 ,1,**, 张婷
,1,**, 张婷 ,1,**, 彭文静2, 段瑶瑶1, 许哲昕1, 林艺华1, 高三基
,1,**, 彭文静2, 段瑶瑶1, 许哲昕1, 林艺华1, 高三基 ,1,*
,1,*Identification of resistance to leaf scald in newly released sugarcane varieties at seedling stage by artificial inoculation
FU Hua-Ying ,1,**, ZHANG Ting
,1,**, ZHANG Ting ,1,**, PENG Wen-Jing2, DUAN Yao-Yao1, XU Zhe-Xin1, LIN Yi-Hua1, GAO San-Ji
,1,**, PENG Wen-Jing2, DUAN Yao-Yao1, XU Zhe-Xin1, LIN Yi-Hua1, GAO San-Ji ,1,*
,1,*通讯作者: * 高三基, E-mail:gaosanji@fafu.edu.cn
第一联系人:
收稿日期:2020-09-4接受日期:2020-12-1网络出版日期:2021-08-12
| 基金资助: |
Received:2020-09-4Accepted:2020-12-1Online:2021-08-12
| Fund supported: |
作者简介 About authors
傅华英,E-mail:mddzyfhy@163.com;
张婷,E-mail:Zhang_970907@163.com

摘要
由白条黄单胞杆菌(Xanthomonas albilineans)引起的甘蔗白条病是甘蔗生产中的重要细菌性病害之一,选用抗病品种是甘蔗白条病防控最经济、最有效的措施。为明确国内甘蔗新品种(系)对白条病菌的抗性水平, 本研究选用抗白条病品种LCP 85-384和感白条病品种新台糖20号为对照种, 对我国各育种单位新选育的49个甘蔗新品种(系)进行苗期人工接种抗白条病鉴定。研究结果显示, 从白条病菌Xa-FJ1菌液接种后第7天开始, 随着时间的推移, 甘蔗品种白条病病情指数不断提高, 到接种后21~28 d时, 各品系的病情指数进入平稳增长时期。对接种后的病叶进行病原菌分离和叶片总DNA的提取, 利用甘蔗白条病菌特异性引物XAF1/XAR1进行PCR检测和鉴定, 结果证实白条病菌Xa-FJ1通过人工接种方式成功侵染供试材料。基于病情指数评价甘蔗抗病性水平, 将51份供试材料划分为抗、中抗、感、高感4个不同抗病等级。抗病至中抗水平的品种(系)有19份, 占37.3%, 其中抗病等级只有3份, 分别为粤甘50、福农09-7111和中蔗10号; 感病至高感水平的品种(系)有32份, 占62.7%。上述抗性鉴定结果表明, 本研究参试国内新选育的甘蔗品种(系)苗期对白条病的抗性水平没有达到高抗的品种(系), 建议加强甘蔗白条病抗病基因资源的挖掘和利用。
关键词:
Abstract
Sugarcane leaf scald caused by the pathogen of Xanthomonas albilineans is one of important bacterial diseases. The utilization of sugarcane resistant varieties is the most economical and effective measure for control of this disease. In this study, to identify the level of resistance to leaf scald for domestic sugarcane varieties, a total of 49 newly-released sugarcane varieties from different sugarcane breeding institutions in China were identified upon 3-5 fully expanding leaf stage by artificial inoculation with Xa-FJ1 strain. The leaf scald resistant variety LCP 85-384 and the leaf scald susceptible variety ROC20 were used as controls. Our results indicated that the disease indexes of these varieties were continually increased from the 7th day post-inoculation (dpi), while the disease indexes reached a steady plateau at 21-28 dpi. These re-isolated pathogenic bacteria in leaf tissues of the inoculated plants were detected by PCR assay with the XAF1/XAR1 primers specific to X. albilineans. PCR results confirmed that the pathogen Xa-FJ1 was successfully infected with all the tested varieties after inoculation. Based on the disease indexes, 51 sugarcane varieties were divided into four different groups, resistant, medium resistant, susceptible, and high susceptible grades. Of all the tested varieties, 37.3% (19/51) varieties were resistant or medium resistant to leaf scald, among which only three varieties (Yuegan 50, Funong 09-7111, and Zhongzhe 10) reached resistant levels. Meanwhile, 62.7% (32/51) varieties were susceptible or highly susceptible to leaf scald. Our results revealed the lack of high resistance germplasms in the newly released varieties in China, and it is urgent to create more germplasm resources resistant to leaf scald.
Keywords:
PDF (2705KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
傅华英, 张婷, 彭文静, 段瑶瑶, 许哲昕, 林艺华, 高三基. 甘蔗新品种(系)苗期白条病人工接种抗性鉴定与评价. 作物学报[J], 2021, 47(8): 1531-1539 DOI:10.3724/SP.J.1006.2021.04203
FU Hua-Ying, ZHANG Ting, PENG Wen-Jing, DUAN Yao-Yao, XU Zhe-Xin, LIN Yi-Hua, GAO San-Ji.
甘蔗(Saccharum spp. hybrid)是世界上重要的糖料作物, 也是一种重要的可再生能源作物[1]。我国甘蔗主要分布在广西、云南、广东、海南等省(区), 形成了桂中南、滇西南、粤西、琼北等甘蔗主产区[2]。近几年来, 我国食糖年蔗糖产量在1000万吨左右, 其中甘蔗糖约占我国食糖总量的90%[3]。我国甘蔗产业健康稳定发展关系国计民生。甘蔗在生产过程中常遭受各种生物和非生物逆境胁迫, 导致甘蔗品种种性退化, 从而引起甘蔗减产、品质变劣[4]。据ISSCT统计, 甘蔗病原微生物为害至少造成甘蔗减产10%~15% [5]。
由白条黄单胞杆菌Xanthomonas albilineans引致的甘蔗白条病是影响甘蔗生产安全的重大生物灾害, 对甘蔗生产具有毁灭性危害, 是甘蔗三大细菌性病害之一[6,7,8]。近年来, 该病害在我国广西、广东、云南、海南等蔗区已有报道[9,10,11]。X. albilineans早在2007年被列入《中华人民共和国进口植物检疫性有害生物》名录。甘蔗白条病在发病初期的症状表现为叶片出现铅笔状细长的白色条纹,在发病中后期甘蔗叶片出现褪绿白化、枯萎、坏死, 感病植株出现枯萎死亡[6,8]。该病害可以通过感染病菌种茎、砍刀等方式进行传播, 也可通过植株间的接触、土壤和气流传播[12,13]。含有病菌的甘蔗容易通过调种或引种的方式在不同国家和国内不同的蔗区之间传播, 这给甘蔗白条病防控工作带来挑战。甘蔗白条病发生严重影响甘蔗的产量和蔗糖分, 尤其在感病品种上为害更加明显[14]。
培育优良的抗病品种是病害防控最经济、最有效的方法, 也是一项重要的绿色防控措施。但是, 建立高效、准确的抗病鉴定与评价技术体系是抗病育种实践中的关键环节之一。Rott等[15]在大田条件下采用截头法评价7个甘蔗品种白条病的抗性, 1年新植和2年宿根的田间试验结果表明, B69379为感病品种, 减产21%, 建议该品种不适宜在瓜德罗普岛种植。随后, Rott等[16]在温室和大田2种试验条件下, 同样采用截头法对40个品种和15个品系的抗病性作了评价。Lopes等[17]采用不同浓度白条病菌菌液浸种单芽蔗种, 利用EC50值(感染50%接种植株所需细菌浓度的log10对数值)评价甘蔗品种白条病抗病性。Garces等[18]和Gutierrez等[19]采用截头法接种技术和实时荧光定量PCR (qPCR)技术, 用甘蔗植株体内白条病原菌的含量高低来评价品种的抗病性。最近, 国内****采用田间自然发病率对广西的北海、来宾、百色等蔗区甘蔗品种白条病的抗性表现做了评价[20,21]。福建农林大学国家甘蔗工程技术研究中心病虫害绿色防控课题组采用剪叶法接种技术开展白条病菌在甘蔗[9]和象草[22]上的致病性测定。
甘蔗是一种高光效、高生物量的C4作物, 其收获物是营养器官蔗茎。甘蔗产量性状、糖分性状和抗病表现受环境影响较大。甘蔗抗病鉴定通常采用人工接种鉴定技术和田间抗病性表现, 需要多年多点试验结果来综合评价, 这种鉴定和评价方法耗时费力。为此, 本研究首先确立一套精准快速的甘蔗品种白条病室内抗性鉴定及评价方法, 然后对我国各育种单位新选育出来的甘蔗新品种(系)进行鉴定和评价, 以期为甘蔗白条病抗病育种和品种多系布局提供参考。
1 材料与方法
1.1 试验材料
选用我国各主要甘蔗育种单位选育的新品种(系)为白条病抗性鉴定材料, 以抗病品种LCP 85-384和感病品种新台糖20号(ROC20)为对照种[23], 合计51份。各品种(系)种茎材料采自福建农林大学国家甘蔗工程技术研究中心福州试验基地。本研究所用甘蔗白条病菌Xa-FJ1菌株由作者所在课题组分离保存[9]。1.2 试验方法
1.2.1 甘蔗种苗培育 2019年8月下旬挑选健康、蔗芽饱满的甘蔗种茎, 砍成3~5 cm单芽茎段, 放入尼龙种子袋中, 于室温水浸泡24 h之后, 50℃恒温循环水浴处理2 h进行温汤脱毒(菌)。随后, 以营养土为介质, 把单芽茎段种植于边长6 cm宽的正方形小花盆中, 然后移入育苗盘, 每盘35个小花盆。在智能人工气候培养箱催芽和育苗, 培养条件为: 温度28℃, 湿度60%, 光照强度为3000 μmol m-2 s-1, 光照时间为12 h d-1。定期浇水, 保持土壤湿润。待甘蔗植株长到3~5片叶片完全展开时, 每个品种挑选长势一致的植株35株, 用于白条病菌菌液人工接种抗性鉴定。1.2.2 白条病原菌的活化和接种 Xa-FJ1菌株用平板划线法在XAS固体培养基上活化2次, 于恒温培养箱28℃培养4~5 d。接种前, 挑取单菌落置于XAS液体培养基中, 放入摇床28℃、200转 min-1培养约12 h, 用血球计数板观察计数菌液浓度, 用无菌XAS液体培养基稀释成1×108 CFU mL-1后立即用于接种。XAS培养基配方参考Davis等[24]文献所述成分。采用剪叶接种法对参试甘蔗品种进行接种[9], 即用消过毒的手术刀沾取Xa-FJ1菌株悬浮菌液, 剪掉植株叶片叶尖1/3部位。每接种1株后, 手术刀要重新沾取菌液。最后, 用菌液喷雾1次。接种后的甘蔗植株继续在智能人工气候培养箱里培养。
1.2.3 白条病严重度分级和病情指数计算 甘蔗白条病严重度分级参考Rott等报道的白条病严重度划分标准[16], 并略作修改(表1)。在接种白条病原菌后的第7天, 进行第1次调查, 之后每隔7 d调查1次, 共调查4次。观察和记录每个甘蔗品种植株白条病的发病严重度, 根据发病率和发病严重度计算病情指数。按表2所示划分标准评价甘蔗品种白条病的抗性。
Table 1
表1
表1甘蔗植株白条病发病严重度评判标准
Table 1
| 分级Grade | 症状描述Symptom |
|---|---|
| 0级 Score 0 | 无症状Asymptomatic |
| 1级 Score 1 | 1-2条铅笔状白色条纹One or two white pencil lines |
| 2级 Score 2 | 超过2条铅笔状白色条纹More than two white pencil lines |
| 3级 Score 3 | 叶片变白或者黄化Chlorotic or yellowing leaf |
| 4级 Score 4 | 叶片坏死Leaf necrosis |
| 5级 Score 5 | 植株死亡Plant death |
新窗口打开|下载CSV
Table 2
表2
表2基于病情指数评价白条病抗性标准
Table 2
| 抗性评价Resistance evaluation | 病情指数分级Disease index (%) |
|---|---|
| 高抗High resistant | 病情指数 ≤ 5.0 Disease index ≤ 5.0 |
| 抗病Resistant | 5.0 < 病情指数 ≤ 15.0 5.0 < Disease index ≤ 15.0 |
| 中抗Medium resistant | 15.0 < 病情指数 ≤ 30.0 15.0 < Disease index ≤ 30.0 |
| 感病Susceptible | 30.0 < 病情指数 ≤ 50.0 30.0 < Disease index ≤ 50.0 |
| 高感High susceptible | 病情指数 > 50.0 Disease index > 50.0 |
新窗口打开|下载CSV
病情指数计算公式:
病情指数(%) = [∑(发病等级数×相应等级的发病株数)/(最高发病等级数×总调查株数)]×100
1.2.4 接种后病原分离与鉴定 接种后第28天, 挑取3种有代表性的不同严重度(铅笔状白色条纹、叶片褪绿白化、叶片褪绿黄化)甘蔗植株叶片进行病原菌分离与鉴定, 参考Lin等[9]描述方法完成。具体步骤如下: 将叶片的病健交界处用75%的酒精消毒后放入无菌的培养皿中, 加入少量无菌水, 用解剖刀剁成匀浆, 在超净工作台中静置1 h以上。用移液枪吸取混合液在XAS固体培养基上划线, 28℃恒温培养箱中培养4~5 d。挑选形状为圆形、凸起、光滑、浅黄色的单菌落, 再次在XAS固体培养基上进行平板划线。每个平板随机挑取3个单菌落, 用特异性引物XAF1 (5'-CCTGGTGATGACGCTGGGTT-3')和XAR1 (5'-CGATCAGCGATGCACGCAGT-3')进行菌液PCR鉴定[25], 以Xa-FJ1菌株为阳性对照, 无菌水为空白对照。
1.2.5 接种后叶片收集和总DNA提取 接种后第28天, 剔除白条病发病严重度为4级和5级的植株, 其余植株叶片收集一起, 剪碎混匀后, 分成三等份。采用CTAB法提取接种后的甘蔗叶片总DNA, 通过电泳检测DNA质量, 并用BioTek ELx800酶标仪测定DNA浓度, 样本DNA稀释至100 ng μL-1工作浓度。
1.2.6 菌液和叶片总DNA检测 用已报道的甘蔗白条病菌特异性引物XAF1和XAR1[25], 对上述接种后从叶片分离获得的单菌落和接种后叶片总DNA进行PCR检测。PCR反应总体积为25 μL, 包含Ex Taq 12.5 μL、上游引物和下游引物各1.0 μL、模板DNA 1.0 μL、无菌水9.5 μL。反应程序为94℃预变性1 min; 94℃变性30 s, 56℃退火1 min, 72℃延伸30 s, 35个循环; 72℃终延伸5 min。取5.0 μL的PCR产物在1%琼脂糖凝胶中进行电泳检测, 用凝胶成像仪观察扩增出的目的条带(608 bp)。
1.2.7 PCR产物克隆和测序 将上述扩增后的PCR产物用琼脂糖凝胶电泳后回收目的条带, 与pMD19-T载体连接后, 质粒转化DH5α感受态细胞, 涂布平板。随机挑取3个单菌落, 经过菌液PCR验证为阳性后, 送生工生物工程(上海)有限公司测序。测序结果进行BLAST序列比对分析, 根据同源性判断病原菌的信息。
1.3 统计分析
在接种后第28天, 计算51份供试材料的病情指数(%), 根据表2所述标准进行抗性等级分组, 采用单因素组内数目不等重复数进行方差分析, 平均值多重比较采用q检验法。病情指数原始数据进行平方根反正弦变换后, 运用重心法(Centriod method)计算欧几里得距离(Euclidean metric)进行品种聚类分析。以上统计分析均由SAS 9.2软件完成。2 结果与分析
2.1 接种后的甘蔗白条病症状表现
用甘蔗白条病菌Xa-FJ1菌株通过剪叶法对51个甘蔗新品种(系)进行人工接种。在接种第7天之前, 多数品种叶片没有典型的狭窄型铅笔状白色褪绿条纹, 只有少数品种叶片在接种后第3天出现长度为1 cm左右的白色褪绿条纹。在接种第7天之后, 各品种叶片陆续出现典型白条病症状, 从接种后的第21天开始, 有些植株出现叶片白化或者黄化现象, 发病严重的甘蔗品种出现叶片坏死, 甚至整株死亡。参考甘蔗白条病严重度划分等级(表1和图1), 白条病症状表现为无症状(0级)、1~2条白色褪绿条纹(1级)、2条以上白色褪绿条纹(2级)、叶片白化或黄化(3级)、叶片坏死(4级)和植株死亡(5级)。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1甘蔗白条病不同发病严重度
Score 0: 严重度0级; Score 1: 严重度1级; Score 2: 严重度2级; Score 3: 严重度3级; Score 4: 严重度4级; Score 5: 严重度5级。
Fig. 1Different disease severity grades of sugarcane leaf scald
Score 0: grade 0; Score 1: grade 1; Score 2: grade 2; Score 3: grade 3; Score 4: grade 4; Score 5: grade 5.
2.2 接种后的叶片病原分离和分子鉴定
对153份接种后的甘蔗叶片总DNA样本, 采用特异性引物XAF1/XAR1进行PCR检测, 结果显示, 在每个甘蔗品种中, 至少有一个生物学重复的叶片总DNA样本检测为阳性。这些PCR产物测序结果表明, PCR检测目的片段序列与Xa-FJ1菌株对应的序列同源性均为100%。另外, 挑取3种不同发病严重度的病叶, 铅笔状白色条纹、叶片褪绿白化和叶片褪绿黄化的叶片样品5个, 进行白条病菌分离。成功分离纯化的这些白条病菌单菌落形态与Xa-FJ1相似, 为黄色、平滑、圆整、光亮、黏稠状(图2-A~E)。这些菌液PCR产物测序结果表明, 分离获得这些菌株与Xa-FJ1菌株一致(图2-F)。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2接种后的病叶白条病菌分离和PCR检测
A: 白色褪绿条纹样品(新台糖22号)分离出来的菌落; B: 白色褪绿条纹样品(闽糖11-610)分离出来的菌落; C: 白化叶片(柳城09-15)出来的菌落; D: 黄化叶片(粤甘49)分离出来的菌落; E: 黄化叶片(福农11-2907)分离出来的菌落; F: 从不同品种分离出来的白条病菌单菌落PCR检测。1: D15000+2000 DNA marker; 2: 空白对照, 无菌水; 3: 阴性对照, 健康植株叶片DNA; 4: 阳性对照, Xa-FJ1菌株; 5~9: 上述分离出来的5个菌株的DNA样品。
Fig. 2Pathogen isolation and PCR detection of inoculated leaf samples
A: colonies isolated from ROC22 with white pencil lines; B: colonies isolated from Mintang 11-610 with white striped leaves; C: colonies isolated from Liucheng 09-15 with chlorotic leaf; D: colonies isolated from Yuegan 49 with yellowing leaf; E: colonies isolated from Funong 11-2907 with yellowing leaf; F: PCR detection for colonies isolated from the above-mentioned samples. 1: D15000+2000 DNA marker; 2: the blank control, sterile H2O; 3: the negative control, healthy leaf sample; 4: the positive control, Xa-FJ1 strain; 5-9: DNA samples from colonies isolated from the above-mentioned samples.
2.3 甘蔗新品种(系)白条病病情指数分析
51个甘蔗品种接种后的不同时间点的病情指数如图3所示, 病情指数总体趋势随着时间的延长不断升高。在接种后的第7天时, 病情指数比较低, 为0~6.9%; 在接种后的第14天时, 病情指数为0~34.3%; 在接种后第21天开始, 多数品种的病情指数趋于稳定, 在接种后的第28天时, 病情指数为10.6%~58.2%。图3
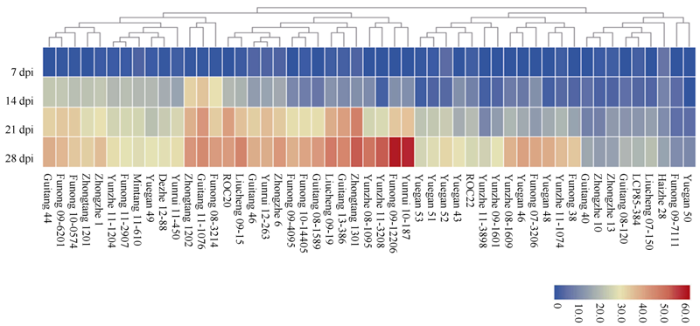 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3接种后不同时间点的51个甘蔗品种病情指数
Fig. 3Disease indexes of 51 sugarcane varieties at different day post-inoculation (dpi)
按照表2所示的白条病抗性评价标准, 以接种后第28天时的病情指数将51个甘蔗品种白条病抗性划分为4个等级(表3)。抗病等级组包括粤甘50、福农09-7111和中蔗10号3个品种, 病情指数在10.6%~14.7%之间。中抗等级组包括LCP85-384对照种等在内的16个品种, 病情指数在15.4%~28.7%之间, 其中LCP85-384病情指数为19.4%。感病等级组包括新台糖20号对照种在内的28个品种, 病情指数在30.3%~48.4%之间, 其中新台糖20号病情指数为41.2%。高感等级组包括云蔗11-3208、中糖1301、云瑞10-187、福农09-12206共4个品种, 病情指数变化幅度为51.6%~58.2%。不同抗性等级组之间的甘蔗品种的病情指数达极显著差异(P<0.01)。
Table 3
表3
表3甘蔗新品种人工接种白条病抗性等级
Table 3
| 抗病等级 Grade of resistance | 品种(系)名称 Variety (line) name | 品种(系)数量 Number of variety | 病情指数 Disease index (%) | 平均值多重比较 Mean multiple comparison |
|---|---|---|---|---|
| 抗 Resistant | 粤甘50, 福农09-7111, 中蔗10号 Yuegan 50 , Funong 09-7111, Zhongzhe 10 | 3 | 10.6-14.7 | 12.2 A |
| 中抗 Medium resistant | 中蔗13号, 海蔗28号, 桂糖40号, LCP85-384, 桂糖08-120, 柳城07-150, 云蔗11-3898, 粤甘53, 新台糖22号, 闽糖11-610, 粤甘51, 德蔗12-88, 福农11-2907, 粤甘49, 云蔗09-1601, 粤甘43 Zhongzhe 13, Haizhe 28, Guitang 40, LCP85-384, Guitang 08-120, Liucheng 07-150, Yunzhe 11-3898, Yuegan 53, ROC22, Mintang 11-610, Yuegan 51, Dezhe 12-88, Funong 11-2907, Yuegan 49, Yunzhe 09-1601, Yuegan 43 | 16 | 15.4-28.7 | 23.9 B |
| 感病 Susceptible | 云蔗11-1204, 云瑞11-450, 粤甘52, 中糖1201, 福农10-0574, 福农38, 中蔗1号, 云蔗11-1074, 福农09-6201, 云蔗08-1609, 福农09-4095, 云瑞12-263, 桂糖46号, 桂糖44号, 粤甘48, 福农07-3206, 福农10-14405, 粤甘46, 福农08-3214, 新台糖20号, 桂糖08-1589, 中蔗6号, 中糖1202, 桂糖11-1076, 桂糖13-386, 柳城09-15, 柳城09-19, 云蔗08-1095 Yunzhe 11-1204, Yunrui 11-450, Yuegan 52, Zhongtang 1201, Funong 10-0574, Funong 38, Zhongzhe 1, Yunzhe 11-1074, Funong 09-6201, Yunzhe 08-1609, Funong 09-4095, Yunrui 12-263, Guitang 46, Guitang 44, Yuegan 48, Funong 07-3206, Funong 10-14405, Yuegan 46, Funong 08-3214, ROC20, Guitang 08-1589, Zhongzhe 6, Zhongtang 1202, Guitang 11-1076, Guitang 13-386, Liucheng 09-15, Liucheng 09-19, Yunzhe 08-1095 | 28 | 30.3-48.4 | 38.7 C |
| 高感 High susceptible | 云蔗11-3208, 中糖1301, 云瑞10-187, 福农09-12206 Yunzhe 11-3208, Zhongtang 1301, Yunrui 10-187, Funong 09-12206 | 4 | 51.6-58.2 | 54.6 D |
新窗口打开|下载CSV
2.4 甘蔗新品种(系)白条病病情指数聚类分析
对接种后第28天的甘蔗品种病情指数进行聚类分析表明, 当阈值为0.125时, 51份供试材料白条病抗病性可分为四大组群, 第I组为抗病等级, 包括对照种LCP85-384在内9个品种; 第II组为中抗等级, 包括国内主栽品种新台糖22在内10个品种; 第III组为感病等级, 包括对照种新台糖20号在内23个品种; 第IV组为高感等级, 包括9个品种。聚类分析结果与病情指数划分结果略有差异。如柳城07-150、桂糖08-120、LCP85-384、桂糖40号、中蔗13号和海蔗28号在病情指数划分为中抗品种, 但在聚类分析结果中显示为抗; 福农09-6201、桂糖11-1076、桂糖13-386、柳城09-19和云蔗08-1095在病情指数划分为感病品种, 但在聚类分析结果中划分为高感。图4
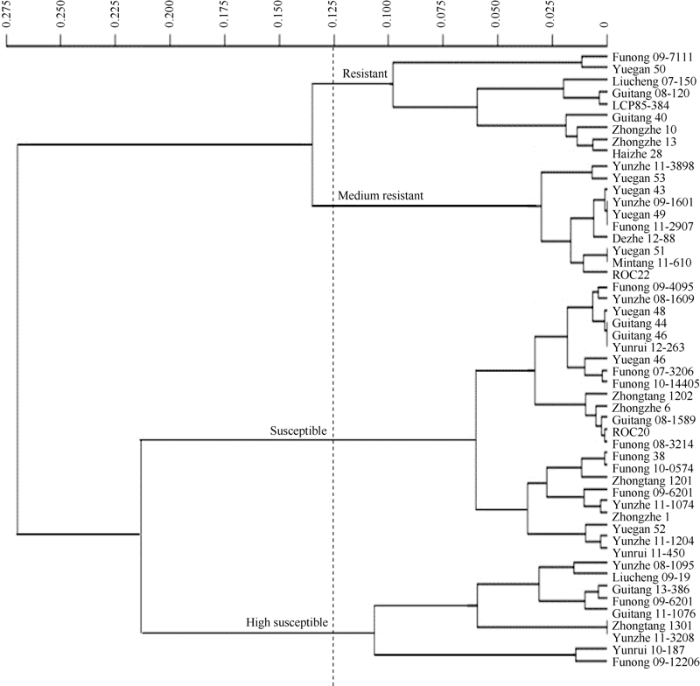 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4供试甘蔗品种白条病抗性的聚类分析树状图
Fig. 4Cluster analysis of disease resistance to leaf scald in sugarcane cultivars
3 讨论
甘蔗栽培种Saccharum spp. hybrid经过100年前甘蔗高贵化育种选育出来的种间杂交后代, 其基因组80%的血缘来自热带种S. officinarum, 10%~15%来自割手密S. spontaneum, 还有5%~10%来自其他原始种的重组[26]。当前甘蔗栽培种血缘可追溯到20世纪20~30年代选育出来的几个亲本, 如POJ2878、Co419和NCo310等, 导致国内外甘蔗亲本资源匮乏、血缘相近、遗传基础狭窄[27]; 另外, 甘蔗为异源多倍体作物, 遗传背景复杂, 利用传统杂交法改良甘蔗品种特性周期长、优异性状聚合育种几率小, 限制了当前甘蔗新品种进一步改良的潜力[28]。新品种(系)抗病性鉴定是甘蔗抗病育种工作重要环节之一。甘蔗白条病田间接种鉴定方法主要有浸种法、剪叶法和截头法[8]。本研究建立了甘蔗新品种(系)白条病室内苗期剪叶法人工接种抗性鉴定与评价技术体系, 具有操作简便、节省土地资源和人力物力、缩短了抗病鉴定的周期、受外界环境条件影响小等优点, 为甘蔗早代育种材料的大规模抗病性鉴定提供技术支持。国外有文献报道采用截头法开展甘蔗品种白条病抗病鉴定与评价, 最好的接种时期是甘蔗伸长期的初期阶段, 适合于田间抗性鉴定, 但受到甘蔗生长季节限制和外界环境变化的影响较大[16,29]。本研究以国内主要流行株系Xa-FJ1菌株作为甘蔗白条病菌接种源, 该菌株属于PFGE-B组群, 该组群株系是20世纪80至90年代在美国佛罗里达、墨西哥、多米尼加、加勒比海东部的瓜德鲁普岛、古巴等蔗区曾经暴发流行的株系[8,9,10]。本研究采用甘蔗苗期室内剪叶法接种甘蔗品种, 随着接种时间的延长, 病情指数不断升高, 但是, 在接种后第21天和第28天, 病情指数基本维持在一个相对稳定状态, 为此, 建议这一时期的甘蔗品种病情指数可作为抗性鉴定评价指标。本研究采用LCP 85-384和新台糖20号为抗性鉴定对照种, 抗性鉴定结果与文献报道的田间抗病性结果相一致[18-19,23]。而且, 本方法鉴定认为桂糖46号为感病品种, 这与国内蔗区田间调查结果一致[21]。值得注意的是, 国内各研究单位新选育出来的这些甘蔗新品系, 没有发现高抗白条病的品系, 37.3%的品系为抗病和中抗等级, 而62.7%的品系为感病和高感等级。这说明国内甘蔗白条病抗性种质缺乏, 加快甘蔗抗病种质的收集、挖掘和利用迫在眉睫。本研究共筛选出19个甘蔗白条病中抗以上品种(系), 包括国内主栽品种新台糖22号在内, 这些新品种(系)为甘蔗白条病的抗病品种合理的布局提供参考。
在接种后第28天时, 多数品种甘蔗叶片样品的3份生物学重复常规PCR检测均有阳性条带, 但是, 有的品系只有1个生物学重复样品PCR检测为阳性, 这一现象的原因可能是抗病品种的抗病能力强, 不易受白条病菌侵染和在体内繁殖, 导致病菌含量低。有报道甘蔗白条病抗病性与植株体内病原菌浓度呈显著负相关[18,19]。另一原因可能是感病品种在接种后21~28 d时, 多数叶片已出现白化、黄化症状, 有的叶片组织已坏死, 导致这种半活体营养型的甘蔗白条病菌含量低, 不易检测出来。我们预实验结果发现, 接种21 d后的坏死的叶片组织和死亡的植株分离不出来甘蔗白条病菌, 用CTAB法提取的这些类型的叶片总DNA质量极差, 几乎已降解, 且含量极低。为此, 我们建议在接种后第14天之前采样供核酸分子检测。另外, 对于病菌含量低的样品, 可以采用灵敏度更高的荧光定量qPCR[18-19,30]或者环介导等温扩增(LAMP)技术[31]。国外****利用qPCR定量检测接种后的甘蔗植株X. albilineans病原菌含量来评价甘蔗白条病抗病性[18,19], 这种抗性鉴定方法也值得借鉴。综上所述, 本研究建立的甘蔗新品种(系)苗期白条病人工接种抗性鉴定与评价体系为甘蔗抗白条病育种提供技术支撑。
4 结论
本研究建立了甘蔗新品种(系)苗期白条病苗期人工接种抗性鉴定与评价体系, 具有快速、简便, 鉴定结果受环境因素影响较小等优点, 且能真实反映出品种的抗病性水平。运用这一方法, 对来自国内甘蔗育种单位新选育的49个优良品种(系)进行白条病抗性鉴定和评价, 初步筛选了一批抗病品种, 为蔗区品种多系布局和抗性种质的利用提供了重要参考。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOIURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIPMID [本文引用: 1]

Sugarcane is one of the most important commercial crops cultivated worldwide for the production of crystal sugar, ethanol, and other related by-products. Unlike other comparable monocots like sorghum, maize, and rice, sugarcane genome by virtue of its polyploidy nature remains yet to be fully deciphered. Proteomics-an established complementary tool to genomics is at its infancy in sugarcane as compared to the other monocots. However, with the surge in genomics research accomplished by next-generation sequencing platforms, sugarcane proteomics has gained momentum. This review summarizes the available literature from 1970 to 2014, which ensures a comprehensive coverage on sugarcane proteomics-a topic first of its kind to be reviewed. We herewith compiled substantial contributions in different areas of sugarcane proteomics, which include abiotic and biotic stresses, cell wall, organelle, and structural proteomics. The past decade has witnessed a paradigm shift in the pace with which sugarcane proteomics is progressing, as evident by the number of research publications. In addition to extensively reviewing the progress made thus far, we intend to highlight the scope in sugarcane proteomics, with an aspiration to instigate focused research on sugarcane to harness its full potential for the human welfare. ? 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
[本文引用: 2]
[本文引用: 1]
[本文引用: 4]
[本文引用: 4]
DOIURL [本文引用: 6]
DOI [本文引用: 2]

Xanthomonas albilineans is the causal agent of leaf scald, a disease that can cause considerable damage to sugarcane industries. This study analysed the phylogenetic relationship of 14 samples of X. albilineans from China and 13 reference strains retrieved from the GenBank database by multilocus sequence analysis (MLSA). To reach this goal, five housekeeping genes of X. albilineans were amplified from diseased leaves and sequenced: gyrB, abc, rpoD, atpD and glnA. Based on the concatenated sequence of these genes (4473 nt), the 14 samples of X. albilineans from China had 99.9-100% sequence identity with one another and with five strains of the pathogen from the French West Indies and the USA (Florida). The 27 samples or strains of X. albilineans were distributed in two distinct clades in the MLSA-based phylogenetic tree. Clade 1 was formed by four strains of the pathogen from Fiji, Papua New Guinea and the USA. All the other strains from worldwide locations, including the 14 samples from China, were grouped in clade 2. This latter clade included all strains of the pathogen that were associated with outbreaks of leaf scald that have occurred over the last two decades, especially in the Caribbean islands and the USA. The very low diversity of X. albilineans in four Chinese provinces suggests recent spread of a single strain (from genetic group PFGE-B) of the leaf scald pathogen within China.
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIPMID [本文引用: 3]

A streptomycin- and rifampicin-resistant mutant of Xanthomonas al-bilineans was used to study symptom expression of leaf scald disease (LSD) and colonization of sugarcane (Saccharum spp.) and its wild relatives by this bacterial pathogen. A total of 40 sugarcane cultivars and 15 clones from the Saccharum complex that differed in resistance to LSD were inoculated by a decapitation technique in both field and greenhouse experiments. In the plant crop, disease severity varied between 0 for the most resistant genotypes and 100 for the most susceptible ones. Resistance to LSD was characterized by limited colonization of the host plant by X. albilineans. Although almost all genotypes were colonized by the pathogen, the greatest bacterial population densities were found in the susceptible cultivars. There was a high correlation between disease severity and pathogen population in the apex. Several genotypes exhibited no or slight symptoms even though they were highly colonized in the upper and/or basal nodes of stalks. Two mechanisms, therefore, may play an important role in resistance to LSD: resistance to colonization of the apex, which is characterized by absence of symptoms, and resistance to colonization of the upper and lower parts of the stalk. In contrast, disease severity and pathogen population densities in the first ratoon crop in the field were nil or very low in the stalks, except for the highly susceptible cv. CP68-1026. Sugarcane ratoons, therefore, may recover from the disease after plant cane infection. Nevertheless, because low levels of the pathogen were still detected in some stalks, it is possible that LSD could develop from latent infections if favorable environmental conditions occur.
DOIURL [本文引用: 1]
DOIPMID [本文引用: 5]

Leaf scald is an important disease of sugarcane with erratic symptom expression. Latency represents a threat to germplasm exchange, and erratic symptom development makes accurate evaluation of disease resistance during breeding and selection problematic. Real-time quantitative polymerase chain reaction (qPCR) assays for Xanthomonas albilineans, the causal agent of leaf scald, were developed and evaluated for the sensitive, specific detection and quantification of the pathogen. Assays with SYBR Green primers and TaqMan probe and primers derived from the albicidin toxin biosynthesis gene cluster efficiently and reproducibly amplified X. albilineans. Detection was more sensitive with qPCR compared with conventional PCR. Assays were specific for X. albilineans and sap extracts did not inhibit the qPCR reaction. Leaf-scald-resistant and -susceptible cultivars were distinguished by infection incidence, disease severity, and X. albilineans population determined by SYBR Green qPCR in both greenhouse and field experiments. Populations of X. albilineans varied in different tissues. Differences were the greatest within tissues in resistant cultivars, and bacterial populations in systemically infected, young, not yet fully emerged leaves exhibited the greatest differences between resistant and susceptible cultivars. The results demonstrate that qPCR is a highly sensitive method for the detection of X. albilineans that could provide a reliable method for leaf scald resistance screening.
DOIPMID [本文引用: 5]

Leaf scald, caused by Xanthomonas albilineans, is a major sugarcane disease controlled primarily with host resistance. Because visual evaluation can be uncertain due to erratic symptom expression, a reliable resistance screening method is needed. A quantitative polymerase chain reaction (qPCR) with potential for resistance screening was used to compare bacterial populations in 31 clones at different times after inoculation, and the correlation with the visual symptom rating method was determined. Comparisons of bacterial populations quantified by qPCR and visual symptom severity ratings in systemically infected leaves showed variable results, with the highest correlation at 8 weeks after inoculation. To measure consistency, the correlation was determined among three different field experiments for data obtained with the same method at different times after inoculation. The qPCR assay was more consistent among experiments compared with visual symptom rating at 8 weeks after inoculation. Susceptible check cultivars always had high bacterial populations but the severe inoculation resulted in moderate to high bacterial populations in two of three resistant checks in some experiments. The results suggest that qPCR can provide an improved method to evaluate resistance to leaf scald in sugarcane; however, multiple experiments will be needed to accurately determine clone resistance levels.
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
DOIURL [本文引用: 1]
DOIURL [本文引用: 2]
DOIURL [本文引用: 1]
DOIURL [本文引用: 2]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
DOIURL [本文引用: 1]
