 ,1, 吴洁1, 王欣1, 白冬梅2, 胡美玲1, 晏立英1, 陈玉宁1, 康彦平1, 王志慧1, 淮东欣
,1, 吴洁1, 王欣1, 白冬梅2, 胡美玲1, 晏立英1, 陈玉宁1, 康彦平1, 王志慧1, 淮东欣 ,1,*, 雷永1, 廖伯寿
,1,*, 雷永1, 廖伯寿 ,1,*
,1,*Effects of cold stress on germination in peanut cultivars with normal and high content of oleic acid
XUE Xiao-Meng ,1, WU JIE1, WANG Xin1, BAI Dong-Mei2, HU Mei-Ling1, YAN Li-Ying1, CHEN Yu-Ning1, KANG Yan-Ping1, WANG Zhi-Hui1, HUAI Dong-Xin
,1, WU JIE1, WANG Xin1, BAI Dong-Mei2, HU Mei-Ling1, YAN Li-Ying1, CHEN Yu-Ning1, KANG Yan-Ping1, WANG Zhi-Hui1, HUAI Dong-Xin ,1,*, LEI Yong1, LIAO Bo-Shou
,1,*, LEI Yong1, LIAO Bo-Shou ,1,*
,1,*通讯作者: * 淮东欣, E-mail:dxhuai@caas.cn;廖伯寿, E-mail:lboshou@hotmail.com
收稿日期:2020-07-26接受日期:2021-01-21网络出版日期:2021-02-23
| 基金资助: |
Corresponding authors: * E-mail:dxhuai@caas.cn;E-mail:lboshou@hotmail.com
Received:2020-07-26Accepted:2021-01-21Online:2021-02-23
| Fund supported: |
作者简介 About authors
E-mail:xiaomengxue1991@163.com

摘要
高油酸花生以其营养价值高和耐储藏等特点深受广大消费者和加工企业的喜爱。近年来, 随着高油酸花生品种在我国的推广应用, 高油酸花生在高海拔、高纬度地区的发芽期耐寒性成为关注热点。为探究花生种子的油酸含量与其萌发期耐寒性的相关性, 本研究调查6组不同遗传背景的花生品种及其高油酸回交后代品系(BC4F8)在低温条件下的发芽率和发芽指数发现, 在低温胁迫下花生的萌发期耐寒性与其油酸含量无显著相关性。在泉花551高油酸后代品系(Quanhua 551-HO)低温发芽率显著低于其普通油酸含量亲本(Quanhua 551-NO)的组合中, 追踪分析8种主要脂肪酸在低温胁迫萌发过程中含量的变化发现, 在低温胁迫下Quanhua 551-NO中油酸含量显著减少且亚油酸含量显著增加, 而Quanhua 551-HO中也表现出了油酸含量减少、亚油酸含量增加的趋势, 但是未达到显著水平。进而分析上述2种材料中低温胁迫下各脂肪酸脱氢酶(fatty acid desaturase 2, FAD2)基因的表达模式发现, Quanhua 551-NO中AhFAD2-1A/B受低温诱导显著上调表达, 而AhFAD2-4A/B显著下调表达; 在Quanhua 551-HO中AhFAD2-4A/B受低温诱导持续显著上调表达, 而AhFAD2-1A/B显著下调表达, 推测由于高油酸花生中AhFAD2-1A/B编码蛋白失活, AhFAD2-4A/B在低温诱导下高量表达, 部分弥补了AhFAD2-1A/B缺失的功能。综上所述, 花生种子中油酸含量并不是决定其萌发期耐寒性的关键因素。
关键词:
Abstract
High oleate (HO) peanut is highly popular for its improved nutrient value and strengthened storage stability among customers and peanut processing enterprises. In recent years, with the adoption of HO peanut in our country, the cold tolerance of peanut at germination stage in high altitude or high latitude area has become a major concern. To figure out the relationship between the seed oleic acid content and the cold tolerance at germination stage in peanut, the germination rate and germination index of seeds under cold stress were investigated among six peanut cultivars with normal content of oleic acid (NO) and their backcross-derived HO lines, respectively. The results showed that the oleic acid content was not significantly correlated with the cold tolerance at germination stage. The contents of eight main fatty acids under cold stress at germination stage were tracked in Quanhua 551 (Quanhua 551-NO) and its HO offspring line (Quanhua 551-HO), and the germination rate of Quanhua 551-HO was significantly lower than that of Quanhua 551-NO under cold stress. The oleic acid content of Quanhua 551-NO was significantly decreased while the linoleic acid content was significantly increased under cold stress. However, the contents of oleic acid and linoleic acid exhibited the same trend in Quanhua 551-HO, and there was no significant difference. The expression profiles offatty acid desaturase 2 (AhFAD2) genes in both Quanhua 551-NO and Quanhua 551-HO under cold stress revealed that the relative expression level of AhFAD2-1A/B was significantly up-regulated, while that of AhFAD2-4A/B was significantly down-regulated under cold stress in Quanhua 551-NO. Conversely, the relative expression level of AhFAD2-1A/B was significantly decreased, but the relative expression level of AhFAD2-4A/B was significantly increased under cold stress in Quanhua 551-HO. These results implied that the up-regulation of AhFAD2-4A/Bin HO peanut may partly compensate for lost function of AhFAD2-1A/Bin response to cold stress. In conclusion, the oleic acid content in seed was not the main factor to determinate the cold tolerance at germination stage.
Keywords:
PDF (1898KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
薛晓梦, 吴洁, 王欣, 白冬梅, 胡美玲, 晏立英, 陈玉宁, 康彦平, 王志慧, 淮东欣, 雷永, 廖伯寿. 低温胁迫对普通和高油酸花生种子萌发的影响. 作物学报[J], 2021, 47(9): 1768-1778 DOI:10.3724/SP.J.1006.2021.04170
XUE Xiao-Meng, WU JIE, WANG Xin, BAI Dong-Mei, HU Mei-Ling, YAN Li-Ying, CHEN Yu-Ning, KANG Yan-Ping, WANG Zhi-Hui, HUAI Dong-Xin, LEI Yong, LIAO Bo-Shou.
花生(Arachis hypogaea L.)是全世界范围广泛种植的油料作物和经济作物之一。花生是我国重要的食用植物油和蛋白质来源, 2018年我国花生总产量和产油量分别达到了1733万吨和294万吨[1]。近年来, 高油酸花生以其营养价值高、稳定性强、货架期长等优势, 越来越受到消费者和加工企业的喜爱[2,3]。高油酸花生是指油酸含量超过73%的花生品种[4], 与普通花生品种(油酸含量约35%~65%)相比, 高油酸花生中油酸含量显著提高。油酸(C18:1)是一种单不饱和脂肪酸, 能选择性降低人体血液中有害的低密度胆固醇, 而保持有益的高密度胆固醇, 有助于预防心脑血管疾病[5,6]。高油酸花生中亚油酸(C18:2)含量显著降低(约2%), 亚油酸具有2个双键, 在加工和储存过程中易被氧化变质, 其含量的大幅降低提升了高油酸花生的加工和储藏品质。因此, 高油酸花生已逐渐成为我国当前的主推品种。
低温是影响农作物生长和产量的重要非生物胁迫因素之一。花生是一种喜温作物, 整个生育期都需要充足的光照和适宜的温度, 花生种子萌发所需的最低温度为12~15℃[7,8]。随着高油酸花生品种的种植面积不断扩大, 高油酸花生品种对低温敏感, 低温耐受能力较差, 尤其在播种发芽期, 易出现出苗不齐、冷害死苗等现象, 低温成为了限制高油酸花生在我国北部以及一些高纬度、高海拔地区推广和种植的重要影响因素[9,10,11]。花生高油酸性状是由2对Δ12脂肪酸脱氢酶(fatty acid desaturase 2, FAD2)基因(AhFAD2-1A和AhFAD2-1B)发生隐性突变导致的[12]。已有研究报道, FAD2基因功能与植物的耐寒性密切相关[13,14,15], 但是, 高油酸花生的耐寒性是否有影响尚不明确。薛晓梦等[16]比较普通油酸花生中花16和高油酸花生中花413在15℃下的发芽率发现, 中花413的发芽率未显著下降, 而中花16的发芽率显著降低。花生基因组中共鉴定到7个AhFAD2基因, 不同AhFAD2基因在花生不同发育阶段和不同组织中特异表达, 行使其功能[17]。特别是AhFAD2-1A/B和AhFAD2-4A/B都受到低温胁迫诱导表达, 虽然在高油酸花生中AhFAD2-1A/B编码蛋白失活, 但AhFAD2-4A/B的高量表达在一定程度上可以弥补这部分功能[16]。但是, 由于上述研究中使用材料较少, 不具有普遍性, 且高油酸花生和普通油酸花生间的遗传背景相差较大, AhFAD2-1A/B基因突变对花生耐寒性的影响有待进一步研究。
为明确花生中AhFAD2-1A/B基因突变对萌发期耐寒性的影响, 本研究利用6组不同遗传背景的普通油酸花生品种及其高油酸回交后代BC4F8品系为试验材料, 系统调查了其在低温胁迫下的发芽情况, 并分析了泉花551及其高油酸后代品系中8种主要脂肪酸在低温发芽过程中的含量变化以及AhFAD2家族基因的表达模式, 进一步探讨花生油酸含量与花生耐寒性的相关性, 以期为解析高油酸花生耐寒机制以及高油酸花生在高海拔、高纬度地区的推广提供理论和技术支持。
1 材料与方法
1.1 试验材料
供试材料为6组普通油酸(normal oleate, NO)花生品种及其高油酸(high oleate, HO)回交后代(BC4F8)品系(表1)。上述材料种植于中国农业科学院油料作物研究所试验基地(湖北阳逻), 2019年5月初播种, 8月底收获。发芽试验在中国油料作物研究所温度梯度恒温箱(MTI-201, Tokyo Rikakikai, Co. Ltd)内完成。Table 1
表1
表1供试材料及其油酸含量
Table 1
| 品种 Cultivar | 油酸含量 Oleic acid content (%) | 品种 Cultivars | 油酸含量 Oleic acid content (%) | ||
|---|---|---|---|---|---|
| 徐花13号 Xuhua 13 | 普通油酸NO | 45.58 | 中花21 Zhonghua 21 | 普通油酸NO | 38.40 |
| 高油酸HO | 81.14 | 高油酸HO | 80.83 | ||
| 徐花9号 Xuhua 9 | 普通油酸NO | 49.73 | 泉花551 Quanhua 551 | 普通油酸NO | 50.72 |
| 高油酸HO | 81.60 | 高油酸HO | 80.62 | ||
| 中花16 Zhonghua 16 | 普通油酸NO | 50.56 | 漯花9号 Luohua 9 | 普通油酸NO | 47.90 |
| 高油酸HO | 80.70 | 高油酸HO | 80.50 | ||
新窗口打开|下载CSV
1.2 试验方法
1.2.1 种子发芽情况调查 试验设置15℃和25℃两个温度梯度。每份材料选取籽粒饱满、种胚完整、无霉变的花生种子120粒, 用多菌灵(80% WP可湿性粉剂, 天津市农丰农业科技有限公司)和卫福(总有效成分含量: 400 g L-1, 爱利思达生物化学品有限公司)的1000倍稀释液浸泡花生5 min, 75%的酒精进行表面消毒3 min, 再用去灭菌离子水冲洗3~4次后, 置于培养皿中, 每皿20粒, 分别置于15℃和25℃培养箱中暗培养, 培养3 d后, 将15℃培养箱中种子移至25℃培养箱, 继续培养3 d。以上试验重复3次, 每天统计发芽种子数量, 并分别计算发芽率和发芽指数。发芽率(%) = (发芽种子数/供试花生种子数)×100;
发芽指数(GI) = ∑ Gt/Dt
式中, Gt表示第t天的种子发芽率, Dt表示相对应的种子发芽天数[18,19]。
1.2.2 种子发芽过程中脂肪酸含量测定 利用内标法[20]定量测定花生种子在发芽过程中各脂肪酸含量, 内标物为混合脂肪酸甲酯标准品和十五烷酸甲酯标准品(上海安谱实验科技股份有限公司), 配成10 mg mL-1的十五烷酸甲酯/甲醇溶液, 分别取普通油酸(Quanhua 551-NO)和高油酸回交品系(Quanhua 551-HO)在不同处理条件下发芽第1、3和6天的种子各5粒, 液氮研磨, 放置冷冻干燥机(CHRIST Alpha 2-4 LD plus, 德国CHRIST公司)中冷冻干燥, 称取50 mg花生粉末至干净的玻璃试管中, 设置3个重复, 加入3 mL 2.5%硫酸/甲醇溶液, 再加入300 μL十五烷酸甲酯/甲醇溶液(10 mg mL -1)和400 μL甲苯溶液, 密封紧密, 放置95℃水浴锅中水浴40 min, 取出玻璃试管, 冷却至室温后加入1 mL ddH 2O和1 mL正己烷混匀, 2000 × g离心10 min, 取1 mL上清液装入进样瓶, 使用Agilent7890B气相色谱仪(美国Agilent公司)进行气相色谱分析。色谱柱为DB-23毛细管柱(30 m × 0.25 mm × 0.25 μm, 美国Agilent公司), 检测器为氢火焰离子化检测器(FID), 进样口温度为280℃, 进样口压力为1.7×10 5 Pa, 进样量为1.0 μL, 分流比为1:40。温度程序为180℃保持1 min, 再以10℃ min -1升至220℃, 保持9 min。对测试花生样品的色谱图进行解析, 通过内标法公式[19]计算花生中8种主要脂肪酸(棕榈酸、硬脂酸、油酸、亚油酸、花生酸、花生烯酸、山嵛酸和木蜡酸)的含量, 公式如下:
${{X}_{\text{FAM}{{\text{E}}_{i}}}}={{F}_{i}}\frac{C{{c}_{15}}\times V{{c}_{15}}}{m}\times 100%$
${{F}_{i}}=\frac{C{{s}_{i}}\times A{{c}_{15}}}{A{{s}_{i}}\times C{{c}_{15}}}$
${{F}_{\text{F}{{\text{A}}_{i}}}}={{X}_{\text{FAM}{{\text{E}}_{i}}}}\times \frac{{{M}_{F{{A}_{i}}}}}{{{M}_{\text{FAM}{{\text{E}}_{i}}}}}$
式中, ${{F}_{\text{F}{{\text{A}}_{i}}}}$为脂肪酸i含量; ${{M}_{\text{F}{{\text{A}}_{i}}}}$为脂肪酸i的分子质量; ${{M}_{\text{FAM}{{\text{E}}_{i}}}}$为脂肪酸甲酯i的分子质量;${{X}_{\text{FAM}{{\text{E}}_{i}}}}$为样品中脂肪酸甲酯i含量;${{F}_{i}}$为脂肪酸甲酯i的响应因子; $C{{c}_{15}}$为十五烷酸甲酯i的浓度(mg mL-1); $V{{c}_{15}}$为试样中加入十五烷酸甲酯的体积; m为试样的质量(mg); $C{{s}_{i}}$为混标中各脂肪酸甲酯i的浓度(mg mL-1); $A{{c}_{15}}$为十五烷酸甲酯峰面积; $A{{s}_{i}}$为脂肪酸甲酯i的峰面积; $C{{c}_{15}}$为混标中十五烷酸甲酯浓度(mg mL-1)。
1.2.3 AhFAD2家族基因的表达模式分析 利用在线工具Integrated DNA Technologies (
Table 2
表2
表2Real time PCR引物及其碱基序列
Table 2
| 基因 Gene | 引物名称 Primer name | 引物序列 Primer sequence (5°-3°) |
|---|---|---|
| AhFAD2-1A, AhFAD2-1B | RTAhFAD2-1-F | ATCTGCTATATCACATAGCAACTCT |
| RTAhFAD2-1-R | ACTGTTGCCAATGCTCCTCT | |
| AhFAD2-3A, AhFAD2-3B | RTAhFAD2-3-F | GGTCTTATCCGTCTTGTCATGG |
| RTAhFAD2-3-R | AGATGAATCGTAATGTGGCAATG | |
| AhFAD2-4A, AhFAD2-4B | RTAhFAD2-4-F | TCATTCTGCCGGGAAGAGG |
| RTAhFAD2-4-R | ATGGCGACATAGGCGAAAAT | |
| AhActin | Actin-F | TAAGAACAATGTTGCCATACAGA |
| Actin-R | GTTGCCTTGGATTATGAGC |
新窗口打开|下载CSV
2 结果与分析
2.1 低温胁迫对普通油酸和高油酸花生发芽率和发芽指数的影响
分别调查了6组遗传背景各异的花生品种徐花13号、徐花9号、中花16、中花21、泉花551和漯花9号及其高油酸回交后代品系在常温处理(25℃培养6 d)和低温处理(15℃培养3 d, 再恢复到25℃培养3 d)的发芽率和发芽指数(图1)。在常温处理条件下, 所有材料的发芽率在前3 d增速最快; 而在低温处理条件下, 所有材料的发芽率在15℃条件下均增长缓慢, 当温度恢复至25℃后, 在第3~4天发芽率迅速提高, 表明低温处理延缓了种子的萌发速率。图1
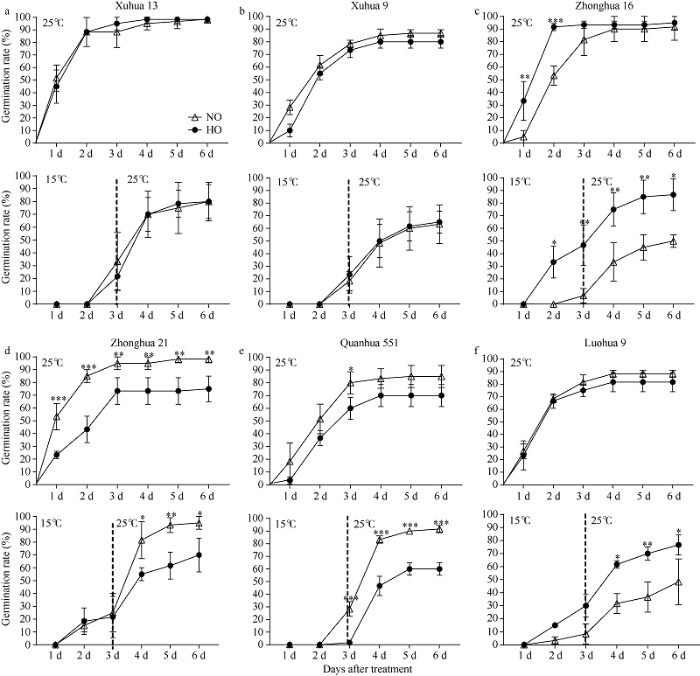 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1低温胁迫对不同品种花生的高油酸和普通油酸种子发芽率影响
NO: 普通油酸花生; HO: 高油酸花生。*、**和***分别表示在0.05、0.01和0.001水平显著差异。
Fig. 1Effects of cold stress on germination rate and its corresponding normal oleate seeds in high oleate seeds
NO: normal oleate peanut; HO: high oleate peanut. *, **, and *** mean significant difference at the 0.05, 0.01, and 0.001 probability levels, respectively.
在常温处理条件下, 徐花13号、徐花9号、中花16、泉花551和漯花9号组合中普通油酸花生和高油酸花生在第6天的发芽率无显著差异; 而中花21组合中普通油酸花生种子发芽率显著高于高油酸花生。在低温处理条件下, 徐花13号和徐花9号组合中普通油酸花生和高油酸花生的第6天发芽率无显著差异; 中花16和漯花9号组合中高油酸花生种子的第6天发芽率显著高于其对应的普通油酸花生; 中花21和泉花551的普通油酸花生种子的第6天发芽率显著高于其对应的高油酸花生。由此可见, 徐花13号和徐花9号的普通油酸花生和高油酸花生萌发期耐寒性差异不显著, 而中花16和漯花9号的高油酸花生萌发期耐寒性优于其普通油酸花生, 仅泉花551的高油酸花生耐寒性显著低于其普通油酸花生。利用各材料种子中油酸含量与其低温处理6 d后的发芽率做相关性分析表明, 油酸含量与其低温处理发芽率无显著相关性( r = -0.031, P = 0.924)。
低温胁迫对花生的发芽指数影响较大, 通过比较各组高油酸花生和普通油酸花生在常温和低温处理条件下的发芽指数发现, 除Zhonghua 21-HO以外, 其余材料在低温处理条件下发芽指数均显著低于其在常温条件下的发芽指数。在常温条件下, 徐花9号和泉花551的高油酸花生发芽指数显著低于其对应的普通油酸花生的发芽指数; 而在低温处理条件下, 各组中高油酸花生发芽指数与普通油酸花生的发芽指数无显著差异(图2)。低温胁迫显著降低了高油酸花生和普通油酸的发芽指数, 但是在低温胁迫条件下高油酸花生发芽指数并没有显著低于其普通油酸花生的发芽指数, 表明花生的油酸含量与其在低温胁迫下的发芽指数无显著相关性(r= 0.072, P= 0.823)。
图2
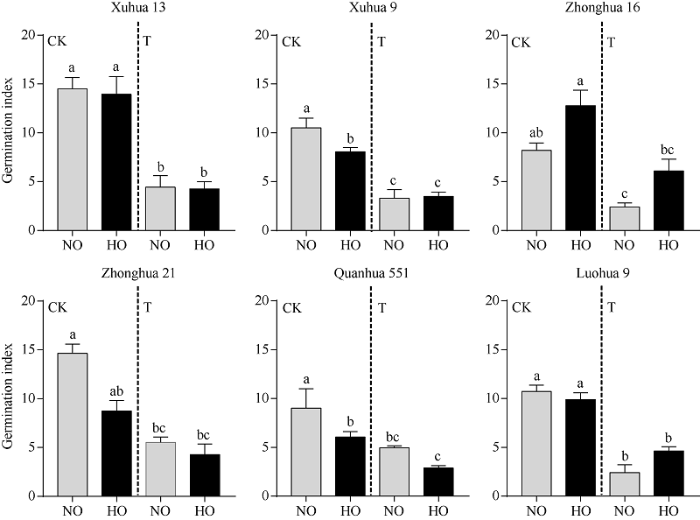 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2低温胁迫下不同品种花生高油酸和普通油酸种子的发芽指数
CK: 种子在25℃条件下萌发6 d; T: 种子第1~3天在15℃条件下萌发, 第4~6天在25℃条件下萌发; NO: 普通油酸花生; HO: 高油酸花生。经单因素方差分析差异达到显著水平, 标以不同小写字母的柱值表示经单因素方差分析和最小显著差异法(LSD)检验差异达到显著水平(P < 0.05)。
Fig. 2Germination index of high oleate seeds and normal oleate seeds in different peanut varieties under cold stress
CK: control group, germination of seed at 25℃ for six days; T: treatment group, germination of seed at 15℃ for 1-3 days, and then 25℃ for 4-6 days; NO: normal oleate peanut; HO: high oleate peanut. Bars superscripted by different lowercase letters are significantly different at P < 0.05 by one-way ANOVA and least significant difference (LSD) test.
综合以上结果表明, 种子萌发初期进行低温处理会推迟种子萌发时间, 延缓种子萌发速率, 降低种子发芽指数。在低温处理条件下, 花生的发芽率和发芽指数均与自身的油酸含量无显著相关性。而不同遗传背景的花生的耐寒性各不相同, 无论高油酸花生还是普通油酸花生均有对低温耐受和敏感的材料, 例如Xuhua 13-NO和Xuhua 13-HO的低温耐受性都较高, 而Quanhua 551-HO和Xuhua 9-NO都对低温较敏感, 表明花生的油酸含量不是影响其萌发期耐寒性的关键因素, 而花生自身的遗传背景对其耐寒性的影响更重要。
2.2 低温胁迫对普通油酸和高油酸花生种子萌发期脂肪酸含量的影响
为进一步探究花生的萌发期耐寒性与其脂肪酸含量变化的关系, 我们选择低温处理条件下发芽率显著降低的Quanhua 551-HO为研究对象, 发芽率未显著降低的Quanhua 551-NO为对照(图1和图3), 检测了Quanhua 551-NO和Quanhua 551-HO在低温处理萌发过程中8种脂肪的含量变化情况(图4)。在常温条件下种子萌发过程中, Quanhua 551-NO中各脂肪酸含量和含油量在第1~3天均无显著变化, 而在第6天均显著降低; 而Quanhua 551-HO中, 直至第6天, 8种脂肪酸的含量以及含油量均无显著变化。在低温处理3 d的条件下, Quanhua 551-NO中C18:1含量显著降低, 而C18:2的含量显著增加; 而在Quanhua 551-HO中C18:1和C18:2的含量表现出了相同的趋势, 但是变化未达到显著水平。在低温胁迫种子发芽的过程中, 普通油酸花生种子中C18:1含量降低, 向C18:2转化, 但在高油酸花生中变化幅度较小, 未达到显著水平。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3泉花551高油酸花生和普通油酸花生在不同温度处理下的发芽情况
25-NO: 泉花551普通油酸花生在25℃条件下萌发6 d; 15-NO: 第1~3天泉花551普通油酸花生在15℃条件下萌发, 第4~6天在25℃条件下萌发; 25-HO: 泉花551高油酸花生在25℃条件下萌发6 d; 15-HO: 第1~3天泉花551高油酸花生在15℃条件下萌发, 第4~6天在25℃条件下萌发。
Fig. 3Seed germination of peanut with high oleate and normal oleate in Quanhua 551 at different temperatures
25-NO: the normal oleate peanut of Quanhua 551 germinated at 25℃ for six days; 15-NO: the normal oleate peanut of Quanhua 551 germinated at 15℃ from the 1st to 3rd day then at 25℃ for the last three days; 25-HO: the high oleate peanut of Quanhua 551 germinated at 25℃ for six days; 15-HO: the high oleate peanut of Quanhua 551 germinated at 15℃ from the 1st to 3rd day then at 25℃ from the 4th to 6th day.
图4
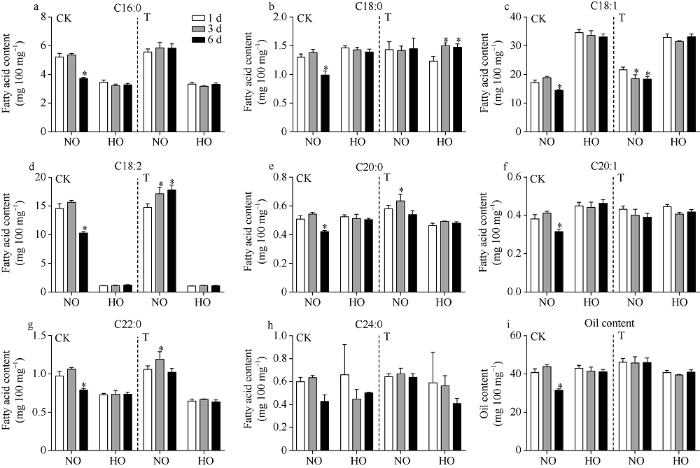 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4低温胁迫下Quanhua 551种子发芽过程中脂肪酸含量的变化情况
C16:0: 棕榈酸; C18:0: 硬脂酸; C18:1: 油酸; C18:2: 亚油酸; C20:0: 花生酸; C20:1: 花生烯酸; C22:0: 山嵛酸; C24:0: 木蜡酸。CK: 种子在25℃条件下萌发6 d; T: 种子第1~3天在15℃条件下萌发, 第4~6天在25℃条件下萌发; NO: 普通油酸花生; HO: 高油酸花生。*表示在0.05水平差异显著。
Fig. 4Changes of fatty acid contents at seed germination stage of Quanhua 551 under cold stress
C16:0: palmitic acid; C18:0: stearic acid; C18:1: oleic acid; C18:2: linoleic acid; C20:0: arachidic acid; C20:1: gadoleic acid; C22:0: behenic acid; C24:0: lignoceric acid. CK: control group, germination of seed at 25℃ for six days; T: treatment group, germination of seed at 15℃ from the 1st to 3rd day, and then 25℃ from 4th to 6th day; NO: normal oleate peanut; HO: high oleate peanut. * means significant difference at the 0.05 probability levels.
2.3 低温胁迫对普通油酸和高油酸花生种子中AhFAD2家族基因表达模式的影响
已有研究表明AhFAD2基因参与了花生的低温响应过程[16], 但是Quanhua 551-HO在低温胁迫下C18:2的含量增加未达到显著水平, 因此我们利用荧光定量PCR分析了Quanhua 551-NO和Quanhua 551-HO在常温和低温处理萌发过程中AhFAD2家族基因的表达量(图5)。AhFAD2-1A/B的表达量在Quanhua 551-NO中15℃处理1 d与其在25℃处理1 d相比, 显著上调表达; 而在Quanhua 551-HO中15℃处理1 d与其在25℃处理1 d相比, 则显著下调表达(图5-a, d)。当Quanhua 551-NO在15℃处理3 d与其在25℃处理3 d相比, AhFAD2-1A/B的表达量无显著变化; 而且当Quanhua 551-HO在15℃处理3 d与其在25℃处理3 d相比, AhFAD2-1A/B的表达量也无显著差异(图5-a, d)。当Quanhua 551-NO恢复至25℃再处理3 d与其在25℃处理6 d相比, AhFAD2-1A/B的表达量显著上调; 当Quanhua 551-HO恢复至25℃再处理3 d与其在25℃处理6 d相比,AhFAD2-1A/B的表达量也显著上调(图5-a, d)。由此可见, AhFAD2-1A/B在Quanhua 551-NO中受低温胁迫诱导始终上调表达, 而在Quanhua 551-HO中先下调、再上调表达。图5
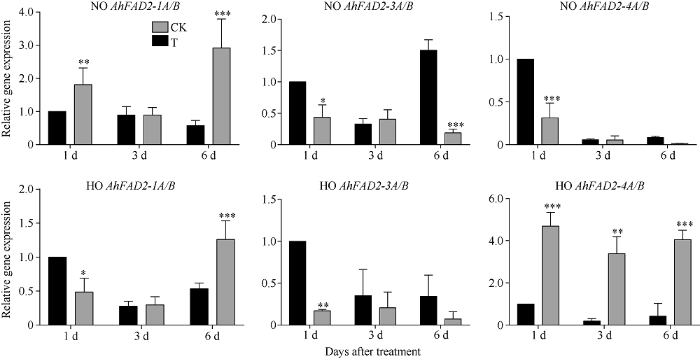 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5低温胁迫下各AhFAD2基因在泉花551高油酸花生和普通油酸花生中的表达模式
a: 在不同温度处理条件下, AhFAD2-1A/B在Quanhua 551-NO中的表达模式; b: 在不同温度处理条件下, AhFAD2-3A/B在Quanhua 551-NO中的表达模式; c: 在不同温度处理条件下, AhFAD2-4A/B在Quanhua 551-NO中的表达模式; d: 在不同温度处理条件下, AhFAD2-1A/B在Quanhua 551-HO花生中的表达模式; e: 在不同温度处理条件下, AhFAD2-3A/B在Quanhua 551-HO中的表达模式; f: 在不同温度处理条件下, AhFAD2-4A/B在Quanhua 551-HO中的表达模式。CK: 种子在25℃条件下萌发6 d; T: 种子第1~3 天在15℃条件下萌发, 第4~6天在25℃条件下萌发; NO: 普通油酸花生; HO: 高油酸花生。*、**和***分别表示在0.05、0.01和0.001水平显著差异。
Fig. 5Relative expression levels of AhFAD2 genes in high oleate and normal oleate of Quanhua 551 under cold stress
a: the expression pattern of AhFAD2-1A/B in Quanhua 551-NO at different temperatures; b: the expression pattern of AhFAD2-3A/B in Quanhua 551-NO at different temperatures; c: the expression pattern of AhFAD2-4A/B in Quanhua 551-NO at different temperatures; d: the expression pattern ofAhFAD2-1A/B in Quanhua 551-HO at different temperatures; e: the expression pattern ofAhFAD2-3A/B in Quanhua 551-HO at different temperatures; f: the expression pattern of AhFAD2-4A/B in Quanhua 551-HO at different temperatures. CK: control group, germination of seed at 25℃ for six days; T: treatment group, germination of seed at 15℃ from 1st to 3rd day, and then 25℃ from the 4th to 6th day; NO: normal oleate peanut; HO: high oleate peanut. *, **, and *** mean significant difference at the 0.05, 0.01, and 0.001 probability levels, respectively.
AhFAD2-3A/B的表达量在Quanhua 551-NO中15℃处理1 d与其在25℃处理1 d相比, 显著下调表达; 同样, 在Quanhua 551-HO中15℃处理1天与其在25℃处理1天相比, 也显著下调表达(图5-b, e)。当Quanhua 551-NO在15℃处理3 d与其在25℃处理3 d相比, AhFAD2-3A/B的表达量无显著变化; 而且当Quanhua 551-HO在15℃处理3 d与其在25℃处理3 d相比,AhFAD2-3A/B的表达量也无显著差异(图5-b, e)。当Quanhua 551-NO恢复至25℃再处理3 d与其在25℃处理6 d相比, AhFAD2-3A/B的表达量仍然显著下调; 而AhFAD2-3A/B的表达量在Quanhua 551-HO恢复至25℃再处理3 d与其在25℃处理6 d相比, 则无显著差异(图5-b, e)。表明, 在低温胁迫种子萌发过程中, AhFAD2-3A/B显著下调表达。
AhFAD2-4A/B的表达量在Quanhua 551-NO中15℃处理1 d与其在25℃处理1 d相比, 显著下调表达; 而在Quanhua 551-HO中15℃处理1 d与其在25℃处理1 d相比, 则显著上调表达(图5-c, f)。当Quanhua 551-NO在15℃处理3 d与其在25℃处理3 d相比, AhFAD2-4A/B的表达量无显著变化; 而在Quanhua 551-HO中, AhFAD2-4A/B的表达量在15℃处理3 d比其在25℃处理3 d依然显著上调表达, 且维持在较高水平(图5-c, f)。当Quanhua 551-NO恢复至25℃再处理3 d与其在25℃处理6 d相比, AhFAD2-4A/B的表达量无显著差异; 而在Quanhua 551-HO恢复至25℃再处理3 d与其在25℃处理6 d相比, AhFAD2-4A/B的表达量仍然显著上调, 始终维持在较高水平(图5-e, f)。由此可见, AhFAD2-4A/B在Quanhua 551-NO中受低温胁迫诱导下调表达, 而在Quanhua 551-HO中显著上调表达, 表明高油酸花生中AhFAD2-1A/B功能的丧失, 导致AhFAD2-4A/B基因表达模式发生改变, 即在高油酸花生中AhFAD2-4A/B受低温胁迫诱导持续高水平上调表达。
综合以上结果表明, 在低温胁迫种子萌发过程中, AhFAD2-1A/B、AhFAD2-3A/B和AhFAD2- 4A/B表达量都收到低温胁迫的影响, 不同的是, AhFAD2-1A/B在普通油酸花生中受低温胁迫诱导始终上调表达, 而在高油酸花生中先下调、再上调表达; AhFAD2-3A/B无论在普通油酸花生还是高油酸花生中都受低温诱导下调表达; 而AhFAD2- 4A/B在普通油酸花生中受低温胁迫诱导下调表达, 而在高油酸花生中显著上调表达, 且表达量一直维持在较高水平, 推测高油酸花生在受到低温胁迫的过程中, AhFAD2-4A/B高量表达可以部分弥补突变后的AhFAD2-1A/B基因的功能, 保障高油酸花生的耐寒性。
3 讨论
随着人们对花生品质要求的不断提高, 高油酸花生越来越受到消费者和加工企业的喜爱, 在我国的种植面积也在不断扩大, 但由于我国辽宁、吉林和黑龙江等东北地区春季播种温度较低, 限制了高油酸花生在我国北部地区的全面推广。近年来, 关于高油酸花生的耐寒性是否比普通油酸花生差以及高油酸花生是否适合在我国东北部地区推广的话题成为专家们关注和讨论的热点。本研究选用6组普通油酸花生品种以及该品种回交BC4F8代高油酸品系为研究对象, 比较各组合中普通油酸和高油酸花生在低温胁迫下种子的发芽率发芽指数, 并分析种子在萌发过程中脂肪酸组分含量的变化, 以及决定高油酸性状的AhFAD2家族基因的表达模式, 来探究花生的油酸含量与耐寒性的相关性。低温胁迫延长了花生种子的萌发时间, 减缓了萌发速率, 降低了所有材料的发芽指数(图2)。通过对比种子萌发情况发现, 徐花13号和徐花9号的高油酸花生和普通油酸花生在低温处理条件下发芽率无显著下降(图1-a, b), 中花21和泉花551的普通油酸花生在低温处理条件下种子发芽率显著高于高油酸花生种子(图1-d, e), 而漯花9号和中花16的高油酸花生在低温处理条件下的种子发芽率显著高于普通油酸花生种子(图1-c, f)。不同遗传背景下的高油酸花生种子在低温处理条件下并没有表现出一致低于其普通油酸亲本的现象, 而且相关性分析也表明花生的低温发芽率与其中油酸含量无相关性(r= -0.031, P = 0.924)。通过进一步分析各组中普通油酸和高油酸花生低温胁迫下的发芽指数发现, 花生的低温发芽指数与其油酸含量无相关性(r = 0.072, P= 0.823)。综上所述, 花生的萌发期耐寒性与其种子中油酸含量无相关性, 即油酸含量并不是决定其萌发期耐寒性的关键因素。不同品种的高油酸品系耐寒性也各不相同, 表明高油酸花生种子的耐寒性与其自身的遗传背景更为相关。钟鹏等[20]通过比较黑龙江省种植面积较大的多粒型吉花3号和四粒红以及珍珠型白沙1016和宏花1号在低温胁迫下的发芽情况发现, 多粒型四粒红和吉花3号的抗寒性比珍珠豆型白沙1016和宏花1号强, 上述研究也表明遗传背景对花生的耐寒性影响更重要。
通过分析泉花551的高油酸花生和普通油酸花生在低温胁迫种子发芽过程中籽仁中脂肪酸含量的变化发现, 在常温条件下种子发芽过程中, 各种脂肪酸含量在种子萌发前3 d并未发生显著变化, 至第6天才显著降低(图4), 表明在种子萌发过程中脂肪酸的利用发生在种子萌发后期。有研究表明, 黄芪种子萌发过程中, 优先利用子叶中储藏的淀粉类物质, 脂质则主要在萌发后生长阶段被动员[23]。王允等[24]通过分别测定萌发后第5、10、15、20、25和30天后花生中粗脂肪含量发现, 在种子萌发后的5~30 d内, 粗脂肪不断降解, 为植物生长提供能量。而本研究中种子萌发过程中高油酸花生脂肪酸含量未见明显改变, 可能是由于低温胁迫导致种子萌发缓慢, 种子仍然处于萌发初期, 还未启动消耗子叶中储存脂质的能量。在低温胁迫种子发芽过程中, 普通油酸花生中C18:1含量降低, 而C18:2含量增高, 表明花生种子中部分C18:1受低温诱导转化为C18:2 (图4-c, d)。高油酸花生中脂肪酸存在同样的变化趋势, 但变化幅度较小, 未达到显著水平(图4-c, d)。
FAD2基因的功能是催化C18:1去饱和生成C18:2 [23], AhFAD2-4A/B与AhFAD2-1A/B的DNA序列同源性为83.01%, 氨基酸序列的同源性为83.25%, 其蛋白二级结构相似, 且都具有3个高度保守的组氨酸富集区, 可以与铁原子组成酶活性中心, 该保守结构域是脂肪酸脱氢酶的关键活性域[16,17]。高油酸花生中AhFAD2-1A/B基因功能的失活, 使得种子中C18:1转化为C18:2的能力大幅下降, 但是AhFAD2-4A/B在受到低温诱导持续显著上调表达部分弥补了AhFAD2-1A/B基因的功能, 因此, 我们观察到在低温胁迫第3天高油酸花生中C18:1的含量比第1天低, 且C18:2的含量也比第1天高(图4-c, d), 但是变化的幅度都没有达到显著水平。薛晓梦等[16]以普通油酸花生中花16和高油酸花生中花413为试验材料, 同样发现在低温胁迫下高油酸花生种子萌发过程中, AhFAD2-4A/B的上调表达部分弥补了AhFAD2-1A/B缺失的功能, 而且由于中花413在低温胁迫下发芽率未发生显著下降, 检测到其AhFAD2-4A/B基因表达量的上调倍数达到了20倍以上。而在本研究中, 由于选取的研究材料Quanhua 551-HO在低温胁迫下发芽率是显著下降的, 而Quanhua 551-NO的低温发芽率并没有显著下降, AhFAD2-4A/B在Quanhua 551-HO中的上调倍数仅为5倍, 因此观察到的弥补效应较小; 如果本研究选择在低温胁迫下发芽率并未发生显著下降的材料为研究对象, 也许可以观察到更显著的AhFAD2- 4A/B基因的弥补效应。
综上所述, 花生种子油酸含量并不是影响其耐寒性的决定因素, 而遗传背景对花生耐寒性的影响更大。于树涛等[26]以高产和抗叶班病强的辽宁省沙地改良品系01-2为母本, 以山东高油酸小花生品系9506为父本, 在2018年成功选育出适应于东北地区的高油酸花生新品种阜花22号, 为辽宁高油酸花生品种填补了空白; 于树涛等[27]通过以抗逆性强阜12E3-1为父本, 以山东省农科院创制的高油酸辐射突变体FB4为母本, 成功培育出适合北方地区种植的高油酸花生阜花27。潘丽娟等[28]也成功培育出适合东北地区种植的花育32和花育917等高油酸花生新品种。因此, 利用抗寒能力强的花生品种为亲本培育耐寒的高油酸花生新品种是促进高油酸花生在高纬度、高海拔地区推广的重要途径。
4 结论
本研究通过分析6组不同遗传背景的花生品种及其高油酸回交后代品系(BC4F8)在低温条件下的发芽率和发芽指数表明, 在低温胁迫下花生萌发期的耐寒性与其油酸含量无显著相关性。低温处理高油酸花生种子萌发时, AhFAD2-4A/B受低温诱导显著上调表达, 而非决定花生高油酸性状的AhFAD2- 1A/B, 表明高油酸花生中AhFAD2-1A/B功能的缺失, 并不是决定高油酸花生耐寒性的关键因素。本研究为培育耐寒能力强的高油酸花生品种以及在我国高纬度、高海拔地区推广应用提供了理论依据。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
DOIPMID [本文引用: 1]

Background: Fatty acid composition of oil extracted from peanut (Arachis hypogaea L.) seed is an important quality trait because it may affect the flavor and shelf life of resulting food products. In particular, a high ratio of oleic (C18:1) relative to linoleic (C18:2) fatty acid (O/L >= 10) results in a longer shelf life. Previous reports suggest that the high oleic (similar to 80%) trait was controlled by recessive alleles of ahFAD2A and ahFAD2B, the former of which is thought to have a high frequency in US runner-and virginia-type cultivars. Functional mutations, G448A in ahFAD2A and 442insA in ahFAD2B eliminate or knock down desaturase activity and have been demonstrated to produce peanut oil with high O/L ratios. In order to employ marker assisted selection (MAS) to select a high oleic disease resistant peanut and to evaluate genotypic and phenotypic variation, crosses were made between high oleic (similar to 80%) and normal oleic (similar to 50%) peanuts to produce segregating populations.;Results: A total of 539 F-2 progenies were randomly selected to empirically determine each ahFAD2 genotype and the resulting fatty acid composition. Five of the six crosses segregated for the high oleic trait in a digenic fashion. The remaining cross was consistent with monogenic segregation because both parental genotypes were fixed for the ahFAD2A mutation. Segregation distortion was significant in ahFAD2A in one cross; however, the remaining crosses showed no distortion. Quantitative analyses revealed that dominance was incomplete for the wild type allele of ahFAD2, and both loci showed significant additive effects. Oleic and linoleic acid displayed five unique phenotypes, based on the number of ahFAD2 mutant alleles. Further, the ahFAD2 loci did exhibit pleiotropic interactions with palmitic (C16:0), oleic (C18:1), linoleic (C18:2) acids and the O/L ratio. Fatty acid levels in these progeny were affected by the parental genotype suggesting that other genes also influence fatty acid composition in peanut. As far as the authors are aware, this is the first study in which all of the nine possible ahFAD2 genotypes were quantitatively measured.;Conclusions: The inheritance of the high oleic trait initially was suggested to be controlled by dominant gene action from two homoeologous genes (ahFAD2A and ahFAD2B) exhibiting complete recessivity. Analyzing the ahFAD2 genotypes and fatty acid compositions of these segregating peanut populations clearly demonstrated that the fatty acid contents are quantitative in nature although much of the variability in the predominant fatty acids (oleic, linoleic, and palmitic) is controlled by only two loci.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
PMID [本文引用: 1]

To investigate how the fatty acid composition of membrane lipids influences cell growth and mitochondrial respiration, in particular the expression and capacity of alternative oxidase (AOX), under cold stress, we used the Arabidopsis thaliana fad2 knockout and FAD3+ -overexpressing cultured cells lines affected in extrachloroplastic fatty acid desaturation activities. At 22 degrees C, fad2 mitochondria exhibited a low polyunsaturated fatty acid content and low protein to lipid ratio, while mitochondria from FAD3+ were enriched in linolenic acid and in total membrane protein. As a consequence, both mutants showed a higher membrane microviscosity than the wild type. After exposure to 9 degrees C, FAD3+ mitochondria exhibited lower microviscosity and lower rigidification upon a temperature downshift than fad2. Furthermore, the extent of reduction of cell growth and respiratiory rates in the phosphorylating state was positively related to the cold sensitivity of each cell line, being more pronounced in fad2 that in the wild type, whereas the stability of those parameters reflected the cold resistance of FAD3+. In contrast, an increase in AOX capacity was observed in the three cell lines at 9 degrees C. These inductions were correlated to AOX protein amounts and seem to result from an accumulation of AOX1c transcripts in the three cell lines and of AOX1a transcripts in wild-type and fad2 cells. The fact that there is no direct relationship between the degree of cold tolerance of each cell line and their ability to enhance their AOX capacity suggests that the participation of AOX in the response of Arabidopsis cells to cold stress does not necessarily favor cold tolerance.
DOIPMID [本文引用: 1]

Lipid modifying enzymes play a key role in the development of cold stress tolerance in cold-resistant plants such as cereals. However, little is known about the role of the endogenous enzymes in cold-sensitive species such as cotton. Delta 12 fatty acid desaturases (FAD2), known to participate in adaptation to low temperatures through acyl chain modifications were used in gene expression studies in order to identify parameters of plant response to low temperatures. The induction of microsomal delta 12 fatty acid desaturases at an mRNA level under cold stress in plants is shown here for first time. Quantitative PCR showed that though both delta 12 omega 6 fatty acid desaturase genes FAD2-3 and FAD2-4 identified in cotton are induced under cold stress, FAD2-4 induction is significantly higher than FAD2-3. The induction of both isoforms was light regulated, in contrast a third isoform FAD2-2 was not affected by cold or light. Stress tolerance and light regulatory elements were identified in the predicted promoters of both FAD2-3 and FAD2-4 genes. Di-unsaturated fatty acid species rapidly increased in the microsomal fraction isolated from cotton leaves, following cold stress. Expression analysis patterns were correlated with the observed increase in both total and microsomal fatty acid unsaturation levels suggesting the direct role of the FAD2 genes in membrane adaptation to cold stress.
DOIURL [本文引用: 1]
[本文引用: 5]
[本文引用: 5]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
