 ,1, 杨乃科1, 商丽丽2, 高晓茹1, 刘庆昌1, 翟红1, 高少培1, 何绍贞
,1, 杨乃科1, 商丽丽2, 高晓茹1, 刘庆昌1, 翟红1, 高少培1, 何绍贞 ,1,*
,1,*Cloning and functional analysis of a drought tolerance-related gene IbNAC72 in sweet potato
ZHANG Huan ,1, YANG Nai-Ke1, SHANG Li-Li2, GAO Xiao-Ru1, LIU Qing-Chang1, ZHAI Hong1, GAO Shao-Pei1, HE Shao-Zhen
,1, YANG Nai-Ke1, SHANG Li-Li2, GAO Xiao-Ru1, LIU Qing-Chang1, ZHAI Hong1, GAO Shao-Pei1, HE Shao-Zhen ,1,*
,1,*通讯作者:
收稿日期:2020-02-29接受日期:2020-07-2网络出版日期:2020-11-12
| 基金资助: |
Received:2020-02-29Accepted:2020-07-2Online:2020-11-12
| Fund supported: |
作者简介 About authors
E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (4569KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
张欢, 杨乃科, 商丽丽, 高晓茹, 刘庆昌, 翟红, 高少培, 何绍贞. 甘薯抗旱相关基因IbNAC72的克隆与功能分析[J]. 作物学报, 2020, 46(11): 1649-1658. doi:10.3724/SP.J.1006.2020.04051
ZHANG Huan, YANG Nai-Ke, SHANG Li-Li, GAO Xiao-Ru, LIU Qing-Chang, ZHAI Hong, GAO Shao-Pei, HE Shao-Zhen.
甘薯[Ipomoea batatas (L.) Lam.]属旋花科(Convolvulaceae)甘薯属(Ipomoea)甘薯组(Section Batatas), 是一种重要的粮食、饲料、工业原料和新型能源用作物。甘薯广泛种植于世界上100多个国家和地区, 在世界粮食生产中排第8位[1]。中国是世界上最大的甘薯生产国, 年产量5324.57万吨, 占世界甘薯总产量的57.91%, 年种植面积237.93万公顷, 占世界甘薯总种植面积的29.51%[1]。
随着全球气候的变暖和生态平衡的破坏, 干旱灾害成为全球最大的自然灾害之一。干旱土地多数肥力较低, 土壤瘠薄, 长期生长在干旱土壤的作物会因干旱而生长缓慢、生长停滞、甚至死亡, 导致作物减产, 加剧全球粮食危机, 因此, 提高植物抗旱性具有战略性意义[2]。同时, 我国干旱、半干旱地区面积占国土面积的52.5%, 主要集中在西北和华北地区, 科学合理、可持续地开发利用这些土地, 对国家生态环境建设和后备耕地资源拓展具有重要意义[3]。植物抗旱性是复杂的数量性状, 挖掘并克隆抗旱相关基因、培育抗旱作物新品种是解决土壤干旱减产并确保粮食安全的重要途径[4]。
近年来, 随着植物抗逆分子机制研究的不断深入, 一些与抗旱应答有关的转录因子相继被鉴定, 包括NAC类[5]、MYB类[6]、DREB类[7]、WRKY类[8]、锌指蛋白类[9]等。其中, NAC是植物中最大的转录因子家族之一, 也是植物特有的转录因子。NAC结构域最初的特征在于来自矮牵牛NAM、拟南芥ATAF1/2和CUC2的共有高度保守的氨基酸序列, 其大约含有150个氨基酸, 位于蛋白质的N末端, 通常分为A~E 5个亚结构域, 其中A、C、D亚结构域高度保守, 亚结构域B和E不保守, 参与NAC蛋白的多种特异的功能[10]。水稻中过表达OsNAC6或ONAC045基因能够通过其激活抗氧化等抗逆相关途径, 来提高水稻转基因植株的耐盐性和抗旱性[11,12]。水稻中过表达SNAC1基因, 干旱胁迫条件下, 转基因水稻在生殖生长阶段的结实率比对照显著提高22%~34%, 在营养生长阶段, 相比于对照, 转基因水稻对脱落酸更敏感, 促进关闭叶片气孔, 减少水分丧失速率, 提高了水稻的抗旱性[13]。水稻中过表达ONAC066基因能够提高水稻的抗旱和抗氧化性[14]。在拟南芥中过表达显著响应干旱胁迫的NAC转录因子基因RD26后, ABA和胁迫诱导相关基因表达上调, ABA敏感性显著增加, RD26基因参与到防御响应和ABA响应的信号途径中, 并发挥重要作用[15]。在拟南芥中过表达玉米ZmSNAC1基因可以显著提高转基因拟南芥植株的抗旱性和耐盐性[16]。拟南芥ANAC096与ABF2互作, 协同激活抗旱相关基因RD29A的转录, 同时在脱水和渗透胁迫下激活ABA诱导型基因的转录, 从而调控植株的抗旱和抗氧化性[17]。拟南芥NAC016通过直接结合ABA信号途径关键调控因子AREB1的启动子并抑制其表达, 从而降低植株的抗旱性[18]。番茄中的NAC转录因子JUN-GBRUNNEN1 (JUB1)通过直接结合抗旱相关基因SlDREB1、SlDREB2和SlDELLA的启动子, 调控它们的表达来提高番茄的耐旱性[19]。干旱条件下, 过表达大麦HvSNAC1能够使植株失水率显著降低, 维持田间产量[20]。
甘薯是一种比较耐旱、耐瘠薄的作物, 且其高产、稳产、富含淀粉, 可以转化为燃料乙醇而被视为重要的粮食和新型生物质能源作物。加强对甘薯抗旱性的研究, 克隆抗旱相关基因, 创制抗旱新材料及培育抗旱新品种, 有望充分利用我国广阔的干旱瘠薄的丘陵, 而又不与粮食争地, 对国家的粮食安全和生物质能源发展意义重大。甘薯抗旱基因IbNHX2[21]、IbMIPS1[22]、IbLCYB2[23]、IbC3H18[9]等被相继报道。Zhu等[24]对抗旱甘薯品种Xushu 55-2的抗旱转录组分析表明, 部分NAC类、MYB类、bHLH类和WRKY类转录因子在PEG-6000处理下显著上调。
目前NAC家族基因在甘薯逆境胁迫反应中的功能研究尚未见报道。本研究从甘薯抗逆SSH差减文库中筛选克隆获得IbNAC72基因, 其被干旱和盐胁迫显著诱导表达。为了研究IbNAC72基因的功能, 构建了过表达载体, 转化并获得稳定的过表达烟草植株, 通过抗旱鉴定证明, 过表达IbNAC72基因能够显著提高转基因烟草植株的抗旱性。表明IbNAC72基因在干旱胁迫应答过程中发挥作用。
1 材料与方法
1.1 植物材料
甘薯品种栗子香和烟草品种Wisconsin 38均来自中国农业大学甘薯研究室。1.2 菌株和质粒载体
大肠杆菌DH5α购自北京全式金生物技术有限公司; 农杆菌EHA105由本实验室提供。pEASY-Blunt Simple载体购自北京全式金生物技术有限公司, pMD19-T载体购自宝生物工程(大连)有限公司。pBI121和pCAMBIA3301载体由本实验室提供。1.3 IbNAC72基因的克隆
利用本实验室构建的抗逆SSH差减文库筛选到的差异基因EST序列, 通过NCBI BLAST (https:// blast.ncbi.nlm.nih.gov/Blast.cgi)分析, 得到一段带有NAM保守结构域的序列。根据已经获得的EST序列设计3′-RACE引物NAC-1和NAC-2 (表1), 以及5′-RACE引物NAC-3和NAC-4 (表1)。分别使用3′-Full RACE Core Set Ver.2.0 Kit试剂盒(宝生物工程(大连)有限公司, D314)和5′-Full RACE Kit试剂盒, 进行3′-RACE和5′-RACE的扩增。根据RACE方法克隆得到的IbNAC72基因3′和5′序列, 设计IbNAC72基因ORF全长引物NAC-F和NAC-R (表1)。Table 1
表1
表1IbNAC72基因克隆和功能分析所用引物
Table 1
| 引物名称 Primer name | 引物序列 Primer sequence (5′-3′) | 用途 Function |
|---|---|---|
| NAC-1 | CGGAAAGTTGGGATCAAGAA | 3′-RACE扩增 |
| NAC-2 | CAGGAAGACCGGAAGTTCAA | 3′-RACE amplification |
| NAC-3 | TTGAACTTCCGGTCTTCCTG | 5′-RACE扩增 |
| NAC-4 | ATTCGCCCTGCTAGGAAGAT | 5′-RACE amplification |
| NAC-F | ATGGGTGTGAAAGATATGGACC | ORF、DNA序列扩增 |
| NAC-R | TTACTGCCTGAACGCTAAGCTAC | ORF and DNA sequence amplification |
| NAC-QF | TCTTCAACAAGACGACAAGTCAAG | RT-qPCR检测 |
| NAC-QR | AGGAAAACTCAATGCCATTG | RT-qPCR detection |
| Actin-F | AGCAGCATGAAGATTAAGGTTGTAGCAC | 甘薯内参基因检测 |
| Actin-R | TGGAAAATTAGAAGCACTTCCTGTGAAC | Sweet potato internal control |
| NAC-OE-F (Xba I) | GCTCTAGAATGGGTGTGAAAGATATGGACC | 过表达载体构建 |
| NAC-OE-R (Sac I) | CGAGCTCTTACTGCCTGAACGCTAAGCTAC | Construction of overexpression vector |
新窗口打开|下载CSV
使用RNA preppure PlantKit (TIANGEN, Beijing)提取栗子香甘薯叶片总RNA, 利用QuantScript RT Kit (TIANGEN, Beijing) QuantcDNA第1链合成试剂盒(目录号为KR103)反转录成cDNA。以cDNA为模板扩增IbNAC72, 回收纯化, 将其连接到pMD19-T (TaKaRa)载体上, 挑单克隆进行测序及分析, 获得IbNAC72的ORF序列。同时, 以栗子香叶片基因组DNA做底物进行扩增, 获得IbNAC72的基因组DNA序列。
1.4 IbNAC72基因的序列分析
利用Primer Premier 5软件推导IbNAC72基因编码的氨基酸序列。利用NCBI Conserved Domain Search (1.5 IbNAC72基因在甘薯中的表达分析
分别提取4周大小栗子香试管苗的叶片、茎段和根系, 以及3个月大小的栗子香大田苗的叶片、茎段、块根、梗根和须根各个组织中的总RNA, 并反转录成cDNA, 对IbNAC72基因在甘薯各个组织中的表达量进行分析。同时, 将试管苗洗净放入1/2霍格兰溶液中驯化3 d, 然后分别浸入含有100 mmol L-1 NaCl和20% PEG-6000的1/2霍格兰溶液中, 处理0、2、4、6、12、24、48 h后取材, 提取叶片总RNA并反转录成cDNA, 对IbNAC72基因在盐、旱处理后的表达量进行分析。根据IbNAC72基因的ORF非保守区间, 用Primer Premier 5软件设计其荧光定量引物NAC-QF和NAC-QR (表1)。内标基因为甘薯肌动蛋白基因(β-Actin)(表1)。使用SYBR Premix Ex Taq (Tli RNaseH Plus)荧光定量试剂盒(宝生物工程(大连)有限公司, RR420)和7500 Real-Time PCR System仪器(Applied Biosystems, Foster City, CA, USA)进行RT-qPCR分析。1.6 植物表达载体的构建
选择pBI121和pCAMBIA3301载体作为原始载体。设计IbNAC72基因过表达载体引物NAC-OE-F和NAC-OE-R(表1)。使用东洋纺(上海)生物科技有限公司生产的高保真酶KOD-Plus对IbNAC72基因的ORF进行扩增, 将扩增片段连接在克隆测序载体pEASY-Blunt simple上, 得到pEASY-IbNAC72。用EcoR I和Hind III对pCAMBIA3301和pBI-121进行双酶切, 回收pCAMBIA3301载体和pBI121表达盒, 连接转化得到克隆中间载体pC3301-121, 并酶切鉴定。用Xba I和Sac I对pC3301-121和pEASY- IbNAC72双酶切, 回收pC3301-121载体大片段及IbNAC72基因, 连接、转化并测序。提取测序正确的质粒, 对构建好的植物表达载体进行酶切鉴定, 最后得到植物表达载体pC3301-121-IbNAC72。将该重组质粒转化农杆菌EHA105感受态, 并筛选出阳性单克隆保存备用。1.7 转基因烟草植株的获得与鉴定
利用农杆菌介导的叶盘法转化烟草, 方法参见《植物基因工程》[25]。超净工作台中切下不含主脉5 mm × 5 mm的烟草叶盘, 放置在再生培养基(MS+ 1.0 mg L-1 6-BA+0.1 mg L-1 NAA)上, 背面贴培养基, 黑暗28℃培养2~3 d。用OD600为0.4~0.6农杆菌菌液振荡侵染叶盘10 min。黑暗28℃放置在再生培养基上共培养2~3 d。接着用无菌蒸馏水洗3次, 500 mg L-1羧苄青霉素洗1次后, 放置在筛选培养基(MS+1.0 mg L-1 6-BA+0.1 mg L-1 NAA+300 mg L-1 Carbenicillin)上分化出芽(光照: 45 μmol m-2 s-1, 28℃)。随后, 切下长度为1 cm的芽, 放置在MS培养基上生长成完整植株。参照Jefferson等[26]的方法, 对获得的拟转基因烟草植株的叶片、茎段和根系进行GUS检测。将烟草植株的不同组织浸入GUS检测液中, 37℃过夜; 将待检材料取出, 转入FAA固定液中固定1 h以上; 依次用70%、90%、100%的乙醇进行脱色; 记录并照相。同时, 提取拟转基因植株和对照植株叶片的基因组DNA, 以载体质粒DNA为阳性对照, 水和非转基因植株DNA为阴性对照进行PCR检测。引物序列NAC-F和NAC-R (表1)。
1.8 转基因烟草植株的抗旱性鉴定
选取1个月大小的长势良好、生长状态一致的转基因烟草植株和野生型对照植株, 一次性浇足水后停止浇水, 进行4周自然干旱处理。4周后观察表型, 测量植株的株高、根的鲜重、超氧化物歧化酶(SOD)活性及丙二醛(MDA)含量。干旱胁迫下, 参照Zhang等[9]的方法测量烟草叶片的SOD活性及MDA含量。2 结果与分析
2.1 IbNAC72基因的特性分析
前期在甘薯抗逆SSH差减文库中筛选到一个在干旱处理下表达差异的EST序列。利用RACE方法, 分别获得该基因的5′-RACE和3′-RACE片段, 利用DNAMAN进行拼接后, 设计引物扩增出其ORF序列。经NCBI比对发现, 该基因序列与其他物种的NAC72基因的同源性较高, 因此命名为IbNAC72。IbNAC72基因cDNA长为1319 bp, ORF为1008 bp, 编码335个氨基酸; 含有129 bp 5′非翻译区、169 bp 3′非翻译区和13 bp多聚腺苷酸尾。预测的分子量为37.4 kD, 等电点(pI)为8.76。同源蛋白序列多重比对结果表明, IbNAC72在N端15~139 aa的位置存在一个NAC家族具有的NAM保守结构域, 分为A~E 5个亚结构域, 其中B亚结构域与其他同源基因同源性较低, 各同源蛋白在C端的保守性也较低(图1-A)。系统进化树分析结果图1
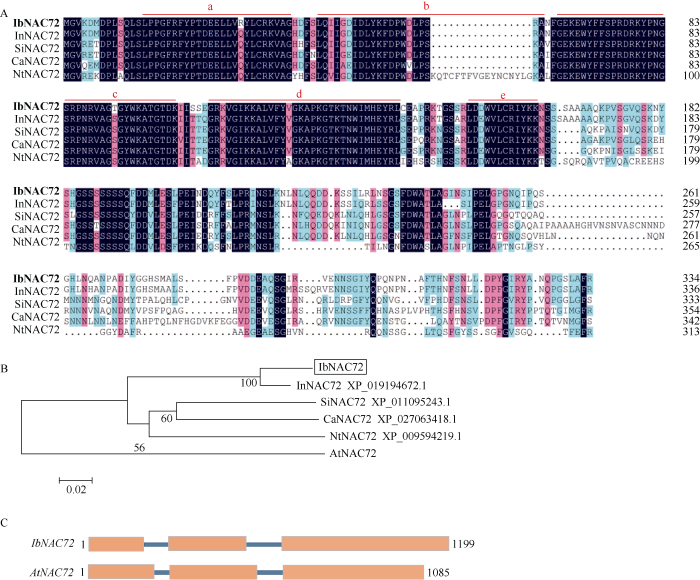 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1IbNAC72基因的序列分析
A: 不同物种中NAC72同源蛋白序列的多重比较; 红色线标注为a~e 5个亚结构域。B: 不同物种中NAC72蛋白序列的同源进化树分析。C: IbNAC72与拟南芥AtNAC72的基因组结构比较; 方框表示外显子, 直线表示内含子。
Fig. 1Sequence analysis of IbNAC72
A: multiple protein sequence alignment of IbNAC72 with other plant NAC72 proteins; the red lines indicate the five subdomains a to e. B: phylogenetic analysis of NAC72 proteins. C: comparison of the genomic structures of IbNAC72 and AtNAC72; boxes indicate exons, and lines indicate introns.
表明, IbNAC72与牵牛花中的同源蛋白(Ipomoea nil, XP_019194672.1)亲缘关系最近, 达到90.88%, 与拟南芥的AtNAC72的同源性为57.10% (图1-B)。同时, 扩增得到1199 bp的IbNAC72基因组DNA全长序列, 包含3个外显子和2个内含子。将该基因与拟南芥AtNAC72的基因组DNA全长的外显子与内含子的组成进行比较分析发现, IbNAC72与AtNAC72基因组结构一致, 含有相同数目的外显子和内含子(图1-C)。
2.2 IbNAC72基因的表达分析
以甘薯肌动蛋白基因Actin (GenBank登录号为AY905538)为内参基因, qRT-PCR分析结果表明, IbNAC72基因在栗子香试管苗的根系、茎段、叶片中均有不同程度的表达, 其中在叶片中的表达量显著高于在根系和茎段中(图2-A); IbNAC72基因在栗子香大田植株的叶片、茎段、块根、梗根和须根中均有不同程度的表达, 其中在块根中的表达量显著高于在其他组织中(图2-B)。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2IbNAC72基因的表达分析
A: IbNAC72基因在栗子香试管苗不同组织中的表达分析; B: IbNAC72基因在栗子香大田植株不同组织中的表达分析; C: IbNAC72基因在100 mmol L-1 NaCl胁迫处理下的表达分析; D: IbNAC72基因在20% PEG-6000胁迫处理下的表达分析。数据表示为平均值 ± SD (n = 3)。采用单因素方差分析法对数据进行统计分析, 柱上不同的小写字母代表柱值在0.05水平差异显著。
Fig. 2Expression analysis of IbNAC72
A: transcriptional levels of IbNAC72 in different tissues of 4-week-old in vitro-grown Lizixiang plants; B: transcriptional levels of IbNAC72 in different tissues of 3-month-old field-grown Lizixiang plants; C: expression level of IbNAC72 in the Lizixiang plants under 100 mmol L-1 NaCl treatment; D: expression level of IbNAC72 in the Lizixiang plants under 20% PEG-6000 treatment. The error bars indicate ± SD (n = 3). Different lowercase letters indicate significant difference at the 0.05 probability level based on one-way ANOVA.
IbNAC72基因分别受到盐、旱胁迫诱导表达。在100 mmol L-1 NaCl胁迫处理2~48 h期间均处于基因诱导上调状态, 其中IbNAC72基因的表达量在48 h达到最高(5.25倍) (图2-C)。在20% PEG-6000胁迫处理2~48 h期间均处于基因诱导上调状态, 其中IbNAC72基因的表达量在2 h达到峰值(7.96倍) (图2-D)。盐、旱胁迫下, IbNAC72基因的表达同时存在被诱导和波动的情况, 这可能与胁迫环境下基因表达的时空特异性、上游基因的应答调控以及取样时间点的疏密设置相关。
2.3 过表达IbNAC72转基因烟草转化及阳性植株的鉴定
扩增IbNAC72 (图3-A)并构建获得植物表达载体pC3301-121-IbNAC72。对构建好的植物表达载体进行酶切鉴定, 如图3-B所示, 切下的小片段即为IbNAC72基因的ORF, 证明植物表达载体构建成功。将该重组质粒转化农杆菌EHA105感受态, 利用农杆菌介导的叶盘法转化烟草。暗培养2~3 d后, 转至分化培养基进行光照培养, 3~5周后形成抗性愈伤组织, 并逐渐分化出抗性芽, 当抗性芽长到约1 cm时, 将其切下并转移至1/2 MS生根培养基上, 最终生长成完整植株(图4-A~D)。随后, 将获得的87株拟转基因植株的叶片、茎段和根分别进行GUS检测发现, 有36株表现GUS阳性, 阳性率为41%, GUS基因已经在这些植株的叶片、茎段和根系中稳定表达(图4-E~G)。接着, 提取GUS检测呈阳性的36株拟转基因植株基因组DNA, 以质粒为阳性对照, 以非转基因植株为阴性对照, 进行PCR检测。结果表明, 36株拟转基因植株以及阳性对照扩增得到1008 bp的条带, 非转基因植株没有得到扩增条带(图4-H)。该结果与GUS染色结果完全一致, 从而进一步验证了这36株植株为转基因植株。图3
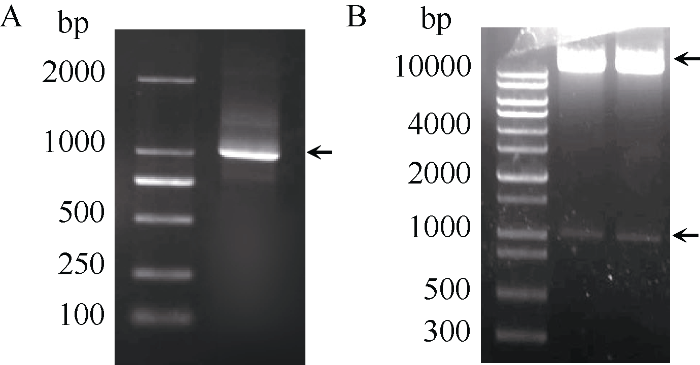 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3IbNAC72基因过表达载体的构建与鉴定
A: IbNAC72基因ORF的PCR扩增; B: 过表达载体pC3301- 121-IbNAC72的酶切鉴定。
Fig. 3Construction and identification of IbNAC72 overexpression vector
A: PCR amplification of IbNAC72 gene; B: double enzyme digestion detection of pC3301-121-IbNAC72.
图4
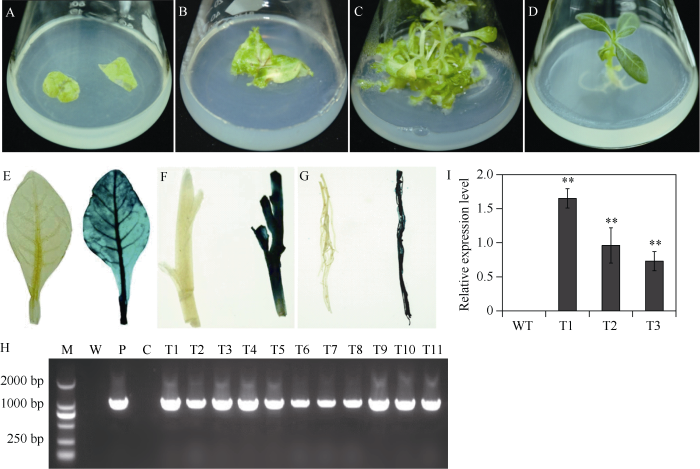 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4IbNAC72转基因烟草植株的获得及鉴定
A~D: IbNAC72基因转化烟草的转化、筛选和再生过程; E~I: 转基因烟草植株叶片、茎段和根系的GUS染色呈阳性, WT呈阴性; H: IbNAC72转基因烟草植株的PCR分析; I: 转基因烟草植株IbNAC72基因的表达分析。M: DL2000 marker; W: 水作为阴性对照; P: 质粒pCAMBIA3301-121-IbNAC72作为阳性对照; C: WT作为阴性对照; WT: 野生型烟草植株; T1~T3: 过表达IbNAC72烟草株系。数据表示为平均值 ± SD (n = 3)。** 表示用Student’s t-test在0.01水平差异显著。
Fig. 4Production and identification of IbNAC72 transgenic tobacco plants
A-D: transformation, screening and regeneration of IbNAC72 transgenic tobacco plants; E-I: the leaves, stems, and roots of the overexpression plants show positive reactions in the GUS assay, whereas no GUS expression is detected in WT; H: PCR analysis of GUS-positive plants; I: transcript levels of IbNAC72 in transgenic and WT plants. M: BL2000 DNA markers; W: water as a negative control; P: pCAMBIA3301-121-IbNAC72 as a positive control; C: WT as a negative control; WT: wild-type tobacco plants; T1-T3: IbNAC72-overexpressing tobacco lines. The error bars indicate ± SD (n = 3). ** indicates significant difference at the 0.01 probability level based on Student’s t-test.
对经GUS染色和PCR鉴定呈阳性的3个过表达株系进行IbNAC72基因的RT-qPCR分析发现, IbNAC72基因在野生型对照植株中没有表达, 而在3个转基因植株中均有不同程度的表达, 表达水平显著高于野生型对照植株(图4-I)。
2.4 过表达IbNAC72烟草植株的抗旱性鉴定
过表达IbNAC72烟草植株无形态变异。选取长势良好、生长状态一致的转基因烟草植株和野生型植株, 进行干旱处理。对转基因株系和野生型对照植株连续进行4周的干旱胁迫处理后发现, 野生型对照植株叶片严重萎蔫失水下垂, 而过表达IbNAC72烟草植株表现出较好的生长状态(图5-A), 植株高度比对照植株提高了56.10%~65.85% (图5-B)。此外, 干旱胁迫处理后, 过表达IbNAC72烟草植株的根系更加发达, 根系鲜重较野生型对照提高了64.29%~75.00% (图5-C, D)。表明通过IbNAC72基因的过表达显著提高了烟草转基因植株抗旱性。图5
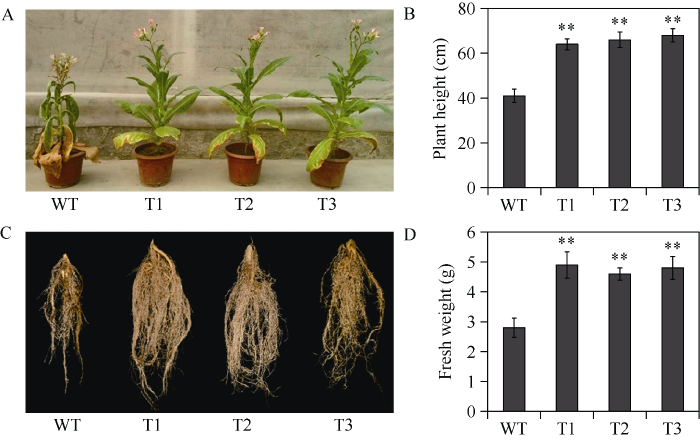 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5IbNAC72基因的过表达提高了转基因烟草植株的抗旱性
A: 干旱胁迫4周后, 转基因烟草和野生型烟草植株的生长状态比较; B: 干旱胁迫4周后, 转基因烟草和野生型烟草植株株高的比较分析; C: 干旱胁迫4周后, 转基因烟草和野生型烟草植株根系的生长状态比较; D: 干旱胁迫4周后, 转基因烟草和野生型烟草植株根系鲜重的比较分析。数据表示为平均值 ± SD (n = 3)。** 表示用Student’s t-test在0.01水平差异显著。缩写同
Fig. 5Overexpression of IbNAC72 enhances drought tolerance of transgenic tobacco plants
A: phenotypes of transgenic plants and WT grown for four weeks under drought stress; B: plant height of transgenic plants and WT grown for four weeks under drought stress; C: root phenotype of transgenic plants and WT grown for four weeks under drought stress; D: fresh weight of the root of transgenic plants and WT grown for four weeks under drought stress. The error bars indicate ± SD (n = 3). ** indicates significant difference at the 0.01 probability level based on Student’s t-test. Abbreviations are the same as those given in
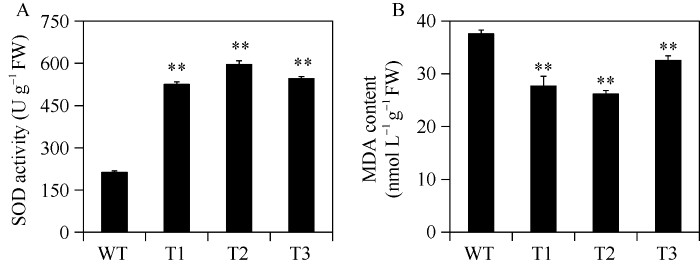
2.5 过表达IbNAC72烟草植株的SOD活性和MDA含量分析
干旱胁迫下, 过表达株系的SOD活性显著高于对照植株(图6-A), 而过表达株系的MDA含量显著低于对照植株(图6-B)。图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6干旱胁迫对转基因植株中SOD活性和MDA积累量的影响
A: 干旱胁迫4周后, 转基因烟草和野生型烟草植株的超氧化物歧化酶(SOD)活性; B: 干旱胁迫4周后, 转基因烟草和野生型烟草植株的丙二醛(MDA)含量。数据表示为means ± SD (n = 3)。** 表示用Student’s t-test在0.01水平差异显著。缩写同
Fig. 6Effects of drought stress on SOD activity and MDA accumulation in transgenic plants
A: the superoxide dismutase (SOD) activity in transgenic plants and WT grown for four weeks under drought stress; B: the malondialdehyde (MDA) content in transgenic plants and WT grown for four weeks under drought stress. The error bars indicate ± SD (n = 3). ** indicate significant difference at the 0.01 probability level based on Student’s t-test. Abbreviations are the same as those given in
3 讨论
大多数NAC类转录因子成员响应干旱、高盐、低温、机械损伤、病原菌等各种非生物和生物胁迫环境的诱导表达, 是一类重要的逆境调控因子[27]。本研究从甘薯中筛选克隆了一个NAC转录因子IbNAC72, 与牵牛花中的同源蛋白的同源性最高(图1-B)。与之前的报道结果一致, IbNAC72的NAC结构域中的B亚结构域与其他同源基因同源性较低, 不保守(图1-A), 推测其参与介导该基因特有的功能。IbNAC72受到高盐、干旱的显著诱导表达(图2-C, D), 由此推测其在抗旱过程中发挥调控作用。利用农杆菌介导法转化烟草, 获得了过表达IbNAC72的烟草转基因植株。在长时间干旱条件下, IbNAC72转基因烟草植株的根系更加发达, 抗旱性显著提高, 证明IbNAC72是一个正调控甘薯抗旱性的NAC家族转录因子。目前其他物种中也有NAC72同源基因的相关报道, 如拟南芥中NAC072与ABF3互作, 作为一种辅助因子, 参与ABF3介导的ABA应答调控[28]。在拟南芥中过表达黄花棘豆的OoNAC72导致了ABA超敏反应, 上调了应激抗逆相关基因如RD29A、RD29B、RD26、LEA14、ANACOR19、ZAT10、PP2CA、NCED3的表达, 增强了转基因植株种子萌发和萌发后生长时期对干旱和盐胁迫的耐受性[29]。柑橘中的PtrNAC72负调控精氨酸脱羧酶(ADC)介导的腐胺生物合成, 而腐胺在活性氧稳态中起着重要作用。过表达PtrNAC72的转基因烟草植株活性氧升高, 对干旱的敏感性增加, 拟南芥nac72突变体表现出活性氧积累量降低, 抗旱性提高[30]。这些研究结果表明, 不同物种中的NAC72转录因子对逆境的调控作用存在差异。
植物根系是吸收水分和营养离子的重要器官。健壮的根系能够提高植物对水分的吸收, 特别是干旱条件下对土壤深层水分的吸收, 能够在叶片蒸腾速率不变的情况下提高植物的抗旱性。之前的研究结果表明, 过表达TaSNAC8-6A小麦和拟南芥转基因植株通过诱导生长素和干旱响应相关途径, 刺激侧根发育, 提高水分利用效率, 从而表现出更强的耐旱性[31]。小麦中过表达TaRNAC1可以提高根长和生物量, 增强小麦的耐旱性[32]。而本研究中, 在长时间干旱条件下, IbNAC72转基因烟草植株的根系更加健壮发达, 鲜重显著提高(图5-C, D), 从而维持了较高的水分吸收与水分利用效率, 使转基因植株的抗旱性显著提高。
干旱胁迫导致植物体内产生过量的活性氧(reactive oxygen species, ROS), ROS的毒性能够损伤蛋白质、脂类、碳水化合物和DNA, 导致氧化胁迫。SOD被逆境胁迫诱导, 迅速将过氧化物歧化为O2和H2O2, 后者将通过其他途径被清除掉[33]。ROS对植物功能分子的破坏作用包括膜脂过氧化作用和产生MDA, MDA是膜脂氧化作用的最终分解产物, 可以准确反映植物遭受逆境伤害的程度, 含量越高, 受伤害程度越大[34]。在本研究中, IbNAC72转基因烟草植株在干旱胁迫下的SOD活性显著高于野生型对照植株, MDA的积累量显著低于野生型对照植株, 进一步说明过表达株系的ROS清除系统增强, 抗旱性提高。通过对该基因进行深入的功能研究, 将有助于进一步阐明甘薯中NAC类转录因子的功能, 同时也为通过基因工程改良甘薯等重要农作物的抗旱性提供一个新的候选基因。
4 结论
从甘薯中筛选克隆得到NAC类转录因子基因IbNAC72。IbNAC72在甘薯叶片中表达量最高, 且受到高盐和干旱胁迫的显著诱导表达。在长时间干旱条件下, IbNAC72转基因烟草植株的根系更加发达, 维持较高的水分利用效率, 其抗氧化性和抗旱性较野生型植株显著提高。证明IbNAC72是一个正调控甘薯抗旱性的NAC家族转录因子基因。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URL [本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:28684428 [本文引用: 1]
DOI:10.3390/ijms160715811URLPMID:26184177 [本文引用: 1]

Water scarcity is one of the major causes of poor plant performance and limited crop yields worldwide and it is the single most common cause of severe food shortage in developing countries. Several molecular networks involved in stress perception, signal transduction and stress responses in plants have been elucidated so far. Transcription factors are major players in water stress signaling. In recent years, different MYB transcription factors, mainly in Arabidopsis thaliana (L.) Heynh. but also in some crops, have been characterized for their involvement in drought response. For some of them there is evidence supporting a specific role in response to water stress, such as the regulation of stomatal movement, the control of suberin and cuticular waxes synthesis and the regulation of flower development. Moreover, some of these genes have also been characterized for their involvement in other abiotic or biotic stresses, an important feature considering that in nature, plants are often simultaneously subjected to multiple rather than single environmental perturbations. This review summarizes recent studies highlighting the role of the MYB family of transcription factors in the adaptive responses to drought stress. The practical application value of MYBs in crop improvement, such as stress tolerance engineering, is also discussed.
[本文引用: 1]
URLPMID:28576847 [本文引用: 1]
URLPMID:31091337 [本文引用: 3]
[本文引用: 1]
[本文引用: 1]
URLPMID:17587305 [本文引用: 1]
DOI:10.1016/j.bbrc.2008.12.163URLPMID:19135985 [本文引用: 1]

The plant-specific NAC (NAM, ATAF1/2, CUC2) transcription factors play diverse roles in plant development and stress responses. In this study, a rice NAC gene, ONAC045, was functionally characterized, especially with regard to its role in abiotic stress resistance. Expression analysis revealed that ONAC045 was induced by drought, high salt, and low temperature stresses, and abscisic acid (ABA) treatment in leaves and roots. Transcriptional activation assay in yeast indicated that ONAC045 functioned as a transcriptional activator. Transient expression of GFP-ONAC045 in onion epidermal cells revealed that ONAC045 protein was localized in the nucleus. Transgenic rice plants overexpressing ONAC045 showed enhanced tolerance to drought and salt treatments. Two stress-responsive genes were upregulated in transgenic rice. Together, these results suggest that ONAC045 encodes a novel stress-responsive NAC transcription factor and is potential useful for engineering drought and salt tolerant rice.
URLPMID:16924117 [本文引用: 1]
DOI:10.1186/s12870-019-1883-yURLPMID:31238869 [本文引用: 1]

BACKGROUND: NAC (NAM, ATAF and CUC) transcriptional factors constitute a large family with more than 150 members in rice and several members of this family have been demonstrated to play crucial roles in rice abiotic stress response. In the present study, we report the function of a novel stress-responsive NAC gene, ONAC066, in rice drought and oxidative stress tolerance. RESULTS: ONAC066 was localized in nuclei of cells when transiently expressed in Nicotiana benthamiana and is a transcription activator with the binding ability to NAC recognition sequence (NACRS) and AtJUB1 binding site (JBS). Expression of ONAC066 was significantly induced by PEG, NaCl, H2O2 and abscisic acid (ABA). Overexpression of ONAC066 in transgenic rice improved drought and oxidative stress tolerance and increased ABA sensitivity, accompanied with decreased rate of water loss, increased contents of proline and soluble sugars, decreased accumulation of reactive oxygen species (ROS) and upregulated expression of stress-related genes under drought stress condition. By contrast, RNAi-mediated suppression of ONAC066 attenuated drought and oxidative stress tolerance and decreased ABA sensitivity, accompanied with increased rate of water loss, decreased contents of proline and soluble sugars, elevated accumulation of ROS and downregulated expression of stress-related genes under drought stress condition. Furthermore, yeast one hybrid and chromatin immunoprecipitation-PCR analyses revealed that ONAC066 bound directly to a JBS-like cis-elements in OsDREB2A promoter and activated the transcription of OsDREB2A. CONCLUSION: ONAC066 is a nucleus-localized transcription activator that can respond to multiple abiotic stress factors. Functional analyses using overexpression and RNAi-mediated suppression transgenic lines demonstrate that ONAC066 is a positive regulator of drought and oxidative stress tolerance in rice.
DOI:10.1111/j.1365-313X.2004.02171.xURLPMID:15341629 [本文引用: 1]

Arabidopsis thaliana RD26 cDNA, isolated from dehydrated plants, encodes a NAC protein. Expression of the RD26 gene was induced not only by drought but also by abscisic acid (ABA) and high salinity. The RD26 protein is localized in the nucleus and its C terminal has transcriptional activity. Transgenic plants overexpressing RD26 were highly sensitive to ABA, while RD26-repressed plants were insensitive. The results of microarray analysis showed that ABA- and stress-inducible genes are upregulated in the RD26-overexpressed plants and repressed in the RD26-repressed plants. Furthermore, RD26 activated a promoter of its target gene in Arabidopsis protoplasts. These results indicate that RD26 functions as a transcriptional activator in ABA-inducible gene expression under abiotic stress in plants.
[本文引用: 1]
URLPMID:24285786 [本文引用: 1]
URLPMID:26059204 [本文引用: 1]
DOI:10.1111/pbi.12776URLPMID:28640975 [本文引用: 1]

Water deficit (drought stress) massively restricts plant growth and the yield of crops; reducing the deleterious effects of drought is therefore of high agricultural relevance. Drought triggers diverse cellular processes including the inhibition of photosynthesis, the accumulation of cell-damaging reactive oxygen species and gene expression reprogramming, besides others. Transcription factors (TF) are central regulators of transcriptional reprogramming and expression of many TF genes is affected by drought, including members of the NAC family. Here, we identify the NAC factor JUNGBRUNNEN1 (JUB1) as a regulator of drought tolerance in tomato (Solanum lycopersicum). Expression of tomato JUB1 (SlJUB1) is enhanced by various abiotic stresses, including drought. Inhibiting SlJUB1 by virus-induced gene silencing drastically lowers drought tolerance concomitant with an increase in ion leakage, an elevation of hydrogen peroxide (H2 O2 ) levels and a decrease in the expression of various drought-responsive genes. In contrast, overexpression of AtJUB1 from Arabidopsis thaliana increases drought tolerance in tomato, alongside with a higher relative leaf water content during drought and reduced H2 O2 levels. AtJUB1 was previously shown to stimulate expression of DREB2A, a TF involved in drought responses, and of the DELLA genes GAI and RGL1. We show here that SlJUB1 similarly controls the expression of the tomato orthologs SlDREB1, SlDREB2 and SlDELLA. Furthermore, AtJUB1 directly binds to the promoters of SlDREB1, SlDREB2 and SlDELLA in tomato. Our study highlights JUB1 as a transcriptional regulator of drought tolerance and suggests considerable conservation of the abiotic stress-related gene regulatory networks controlled by this NAC factor between Arabidopsis and tomato.
[本文引用: 1]
[本文引用: 1]
URLPMID:26011089 [本文引用: 1]
DOI:10.1016/j.plantsci.2018.05.005URLPMID:29807598 [本文引用: 1]

Lycopene beta-cyclase (LCYB) is an essential enzyme that catalyzes the conversion of lycopene into alpha-carotene and beta-carotene in carotenoid biosynthesis pathway. However, the roles and underlying mechanisms of the LCYB gene in plant responses to abiotic stresses are rarely known. This gene has not been used to improve carotenoid contents of sweetpotato, Ipomoea batatas (L.) Lam.. In the present study, a new allele of the LCYB gene, named IbLCYB2, was isolated from the storage roots of sweetpotato line HVB-3. Its overexpression significantly increased the contents of alpha-carotene, beta-carotene, lutein, beta-cryptoxanthin and zeaxanthin and enhanced the tolerance to salt, drought and oxidative stresses in the transgenic sweetpotato (cv. Shangshu 19) plants. The genes involved in carotenoid and abscisic acid (ABA) biosynthesis pathways and abiotic stress responses were up-regulated in the transgenic plants. The ABA and proline contents and superoxide dismutase (SOD) activity were significantly increased, whereas malonaldehyde (MDA) and H2O2 contents were significantly decreased in the transgenic plants under abiotic stresses. The overall results indicate that the IbLCYB2 gene enhances carotenoid contents and abiotic stress tolerance through positive regulation of carotenoid and ABA biosynthesis pathways in sweetpotato. This gene has the potential to improve carotenoid contents and abiotic stress tolerance in sweetpotato and other plants.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:3327686 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:27486475 [本文引用: 1]
URLPMID:31354764 [本文引用: 1]
URLPMID:27663409 [本文引用: 1]
[本文引用: 1]
URLPMID:29079898 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:32034034 [本文引用: 1]
