 ,1, 丁鑫超1, 卢海1, 胡亚丽1, 岳娇1, 黄震1, 莫良玉1, 陈立1, 陈涛2, 陈鹏
,1, 丁鑫超1, 卢海1, 胡亚丽1, 岳娇1, 黄震1, 莫良玉1, 陈立1, 陈涛2, 陈鹏 ,1,*
,1,*Physiological characteristics and DNA methylation analysis under lead stress in kenaf (Hibiscus cannabinus L.)
LI Zeng-Qiang ,1, DING Xin-Chao1, LU Hai1, HU Ya-Li1, YUE Jiao1, HUANG Zhen1, MO Liang-Yu1, CHEN Li1, CHEN Tao2, CHEN Peng
,1, DING Xin-Chao1, LU Hai1, HU Ya-Li1, YUE Jiao1, HUANG Zhen1, MO Liang-Yu1, CHEN Li1, CHEN Tao2, CHEN Peng ,1,*
,1,*通讯作者:
收稿日期:2020-05-8接受日期:2020-08-19网络出版日期:2021-06-12
| 基金资助: |
Received:2020-05-8Accepted:2020-08-19Online:2021-06-12
| Fund supported: |
作者简介 About authors
E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (1137KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
李增强, 丁鑫超, 卢海, 胡亚丽, 岳娇, 黄震, 莫良玉, 陈立, 陈涛, 陈鹏. 铅胁迫下红麻生理特性及DNA甲基化分析[J]. 作物学报, 2021, 47(6): 1031-1042. doi:10.3724/SP.J.1006.2021.04104
LI Zeng-Qiang, DING Xin-Chao, LU Hai, HU Ya-Li, YUE Jiao, HUANG Zhen, MO Liang-Yu, CHEN Li, CHEN Tao, CHEN Peng.
土壤重金属污染严重影响农作物的产量和品质, 并且通过食物链传递严重危害人类的健康。铅是最严重的土壤污染重金属之一, 有效治理土壤中的铅污染是目前亟待解决的重大课题[1]。红麻是世界上重要的纤维作物, 其生长迅速、生物量大、对重金属耐受性强, 并且不进入食物链, 是重金属污染土壤修复的理想作物[2]。
DNA甲基化在调控植物生长发育和响应生物及非生物胁迫中起重要作用[3,4]。研究表明, 重金属胁迫下, 植物的DNA甲基化水平和模式会发生改变, 这可能是植物对逆境的应急响应[5]。甲基化敏感扩增多态性(methylation sensitive amplified polymorphism, MSAP)技术是基于扩增片段长度多态性(amplified fragment length polymorphism, AFLP)技术的基础上发展起来的, 它的主要原理是采用限制性内切酶Hpa II和Msp I对5′-CCGG-3′位点进行酶切, 由于这2种酶对该位点DNA甲基化的敏感性不同, 因此可以通过切割产生不同的片段来揭示DNA甲基化的程度, 该技术已广泛用于植物基因组DNA甲基化水平的检测[6,7]。何玲莉等[8]利用MSAP技术检测不同浓度铅胁迫对萝卜肉质根基因组DNA甲基化水平的变化发现, 其甲基化水平随着铅浓度的提高而上升, 推测可能是由于铅胁迫产生了大量的甲基自由基所致, 并且DNA甲基化水平的变化可以激活或者抑制相关基因的表达, 进而提高萝卜对铅胁迫的适应性。郭丹蒂[9]运用MSAP技术发现, 铅和镉胁迫均导致中华水韭DNA甲基化水平提高, 但与重金属的浓度无显著相关性。殷欣[10]也运用MSAP技术发现, 大豆基因组DNA甲基化水平随镉浓度的增加而逐渐提高, 并且甲基化差异基因广泛参与了植物响应重金属胁迫的过程。然而, 也有研究发现, 重金属胁迫导致基因组DNA甲基化水平降低的现象, 表明重金属胁迫下DNA甲基化水平变化和响应机制的复杂性[11]。
有关DNA甲基化响应红麻重金属胁迫的研究尚未见报道。本研究利用不同浓度PbCl2对红麻材料P3A幼苗进行胁迫处理, 研究幼苗农艺性状、ROS含量和抗氧化酶活性变化, 检测铅胁迫下基因组DNA甲基化水平变化, 并对甲基化差异片段进行克隆测序和表达水平分析, 以期为明确铅胁迫对红麻生长的影响, 揭示DNA甲基化响应红麻铅胁迫的潜在机制具有重要意义。
1 材料与方法
1.1 试验材料及处理
红麻材料P3A由广西大学农学院周瑞阳教授提供。挑选适量籽粒饱满的种子, 常温条件下用蒸馏水浸泡1 h, 经3%过氧化氢消毒10 min, 再用蒸馏水冲洗3次, 均匀地摆放在铺有2层纱布(注意定期定量添加1/4 Hoagland营养液, 始终保持纱布湿润)的塑料盒(27 cm × 18 cm × 9 cm)中, 放置于恒温光照培养箱(光/暗周期为10 h/14 h, 温度为白天27℃/夜晚25℃, 下同)中培养7 d后, 选取长势一致的幼苗240株, 随机分成12组, 每组20株, 移栽于12个底部含有托盘的育苗盘(21孔花卉育苗盘)中, 即为4个处理, 每个处理3次重复。向托盘中分别添加含有不同PbCl2浓度(0、200、400、600 μmol L-1)的1/4 Hoagland处理液, 注意每天定量地更换处理液。1.2 农艺性状及生理指标的测定
胁迫处理7 d后, 全部材料用蒸馏水清洗干净并用吸水纸擦干, 分别用直尺、游标卡尺和电子天平测定各单株的株高、茎粗、全鲜重, 并利用根系扫描分析仪(EPSON EXPRESSION 10000XL)对根系进行扫描分析。之后将材料用液氮速冻后保存于-80℃冰箱, 用于后续试验。分别采用羟胺氧化法[12]、硫代巴比妥酸法[13]测定根系的超氧阴离子(superoxide anion, O2?)和丙二醛(malondialdehyde, MDA)含量; 分别采用氮蓝四唑法[13]、愈创木酚法[13]、紫外分光光度法[12]测定根系的超氧化物歧化酶(superoxide dismutase, SOD)、过氧化物酶(peroxidase, POD)、过氧化氢酶(catalase, CAT)活性。
1.3 甲基化敏感扩增多态性(MSAP)分析
参考Tang等[14]报道的MSAP方法并加以改良, 对部分处理(0、600 μmol L-1)的根系进行DNA甲基化水平分析。首先采用改良CTAB法[15]提取上述材料的基因组DNA, 之后分别用EcoR I/Hpa II和EcoR I/Msp I 两种酶组合进行双酶切, 连接, 预扩增、选择性扩增, 6%聚丙烯酰胺凝胶电泳, 银染、显色, 拍照保存并进行MSAP多态性片段的统计。双酶切体系(3种酶均购于NEB公司)为EcoR I 0.5 μL、Hpa II或Msp I 0.5 μL、CutSmart buffer 2 μL、模板DNA (100 ng μL-1) 4 μL、ddH2O 13 μL; 程序为37℃ 6 h, 80℃ (EcoR I/Hpa II)或65℃ (EcoR I/Msp I) 20 min。连接体系为正反向引物(10 μmol L-1)各1 μL、T4 DNA Ligase (350 U μL-1, TaKaRa公司) 2 μL、10×T4 DNA Ligase buffer 2 μL、酶切产物14 μL; 程序为16℃ 14 h, 65℃ 20 min。预扩增和选择性扩增体系均为稀释10倍后的连接产物或预扩增产物5 μL、正反向引物(10 μmol L-1)各1 μL、2×Rapid Taq Master Mix (南京诺唯赞生物科技有限公司) 10 μL、ddH2O 3 μL; 程序均为95℃ 3 min; 95℃ 15 s, 60℃ (以每对引物的平均Tm值为准) 15 s, 72℃ 30 s, 35个循环; 72℃ 5 min。接头序列、预扩增和选择性扩增引物序列见表1。Table 1
表1
表1接头和引物序列
Table 1
| 引物类型 Primer type | 引物名称及序列Primer name and sequence (5′-3′) | |||
|---|---|---|---|---|
| EcoR I (E) | Hpa II/Msp I (HM) | |||
| 接头 Adapter | EA1 | CTCGTAGACTGCGTACC | HMA1 | GACGATGAGTCTAGAA |
| EA2 | AATTGGTACGCAGTC | HMA2 | CGTTCTAGACTCATC | |
| 预扩增 Pre-amplification | E0 | GACTGCGTACCAATTCA | HM0 | GATGAGTCTAGAACGGT |
| E1 | GACTGCGTACCAATTCAAC | HM1 | GATGAGTCTAGAACGGTAG | |
| E2 | GACTGCGTACCAATTCAAG | HM2 | GATGAGTCTAGAACGGTAC | |
| E3 | GACTGCGTACCAATTCACA | HM3 | GATGAGTCTAGAACGGTTG | |
| 选择性扩增 | E4 | GACTGCGTACCAATTCACT | HM4 | GATGAGTCTAGAACGGTTC |
| Selective amplification | E5 | GACTGCGTACCAATTCACC | HM5 | GATGAGTCTAGAACGGTGT |
| E6 | GACTGCGTACCAATTCACG | HM6 | GATGAGTCTAGAACGGTGC | |
| E7 | GACTGCGTACCAATTCAGC | HM7 | GATGAGTCTAGAACGGTCT | |
| E8 | GACTGCGTACCAATTCAGG | HM8 | GATGAGTCTAGAACGGTCG | |
新窗口打开|下载CSV
MSAP多态性条带标记方法如下: 同一水平线上, EcoR I/Hpa II和EcoR I/Msp I酶切都有带, 记为I型(无甲基化); EcoR I/Hpa II酶切有带而EcoR I/ Msp I酶切无带, 记为II型(半甲基化); EcoR I/Hpa II酶切无带而EcoR I/Msp I酶切有带, 记为III型(全甲基化); EcoR I/Hpa II和EcoR I/Msp I酶切都无带, 记为IV型(全甲基化)。DNA甲基化水平统计方法如下: 甲基化条带数 = II型+III型+IV型, 甲基化率 = (II型+III型+IV型)/(I型+II型+III型+IV型)×100%; 半甲基化条带数 = II型, 半甲基化率 = II型/(I型+II型+III型+IV)×100%; 全甲基化条带数 = III型+IV型, 全甲基化率 = (III型+IV型)/(I型+II型+III型+IV型)×100% [7]。
1.4 甲基化差异片段的回收、克隆和测序分析
对显色后的6%变性聚丙烯酰胺凝胶结果进行照相和MSAP多态性片段统计后, 切下观察到的甲基化差异片段于1.5 mL离心管中, 加入30 μL ddH2O, 煮沸10 mim, 用枪头将胶块捣碎, 短暂离心后取上清进行PCR扩增。体系包含上清10 μL、选择性扩增时的正反向引物(10 μmol L-1)各4 μL、2×Rapid Taq Master Mix 50 μL、ddH2O 32 μL, PCR程序同选择性扩增。之后进行2%琼脂糖凝胶电泳及胶回收。使用北京全式金生物技术有限公司的T1 Cloning Kit进行克隆, 阳性克隆经PCR方法鉴定后, 送深圳华大基因科技服务有限公司测序。测序结果在红麻基因组数据库[16] (https://bigd.big.ac.cn/gwh)及NCBI网站上进行Nucleotide BlAST同源性比对。1.5 实时荧光定量PCR (qRT-PCR)分析
利用改良异硫氰酸胍法[17]分别提取相应根系材料(0, 600 μmol L-1)的RNA, 并进行1%琼脂糖凝胶电泳, 采用超微量紫外分光光度计检测RNA的质量和浓度, 之后使用诺唯赞反转录试剂盒(货号: R223-01)反转录成cDNA, 并以此为模板进行qRT-PCR分析。以组蛋白基因His3为内参基因, 采用2-ΔΔCT方法[2]计算基因的相对表达量, qRT-PCR引物序列见表2。Table 2
表2
表2实时荧光定量PCR引物序列
Table 2
| 引物名称 Primer name | 正向引物 Forward sequence (5′-3′) | 反向引物 Reverse sequence (5′-3′) |
|---|---|---|
| AHL23 | TCCTCCATCCGAACCAGTGTG | CAAGGGAATCCAGAGAGACCATC |
| CesA2 | CCTAAAAATGCCGAACTCTACGC | TAGAAAATCTGGACTCTCCTGGTGC |
| ETO1 | ATCTTTTCCGAGTTTGGTGTATCCT | CGCTCCCTCCTTTCTTCGTC |
| NPF5.4 | TGAAGTTAGCAGCATCGGTTG | TTCCAACACTTCCAAGAGGGT |
| PLK | TTGCGGCACTACGAGGTTGTT | CCCCAATCATTCAATCTCCATACC |
| PME7 | CGCTGGACAAGAAAGATACCG | GACACGCCGAGTGACATCATAGA |
| RABA1f | ACAAGGCTCACCATTTCCACAT | GGGTCCCAGATACGAGGTTTTC |
| SGT1 | TCATTGCCAACACTAAAGCACACAT | CCTAAGGCTACTCTGGAATCTGGGT |
| VPS13F | TTGAGCCATCGTGGTGAAGGG | GCAGCAGGATTTGCGGTGTT |
| WD40 | ATGCCCTCCTTCAATGCGT | CCCAATACCGATGAACAGCC |
| β-man6 | GATGATTGGTGGAGGAATGAGCA | GTCGTCTGCTGCTTTAGAGGTCAC |
| His3 (reference gene) | GTGGAGTCAAGAAGCCTCACAG | ATGGCTCTGGAAACGCAAA |
新窗口打开|下载CSV
2 结果与分析
2.1 不同浓度PbCl2胁迫对红麻幼苗农艺性状的影响
对不同浓度PbCl2胁迫下红麻幼苗农艺性状的测定发现(表3), 低浓度(200 μmol L-1) PbCl2胁迫对幼苗的株高、全鲜重和根鲜重无显著抑制, 中浓度(400 μmol L-1)及高浓度(600 μmol L-1)胁迫下均出现显著抑制。说明低浓度PbCl2胁迫对红麻幼苗生长无明显的影响, 红麻具有较强的铅耐受性。不同浓度PbCl2胁迫均显著抑制了幼苗的茎粗、根长和根表面积。中浓度和高浓度PbCl2胁迫对幼苗生长的抑制除在株高方面呈显著性差异外, 在其他方面均无显著性差异。Table 3
表3
表3不同浓度PbCl2胁迫对红麻幼苗农艺性状的影响
Table 3
| PbCl2浓度 Concentration of PbCl2 (μmol L-1) | 株高 Plant height (cm) | 茎粗 Stem diameter (mm) | 全鲜重 Fresh weight (g) | 根鲜重 Root fresh weight (g) | 根长 Root length (cm) | 根表面积 Root surface area (cm2) |
|---|---|---|---|---|---|---|
| 0 | 18.12±0.42 a | 2.01±0.02 a | 14.33±0.61 a | 2.71±0.17 a | 105.79±12.13 a | 16.70±1.38 a |
| 200 | 18.18±0.20 a | 1.78±0.01 b | 13.58±0.50 a | 2.48±0.15 a | 76.99±4.53 b | 13.53±0.61 b |
| 400 | 14.90±0.05 b | 1.64±0.06 c | 8.91±0.16 b | 1.27±0.01 b | 34.60±1.09 c | 6.51±0.32 c |
| 600 | 12.18±0.20 c | 1.52±0.03 c | 7.27±0.27 b | 1.00±0.04 b | 28.79±1.84 c | 5.12±0.44 c |
新窗口打开|下载CSV
2.2 不同浓度PbCl2胁迫对红麻幼苗根系铅含量的影响
由图1可知, 随着PbCl2浓度的逐渐增加, 根系中的铅含量显著升高。红麻幼苗根系的铅含量在对照条件下为3.59 mg kg-1, 低浓度(200 μmol L-1)、中浓度(400 μmol L-1)和高浓度(600 μmol L-1) PbCl2胁迫分别使根系的铅含量增加了34、138和203倍。结合对红麻幼苗农艺性状的测定结果得出, 本研究中不同浓度PbCl2胁迫虽然显著增加了幼苗根系中铅的积累, 但是并没有严重影响到红麻的生长, 说明红麻对铅胁迫具有较强的耐受性。图1
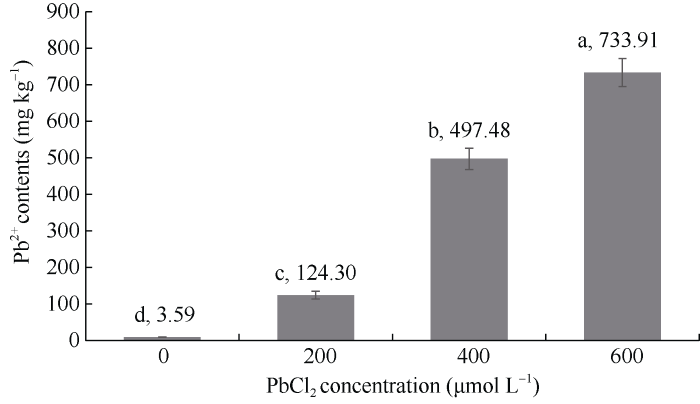 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1不同浓度PbCl2胁迫对根系铅含量的影响
不同小写字母表示在0.05水平差异显著。
Fig. 1Effects of different concentrations of PbCl2 on lead contents in roots
Bars superscripted by different lowercase letters indicate significant differences at the 0.05 probability level.
2.3 不同浓度PbCl2胁迫对红麻幼苗根系抗氧化系统的影响
由图2可知, 随着PbCl2胁迫浓度的升高, 红麻幼苗根系的O2? (图2-A)和MDA含量(图2-B)、以及SOD活性(图2-C)总体上都呈逐渐上升的趋势。POD活性(图2-D)呈先下降后缓慢上升的趋势, 高浓度(600 μmol L-1) PbCl2胁迫下活性最高, 其次为对照条件和中浓度(400 μmol L-1), 低浓度(200 μmol L-1) PbCl2胁迫下活性最低。CAT活性(图2-E)呈先上升后降低的趋势, 中浓度PbCl2胁迫下活性最高, 其次为高浓度和低浓度, 对照条件下活性最低。各项指标在不同浓度PbCl2胁迫下都呈显著性差异。即不同浓度PbCl2胁迫均使红麻幼苗根系的O2?和MDA含量升高, SOD和CAT活性上升。POD活性在高浓度胁迫下升高, 中浓度和低浓度胁迫下降低。说明铅胁迫使红麻幼苗根系发生了膜脂过氧化, 但其自身的抗氧化酶活性也会相应提高, 以清除过量ROS的产生, 使铅胁迫对幼苗的伤害降到最低。图2
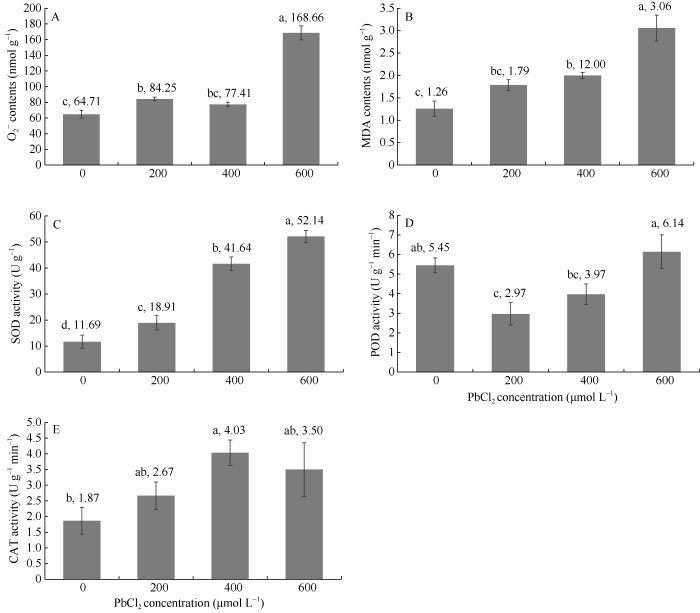 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2不同浓度PbCl2胁迫对根系抗氧化系统的影响
不同小写字母表示在0.05水平差异显著。
Fig. 2Effects of different concentration of PbCl2 on physiological indicators of roots
Bars superscripted by different lowercase letters indicate significant differences at the 0.05 probability level.
2.4 PbCl2胁迫对红麻幼苗根系DNA甲基化水平的影响
运用MSAP技术分析了600 μmol L-1 PbCl2胁迫和对照条件下红麻幼苗根系的DNA甲基化水平, 部分代表性聚丙烯酰胺凝胶电泳结果见图3, 统计结果见表4。对照条件下, 幼苗根系的DNA甲基化率为71.13%, 其中全甲基化率和半甲基化率分别为50.52%和20.62%。600 μmol L-1 PbCl2胁迫下, 幼苗根系的DNA甲基化率为62.2%, 其中全甲基化率和半甲基化率分别为37.8%和24.4%。即600 μmol L-1 PbCl2胁迫使幼苗根系的DNA甲基化率和全甲基化率降低, 半甲基化率升高。表明在整体水平上, 600 μmol L-1 PbCl2胁迫降低了红麻幼苗根系的DNA甲基化水平。图3
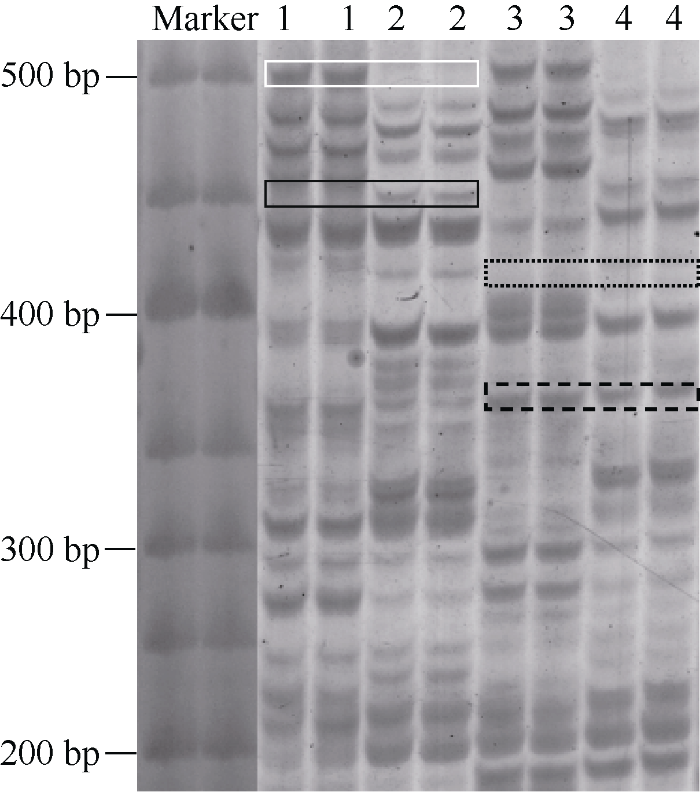 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3MSAP聚丙烯酰胺凝胶电泳图
泳道1和3代表EcoR I/Hpa II酶切, 泳道2和4代表EcoR I/Msp I酶切。1和2: PbCl2-0; 3和4: PbCl2-600。虚线方框: I型(无甲基化); 白色方框: II型(半甲基化); 黑色方框: III型(全甲基化); 虚点方框: IV型(全甲基化)。
Fig. 3MSAP acrylamide gel analysis
The left lane 1, 3 and right lane 2, 4 represent digestion with EcoR I/ Hpa II and EcoR I/Msp I respectively. The lane 1, 2, and 3, 4 represent the PbCl2-0, PbCl2-600 respectively. The dotted line frame, white frame, black frame and virtual point frame represent the type I (no methylation), type II (hemi-methylation), type III (full methylation), and type IV (full methylation), respectively.
Table 4
表4
表4DNA甲基化水平统计分析
Table 4
| 甲基化类型 Methylation type | PbCl2浓度PbCl2 concentration | 变化比率(上升↑, 下降↓) Exchange rate (up↑, down↓) | |
|---|---|---|---|
| 0 μmol L-1 | 600 μmol L-1 | ||
| 类型I (无甲基化) Type I (unmethylation) | 83 | 110 | 32.53↑ |
| 类型II (半甲基化) Type II (hemi-methylation) | 61 | 71 | 16.39↑ |
| 类型III, IV (全甲基化) Type III and IV (full methylation) | 147 | 110 | 25.17↓ |
| 半甲基化率Hemi-methylated ratio (%) | 20.62 | 24.40 | 18.33↑ |
| 全甲基化率Fully methylated ratio (%) | 50.52 | 37.80 | 34.20↓ |
| 甲基化率/MSAP Total methylated ratio/MSAP (%) | 71.13 | 62.20 | 12.55↓ |
新窗口打开|下载CSV
2.5 甲基化差异片段的测序及qRT-PCR分析
本研究共扩增出291条MSAP多态性片段, 回收到97条DNA甲基化差异片段, 测序比对得到38条具有功能的DNA甲基化差异序列。与植物生长发育和响应逆境相关的甲基化差异序列测序结果见表5, 其中14条DNA甲基化差异序列比对到的同源基因与响应植物抗逆性密切相关, 例如AT-hook家族基因(AHL23)、SGT家族基因(SGT1)、S-腺苷-L-蛋氨酸依赖性甲基转移酶家族蛋白(SAM- MET)、NRT1/PTR家族蛋白(NPF5.4)、RaBa家族蛋白(RaBa1f)、Ran GTPase结合蛋白(GTPase)、WD40重复序列蛋白(WD40)、乙烯过表达蛋白(ETO1)、液泡分选蛋白(VPS13F)、β-甘露聚糖酶(β-man6)、类受体蛋白激酶(PLK)、纤维素合成酶(CesA2)、果胶酯酶抑制剂(PME7)、线粒体内膜转位因子(TIM21)。Table 5
表5
表5甲基化差异片段比对分析
Table 5
| 基因名 Gene name | 片段大小 Fragment length (bp) | 基因代号或ID Gene symbol or ID | 基因全称 Gene full name | 功能注释 Functional annotation |
|---|---|---|---|---|
| 7-dlgase | 255 | Hca.05G0009940 | Hca. flavonol-3-O-glycoside-7-O-glucosyltransferase 1 | 将UDP-糖基供体转移到花色素的C7羟基(羧基)[18]。 Transfer UDP glycosyl donor to C7 hydroxy (carboxy) of anthocyanins. |
| AHL23 | 110 | Hca.02G0040470 | Hca. AT hook motif domain containing protein | AT-hook家族蛋白在植物生长发育、激素信号转导和逆境胁迫应答中发挥重要作用[19]。 At-hook family proteins play an important role in plant growth and development, plant hormone signal transduction and stress response. |
| CesA2 | 140 | Hca.03G0045600 | Hca. CESA1-cellulose synthase | CesA影响初生壁和次生壁的生物合成,响应植物的抗病性[20]。 CesA affects cell wall biosynthesis and responds to plant disease resistance. |
| chD12 | 136 | CP023742 | Gossypium hirsutum cultivar TM1 chromosome D12 | 尚未见报道。 Has not been reported. |
| EF1B/S6 | 131 | Hca.09G0002430 | Hca. eukaryotic translation initiation factor 3 subunit D | 尚未见报道。 Has not been reported. |
| ETO1 | 178 | Hca.13G0028050 | Hca. ethylene-responsive protein related | 乙烯调控种子萌发、器官衰老、生物和非生物胁迫等过程。 Ethylene regulates seed germination, organ senescence, biotic and abiotic stress. |
| GTPase | 361 | Hca.01G0050250 | Hca. RAN GTPase-activating protein 1 | 参与调控细胞周期中各个时期的细胞生命活动。 It is involved in the regulation of cell activities at various stages of the cell cycle. |
| Kinase | 165 | Hca.02G0009450 | Hca. protein kinase family protein | 尚未见报道。 Has not been reported. |
| mit-gene | 247 | KR736346 | Gossypium trilobum mitochondrion, complete genome | 尚未见报道。 Has not been reported. |
| NPF5.4 | 269 | LOC108487536 | Gossypium arboreum protein NRT1/ PTR FAMILY 5.4-like | NRT1/PTR家族蛋白参与转运植物激素及次生代谢物合成过程。 NRT1/PTR family proteins are involved in the transport of plant hormones and the synthesis of secondary metabolites. |
| Phosphatase | 248 | Hca.13G0013080 | Hca. phosphatidic acid phosphatase-related | 参与磷酸基团转移、代谢等生理过程。 It is involved in the transfer and metabolism of phosphate groups. |
| PLK | 265 | Hca.15G0014780 | Hca. receptor-like protein kinase | 类受体激酶通过接收和传递胞外信号调控细胞的生理反应,参与植物生长发育过程。 Receptor-like protein kinase regulates cellular physiological responses by receiving and transmitting extracellular signals and are involved in plant growth and development. |
| PME7 | 117 | Hca.01G0006960 | Hca. pectinesterase | 果胶酯酶抑制剂在植物生长发育和响应逆境胁迫中发挥重要作用[21]。 Pectinesterase inhibitor plays an important role in plant growth and response to stress. |
| PMT24 | 171 | Hca.15G0021740 | Hca. methyltransferase | 催化腐胺向N-甲基腐胺的转化。 Catalyze the conversion of putrescine to N-methylputrescine. |
| RaBa1f | 170 | LOC105766406 | Gossypium raimondii ras-related protein RABA1f | RABA家族蛋白在调控根毛扩张?细胞壁组分、响应生物胁迫等方面发挥重要作用。 RABA family protein plays an important role in regulating root hair expansion, cell wall components and responding to biological stress. |
| SAM-MET | 114 | Hca.18G0000220 | Hca. S-adenosyl-L-methionine-dependent methyltransferases | 催化的甲基化修饰对植物信号传导、染色体表达和基因沉默等起重要的调节作用。 Catalytic methylation plays an important role in regulating signal transduction, chromosome expression and gene silencing in plants. |
| SGT1 | 300 | Hca.02G0014470 | Hca. SGT1 protein | SGT1基因与植物与植物响应生物和非生物胁迫密切相关 。 SGT1 gene is closely related to plant response to biotic and abiotic stress. |
| TIM21 | 257 | Hca.12G0027090 | Hca. mitochondrial import inner membrane translocase subunit Tim | 负责线粒体内膜的转运与装配[22]。 Responsible for the transport and assembly of mitochondrial intima. |
| TPR | 353 | Hca.17G0008870 | Hca. tetratricopeptide repeat domain containing protein | 介导与蛋白质的相互作用。 Mediates interactions with proteins. |
| VPS13F | 102 | Hca.05G0005060 | Hca. vacuolar protein sorting-associated protein 16 | 液泡分选相关蛋白参与调控主根的发育和植株生长素响应过程。 Vacuolar protein sorting-associated protein are involved in the regulation of taproot development and auxin response. |
| WD40 | 140 | Hca.01G0001440 | Hca. katanin p80 WD40 repeat-containing subunit B1 homolog 1 | WD40是拟南芥生长发育和胁迫信号传递的关键调控因子[23]。 WD40 is a key regulator of Arabidopsis growth and stress signal transmission. |
| β-man6 | 188 | Hca.02G0010790 | Hca. alpha-mannosidase 2 | α-甘露聚糖酶引导蛋白的跨膜运输。 alpha-mannosidase guides the transmembrane transport of proteins. |
新窗口打开|下载CSV
挑选以上部分DNA甲基化差异基因对600 μmol L-1 PbCl2胁迫和对照条件下的红麻幼苗根系进行qRT-PCR分析。由图4可知, 11个与响应植物抗性密切相关的DNA甲基化差异基因中, 有7个基因在PbCl2胁迫下的表达量与对照呈显著性差异。其中基因AHL23、CesA2、NPF5.4、PME7、SGT1的表达量在PbCl2胁迫下显著降低, 基因PLK、RaBa1f、WD40的表达量在PbCl2胁迫下显著升高。说明这些基因的DNA甲基化情况变化与基因表达水平变化密切相关, 并且DNA甲基化变化对基因表达水平的影响是复杂多样的。DNA甲基化水平的升高大多数情况下抑制基因的表达, 但也存在对基因表达水平起促进作用, 或者无显著影响的情况[4]。
图4
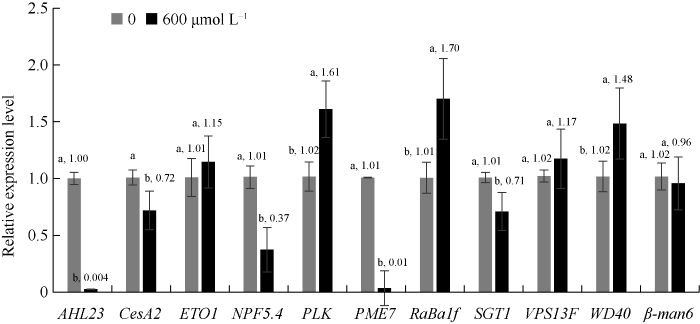 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4PdCl2胁迫下甲基化差异片段的qRT-PCR分析
不同小写字母表示在0.05水平差异显著。
Fig. 4qRT-PCR analysis of the differentially methylated sequences under PdCl2 stress
Bars superscripted by different lowercase letters indicate significant differences at the 0.05 probability level.
3 讨论
3.1 红麻可以作为修复铅污染土壤的潜在作物
研究表明, 禾本科牧草和黄化枫林木的生物量随着土壤中铅浓度的逐渐增加呈先升高后降低的趋势[24,25], 低浓度铅胁迫可以促进小麦种子的萌发[25]。本研究表明, 低浓度(200 μmol L-1) PbCl2胁迫对红麻幼苗的生长无明显的影响, 而在较高浓度(400 μmol L-1以上)则表现出明显的抑制, 并且一定浓度范围内(400~600 μmol L-1)铅胁迫对幼苗生长的抑制并无显著性差异, 表明红麻具有较强的铅耐受性。由于红麻以收获营养器官为目的, 不进入食物链, 因此生产上可以利用红麻能够吸收土壤中的重金属铅, 并且对其产量无明显影响的优势来改良土壤。植物在正常的生长发育条件下, 活性氧的产生与清除之间时常处于良性的动态平衡状态[27]。当植物遭受生物或非生物胁迫后, 这种动态平衡就会被打破, 导致植物体内积累大量的活性氧, 例如积累过量的O2-使膜发生过氧化作用, 产生过量丙二醛(MDA), 使细胞膜及细胞器破坏解体, 最终影响植物的生长发育[23-24,27]。与此同时, 当植物遭受逆境胁迫时, 负责清除活性氧的抗氧化酶等系统的活性会相应提高, 使对植物的伤害降到最低, 但当胁迫程度严重时, 会引起抗氧化酶系统紊乱, 无力清除更多的活性氧, 最终严重影响植物的生长发育[28,29]。本研究表明, 不同浓度PbCl2胁迫均显著提高了红麻幼苗根系的O2?和MDA含量, 以及SOD和CAT的活性, POD活性也在高浓度铅胁迫下显著升高。表明红麻具有较强的抗氧化系统, 可以在一定范围内清除铅胁迫产生的ROS以缓解对红麻生长的影响。
3.2 铅胁迫降低了红麻幼苗根系的DNA甲基化水平
本研究首次检测了红麻幼苗根系的DNA甲基化水平, 并得出600 μmol L-1 PbCl2胁迫使幼苗根系的DNA甲基化率和全甲基化率降低, 半甲基化率升高, 即整体上降低了幼苗根系的DNA甲基化水平。这与李雪林等[30]和高桂珍等[31]在其他作物中的研究结果一致, 但也有相反的结论[8,9]。这可能是由于不同作物和不同非生物胁迫的特性, 或者铅胁迫浓度和处理时间不同而导致的。也有研究表明, 枫树在不同浓度盐胁迫下的DNA甲基化水平变化情况不同, 并且相同浓度盐胁迫对不同组织DNA甲基化水平变化的影响也不尽相同[31], 说明植物响应非生物胁迫时, DNA甲基化情况变化的复杂性。3.3 红麻铅胁迫下DNA甲基化变化参与调控抗性相关基因的表达
本研究发现了7个已报道的与植物抗逆性密切相关的基因在铅胁迫下同时存在DNA甲基化和基因表达水平的差异, 因此我们推测DNA甲基化水平的变化可能参与调控这些基因的表达, 进而调控红麻对铅胁迫的响应。在水稻中已经鉴定出49个与生长发育和响应胁迫相关的果胶酯酶抑制剂PMEI家族基因[21]。PMEI家族基因参与植物油菜素内酯的调节[33], 很多基因也抑制蔗糖转移酶的活性[21], 影响植物的抗逆性。本研究中甲基化差异基因PME7在铅胁迫下几乎不表达, 因此可能对油菜素内酯和蔗糖的合成起到促进作用, 提高红麻幼苗对铅胁迫的适应性。纤维素合酶CesA在纤维素和细胞壁结构的合成中起重要作用[20,34], 拟南芥CesA3及CesA7基因表达水平的下调可以增强对葡萄孢菌的抗病性[35]。DNA甲基化可能通过调控纤维素合成酶基因的表达促进纤维素的合成, 最终可以提高拟南芥的抗盐性[36]。本研究中纤维素合酶CesA2的甲基化情况在铅胁迫下发生了改变, 基因表达量显著下调, 因此推测其甲基化情况的变化在调控基因表达、促进纤维素合成, 进而在响应铅胁迫方面起到了一定的作用。AT-hook家族基因在植物的生长发育、植物激素信号转导和响应逆境胁迫中起重要的作用, 部分AT-hook家族基因表达量的变化很可能参与到了番茄响应逆境胁迫中[19]。NRT1/PTR家族蛋白在植物转运硝酸盐、钾盐、氨基酸和植物激素过程中起重要的作用[37,38]。本研究中AT-hook家族的甲基化差异基因AHL23在铅胁迫下几乎不表达, NRT1/PTR家族基因NPF5.4在铅胁迫下的表达量显著降低, 可能会影响营养元素和植物激素的转运, 使红麻幼苗在铅胁迫下生长受抑。有研究报道SGT1家族基因与植物的抗病性密切相关[39], 李为民等[40]研究发现, 过表达SGT1的转基因烟草对赤星病菌的抗性明显提高, 拟南芥SGT1基因突变体对霜霉病的抗性大大降低[39]。本研究中, 甲基化情况的变化使得抗病基因SGT1的表达量显著降低, 且该基因也可能参与到了植物非生物胁迫的响应中。类受体蛋白激酶PLKs广泛参与植物细胞信号转导和逆境胁迫的响应过程, 水稻PLKN1基因正向调控水稻的抗盐性, 高盐胁迫下其突变体的存活率降低, MDA含量升高, SOD和CAT活性降低[41]。本研究中甲基化差异基因PLK在铅胁迫下的表达量显著升高, 因此可能抑制MDA的积累, 提高抗氧化酶的活性, 进而提高红麻幼苗对铅胁迫的适应性。RaBa家族基因在植物响应生物和非生物胁迫中发挥重要的作用[42], 转PtRabA2f基因拟南芥受胁迫的影响更小[43]。核桃WD40转录因子JrATG18a基因参与到了逆境胁迫的响应当中[44], 夏凯文[23]也发现, WD40重复序列结构域蛋白是拟南芥生长发育和胁迫信号传递的关键调控因子。本研究中甲基化差异基因RaBa1f和WD40在铅胁迫下的表达量均显著提高, 因此推测DNA甲基化水平的变化参与以上抗性基因的表达, 从而启动了红麻幼苗对铅胁迫的应急响应。
4 结论
红麻对铅胁迫具有较强的耐受性, 并且一定浓度范围内的铅胁迫对红麻生长的抑制无显著差异, 生产上可以利用红麻来改良重金属铅污染土壤。一定浓度范围内的铅胁迫下, 红麻幼苗可以通过提高自身的抗氧化酶活性来清除过量ROS的产生。600 μmol L-1 PbCl2胁迫使幼苗根系整体的DNA甲基化水平降低, 与植物抗逆性密切相关的AT-hook家族基因、NRT1/PTR家族蛋白、RaBa家族蛋白、SGT家族基因、WD40重复序列蛋白、类受体蛋白激酶、纤维素合成酶等相关基因在铅胁迫下都发生了DNA甲基化和基因表达水平的显著变化。推测红麻幼苗可以通过自身的抗氧化酶系统, 及DNA甲基化水平变化来响应铅胁迫, 以消除或减轻铅胁迫对红麻生长的影响。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1016/j.foodchem.2013.11.133URLPMID:24444908 [本文引用: 1]

A new adsorbent, polyhydroxybutyrate-b-polyethyleneglycol, was used for the separation and preconcentration of copper(II) and lead(II) ions prior to their flame atomic absorption spectrometric detections. The influences of parameters such as pH, amount of adsorbent, flow rates and sample volumes were investigated. The polymer does not interact with alkaline, alkaline-earth metals and transition metals. The enrichment factor was 50. The detection limits were 0.32 mug L(-1) and 1.82 mug L(-1) for copper and lead, respectively. The recovery values were found >95%. The relative standard deviations were found to be less than 6%. The validation of the procedure was performed by analysing certified reference materials; NIST SRM 1515 Apple leaves, IAEA-336 Lichen and GBW-07605 Tea. The method was successfully applied for the analysis of analytes in water and food samples.
[本文引用: 2]
DOI:10.1016/s0959-437x(00)00061-7URLPMID:10753779 [本文引用: 1]

Recent research has demonstrated that DNA methylation plays an integral role in regulating the timing of flowering and in endosperm development. The identification of key genes controlling these processes, the expression of which is altered in plants with low methylation, opens the way to understanding how DNA methylation regulates plant development.
DOI:10.1016/j.pbi.2008.12.006URLPMID:19179104 [本文引用: 2]

Gene expression driven by developmental and stress cues often depends on nucleosome histone post-translational modifications and sometimes on DNA methylation. A number of studies have shown that these DNA and histone modifications play a key role in gene expression and plant development under stress. Most of these stress-induced modifications are reset to the basal level once the stress is relieved, while some of the modifications may be stable, that is, may be carried forward as 'stress memory' and may be inherited across mitotic or even meiotic cell divisions. Epigenetic stress memory may help plants more effectively cope with subsequent stresses. Comparative studies on stress-responsive epigenomes and transcriptomes will enhance our understanding of stress adaptation of plants.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
URLPMID:14633664 [本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 3]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
[本文引用: 3]
[本文引用: 3]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
URLPMID:21617373 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
