 ,, 凌磊, 张文睿, 王丹, 郭长虹
,, 凌磊, 张文睿, 王丹, 郭长虹 ,*哈尔滨师范大学生命科学与技术学院/黑龙江省分子细胞遗传与遗传育种重点实验室, 黑龙江哈尔滨 150025
,*哈尔滨师范大学生命科学与技术学院/黑龙江省分子细胞遗传与遗传育种重点实验室, 黑龙江哈尔滨 150025Genome-wide identification and expression analysis of B-box gene family in wheat
WANG Yan-Peng ,, LING Lei, ZHANG Wen-Rui, WANG Dan, GUO Chang-Hong
,, LING Lei, ZHANG Wen-Rui, WANG Dan, GUO Chang-Hong ,*Key Laboratory of Molecular Cytogenetics and Genetic Breeding of Heilongjiang Province/College of Life Science and Technology, Harbin Normal University, Harbin 150025, Heilongjiang, China
,*Key Laboratory of Molecular Cytogenetics and Genetic Breeding of Heilongjiang Province/College of Life Science and Technology, Harbin Normal University, Harbin 150025, Heilongjiang, China通讯作者: * 郭长虹, E-mail:kaku3008@126.com
收稿日期:2020-09-18接受日期:2021-01-13网络出版日期:2021-08-12
| 基金资助: |
Received:2020-09-18Accepted:2021-01-13Online:2021-08-12
| Fund supported: |
作者简介 About authors
E-mail:13694609045@163.com

摘要
B-box (BBX)是一类含有1个或2个B-box结构域的锌指蛋白, 在植物生长发育中起着重要作用。本研究明确小麦B-box转录因子的数量、基因结构和分类进化关系, 研究各基因成员在不同组织中的特异性表达以及对非生物胁迫的响应。从小麦全基因组中鉴定得到87个B-box基因家族成员, 所有TaBBXs蛋白均含有B-box结构域。TaBBXs编码146~489个氨基酸, 理论等电点为4.32~10.42。染色体定位分析表明, TaBBXs分布在除1A、1B和1D之外的18条小麦染色体上。通过系统发育分析将TaBBXs划分为5个亚家族, 有0~4个内含子。在同组内同一个系统进化树分支中的亚族成员具有高度相似的基因结构。qRT-PCR分析的20个TaBBXs基因, 具有不同的组织表达模式, 16个基因在叶中有较高表达, TaBBX10和TaBBX39仅在叶中有较高表达, 而TaBBX74在穗中表达, TaBBX43在根中特异性表达。在不同逆境胁迫下, TaBBXs呈现不同表达模式, 11个基因在低温胁迫后上调表达, 12个基因在ABA处理后下调表达, 盐胁迫后10个基因出现上调表达, 干旱胁迫后7个基因出现下调表达, TaBBX10、TaBBX39、TaBBX60、TaBBX67和TaBBX74基因在2种或2种以上胁迫下有显著的上调表达。
关键词:
Abstract
B-box (BBX) is a class of zinc finger proteins that contain one or two B-box domains and play important roles in plant growth and development. The number, gene structure and phylogenetic relationship of wheat B-box transcription factors, as well as their expression specificity in different tissues and response to abiotic stress were investigated. A total of 87 members of B-box gene family were identified from wheat genome and all contained the B-box domain. TaBBXs encoded 146 to 489 amino acids and the isoelectric points ranged from 4.32 to 10.42. Chromosome mapping showed that these genes were distributed on 18 wheat chromosomes except 1A, 1B, and 1D. Based on phylogenetic analysis, TaBBXs were divided into five subfamilies, with 0-4 introns. The members of the subfamily in the same phylogenetic tree branch in the same group had highly similar gene structures. The qRT-PCR revealed that the investigated 20 genes had different expression patterns, and most genes were highly expressed in leaves, and TaBBX10 and TaBBX39 were only highly expressed in leaves, while TaBBX74 was expressed in spikes, TaBBX43 was specifically expressed in roots. These genes showed different expression patterns under different stress. 11 genes were up-regulated after low temperature stress, 13 genes were down-regulated after ABA treatment, 10 genes were up-regulated after salt stress, and 7 genes were down-regulated after drought stress. TaBBX10, TaBBX39, TaBBX60, TaBBX67, and TaBBX74 were significantly up-regulated under two or more stresses.
Keywords:
PDF (3123KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
王艳朋, 凌磊, 张文睿, 王丹, 郭长虹. 小麦B-box基因家族全基因组鉴定与表达分析. 作物学报[J], 2021, 47(8): 1437-1449 DOI:10.3724/SP.J.1006.2021.01077
WANG Yan-Peng, LING Lei, ZHANG Wen-Rui, WANG Dan, GUO Chang-Hong.
小麦(Triticum aestivum L.)是世界上最重要的粮食作物之一, 占谷物种植面积的30% [1,2]。其基因组十分复杂, 由A、B、D 3个亚基因组整合而形成的异源六倍体(AABBDD), 重复序列高达85%, 大小约为15 GB [3,4]。小麦在生长发育过程中面临多种环境胁迫, 其中盐、干旱、低温等非生物胁迫严重影响小麦的产量和品质[5]。植物B-box蛋白可参与逆境响应, 但目前尚未对小麦B-box基因家族进行系统分析。因此, 本研究基于小麦全基因组对B-box基因家族进行分析, 为进一步研究该基因家族成员功能提供参考, 研究结果对进一步培育或改良小麦抗性品种具有重要意义。
转录因子(transcription factors, TF)能够调节植物的生长发育, 在植物对高盐、干旱和高温等逆境的响应及应答过程中具有重要作用[6]。B-box转录因子家族是一个具有B-box结构域的锌指蛋白家族。1995年首次在拟南芥(Arabidopsis thaliana)的一个晚花突变体中鉴定得到B-box基因, 命名为CONSTANS (CO), 并证实了该基因参与植物开花和其他生命活动的调节[7]。随后的研究进一步揭示, 植物B-box转录因子在调节种子萌发[8]、开花[9]、避荫反应[10]、生物或非生物应激反应[11]、植物激素信号转导[12]等多种生命活动中发挥着重要的作用。如AtBBX19可以通过诱导ABI5抑制种子萌发[13], AtBBX31能促进UV-B辐射下的光形态发生, 增强对高剂量UV-B辐射的耐受性[14]。Gangappa等[15]指出, AtBBX25通过与HY5形成二聚体并抑制其功能, 参与植物光形态发生的负调控。最新研究表明, 苹果中的MdBBX37能够和MdMYB1和MdMYB9相互作用, 抑制这两种蛋白与目标基因的结合, 从而对花青素生物合成产生负调控作用[16]。非生物胁迫方面, AtBBX24 (最初被称为STO)参与盐胁迫的信号转导, 可以使盐敏感型的突变体酵母具有更强的耐盐能力[17,18]。AtBBX5通过ABA途径参与胁迫响应, 在受到外源的ABA、盐和渗透胁迫时, 该基因上调表达[19]。AtBBX18的下调表达可以使植物的耐热性增加, 过表达该基因可以降低植物的耐热性[11]。MdBBX10能够增强拟南芥的耐盐耐旱性, 在大肠杆菌(E. coli)中表达的MdBBXs分别增强了细胞对盐胁迫和渗透胁迫的耐受性[20,21]。梨PuBBX24启动子显著响应ABA、光、低温、渗透以及盐胁迫处理[22]。
目前B-box基因家族的研究主要集中在模式植物拟南芥、水稻和蒺藜苜蓿中, 而在小麦中尚未见报道。随着小麦基因组的公布[4], 使得对小麦B-box基因家族分析成为可能。通过生物信息学方法对全基因组中小麦B-box基因家族成员进行鉴定, 对所有家族成员的理化信息、结构功能、表达模式进行分析。利用qRT-PCR实验分析20个B-box基因在不同组织中以及非生物胁迫条件下的表达模式, 为解析B-box基因家族在小麦中的功能提供参考。
1 材料与方法
1.1 材料与处理
植物试验材料为小麦中国春(Triticum aestivum L., Chinese Spring), 将小麦种子表面用15%次氯酸钠消毒5 min, 蒸馏水冲洗3次, 消毒后的种子置于无菌培养皿中进行发芽培养24 h, 将幼芽移栽至小麦水培盒中, 种植在1/2 Hoagland营养液中, 在22℃光照16 h的温室中生长。将2周龄小麦幼苗分别用0.2 mol L-1 NaCl、20% PEG、100 μmol L-1 ABA和4℃进行胁迫处理, 以正常条件下的幼苗为对照。处理6 h后对处理组和对照组植株进行取材, 每个样品3个重复。此外, 采集在4月至7月大田中培养的小麦组织: 根、茎、叶、穗(开花前1 d)和籽粒(授粉后10 d)进行组织表达分析, 每个样品3个重复。将所有样品液氮速冻后, -80℃保存备用。1.2 小麦B-box基因家族成员的鉴定
小麦全基因组数据、蛋白序列和注释文件从Ensembl Plants数据库中下载, 拟南芥和水稻B-box基因序列和蛋白序列下载自NCBI (1.3 小麦B-box蛋白理化性质及结构域预测分析
利用ExPASy网站(1.4 小麦B-box基因家族的系统进化分析
使用ClustalX软件对小麦B-box氨基酸序列进行多序列比对, 通过MEGA7.0软件构建系统发育树: 采用邻接法(Neighbor-Joining algorithm), 泊松校正(Poission correction), 成对删除(pairwise deletion), Bootstrap重复值1000次。参考拟南芥和水稻B-box基因家族的亚族分类结果对小麦B-box基因家族进行亚族分类。1.5 小麦B-box基因结构及染色体定位分析
从小麦基因信息GFF3文件中提取TaBBXs的染色体位置和基因结构信息, 分别使用MapDraw和GSDS (1.6 小麦B-box基因家族的复制事件及同源性分析
使用BLASTp、OrthoMCL、多重共线扫描工具包(MCScanX)和默认参数分析基因复制事件(E<1e-5), 如果2个同源基因被5个或更少的基因分开, 则它们被鉴定为串联重复, 如果2个基因被5个以上的基因分开或分布在不同的染色体上, 则称为片段重复。同源基因结合系统发育树进一步鉴定, 鉴定标准为: 同源序列覆盖率>75%, 同源性>75%。1.7 小麦B-box基因家族的启动子分析
从小麦全基因组数据库中提取每个小麦B-box基因启动子区域(上游2000 bp), Plant CARE数据库(1.8 RNA提取和实时荧光定量PCR分析
采用RNAprep Pure Plant Kit试剂盒提取小麦总RNA, 将提取出的总RNA作为模板, 利用PrimeScript RT Reagent Kit with gDNA Eraser (Perfect Real Time) (RR047, TaKaKa)反转录获得cDNA。利用软件Primer 3和Primer Premier 6设计引物, 具体引物序列见表1, 其中小麦Actin为内参基因。PCR反应体系为cDNA 2 μL, 2×SYBR Premix ExTaq 10 μL, 50×ROX Reference Dye 0.4 μL, 正、反向引物各0.8 μL和ddH2O 6 μL。扩增程序为94℃ 30 s; 94℃ 5 s, 54℃ 15 s, 72℃ 31 s, 40个循环。每个处理3个生物学重复, 并使用2-ΔΔCT方法计算基因的相对表达量。用SPSS软件进行差异显著性分析, P < 0.05表示差异显著, P < 0.01表示差异极显著。Table 1
表1
表1本试验所用引物
Table 1
| 基因 Gene | 正向引物 Forward primer (5′-3′) | 反向引物 Reverse primer (5′-3′) |
|---|---|---|
| TaBBX01 | AACGGGGGAGTGTTACTTCT | AGTTGGAGCAGAGGAACCGA |
| TaBBX06 | AACTCGCCAAGTCGGAGGA | AGCTGCTGATACGGAGGTACA |
| TaBBX08 | TACCGCCGACGTGTTCTT | TCAGAGGAGGATACGCTGTG |
| TaBBX10 | CTCGCATCGTCCTCTCCAA | TCTGCTGGCATGAAGGTACA |
| TaBBX12 | CACAGCGTATCGTCGTCTGA | ATCTCCTCGTGCTCCTCCAT |
| TaBBX13 | TCGAACAAGCCGTATCAGCA | AGAAGGAGGAGCCGAGAGAC |
| TaBBX14 | AGCCGGAGGTAATCAAAGCC | GAGGAGGACGACAACGATCC |
| TaBBX18 | GGGTTCTCCGGGTTCGAC | CCTCTAACTCTTGCTCCGGC |
| TaBBX35 | TGACATTGAAAGGCTGCGT | TGACATTGAAAGGCTGCGT |
| TaBBX39 | ACCGCCGATTCCTCATCAC | CCAATGTCGTCTTCTCCTCCT |
| TaBBX43 | CTCCGAGTACCTCACCAAGAC | CAATGCTCCTGCCTCATCCA |
| TaBBX46 | CACGCGGTACATGGCACG | CCGGTGCGCTTGACGAAG |
| TaBBX52 | CCGAACTGCGAGGAAGGAATA | GCTGGCTGATGTGTAGGAAGT |
| TaBBX60 | GCCGAACTGTGAGGAAGGAA | AGGCTGATGCGTAGGAAGTG |
| TaBBX62 | CACGACGGGCGGGTAAAG | GGCTCCTTTTCAAGAACTGCG |
| TaBBX67 | GAGAAGGAAGGGAGCGAGTG | GCTGGACTGGACCGTATTGT |
| TaBBX74 | GCAACCAAGAGCAGTATGTGAT | TGTTGACGGAATCTGTGTAAGC |
| TaBBX76 | AGGTAAGCTCATGCACCTCG | CGTCTCGCTGTCGATCCTTG |
| TaBBX77 | GACGAGCCCATTCACAGCG | GGCAGATGTTGGTGAGGTAGTC |
| TaBBX86 | AGGGCGGGAAGATGGACTAC | ATGAGGAGCTGTAGGTCTGC |
| TaActin | TACTCCCTCACAACAACCG | AGAACCTCCACTGAGAACAA |
新窗口打开|下载CSV
2 结果与分析
2.1 小麦基因组中B-box家族基因鉴定
参考AtBBX、OsBBX基因, 共获得87个小麦B-box基因家族成员, 根据它们在染色体上的相对位置将其命名为TaBBX1-87。预测结果显示, 大多数TaBBXs蛋白的等电点都小于7, 只有TaBBX27、TaBBX28、TaBBX33、TaBBX34、TaBBX38、TaBBX64和TaBBX65七个成员在7以上; TaBBXs基因的长度有很大差距, 从441 bp (TaBBX26)到1473 (TaBBX69) bp, 分子量从15,371.44 Da (TaBBX26)到52,005.04 (TaBBX69) Da (附表1)。Table S1
附表1
附表1B-box家族的基本信息分析
Table S1
| 基因名 Gene name | 基因登录号 Gene ID | 染色体位点 Chromosome location | 基因长度 Length (bp) | 氨基酸长度 Length (aa) | 等电点 Isoelectric point (pI) | 分子量 Molecular weight (kD) | 外显子个数 Exon number | 分组 Group | 结构域类型 Domain type |
|---|---|---|---|---|---|---|---|---|---|
| TaBBX01 | TraesCS2A02G119600 | 2A:70102039-70104699 | 1149 | 382 | 5.73 | 40010.83 | 4 | Ⅲ | 1BBX + CCT |
| TaBBX02 | TraesCS2A02G348900 | 2A:587750669-587751938 | 765 | 254 | 4.95 | 27209.52 | 2 | Ⅳ | 2BBX |
| TaBBX03 | TraesCS2A02G353900 | 2A:594579959-594581671 | 951 | 316 | 5.56 | 33724.51 | 2 | Ⅰ | 2BBX+ CCT |
| TaBBX04 | TraesCS2A02G366100 | 2A:610710308-610712939 | 789 | 262 | 4.79 | 28237.51 | 2 | Ⅳ | 2BBX |
| TaBBX05 | TraesCS2A02G388500 | 2A:635722864-635724553 | 777 | 258 | 5.82 | 27357.5 | 3 | Ⅳ | 2BBX |
| TaBBX06 | TraesCS2B02G140300 | 2B:106954443-106956656 | 1164 | 387 | 5.44 | 40384.32 | 4 | Ⅲ | 1BBX + CCT |
| TaBBX07 | TraesCS2B02G367300 | 2B:523760095-523761742 | 774 | 257 | 4.89 | 27492.85 | 2 | Ⅳ | 2BBX |
| TaBBX08 | TraesCS2B02G372000 | 2B:530306217-530307895 | 960 | 319 | 5.56 | 33908.66 | 2 | Ⅰ | 2BBX+ CCT |
| TaBBX09 | TraesCS2B02G406600 | 2B:575903665-575905255 | 795 | 264 | 5.82 | 27823.99 | 3 | Ⅳ | 2BBX |
| TaBBX10 | TraesCS2D02G121400 | 2D:70622968-70625567 | 1146 | 381 | 5.72 | 39968.91 | 4 | Ⅲ | 1BBX + CCT |
| TaBBX11 | TraesCS2D02G347300 | 2D:445396017-445397519 | 777 | 258 | 4.81 | 27494.76 | 2 | Ⅳ | 2BBX |
| TaBBX12 | TraesCS2D02G351900 | 2D:450234732-450236192 | 960 | 319 | 5.56 | 33936.74 | 2 | Ⅰ | 2BBX+ CCT |
| TaBBX13 | TraesCS2D02G386300 | 2D:491429370-491431171 | 789 | 262 | 5.82 | 27600.81 | 3 | Ⅳ | 2BBX |
| TaBBX14 | TraesCS3A02G139300 | 3A:117227850-117230216 | 1050 | 349 | 5.15 | 37168.47 | 3 | Ⅳ | 2BBX |
| TaBBX15 | TraesCS3B02G156900 | 3B:150154692-150156947 | 1050 | 349 | 5.22 | 37477.8 | 3 | Ⅳ | 2BBX |
| TaBBX16 | TraesCS3D02G139600 | 3D:99748881-99751184 | 1050 | 349 | 5.32 | 37482.8 | 3 | Ⅳ | 2BBX |
| TaBBX17 | TraesCS4A02G140600 | 4A:216145053-216148012 | 1239 | 412 | 5.5 | 45256.44 | 5 | Ⅲ | 1BBX + CCT |
| TaBBX18 | TraesCS4A02G268500 | 4A:580733925-580736107 | 1233 | 410 | 5.85 | 44829.19 | 2 | Ⅱ | 1BBX + CCT |
| TaBBX19 | TraesCS4B02G045700 | 4B:32896523-32898695 | 1236 | 411 | 6.04 | 44870.33 | 2 | Ⅱ | 1BBX + CCT |
| TaBBX20 | TraesCS4B02G155800 | 4B:281732350-281735921 | 1194 | 397 | 6.06 | 43627.8 | 3 | Ⅲ | 1BBX + CCT |
| TaBBX21 | TraesCS4D02G046200 | 4D:21774703-21776886 | 1233 | 410 | 5.85 | 44816.25 | 2 | Ⅱ | 1BBX + CCT |
| TaBBX22 | TraesCS4D02G167300 | 4D:288428472-288432595 | 1194 | 397 | 6.1 | 43454.51 | 3 | Ⅲ | 1BBX + CCT |
| TaBBX23 | TraesCS5A02G166100 | 5A:355071283-355072804 | 981 | 326 | 5.78 | 35576.36 | 2 | Ⅰ | 1BBX + CCT |
| TaBBX24 | TraesCS5A02G317100 | 5A:528110873-528114741 | 636 | 211 | 5.74 | 23146.7 | 5 | Ⅳ | 2BBX |
| TaBBX25 | TraesCS5A02G336000 | 5A:545058736-545059411 | 540 | 179 | 5.29 | 19206.94 | 2 | Ⅲ | 1BBX |
| TaBBX26 | TraesCS5A02G336100 | 5A:545073760-545074317 | 441 | 146 | 4.57 | 15371.44 | 2 | Ⅲ | 1BBX |
| TaBBX27 | TraesCS5A02G339000 | 5A:546563819-546564523 | 705 | 234 | 9.27 | 24319.82 | 1 | Ⅴ | 1BBX |
| TaBBX28 | TraesCS5A02G339100 | 5A:546575974-546576576 | 603 | 200 | 10.42 | 21432.56 | 1 | Ⅴ | 1BBX |
| TaBBX29 | TraesCS5B02G163500 | 5B:302753429-302754975 | 978 | 325 | 5.96 | 35477.32 | 2 | Ⅰ | 1BBX + CCT |
| TaBBX30 | TraesCS5B02G317700 | 5B:501954375-501958483 | 636 | 211 | 5.85 | 23088.66 | 5 | Ⅳ | 2BBX |
| TaBBX31 | TraesCS5B02G335200 | 5B:518472466-518473347 | 468 | 155 | 5 | 16721.09 | 2 | Ⅲ | 1BBX |
| TaBBX32 | TraesCS5B02G335300 | 5B:518481548-518484145 | 1107 | 368 | 6.23 | 39645.26 | 4 | Ⅲ | 1BBX + CCT |
| TaBBX33 | TraesCS5B02G337400 | 5B:520970152-520970850 | 699 | 232 | 9.02 | 24101.56 | 1 | Ⅴ | 1BBX |
| TaBBX34 | TraesCS5B02G337500 | 5B:521031958-521032572 | 615 | 204 | 9.65 | 21590.87 | 1 | Ⅴ | 1BBX |
| TaBBX35 | TraesCS5D02G170700 | 5D:267762539-267764201 | 978 | 325 | 5.96 | 35519.42 | 2 | 1 | 1BBX + CCT |
| TaBBX36 | TraesCS5D02G323400 | 5D:415613354-415617623 | 636 | 211 | 5.85 | 23084.67 | 5 | Ⅳ | 2BBX |
| TaBBX37 | TraesCS5D02G341000 | 5D:429289528-429291886 | 1083 | 360 | 6.33 | 38690.35 | 4 | Ⅲ | 1BBX + CCT |
| TaBBX38 | TraesCS5D02G343300 | 5D:431251824-431252543 | 720 | 239 | 9.03 | 25267.96 | 1 | Ⅴ | 1BBX |
| TaBBX39 | TraesCS6A02G143900 | 6A:121616223-121619829 | 645 | 214 | 4.72 | 22147.27 | 2 | Ⅳ | 2BBX |
| TaBBX40 | TraesCS6A02G150900 | 6A:134947729-134949478 | 1095 | 364 | 5.68 | 37982.35 | 3 | Ⅰ | 2BBX+ CCT |
| TaBBX41 | TraesCS6A02G216400 | 6A:398252913-398254425 | 771 | 256 | 5.14 | 27782.32 | 2 | Ⅳ | 2BBX |
| TaBBX42 | TraesCS6A02G218900 | 6A:405166583-405168134 | 966 | 321 | 5.24 | 33625.42 | 2 | Ⅰ | 2BBX+ CCT |
| TaBBX43 | TraesCS6A02G239300 | 6A:449584187-449585880 | 711 | 236 | 5.77 | 25104.96 | 3 | Ⅳ | 2BBX |
| TaBBX44 | TraesCS6A02G286400 | 6A:518666410-518670650 | 972 | 323 | 6.65 | 35122.47 | 3 | Ⅲ | 2BBX+ CCT |
| TaBBX45 | TraesCS6A02G289400 | 6A:521451709-521454099 | 1110 | 369 | 5.91 | 41051.82 | 2 | Ⅰ | 2BBX+ CCT |
| TaBBX46 | TraesCS6A02G293200 | 6A:524718099-524720326 | 1281 | 426 | 6.81 | 46496.91 | 2 | Ⅱ | 1BBX + CCT |
| TaBBX47 | TraesCS6B02G172300 | 6B:184884215-184886653 | 645 | 214 | 4.89 | 22154.35 | 2 | Ⅳ | 2BBX |
| TaBBX48 | TraesCS6B02G179000 | 6B:198984566-198986312 | 1125 | 374 | 5.28 | 38846.14 | 3 | Ⅰ | 2BBX+ CCT |
| TaBBX49 | TraesCS6B02G246500 | 6B:439021199-439022376 | 783 | 260 | 4.98 | 28026.56 | 2 | Ⅳ | 2BBX |
| TaBBX50 | TraesCS6B02G248400 | 6B:445852206-445853977 | 966 | 321 | 5.34 | 33887.8 | 2 | Ⅰ | 2BBX+ CCT |
| TaBBX51 | TraesCS6B02G285200 | 6B:514117744-514119320 | 717 | 238 | 6.04 | 25141.06 | 3 | Ⅳ | 2BBX |
| TaBBX52 | TraesCS6B02G315400 | 6B:563320471-563324616 | 1173 | 390 | 4.96 | 42531.41 | 4 | Ⅲ | 2BBX+ CCT |
| TaBBX53 | TraesCS6B02G319500 | 6B:567397520-567399497 | 1113 | 370 | 5.58 | 41154.91 | 2 | Ⅰ | 2BBX+ CCT |
| TaBBX54 | TraesCS6B02G323400 | 6B:572391940-572393940 | 1287 | 428 | 6.4 | 46597.09 | 2 | Ⅱ | 1BBX + CCT |
| TaBBX55 | TraesCS6D02G133100 | 6D:100871867-100874382 | 645 | 214 | 4.97 | 22054.19 | 2 | Ⅳ | 2BBX |
| TaBBX56 | TraesCS6D02G140900 | 6D:110442898-110444721 | 1095 | 364 | 5.68 | 38019.41 | 3 | Ⅰ | 2BBX+ CCT |
| TaBBX57 | TraesCS6D02G199200 | 6D:277099701-277101029 | 783 | 260 | 5.14 | 28221.83 | 2 | Ⅳ | 2BBX |
| TaBBX58 | TraesCS6D02G202000 | 6D:284849813-284850866 | 954 | 317 | 5.17 | 33352.08 | 2 | Ⅰ | 2BBX+ CCT |
| TaBBX59 | TraesCS6D02G221700 | 6D:312763126-312764854 | 717 | 238 | 5.77 | 25258.08 | 3 | Ⅳ | 2BBX |
| TaBBX60 | TraesCS6D02G267100 | 6D:377220309-377224969 | 1179 | 392 | 4.79 | 43027.95 | 4 | Ⅲ | 2BBX+ CCT |
| TaBBX61 | TraesCS6D02G269500 | 6D:379572086-379574125 | 1110 | 369 | 5.5 | 40998.72 | 2 | Ⅰ | 2BBX+ CCT |
| TaBBX62 | TraesCS6D02G274100 | 6D:382588127-382590143 | 1305 | 434 | 6.12 | 47026.54 | 2 | Ⅱ | 1BBX + CCT |
| TaBBX63 | TraesCS7A02G108400 | 7A:65969778-65975971 | 1080 | 359 | 5.1 | 38311.73 | 3 | Ⅳ | 2BBX |
| TaBBX64 | TraesCS7A02G108700 | 7A:66173971-66174456 | 486 | 161 | 8.98 | 17172.82 | 1 | Ⅴ | 1BBX |
| TaBBX65 | TraesCS7A02G132100 | 7A:85159979-85160728 | 750 | 249 | 7.65 | 25502.35 | 1 | Ⅰ | 1BBX + CCT |
| TaBBX66 | TraesCS7A02G206200 | 7A:168812422-168813720 | 1299 | 432 | 5.32 | 47132.21 | 1 | Ⅱ | 1BBX + CCT |
| TaBBX67 | TraesCS7A02G211300 | 7A:174203246-174205720 | 1158 | 385 | 6.06 | 42218.87 | 2 | Ⅰ | 2BBX+ CCT |
| TaBBX68 | TraesCS7A02G218600 | 7A:185642724-185648145 | 1191 | 396 | 4.92 | 43615.36 | 4 | Ⅲ | 2BBX+ CCT |
| TaBBX69 | TraesCS7A02G263500 | 7A:261221617-261225670 | 1473 | 490 | 6.1 | 52005.04 | 4 | Ⅲ | 1BBX + CCT |
| TaBBX70 | TraesCS7A02G383400 | 7A:558402604-558404184 | 852 | 283 | 5.04 | 29956.59 | 3 | Ⅳ | 2BBX |
| TaBBX71 | TraesCS7A02G497200 | 7A:686936894-686938479 | 1119 | 372 | 5.96 | 39566.55 | 2 | Ⅰ | 2BBX+ CCT |
| TaBBX72 | TraesCS7B02G006400 | 7B:3684186-3689427 | 1017 | 338 | 4.83 | 36159.24 | 4 | Ⅳ | 2BBX |
| TaBBX73 | TraesCS7B02G113400 | 7B:131376607-131378427 | 1299 | 432 | 5.3 | 47173.18 | 1 | Ⅱ | 1BBX + CCT |
| TaBBX74 | TraesCS7B02G118300 | 7B:137793769-137796142 | 1152 | 383 | 6.24 | 42114.89 | 2 | Ⅰ | 2BBX+ CCT |
| TaBBX75 | TraesCS7B02G125500 | 7B:146898679-146904866 | 1014 | 337 | 4.32 | 36449.17 | 3 | Ⅲ | 2BBX |
| TaBBX76 | TraesCS7B02G161500 | 7B:220866464-220870459 | 1467 | 488 | 6.44 | 51810.89 | 4 | Ⅲ | 1BBX + CCT |
| TaBBX77 | TraesCS7B02G286300 | 7B:521577849-521579171 | 936 | 311 | 5.69 | 33195.49 | 2 | Ⅳ | 2BBX |
| TaBBX78 | TraesCS7B02G400600 | 7B:667070044-667071768 | 1119 | 372 | 5.9 | 39407.46 | 2 | Ⅰ | 2BBX+ CCT |
| TaBBX79 | TraesCS7D02G103300 | 7D:63325536-63331403 | 1092 | 363 | 5.03 | 38772.21 | 3 | Ⅳ | 2BBX |
| TaBBX80 | TraesCS7D02G131700 | 7D:83472836-83474058 | 753 | 250 | 6.09 | 25596.33 | 1 | Ⅰ | 1BBX + CCT |
| TaBBX81 | TraesCS7D02G209000 | 7D:167228163-167229829 | 1299 | 432 | 5.26 | 47125.17 | 1 | Ⅱ | 1BBX + CCT |
| TaBBX82 | TraesCS7D02G213000 | 7D:171311981-171314465 | 1119 | 372 | 6.31 | 41073.77 | 2 | Ⅰ | 2BBX+ CCT |
| TaBBX83 | TraesCS7D02G220300 | 7D:180685905-180691434 | 1191 | 396 | 4.92 | 43609.35 | 4 | Ⅲ | 2BBX+ CCT |
| TaBBX84 | TraesCS7D02G264300 | 7D:244952312-244956390 | 1470 | 489 | 6.21 | 51903.89 | 4 | Ⅲ | 1BBX + CCT |
| TaBBX85 | TraesCS7D02G379900 | 7D:492148581-492150240 | 858 | 285 | 5.02 | 30133.81 | 3 | Ⅳ | 2BBX |
| TaBBX86 | TraesCS7D02G484400 | 7D:594729348-594730950 | 1119 | 372 | 5.86 | 39527.57 | 2 | Ⅰ | 2BBX+ CCT |
| TaBBX87 | TraesCSU02G091500 | Un:81434193-81437118 | 1110 | 369 | 6.21 | 40011.43 | 4 | Ⅲ | 1BBX + CCT |
新窗口打开|下载CSV
2.2 小麦B-box基因家族蛋白序列及系统进化分析
TaBBXs蛋白的分子长度在146~490个氨基酸不等。在87个TaBBXs中, 23个TaBBXs包含2个B-box结构域和1个保守的CCT结构域。29个成员包含2个B-box结构域, 但没有CCT结构域。9个TaBBXs只包含1个B-box结构域, 剩下的26个包含1个B-box域和1个CCT结构域(附表1)。蛋白质序列比对显示, TaBBXs的B-box1和B-box2结构域具有相似的保守序列, TaBBXs蛋白质中的CCT结构域是高度保守的(图1)。此外, 特定位点的氨基酸残基高度保守, 暗示其保守的功能, 例如1、4、13、21和24位点的半胱氨酸残基在B-box结构域中高度保守。图1
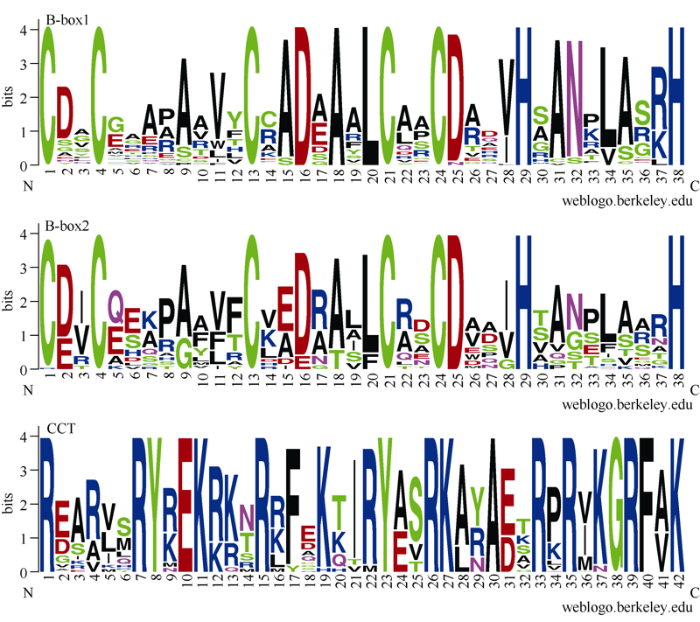 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1小麦B-box基因家族的保守结构域
Fig. 1Conserved domains of B-box genes family in wheat
为了详细研究TaBBXs成员的系统发育关系和功能差异, 对87个TaBBXs蛋白质序列构建系统发育树, 包括AtBBXs和OsBBXs系统发育树(附图1)。根据Khanna等[23]的分组研究和分析, 将TaBBXs进一步划分为5个亚家族(图2), 5个亚家族成员数量分别为23、9、21、28、6。亚家族I、II和III的成员是同时包含B-box结构域和CCT结构域的TaBBXs。亚家族I成员包含1~2个B-box结构域和1个CCT结构域, 亚家族II成员包含1个B-box结构域和1个CCT结构域, 亚家族III成员包含1~2个B-box结构域和0~1个CCT结构域。小麦有两类在拟南芥中没有发现的B-box基因, 亚族I成员中有一类, 它包含1个B-box结构域(TaBBX23、TaBBX29、TaBBX35、TaBBX65和TaBBX80); 另一类是亚族III成员, 其中大多数成员具有1个B-box结构域和1个CCT结构域, 但TaBBX44、TaBBX52、TaBBX60、TaBBX68和TaBBX75拥有B-box2结构域, 这与包含2个B-box结构域的其他成员分组不同。第IV组和第V组的成员没有CCT结构域, 分别有2个和1个B-box结构域。
附图1
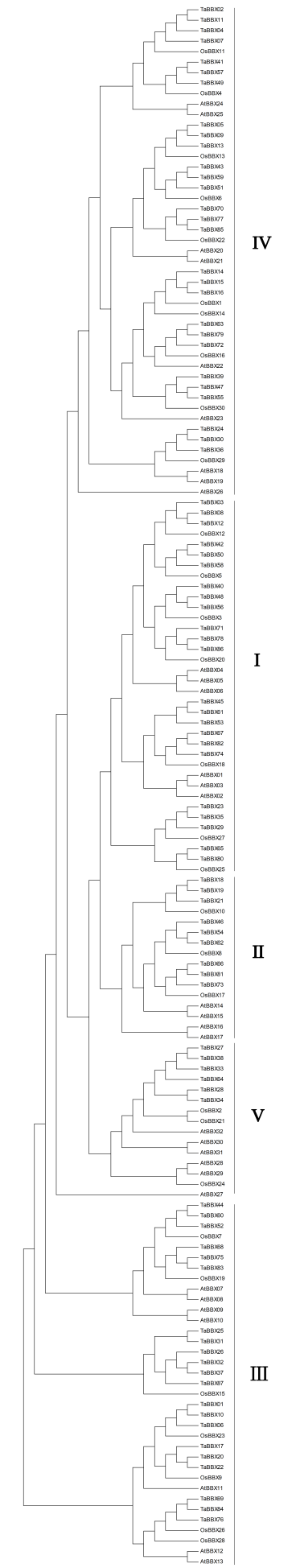 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT附图1小麦、拟南芥和水稻B-box基因系统进化树
Fig. S1Phylogenetic tree of B-box genes in wheat, A. thaliana and Oryza sativa
图2
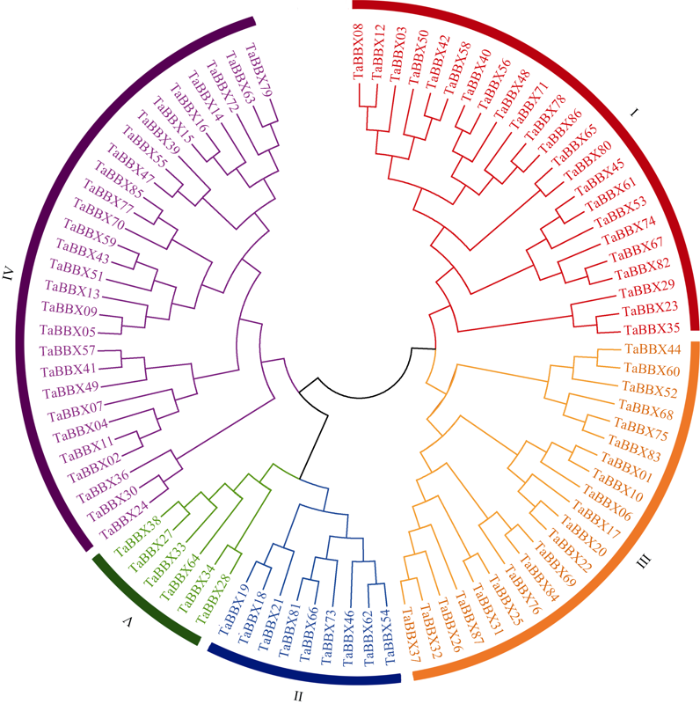 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2小麦B-box基因家族进化树
Fig. 2Phylogenetic tree of B-box genes family in wheat
2.3 小麦B-box基因家族的染色体定位与基因复制
小麦B-box基因不均等地分布在小麦21条染色体中的18条上, 1A、1B、1D染色体上没有发现B-box基因, 其中的1个成员(TaBBX87)无法进一步定位到染色体(图3)。在7A染色体上分布的基因最多, 共含有9个基因; 其次是6A、6B、6D和7D染色体上分别含有8个基因; 其余染色体上均含有1~7个基因不等。有92% (80/87)的小麦B-box成员显示出重复事件, 在122个复制事件中并未发现串联重复事件, 在不同的染色体中发现了高度相似的基因, 出现了片段重复事件。如图3所示, 重复事件主要发生在染色体6A、6B和6D上, 而3A、3B和3D上则较少。此外, 我们对同源基因进行进一步聚类分析发现, 所有同源基因都处于同一进化分支中(附图2), 除TaBBX17、TaBBX20、TaBBX65和TaBBX80基因外, 在3个部分同源染色体组(A、B、D)上都有同源位点, 如: TaBBX03、TaBBX08和TaBBX12为同源基因位于同一进化分支中, 且分别位于2A、2B和2D染色体上, 表明小麦B-box基因具有大量的同源位点, 同源保留率高。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3小麦B-box基因家族成员在染色体上的位置和基因复制
每个彩色条代表一条染色体, 基因命名是根据它们在染色体上的位置来标记的, 片段复制基因用彩色线条连接。
Fig. 3Chromosome location and gene duplications of the B-box gene family in wheat
Each colored bar represents a chromosome, gene names are labeled on the basis of their positions on the chromosomes, segmental duplication genes are linked by colored lines.
附图2
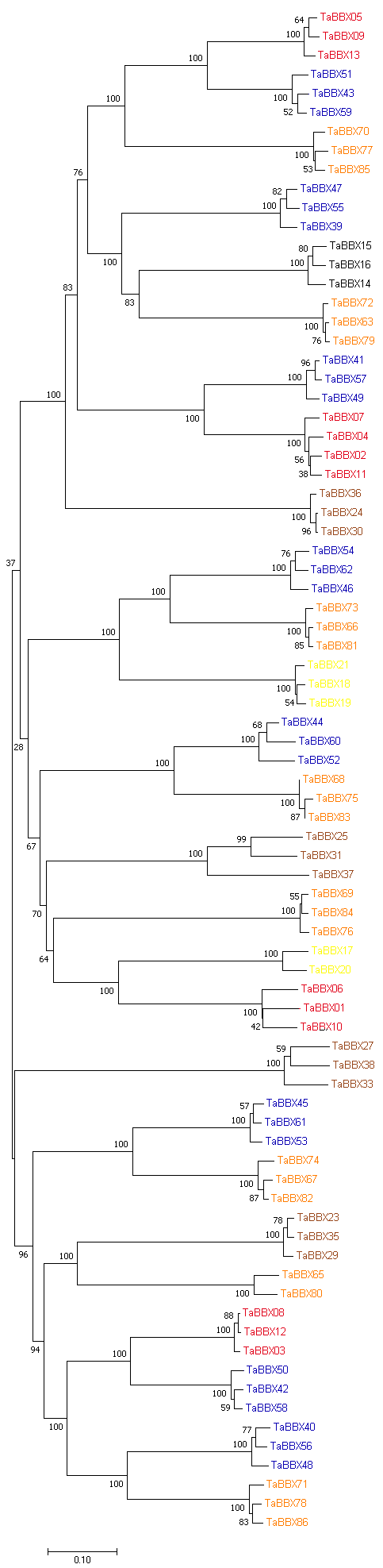 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT附图2小麦B-box同源基因进化树
红色:位于2A、2B和2D染色体上的基因;黑色:位于3A、3B和3D染色体上的基因;黄色:位于4A、4B和4D染色体上的基因;棕色:位于5A、5B和5D染色体上的基因;蓝色:位于6A、6B和6D染色体上的基因;橘色:位于7A、7B和7D染色体上的基因。
Fig. S2Phylogenetic tree of B-box homoeologs genes in wheat
Red: gene on chromosome 2A, 2B and 2D; Black: gene on chromosome 3A, 3B and 3D; Yellow: gene on chromosome 4A, 4B and 4D; Brown: gene on chromosome 5A, 5B and 5D; Blue: gene on chromosome 6A, 6B and 6D; Orange: gene on chromosome 7A, 7B and 7D.
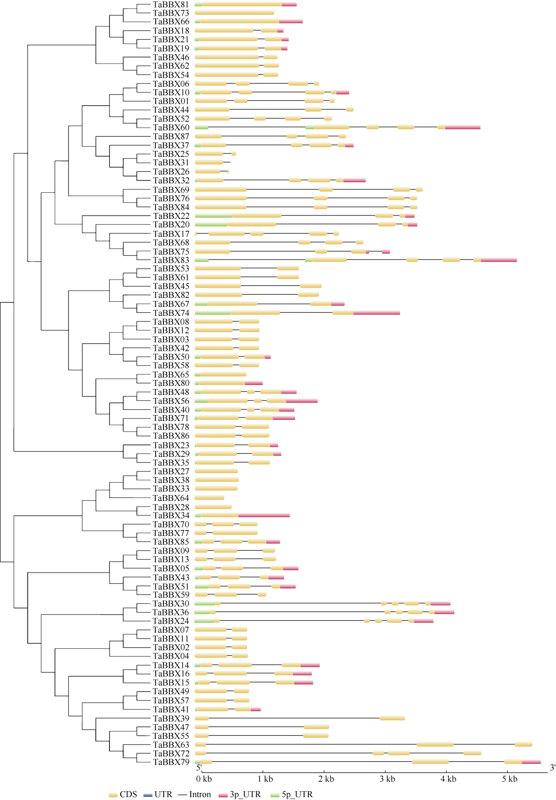
2.4 小麦B-box基因家族的基因结构分析
小麦B-box家族成员的外显子数目为1~5个, 内含子数目为0~4个(图4)。对基因结构进一步分析发现, I组的大多数成员具有1个内含子; II组中TaBBX66、TaBBX73和TaBBX81不含内含子, 其余基因含1个内含子; III组和IV组的成员结构较相似, 含1~4个内含子; V组中的基因结构较简单, 所有成员都不含内含子。图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4小麦B-box基因家族基因结构
Fig. 4Gene structure of B-box gene family in wheat
2.5 小麦B-box基因家族的启动子分析
从TaBBXs中鉴定出101种顺式作用元件, 其中40种在大多数成员中被检测出来。除了2种传统的启动子元件(TATA-box, CAAT-box)外, 其余38个顺式作用元件可分为4组: 14个具有光响应性, 包括MRE、G-Box、ACE、AE-box、Sp1、GT1-motif、GATA-motif、TCCC-motif、I-box、TCT-motif、GA-motif、Box I、Box 4和ATCT-motif; 8个是激素反应性的, 包括CGTCA-motif、TGACG-motif、ABRE、P-box、ERE、GARE-motif、AuxRR-core和TGA-element; 9个是胁迫响应元件: ARE、MBS、LTR、W box、GC-motif、CCAAT-box、TCA-element、WUN-motif和TC-rich repeats。第4组为其他顺式作用元件, 如: 胚乳表达所需的顺式作用元件(GCN4_motif), 与分生组织表达相关的顺式作用调控元件(CAT-box)等。下表展示了与胁迫和激素响应相关的元件(表2)。Table 2
表2
表2预测小麦B-box家族基因启动子中顺式调控元件
Table 2
| 顺式作用元件 cis-elements | 基因数目 Number of genes | 顺式作用元件的功能 Functions of cis-elements |
|---|---|---|
| CAAT-box | 87 | Common cis-acting element in promoter and enhancer regions |
| TATA-box | 87 | Core promoter element around -30 of transcription start |
| CGTCA-motif | 75 | cis-acting regulatory element involved in the MeJA-responsiveness |
| TGACG-motif | 75 | cis-acting regulatory element involved in the MeJA-responsiveness |
| ABRE | 84 | cis-acting element involved in the abscisic acid responsiveness |
| ARE | 67 | cis-acting regulatory element essential for the anaerobic induction |
| MBS | 49 | MYB binding site involved in drought-inducibility |
| LTR | 57 | cis-acting element involved in low-temperature responsiveness |
| W box | 51 | Wounding and pathogen responsiveness. |
| TGA-element | 42 | Auxin-responsive element |
| GC-motif | 51 | Enhancer-like element involved in anoxic specific inducibility |
| CCAAT-box | 24 | MYBHv1 binding site |
| TCA-element | 30 | cis-acting element involved in salicylic acid responsiveness |
| P-box | 31 | Gibberellin-responsive element |
| WUN-motif | 20 | Wound-responsive element |
| ERE | 18 | Ethylene-responsive element |
| GARE-motif | 23 | Gibberellin-responsive element |
| TC-rich repeats | 27 | cis-acting element involved in defense and stress responsiveness |
| AuxRR-core | 18 | cis-acting regulatory element involved in auxin responsiveness |
新窗口打开|下载CSV
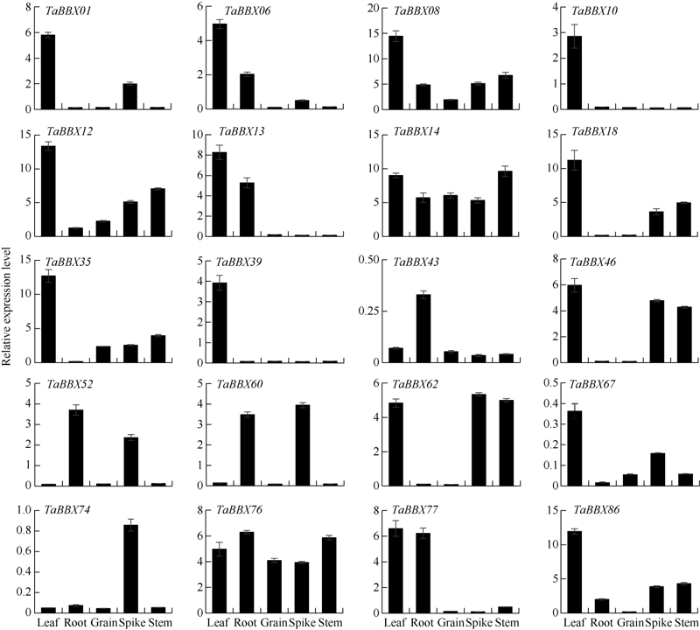
2.6 小麦B-box基因家族在不同组织和非生物胁迫及激素处理下的表达
采用qRT-PCR分析了20个小麦B-box基因在不同组织和干旱、低温、盐和ABA处理下的表达模式(图5)。20个基因在所有检测组织中均有不同程度的表达, TaBBX08、TaBBX12、TaBBX14、TaBBX43、TaBBX76在所有组织中均有表达, 大多数(16个)基因在叶中有较高的表达, 其次是在穗、根和茎中表达, 而较少在籽粒中表达。TaBBX10和TaBBX39在叶中有较高表达, TaBBX74在穗中特异性表达, TaBBX43在根中特异性表达, TaBBX52和TaBBX60在穗和根中表达量较高。图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5小麦B-box基因家族在不同组织中的表达分析
Fig. 5Expression profile of B-box gene family in different tissues of wheat
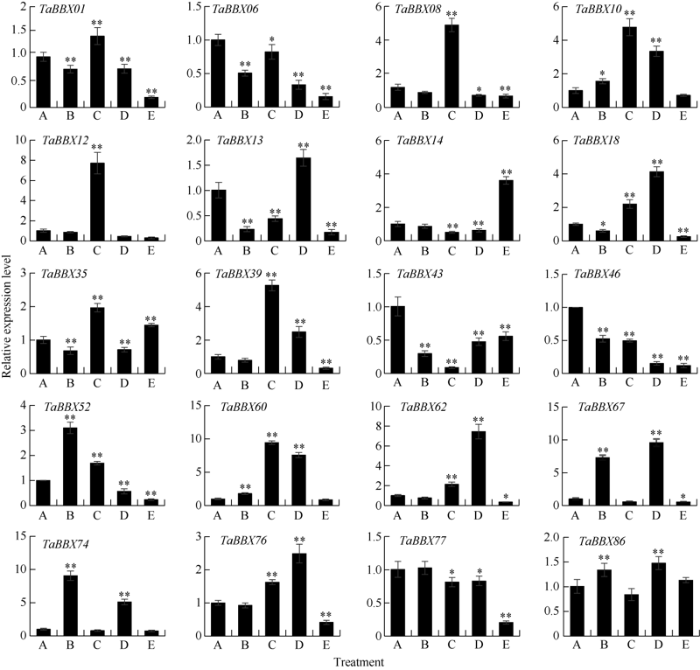
TaBBXs响应非生物胁迫, 但表达水平不同(图6)。在干旱胁迫下, TaBBX10、TaBBX52、TaBBX60、TaBBX67、TaBBX74和TaBBX86显著上调表达; 大多数基因在低温胁迫下上调表达, TaBBX08、TaBBX10、TaBBX12、TaBBX39和TaBBX60等11个基因的表达量与对照组相比显著上调; 在盐胁迫处理下, 基因出现上调或下调表达, 其中TaBBX60、TaBBX62、TaBBX67和TaBBX76等10个基因显著上调表达; 在ABA处理下, 除TaBBX14、TaBBX35和TaBBX86外, 所有小麦B-box基因成员的表达水平都很低; TaBBX10、TaBBX39、TaBBX60、TaBBX67和TaBBX74在2种或2种以上胁迫下相比对照有显著的上调表达。
图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6小麦B-box基因家族在不同处理下的表达分析
A: 对照; B: 20% PEG处理6 h; C: 4℃处理6 h; D: 0.2 mol L-1 NaCl处理6 h; E: 100 μmol L-1 ABA处理6 h。*表示在0.05水平上显著; **表示在0.01水平上显著。
Fig. 6Expression profile of B-box gene family under different treatments in wheat
A: control; B: six hours of 20% PEG treatment; C: six hours of 4℃ treatment; D: six hours of 0.2 mol L-1 NaCl treatment; E: six hours of 100 μmol L-1 ABA treatment. * and ** indicate significantly different at P < 0.05 and P < 0.01, respectively.
3 讨论
B-box基因家族是锌指转录因子中的一个家族, 包含B-box和CCT结构域[23,25]。目前, B-box基因家族已经在多个物种中被鉴定出来, 比如在拟南芥[23]、水稻[24]、番茄[26]、马铃薯[27]、苹果[28,29]、梨[30]、葡萄[31]分别鉴定出32、30、29、30、64、37、24个B-box家族成员。本研究从小麦全基因组数据库中获得了87个B-box基因成员, 均高于上述物种中的成员数, 可能是因为小麦是异源六倍体, 具有3个部分同源基因组, 存在更多的同源基因(homoeologs), 高同源保留率也可以部分解释小麦B-box基因数量多的原因。根据B-box结构域的数目和序列特征以及CCT结构域的存在特征, B-box在拟南芥中被分为5组, I组和II组中的所有成员都包含2个B-box和CCT结构域, 第III组具有1个B-box和CCT结构域, 第IV组和V组分别具有2个和1个B-box结构域[23]。在小麦中同样被分为5组, 有1个或2个B-box结构域和1个CCT结构域的53个TaBBXs被分为I组、II组和III组, 具有2个B-box结构域的28个成员为IV组, V组6个成员含有1个B-box结构域。但其中III组中有4个TaBBXs (TaBBX25、TaBBX26、TaBBX31和TaBBX75)不具有CCT结构域, 这与拟南芥分组不一致, 在其他物种中也发现了类似的不一致, 例如, 水稻中的OsBBX25和OsBBX27属于第I组, 玉米中的ZmBBX7属于第II组, 尽管它们都缺少1个B-box结构域[25,32]。番茄中的SlBBX9、SlBBX11和SlBBX12以及水稻中的OsBBX7和OsBBX19属于第III组, 尽管它们有2个B-box结构域[25,26]。可能是进化缺失或重复导致了分组的差异, 与之前在水稻研究中的分类相似[25]。
基因结构的多样性在基因家族的进化中也起着重要的作用。本研究结果显示, 小麦B-box基因在同组内同一个系统进化树分支中的亚族成员具有高度相似的基因结构, 总体内含子数目较少, 显示出进化的保守性。V组中的一些同源基因, 如TaBBX24、TaBBX30和TaBBX36显示了一个保守的基因结构, 与同组成员有所差异。这种差异可能不是偶然的突变事件, 而是植物B-box转录因子进化的一种保守模式[33]。此外, 保守序列分析发现, B-box1与B-box2相比具有更高的保守性, CCT结构域是高度保守的[32]。前人报道中, 有一种理论认为, 最初仅有的B-box2结构域在进化过程中发生复制事件随后又发生删除事件, 导致出现B-box结构域数目不同的B-box基因[32]。本研究中B-box1比B-box2具有更高的保守性, 所以可能是B-box2中发生了删除事件并产生B-box1, 与Crocco和Botto提出的进化模型一致[32]。
复制事件对植物进化过程中基因家族成员的扩增至关重要[34]。植物中的基因复制主要有片段复制和串联复制[35,36]。在本研究中, 没有发现串联复制事件只发生了片段复制事件。表明片段复制事件可能是小麦B-box基因家族成员扩增的主要原因, 这与玉米、水稻等物种相类似[37]。
基因表达模式可以提供相关基因功能的重要信息。在拟南芥中, B-box基因(BBX6, COL5)的过表达促进开花[38], 而过表达COL9 (BBX7)则延迟开花[39], 在小麦中与BBX6处于同一组的基因有TaBBX08、TaBBX12、TaBBX35、TaBBX67、TaBBX74和TaBBX86, 其中TaBBX08、TaBBX12和TaBBX35基因在低温胁迫下显著上调表达。小麦开花主要受春化影响, 即小麦开花需经过长时间环境低温诱导的生理过程, 同时在TaBBX12和TaBBX35启动子区发现了参与低温响应的顺式作用元件LTR (表2), 因此TaBBX12和TaBBX35基因可能参与了小麦开花调控过程。在玉米中, B-box同源基因参与不同的生物学过程, 在基因表达上具有明显的组织特异性[40]; B-box基因家族成员在番茄各组织中均有不同程度的表达[26], 马铃薯B-box家族成员在不同器官中表达模式不同[27]。小麦B-box基因在不同组织中有不同的表达模式, TaBBX10、TaBBX39等16个基因在叶中有较高的表达, TaBBX43、TaBBX52、TaBBX60和TaBBX74在穗、根等组织中表达量较高, 说明小麦B-box基因在植物生长发育过程中可能发挥重要作用, 在不同发育阶段可能具有独特的功能。
非生物胁迫, 如盐、干旱、极端温度, 都会对植物的生长和发育产生负面影响[41,42]。本研究发现, 在B-box基因启动子区存在胁迫反应顺式作用元件, 如ARE、LTR、MBS和TC-rich, 与干旱、盐和低温有关。79个B-box基因都至少具有1个胁迫响应顺式作用元件, 表明它们在逆境反应中可能发挥着重要作用。在盐、低温、干旱胁迫和ABA处理下, 小麦B-box家族成员均有不同程度的诱导表达。之前的研究结果表明, 耐盐蛋白AtBBX24在拟南芥的高盐度条件下促进根的生长, 耐盐活性也在酵母细胞中触发[17], 小麦TaBBX13、TaBBX14、TaBBX39、TaBBX43和TaBBX77与AtBBX24处于同一分组内, 这些基因受到盐胁迫诱导, 因此TaBBX13、TaBBX14、TaBBX39、TaBBX43和TaBBX77可能参与了盐胁迫调控。菊花CmBBX24能够提高植物的耐寒性或耐旱性[43], OsBBX25在拟南芥中过表达, 可以增强转基因植物对干旱胁迫的抗性[44], 小麦TaBBX08、TaBBX12、TaBBX35、TaBBX67、TaBBX74和TaBBX86与OsBBX25处于同一分组内, 干旱胁迫显著诱导TaBBX35、TaBBX67、TaBBX74和TaBBX86的表达, 并且在TaBBX67和TaBBX74基因启动子中发现含有响应干旱胁迫的顺式作用元件MBS (表2), 表明TaBBX67和TaBBX74在干旱胁迫过程中可能起着重要的作用。我们还发现, TaBBX10和TaBBX60在盐、低温和干旱胁迫下都上调表达, 表明这2个基因可能整合了不同的非生物胁迫信号。TaBBX01、TaBBX08和TaBBX13等11个基因在盐、低温和干旱胁迫下表现出不同的表达模式, 说明这些基因可能在小麦响应不同的非生物胁迫过程中发挥作用。
4 结论
本研究对小麦B-box基因家族进行了全基因组鉴定, 共筛选获得87个TaBBXs家族成员, 可分为5组。除了1A、1B和1D号染色体外, 其他的18条小麦染色体上均有TaBBXs分布, TaBBXs内含子数目0~4个。小麦B-box基因家族表达具有组织特异性, TaBBXs不同程度地响应盐、干旱和低温胁迫, 推测TaBBXs可能参与调控小麦的盐、 干旱和低温等逆境响应过程。附表和附图 请见网络版: 1) 本刊网站
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 2]
[本文引用: 1]
[本文引用: 1]
PMID [本文引用: 1]

The term zinc finger was first used to describe a 30-residue, repeated sequence motif found in an unusually abundant Xenopus transcription factor. It was proposed that each motif is folded around a central zinc ion to form an independent minidomain and that adjacent zinc fingers are combined as modules to make up a DNA-binding domain with the modules "gripping" the DNA (hence the term finger). We now know that these proposals were correct and that these DNA-binding motifs are found in many eukaryotic DNA-binding proteins. More recently, crystal structures of three different complexes between zinc finger domains and their target DNA binding sites have revealed a remarkably simple mode of interaction with DNA. The simplicity of the zinc finger structure, and of its interaction with DNA, is a very striking feature of this protein domain. After the discovery of the zinc finger motif, patterns of potential zinc ligands have been found in several other proteins, some of which also bind to DNA. Structural studies of these domains have revealed how zinc can stabilize quite diverse protein architectures. In total, 10 such small zinc-binding domains have been studied structurally. These form a diverse collection, but each in turn has been termed a zinc finger motif-although clearly what they have in common is only their zinc-binding property, which stabilizes an apparently autonomously folded unit.
PMID [本文引用: 1]

The vegetative and reproductive (flowering) phases of Arabidopsis development are clearly separated. The onset of flowering is promoted by long photoperiods, but the constans (co) mutant flowers later than wild type under these conditions. The CO gene was isolated, and two zinc fingers that show a similar spacing of cysteines, but little direct homology, to members of the GATA1 family were identified in the amino acid sequence. co mutations were shown to affect amino acids that are conserved in both fingers. Some transgenic plants containing extra copies of CO flowered earlier than wild type, suggesting that CO activity is limiting on flowering time. Double mutants were constructed containing co and mutations affecting gibberellic acid responses, meristem identity, or phytochrome function, and their phenotypes suggested a model for the role of CO in promoting flowering.
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 2]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
PMID [本文引用: 2]

The salt-sensitive phenotype of yeast cells deficient in the phosphoprotein phosphatase, calcineurin, was used to identify genes from the higher plant Arabidopsis thaliana that complement this phenotype. cDNA clones corresponding to two different sequences, designated STO (salt tolerance) and STZ (salt tolerance zinc finger), were found to increased tolerance of calcineurin mutants and of wild-type yeast to both Li+ and Na+ ions. STZ is related to Cys2/His2-type zinc-finger proteins found in higher plants, and STO is similar to the Arabidopsis CONSTANS protein in regions that may also be zinc fingers. Although neither protein has sequence similarity to any protein phosphatase, STO was able to at least partially compensate for all tested additional phenotypic effects of calcineurin deficiency, and STZ compensated for a subset of these effects. Salt tolerance produced by STZ appeared to be partially dependent on ENA1/PMR2, a P-type ATPase required for Li+ and Na+ efflux in yeast, whereas the effect of STO on salt tolerance was independent of ENA1/PMR2. STZ and STO were found to be expressed in Arabidopsis roots and leaves, whereas only STO message was detectable in flowers. An apparent increase in the level of STZ mRNA was observed in response NaCl exposure in Arabidopsis seedlings, but the level of STO mRNA was not altered by this treatment.
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 5]
DOIURL [本文引用: 2]
DOIURL [本文引用: 4]
[本文引用: 3]
DOIURL [本文引用: 2]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 4]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIPMID [本文引用: 1]

To ensure that the initiation of flowering occurs at the correct time of year, plants need to integrate a diverse range of external and internal signals. In Arabidopsis, the photoperiodic flowering pathway is controlled by a set of regulators that include CONSTANS (CO). In addition, Arabidopsis plants also have a family of genes with homologies to CO known as CO-LIKE (COL) about which relatively little is known. In this paper, we describe the regulation and interactions of a novel member of the family, COL5. The expression of COL5 is under circadian and diurnal regulation, but COL5 itself does not appear to affect circadian rhythms. COL5, like CO, is regulated by GIGANTEA. Furthermore, COL5 is expressed in the vascular tissue. Using COL5 over-expressing lines we show that, under short days, constitutive expression of COL5 affects flowering time and the expression of the floral integrator genes, FLOWERING LOCUS T and SUPPRESSOR OF OVEREXPRESSION OF CO 1. Constitutive expression of COL5 partially suppresses the late flowering phenotype of the co-mutant plants. However, plants with loss of COL5 function do not show altered flowering. Taken together, our results suggest that COL5 has COL activity, but may either not have a role in regulating flowering in wild-type plants or may act redundantly with other flowering regulators.
DOIURL [本文引用: 1]
[本文引用: 1]
PMID [本文引用: 1]

Salinization is the accumulation of water-soluble salts in the soil solum or regolith to a level that impacts on agricultural production, environmental health, and economic welfare. Salt-affected soils occur in more than 100 countries of the world with a variety of extents, nature, and properties. No climatic zone in the world is free from salinization, although the general perception is focused on arid and semi-arid regions. Salinization is a complex process involving the movement of salts and water in soils during seasonal cycles and interactions with groundwater. While rainfall, aeolian deposits, mineral weathering, and stored salts are the sources of salts, surface and groundwaters can redistribute the accumulated salts and may also provide additional sources. Sodium salts dominate in many saline soils of the world, but salts of other cations such as calcium, magnesium, and iron are also found in specific locations. Different types of salinization with a prevalence of sodium salts affect about 30% of the land area in Australia. While more attention is given to groundwater-associated salinity and irrigation salinity, which affects about 16% of the agricultural area, recent investigations suggest that 67% of the agricultural area has a potential for "transient salinity", a type of non-groundwater-associated salinity. Agricultural soils in Australia, being predominantly sodic, accumulate salts under seasonal fluctuations and have multiple subsoil constraints such as alkalinity, acidity, sodicity, and toxic ions. This paper examines soil processes that dictate the exact edaphic environment upon which root functions depend and can help in research on plant improvement.
DOIPMID [本文引用: 1]

The natural environment for plants is composed of a complex set of abiotic stresses and biotic stresses. Plant responses to these stresses are equally complex. Systems biology approaches facilitate a multi-targeted approach by allowing one to identify regulatory hubs in complex networks. Systems biology takes the molecular parts (transcripts, proteins and metabolites) of an organism and attempts to fit them into functional networks or models designed to describe and predict the dynamic activities of that organism in different environments. In this review, research progress in plant responses to abiotic stresses is summarized from the physiological level to the molecular level. New insights obtained from the integration of omics datasets are highlighted. Gaps in our knowledge are identified, providing additional focus areas for crop improvement research in the future.
DOIURL [本文引用: 1]
DOI [本文引用: 1]

锌指蛋白在调控植物生长发育和应对逆境过程中发挥着重要作用。为进一步研究锌指类蛋白参与植物非生物胁迫响应的分子机制, 对水稻(Oryza sativa)中一个编码含有B-box锌指结构域蛋白的OsBBX25基因进行了功能分析。OsBBX25受盐、干旱和ABA诱导表达。异源表达OsBBX25的转基因拟南芥(Arabidopsis thaliana)与野生型相比对盐和干旱的耐受性增强, 且盐胁迫条件下转基因植物中KIN1、RD29A和COR15的表达上调, 干旱胁迫下KIN1、RD29A和RD22的表达上调。外源施加ABA时, 转基因植物的萌发率与野生型之间没有明显差异。OsBBX25可能作为转录调控的辅助因子调节胁迫应答相关基因的表达, 进而参与植物对非生物胁迫的响应。
[本文引用: 1]
