 ,1, 刘莹1,2, 张豪杰1, 王璐1, 郝心愿1, 张伟富1, 王玉春1, 熊飞1,3, 杨亚军
,1, 刘莹1,2, 张豪杰1, 王璐1, 郝心愿1, 张伟富1, 王玉春1, 熊飞1,3, 杨亚军 ,1,*, 王新超
,1,*, 王新超 ,1,*
,1,*Promoter cloning and expression analysis of the hexokinase gene CsHXK2 in tea plant (Camellia sinensis)
LI Na-Na ,1, LIU Ying1,2, ZHANG Hao-Jie1, WANG Lu1, HAO Xin-Yuan1, ZHANG Wei-Fu1, WANG Yu-Chun1, XIONG Fei1,3, YANG Ya-Jun
,1, LIU Ying1,2, ZHANG Hao-Jie1, WANG Lu1, HAO Xin-Yuan1, ZHANG Wei-Fu1, WANG Yu-Chun1, XIONG Fei1,3, YANG Ya-Jun ,1,*, WANG Xin-Chao
,1,*, WANG Xin-Chao ,1,*
,1,*通讯作者:
收稿日期:2019-11-6接受日期:2020-06-2网络出版日期:2020-07-03
| 基金资助: |
Received:2019-11-6Accepted:2020-06-2Online:2020-07-03
| Fund supported: |
作者简介 About authors
E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (21041KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
李娜娜, 刘莹, 张豪杰, 王璐, 郝心愿, 张伟富, 王玉春, 熊飞, 杨亚军, 王新超. 茶树己糖激酶基因CsHXK2的启动子克隆及表达特性分析[J]. 作物学报, 2020, 46(10): 1628-1638. doi:10.3724/SP.J.1006.2020.94166
LI Na-Na, LIU Ying, ZHANG Hao-Jie, WANG Lu, HAO Xin-Yuan, ZHANG Wei-Fu, WANG Yu-Chun, XIONG Fei, YANG Ya-Jun, WANG Xin-Chao.
植物己糖激酶HXK (hexokinase, EC: 2.7.1.1)是兼职双功能蛋白。源库组织内, 由淀粉和蔗糖产生的葡萄糖和果糖, 经HXK磷酸化形成葡萄糖-6-磷酸和果糖-6-磷酸, 进而参与糖酵解、呼吸作用、分解与合成等代谢过程, 为植物的生理活动提供能量和中间代谢产物; 此外, HXK在植物糖信号感知中具有重要作用[1,2], 其作为糖传感蛋白, 参与糖信号转导, 感知胁迫、光照、激素和养分等条件, 进而调控植株的基因表达与生长发育[3]。
Dai等[4]利用酵母三重突变体(hxk1/hxk2/glk1), 通过功能互补策略, 首次分离获得植物拟南芥HXK基因。随后, 玉米、番茄、水稻、葡萄、马铃薯、烟草、木薯、油菜、梨、麻风树等多种植物的HXK基因相继被分离克隆[5,6,7,8,9]。HXK在双子叶和单子叶植物中均以多基因家族形式存在, 拟南芥有6个AtHXKs[10], 烟草有9个NtHXKs[11], 木薯有7个MeHXKs[6], 水稻有10个OsHXKs[12]。根据N-末端氨基酸序列的保守性和定位信号肽的预测结果, 植物中的HXK蛋白可分为2种类型, 即含有叶绿体转运肽的type A类和具有膜锚定区域的type B类[13,14]。依据HXK是否具有己糖磷酸化功能, HXK蛋白划分为具有磷酸化活性的HXK和缺乏磷酸化活性的HXKL(HXK-like) 2类。如AtHXK1-3蛋白属催化活跃的HXK类, AtHXKL1-3蛋白和NtHXKL1蛋白属催化失活的HXKL类[10,11]。
目前, 对植物己糖激酶的研究主要集中于HXK基因在植物生长发育过程中的作用, 关于其在抗逆胁迫方面的研究则相对较少。组织内超表达拟南芥AtHXK1基因, 植株表现出生长抑制、叶绿素含量减少、光合作用减弱、可溶性固形物和淀粉含量降低、叶片加速衰老以及光合作用相关基因CAB1和RBCS表达下调等现象[15,16]。利用拟南芥葡萄糖非敏感突变体(gin2)研究AtHXK1基因的生理功能[17], 缺乏HXK1葡萄糖催化功能的突变体仍具有多种信号功能, 可调控光合作用基因CAB1和RBCS表达、细胞增殖、根叶生长、开花和衰老等, gin2突变体表现出对生长素(IAA)不敏感而对细胞分裂素2IP超敏感, 表明植物利用HXK为葡萄糖感受体, 使得养分、光照和激素信号网络相互关联, 从而调控植物的生长与发育。通过RNAi技术抑制BnHXK9基因在油菜籽苗内的表达, 植株具有生长迟缓、矮化、叶片卷曲特征, 表明BnHXK9参与植物的生长发育过程[7]。胁迫过程中, NbHXK1、AtHXK1和AtHXK2基因能响应甲基紫精和病原体侵染诱导的氧化胁迫, 高水平的HXK能增强对氧化胁迫的抵抗力[18]; AtHXK2和AtHXKL3基因能在低温、高盐处理下表达上调[14]; 麻风树JcHXK1、JcHXK2和JcHKL1基因在叶片内同样能响应低温而诱导表达[9]; 油菜BnHXK1、BnHXK3和BnHXK9基因能响应核盘菌在抗性品种内的侵染而表达显著上调[7]。
茶树[Camellia sinensis (L.) O. Kuntze]是我国重要的经济叶用型作物, 在其生长过程中频受低温、干旱、高盐、病原菌等胁迫。因此, 挖掘茶树重要抗性基因、阐明茶树抗逆分子机制、选育强抗逆茶树品种, 是减少环境胁迫造成茶产业经济损失的重要举措。基于HXK基因在拟南芥等植物中的重要作用, 本研究开展了茶树HXK基因的分析研究, 以期获得HXK基因在茶树生长发育及抵御胁迫过程中的生理功能。本课题组前期已从茶树‘龙井43’组织内克隆获得4个己糖激酶基因CsHXK1~ CsHXK4。CsHXK2基因(NCBI登录号为KX159478)的开放阅读框(open reading frame, ORF)长度为1479 bp, 编码492个氨基酸; CsHXK2蛋白含有叶绿体转运信号肽而不具备跨膜螺旋结构, 且具有保守的ATP结合和糖结合区域, 属于type A类和磷酸化功能活跃的HXK类[19]。本研究进而对CsHXK2基因启动子进行克隆, 并深入分析该段启动子的顺式作用元件构成, 探明该编码蛋白的亚细胞作用位点以及己糖磷酸化活性, 并检测该基因在茶树不同组织器官内以及低温、炭疽菌、赤霉素处理下的表达变化。以期为阐明CsHXK2基因在茶树中的生理功能提供理论依据。
1 材料与方法
1.1 试验材料
茶树苗栽培于中国农业科学院茶叶研究所试验地(30.18°N, 120.09°E)。室内赤霉素、低温处理以及不同组织器官取样的茶苗为同一批盆栽的‘龙井43’, 即于2015年移栽入花盆内的一年生茶苗。2016年, 待盆栽苗在自然条件下新梢萌展至一芽二叶, 将茶苗移至人工气候室内适应性培养14 d [14 h光照/10 h黑暗; (24 ± 0.5)℃; 相对湿度为75%], 把配置好的浓度为50 μmol L-1的GA3溶液均匀喷施至新梢蓬面, 同时以喷施ddH2O作为对照处理, 采摘处理0、1、2、3、5 d的一芽二叶, 用于基因表达分析。2017年, 待盆栽苗在自然环境下新梢萌展至一芽三叶, 将茶苗移至人工气候室内适应性生长7 d [14 h光照/10 h黑暗; (25 ± 0.5)℃; 相对湿度为75%], 随后将温度降至4℃, 低温处理8 d, 再将温度恢复至25℃生长2 d, 采摘低温和常温恢复阶段的一芽三/四叶, 用于基因表达分析。2018年4月, 采集盆栽苗顶芽、第一叶、第二叶、第三叶、老叶、茎和根, 11月采集顶芽、腋芽、花蕾、盛花、老叶、茎和根, 用于基因启动子克隆和组织特异性表达检测。室内炭疽菌接种的材料为2013年移栽入花盆内的一年生‘龙井43’茶苗。2018年, 待盆栽苗新梢萌展至一芽二叶, 采集离体枝条做针刺叶片有伤处理, 将浓度为1×107个 mL-1的茶树炭疽菌(Colletotrichum camelliae)孢子悬浮液均匀喷施至供试枝条, 并以喷施无菌ddH2O作为对照, 随后保湿培养于光照培养箱内[12 h光照/12 h黑暗; (25 ± 0.4)℃], 处理0、12和24 h后取样, 用于基因表达检测。2017年11月至2018年3月, 每1个月左右, 于上午9: 00—10: 00采集成龄茶树品种‘龙井43’、‘大面白’、‘浙农12’、‘浙农113’的健康成熟叶片(第二、三、四叶), 用于基因表达检测。以上试验均设置3个生物学重复。所有采集的样品立即用液氮速冻, 置于-80°C保存待用。
1.2 基因组DNA、总RNA的提取及cDNA的合成
使用植物基因组DNA提取试剂盒(DP305, 北京天根生化科技有限公司)提取茶树组织DNA, 使用RNAprep Pure多糖多酚植物总RNA提取试剂盒(DP441, 北京天根生化科技有限公司)提取总RNA。利用分光光度计NanoDrop 2000C (Thermo Scientific, 美国)检测DNA和RNA的浓度和纯度, 并分别用1.0%和1.5%琼脂糖凝胶电泳检测DNA和RNA的完整性。随后, 使用PrimeScript RT Reagent Kit with gDNA Eraser试剂盒(TaKaRa, 中国), 取1 μg总RNA为模板合成cDNA第1链。1.3 CsHXK2基因启动子克隆及生物信息学分析
根据已公布的茶树基因组信息[20], 查找获得CsHXK2基因上游启动子模板序列, 并设计扩增引物(表1)。以‘龙井43’组织DNA为模板, 使用高保真酶PrimeSTAR HS DNA Polymerase (TaKaRa, 中国)进行PCR扩增。PCR产物经琼脂糖凝胶电泳、目的条带回收纯化、连接至PMD18-T载体、转化至感受态细胞后, 取适量转化产物涂板于LB+氨苄青霉素(Amp)的固体培养基, 随机挑选阳性单克隆送测序, 获得目的启动子序列。在New PLACE数据库(A Database of Plant Cis-acting Regulatory DNA Elements; https://www.dna.affrc.go.jp/PLACE/?action=newplace)[21]预测分析启动子序列所含的顺式作用元件。Table 1
表1
表1引物信息
Table 1
| 序列名称 Sequence name | 上游引物序列 Forward primer (5'-3') | 下游引物序列 Reverse primer (5'-3') | 用途 Application |
|---|---|---|---|
| CsPTB ORF | ACCAAGCACACTCCACACTATCG | TGCCCCCTTATCATCATCCACAA | 荧光定量PCR qRT-PCR |
| CsHXK2 promoter | GATTTTGAGTGCATAAATTGAAAACATCGAG | TTGAAACAGAGCGAGAGCGAGA | 启动子扩增 Promoter amplification |
| CsHXK2 ORF | ATTTCCGAGTGCTGAGGGTGCAA | TTTCCAGCCGTTCCAGAGACTGC | 荧光定量PCR qRT-PCR |
| CsHXK2 ORF | GGTACCATGTCCGTCACCGTAAGTCCA | GGATCCAAAATTGTGTTCATACTTCGAGTTTGT | 亚细胞定位 Subcellular localization |
| CsHXK2 ORF-cTP | GGTACCATGAACGTTGTCACCGTCGCC | TCTAGAAAAATTGTGTTCATACTTCGAGTT | 亚细胞定位 Subcellular localization |
| CsHXK2 ORF | ACTAGTATGTCCGTCACCGTAAGTCCA | CCCGGGCTAAAAATTGTGTTCATACTT | 酵母表达 Yeast expression |
| CsHXK2 ORF-cTP | ACTAGTATGAACGTTGTCACCGTCGCC | CCCGGGCTAAAAATTGTGTTCATACTT | 酵母表达 Yeast expression |
| pDR196 | CTCTTTTATACACACATTCA | CTGGCGAAGAAGTCCAAAGC | 菌液PCR Colony PCR |
新窗口打开|下载CSV
1.4 CsHXK2基因系列载体构建
以‘龙井43’组织cDNA为模板, 表1对应序列为载体构建引物, 利用高保真酶PrimeSTAR HS DNA Polymerase (TaKaRa, 中国)进行qRT-PCR, 扩增片段经琼脂糖凝胶电泳后切割、回收, 连接至pEASY-Blunt Zero中间载体(CB501, Transgen, 中国), 连接反应详见说明书。连接产物转化至感受态细胞DH5α, 菌液涂板于LB+卡那霉素(Kan)的固体培养基上, 筛选阳性单克隆菌液送测序。使用相对应的限制性内切酶(Kpn I和BamH I, Kpn I和Xba I, Bcu I和Cfr9 I; Thermo scientific, 美国)对中间连接载体CsHXK2::pEASY-Blunt Zero、CsHXK2-cTP::pEASY- Blunt Zero和终载体35S::sGFP、pDR196进行双酶切, 反应体系详见说明书。酶切产物进行琼脂糖凝胶电泳, 切割、回收目的片段。使用T4 DNA连接酶(M0202, New England Biolabs, 美国)将目的片段与终载体骨架按照3∶1摩尔比进行连接反应, 反应体系详见说明书。连接产物转化至感受态细胞DH5α, 菌液涂板于LB+Kan/Amp的固体培养基上, 鉴定阳性单克隆菌液并送测序。最后, 提取正确的终载体质粒, 即35S::CsHXK2::sGFP、35S:: CsHXK2-cTP::sGFP、CsHXK2::pDR196和CsHXK2- cTP:: pDR196。
1.5 CsHXK2蛋白亚细胞定位
采用农杆菌介导的烟草瞬时表达方法。用冻融法将表达载体35S::CsHXK2::sGFP、35S::CsHXK2-cTP::sGFP以及空载体35S::sGFP转化至感受态农杆菌GV3101; 然后将携带目的质粒的农杆菌在10 mL LB+Kan+利福平(Rif)的液体培养基中震荡培养(28℃和220×g), 使OD600=1.0~1.2; 取适量菌液, 室温5000×g离心5 min后弃上清; 用转化缓冲液(50 mmol L-1 MES, 2 mmol L-1 Na3PO4, 0.5%葡萄糖, 100 μmol L-1乙酰丁香酮)重悬, 使OD600=0.8~1.0; 用无菌针管将悬浮液注射至烟草叶片, 正常条件下培养48 h后, 用激光共聚焦显微镜(Zeiss, 德国)观察并照相。1.6 CsHXK2蛋白酵母功能互补验证
采用化学转化法将载体CsHXK2::pDR196、CsHXK2-cTP::pDR196以及空载体pDR196转化至新鲜制备的感受态酵母突变体菌株YSH7.4-3C(己糖激酶hxk1/hxk2/glk1三重缺失)[22]。随后, 将含有重组质粒和空载体质粒的酵母分别稀释至OD600=0.2、OD600=0.04、OD600=0.008和OD600=0.0016, 然后点板生长于含有不同碳源的SD/-Ura选择性培养基上(0.67% yeast nitrogen base, 0.077%-Ura DO Supplement, 2%琼脂, 2% ddH2O/2%半乳糖/2%葡萄糖/2%果糖)[12]。置于30℃恒温培养箱内暗培养4 d后观察、记录并照相。1.7 CsHXK2基因表达分析
采用实时荧光定量PCR法(qRT-PCR)检测CsHXK2在茶树不同组织器官内和不同处理下的表达情况。基于CsHXK2基因序列[19], 在NCBI的Primer-BLAST网页(http://www.ncbi.nlm.nih.gov/tools/primer-blast/)设计CsHXK2 荧光定量引物(表1), 并用常规qRT-PCR和琼脂糖凝胶电泳检测引物的特异性。选用CsPTB作为内参基因[23]; 使用LightCycler 480 SYBR Green I Master试剂和LightCycler 480 II仪器(Roche, 瑞士)进行qRT-PCR反应。采用2-?CT或2-??CT法[24]计算该基因相对表达水平。1.8 数据获取、处理及分析
使用TPIA数据库http://tpia.teaplant.org/index.html[25]下载茶树品种‘舒茶早’不同组织器官内CsHXK2基因(TEA022532)表达量数据。采用“平均值±标准误”表示; 使用Prism 6.0 (GraphPad, 美国)制作柱状图; 使用SPSS Statistics 20.0软件(IBM, 美国)对数据进行单因素方差分析。2 结果与分析
2.1 CsHXK2基因启动子克隆及分析
通过qRT-PCR扩增, 获得了特异性的目的条带(图1-A)。经克隆、测序得到CsHXK2基因起始密码子ATG上游-2097 bp至-69 bp启动子序列(图1-B), 其中腺嘌呤(A)数为785个, 占38.69%; 鸟嘌呤(G)数为193, 占9.51%; 胞嘧啶(C)数为228, 占11.24%; 胸腺嘧啶(T)数为823, 占40.56%。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1茶树CsHXK2基因启动子扩增及序列
A: CsHXK2启动子PCR扩增产物电泳图; B: CsHXK2启动子序列。
Fig. 1Amplification and sequence of CsHXK2 promoter in the tea plant
A: electrophoresis of PCR products of CsHXK2 promoter; B: sequence of CsHXK2 promoter.
利用New PLACE数据库分析该段2029 bp序列所含的关键顺式作用调控DNA元件。由表2可知, 该启动子含有多种调控茶树响应外在信号的顺式作用元件, 如感知光照、热击、氧气、病菌、创伤、低温、干旱的响应元件, 即-10PEHVPSBD、GATABOX、CCAATBOX1、CURECORECR、ELRECOREPCRP1、LTRE1HVBLT49、MYCCONSENSUSAT等; 如响应生长素、细胞分裂素、水杨酸、乙烯、赤霉素、脱落酸的作用元件, 即ARFAT、ARR1AT、ELRECOREPCRP1、ERELEE4、GARE1OSREP1、MYB1AT等; 多个糖信号响应元件, 即MYBGAHV、SREATMSD、SURE1STPAT21、WBOXHVISO1; 且有ARF、Dof、WRKY、MYB、MYC、RAV1转录因子结合位点, 即ARFAT、DOFCOREZM、ELRECOREPCRP1、MYB1AT、MYCCONSENSUSAT、RAV1AAT等。此外, 还具有调控叶肉、种子、花、根系、腋芽、保卫细胞组织基因表达的元件, 即CACTFTPPCA1、CANBNNAPA、CARGATCON SENSUS、RHERPATEXPA7、SREATMSD、TAAAGSTKST1等。说明茶树CsHXK2基因启动子具备多种调控作用元件, 可能通过参与糖代谢和转导糖信号而调控茶树的生长发育以及逆境胁迫应答。
Table 2
表2
表2茶树CsHXK2基因启动子的主要顺式作用元件
Table 2
| 位点名称 Site name | 序列 Sequence | 功能 Function | |||
|---|---|---|---|---|---|
| -10PEHVPSBD | TATTCT | 叶绿体基因表达; 生理节律; 光调节。 Chloroplast gene expression; circadian rhythms; light regulation. | |||
| ARFAT | TGTCTC | ARF (生长素响应因子)结合位点; 生长素信号。 ARF (auxin response factor) binding site; auxin signaling. | |||
| ARR1AT | NGATT | ARR1结合元件; 细胞分裂素响应元件。 ARR1-binding element; cytokinin-responsive element. | |||
| BOXLCOREDCPAL | ACCWWCC | MYB结合位点; 苯丙氨酸解氨酶基因; 激发处理; UV-B辐射; 稀释效应。 MYB binding site; phenylalanine ammonia-lyase gene; elicitor treatment; UV-B irradiation; dilution effect. | |||
| CACTFTPPCA1 | YACT | 叶肉特异性基因表达。 Mesophyll-specific gene expression. | |||
| CANBNNAPA | CNAACAC | 胚和胚乳特异转录元件; 种子; 贮藏蛋白。 Element required for embryo and endosperm-specific transcription; seed; storage protein. | |||
| CARGATCONSENSUS | CCWWWWWWGG | 开花时间。 Flowering time. | |||
| CCAATBOX1 | CCAAT | 热激元件。 Heat shock element. | |||
| CURECORECR | GTAC | 铜响应元件; 氧响应元件。 Copper-responsive element; oxygen-response element. | |||
| DOFCOREZM | AAAG | Dof蛋白核心结合位点; 碳代谢调控。 Core site required for binding of Dof proteins; regulating the carbon metabolism. | |||
| ELRECOREPCRP1 | TTGACC | 激发响应元件; WRKY结合位点; 水杨酸/病菌/创伤诱导信号。 Elicitor responsive element; WRKY protein binding site; salicylic acid/pathogen/wound- induced signaling. | |||
| ERELEE4 | AWTTCAAA | 乙烯响应元件; 衰老。 Ethylene responsive element; senescence. | |||
| GARE1OSREP1 | TAACAGA | 赤霉素响应元件; 种子。 Gibberellin-responsive element; seed. | |||
| GATABOX | GATA | 光响应元件。 Light-responsive element. | |||
| GT1CONSENSUS | GRWAAW | 光响应元件。 Light-responsive element. | |||
| LTRE1HVBLT49 | CCGAAA | 低温响应元件。 Low-temperature-responsive element. | |||
| MYB1AT | WAACCA | MYB识别位点; 干旱/ABA响应元件。 MYB recognition site; dehydration/ABA-responsive element. | |||
| MYBCORE | CNGTTR | MYB结合位点; 干旱/水响应元件; 黄酮类生物合成。 MYB binding site; dehydration/water-responsive element; flavonoid biosynthesis. | |||
| MYBGAHV | TAACAAA | 赤霉素响应元件; MYB结合位点; α-淀粉酶。 Gibberellin response element; MYB binding site; α-amylase. | |||
| MYCCONSENSUSAT | CANNTG | MYC识别位点; 低温/干旱/ABA响应元件。 MYC recognition site; cold/dehydration/ABA-responsive element. | |||
| NTBBF1ARROLB | ACTTTA | 组织特异性表达与生长素诱导。 Tissue-specific expression and auxin induction. | |||
| POLLEN1LELAT52 | AGAAA | 花粉特异性表达。 Pollen specific expression. | |||
| RAV1AAT | CAACA | RAV1结合位点; 莲座叶和根。 RAV1 binding site; rosette leaves and roots. | |||
| RHERPATEXPA7 | KCACGW | 根毛特异性元件; 根; 毛。 Root hair-specific cis-element; root; hair. | |||
| 位点名称 Site name | 序列 Sequence | 功能 Function | |||
| SREATMSD | TTATCC | 糖抑制元件; 腋芽生长。 Sugar-repressive element; axillary bud outgrowth. | |||
| SURE1STPAT21 | AATAGAAAA | 蔗糖响应元件。 Sucrose responsive element. | |||
| TAAAGSTKST1 | TAAAG | 保卫细胞特异性基因表达。 Guard cell-specific gene expression. | |||
| WBBOXPCWRKY1 | TTTGACY | WRKY结合位点。 WRKY binding site. | |||
| WBOXHVISO1 | TGACT | 糖响应元件; 糖信号; WRKY结合位点。 Sugar-responsive element; sugar signaling; WRKY binding site. | |||
新窗口打开|下载CSV
2.2 CsHXK2蛋白亚细胞定位
将携带35S::sGFP和35S::CsHXK2::sGFP载体的农杆菌注射至烟叶片内。经显微镜观察发现, 注射空载体的叶片在细胞质、细胞膜、细胞核内均具有GFP绿色荧光信号(图2-A, C); 含有CsHXK2融合GFP蛋白的叶片, 细胞内的绿色荧光(图2-D)可以与叶绿素自发红色荧光(图2-E)相互重叠, 呈现出黄色荧光信号(图2-G)。随后, 我们将去除N末端叶绿体转运肽的CsHXK2蛋白融合GFP表达载体(即35S:: CsHXK2-cTP::sGFP)注射至含有RFP核定位marker的烟草叶片内发现, 含有CsHXK2-cTP融合GFP蛋白的叶片与含有空载体的叶片所发射的绿色荧光信号具有相同的来源(图2-L, O, H, K), 即来源于细胞质、细胞膜、细胞核。表明CsHXK2蛋白定位于叶绿体, 且其蛋白质序列上存在的N末端叶绿体转运信号肽对其定位起到了决定性的作用。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2茶树CsHXK2蛋白亚细胞定位
A~C和H~K: 35S::sGFP; D~G: 35S::CsHXK2::sGFP; L~O: 35S::CsHXK2-cTP::sGFP。A, D, H, L: GFP绿色荧光信号; E: 叶绿素自发荧光; I, M: 细胞核RFP红色荧光信号; B, F, J, N: 明场; C, G, K, O: 信号融合。
Fig. 2Subcellular localization of CsHXK2 protein in the tea plant
A-C and H-K: 35S::sGFP; D-G: 35S::CsHXK2::sGFP; L-O: 35S::CsHXK2-cTP::sGFP. A, D, H, L: GFP green fluorescent signal; E: chlorophyll autofluorescence; I, M: RFP red fluorescent signal of nucleus; B, F, J, N: bright field; C, G, K, O: merged signal.
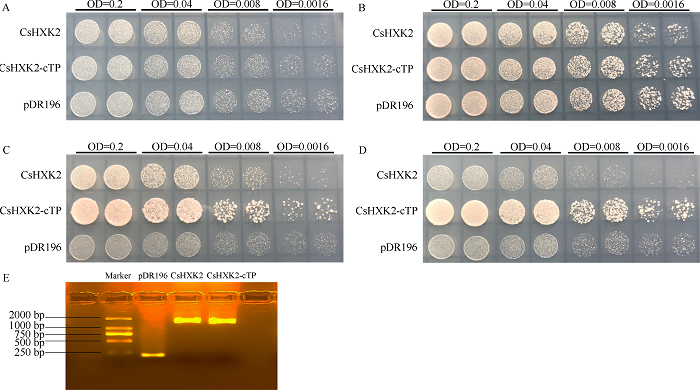
2.3 CsHXK2蛋白酵母功能互补验证
本研究将CsHXK2::pDR196、CsHXK2-cTP::pDR196酵母表达载体以及空载体pDR196分别转化至缺乏己糖激酶(hxk1/hxk2/glk1)活性的酵母三重突变体YSH7.4-3C, 转化片段经酵母菌液PCR和电泳跑胶验证正确(图3-E)。点板试验结果显示, 转化空载体、CsHXK2和CsHXK2-cTP的酵母均可在无碳源而仅有yeast nitrogen base、-Ura DO Supplement、琼脂成分的培养基上进行缓慢生长(图3-A), 而在含半乳糖为碳源的培养基上迅速生长(图3-B); 在含葡萄糖为碳源的培养基上, 转化CsHXK2-cTP的酵母长势显著优于转化CsHXK2的酵母, 且二者长势明显优于转化空载体pDR196的酵母(图3-C); 在含果糖为碳源的培养基上, 转化CsHXK2的酵母与转化空载体pDR196的酵母生长势相似, 而转化CsHXK2-cTP的酵母长势显著性优于二者(图3-D)。说明CsHXK2蛋白具有的叶绿体转运肽能显著影响其磷酸化催化活性; CsHXK2成熟蛋白可以磷酸化葡萄糖和果糖。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3CsHXK2蛋白酵母功能互补验证
A: ddH2O; B: 2%半乳糖; C: 2%葡萄糖; D: 2%果糖; E: 酵母转化片段电泳检测。
Fig. 3Complementation of the hexokinase-deficient yeast triple mutant with CsHXK2 protein
A: ddH2O; B: 2% galactose; C: 2% glucose; D: 2% fructose; E: electrophoretic detection of the transformed fragments in yeast.
2.4 CsHXK2基因组织表达特异性
本研究利用qRT-PCR检测了茶树品种‘龙井43’春季和秋季不同组织中CsHXK2基因的表达情况, 并用TPIA数据库[25]获取了CsHXK2基因在茶树品种‘舒茶早’不同组织器官内的表达水平(图4)。‘龙井43’春季组织中, CsHXK2基因表达量在根系中最高, 其次在茎中; 此外, 其表达量随着顶芽生长发育至第三叶而显著性上升; 但在老叶中呈现出最低表达量(图4-A)。‘龙井43’秋季组织中, CsHXK2基因同样在根系内表达量最高, 其次在茎和未绽放的花蕾中, 3个组织内的表达量无显著性差异; CsHXK2基因在腋芽内的表达量显著性高于顶芽, 而在老叶和盛开的花中表达量最低(图4-B)。CsHXK2基因在‘舒茶早’各组织器官内的表达水平呈现出与‘龙井43’组织内相似的表达特异性, 即表达量在根系中最高, 其次在茎中, 而在老叶中最低(图4-C)。表明CsHXK2基因在茶树源库组织内均具有一定的作用, 尤其在库组织根和茎的生长发育和物质代谢中起到重要的作用。图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4茶树CsHXK2基因的组织特异性表达模式
A: 春季‘龙井43’不同组织; B: 秋季‘龙井43’不同组织; C: ‘舒茶早’不同组织。柱上标以不同字母表示数据间的显著性差异(P < 0.05)。
Fig. 4Tissue-specific expression patterns of CsHXK2 in the tea plant
A: different tissues of ‘Longjing 43’ in spring; B: different tissues of ‘Longjing 43’ in autumn; C: different tissues of ‘Shuchazao’. Different letters on the column indicate significant difference between the data (P < 0.05).
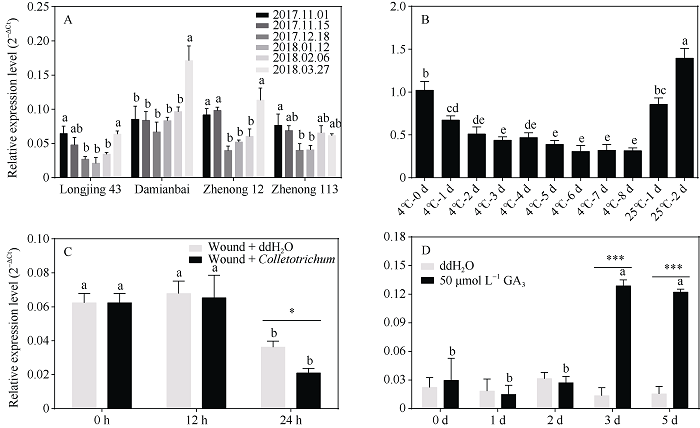
2.5 CsHXK2基因不同处理下的表达模式
对茶树品种‘龙井43’、‘大面白’、‘浙农12’和‘浙农113’自然冷驯化(越冬期)和脱驯化过程中的CsHXK2基因表达情况的分析发现, CsHXK2基因在4个不同茶树品种内具有相似的表达模式, 且在‘龙井43’、‘浙农12’和‘浙农113’内的表达随着环境气温的降低(2017.12.18, 2018.01.12)[26]而显著下调, 并随着气温的升高(2018-03-27)而显著上调(图5-A)。室内4℃低温处理能显著降低茶树叶片内CsHXK2基因的表达量, 且随着处理时间的延长而逐渐降低; 而CsHXK2基因的表达水平随着处理温度恢复至25℃而快速上升至处理前表达量(图5-B)。表明CsHXK2基因的表达受到低温的抑制。图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5茶树CsHXK2基因在不同处理下的表达分析
A: 自然冷驯化; B: 4℃低温及25℃恢复; C: 炭疽菌接种; D: 50 μmol L-1外源GA3处理。柱上标以不同字母表示数据间的显著性差异(P < 0.05)。*表示0.05水平显著; ***表示0.001水平显著。
Fig. 5Expression analysis of CsHXK2 under different treatments in the tea plant
A: natural cold acclimation; B: 4℃ cold and 25℃ recovery; C: inoculation with Colletotrichum; D: 50 μmol L-1 exogenous GA3. Different letters on the column indicate significant difference between the data (P < 0.05). * indicates significant at the 0.05 probability level; *** indicates significant at the 0.001 probability level.
采用有伤接种的方式, 对‘龙井43’叶片进行针头刺伤后分别喷施ddH2O和炭疽菌孢子悬浮液, 取样后检测CsHXK2基因的表达变化。由图5-C可知, 在刺伤喷洒ddH2O和炭疽菌的叶片中, CsHXK2基因的表达均随着处理时间延长至24 h而显著下调; 在24 h时间点, 炭疽菌处理叶片内的CsHXK2基因表达量显著低于对照。表明CsHXK2基因响应炭疽菌生物胁迫侵染而抑制表达。
对盆栽‘龙井43’叶片分别喷洒ddH2O和50 μmol L-1 GA3, 处理后取样并进行CsHXK2基因表达分析。喷洒ddH2O叶片内的CsHXK2基因表达无显著性变化; 而50 μmol L-1 GA3处理的叶片内, CsHXK2基因表达量在第3天和第5天显著上升, 且显著高于同一时间点的对照(图5-D)。说明CsHXK2基因参与响应赤霉素信号转导途径。
3 讨论
六碳糖己糖(Hexose), 如葡萄糖和果糖, 是植物中大部分代谢途径和有机物质的最初底物。己糖在参与呼吸作用、合成代谢等过程之前必须被磷酸化[27]。己糖激酶HXKs可以ATP依赖性地磷酸化一系列己糖(通常为葡萄糖和果糖)转换成己糖-6-磷酸; 此外, 许多HXKs如拟南芥AtHXK1, 具有感知糖浓度水平, 进而调控光合系统基因表达、细胞增殖、根和花序生长、叶片扩展和衰老[16,17]。因此, HXKs是参与碳水化合物代谢和糖信号转导的双功能酶, 在植物代谢以及生长发育中起着不可或缺的作用。与茶树CsHXK2关系密切的亲缘蛋白有拟南芥AtHXK3、水稻OsHXK4、烟草NtHXK2及番茄LeHXK4, 均属于具有叶绿体转运肽的type A类HXKs[19], 且均作用于叶绿体基质[10,12,28-29]。基于酵母己糖激酶功能缺失互补试验发现, LeHXK4全长蛋白不能磷酸化葡萄糖和果糖, 但其去除叶绿体转运肽的成熟蛋白能磷酸化葡萄糖和果糖; 而利用酶活动力学试验发现, LeHXK4具有葡萄糖和果糖磷酸化活性[29]。因此, 转化LeHXK4的酵母突变体不能利用己糖为碳源, 主要是由于叶绿体转运肽所引起的蛋白细胞内定位而造成, 转运肽本身并没有改变酶的催化活性。本研究中, CsHXK2蛋白定位于叶绿体(图2-D和G)内, 具有叶绿体转运肽的CsHXK2蛋白仅能以磷酸化葡萄糖为碳源, 而去除转运肽的CsHXK2成熟蛋白则能催化葡萄糖和果糖为碳源(图3-C和D)。说明CsHXK2成熟蛋白具备己糖磷酸化活性, 而完整CsHXK2蛋白的催化能力仍有待用酶活试验进行进一步的探究。
拟南芥AtHXK3基因在根和种子(长角果)中具有相对丰富的表达量[10,14]; 水稻OsHXK4基因在根、花、幼嫩种子内的表达量明显高于叶片, 且在胚乳内的表达显著高于种皮[12]; 烟草pNtHXK2::GUS活性可在淀粉鞘、木质部薄壁组织、保卫细胞和根尖组织中被检测到, 而在成熟花药内无活性[28]; 质体定位的番茄LeHXK4编码基因可在非光合作用库组织内表达, 包括根、茎、花、绿果、粉果、红果[29]。与同为type A类的AtHXK3、OsHXK4、NtHXK2、LeHXK4基因组织表达特异性相似, CsHXK2基因在茶树的根、茎、嫩叶(芽)、花、腋芽内具有较高的表达量(图4)。这可能归因于CsHXK2基因启动子上具有的根(RAV1AAT和RHERPATEXPA7)、花(CARGATCONSENSUS和POLLEN1LELAT52)、叶肉(CACTFTPPCA1)以及腋芽(SREATMSD)特异性表达元件(表2)。说明CsHXK2基因主要在茶树根、茎等库组织的生长发育中发挥作用, 可能通过磷酸化质体内的葡萄糖和果糖而促进糖代谢, 进而为组织生长发育提供能量和中间代谢物。
茶树原产于热带及亚热带地区, 性喜温暖, 冬季低温和春季倒春寒是威胁茶叶生产的主要气候因素。处于温暖湿润的茶树叶片极易感染由炭疽菌而引发的茶炭疽病, 造成茶叶品质及产量降低。自然冷驯化低温条件下, 茶树叶片内可溶性总糖、蔗糖、葡萄糖及果糖含量显著上升[30,31]。同样, 经炭疽菌侵染的植物叶片内总可溶性碳水化合物、蔗糖、己糖含量明显积累[32,33]。增加的可溶性糖, 包括葡萄糖和果糖, 既能参与细胞内碳和能量代谢, 又能作为信号分子而参与调控植物胁迫响应和生长发育[34]。本研究中, CsHXK2基因在低温及炭疽菌处理后叶片内的表达情况表明, 该基因响应低温及炭疽菌胁迫而表达显著下调(图5-A~C)。拟南芥AtHXK3基因响应低温、渗透和盐胁迫而在根系或嫩梢组织内表达降低[14]; 同样为type A类的麻风树JcHXK2基因, 在低温处理24 h的叶片内表达上调, 而在根系内表达下调[9]。因此, 感应低温和炭疽菌而表达抑制的CsHXK2基因是如何参与调节茶树适应外在刺激, 仍有待深入研究。
赤霉素(GA3)作为一种植物激素, 参与调控多种生长发育过程。赤霉素信号途径与糖信号之间存在着紧密关联, 其中HXKs是激素与糖联系的一个关键元件。离体矮牵牛花冠内, GA3对赤霉素诱导基因gip (gibberellin-induced gene)和查尔酮合成酶基因chs (chalcone synthase gene)表达的诱导受到葡萄糖的促进, 但这种葡萄糖引起的促进效果可以被HXK竞争性抑制剂甘露庚酮糖所完全摧毁, 表明HXKs参与的糖磷酸化相关信号转导与赤霉素信号相互作用, 进而诱导矮牵牛花冠发育过程中的基因表达和花青素积累[35]。阿洛糖作为葡萄糖的差向异构体, 能强烈抑制水稻赤霉素依赖性反应, 如第二叶鞘的伸长以及无胚半稻种子α-淀粉酶的诱导, 也能抑制具有赤霉素组成型响应表型的slr1水稻突变体的生长, 而这些抑制能被HXK抑制剂甘露庚酮糖所阻止, 表明阿洛糖通过HXK依赖途径抑制赤霉素信号传导[36]。本研究检测GA3处理的茶树叶片内CsHXK2基因的表达发现, 该基因转录本在处理样品中有显著的诱导富集(图5-D)。表明茶树赤霉素信号途径和CsHXK2基因表达关系密切, 可能与HXK介导的糖信号途径共同调控茶树的生长发育与逆境胁迫响应有关。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1023/A:1026501430422URL [本文引用: 1]

Previous studies have revealed a central role of Arabidopsis thaliana hexokinases (AtHXK1 and AtHXK2) in the glucose repression of photosynthetic genes and early seedling development. However, it remains unclear whether HXK can modulate the expression of diverse sugar-regulated genes. On the basis of the results of analyses of gene expression in HXK transgenic plants, we suggest that three distinct glucose signal transduction pathways exist in plants. The first is an AtHXK1-dependent pathway in which gene expression is correlated with the AtHXK1-mediated signaling function. The second is a glycolysis-dependent pathway that is influenced by the catalytic activity of both AtHXK1 and the heterologous yeast Hxk2. The last is an AtHXK1-independent pathway in which gene expression is independent of AtHXK1. Further investigation of HXK transgenic Arabidopsis discloses a role of HXK in glucose-dependent growth and senescence. In the absence of exogenous glucose, plant growth is limited to the seedling stage with restricted true leaf development even after a 3-week culture on MS medium. In the presence of glucose, however, over-expressing Arabidopsis or yeast HXK in plants results in the repression of growth and true leaf development, and early senescence, while under-expressing AtHXK1 delays the senescence process. These studies reveal multiple glucose signal transduction pathways that control diverse genes and processes that are intimately linked to developmental stages and environmental conditions.]]>
DOI:10.1042/BST0330269URLPMID:15667323 [本文引用: 1]

Plant sugar signalling operates in a complex network with plant-specific hormone signalling pathways. Hexokinase was identified as an evolutionarily conserved glucose sensor that integrates light, hormone and nutrient signalling to control plant growth and development.
DOI:10.1016/j.tplants.2008.06.006URLPMID:18701338 [本文引用: 1]

Plants are constantly confronted by multiple types of stress. Despite their distinct origin and mode of perception, nutrient deprivation and most stresses have an impact on the overall energy status of the plant, leading to convergent downstream responses that include largely overlapping transcriptional patterns. The emerging view is that this transcriptome reprogramming in energy and stress signaling is partly regulated by the evolutionarily conserved energy sensor protein kinases, SNF1 (sucrose non-fermenting 1) in yeast, AMPK (AMP-activated protein kinase) in mammals and SnRK1 (SNF1-related kinase 1) in plants. Upon sensing the energy deficit associated with stress, nutrient deprivation and darkness, SnRK1 triggers extensive transcriptional changes that contribute to restoring homeostasis, promoting cell survival and elaborating longer-term responses for adaptation, growth and development.
DOI:10.1104/pp.108.2.879URLPMID:7610198 [本文引用: 1]
DOI:10.1093/pcp/pcx062URLPMID:28449056 [本文引用: 1]

Sugars are the main carbon and energy source in cells, but they can also act as signaling molecules that affect the whole plant life cycle. Certain tissues can produce sugars and supply them to others, and this plant tissue heterogeneity makes sugar signaling a highly complex process that requires elements capable of perceiving changes in sugar concentrations among different tissues, cell compartments and developmental stages. In plants, the regulatory effects of glucose (Glc) have been the most studied to date. The first Glc sensor identified in plants was hexokinase (HXK), which is currently recognized as a dual-function protein. In addition to its catalytic activity, this enzyme can also repress the expression of some photosynthetic genes in response to high internal Glc concentrations. Additionally, the catalytic activity of HXKs has a profound impact on cell metabolism and other sugar signaling pathways that depend on phosphorylated hexoses and intermediate glycolytic products. HXKs are the only proteins that are able to phosphorylate Glc in plants, since no evidence has been provided to date concerning the existence of a glucokinase. Moreover, the intracellular localization of HXKs seems to be crucial to their activity and sensor functions. Recently, two new and surprising functions have been described for HXKs. In this review, we discuss the versatility of HXKs in regard to their catalytic and glucose sensor activities, intracellular location, protein-protein and hormone interactions, as well as how these HXK characteristics influence plant growth and development, in an effort to understand this enzyme's role in improving plant productivity.
DOI:10.3390/ijms18051041URL [本文引用: 2]
DOI:10.1007/s00425-018-2888-8URLPMID:29644447 [本文引用: 3]

MAIN CONCLUSION: Genome-wide identification, expression analysis, and functional characterization of previously uncharacterized hexokinase family of oil crop, Brassica napus, underscore the importance of this gene family in plant growth and development. In plants, the multi-gene family of dual-function hexokinases (HXKs) plays important roles in sugar metabolism and sensing that affect growth and development. Rapeseed (Brassica napus L.) is an important oil crop; however, little is known about the B. napus HXK gene family. We identified 19 putative HXKs in B. napus genome. B. rapa and B. oleracea, the two diploid progenitors of B. napus, contributed almost equally to the BnHXK genes. Phylogenetic analysis divided the 19 BnHXKs into four groups. The exon-intron structures of BnHXKs share high similarity to those of HXKs in Arabidopsis and rice. The group III and IV BnHXKs are highly expressed in roots, whereas group I members preferentially express in leaves. Analysis of seed transcriptomes at different developmental stages showed that most of group I and IV HXKs are highly expressed 2-weeks after pollination (2WAP), compared to 4WAP for group III. BnHKXs are differentially expressed in susceptible and tolerant B. napus cultivars after fungal infection, suggesting the possible involvement in defense response. We generated rapeseed RNAi lines for BnHXK9, a member of relatively less characterized group IV, by pollen-mediated gene transformation. The seedlings of BnHXK9-RNAi lines showed delayed growth compared to the wild type. The RNAi plants were dwarf with curly leaves, suggesting the involvement of BnHXK9 in plant development. Collectively, our findings provides a comprehensive account of BnHXK gene family in an important crop and a starting point for further elucidation of their roles in sugar metabolism and sensing, as well as plant growth and development.
DOI:10.1016/j.gene.2019.06.022URLPMID:31202905 [本文引用: 1]

Hexokinase (HXK) is a multifunctional protein that serves as a sugar sensor for glucose signaling and a catalyst for glycolysis. It has been well studied in many species, however, there is far less information about this family in pear. To investigate the roles of HXK in the growth and development of pear fruit, we performed a genome-wide analysis and identified the HXK gene family members in pear. In addition, we functionally characterized a glucose sensor gene, PbHXK1, in P. bretschneideri. In total, 10 HXK genes were identified in pear, and a multiple sequence alignment and phylogenetic analysis showed that PbHXK1 is a Type B HXK that contains four conserved domains, phosphate 1 and 2, sugar binding and adenosine, which are specific to plant HXKs and essential for enzymatic functions. A qRT-PCR analysis revealed that the relative expression levels of PbHXK1 were negatively correlated with sugar content but significantly positively correlated with HXK activity during pear fruit development. Furthermore, the overexpression of PbHXK1 in tomatoes significantly enhanced the HXK activity and decreased the sugar content. In addition, the growth of transgenic tomato plants overexpressing PbHXK1 was inhibited, leading to shortened internodes and smaller leaves. Thus, in pear, PbHXK1 encodes HXK, which regulated the sugar content in fruit and affected the growth and development of plants.
DOI:10.1007/s11033-019-04613-0URLPMID:30756333 [本文引用: 3]

Hexokinase, the key rate-limiting enzyme of plant respiration and glycolysis metabolism, has been found to play a vital role in plant sugar sensing and sugar signal transduction. Using Jatropha curcas genome database and bioinformatics method, J. curcas HXK gene family (JcHXK) was identified and its phylogenetic evolution, functional domain, signal peptide at the N-terminal, and expression analysis were conducted. The results showed that a total of 4 HXK genes (JcHXK1, JcHXK2, JcHXK3, and JcHKL1) with 9 exons were systematically identified from J. curcas. JcHXK1, JcHXK3, and JcHKL1 with putative transmembrane domain at the N-terminal belonged to the type of secretory pathway protein, and JcHXK2 contained putative chloroplast targeting peptide. Quantitative real-time PCR (qRT-PCR) analysis revealed that all the four JcHXKs were expressed in different tissues of the leaves, roots, and seeds; however, JcHXK1 and JcHKL1 expression were higher in the roots, whereas JcHXK2 and JcHXK3 showed over-expression in the leaves and seeds, respectively. Furthermore, all the four JcHXKs were up-regulated in the leaves after cold stress at 12 degrees C; however, only JcHXK3 remarkably demonstrated cold-induced expression in the roots, which reached the highest expression level at 12 h (2.28-fold). According to the cis-acting element analysis results, JcHXK2 contained the most low temperature responsive elements, which was closely related to the cold resistance in J. curcas. A pET-28a-JcHXK2 prokaryotic recombinant expression vector was successfully constructed and a 57.0 kDa protein was obtained, JcHXK2 revealed catalytic activity towards glucose and fructose, with a higher affinity for glucose than fructose. The subcellular localization assays revealed that JcHXK2 was localized in the chloroplast. The results of this study might provide theoretical foundation for further studies on gene cloning and functional verification of HXK family in J. curcas.
DOI:10.1007/s00425-008-0746-9URL [本文引用: 4]

Arabidopsis hexokinase1 (HXK1) is a moonlighting protein that has separable functions in glucose signaling and in glucose metabolism. In this study, we have characterized expression features and glucose phosphorylation activities of the six HXK gene family members in Arabidopsis thaliana. Three of the genes encode catalytically active proteins, including a stromal-localized HXK3 protein that is expressed mostly in sink organs. We also show that three of the genes encode hexokinase-like (HKL) proteins, which are about 50% identical to AtHXK1, but do not phosphorylate glucose or fructose. Expression studies indicate that both HKL1 and HKL2 transcripts occur in most, if not all, plant tissues and that both proteins are targeted within cells to mitochondria. The HKL1 and HKL2 proteins have 6–10 amino acid insertions/deletions (indels) at the adenosine binding domain. In contrast, HKL3 transcript was detected only in flowers, the protein lacks the noted indels, and the protein has many other amino acid changes that might compromise its ability even to bind glucose or ATP. Activity measurements of HXKs modified by site-directed mutagenesis suggest that the lack of catalytic activities in the HKL proteins might be attributed to any of numerous existing changes. Sliding windows analyses of coding sequences in A. thaliana and A. lyrata ssp. lyrata revealed a differential accumulation of nonsynonymous changes within exon 8 of both HKL1 and HXK3 orthologs. We further discuss the possibility that the non-catalytic HKL proteins have regulatory functions instead of catalytic functions.]]>
DOI:10.1111/pce.12060URLPMID:23305564 [本文引用: 2]

Hexokinase (HXK) is present in all virtually living organisms and is central to carbohydrate metabolism catalysing the ATP-dependent phosphorylation of hexoses. In plants, HXKs are supposed to act as sugar sensors and/or to interact with other enzymes directly supplying metabolic pathways such as glycolysis, the nucleotide phosphate monosaccharide (NDP-glucose) pathway and the pentose phosphate pathway. We identified nine members of the tobacco HXK gene family and observed that among RNAi lines of these nine NtHXKs, only RNAi lines of NtHXK1 showed an altered phenotype, namely stunted growth and leaf chlorosis. NtHXK1 was also the isoform with highest relative expression levels among all NtHXKs. GFP-tagging and immunolocalization indicated that NtHXK1 is associated with mitochondrial membranes. Overexpression of NtHXK1 resulted in elevated glucose phosphorylation activity in leaf extracts or chloroplasts. Moreover, NtHXK1 was able to complement the glucose-insensitive Arabidopsis mutant gin2-1 suggesting that NtHXK1 can take over glucose sensing functions. RNAi lines of NtHXK1 showed severely damaged leaf and chloroplast structure, coinciding with an excess accumulation of starch. We conclude that NtHXK1 is not only essential for maintaining glycolytic activity during respiration but also for regulating starch turnover, especially during the night.
DOI:10.1007/s00425-006-0251-yURL [本文引用: 4]

Hexokinase (HXK) is a dual-function enzyme that both phosphorylates hexose to form hexose 6−phosphate and plays an important role in sugar sensing and signaling. To investigate the roles of hexokinases in rice growth and development, we analyzed rice sequence databases and isolated ten rice hexokinase cDNAs, OsHXK1 (Oryza sativa Hexokinase 1) through OsHXK10. With the exception of the single-exon gene OsHXK1, the OsHXKs all have a highly conserved genomic structure consisting of nine exons and eight introns. Gene expression profiling revealed that OsHXK2 through OsHXK9 are expressed ubiquitously in various organs, whereas OsHXK10 expression is pollen-specific. Sugars induced the expression of three OsHXKs, OsHXK2, OsHXK5, and OsHXK6, in excised leaves, while suppressing OsHXK7 expression in excised leaves and immature seeds. The hexokinase activity of the OsHXKs was confirmed by functional complementation of the hexokinase-deficient yeast strain YSH7.4-3C (hxk1, hxk2, glk1). OsHXK4 was able to complement this mutant only after the chloroplast-transit peptide was removed. The subcellular localization of OsHXK4 and OsHXK7, observed with green fluorescent protein (GFP) fusion constructs, indicated that OsHXK4 is a plastid-stroma-targeted hexokinase while OsHXK7 localizes to the cytosol.]]>
DOI:10.1074/jbc.M306265200URLPMID:12941966 [本文引用: 1]

Hexokinase catalyzes the first step in the metabolism of glucose but has also been proposed to be involved in sugar sensing and signaling both in yeast and in plants. We have cloned a hexokinase gene, PpHXK1, in the moss Physcomitrella patens where gene function can be studied directly by gene targeting. PpHxk1 is a novel type of chloroplast stromal hexokinase that differs from previously studied membrane-bound plant hexokinases. Enzyme assays on a knock-out mutant revealed that PpHxk1 is the major glucose-phosphorylating enzyme in Physcomitrella, accounting for 80% of the total activity in protonemal tissue. The mutant is deficient in the response to glucose, which in wild type moss induces the formation of caulonemal filaments that protrude from the edge of the colony. Growth on glucose in the dark is strongly reduced in the mutant. Sequence data suggest that most plants including Physcomitrella and Arabidopsis have both chloroplast-imported hexokinases similar to PpHxk1 and traditional membrane-bound hexokinases. We propose that the two types of plant hexokinases have distinct physiological roles.
DOI:10.1016/j.phytochem.2006.12.001URLPMID:17234224 [本文引用: 4]

Hexokinase (HK) occurs in all phyla, as an enzyme of the glycolytic pathway. Its importance in plant metabolism has emerged with compelling evidence that its preferential substrate, glucose, is both a nutrient and a signal molecule that controls development and expression of different classes of genes. A variety of plant tissues and organs have been shown to express multiple HK isoforms with different kinetic properties and subcellular localizations. Although plant HK is known to fulfill a catalytic function and act as a glucose sensor, the physiological relevance of plural isoforms and their contribution to either function are still poorly understood. We review here the current knowledge and hypotheses on the physiological roles of plant HK isoforms that have been identified and characterized. Recent findings provide hints on how the expression patterns, biochemical properties and subcellular localizations of HK isoforms may relate to their modes of action. Special attention is devoted to kinetic, mutant and transgenic data on HKs from Arabidopsis thaliana and the Solanaceae potato, tobacco, and tomato, as well as HK gene expression data from Arabidopsis public DNA microarray resources. Similarities and differences to known properties of animal and yeast HKs are also discussed as they may help to gain further insight into the functional adaptations of plant HKs.
DOI:10.1105/tpc.9.1.5URLPMID:9014361 [本文引用: 1]

The mechanisms by which higher plants recognize and respond to sugars are largely unknown. Here, we present evidence that the first enzyme in the hexose assimilation pathway, hexokinase (HXK), acts as a sensor for plant sugar responses. Transgenic Arabidopsis plants expressing antisense hexokinase (AtHXK) genes are sugar hyposensitive, whereas plants overexpressing AtHXK are sugar hypersensitive. The transgenic plants exhibited a wide spectrum of altered sugar responses in seedling development and in gene activation and repression. Furthermore, overexpressing the yeast sugar sensor YHXK2 caused a dominant negative effect by elevating HXK catalytic activity but reducing sugar sensitivity in transgenic plants. The result suggests that HXK is a dual-function enzyme with a distinct regulatory function not interchangeable between plants and yeast.
DOI:10.1105/tpc.11.7.1253URLPMID:10402427 [本文引用: 2]

Sugars are key regulatory molecules that affect diverse processes in higher plants. Hexokinase is the first enzyme in hexose metabolism and may be a sugar sensor that mediates sugar regulation. We present evidence that hexokinase is involved in sensing endogenous levels of sugars in photosynthetic tissues and that it participates in the regulation of senescence, photosynthesis, and growth in seedlings as well as in mature plants. Transgenic tomato plants overexpressing the Arabidopsis hexokinase-encoding gene AtHXK1 were produced. Independent transgenic plants carrying single copies of AtHXK1 were characterized by growth inhibition, the degree of which was found to correlate directly to the expression and activity of AtHXK1. Reciprocal grafting experiments suggested that the inhibitory effect occurred when AtHXK1 was expressed in photosynthetic tissues. Accordingly, plants with increased AtHXK1 activity had reduced chlorophyll content in their leaves, reduced photosynthesis rates, and reduced photochemical quantum efficiency of photosystem II reaction centers compared with plants without increased AtHXK1 activity. In addition, the transgenic plants underwent rapid senescence, suggesting that hexokinase is also involved in senescence regulation. Fruit weight, starch content in young fruits, and total soluble solids in mature fruits were also reduced in the transgenic plants. The results indicate that endogenous hexokinase activity is not rate limiting for growth; rather, they support the role of hexokinase as a regulatory enzyme in photosynthetic tissues, in which it regulates photosynthesis, growth, and senescence.
DOI:10.1126/science.1080585URLPMID:12690200 [本文引用: 2]

Glucose modulates many vital processes in photosynthetic plants. Analyses of Arabidopsis glucose insensitive2 (gin2) mutants define the physiological functions of a specific hexokinase (HXK1) in the plant glucose-signaling network. HXK1 coordinates intrinsic signals with extrinsic light intensity. HXK1 mutants lacking catalytic activity still support various signaling functions in gene expression, cell proliferation, root and inflorescence growth, and leaf expansion and senescence, thus demonstrating the uncoupling of glucose signaling from glucose metabolism. The gin2 mutants are also insensitive to auxin and hypersensitive to cytokinin. Plants use HXK as a glucose sensor to interrelate nutrient, light, and hormone signaling networks for controlling growth and development in response to the changing environment.
DOI:10.1007/BF03036136URL [本文引用: 1]

Previously, we reported that mitochondria-associated hexokinases are active in controlling programmed cell death in plants (Plant Cell 18, 2341-2355). Here, we investigated their role under abiotic- and biotic-stress conditions. Expression ofNbHxk1, aNicotiana benthamiana hexokinase gene, was stimulated by treatment with salicylic acid or methyl viologen (MV), and was also up-regulated by pathogen infection. In response to MV-induced oxidative stress, NbHxk1-silenced plants exhibited increased susceptibility, while the HXK1— and HXK2-overexpressingArabidopsis plants had enhanced tolerance. Moreover, those overexpressing plants showed greater resistance to the necrotrophic fungal pathogenAlternaria brassicicola. HXK-over-expression also mildly protected plants against the bacterial pathogenPseudomonas syringae pv.tomato DC3000, a response that was accompanied by increased H2O2 production and elevatedPR1 gene expression. These results demonstrate that higher levels of hexokinase confer improved resistance to MV-induced oxidative stress and pathogen infection.]]>
DOI:10.1016/j.jplph.2016.11.007URLPMID:28013175 [本文引用: 3]

Hexokinases (HXKs, EC 2.7.1.1) and fructokinases (FRKs, EC 2.7.1.4) play important roles in carbohydrate metabolism and sugar signaling during the growth and development of plants. However, the HXKs and FRKs in the tea plant (Camellia sinensis) remain largely unknown. In this manuscript, we present the molecular characterization, phylogenetic relationships, conserved domains and expression profiles of four HXK and seven FRK genes of the tea plant. The 11 deduced CsHXK and CsFRK proteins were grouped into six main classes. All of the deduced proteins, except for CsFKR7, possessed putative ATP-binding motifs and a sugar recognition region. These genes exhibited tissue-specific expression patterns, which suggests that they play different roles in the metabolism and development of source and sink tissues in the tea plant. There were variations in CsHXKs and CsFRKs transcript abundance in response to four abiotic stresses: cold, salt, drought and exogenous abscisic acid (ABA). Remarkably, CsHXK3 and CsHXK4 were significantly induced in the leaves and roots under cold conditions, CsHXK1 was apparently up-regulated in the leaves and roots under salt and drought stresses, and CsHXK3 was obviously stimulated in the leaves and roots under short-term treatment with exogenous ABA. These findings demonstrate that CsHXKs play critical roles in response to abiotic stresses in the tea plant. Our research provides a fundamental understanding of the CsHXK and CsFRK genes of the tea plant and important information for the breeding of stress-tolerant tea cultivars.
DOI:10.1016/j.molp.2017.04.002URLPMID:28473262 [本文引用: 1]

Tea is the world's oldest and most popular caffeine-containing beverage with immense economic, medicinal, and cultural importance. Here, we present the first high-quality nucleotide sequence of the repeat-rich (80.9%), 3.02-Gb genome of the cultivated tea tree Camellia sinensis. We show that an extraordinarily large genome size of tea tree is resulted from the slow, steady, and long-term amplification of a few LTR retrotransposon families. In addition to a recent whole-genome duplication event, lineage-specific expansions of genes associated with flavonoid metabolic biosynthesis were discovered, which enhance catechin production, terpene enzyme activation, and stress tolerance, important features for tea flavor and adaptation. We demonstrate an independent and rapid evolution of the tea caffeine synthesis pathway relative to cacao and coffee. A comparative study among 25 Camellia species revealed that higher expression levels of most flavonoid- and caffeine- but not theanine-related genes contribute to the increased production of catechins and caffeine and thus enhance tea-processing suitability and tea quality. These novel findings pave the way for further metabolomic and functional genomic refinement of characteristic biosynthesis pathways and will help develop a more diversified set of tea flavors that would eventually satisfy and attract more tea drinkers worldwide.
DOI:10.1093/nar/27.1.297URLPMID:9847208 [本文引用: 1]

PLACE (http://www.dna.affrc.go.jp/htdocs/PLACE/) is a database of nucleotide sequence motifs found in plant cis-acting regulatory DNA elements. Motifs were extracted from previously published reports on genes in vascular plants. In addition to the motifs originally reported, their variations in other genes or in other plant species in later reports are also compiled. Documents for each motif in the PLACE database contains, in addition to a motif sequence, a brief definition and description of each motif, and relevant literature with PubMed ID numbers and GenBank accession numbers where available. Users can search their query sequences for cis-elements using the Signal Scan program at our web site. The results will be reported in one of the three forms. Clicking the PLACE accession numbers in the result report will open the pertinent motif document. Clicking the PubMed or GenBank accession number in the document will allow users to access to these databases, and to read the of the literature or the annotation in the DNA database. This report summarizes the present status of this database and available tools.
DOI:10.1111/j.1432-1033.1996.00633.xURLPMID:8917466 [本文引用: 1]

Addition of rapidly fermentable sugars to cells of the yeast Saccharomyces cerevisiae grown on nonfermentable carbon sources causes a variety of short-term and long-term regulatory effects, leading to an adaptation to fermentative metabolism. One important feature of this metabolic switch is the occurrence of extensive transcriptional repression of a large group of genes. We have investigated transcriptional regulation of the SUC2 gene encoding repressible invertase, and of HXK1, HXK2 and GLK1 encoding the three known yeast hexose kinases during transition from derepressed to repressed growth conditions. Comparing yeast strains that express various combinations of the hexose kinase genes, we have determined the importance of each of these kinases for establishing the catabolite-repressed state. We show that catabolite repression involves two distinct mechanisms. An initial rapid response is mediated through any kinase, including Glk1, which is able to phosphorylate the available sugar. In contrast, long-term repression specifically requires Hxk2 on glucose and either Hxk1 or Hxk2 on fructose. Both HXK1 and GLK1 are repressed upon addition of glucose or fructose. However, fructose repression of Hxk1 is only transient, which is in line with its preference for fructose as substrate and its requirement for long-term fructose repression. In addition, expression of HXK1 and GLK1 is regulated through cAMP-dependent protein kinase. These results indicate that sugar sensing and establishment of catabolite repression are controlled by an interregulatory network, involving all three yeast sugar kinases and the Ras-cAMP pathway.
DOI:10.3390/ijms151222155URLPMID:25474086 [本文引用: 1]

Reliable reference selection for the accurate quantification of gene expression under various experimental conditions is a crucial step in qRT-PCR normalization. To date, only a few housekeeping genes have been identified and used as reference genes in tea plant. The validity of those reference genes are not clear since their expression stabilities have not been rigorously examined. To identify more appropriate reference genes for qRT-PCR studies on tea plant, we examined the expression stability of 11 candidate reference genes from three different sources: the orthologs of Arabidopsis traditional reference genes and stably expressed genes identified from whole-genome GeneChip studies, together with three housekeeping gene commonly used in tea plant research. We evaluated the transcript levels of these genes in 94 experimental samples. The expression stabilities of these 11 genes were ranked using four different computation programs including geNorm, Normfinder, BestKeeper, and the comparative CT method. Results showed that the three commonly used housekeeping genes of CsTUBULIN1, CsACINT1 and Cs18S rRNA1 together with CsUBQ1 were the most unstable genes in all sample ranking order. However, CsPTB1, CsEF1, CsSAND1, CsCLATHRIN1 and CsUBC1 were the top five appropriate reference genes for qRT-PCR analysis in complex experimental conditions.
DOI:10.1006/meth.2001.1262URLPMID:11846609 [本文引用: 1]

The two most commonly used methods to analyze data from real-time, quantitative PCR experiments are absolute quantification and relative quantification. Absolute quantification determines the input copy number, usually by relating the PCR signal to a standard curve. Relative quantification relates the PCR signal of the target transcript in a treatment group to that of another sample such as an untreated control. The 2(-Delta Delta C(T)) method is a convenient way to analyze the relative changes in gene expression from real-time quantitative PCR experiments. The purpose of this report is to present the derivation, assumptions, and applications of the 2(-Delta Delta C(T)) method. In addition, we present the derivation and applications of two variations of the 2(-Delta Delta C(T)) method that may be useful in the analysis of real-time, quantitative PCR data.
DOI:10.1111/pbi.13111URLPMID:30913342 [本文引用: 2]

Tea is the world's widely consumed nonalcohol beverage with essential economic and health benefits. Confronted with the increasing large-scale omics-data set particularly the genome sequence released in tea plant, the construction of a comprehensive knowledgebase is urgently needed to facilitate the utilization of these data sets towards molecular breeding. We hereby present the first integrative and specially designed web-accessible database, Tea Plant Information Archive (TPIA; http://tpia.teaplant.org). The current release of TPIA employs the comprehensively annotated tea plant genome as framework and incorporates with abundant well-organized transcriptomes, gene expressions (across species, tissues and stresses), orthologs and characteristic metabolites determining tea quality. It also hosts massive transcription factors, polymorphic simple sequence repeats, single nucleotide polymorphisms, correlations, manually curated functional genes and globally collected germplasm information. A variety of versatile analytic tools (e.g. JBrowse, blast, enrichment analysis, etc.) are established helping users to perform further comparative, evolutionary and functional analysis. We show a case application of TPIA that provides novel and interesting insights into the phytochemical content variation of section Thea of genus Camellia under a well-resolved phylogenetic framework. The constructed knowledgebase of tea plant will serve as a central gateway for global tea community to better understand the tea plant biology that largely benefits the whole tea industry.
DOI:10.1016/j.envexpbot.2018.11.011URL [本文引用: 1]
DOI:10.3389/fpls.2013.00001URLPMID:23346092 [本文引用: 1]

Phosphatidic acid (PtdOH) is emerging as an important signaling lipid in abiotic stress responses in plants. The effect of cold stress was monitored using (32)P-labeled seedlings and leaf discs of Arabidopsis thaliana. Low, non-freezing temperatures were found to trigger a very rapid (32)P-PtdOH increase, peaking within 2 and 5 min, respectively. In principle, PtdOH can be generated through three different pathways, i.e., (1) via de novo phospholipid biosynthesis (through acylation of lyso-PtdOH), (2) via phospholipase D hydrolysis of structural phospholipids, or (3) via phosphorylation of diacylglycerol (DAG) by DAG kinase (DGK). Using a differential (32)P-labeling protocol and a PLD-transphosphatidylation assay, evidence is provided that the rapid (32)P-PtdOH response was primarily generated through DGK. A simultaneous decrease in the levels of (32)P-PtdInsP, correlating in time, temperature dependency, and magnitude with the increase in (32)P-PtdOH, suggested that a PtdInsP-hydrolyzing PLC generated the DAG in this reaction. Testing T-DNA insertion lines available for the seven DGK genes, revealed no clear changes in (32)P-PtdOH responses, suggesting functional redundancy. Similarly, known cold-stress mutants were analyzed to investigate whether the PtdOH response acted downstream of the respective gene products. The hos1, los1, and fry1 mutants were found to exhibit normal PtdOH responses. Slight changes were found for ice1, snow1, and the overexpression line Super-ICE1, however, this was not cold-specific and likely due to pleiotropic effects. A tentative model illustrating direct cold effects on phospholipid metabolism is presented.
DOI:10.1016/j.febslet.2004.12.071URLPMID:15670855 [本文引用: 2]

Due to their central role in sugar metabolism and signalling plant hexokinases have been studied in great detail, however, little is known about the spatial and temporal expression and the sub-cellular distribution of individual hexokinase isoforms. Based on in planta and in vitro studies the recently isolated tobacco hexokinase 2 (Hxk2) could be located in the chloroplast stroma and biochemically characterized. Hxk2 represents the first innerplastidic hexokinase described from higher plants. Promoter studies indicate that Hxk2 is mainly expressed in cells of the vascular starch sheath and xylem parenchyma, in guard cells and root tips. We propose a role for Hxk2 in starch and secondary metabolism in the mentioned tissues.
DOI:10.1007/s00425-006-0318-9URL [本文引用: 3]

Two new tomato hexokinase genes, LeHXK3 and LeHXK4, were cloned and characterized, placing tomato as the first plant with four characterized HXK genes. Based on their sequence, LeHXK3 is the third membrane-associated (type-B) and LeHXK4 is the first plastidic (type-A) HXK identified in tomato. Expression of HXK-GFP fusion proteins in protoplasts indicated that the LeHxk3 enzyme is associated with the mitochondria while LeHxk4 is localized in plastids. Furthermore, LeHxk4::GFP fusion protein is found within stromules, suggesting transport of LeHxk4 between plastids. Structure prediction of the various plant HXK enzymes suggests that unlike the plastidic HXKs, the predicted membrane-associated HXKs are positively charged near their putative N-terminal membrane anchor domain, which might enhance their association with the negatively charged membranes. LeHxk3 and LeHxk4 were analyzed following expression in yeast. Both enzymes have higher affinity for glucose relative to fructose and are inhibited by ADP. Yet, unlike the other HXKs, the stromal HXK has higher Vmax with glucose than with fructose. Expression analysis of the four HXK genes in tomato tissues demonstrated that LeHXK1 and LeHXK4 are the dominant HXKs in all tissues examined. Notably, the plastidic LeHXK4 is expressed in all tissues including starchless, non-photosynthetic sink tissues, such as pink and red fruits, implying phosphorylation of imported hexoses in plastids. It has been suggested that trehalose 6-phosphate (T6P) might inhibit HXK activity. However, none of the yeast-expressed tomato HXK genes was sensitive either to T6P or to trehalose, suggesting that unlike fungi HXKs, plant HXKs are not regulated by T6P.]]>
DOI:10.1007/s11103-015-0345-7URLPMID:26216393 [本文引用: 1]

Sugar plays an essential role in plant cold acclimation (CA), but the interaction between CA and sugar remains unclear in tea plants. In this study, during the whole winter season, we investigated the variations of sugar contents and the expression of a large number of sugar-related genes in tea leaves. Results indicated that cold tolerance of tea plant was improved with the development of CA during early winter season. At this stage, starch was dramatically degraded, whereas the content of total sugars and several specific sugars including sucrose, glucose and fructose were constantly elevated. Beyond the CA stage, the content of starch was maintained at a low level during winter hardiness (WH) period and then was elevated during de-acclimation (DC) period. Conversely, the content of sugar reached a peak at WH stage followed by a decrease during DC stage. Moreover, gene expression results showed that, during CA period, sugar metabolism-related genes exhibited different expression pattern, in which beta-amylase gene (CsBAM), invertase gene (CsINV5) and raffinose synthase gene (CsRS2) engaged in starch, sucrose and raffinose metabolism respectively were solidly up-regulated; the expressions of sugar transporters were stimulated in general except the down-regulations of CsSWEET2, 3, 16, CsERD6.7 and CsINT2; interestingly, the sugar-signaling related CsHXK3 and CsHXK2 had opposite expression patterns at the early stage of CA. These provided comprehensive insight into the effects of CA on carbohydrates indicating that sugar accumulation contributes to tea plant cold tolerance during winter season, and a simply model of sugar regulation in response to cold stimuli is proposed.
[本文引用: 1]
[本文引用: 1]
DOI:10.17221/PSEURL [本文引用: 1]
DOI:10.1104/pp.112.209676URL [本文引用: 1]

Colletotrichum higginsianum is a hemibiotrophic ascomycete fungus that is adapted to Arabidopsis (Arabidopsis thaliana). After breaching the host surface, the fungus establishes an initial biotrophic phase in the penetrated epidermis cell, before necrotrophic growth is initiated upon further host colonization. We observed that partitioning of major leaf carbohydrates was shifted in favor of sucrose and at the expense of starch during necrotrophic fungal growth. Arabidopsis mutants with impaired starch turnover were more susceptible toward C. higginsianum infection, exhibiting a strong negative correlation between diurnal carbohydrate accumulation and fungal proliferation for the tested genotypes. By altering the length of the light phase and employing additional genotypes impaired in nocturnal carbon mobilization, we revealed that reduced availability of carbon enhances susceptibility in the investigated pathosystem. Systematic starvation experiments resulted in two important findings. First, we showed that carbohydrate supply by the host is dispensable during biotrophic growth of C. higginsianum, while carbon deficiency was most harmful to the host during the necrotrophic colonization phase. Compared with the wild type, the increases in the total salicylic acid pool and camalexin accumulation were reduced in starch-free mutants at late interaction stages, while an increased ratio of free to total salicylic acid did not convey elevated pathogenesis-related gene expression in starch-free mutants. These observations suggest that reduced carbon availability dampens induced defense responses. In contrast, starch-free mutants were more resistant toward the fungal biotroph Erysiphe cruciferarum, indicating that reduced carbohydrate availability influences susceptibility differently in the interaction with the investigated hemibiotrophic and biotrophic fungal pathogens.
[本文引用: 1]
DOI:10.1034/j.1399-3054.2000.100212.xURL [本文引用: 1]
DOI:10.1007/s00425-011-1463-3URL [本文引用: 1]

One of the rare sugars, d-allose, which is the epimer of d-glucose at C3, has an inhibitory effect on rice growth, but the molecular mechanisms of the growth inhibition by d-allose were unknown. The growth inhibition caused by d-allose was prevented by treatment with hexokinase inhibitors, d-mannoheptulose and N-acetyl-d-glucosamine. Furthermore, the Arabidopsis glucose-insensitive2 (gin2) mutant, which is a loss-of-function mutant of the glucose sensor AtHXK1, showed a d-allose-insensitive phenotype. d-Allose strongly inhibited the gibberellin-dependent responses such as elongation of the second leaf sheath and induction of alpha-amylase in embryo-less half rice seeds. The growth of the slender rice1 (slr1) mutant, which exhibits a constitutive gibberellin-responsive phenotype, was also inhibited by d-allose, and the growth inhibition of the slr1 mutant by d-allose was also prevented by d-mannoheptulose treatment. The expressions of gibberellin-responsive genes were down-regulated by d-allose treatment, and the down-regulations of gibberellin-responsive genes were also prevented by d-mannoheptulose treatment. These findings reveal that d-allose inhibits the gibberellin-signaling through a hexokinase-dependent pathway.
