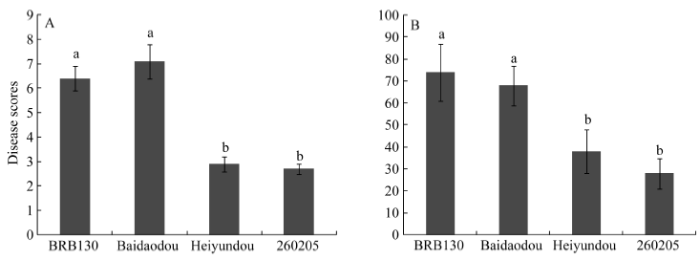 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图6普通菜豆接种FOP-DM01菌株后的病情评价
A: 接种FOP-DM01菌株3周后发病情况; B: 接种FOP-DM01菌株3周后的病情指数。相同时间点中标以不同字母的柱值在不同品种间差异显著(P < 0.05)。
-->Fig. 6Disease assessment of the common bean varieties after inoculating with FOP-DM01
A: Disease progress of the common bean varieties at three weeks post inoculation with FOP-DM01; B: Disease index of the common bean varieties plants at three weeks post inoculation with FOP-DM01. Bars represented by different letters are significantly different (P < 0.05) among four common bean varieties at the same time point.
-->
3 讨论
SA作为典型的植物信号分子, 通过激活抗病相关基因的表达提高寄主抗病性, 在植物系统抗性体系中具有非常重要的作用[22,23,24]。Spletzer和Enyedi[25]研究表明SA处理番茄显著地提升了寄主对链格孢菌Alternaria solani的系统抗性。Sz?ke等[26]研究表明禾谷镰孢菌Fusarium graminearum侵染感病或者中抗的玉米品种并不会导致寄主内SA含量明显变化, 而被其侵染的抗病品种体内的SA水平则显著升高。Wu等[27]揭示出香蕉受Fusarium oxysporum f. sp. cubense侵染后细胞内SA含量显著升高, 并激活苯丙氨酸解氨酶(phenylalanine ammonia lyase, PAL)和过氧化物酶(peroxidase, POX)等相关防御因子的表达, 从而诱导寄主系统获得性抗性(system acquired resistance, SAR)反应的发生。在植物细胞中MeSA是SA稳定的的无活性衍生物, 是植物体内SA主要的运输形式, 可经植物韧皮部从病原菌感染位点运送到效应部位。植物遭受病原菌侵袭后应激产生一些SA信号分子, 在SA甲基转移酶作用下生成MeSA, MeSA对SA水解酶不敏感(SA易受SA水解酶降解), 故可作为可移动的SAR信号, 从病原菌感染的局部位点运送到植物远端组织, 以激发起全植株水平的抵御病原菌的抗性[4-5,10]。因此, MES对于调控植物细胞内SA水平和建立植物抵御病原物侵染的抗性反应, 均是必不可少的调控因子。比较基因组学的研究为揭示普通菜豆基因结构和功能提供了捷径[28]。种间的比较基因组学主要揭示物种间在基因组结构上的差异, 解析基因功能, 探索物种进化关系, 将模式植物基因组的相关信息转移到未知植物基因组中, 精确定位和克隆基因。因此, 通过分析普通菜豆与烟草等模式植物部分或全部基因序列的保守程度, 就可以利用普通菜豆与烟草基因组之间编码顺序上和结构上的同源性, 分析序列信息, 从而获得普通菜豆基因组中的相关序列, 进而揭示普通菜豆基因的潜在功能, 阐明物种进化关系及基因组的内在结构。众所周知, 植物细胞内存在一个SA参与的复杂信号调节网络, 而MES基因作为植物内源SA重要的调控节点蛋白, 目前仅有少数几个基因通过功能验证实验被证明参与了植物的抗病反应。烟草SABP2基因在寄主诱导产生系统获得性抗性过程中发挥重要作用, SABP2基因编码蛋白具有MeSA水解活性, 能够水解MeSA生成SA, 从而激活寄主内SA介导的抗病相关反应[11,12,13,14,15]。拟南芥中AtMES基因家族成员是SABP2基因同源基因, 其中AtMES-1、AtMES-2、 AtMES-7和AtMES-9是激活寄主防御反应最关键的基因, AtMES家族基因表达量的升高显著提升了拟南芥的抗病性[16]。此外, 在致病疫霉Phytophthora infestans侵染马铃薯过程中, 抑制StMES1基因的表达显著降低了MeSA转化为SA的效率, 从而显著削弱了马铃薯晚疫病抗病性[17]。本研究利用生物信息学方法搜索到普通菜豆中7个烟草水杨酸结合蛋白2的同源基因PvMES1~PvMES7, 其中PvMES1、PvMES3、PvMES4、PvMES5和PvMES6的表达量受FOP-DM01菌株诱导均呈现不同程度的升高, 抗病品种的基因表达量显著高于感病品种, 并且抗病品种MES活性和游离SA含量也显著高于感病品种。试验结果表明, 在菜豆与病原菌的互作过程中, 抗病品种PvMES基因表达量迅速升高, MES活性显著提升, 从而水解产生游离SA, 激活了寄主内SA介导的相关防御反应。本研究结果有望在普通菜豆抗病分子育种和抗病机理的研究中取得重要进展, 关于PvMES基因家族各成员在菜豆与病原菌的互作中如何发挥抵抗病原菌侵染的功能, 我们将克隆PvMES基因家族各成员基因, 采用转基因技术和基因沉默技术获得PvMES基因过表达和基因沉默的转基因植株, 通过研究转基因植株抗病表型和生理生化指标的变化进一步验证PvMES基因家族各成员的生物学功能。
4 结论
搜索到普通菜豆中烟草水杨酸结合蛋白2的7个同源基因PvMES1~PvMES7, 其中PvMES1、PvMES3、PvMES4、PvMES5和PvMES6的表达量受 FOP-DM01菌株诱导呈不同程度的升高; MES活性及其水解产生的游离SA含量在接种后也相应显著提升, 但接种后抗病品种中MES活性和游离SA含量都显著高于感病品种, 游离SA含量升高激活寄主体内SA介导的相关防御反应, 这是抗病品种的发病级别和病情指数显著低于感病品种的基础。The authors have declared that no competing interests exist.
作者已声明无竞争性利益关系。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
| [1] | . |
| [2] | . |
| [3] | . |
| [4] | . For more than 200 years, the plant hormone salicylic acid (SA) has been studied for its medicinal use in humans. However, its extensive signaling role in plants, particularly in defense against pathogens, has only become evident during the past 20 years. This review surveys how SA in plants regulates both local disease resistance mechanisms, including host cell death and defense gene expression, and systemic acquired resistance (SAR). Genetic studies reveal an increasingly complex network of proteins required for SA-mediated defense signaling, and this process is amplified by several regulatory feedback loops. The interaction between the SA signaling pathway and those regulated by other plant hormones and/or defense signals is also discussed. |
| [5] | . Liu PP, von Dahl CC, Park SW, Klessig DF. |
| [6] | . |
| [7] | . |
| [8] | . |
| [9] | . |
| [10] | . In plants, the mobile signal for systemic acquired resistance (SAR), an organism-wide state of enhanced defense to subsequent infections, has been elusive. By stimulating immune responses in mosaic tobacco plants created by grafting different genetic backgrounds, we showed that the methyl salicylate (MeSA) esterase activity of salicylic acid--binding protein 2 (SABP2), which converts MeSA into salicylic acid (SA), is required for SAR signal perception in systemic tissue, the tissue that does not receive the primary (initial) infection. Moreover, in plants expressing mutant SABP2 with unregulated MeSA esterase activity in SAR signal--generating, primary infected leaves, SAR was compromised and the associated increase in MeSA levels was suppressed in primary infected leaves, their phloem exudates, and systemic leaves. SAR was also blocked when SA methyl transferase (which converts SA to MeSA) was silenced in primary infected leaves, and MeSA treatment of lower leaves induced SAR in upper untreated leaves. Therefore, we conclude that MeSA is a SAR signal in tobacco. |
| [11] | . |
| [12] | . |
| [13] | . We previously demonstrated that salicylic acid-binding protein 2 (SABP2) of tobacco is an integral component of systemic acquired resistance (SAR). SABP2 is a methyl salicylate (MeSA) esterase that has high affinity for SA, which feedback inhibits its esterase activity. MeSA esterase activity is required in distal, healthy tissue of pathogen-infected plants to hydrolyze MeSA, which functions as a long-distance, phloem-mobile SAR signal; this hydrolysis releases the biologically active defense hormone SA. In this study, we examined the inhibitory interaction of SA with SABP2, and identified a synthetic SA analog, 2,2,2,2'-tetra-f luoroacetophenone (tetraFA) that, like SA, competitively inhibits the activity of SABP2 and targets esterases, which utilize MeSA as a substrate. However, in contrast to SA, tetraFA does not induce downstream defense responses and, therefore, is effective in planta at blocking SAR development in tobacco mosaic virus (TMV)-infected tobacco and Pseudomonas syringae-infected Arabidopsis. These results confirm the importance of SABP2 and MeSA for SAR development in tobacco and establish similar roles for MeSA and the orthologs of SABP2 in Arabidopsis. Moreover, they demonstrate that tetraFA can be used to determine whether MeSA and its corresponding esterase(s) play a role in SAR signaling in other plant species. In planta analyses using tetraFA, in conjunction with leaf detachment assays and MeSA quantification, were used to assess the kinetics with which MeSA is generated in pathogen-infected leaves, transmitted through the phloem, and processed in the distal healthy leaves. In TMV-infected tobacco, these studies revealed that critical amounts of MeSA are generated, transmitted, and processed between 48 and 72 h post primary infection. |
| [14] | . ABSTRACT Salicylic acid (SA) is an important signal in various plant processes. It is well known and widely studied for its role in plant disease resistance. Several proteins, which physically interact with SA has been identified and characterized for their possible role in disease resistance signaling. These plant proteins bind to SA with varying affinity and they differ considerably in their structure and activity. The protein, which binds to SA with highest affinity amongst all the characterized SA-binding proteins, is SABP2. It is a 29-kDa protein and has esterase like enzymatic activity. It is able to use plant synthesized methyl salicylate as a substrate and convert it into SA, which triggers disease resistance in plants. Silencing of SABP2 makes plants more susceptible to pathogens and their capacity to induce SAR is severely compromised. The esterase activity of SABP2 is required to process the phloem mobile signal, MeSA in distal uninoculated tissues to induce resistance. The binding of SA to SABP2 is important for activation of SAR in distal tissues. |
| [15] | . Nonhost resistance is a type of broad-spectrum resistance exhibited by a given plant species to most strains of a pathogen which are generally pathogenic to other plant species. In this study, we have examined the role of tobacco SABP2 (Salicylic acid-Binding Protein 2) in nonhost resistance. SABP2, a methyl salicylate esterase is a critical component of SA-signaling pathway in tobacco plants. The transgenic tobacco SABP2-silenced lines treated with tetraFA, a known inhibitor of esterase activity of SABP2 exhibited enhanced susceptibility to nonhost pathogen, Pseudomonas syringae pv. phaseolicola compared to the control plants. The increased accumulation of SABP2 transcripts upon Psp infection supports the involvement of SABP2 in nonhost resistance. The tetra-FA treated plants also showed delayed expression of pathogenesis related-1 gene upon Psp inoculations. The expression of nonhost marker genes CDM1 and HIN1 was also monitored in tobacco plants infected with host-pathogen P.s. pv. tabaci and P.s. pv. phaseolicola. Overall, results presented in this manuscript suggest that SABP2 has a role in nonhost resistance in tobacco plants. |
| [16] | . Summary Salicylic acid-binding protein 2 (SABP2) is essential for the establishment of systemic acquired resistance (SAR) in tobacco; SABP2’s methyl salicylate (MeSA) esterase activity is required in healthy systemic tissues of infected plants to release the active defense phytohormone SA from MeSA, which serves as a long-distance signal for SAR. In the current study, we characterize a new gene family from Arabidopsis thaliana encoding 18 potentially active α/β fold hydrolases that share 32–57% identity with SABP2. Of 14 recombinant AtMES (MES for methyl esterase) proteins tested, five showed preference for MeSA as a substrate and displayed SA inhibition of MeSA esterase activity in vitro ( AtMES1 , - 2 , - 4 , - 7 , and - 9 ). The two genes encoding MeSA esterases with the greatest activity, AtMES1 and - 9 , as well as AtMES7 were transcriptionally upregulated during infection of Arabidopsis with avirulent Pseudomonas syringae . In addition, conditional expression of AtMES1 , - 7 , or - 9 complemented SAR deficiency in SABP2 -silenced tobacco, suggesting that these three members of the AtMES family are SABP2 functional homologs (orthologs). Underexpression by knockout mutation and/or RNAi-mediated silencing of multiple AtMES genes, including AtMES1 , - 2 , - 7 , and - 9 , compromised SAR in Arabidopsis and correlated with enhanced accumulation of MeSA in the systemic tissue of SAR-induced plants. Together, the data show that several members of the AtMES gene family are functionally homologous to SABP2 and redundant for MeSA hydrolysis and probably SAR. These data suggest that MeSA is a conserved SAR signal in Arabidopsis and tobacco. |
| [17] | . Whether salicylic acid (SA) plays a role in systemic acquired resistance (SAR) signaling in potato is currently unclear because potato, unlike tobacco and Arabidopsis, contains highly elevated levels of endogenous SA. Recent studies have indicated that the SA derivative methyl salicylate (MeSA) serves as a long-distance phloem-mobile SAR signal in tobacco and Arabidopsis. Once in the distal, uninfected tissue of these plant species, MeSA must be converted into biologically active SA by the esterase activity of SA-binding protein 2 (SABP2) in tobacco or members of the AtMES family in Arabidopsis. In this study, we have identified the potato ortholog of tobacco SABP2 (StMES1) and shown that the recombinant protein converts MeSA to SA; this MeSA esterase activity is feedback inhibited by SA or its synthetic analog, 2, 2, 2, 2'-tetra-fluoroacetophenone (tetraFA). Potato plants (cv. D sir e) in which StMES1 activity was suppressed, due to either tetraFA treatment or silencing of StMES1 expression, were compromised for arachidonic acid (AA)-induced SAR development against Phytophthora infestans. Presumably due to the inability of these plants to convert MeSA to SA, the SAR-defective phenotype correlated with elevated levels of MeSA and reduced expression of pathogenesis-related (PR) genes in the untreated distal tissue. Together, these results strongly suggest that SAR signaling in potato requires StMES1, its corresponding MeSA esterase activity, and MeSA. Furthermore, the similarities between SAR signaling in potato, tobacco, and Arabidopsis suggest that at least certain SAR signaling components are conserved among plants, regardless of endogenous SA levels. |
| [18] | . 钙调素蛋白(calmodulin,CaM)作为植物细胞内介导多种功能的Ca^2+结合蛋白,在调节植物的生长发育和抗病性方面具有重要作用。利用普通菜豆(PhaseolusvulgarisL.)表达序列标签(EST)克隆了含有编码普通菜豆CaM基因的cDNA序列。序列分析表明,cDNA片段长713bp,命名为PvCaM1,具有一个453bp的开放阅读框(ORF),GenBank登录号为JN418801,该基因编码150个氨基酸,预测蛋白质分子质量为17.16kD。蛋白质结构分析表明,PvCaM1蛋白含有4个Ca^2+结合结构域(EF-hand)。同源分析结果显示,PvCaM1基因与百脉根、西瓜的CaM基因亲缘关系最近,分别达到77%和76%。荧光定量PCR分析表明,PvCaM1基因受尖镰孢菌菜豆专化型FOP-DM01菌株诱导表达,接种病原菌96h,抗病品种260205根中PvCaM1基因的表达量达到最高,而感病品种BRB-130达到最低,260205叶中PvCaM1基因的表达量均高于BRB-130,而且叶中的表达量高于根和茎中的表达量。PvCaM1基因表达量也受外源植物激素脱落酸、茉莉酸甲酯和乙烯利诱导上调,在根、茎、叶中均有不同程度的表达。本研究表明,PvCaM1基因可能通过脱落酸、茉莉酸和乙烯等信号途径参与菜豆对FOP-DM01菌株的防御反应,推测菜豆PvCaM1基因与镰孢菌枯萎病的抗病性有一定关联。 . 钙调素蛋白(calmodulin,CaM)作为植物细胞内介导多种功能的Ca^2+结合蛋白,在调节植物的生长发育和抗病性方面具有重要作用。利用普通菜豆(PhaseolusvulgarisL.)表达序列标签(EST)克隆了含有编码普通菜豆CaM基因的cDNA序列。序列分析表明,cDNA片段长713bp,命名为PvCaM1,具有一个453bp的开放阅读框(ORF),GenBank登录号为JN418801,该基因编码150个氨基酸,预测蛋白质分子质量为17.16kD。蛋白质结构分析表明,PvCaM1蛋白含有4个Ca^2+结合结构域(EF-hand)。同源分析结果显示,PvCaM1基因与百脉根、西瓜的CaM基因亲缘关系最近,分别达到77%和76%。荧光定量PCR分析表明,PvCaM1基因受尖镰孢菌菜豆专化型FOP-DM01菌株诱导表达,接种病原菌96h,抗病品种260205根中PvCaM1基因的表达量达到最高,而感病品种BRB-130达到最低,260205叶中PvCaM1基因的表达量均高于BRB-130,而且叶中的表达量高于根和茎中的表达量。PvCaM1基因表达量也受外源植物激素脱落酸、茉莉酸甲酯和乙烯利诱导上调,在根、茎、叶中均有不同程度的表达。本研究表明,PvCaM1基因可能通过脱落酸、茉莉酸和乙烯等信号途径参与菜豆对FOP-DM01菌株的防御反应,推测菜豆PvCaM1基因与镰孢菌枯萎病的抗病性有一定关联。 |
| [19] | . |
| [20] | |
| [21] | . |
| [22] | . SA has been shown to play an important signaling role in the activation of various plant defense responses following pathogen attack. These responses include the induction of local and systemic disease resistance, the potentiation of host cell death, and the containment of pathogen spread. The mechanisms through which SA mediates these effects are varied and can involve alterations in the activity or synthesis of certain enzymes, increased defense gene expression, potentiation of several defense responses, and/or the generation of free radicals. Through the analysis of mutant plants exhibiting aberrant responses to pathogen infection, many genes encoding products involved in the SA-mediated defense pathway(s) have been isolated. In addition, mounting evidence suggests that certain defense responses can be activated via a SA-independent pathway(s). This review focuses primarily on recent discoveries pertaining to the SA signaling pathway(s) leading to disease resistance; however, a very brief discussion of the SA-independent pathway (s) and its ability to cross-talk with the SA pathway is also presented. |
| [23] | . |
| [24] | . Plant peroxidases (POXs) are one of the most important redox enzymes in the defense responses. However, the large number of different plant POX genes makes it necessary to carefully confirm the function of each paralogous POX gene in specific tissues and disease interactions. Fusarium wilt is a devastating disease of common bean caused by Fusarium oxysporum f. sp. phaseoli . In this study, we evaluated a peroxidase gene, PvPOX1 , from a resistant common bean genotype, CAAS260205 and provided direct evidence for PvPOX1 role in resistance by transforming the resistant allele into a susceptible common bean genotype, BRB130, via hairy root transformation using Agrobacterium rhizogenes . Analysis of PvPOX1 gene over-expressing hairy roots showed it increased resistance to Fusarium wilt both in the roots and the rest of transgenic plants. Meanwhile, the PvPOX1 expressive level, the peroxidase activity and hydrogen peroxide (H 2 O 2 ) accumulation were also enhanced in the interaction. The result showed that the PvPOX1 gene played an essential role in Fusarium wilt resistance through the occurrence of reactive oxygen species (ROS) induced hypersensitive response. Therefore, PvPOX1 expression was proven to be a valuable gene for further analysis which can strengthen host defense response against Fusarium wilt through a ROS activated resistance mechanism. |
| [25] | . ABSTRACT Alternaria solani is the causal agent of early blight disease in tomato and is responsible for significant economic losses sustained by tomato producers each year. Because salicylic acid (SA) is an important signal molecule that plays a critical role in plant defense against pathogen invasion, we investigated if the exogenous application of SA would activate systemic acquired resistance (SAR) against A. solani in tomato leaves. The addition of 200 muM SA to the root system significantly increased the endogenous SA content of leaves. Free SA levels increased 65-fold over basal levels to 5.85 mug g(-1) fresh weight (FW) after 48 h. This level of SA had no visible phytotoxic effects. Total SA content (free SA + SA-glucose conjugate) increased to 108 mug g(-1) FW after 48 h. Concomitant with elevated SA levels, expression of the tomato pathogenesis-related (PR)-1B gene was strongly induced within 24 h of the addition of 200 muM SA. PR-1B expression was still evident after 48 h; however, PR-1B induction was not observed in plants not receiving SA treatment. Challenge inoculation of SA-treated tomato plants using conidia of A. solani resulted in 83% fewer lesions per leaf and a 77% reduction in blighted leaf area as compared with control plants not receiving SA. Our data indicate that root feeding 200 muM SA to tomato plants can (i) significantly elevate foliar SA levels, (ii) induce PR-1B gene expression, and (iii) activate SAR that is effective against A. solani. |
| [26] | . |
| [27] | . Fusarium wilt of banana is caused by the soil-borne fungus Fusarium oxysporum f. sp. cubense (Foc). The fact that there are no economically viable biological, chemical, or cultural measures of controlling the disease in an infected field leads to search for alternative strategies involving activation of the plant's innate defense system. The mechanisms underlying systemic acquired resistance (SAR) are much less understood in monocots than in dicots. Since systemic protection of plants by attenuated or avirulent pathogens is a typical SAR response, the establishment of a biologically induced SAR model in banana is helpful to investigate the mechanism of SAR to Fusarium wilt. This paper described one such model using incompatible Foc race 1 to induce resistance against Foc tropical race 4 in an in vitro pathosystem. Consistent with the observation that the SAR provided the highest level of protection when the time interval between primary infection and challenge inoculation was 10d, the activities of defense-related enzymes such as phenylalanine ammonia lyase (PAL, EC 4.3.1.5), peroxidase (POD, EC 1.11.1.7), polyphenol oxidase (PPO, EC 1.14.18.1), and superoxide dismutase (SOD, EC 1.15.1.1) in systemic tissues also reached the maximum level and were 2.00 2.43 times higher than that of the corresponding controls on the tenth day. The total salicylic acid (SA) content in roots of banana plantlets increased from about 1 to more than 5 gg 1 FW after the second leaf being inoculated with Foc race 1. The systemic up-regulation of MaNPR1A and MaNPR1B was followed by the second up-regulation of PR-1 and PR-3. Although SA and jasmonic acid (JA)/ethylene (ET) signaling are mostly antagonistic, systemic expression of PR genes regulated by different signaling pathways were simultaneously up-regulated after primary infection, indicating that both pathways are involved in the activation of the SAR. |
| [28] | . 普通菜豆是重要的食用豆类之一,在世界各地普遍种植。近年来,普通菜豆在遗传图谱构建、新标 记开发与利用、抗性基因定位以及比较基因组学等方面取得了很大进展。遗传连锁图谱的构建是基因定位与克隆的基础,是遗传研究的重要内容;利用分子连锁图谱 鉴定、标记和定位抗病基因将在种质改良和分子标记辅助育种方面发挥重要作用。豆科植物比较基因组学的研究成果为菜豆遗传连锁图谱的发展提供了新的思路。本 研究从普通菜豆遗传连锁图谱的获得、普通菜豆与大豆同线性比较以及抗炭疽病基因定位等方面进行了综述,以期为普通菜豆遗传改良和抗病育种提供参考。 . 普通菜豆是重要的食用豆类之一,在世界各地普遍种植。近年来,普通菜豆在遗传图谱构建、新标 记开发与利用、抗性基因定位以及比较基因组学等方面取得了很大进展。遗传连锁图谱的构建是基因定位与克隆的基础,是遗传研究的重要内容;利用分子连锁图谱 鉴定、标记和定位抗病基因将在种质改良和分子标记辅助育种方面发挥重要作用。豆科植物比较基因组学的研究成果为菜豆遗传连锁图谱的发展提供了新的思路。本 研究从普通菜豆遗传连锁图谱的获得、普通菜豆与大豆同线性比较以及抗炭疽病基因定位等方面进行了综述,以期为普通菜豆遗传改良和抗病育种提供参考。 |
