 ,1,**, 赵志超
,1,**, 赵志超 ,2,**, 张瑾晖2, 张锋2, 程治军
,2,**, 张瑾晖2, 张锋2, 程治军 ,2,*, 邹德堂
,2,*, 邹德堂 ,1,*
,1,*Genetic analysis and fine mapping of a sheathed panicle mutant sui2 in rice (Oryza sativa L.)
SUN Qi ,1,**, ZHAO Zhi-Chao
,1,**, ZHAO Zhi-Chao ,2,**, ZHANG Jin-Hui2, ZHANG Feng2, CHENG Zhi-Jun
,2,**, ZHANG Jin-Hui2, ZHANG Feng2, CHENG Zhi-Jun ,2,*, ZOU De-Tang
,2,*, ZOU De-Tang ,1,*
,1,*通讯作者:
收稿日期:2020-02-14接受日期:2020-06-2网络出版日期:2020-11-12
| 基金资助: |
Received:2020-02-14Accepted:2020-06-2Online:2020-11-12
| Fund supported: |
作者简介 About authors
孙琦, E-mail:
赵志超, E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (954KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
孙琦, 赵志超, 张瑾晖, 张锋, 程治军, 邹德堂. 水稻包穗突变体sui2的遗传分析和基因精细定位[J]. 作物学报, 2020, 46(11): 1734-1742. doi:10.3724/SP.J.1006.2020.02009
SUN Qi, ZHAO Zhi-Chao, ZHANG Jin-Hui, ZHANG Feng, CHENG Zhi-Jun, ZOU De-Tang.
杂交水稻不育系普遍存在包穗现象, 主要表现在穗颈节缩短、穗部约30%~60%颍花包裹在剑叶叶鞘内而无法伸出[1]。在大田生产实际中, 一般通过外部喷施一定浓度赤霉素(GA3)的方法来解除包穗现象[2]。但喷施GA3不仅在一定程度上增加了制种成本、降低了杂交种质量, 而且其喷施效果在很大程度上依赖喷施者的相关技术、天气情况及施用时期等[3,4]。因此利用包穗突变体, 克隆包穗相关基因, 研究包穗形成的分子生物学机制, 对于降低制种成本, 提高杂交种产量和质量具有重要的理论指导意义。
水稻倒一节间的缩短导致了包穗现象, 该性状不仅受主效基因的控制, 而且同时受微效基因的影响。至今12个控制倒一节间长度的QTL已被检测到, 并且通过自然变异、理化诱变、组织培养和转基因等技术获得了11种包穗突变体, 这些突变体包括A864、shpl、shp2、shp3、shp4、shp5、shp6、M893、fsp、sui1和esp2[5,6,7,8,9,10,11,12,13,14,15,16,17]。根据穗部被包裹的程度可分为全包穗和部分包穗。A864、shp1、shp2、shp3、shp4、shp5和shp6为部分包穗的突变体, M893、fsp、sui1和esp2为全包穗的突变体。其中fsp各节间均表现出不同程度的缩短, 而M893, sui1和esp2的节间缩短仅特异地发生在倒一节间。sui1和esp2均已经被克隆, 且为同一个基因[10,15], SHP5和SHP6分别定位在4号染色体上和2号染色体上[14,16]。除此之外, 有关水稻包穗突变体相关基因的研究报道极少。因此, 通过突变体挖掘新的包穗基因, 为深入探究水稻倒一节间生长发育的分子机制奠定一定的理论基础。
Zhu等[11]报道了2个突变体sui1-1和sui1-2, 其倒一节间发育受阻。Ma等[15]也发现了倒一节间缩短的突变体, 并命名为sui1-4。本实验的突变体材料表型和前人报道突变体表型相似, 因此我们将突变体命名为sui2。突变体sui2在表型上属于1个半包穗和全包穗之间的突变体, 表现出倒一节间缩短, 遗传上表现为显性。通过与正常抽穗品种IRAT129杂交, 构建了对该突变位点的遗传作图群体, 利用图位克隆的方法, 我们最终获得了目标基因。初步结果表明, 该基因表达量增加导致了包穗的表型, 该突变体为深入研究BR (brassinolide)信号通路基因控制水稻包穗表型提供了材料。
1 材料与方法
1.1 供试材料
水稻包穗突变体sui2来源于日本粳稻品种Kitaake的组织培养后代。以粳稻品种IRAT129为父本, 以突变体sui2为母本进行杂交, 杂交种种于中国农业科学院作物科学研究所顺义试验基地。同年, 将F2分离群体种植于海南省三亚实验基地, 常规水肥管理。成熟时, 田间随机选取野生型Kitaake和突变体sui2各10株, 考察株高、分蘖数、倒一节间长度、粒长、粒宽、粒厚、每穗粒数、千粒重、结实率等农艺性状, 进行数据分析。1.2 倒一节间细胞学观察
抽穗后取Kitaake和突变体的倒一节间, 徒手用刀片切3 mm左右的节间组织, 放置于50%甲醇和10%乙酸混合固定液中, 4℃固定12 h。固定后, 转移至80%乙醇中, 80℃孵育5 min, 再移至固定液固定1 h, 用水洗净后置于1%高碘酸中, 室温静置40 min。洗净后置于100 μg mL-1碘化丙啶希夫试剂中染色1~2 h, 再置于水合氯醛溶液过夜透明。用激光共聚焦显微镜(ZEISS Microsystems LSM 700)观察并照相。1.3 包穗突变基因的精细定位与候选基因分析
在田间分别取样2个亲本IRAT129、sui2及总共608个F2分离群体中隐性(正常表型)单株的叶片, 进行突变基因精细定位。叶片全基因组DNA的提取采用改进的CTAB法。首先, 随机选取取样的F2单株9株与2个亲本的DNA一起构建混池, 利用均匀分布于12条染色体上, 且在双亲间有多态性的186个分子标记进行基因连锁分析。随后, 再随机选取66个隐性单株验证连锁的分子标记, 进行初定位。最后, 在初定位的基础上, 用新开发的多态性InDel标记对全部608个隐性单株基因型进行精细定位。新标记开发参考网站Gramene (http://ensembl.gramene.org/genome_browser/index.html)上籼稻和粳稻日本晴的基因组序列, 在初定位区间内进行序列对比寻找插入/缺失位点, 用Premier3在线软件(http: //primer3.ut.ee/)设计InDel分子标记, 委托上海英潍捷基贸易有限公司合成(表1)。采用北京宝日医生物技术有限公司的Premix Taq进行PCR扩增, 产物经8%非变性聚丙烯酰胺凝胶电泳分离, 染色显色后观察。利用网站(http://rice.plantbiology.msu.edu/cgi-bin/ gbrowse/rice/)确定区间内ORF, 利用南京诺唯赞生物技术有限公司的Phanta Max Super-Fidelity DNA Polymerase扩增, 委托北京博迈德基因技术有限公司测序。Table 1
表1
表1新开发的多态性InDel和SNP引物
Table 1
| 标记 Marker | 正向引物 Forward primer (5′-3′) | 反向引物 Reverse primer (5′-3′) |
|---|---|---|
| S4-13 | CGACGATATCCGTGCATCACC | ACGATTGCATCTGCGTCACACC |
| S4-14 | AGTGATGCACTTCTGTTTGTCC | GGTCCTCTTGTTCAAGTCAAACC |
| S4-14.1 | AGAAGACGACGACTTGGACA | TAGACGACCTGGGTTCGAAG |
| S4-14.2 | AAATTCCACATGCCAATTCC | ATTGAGGCTCGATCCATGAC |
| S4-14.3 | CTTTTGCGAGGGGTCTCATA | CATGGGGCTTTTGCACTAAT |
| S4-14.4 | GCCACAAACTCCCAGCTAAC | GTGGAGACTGGAGAGGTGGA |
| S4-15 | ACCTTTTCTTGGCTTGAGGG | GCTTTTGCTACTTTTGGGGG |
新窗口打开|下载CSV
1.4 候选基因及油菜素内酯相关基因表达量分析
成熟期分别取野生型和突变体的倒一节间基部组织。采用TIANGEN公司的RNA试剂盒提取植物组织总RNA, FastKing cDNA试剂盒反转录合成cDNA。采用北京宝日医生物技术有限公司的TB Green Premix Ex TaqII试剂盒进行实时荧光定量PCR, 在Applied Biosystems 7500 Real-time PCR仪器上进行扩增反应, 评价油菜素内酯合成及信号相关基因(引物表2)在野生型和突变体倒一节间基部组织中的表达情况, 内参基因为Ubiquitin (NCBI Locus: XM_020816636), 每个样品3个重复。Table 2
表2
表2qRT-PCR所用引物
Table 2
| 引物名 Primer name | 正向引物 Forward primer (5′-3′) | 反向引物 Reverse primer (5′-3′) |
|---|---|---|
| LOC_Os04g39360 | TGATCTCGATGGCGATCACC | GCTCCTCGCAGTAGACCATC |
| LOC_Os04g39380 | CTAATTCATCGCCACGTACAAACG | TGATCACCGGCTATCAACAGAAAC |
| LOC_Os04g39410 | CGATTGCCATGAGTTCACCAAGC | TGGCGTCTCGGACCACAATTTC |
| LOC_Os04g39420 | GCGGGTATACGCAGGTTTATGC | ACTCGTTGAAGATGGCCACAGC |
| LOC_Os04g39430 | TGAGGTTCCTCAGTCCTCATGC | AAACACCCTCCCATACCTGGAG |
| LOC_Os04g39440 | TGTGTCACACTGCCACTTACCC | TGGAGCAACTACGTTTCAGTCAGC |
| LOC_Os04g39444 | CCAAGATGAAGCTCGTCAGGTTTC | AGTCTTCAAGTGGGTGTTCATGC |
| LOC_Os04g39450 | GGCCTTCTATGCATCTCTGAGC | TGATGGAGTTGCTGCCATCTTC |
| LOC_Os04g39470 | AGCTCACGAATCACATGGTGTAGC | ATGTGGATCATGGCGTGCTTCG |
| LOC_Os04g39489 | TCCTCTTGGAAATTCAGGACACAC | GGACGCCTTCTTCATCGTCTTG |
| BRD1 | ATTATGATCCATTCCTGTACCCTG | TCTTCCTCCCATCTGTATTGAGT |
| BRD2 | TCAAGGCCACACAGGGTGAATC | GCACAGCCACAGTGGATAAACCTC |
| DWARF4 | GATGGGCTCTGAAACAATCTAACCTT | TCCCCTCTTAGCCTTTGTCTCCTT |
| BAK1 | ACTCTGGTCAATCCGTGCACTTG | AGTGCAGCATTCCCAAGATCAAC |
| BRI1 | TCGTTGGCTCAGTTCTTGGAGAG | TCTCTTGGCTAGAACAAGAAGTGC |
| BU1 | CGACGACGAAGCTGCTGAAGGA | AGGAGGCTGCGGATGATCTCG |
| LIC1 | CTGCACCACTTGCTGCCCCTAC | TGTTCCCAACAGATTCCTCAAACATC |
| TUD1 | GTCCGCCTCATCCGCATACTC | CGCACCGATGCTAACAATCAAAC |
| BZR1 | CGTTCCGGCACCCCTTCTTC | TGGCGTCACCCTCCCCTTGT |
| Ubiquitin | AACCAGCTGAGGCCCAAGA | ACGATTGATTTAACCAGTCCATGA |
新窗口打开|下载CSV
2 结果与分析
2.1 包穗突变体sui2的表型分析
在成熟期, 突变体sui2的穗部被剑叶叶鞘包裹, 其包裹的程度介于半包裹的和全包裹之间(图1-A, B)。sui2的包穗表型是由于节间的伸长与叶鞘的伸长不同步造成的, 在突变体中, 除倒三、倒四节间没有表现出明显的差异外, 倒一、倒二节间长度都相应缩短, 尤其是倒一节间的长度仅为对照Kitaake的50% (图1-C, 表3)。此外, 突变体sui2还表现植株矮化、分蘖数增加、每穗粒数减少、结实率下降、籽粒变大和剑叶变窄(表3), 尤为特别的是, sui2的倒一和倒二叶的夹角显著增大(图1-G, H)。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1野生型与突变体sui2的表型比较
A: 野生型野生型(左)与突变体(右)成熟期表型; B: 成熟期野生型(左)与突变体(右)的1个典型的包穗的表型比较; C: 成熟期野生型(左)与突变体(右)的各节间表型比较, 自左至右为倒一、二、三、四节间; D, E: 野生型(D)和突变体(E)倒一节间横切面细胞学比较; F: 野生型和突变体倒一节间纵切面细胞学比较; G, H: 野生型(G)与突变体(H)苗期倒一和倒二叶叶片夹角。标尺: (A) 15 cm; (B) 5 cm; (C) 5 cm, (D, E) 200 μm; (F) 50 μm。
Fig. 1Phenotypic comparison between wild type and mutant sui2
A: Phenotypes of WT (left) and sui2 (right) plants at rippen period; B: Phenotype comparison of the sheathed panicle of WT (left) and sui2 (right) at the maturation period; C: Comparison of internodes between WT (left) and sui2 (right), from left to right is the uppermost internode, penultimate internode, antepenult internode, fourth internode from top down; D, E: Transverse sections of the first internode of WT (D) and sui2 (E); F: Longitudinal sections of the uppermost internode of wild type and sui2; G, H: The leaf angles between the last and penultimate leaf in the WT (G) and sui2 (H) at the seedling stage. Scale bars: (A) 15 cm; (B) 5 cm; (C) 5 cm; (D, E) 200 μm; (F) 50 μm.
Table 3
表3
表3野生型Kitaake和突变体sui2农艺性状比较
Table 3
| 农艺性状Agronomic trait | Kitaake | sui2 | P |
|---|---|---|---|
| 株高Plant height (cm) | 63.73±2.55 | 43.08±3.79 | *P < 0.05 |
| 分蘖数Tiller number | 14.40±2.01 | 25.00±3.43 | **P < 0.01 |
| 每穗粒数Number of spikelets per panicle | 42.75±8.70 | 27.10±3.41 | **P < 0.01 |
| 千粒重1000-grain weight (g) | 4.415±0.02 | 4.852±0.06 | **P < 0.01 |
| 结实率Seed setting rate (%) | 0.969±0.05 | 0.837±0.08 | **P < 0.01 |
| 粒长Seed length (mm) | 7.06±0.33 | 8.10±0.22 | **P < 0.01 |
| 粒宽Seed weight (mm) | 3.26±0.10 | 3.47±0.12 | **P < 0.01 |
| 粒厚Seed thickness (mm) | 2.20±0.07 | 2.14±0.07 | **P < 0.01 |
| 剑叶宽Flag leaf length (cm) | 1.10±0.08 | 0.90±0.08 | **P < 0.01 |
| 倒一节间长Length of the uppermost internode (cm) | 29.61±3.27 | 14.68±2.57 | **P < 0.01 |
新窗口打开|下载CSV
2.2 sui2节间形态分析
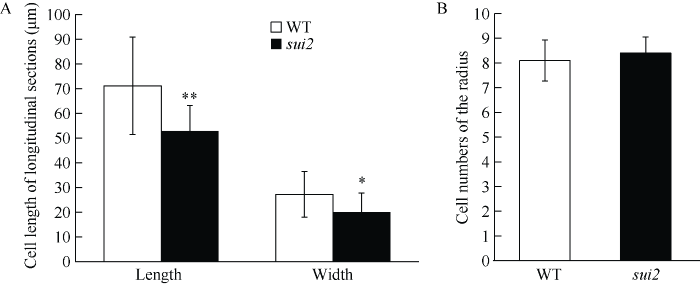
突变体株型矮化, 成熟期株高约43 cm, 只有野生型的80%左右。突变体倒一节间缩短严重, 其长度仅为野生型倒一节间的50% (表3)。由于节间细胞数量减少或细胞伸长受到抑制, 造成了矮秆突变体节间缩短的表型。为了深入研究细胞形态及数目对突变体倒一节间长度的影响, 我们通过PI染色处理成熟期野生型和突变体的倒一节间并进行显微观察。结果发现, 相对于野生型, 在横截面上, 突变体薄壁细胞数目没有明显增多(图2-B), 薄壁细胞的体积变小, 茎秆壁增厚, 空气髓腔明显减小, 维管束数目减少(图1-D, E); 在纵切面上, 突变体的细胞长度明显缩短, 突变体的细胞宽度也变窄(图1-F, 图2-A)。因此, 突变体倒一节间的薄壁细胞伸长出现了障碍, 突变体sui2表现出的矮秆及其包穗表型是细胞伸长受阻所导致。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2野生型和突变体节间组织薄壁细胞体积和数目比较
A: 野生型和突变体倒一节间纵切面薄壁细胞长度及宽度; B: 野生型和突变体倒一节间横截面半径含有的细胞数目。* 表示在P<0.05水平差异显著; ** 表示在P < 0.01水平差异显著。
Fig. 2Comparisons of cell size and number of the parenchyma cells in the uppermost internode between wild type and sui2
A: Comparisons of the length and width of stem parenchyma cells from longitudinal sections of the uppermost internode in wild type and sui2; B: comparison of cell numbers of the radius in the transverse sections of the uppermost internode in wild type and sui2. * represents significantly different at P < 0.05; ** represents significantly different at P < 0.01.
2.3 遗传分析
对sui2与抽穗正常的IRAT129品种的杂交组合后代分析结果表明, F1植株全部包穗, 为突变体表型。F2群体出现明显的性状分离。随机调查927个单株, 217株表现为正常表型, 710株表现为包穗表型, 经卡方(χ2)测验(χ2 = 1.25, χ20.05 = 3.84), 该表型符合1对显性基因3 (显性)∶1 (隐性)的分离比。2.4 基因定位与候选基因分析
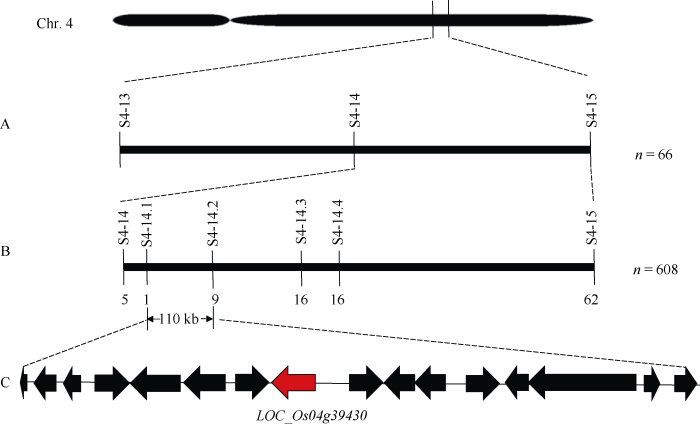
从F2群体中随机选取9个隐性(正常抽穗)单株叶片构建DNA混池。选用实验室186对均匀分布在水稻12条染色体上, 且在双亲间具有多态性InDel或SSR引物, 对DNA混池进行连锁分析。结果发现, 位于4号染色体长臂端的2个标记S4-14和S4-15与混池DNA的单株连锁。之后进一步用66个F2取样单株对这两个标记进行验证, 并确定了物理距离为0.83 Mb初定位区间(图3-A)。利用Gramene网站(http://ensembl.gramene.org/genome_browser/index. html)的籼粳稻基因组序列对比信息, 在初定位区间新开发了2个分子标记S4-14.1和S4-14.4, 利用全部F2单株将基因定位在S4-14.1与S4-14.4之间, 物理距离为287 kb的范围。进一步开发2个SNP分子标记S4-14.2和S4-14.3, 将sui2基因最终定位在了标记S4-14.1和S4-14.2之间物理距离为110 kb区间内(图3-B)。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3候选基因SUI2基因的图位克隆示意图
A: SUI2初步定位在4号染色体标记S4-14和S4-15之间; B: SUI2被精细定位在S4-14.1和S4-14.2之间的110 kb范围内; C: SUI2精细定位区间内包含的14个基因和2个转座子。横线的上方为定位所用的分子标记, 横线下方为交换单株数。
Fig. 3Schematic diagram of map-based cloning on the candidate gene SUI2
A: Rough mapping of the gene SUI2 flanked by two markers S4-14 and S4-15; B: SUI2 was fine mapped to a 110 kb interval delimited by two markers S4-14.1 and S4-14.2 region; C: 14 ORFs and 2 transposons within the fine-mapped fragment. The molecular markers used in linkage analysis were indicated above the bold lines, and the umbers beneath the bold lines represented the recombinants identified by the corresponding markers.
根据水稻基因组注释信息数据库(http://rice. plantbiology.msu.edu/cgi-bin/gbrowse/rice/), 该区间内共包含14个基因和2个转座子(表4和图3-C)。对所有14个基因的基因组测序在野生型和突变体之间均没有发现差异, 说明sui2的突变表型并不是由于DNA水平上的差异引起的。由于基因表达量的差异也会引起植株表型的变化, 我们进一步分析了区间内14个基因在成熟期的表达水平。如图4所示, 与野生型相比, 在sui2中有4个基因明显上调, 1个基因明显下调, 而其他候选基因的表达量在两者之间无明显差异。鉴于该突变体为显性, 我们重点关注表达量差异较大的2个基因LOC_Os04g39420 (上调9倍)和LOC_ Os04g39430 (上调264倍)。经查阅文献, LOC_ Os04g39420编码了6-磷酸果糖激酶-2 (6-phosphofructokinase-2), 可能参与水稻籽粒淀粉的理化性质形成[17]; LOC_Os04g39430编码了1个细胞色素450蛋白, 为D11的1个等位基因, 该基因的过表达植株的表型除了有包穗外, 其他表型也与本实验的突变体sui2相似[18]。因此, 我们认定LOC_Os04g39430是参与调控水稻包穗表型的候选基因。
Table 4
表4
表4定位区间内基因的功能注释
Table 4
| 基因名称 Locus name | 基因注释 Gene annotation |
|---|---|
| LOC_Os04g39360 | Heavy metal transport/detoxification protein, putative, expressed |
| LOC_Os04g39370 | Heavy metal associated domain containing protein, expressed |
| LOC_Os04g39380 | Heavy metal transport/detoxification protein, putative, expressed |
| LOC_Os04g39390 | Retrotransposon protein, putative, unclassified, expressed |
| LOC_Os04g39400 | Retrotransposon protein, putative, unclassified, expressed |
| LOC_Os04g39410 | Pentatricopeptide, putative, expressed |
| LOC_Os04g39420 | 6-phosphofructokinase 2, putative, expressed |
| LOC_Os04g39430 | D11; cytochrome P450; small grain 4; clustered spikelets 4 |
| LOC_Os04g39440 | Ras-related protein, putative, expressed |
| LOC_Os04g39444 | LSM domain containing protein, expressed |
| LOC_Os04g39450 | Expressed protein |
| LOC_Os04g39460 | NBS-LRR type disease resistance protein, putative, expressed |
| LOC_Os04g39470 | Transcription factor with an MYB domain |
| LOC_Os04g39489 | Amino acid transporter, putative, expressed |
| LOC_Os04g39510 | Expressed protein |
| LOC_Os04g39520 | ZOS4-08-C2H2 zinc finger protein, expressed |
新窗口打开|下载CSV
图4
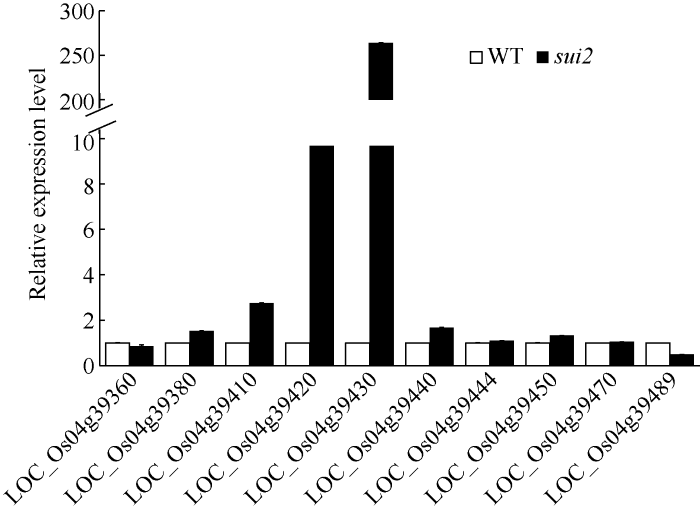 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4候选基因的qRT-PCR检测
Fig. 4Expression level of candidate genes by qRT-PCR
对不同组织中SUI2表达量检测结果表明, SUI2在突变体的幼穗、叶枕、茎秆中表达水平均显著提高, SUI2在幼穗中表达量为514倍, 在茎中表达量为369倍, 在叶枕表达量为214倍(图5)。
图5
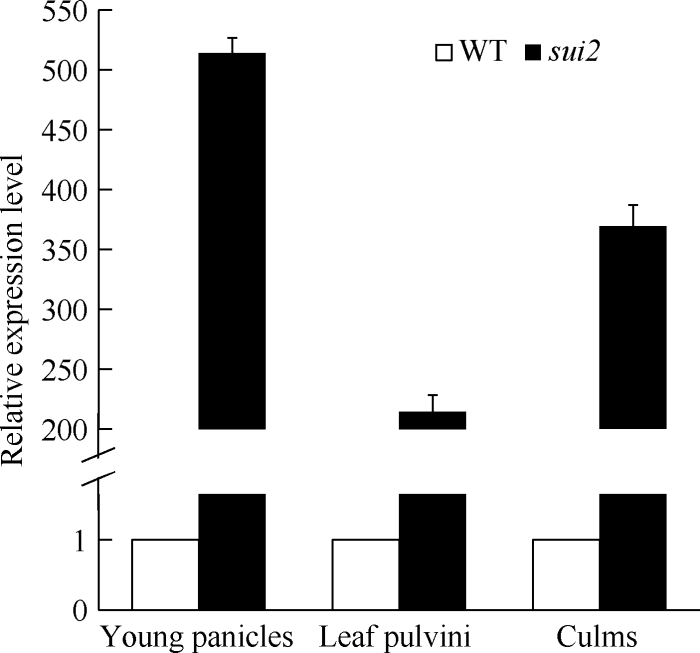 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5SUI2在不同组织中的表达量变化情况
Fig. 5SUI2 expression levels of the different tissues in WT and sui2 mutant
图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6油菜素内酯相关基因在野生型和突变体中的表达量变化情况
A: 野生型及sui2的茎秆中油菜素内酯合成相关基因的表达水平; B: 野生型及sui2的茎秆中油菜素内酯信号相关基因的表达水平。
*表示在P < 0.05水平差异显著; **表示在P < 0.01水平差异显著。
Fig. 6Expression levels of BR-related genes in WT and sui2
A: Relative expression levels of the genes related to BR biosynthesis in WT and sui2 culms. B: Relative expression levels of the genes related to BR signaling in the young WT and sui2 young culms. * represents significantly different at P < 0.05; ** represents significantly different at P < 0.01.
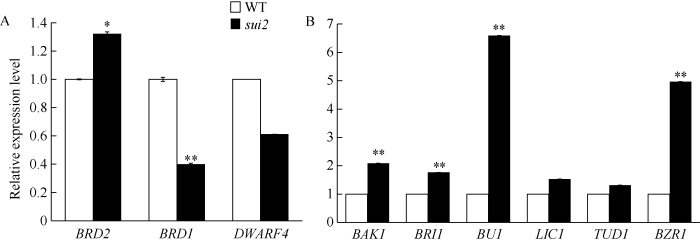
2.5 突变体油菜素内酯合成及信号途径相关基因表达量分析
LOC_Os04g39430编码了1个细胞色素P450酶, 它在油菜素内酯合成通路中发挥作用, 可能参与了油菜素内酯生物合成通路中6-DeoxoTY和TY的合成[18]。为了检测突变体中其他油菜素内酯合成及信号通路基因表达量的变化, 我们利用qRT-PCR分析了野生型和突变体中油菜素内酯合成相关基因BRD1[19]、BRD2[20]、DWARF4[21]及信号通路相关基因BAK1、BRI1[22]、BZR1[23]、LIC1[24]、BU1[25]、TUD1[26]在茎秆中的表达情况。结果表明, 与野生型相比, 3个合成基因在突变体sui2的表达量没有一致变化规律, 1个表达量上调(BRD2)、1个下调(BRD1)、1个没有明显变化(DWARF4)。而被检测的6个信号转导基因中, 有4个基因的表达量明显上调, 尤其是基因BU1的表达量增加了6倍多, 预示着可能一些BR信号转导途径的基因参与了穗颈节的伸长和包穗表型的调控。3 讨论
水稻的穗外露主要由倒一节间的长度决定, 由于突变体倒一节间显著缩短, 导致穗颈节伸出度降低, 从而产生包穗。包穗突变体sui2来自于粳稻品种Kitaake的组织培养后代, 突变体籽粒增大、单穗粒数减少、结实率下降明显。对sui2的茎秆细胞生物学观察发现, 株高变矮和包穗产生的原因可能与细胞的纵向伸长不足有关。对该突变体的遗传分析结果表明, 它为1个显性突变体, 突变基因被定位在第4号染色体的标记S4-14.1和S4-14.2之间, 物理距离为110 kb的区间内。区间内的基因LOC_Os04g39430的表达量在突变体内发生了明显的上调, 高达230倍。由于LOC_Os04g39430是D11的等位基因, 加之该基因的过表达会导致包穗现象[27], 我们认为LOC_Os04g39430是引起sui2包穗表型的关键基因。由于LOC_Os04g39430的基因组和启动子没有DNA序列水平上的差异, 该包穗表型可能不是由于DNA水平上的差异引起的, 我们推测可能是由于表观遗传修饰引起的基因表达量升高而造成包穗表型。表观遗传学修饰涉及到DNA甲基化、非编码RNA的调控、组蛋白修饰、染色质重塑等多种类型[28,29], 具体哪种现象导致sui2突变体的表型差异, 尚不明确, 需要对该基因进行深入研究。基因LOC_Os04g39430最初被Tanabe等[18]命名为D11基因, 它编码了1种细胞色素P450蛋白, 是BR生物合成过程中的1种关键酶。后来研究又陆续报道了几种D11的等位基因, Wu等[27]研究表明cpb1表现为株高降低、叶夹角变小、籽粒变小, 突变体中CPB1表达量上调了5倍; Shi等[30]研究表明, sg4表现籽粒变小且为黄褐色, 植株高于野生型, 叶片直立, 该基因在突变体的叶中的表达量为野生型的2.5倍, 而在茎、根、叶鞘、小穗中该基因表现为下调; Guo等[31]报道cl4突变体表现为半包穗、叶夹角变小、叶宽增加、籽粒发育不正常。以上3个突变体均为隐性突变体。而本实验中sui2为显性突变体, 表现为株高降低, 第1节间缩短, 籽粒变长, 自苗期之后的全生育期叶夹角明显增大, 叶宽减少。这与之前报道的其他几个D11突变体明显不同。Tanabe等[18]又发现D11基因与BR生物合成有关, 本研究对野生型和突变体茎秆组织中的BR合成与信号通路相关基因进行了表达量分析, 结果表明与野生型相比, sui2中BR合成途径中相关基因表达量无一致的变化趋势, 而信号转导途径相关基因BAK1、BRI1、BZR1、BU1表达量显著上调, 因此过表达该类基因可能导致了BR信号途径基因表达量增加。
GA是调节细胞伸长和最上节间长度的主要植物激素[32]。Zhu等[33]和Luo等[34]研究发现, EUI1与具有生物活性赤霉素GA4代谢有关, 同时发现穗下倒一节间伸长与赤霉素积累有关, 穗下倒一节间伸长突变体eui在节间部位积累了过量的赤霉素。Oikawa等[35]研究结果表明Sd1基因突变可以抑制植株体内赤霉素合成, 体内赤霉素含量降低, 最终导致细胞伸长受阻, 使植株矮化, 但喷施外源GA可恢复其表型。此外, 一些研究也指出磷脂酰丝氨酸合酶也参与了倒一节间伸长[15]。但是, 关于BR途径基因对穗下倒一节间伸长影响的报道极少, 它们具体作用的分子机制仍待探究。BR相关途径的基因表达水平分析表明, 有可能信号途径的基因参与了倒一节间伸长的表达调控, sui2也为这方面深入研究提供了难得的材料。
4 结论
通过对来源于组织培养后代的包穗突变体sui2研究发现, 突变体倒一节间长度缩短是包穗的主要原因。此外, sui2还表现植株矮化、分蘖数增加、每穗粒数减少、结实率下降、籽粒变大、剑叶变窄、倒一和倒二叶的夹角显著增大等表型。该性状受1对显性基因控制, SUI2基因被定位在水稻4号染色体上标记S4-14.1和S4-14.2之间物理距离为110 kb区间内, 候选基因LOC_Os04g39430编码了1个细胞色素P450蛋白。本研究同时也证明了油菜素内酯信号途径的基因有可能参与了倒一节间伸长的表达调控, 这为进一步研究BR信号通路基因控制水稻包穗机制奠定了一定的理论基础。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:22956125 [本文引用: 2]
URLPMID:21928114 [本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
.
[本文引用: 2]
DOI:10.1371/journal.pone.0153119URLPMID:27055010 [本文引用: 4]

The uppermost internode is one of the fastest elongating organs in rice, and is expected to require an adequate supply of cell-wall materials and enzymes to the cell surface to enhance mechanical strength. Although it has been reported that the phenotype of shortened uppermost internode 1 (sui1) is caused by mutations in PHOSPHATIDYLSERINE SYNTHASE (OsPSS), the underlying mechanism remains unclear. Here we show that the OsPSS-1, as a gene expressed predominantly in elongating cells, regulates post-Golgi vesicle secretion to intercellular spaces. Mutation of OsPSS-1 leads to compromised delivery of CESA4 and secGFP towards the cell surface, resulting in weakened intercellular adhesion and disorganized cell arrangement in parenchyma. The phenotype of sui1-4 is caused largely by the reduction in cellulose contents in the whole plant and detrimental delivery of pectins in the uppermost internode. We found that OsPSS-1 and its potential product PS (phosphatidylserine) localized to organelles associated with exocytosis. These results together suggest that OsPSS-1 plays a potential role in mediating cell expansion by regulating secretion of cell wall components.
[本文引用: 2]
[本文引用: 2]
URLPMID:15705958 [本文引用: 4]
DOI:10.1371/journal.pone.0030798URLPMID:22292043 [本文引用: 1]

The role of brassinosteroids in plant growth and development has been well-characterized in a number of plant species. However, very little is known about the role of brassinosteroids in maize. Map-based cloning of a severe dwarf mutant in maize revealed a nonsense mutation in an ortholog of a brassinosteroid C-6 oxidase, termed brd1, the gene encoding the enzyme that catalyzes the final steps of brassinosteroid synthesis. Homozygous brd1-m1 maize plants have essentially no internode elongation and exhibit no etiolation response when germinated in the dark. These phenotypes could be rescued by exogenous application of brassinolide, confirming the molecular defect in the maize brd1-m1 mutant. The brd1-m1 mutant plants also display alterations in leaf and floral morphology. The meristem is not altered in size but there is evidence for differences in the cellular structure of several tissues. The isolation of a maize mutant defective in brassinosteroid synthesis will provide opportunities for the analysis of the role of brassinosteroids in this important crop system.
[本文引用: 1]
URLPMID:9490746 [本文引用: 1]
DOI:10.1016/s0092-8674(02)00814-0URLPMID:12150928 [本文引用: 1]

The Arabidopsis BAK1 (BRI1 Associated receptor Kinase 1) was identified by a yeast two-hybrid screen as a specific interactor for BRI1, a critical component of a membrane brassinosteroid (BR) receptor. In yeast, BAK1/BRI1 interaction activates their kinase activities through transphosphorylation. BAK1 and BRI1 share similar gene expression and subcellular localization patterns and physically associate with each other in plants. Overexpression of the BAK1 gene leads to a phenotype reminiscent of BRI1-overexpression transgenic plants and rescues a weak bri1 mutant. In contrast, a bak1 knockout mutation gives rise to a weak bri1-like phenotype and enhances a weak bri1 mutation. We propose that BAK1 and BRI1 function together to mediate plant steroid signaling.
DOI:10.1126/science.1107580URLPMID:15681342 [本文引用: 1]

Brassinosteroid (BR) homeostasis and signaling are crucial for normal growth and development of plants. BR signaling through cell-surface receptor kinases and intracellular components leads to dephosphorylation and accumulation of the nuclear protein BZR1. How BR signaling regulates gene expression, however, remains unknown. Here we show that BZR1 is a transcriptional repressor that has a previously unknown DNA binding domain and binds directly to the promoters of feedback-regulated BR biosynthetic genes. Microarray analyses identified additional potential targets of BZR1 and illustrated, together with physiological studies, that BZR1 coordinates BR homeostasis and signaling by playing dual roles in regulating BR biosynthesis and downstream growth responses.
URLPMID:22570626 [本文引用: 1]
URLPMID:19648232 [本文引用: 1]
DOI:10.1371/journal.pgen.1003391URL [本文引用: 1]
[本文引用: 2]
URLPMID:25674094 [本文引用: 1]
DOI:10.1016/j.cell.2014.02.045URLPMID:24679529 [本文引用: 1]

Since the human genome was sequenced, the term
DOI:10.1007/s11434-015-0798-8URL [本文引用: 1]
DOI:10.1007/s11434-014-0568-zURL [本文引用: 1]
DOI:10.1016/j.tplants.2018.08.007URLPMID:30220494 [本文引用: 1]

Brassinosteroid (BR) regulates many important agronomic traits and thus has great potential in agriculture. However, BR application is limited due to its complex effects on plants. The identification of specific downstream BR components and pathways in the crop plant rice (Oryza sativa) further demonstrates the feasibility of modulating BR responses to obtain desirable traits for breeding. Here, we review advances on how BR regulates various biological processes or agronomic traits such as plant architecture and grain yield in rice. We discuss how these functional specificities of BR can and could be utilized to enhance plant performance and productivity. We propose that unraveling the mechanisms underlying the diverse BR functions will favor BR application in molecular design for crop improvement.
DOI:10.1105/tpc.105.038455URLPMID:16399803 [本文引用: 1]

The recessive tall rice (Oryza sativa) mutant elongated uppermost internode (eui) is morphologically normal until its final internode elongates drastically at the heading stage. The stage-specific developmental effect of the eui mutation has been used in the breeding of hybrid rice to improve the performance of heading in male sterile cultivars. We found that the eui mutant accumulated exceptionally large amounts of biologically active gibberellins (GAs) in the uppermost internode. Map-based cloning revealed that the Eui gene encodes a previously uncharacterized cytochrome P450 monooxygenase, CYP714D1. Using heterologous expression in yeast, we found that EUI catalyzed 16alpha,17-epoxidation of non-13-hydroxylated GAs. Consistent with the tall and dwarfed phenotypes of the eui mutant and Eui-overexpressing transgenic plants, respectively, 16alpha,17-epoxidation reduced the biological activity of GA(4) in rice, demonstrating that EUI functions as a GA-deactivating enzyme. Expression of Eui appeared tightly regulated during plant development, in agreement with the stage-specific eui phenotypes. These results indicate the existence of an unrecognized pathway for GA deactivation by EUI during the growth of wild-type internodes. The identification of Eui as a GA catabolism gene provides additional evidence that the GA metabolism pathway is a useful target for increasing the agronomic value of crops.
DOI:10.1093/pcp/pci233URLPMID:16306061 [本文引用: 1]

Elongation of rice internodes is one of the most important agronomic traits, which determines the plant height and underlies the grain yield. It has been shown that the elongation of internodes is under genetic control, and various factors are implicated in the process. Here, we report a detailed characterization of an elongated uppermost internode1 (eui1) mutant, which has been used in hybrid rice breeding. In the eui1-2 mutant, the cell lengths in the uppermost internodes are significantly longer than that of wild type and thus give rise to the elongated uppermost internode. It was found that the level of active gibberellin was elevated in the mutant, whereas its growth in response to gibberellin is similar to that of the wild type, suggesting that the higher level accumulation of gibberellin in the eui1 mutant causes the abnormal elongation of the uppermost internode. Consistently, the expression levels of several genes which encode gibberellin biosynthesis enzymes were altered. We cloned the EUI1 gene, which encodes a putative cytochrome P450 monooxygenase, by map-based cloning and found that EUI1 was weakly expressed in most tissues, but preferentially in young panicles. To confirm its function, transgenic experiments with different constructs of EUI1 were conducted. Overexpression of EUI1 gave rise to the gibberellin-deficient-like phenotypes, which could be partially reversed by supplementation with gibberellin. Furthermore, apart from the alteration of expression levels of the gibberellin biosynthesis genes, accumulation of SLR1 protein was found in the overexpressing transgenic plants, indicating that the expression level of EUI1 is implicated in both gibberellin-mediated SLR1 destruction and a feedback regulation in gibberellin biosynthesis. Therefore, we proposed that EUI1 plays a negative role in gibberellin-mediated regulation of cell elongation in the uppermost internode of rice.
DOI:10.1007/s11103-004-1692-yURLPMID:15604710 [本文引用: 1]

Gibberellin (GA) 20-oxidase (GA20ox) is a key enzyme that normally catalyzes the penultimate steps in GA biosynthesis. One of the GA20ox genes in rice (Oryza sativa L.), OsGA20ox2 ( SD1 ), is well known as the
