 ,, 倪文荣, 吕尊富, 林燕, 林力卓, 钟子毓, 崔鹏, 陆国权
,, 倪文荣, 吕尊富, 林燕, 林力卓, 钟子毓, 崔鹏, 陆国权 ,*浙江农林大学农业与食品科学学院/浙江省农产品品质改良重点实验室, 浙江杭州 311300
,*浙江农林大学农业与食品科学学院/浙江省农产品品质改良重点实验室, 浙江杭州 311300Identification and index screening of soft rot resistance at harvest stage in sweetpotato
ZHANG Si-Meng ,, NI Wen-Rong, LYU Zun-Fu, LIN Yan, LIN Li-Zhuo, ZHONG Zi-Yu, CUI Peng, LU Guo-Quan
,, NI Wen-Rong, LYU Zun-Fu, LIN Yan, LIN Li-Zhuo, ZHONG Zi-Yu, CUI Peng, LU Guo-Quan ,*College of Agriculture and Food Science, Zhejiang Agriculture and Forest University/Key Laboratory for Quality Improvement of Agricultural Products of Zhejiang Province, Hangzhou 311300, Zhejiang, China
,*College of Agriculture and Food Science, Zhejiang Agriculture and Forest University/Key Laboratory for Quality Improvement of Agricultural Products of Zhejiang Province, Hangzhou 311300, Zhejiang, China通讯作者: * 陆国权, E-mail:lugq10@zju.edu.cn
收稿日期:2020-09-18接受日期:2020-12-1网络出版日期:2021-08-12
| 基金资助: |
Received:2020-09-18Accepted:2020-12-1Online:2021-08-12
| Fund supported: |
作者简介 About authors
E-mail:1826213445@qq.com

摘要
软腐病是甘薯贮藏期最具破坏力的病害之一, 病原菌是匍枝根霉(Rhizopus stolonifer), 匍枝根霉从伤口侵染, 利用伤口的营养物质进行繁殖, 破坏细胞壁, 造成薯块软烂。本研究以不同时期收获的6个甘薯品种块根为试验材料, 通过薯片接菌碟法鉴定软腐病抗性, 测定薯块的质构特性(硬度、黏附性、黏附力、内聚性、弹性、咀嚼性、胶黏性)、营养物质(干率、淀粉、果糖、葡萄糖、蔗糖、粗蛋白、粗纤维)、抗性酶活(POD、PPO、PAL)等生理指标, 对各指标值进行相关分析、灰色关联度分析、隶属函数分析, 筛选和综合评价软腐病抗性指标。根据病斑直径将甘薯软腐病抗性划分等级, 通过病斑直径和各指标值的相关分析、灰色关联度分析, 筛选抗软腐病指标并确定其权重, 再进行隶属函数分析, 得到不同材料软腐病抗性综合评价值(D值), 通过综合评价值和病斑直径比较及相关分析验证指标筛选的可靠性。试验材料的软腐病抗性结果显示, 90 d收获甘薯软腐病抗性表现为抗病, 105 d收获甘薯整体表现为感病和高感, 120、135、150 d收获甘薯软腐病整体抗性为中抗, 同时筛选出果糖含量、咀嚼性、内聚性、弹性、粗蛋白含量、POD、PAL酶活性等7个可评价甘薯软腐病抗性的指标。本研究可为甘薯抗软腐新品种选育提供种质并为甘薯软腐病抗性评价及抗软腐机制研究提供理论依据。
关键词:
Abstract
Soft rot is one of the most destructive diseases during sweetpotato storage. Cell walls were destroyed and soft rot was caused by Rhizopus Stolonifer, which invaded from wounds and propagated with nutrients from wounds. Six varieties of sweetpotato roots in different harvest period were used as the experimental material to identify the resistance index to soft rot, through the inoculation with sweetpotato chips. Physiological indexes including texture of roots (hardness, adhesion, adhesion force, cohesiveness, elasticity, chewiness, and glue viscosity), nutrients (dry matter content, starch, fructose, glucose, sucrose, crude protein and crude fiber), resistance enzyme activity (POD, PPO, PAL) were investigated in this study. Their correlation analysis, grey correlation analysis of each index and subordinate function analysis were applied in screening and comprehensive evaluation of soft rot resistance. The soft rot resistance of sweetpotato roots was graded based on the disease spot diameter. The index and weight of soft rot resistance were conducted by correlation analysis and grey correlation analysis of disease spot diameter and index values. Comprehensive evaluation value (D-value) of six varieties of sweetpotato roots at different harvest stages were calculated using the membership function analysis. The reliability of indicators selection was verified through correlation analysis of D-value based on the disease spot diameter. Soft rot resistance of sweetpotato was high in 90 day at harvest stage, moderate resistance in 120, 135, 150 day of harvest stage, and hypersensitivity and susceptibility in 105 day of harvest stage. Seven indexes, including fructose content selection, chewiness, cohesiveness, elasticity, protein content, POD and PAL enzyme activity, were filtered out to estimate the resistance of soft rot in sweetpotato. These results could provide the germplasm information for selection and breeding of new sweetpotato varieties resistant to soft rot and could serve as a basis for subsequent assessment of sweetpotato resistant to soft rot and their soft rot resistance mechanism.
Keywords:
PDF (1489KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
张思梦, 倪文荣, 吕尊富, 林燕, 林力卓, 钟子毓, 崔鹏, 陆国权. 影响甘薯收获期软腐病发生的指标筛选. 作物学报[J], 2021, 47(8): 1450-1459 DOI:10.3724/SP.J.1006.2021.04213
ZHANG Si-Meng, NI Wen-Rong, LYU Zun-Fu, LIN Yan, LIN Li-Zhuo, ZHONG Zi-Yu, CUI Peng, LU Guo-Quan.
甘薯是世界排名第七的粮食作物[1], 其块根含有丰富营养物质[2,3]。甘薯软腐病分布广泛, 在全球均是甘薯贮藏期的最具破坏力的病害[4]。感染软腐病使薯块组织软烂, 蔓延迅速, 常使全窑腐烂, 造成严重的经挤损失[5], 软腐病的抗性研究对甘薯产业至关重要。甘薯软腐病病原菌是匍枝根霉(Rhizopus stolonifer)[6], 根霉属真菌[7], 匍枝根霉从伤口侵染甘薯, 产生的果胶酶、淀粉酶、纤维素分解酶, 破坏细胞壁[8]。甘薯软腐病病害程度和品种[9]、采前条件[10]、贮藏条件、愈伤处理、甘薯破损程度和类型[11]均有着直接关联。甘薯软腐病抗性研究主要集中在种质资源和外源处理2个方面, 抗性鉴定均采用薯块接菌法, 在薯块上放置同等的菌碟, 一定时间后, 通过薯块的病斑直径来判断甘薯软腐病抗性, 研究表明不同甘薯品种间软腐病抗性差异显著, 甘薯喷施抑菌剂能够提高软腐病抗性[12,13,14,15], 随着粮食药剂管理的日益严格和对有机农作物的推崇, 培育抗病强的甘薯品种是最直接有效的途径。甘薯软腐病抗性鉴定方法已有相关研究, 确定抗软腐指标可以有目的的选择甘薯品种, 加快抗病性育种工作进程。灰色系统理论中的关联度分析是对动态系统进行量化比较的分析方法, 系统中因素之间的关联度大, 说明其变化态势接近, 相互关系密切; 反之, 其相互关系疏远[16]。灰色关联度分析在小麦、玉米等作物方面已经得到较广的运用, 关于指标筛选已取得良好的效果[17,18]。不同甘薯品种对软腐病的抗性存在差异, 而软腐病病菌主要通过伤口侵染, 并利用伤口的营养物质进行繁殖[11], 表明甘薯营养物质对软腐病抗性有着直接的影响, Petsch等[19]发现了匍枝根霉侵染番茄果实都需要经过特定的熟化过程, 甘薯块根的营养物质含量也受到收获期的影响。本研究选取不同收获期的甘薯作为软腐病抗性研究材料, 从甘薯块根的质构特性、营养物质、抗性酶活3个方面进行指标的筛选。本文选取不同甘薯品种, 不同时期收获, 鉴定软腐病抗性的差异, 并进行指标筛选, 旨为甘薯不同收获期软腐病抗性提供依据, 为选育甘薯抗软腐优良品种提供理论依据。
1 材料与方法
1.1 试验材料与处理
试验材料选取紫薯型(‘徐紫薯8号’、‘漯紫薯4号’)、鲜食型(‘烟薯25’、‘心香’)、淀粉型(‘济薯25’、‘浙薯13’)共6个甘薯品种, 均于2019年5月在浙江农林大学东湖校区官塘试验基地统一种植, 甘薯收获期一般为90~150 d, 选取90、105、120、135、150 d 5个收获期进行软腐病抗性研究。选择无病虫害、大小一致、形状相似的薯块, 清洗后进行抗病性鉴定和指标测定, 试验重复3次, 甘薯样品编号如表1。Table 1
表1
表1甘薯样品编号
Table 1
| 品种 Variety | 收获时间Harvest time | ||||
|---|---|---|---|---|---|
| 90 d | 105 d | 120 d | 135 d | 150 d | |
| 徐紫薯8号 Xuzishu 8 | 1-1 | 1-2 | 1-3 | 1-4 | 1-5 |
| 烟薯25 Yanshu 25 | 2-1 | 2-2 | 2-3 | 2-4 | 2-5 |
| 济薯25 Jishu 25 | 3-1 | 3-2 | 3-3 | 3-4 | 3-5 |
| 浙薯13 Zheshu 13 | 4-1 | 4-2 | 4-3 | 4-4 | 4-5 |
| 心香 Xinxiang | 5-1 | 5-2 | 5-3 | 5-4 | 5-5 |
| 漯紫薯4号 Luozishu 4 | 6-1 | 6-2 | 6-3 | 6-4 | 6-5 |
新窗口打开|下载CSV
1.2 指标测定方法
甘薯块根的软腐病抗性鉴定参考杨冬静等[12]方法: 选取3块中等大小的薯块, 经严格消毒晾干后, 切薯块中部薯片, 每薯块切3个薯片, 每薯片厚度约为8 mm, 培养皿中放置湿润纱布, 接种1块6 mm的甘薯软腐病菌菌碟于薯片中央, 将接种好的薯片置于26℃生化培养箱中培养, 接种21 h后将平板取出, 量取薯片横切面上薯片发病的直径, 通过病斑直径计算病情指数, 根据病情指数进行品种的抗感特性评价, 重复试验中出现抗感不一致情况下, 作感病处理, 可将甘薯各样品的软腐病抗性划分为5个等级, 分级及抗感评价标准为, 0级: 薯片正常无任何病症; 1级: 薯片病斑直径≤10 mm; 2级: 10 mm<薯片病斑直径≤20 mm; 3级: 20 mm<薯片病斑直径≤30 mm; 4级: 薯片病斑直径>30 mm。病情指数计算公式:
$病情指数=\frac{\sum{(各级感病薯片数\times 相应级数)}}{ 调查总薯片数\times 最高病级数(4)}\times 100$
软腐病抗性等级划分: 高抗HR (病情指数≤20); 抗病R (20<病情指数≤40); 中抗MR (40<病情指数≤60); 感病S (60<病情指数≤80); 高感HS (80<病情指数)。
采用物性分析仪(美国FTC公司型号为TMS-PRO), 参考Alessandrini等[20]方法, 在甘薯块根上进行质地多面分析(texture profile analysis, TPA)测试。在整薯中部切取1 cm厚的圆片, 采用物性分析仪P/5圆柱形探头(直径5 mm)在圆片的赤道部位进行TPA试验, 由质构特征曲线得到薯块硬度、黏附性、内聚性、弹性、胶黏性和咀嚼性等参数。
采用酸解DNS法[21]测定淀粉含量, 参考李燕平[22]试验方法测定果糖、葡萄糖、蔗糖含量, 取0.2 g甘薯冻干粉, 加4 mL超纯水离心, 取0.5 mL上清液, 加3.5 mL 70%乙腈混合, 取适量样液过0.22 μm有机微孔滤膜, 进样品瓶后进行高效液相色谱-示差折光检测器测定; 采用凯氏定氮法[23]测定粗蛋白含量。
根据Roa等[24]方法测定过氧化物酶(peroxidase, POD)活性; 参照Francesco等[25]方法测定多酚氧化酶(polyphenol oxidase, PPO)活性; 参照Lister等[26]方法测定苯丙氨酸解氨酶(L-phenylalanin ammo-nialyase, PAL)活性。
1.3 数据统计分析
Microsoft Excel 2010和IBM.SPSS Statistics 21.0进行方差分析、相关性分析及隶属函数分析, DPS.9.50标准版进行灰色关联度分析。参考贾小平等[27]方法计算权重系数。
${{W}_{j}}\text{=}{{\gamma }_{j}}/\sum\limits_{j=1}^{n}{{{\gamma }_{j}}}$
式中, γj表示第j个指标的关联度, j = 1, 2, 3, ..., n。
不同编号甘薯各综合指标的隶属函数值和加权综合值计算, 相关系数为正值的指标按公式(1)计算, 负值的则按公式(2)计算:
$D=\sum\nolimits_{j=1}^{n}{\left[ {{\gamma }_{j}}*u({{X}_{j}}) \right]}$ j = 1, 2, 3, ..., n
式中, Xj表示第j个指标, Xmin表示第j个指标的最小值, Xmax表示第j个指标的最大值, u(Xj)表示第j个指标的隶属函数值, D表示综合隶属函数值。
2 结果与分析
2.1 不同收获期甘薯块根软腐病抗性鉴定
通过病斑直径计算病情指数并划分抗性等级, 据图1、表2、表3可知, 不同甘薯品种在不同收获期时的软腐病病斑直径存在差异, 表现的抗性不同, 6个甘薯品种均在90 d收获病斑直径小于1 cm, 病情指数为25, 表现抗病, 收获期105 d时, 病斑直径除‘漯紫薯4号’外均高于2 cm, 病情指数大于60, 抗性表现为高感、感病, ‘漯紫薯4号’抗性为中抗, ‘烟薯25’、‘浙薯13’和‘心香’软腐病抗性为高感; 在120 d收获时, 除‘心香’表现为感病外, 其他甘薯品种均表现为中抗; 135 d收获时, 除‘漯紫薯4号’表现为抗病外, 其他甘薯品种均表现为中抗; 150 d收获时软腐病抗性均为中抗。总体来看, 不同品种间抗性不同, ‘漯紫薯4号’对软腐病抗性最强, ‘烟薯25’、‘心香’、‘浙薯13’的抗性较弱。图1
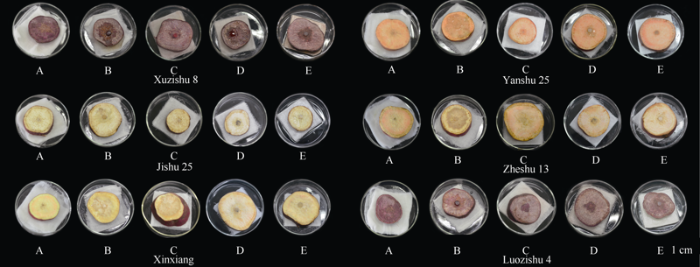 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1不同收获期甘薯块根接匍枝根霉菌碟21 h后发病情况
A: 90 d收获; B: 105 d收获; C: 120 d收获; D: 135 d收获; E: 150 d收获。
Fig. 1Incidence of Rhizopus stolonifera of sweetpotato tubers after 21 hours at different harvest stages
A: harvest in 90 days; B: harvest in 105 days; C: harvest in 120 days; D: harvest in 135 days; E: harvest in 150 days.
Table 2
表2
表2不同收获期甘薯块根软腐病病斑直径
Table 2
| 品种 Variety | 收获时间Harvest time | ||||
|---|---|---|---|---|---|
| 90 d | 105 d | 120 d | 135 d | 150 d | |
| 徐紫薯8号 Xuzishu 8 | 0.82±0.12 d | 2.17±0.06 a | 1.622±0.04 b | 1.79±0.16 b | 1.37±0.22 c |
| 烟薯25 Yanshu 25 | 0.90±0.06 d | 3.58±0.05 a | 1.07±0.03 d | 1.74±0.28 b | 1.41±0.13 c |
| 济薯25 Jishu 25 | 0.59±0.05 c | 2.62±0.14 a | 1.31±0.44 b | 1.29±0.05 b | 1.54±0.11 b |
| 浙薯13 Zheshu 13 | 0.59±0.02 e | 2.79±0.36 a | 1.42±0.11 c | 1.04±0.14 d | 1.84±0.17 b |
| 心香 Xinxiang | 0.83±0.01 d | 3.17±0.17 a | 2.82±0.21 b | 1.10±0.10 d | 2.18±0.17 c |
| 漯紫薯4号 Luozishu 4 | 0.52±0.02 c | 1.64±0.07 a | 1.50±0.15 a | 0.69±0.04 c | 1.31±0.14 b |
新窗口打开|下载CSV
Table 3
表3
表3不同收获期甘薯块根软腐病病情指数及抗性评价结果
Table 3
| 品种 Variety | 收获时间Harvest time | ||||
|---|---|---|---|---|---|
| 90 d | 105 d | 120 d | 135 d | 150 d | |
| 徐紫薯8号 Xuzishu 8 | 25.00 R | 66.67 S | 55.56 MR | 52.78 MR | 50.00 MR |
| 烟薯25 Yanshu 25 | 25.00 R | 97.22 HS | 41.67 MR | 52.78 MR | 50.00 MR |
| 济薯25 Jishu 25 | 25.00 R | 66.67 S | 47.22 MR | 47.22 MR | 50.00MR |
| 浙薯13 Zheshu 13 | 25.00 R | 83.33 HS | 47.22 MR | 50.00 MR | 55.56 MR |
| 心香 Xinxiang | 25.00 R | 94.44 HS | 77.78 S | 44.44 MR | 55.56 MR |
| 漯紫薯4号 Luozishu 4 | 25.00 R | 55.56 MR | 55.56 MR | 25.00 R | 50.00 MR |
新窗口打开|下载CSV
2.2 甘薯块根软腐病抗病性的指标筛选
2.2.1 指标筛选 病情指数是通过病斑直径计算得出, 病斑直径能够更加精确地反映甘薯软腐病抗性。因此选择病斑直径作为甘薯软腐病抗性指标筛选的基础数据。将甘薯块根的病斑直径和质构(硬度、黏附性、黏附力、内聚性、弹性、咀嚼性、胶黏性)、营养品质(干率、淀粉、果糖、葡萄糖、蔗糖、粗蛋白、粗纤维)、抗性酶活(POD、PPO、PAL)等指标进行相关性分析, 内聚性、弹性、咀嚼性、果糖、粗蛋白、POD、PAL和甘薯软腐病病斑直径显著性相关, 其中弹性、粗蛋白、PAL、内聚性、咀嚼性相关系数为-0.439、-0.421、-0.471、-0.314、-0.334 (P<0.01), 果糖和POD相关系数为0.222、-0.279 (P<0.05), 可作为评判甘薯软腐病抗性的初步评价指标。由表4可知, 甘薯块根的内聚性在不同收获期间变化范围分别是0.18~0.23、0.19~0.24、0.13~0.21、0.17~0.28、0.16~0.19、0.17~0.20, ‘济薯25’的内聚性总体较小, ‘心香’、‘漯紫薯4号’内聚性较为稳定, 6个甘薯品种的内聚性整体随着收获期的增加而降低, 且90 d收获甘薯的内聚性均大于105 d, 分别为105 d收获的1.15、1.21、1.31、1.56、1.125、1.18倍。甘薯的弹性变化较小, 总体范围为5.08~7.14, 整体来看, 整个收获期的甘薯弹性均在105 d收获时为较低值, 分别为5.83、5.43、5.72、6.02、5.08、5.51 mm。各甘薯品种不同收获期的咀嚼性为158.95~210.67、92.70~138.82、116.60~150.22、125.59~ 218.32、73.64~117.08、97.46~137.18 N, 品种间差异明显且变化较大, 90 d和105 d收获甘薯的咀嚼性整体分别较高和较低。
Table 4
表4
表4甘薯各样品软腐病相关指标测定值
Table 4
| 样品编号 Sample number | 内聚性 Cohesiveness (ratio) | 弹性 Springiness (mm) | 咀嚼性 Chewiness (N) | 果糖 Fructose content (mg g-1) | 粗蛋白 Crude protein content (%) | 过氧化物酶 POD activity (U) | 苯丙氨酸解氨酶活性 PAL activity (U) |
|---|---|---|---|---|---|---|---|
| 1-1 | 0.23±0.00 a | 6.31±0.78 ab | 210.67±7.10 a | 1.36±0.15 d | 0.95±0.05 a | 7.54±0.24 c | 95.00±5.00 a |
| 1-2 | 0.20±0.01 ab | 5.83±0.25 b | 165.18±6.28 b | 4.64±0.37 a | 0.55±0.02 c | 2.45±0.20 e | 35.33±1.53 c |
| 1-3 | 0.21±0.00 a | 5.81±0.17 b | 158.95±2.34 b | 4.34±0.28 a | 0.62±0.01 b | 3.56±0.42 d | 17.00±1.00 d |
| 1-4 | 0.18±0.02 b | 6.75±0.25 a | 178.27±9.20 b | 2.52±0.30 b | 0.45±0.02 d | 10.55±0.43 b | 33.67±3.06 c |
| 1-5 | 0.21±0.02 a | 5.92±0.32 b | 176.68±17.80 b | 1.87±0.20 c | 0.90±0.03 a | 16.49±0.49 a | 45.00±3.61 b |
| 2-1 | 0.23±0.01 ab | 6.51±0.40 a | 126.80±4.36 b | 15.34±0.85 c | 0.71±0.02 a | 10.56±0.37 a | 86.33±1.15 a |
| 2-2 | 0.19±0.01 c | 5.43±0.69 b | 92.70±1.52 d | 19.09±0.08 ab | 0.54±0.01 c | 4.63±0.44 d | 25.33±3.51 b |
| 2-3 | 0.24±0.01 a | 6.71±0.40 a | 138.82±5.38 a | 19.52±0.13 a | 0.45±0.01 d | 6.49±1.20 c | 25.33±1.53 b |
| 2-4 | 0.21±0.01 bc | 6.68±0.04 a | 126.11±10.85 b | 16.46±1.30 c | 0.66±0.03 b | 9.57±0.43 ab | 22.33±3.21 b |
| 2-5 | 0.20±0.01 c | 6.35±0.32 a | 107.98±4.93 c | 17.9±0.40 b | 0.43±0.02 d | 8.46±0.32 b | 8.00±1.00 c |
| 3-1 | 0.21±0.03 a | 6.31±0.07 b | 126.14±3.82 b | 1.49±0.23 bc | 0.57±0.02 d | 15.13±0.53 b | 73.67±2.52 a |
| 3-2 | 0.16±0.00 b | 5.72±0.25 c | 118.63±4.92 b | 1.97±0.14 bc | 0.44±0.03 e | 3.33±0.05 c | 9.33±0.58 c |
| 3-3 | 0.16±0.02 b | 6.52±0.14 a | 150.22±8.80 a | 2.16±0.31 b | 0.87±0.01 a | 5.42±0.42 c | 25.67±2.08 b |
| 3-4 | 0.14±0.01 b | 6.07±0.33 bc | 116.60±2.94 b | 1.17±0.04 c | 0.81±0.01 b | 5.66±0.44 c | 26.33±2.31 b |
| 3-5 | 0.13±0.01 b | 6.83±0.39 ab | 120.41±2.01 b | 10.33±0.96 a | 0.75±0.01 c | 24.39±3.52 a | 12.00±1.00 c |
| 4-1 | 0.28±0.09 a | 6.86±0.18 a | 218.32±3.87 a | 1.80±0.14 d | 0.95±0.02 a | 9.57±0.92 a | 89.00±3.00 a |
| 4-2 | 0.18±0.00 ab | 6.02±0.18 b | 135.65±6.94 c | 2.65±0.17 c | 0.77±0.01 b | 2.69±0.79 d | 16.33±2.52 bc |
| 4-3 | 0.20±0.04 ab | 5.90±0.34 b | 126.72±3.95 d | 5.22±0.42 a | 0.66±0.09 c | 7.47±0.18 b | 18.67±3.06 c |
| 4-4 | 0.17±0.02 b | 6.10±0.11 b | 125.59±3.53 d | 4.08±0.53 b | 0.99±0.01 d | 5.53±0.49 c | 21.67±1.53 b |
| 4-5 | 0.17±0.01 b | 6.71±0.36 a | 169.24±4.96 b | 1.41±0.14 d | 0.40±0.01 d | 10.23±0.64 a | 17.67±1.15 bc |
| 5-1 | 0.18±0.01 a | 5.49±0.27 a | 86.51±2.96 c | 1.78±0.11 c | 1.00±0.02 a | 49.04±0.44 a | 68.00±2.65 a |
| 5-2 | 0.16±0.01 b | 5.08±0.56 a | 73.64±4.39 d | 6.80±0.59 b | 0.54±0.01 c | 5.38±0.33 d | 36.67±3.06 b |
| 5-3 | 0.19±0.01 a | 5.93±0.68 a | 103.35±4.52 b | 10.03±0.60 a | 0.55±0.01 c | 6.37±0.37 d | 25.33±3.06 c |
| 5-4 | 0.17±0.01 ab | 5.97±0.33 a | 106.20±7.81 b | 6.03±0.61 b | 0.39±0.04 d | 8.53±0.97 c | 30.67±3.79 c |
| 5-5 | 0.16±0.01 b | 5.74±0.24 a | 117.08±3.00 a | 6.14±0.65 b | 0.76±0.10 b | 14.48±0.70 b | 13.00±1.73 d |
| 6-1 | 0.20±0.02 a | 7.14±0.68 a | 137.18±3.47 a | 3.06±0.23 d | 0.83±0.01 b | 2.81±0.11 d | 75.33±4.16 a |
| 6-2 | 0.17±0.02 a | 5.51±0.28 c | 97.46±0.35 c | 6.83±0.46 b | 0.44±0.02 d | 8.36±0.41 b | 21.00±2.00 c |
| 6-3 | 0.20±0.01 a | 6.51±0.21 ab | 133.85±4.09 a | 7.63±0.20 a | 0.54±0.02 c | 2.09±0.18 e | 20.33±4.04 c |
| 6-4 | 0.18±0.01 a | 5.99±0.14 bc | 115.39±2.91 b | 7.53±0.35 c | 0.85±0.06 b | 4.84±0.18 c | 55.67±1.53 b |
| 6-5 | 0.19±0.03 a | 5.80±0.28 c | 109.29±7.51 b | 4.98±0.46 c | 0.97±0.08 a | 8.99±0.28 a | 11.33±2.08 d |
新窗口打开|下载CSV
6个甘薯品种中‘烟薯25’的果糖含量较高, 收获期果糖含量为15.34~19.52 mg g-1; ‘济薯25’在150 d收获时果糖含量较高, 为10.33 mg g-1, 其他甘薯品种有较高果糖含量收获时间为120 d左右, 90 d收获的6个甘薯品种的果糖含量均较低, 分别为1.36、15.34、1.49、1.80、1.78、3.06 mg g-1; 果糖含量会随着收获期的增加而增高, 到达一定时间, 果糖含量开始下降, 该时间存在基因型差异; 甘薯粗蛋白含量变化存在基因型差异, 整体在90 d收获时粗蛋白含量处于较高水平, 在105 d收获时粗蛋白含量均出现显著性降低, 90 d收获的粗蛋白含量分别是105 d收获的1.73、1.31、1.30、1.23、1.85、1.89倍。
甘薯POD活性在90~150 d收获过程中, 整体呈现先下降后上升的趋势, 除‘漯紫薯4号’外, 其他甘薯品种在105 d收获时的活性显著降低; POD活性基本在105~120 d处于较低水平; 不同收获期心香的POD活性为5.38~49.04 U, 变化较大。6个甘薯品种均在90 d收获时PAL活性最强且显著高于其他收获期, PAL活性分别为95.00、86.33、73.67、89.00、68.00、755.33 U, 收获期的PAL活性变化存在基因型差异。
2.2.2 指标权重的计算 采用灰色关联度分析进行计算指标和病斑直径的关联度, 由于各指标的量纲不一致, 需对原始数据作“标准化”处理, 使之无量纲化, 分辨系数设置0.1, 母序列为病斑直径, 关联度分析结果见表5。果糖、POD、咀嚼性的关联度较高, 分别为0.5145、0.4996、0.4566, 参照1.3公式计算其权重系数, 为0.1668、0.1620、0.1480。
Table 5
表5
表5甘薯软腐病病斑直径与各指标的灰色关联度及排序
Table 5
| 序列 Indicator | 测定指标 Index | 关联度γ Correlation degree γ | 权重 Weight | 位次 Rank |
|---|---|---|---|---|
| X4 | 果糖 Fructose | 0.5145 | 0.1668 | 1 |
| X6 | 过氧化物酶 Peroxidase | 0.4996 | 0.1620 | 2 |
| X3 | 咀嚼性 Chewiness | 0.4566 | 0.1480 | 3 |
| X8 | 苯丙氨酸解氨酶 L-phenylalanin ammo-nialyase | 0.4144 | 0.1344 | 5 |
| X1 | 内聚性 Cohesiveness | 0.4248 | 0.1377 | 4 |
| X2 | 弹性 Springiness | 0.4069 | 0.1319 | 6 |
| X5 | 粗蛋白 Crude protein | 0.3675 | 0.1192 | 7 |
新窗口打开|下载CSV
2.2.3 隶属函数加权计算综合评价值 由表6可知, 各甘薯品种不同收获期的各指标加权隶属函数综合值分别为0.23~0.55、0.48~0.81、0.42~0.71、0.19~0.58、0.36~0.77、0.37~0.73。90 d收获的各甘薯品种综合值较低,分别为0.23、0.48、0.42、0.19、0.36、0.37; 105 d收获的各甘薯品种综合值较高, 分别为0.55、0.81、0.71、0.57、0.77、0.73, 该方法是以病斑直径作为母序列, 表现为综合值越高, 其软腐病抗性越弱, 因此, 根据综合值来判定的软腐病抗性为90 d收获>120、135、150 d收获>105 d收获, 与薯片接菌碟法鉴定结果一致, 表明筛选出的指标能够较好的评价甘薯块根的软腐病抗性。
Table 6
表6
表6各指标隶属函数值及加权综合值
Table 6
| 样品编号 Sample number | 内聚性 Cohesiveness (ratio) | 弹性 Springiness (mm) | 咀嚼性 Chewiness (N) | 果糖 Fructose content (mg g-1) | 粗蛋白 Crude protein content (%) | 过氧化物酶 POD activity (U) | 苯丙氨酸解氨酶活性 PAL activity (U) | 加权综合值 Weighted composite value |
|---|---|---|---|---|---|---|---|---|
| 1-1 | 0.09 | 0.40 | 0.05 | 0.12 | 0.08 | 0.88 | 0.00 | 0.23 |
| 1-2 | 0.32 | 0.64 | 0.37 | 0.33 | 0.73 | 0.99 | 0.69 | 0.55 |
| 1-3 | 0.21 | 0.65 | 0.41 | 0.30 | 0.62 | 0.97 | 0.90 | 0.55 |
| 1-4 | 0.53 | 0.19 | 0.28 | 0.20 | 0.91 | 0.82 | 0.70 | 0.49 |
| 1-5 | 0.24 | 0.59 | 0.29 | 0.12 | 0.15 | 0.69 | 0.57 | 0.37 |
| 2-1 | 0.09 | 0.31 | 0.63 | 0.83 | 0.47 | 0.82 | 0.10 | 0.48 |
| 2-2 | 0.45 | 0.83 | 0.87 | 0.92 | 0.76 | 0.95 | 0.80 | 0.81 |
| 2-3 | 0.00 | 0.21 | 0.55 | 1.00 | 0.90 | 0.91 | 0.80 | 0.64 |
| 2-4 | 0.27 | 0.22 | 0.64 | 0.80 | 0.55 | 0.84 | 0.84 | 0.61 |
| 2-5 | 0.32 | 0.38 | 0.76 | 0.95 | 0.94 | 0.86 | 1.00 | 0.75 |
| 3-1 | 0.25 | 0.40 | 0.64 | 0.08 | 0.70 | 0.72 | 0.25 | 0.42 |
| 3-2 | 0.78 | 0.69 | 0.69 | 0.11 | 0.92 | 0.97 | 0.98 | 0.71 |
| 3-3 | 0.71 | 0.30 | 0.47 | 0.12 | 0.21 | 0.93 | 0.80 | 0.50 |
| 3-4 | 0.91 | 0.52 | 0.70 | 0.05 | 0.31 | 0.92 | 0.79 | 0.59 |
| 3-5 | 1.00 | 0.15 | 0.68 | 0.42 | 0.40 | 0.53 | 0.95 | 0.60 |
| 4-1 | 0.09 | 0.13 | 0.00 | 0.09 | 0.07 | 0.84 | 0.07 | 0.19 |
| 4-2 | 0.51 | 0.55 | 0.57 | 0.14 | 0.37 | 0.99 | 0.90 | 0.57 |
| 4-3 | 0.34 | 0.60 | 0.63 | 0.31 | 0.56 | 0.89 | 0.88 | 0.58 |
| 4-4 | 0.64 | 0.51 | 0.64 | 0.25 | 0.01 | 0.93 | 0.84 | 0.54 |
| 4-5 | 0.64 | 0.21 | 0.34 | 0.06 | 0.98 | 0.83 | 0.89 | 0.54 |
| 5-1 | 0.50 | 0.80 | 0.91 | 0.10 | 0.00 | 0.00 | 0.31 | 0.36 |
| 5-2 | 0.76 | 1.00 | 1.00 | 0.31 | 0.75 | 0.93 | 0.67 | 0.77 |
| 5-3 | 0.46 | 0.59 | 0.79 | 0.52 | 0.73 | 0.91 | 0.80 | 0.68 |
| 5-4 | 0.61 | 0.57 | 0.77 | 0.31 | 1.00 | 0.86 | 0.74 | 0.68 |
| 5-5 | 0.75 | 0.68 | 0.70 | 0.00 | 0.39 | 0.74 | 0.94 | 0.63 |
| 6-1 | 0.37 | 0.00 | 0.56 | 0.20 | 0.27 | 0.98 | 0.23 | 0.37 |
| 6-2 | 0.62 | 0.79 | 0.84 | 0.52 | 0.92 | 0.87 | 0.85 | 0.73 |
| 6-3 | 0.38 | 0.31 | 0.58 | 0.57 | 0.75 | 1.00 | 0.86 | 0.60 |
| 6-4 | 0.58 | 0.56 | 0.71 | 0.61 | 0.24 | 0.94 | 0.45 | 0.56 |
| 6-5 | 0.44 | 0.65 | 0.75 | 0.34 | 0.04 | 0.85 | 0.96 | 0.56 |
新窗口打开|下载CSV
3 讨论
软腐病是甘薯贮藏期最具破坏力的病害之一[4], 通过伤口侵染甘薯, 软腐病的防治管理选择有限, 培育抗病品种是试验的重点, 目前没有完全抵抗软腐病的甘薯品种, 所有甘薯品种都可能在一定程度上感病[15]。甘薯软腐病抗性评价是改良软腐病抗性的基础。常见的甘薯抗性鉴定有薯块伤口接菌碟法[13]和薯片接菌碟法[28,29,30], 杨冬静等[12]对其进行比较发现, 薯片接菌碟法更能够直观准确的评价甘薯软腐病抗性, 本研究采用薯片接菌碟法鉴定软腐病抗性。甘薯软腐病抗性是一个复杂的综合性状, 由许多因素共同作用决定的。研究表明通过灰色关联度分析法在许多作物抗病性指标筛选均起到良好的效果[17,31-32], 在客观系统中, 灰色是绝对的, 正是基于此, 灰色系统理论可以用于甘薯软腐病抗性研究, 利用隶属函数分析对试验材料抗性综合评价具有较好的应用效果[33,34], 本试验通过灰色关联度和隶属函数综合分析, 得到的评价结果和软腐病抗性鉴定一致, 收获期不同会导致甘薯软腐病抗性差异, 90 d收获甘薯软腐病抗性最强, 105 d收获抗性最弱。
本研究对甘薯质地、营养物质和抗性酶活是3个方面的指标筛选结果和权重排序为果糖>POD>咀嚼性>内聚性>PAL>弹性>粗蛋白, 果糖、POD、咀嚼性的关联系数大于0.45, 为软腐病抗性指标的重点, 可将筛选结果分为物理和生理生化2个方面。
物理指标包括内聚性、弹性和咀嚼性。内聚性是指甘薯块根在破裂之前可以变形的水平, 是内部凝聚强度的量度, 反映了块根内部结合力的大小和保持完整性的能力; 弹性是指甘薯块根在第1次挤压结束后, 第2次挤压开始前样品恢复的高度; 咀嚼性反映了薯块对咀嚼的持续抵抗性[35]。这3个指标均反映薯块对外部压力的抵抗程度, 能够一定程度上代表薯块的耐破损程度。
生理生化指标包括粗蛋白含量、果糖含量、POD和PAL酶活性。粗蛋白是指材料中的含氮化合物总量, 在玉米等研究表明, 粗蛋白含量和植物抗病性存在显著性正相关[36], 与本试验结论一致。果糖是含6个碳原子的单糖, 不易结晶, 通常为黏稠性液体, 具有良好的吸湿性, 分子量小, 渗透压较高, 能较快地穿透细胞组织, 分解速度较快[37], 这些特点导致匍枝根霉更易附着在果糖含量较高的薯块上, 更利于匍枝根霉对薯块营养物质的吸收和自身生长。Muhanna等[38]研究表明, 果糖含量和甘薯块根软腐病抗性相关。90 d收获的薯块果糖含量较低, 软腐病抗性强。POD是催化底物氧化的酶, 主要存在细胞的过氧化物酶体中, 和木质素的合成及组织的衰老有着密切的联系[39], 幼嫩组织活性低于老化组织, 具有较强抗病性的试验材料POD活性高于抗性弱的[40], 本研究结果表明抗性强的90 d收获薯块具有较高的POD活性。苯丙氨酸解氨酶是植物抗病反应次生代谢途径之一苯丙烷类代谢途径的关键酶和限速酶, PAL活性升高会导致木质素的积累及酚类物质和植保素的合成[41], 与植物抗病性有着密切的关系, 赵亚婷等[42]研究表明, 高PAL活性的具有较强的抗病性, 本研究中90 d收获的薯块具有较高的PAL活性, 与其结论一致。
90 d收获的甘薯较105 d有较强的软腐病抗性, 期间薯块的物理特性(内聚性、弹性、咀嚼性)均显著性降低, 且105 d收获咀嚼性和弹性达到最低值, 造成薯块对外部压力的抵抗能力显著降低, 组织易破损; 生理生化方面, 90 d收获甘薯的果糖含量整体为最小值, 105 d收获显著升高且达到较高水平, 粗蛋白含量、POD和PAL活性在90 d整体达到较高水平, 105 d收获降低到较低水平。果糖含量升高易于病菌的附着和生长, POD和PAL活性低表明有较低的抗病性。各指标共同作用下, 使得105 d收获薯块的软腐病抗性显著低于90 d收获。
4 结论
甘薯不同收获期软腐病抗性存在差异, 在90~150 d收获过程中, 90 d收获对软腐病抗性最强, 105 d收获抗性最弱; 甘薯果糖、POD、咀嚼性、内聚性、PAL、弹性、粗蛋白可作为评价甘薯软腐病抗性的指标, 在抗性育种工作中, 可利用这些指标更便于抗软腐性较强的材料的准确选择。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOIURL [本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
DOIPMID [本文引用: 1]

Postharvest soft rots of sweetpotato caused by Rhizopus stolonifer (Rhizopus soft rot) and Dickeya dadantii (bacterial root rot) occur sporadically and can result in significant losses. A 3-year field study related preharvest conditions, including soil texture, chemistry, and fertility; air temperature; soil temperature and moisture; and various cultural practices (153 total variables), to postharvest susceptibility to both diseases in 75 sweetpotato fields in North Carolina and 63 sweetpotato fields in Louisiana. Storage roots were sampled from each field, cured, stored, and inoculated with each pathogen after 100 to 120 days in storage. Disease susceptibility was measured as incidence of diseased storage roots 10 days following inoculation. There was wide variation from field to field in incidence of both diseases (0 to 100% for Rhizopus soft rot and 5 to 95% for bacterial root rot) in both states in each year. Correlations between disease incidence and each of the preharvest variables revealed numerous significant correlations but the variables that correlated with disease incidence were different between North Carolina and Louisiana. Models for both diseases were built by first using forward stepwise regression to identify variables of interest, followed by a mixed-model analysis to produce a final reduced model. For North Carolina fields, postharvest Rhizopus soft rot susceptibility was described by the percentage of the soil cation exchange capacity occupied by calcium, amount of plant-available soil phosphorus, percent soil humic matter, mean air temperature, mean volumetric soil moisture at 40 cm in depth, and mean soil temperature at 2 cm in depth. Postharvest bacterial soft rot susceptibility was described by soil pH and the number of days of high soil temperature late in the season. For Louisiana fields, Rhizopus soft rot susceptibility was described by a complex of variables, including late-season air and soil temperature and late-season days of extreme soil moisture. For bacterial root rot, days of low air temperature and days of high soil temperature late in the season as well as days of low soil moisture best described variation. Although the influence of preharvest variables on postharvest susceptibility was profound for each disease, the complexity of factors involved and differences between the data for the two states makes development of a predictive system extremely difficult.
DOIURL [本文引用: 2]
[本文引用: 3]
[本文引用: 3]
DOIPMID [本文引用: 2]

Rhizopus soft rot, caused primarily by Rhizopus stolonifer, is one of the most common postharvest diseases of sweetpotato and is often considered the most devastating. Traditionally, Rhizopus soft rot has been effectively controlled using postharvest dips in dicloran fungicides; however, due to changes in market preferences, use of these fungicides is now limited. This, along with the lack of labeled and effective fungicides for control of Rhizopus soft rot in sweetpotato, creates the need for integrated strategies to control the disease. The effects of storage temperature (13, 23, and 29°C), relative humidity (80, 90, and 100%), and initial inoculum levels (3-, 5-, and 7-mm-diameter mycelial plugs) on progression of Rhizopus soft rot in 'Covington' sweetpotato were examined. Percent decay due to Rhizopus soft rot infection was significantly reduced (P < 0.0001) at a low temperature (13°C) but was not significantly affected by changes in relative humidity or initial inoculum level (P >0.05). Sporulation of R. stolonifer was also significantly reduced at the lowest temperature of 13°C. High relative humidity (>95%) significantly increased sporulation of R. stolonifer and sporulation also increased as initial inoculum level increased. Efficacy of chlorine dioxide (ClO) fumigation, UV-C irradiation, and postharvest dips in alternative control products were also investigated for control of Rhizopus soft rot. Static ClO treatments were effective in reducing sporulation on treated roots but had no significant impact on incidence of Rhizopus soft rot. UV irradiation at 3.24 KJ/m 1 h after inoculation as well as dips in aqueous ClO and StorOx 2.0 significantly (P < 0.05) reduced disease incidence. Understanding the epidemiological factors favoring Rhizopus soft rot and identifying alternative control strategies allow for improved recommendations to limit postharvest losses in sweetpotato.
DOIURL [本文引用: 1]
DOIURL [本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
