 ,*华中农业大学作物遗传改良国家重点实验室, 湖北武汉 430070
,*华中农业大学作物遗传改良国家重点实验室, 湖北武汉 430070Transcriptional regulation of oil biosynthesis in different parts of Wanyou 20 (Brassica napus) seeds
ZHANG Yu-Ting, LU Shao-Ping, JIN Cheng, GUO Liang ,*National Key Laboratory of Crop Genetic Improvement, Huazhong Agricultural University, Wuhan 430070, Hubei, China
,*National Key Laboratory of Crop Genetic Improvement, Huazhong Agricultural University, Wuhan 430070, Hubei, China通讯作者:
第一联系人:
收稿日期:2018-07-28接受日期:2018-12-24网络出版日期:2019-01-03
| 基金资助: |
Received:2018-07-28Accepted:2018-12-24Online:2019-01-03
| Fund supported: |

摘要
关键词:
Abstract
Keywords:
PDF (3015KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
张宇婷, 鲁少平, 金诚, 郭亮. 甘蓝型油菜皖油20号种子不同部位油脂合成的转录调控分析[J]. 作物学报, 2019, 45(3): 381-389. doi:10.3724/SP.J.1006.2019.84105
ZHANG Yu-Ting, LU Shao-Ping, JIN Cheng, GUO Liang.
油菜是世界第三大油料作物, 也是我国第一大油料作物, 菜籽油是我国最主要的食用植物油来源之一[1]。油料作物的含油量是一项重要的生产指标, 是油料作物产量的重要因素之一。油菜种子含油量每提高1个百分点, 单位面积油菜的产油量就能提高2.3~2.5个百分点[2]。近年来, 我国油菜单产已达到世界平均水平, 但油菜种子中的油脂含量偏低, 导致国产菜籽油在国际市场缺乏竞争力且受到进口植物油的冲击。因此, 提高油菜籽含油量对我国油菜产业发展极其重要[3,4,5]。油菜种子中各类脂肪酸的组成影响菜籽油的品质及营养价值。脂肪酸根据结构分为饱和脂肪酸和不饱和脂肪酸, 其中饱和脂肪酸(C16:0和C18:0等)熔点较高, 人体不易消化吸收, 易凝固在血管壁上。不饱和脂肪酸(C18:1、C18:2、C18:3等)熔点较低, 易被人体消化和吸收, 不易凝固或沉淀在血管壁上[6]。我国市场上销售的食用植物油有很多种, 其中菜籽油的饱和脂肪酸的含量是所有植物油中最低的, 其油酸、亚油酸和亚麻酸含量比例合理, 是最健康的食用植物油之一[7]。
植物细胞中, 质体和内质网是脂肪酸合成的主要场所。光合作用产生的糖类在细胞质中经糖酵解转化为丙酮酸进入质体, 丙酮酸在丙酮酸脱氢酶的作用下生成乙酰辅酶A, 此过程为脂肪酸从头合成的第一步[8]。乙酰辅酶A再经过一系列的缩合、还原及脱水反应生成丁酰-酰基转运蛋白, 完成脂肪酸链的第一次延伸[9]。接着, 酰基转运蛋白继续与乙酰辅酶A反应并重复上述过程, 每反应一次碳链增加2个碳原子, 直至碳链上碳原子数为16时停止延伸[10]。此时, 合成的16:0-ACP在酮脂酰基ACP合酶(KASII)和硬脂酰ACP脱氢酶(SAD)的作用下生成18:1-ACP。然后, 在酰基-ACP硫脂酶(FATA和FATB)催化下, 脂肪酸从脂酰ACP脱离并在长链脂酰-CoA合成酶(LACS)作用下合成酰基CoA[11]。酰基CoA被转运到内质网或胞质中的酰基CoA池中, 一部分酰基CoA经3-磷酸甘油酰基转移酶(GPAT)、溶血性磷脂酸酰基转移酶(LPAAT)、磷脂酸磷酸酶(PAP)和二酰甘油转酰酶(DGAT)途径最后合成三酰基甘油(TAG), 另一部分酰基CoA经溶血卵磷脂酰基转移酶(LPCAT)作用将脂肪酸转移到卵磷脂(PC)上, 经过脂肪酸脱氢酶FAD2和FAD3对脂肪酸链修饰产生带有多不饱和脂肪酸酸链的PC[12]。此时, PC在磷脂二酯酰甘油酰基转移酶(PDAT)的催化下与二酰基甘油(DAG)反应生成TAG, 或者在磷脂酰胆碱: 二酰甘油磷酸胆碱转移酶(PDCT)和磷酸胆碱转移酶(CPT)的作用下生成DAG, 然后再合成TAG[13]。近年来研究表明, TAG和其他脂类分子在棉花、亚麻芥、油菜和拟南芥等种子中的含量是不均一的, 暗示脂质代谢途径在不同种子中存在着差异[14,15,16,17]。推测转录水平或酶活水平的调控是造成种子不同部位中脂质代谢差异的原因, 然而具体的机制并不明确[18]。
随着测序技术的发展, 转录组测序已广泛应用于生命科学的各个领域[19,20]。RNA测序(RNA-seq)能揭示在特定时间内或部位中RNA的存在和数量, 是一种高通量的分析基因表达的手段[21,22,23]。Manuel等[24]基于深度表达序列标记测序对4种油料作物种子的4个发展阶段的基因表达进行转录组分析, 揭示了参与合成油脂的基因具有保守性和物种特异性。Ste?phane等[25]对油棕果实和种子的3个不同部位的转录组进行了比较, 发现EgWRI1-1和EgWRI1-2转录因子在果皮和胚乳的油脂积累过程中被大量转录。Lu等[26]比较高、低含油量油菜种子不同部位中脂质代谢物与油脂合成相关基因表达, 结果表明种子不同部位的脂质含量存在明显差异, 这些差异主要由种子不同部位中参与油脂合成相关基因表达量的不同造成。但该研究没有对造成种子不同部位脂质含量差异的相关差异表达基因进行深入挖掘和分析。本研究从转录组水平分析并鉴定调控油菜种子不同部位含油量和脂肪酸组成的代谢网络和关键基因, 从转录水平解析油菜种子不同部位中油脂合成的调控机制。
1 材料与方法
1.1 材料及处理方法
供试材料为甘蓝型低芥酸油菜皖油20 (WY20)种子。显微镜下分离WY20发育34 d种子的内子叶(IC)、外子叶(OC)、胚轴(EA)和种皮(SC), 设置5个生物学重复。参考Lu等[26]的方法用脂肪酸甲酯化和气质联用仪(GC-MS)分析种子各部位的油脂含量和脂肪酸组成。提取IC、OC和EA总RNA进行转录组测序, 设每组3个生物学重复。1.2 文库构建与测序
使用植物RNA提取试剂盒(DP432, http://www. tiangen.com/)提取种子各个部位的总RNA, 将提取的RNA样品送GenoSeq公司(http://www.genoseq. cn/), 用Illumina Hiseq进行转录组测序, 每个样品6G数据量。1.3 转录组数据分析
用fastp软件去除接头序列和低质量读数, 测序数据质量控制和过滤后, 通过hisat2软件将9组转录组数据比对到油菜参考基因组(http://www.genoscope. cns.fr/brassicanapus/)。利用featureCounts软件定量得到TPM值(transcripts per kilobase of exon model per million mapped reads)来计算基因的表达量[27,28]。再用DESeq2软件包进行差异表达基因的筛选, 筛选条件为P-value <0.01, |log2 fold change|> 1, 并对所筛选出的差异表达基因进行GO富集分析[29,30]。1.4 候选基因的鉴定及筛选
利用blastp工具将油菜蛋白序列与数据库中拟南芥蛋白序列对比, 设置E-value为1e-5, Coverage> 50%, 找到油菜在拟南芥中对应的同源基因及基因ID。利用拟南芥约700个与脂质代谢相关的基因和甘蓝型油菜全基因组测序得到的1000余个与油脂相关的基因作为参考, 进行油菜基因同源性分析从而构建本研究中油菜油脂合成基因数据库。将差异表达分析的结果与数据库相结合, 筛选出与油脂合成相关的差异表达基因, 进一步对油脂合成相关的差异表达基因进行功能注释和表达数据的分析。最后将与油脂合成相关的差异表达基因比对到油脂合成的代谢途径中, 并在代谢途径中标记出差异基因在IC、OC和EA两两之间表达量差异的倍数。2 结果与分析
2.1 WY20种子不同部位脂肪酸组成和含油量分析
WY20种子切片在显微镜下可以明显地看到OC、IC、EA和SC四个不同部位(图1-A)。脂肪酸分析结果表明, 不同部位之间含油量存在显著差异, OC的含油量最高, SC的含油量最低(图1-B)。比较种子不同部位所占种子重量的百分比, 含油量最高的OC的比例远大于其他3个部位, 含油量最少的SC比例高于EA (图1-C)。WY20种子不同部位的脂肪酸组成存在显著差异, 其中C18脂肪酸在整颗种子中的脂肪酸含量比较高(图1-D, E)。C16:0、C18:2和C20:0在胚轴中的含量均显著高于子叶, 特别是C16:0在EA中的含量约为子叶的2倍。C18:1和C20:1在子叶中的含量则均显著高于EA, C18:0在OC中含量显著低于IC和EA, 而在IC和EA中无差别。C18:3则在OC中含量最高, 在IC和EA中无差异(图1-D)。图1
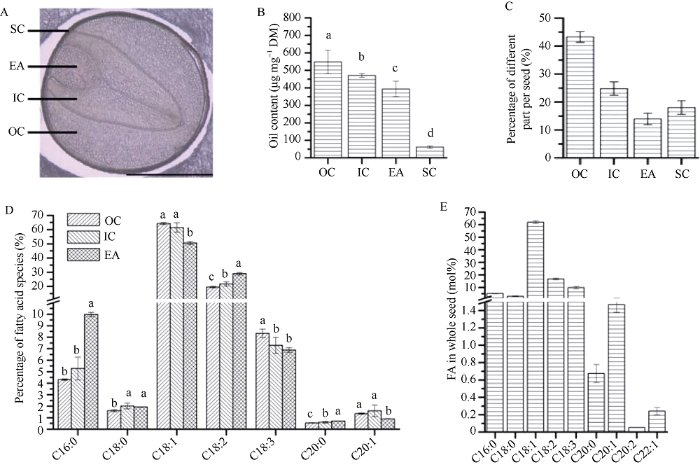 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1甘蓝型油菜WY20种子不同部位含油量和脂肪酸组成
A: 显微镜明视野下WY20种子部位; B: 种子不同部位含油量; C: 种子不同部位重量百分比; D: 种子不同部位脂肪酸组成; E: 整颗种子脂肪酸组成。
Fig. 1Oil content and fatty acid composition in different parts of WY20 seed
A: bright-field image in different parts of WY20 seed under microscope; B: oil content in different parts of seed; C: weight percentage in different parts of seed; D: fatty acid composition in different parts of seed; E: whole seed fatty acid composition.
2.2 转录组质量控制及reads的比对
数据统计约有3.08亿条原始数据, 平均每个部位有1.02亿条。经质量控制和过滤后, 得到质量较好的读段(reads), reads和全基因组比对有93.87%被映射到油菜参考基因组。总读数中约有6.13%的reads可能由于筛选参数设定较严格、测序组装错误或者参考基因组不完整而匹配不上。2.3 基因差异表达分析
调查3个部位中基因表达量的分布并对样品3个生物学重复之间的相关性进行检测和主成分分析(PCA)(图2-A)表明, IC和OC三个生物学重复之间的皮尔森系数均高于0.98, EA三个生物学重复之间的皮尔森系数高于0.92, 但生物学重复之间差异均不显著, 证明3个生物学重复的重复性较好。对3个部位的基因表达量进行两两间的差异分析表明, IC和OC间比较得到了525个差异基因, 其中有233个基因表达上调, 292个表达下调。IC和EA间比较得到了5436个差异基因, 表达上调的有2520个, 下调的有2916个。OC和EA间比较得到了5749个差异基因, 表达上调的有2535个, 下调的有3214个(图2-B)。3个组合去重复后的差异表达基因有7192个, 有116个基因在EA与IC, EA与OC, IC与OC 3个比较组合中均有表达上的差异(图2-C)。此结果也表明内子叶和外子叶间差异基因较少, 暗示着OC和IC的基因表达模式比较一致。图2
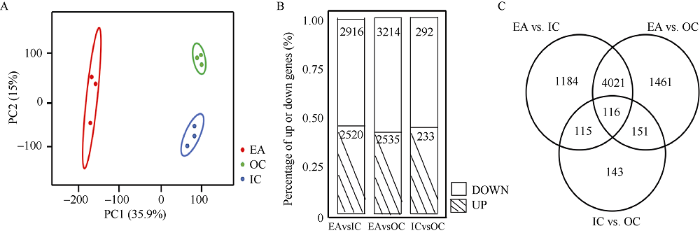 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图23个种子部位差异表达基因分布情况
A: 样本间PCA分析; B: IC、OC和EA差异表达基因数目及百分比; C:差异表达基因维恩图。
Fig. 2Distribution of differentially expressed genes in three parts of seed
A: PCA analysis of different samples; B: differentially expressed genes in IC, OC, and EA; C: Venn diagram of differentially expressed genes.
2.4 GO富集分析
GO富集分析发现, OC与EA和IC与EA之间的差异基因主要富集在光合作用、一元羧酸生物合成过程和脂肪酸生物合成过程。子叶与EA间存在较多参与脂质合成相关的差异表达基因, 这些基因将成为我们后续分析的重点。而OC与IC中的差异表达基因很少, 且主要富集在胞外区和核仁等与油脂合成不直接相关的生物学过程。我们列出了每组比较差异表达基因GO富集到的前10个代谢通路(图3)。图3
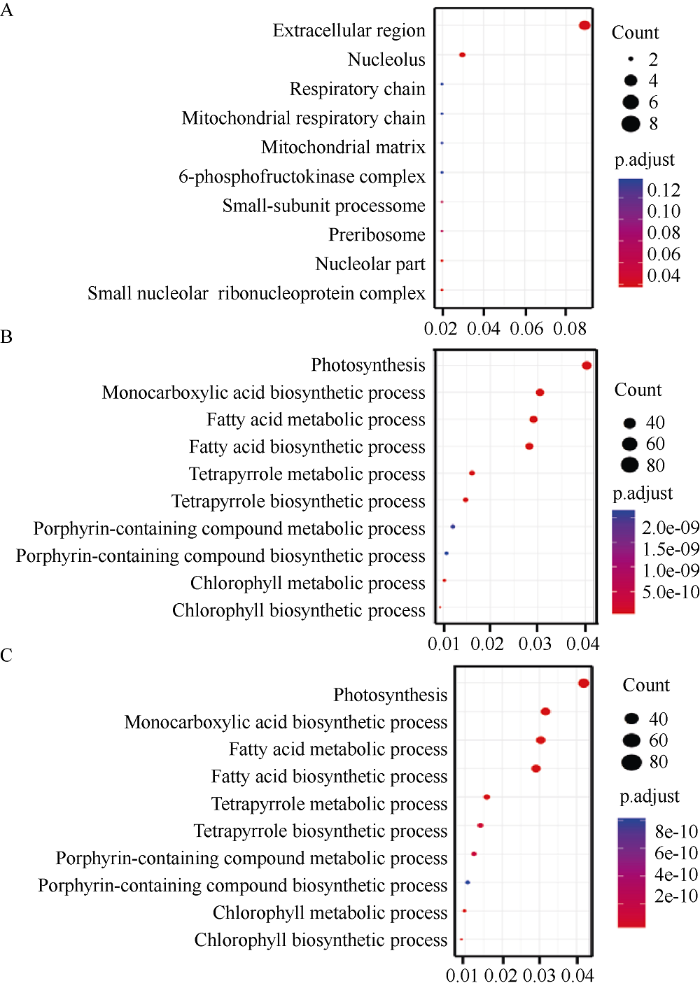 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3油菜胚的3个部位差异表达基因GO功能分布
A: IC和OC差异表达基因功能分布; B: IC和EA差异表达基因功能分布; C: OC和EA差异表达基因功能分布。
Fig. 3GO analysis of differentially expressed genes in three different parts of Brassica napus embryo
A: GO analysis of differentially expressed genes between IC and OC; B: GO analysis of differentially expressed genes between IC and EA; C: GO analysis of differentially expressed genes between OC and OC.
2.5 候选基因的鉴定与筛选
在油菜种子不同部位之间筛选的7192个差异表达基因中, IC与OC之间的差异表达基因中有18个油脂合成相关基因, IC与EA之间的差异表达基因中有268个油脂合成相关基因, OC与EA之间的差异表达基因中有286个油脂合成相关基因。去除相互之间重复基因, 一共筛选出355个和油脂合成相关的差异基因。为了确保差异基因在种子中有表达, 去除这些基因的表达量(TPM值)在3个部位中均小于1的基因, 最终剩余336个基因。再将336个基因匹配到油菜种子油脂合成途径中, 共得到53个可以匹配到代谢通路上的基因, 我们将同一基因的不同拷贝算做1类基因。IC与OC间得到了4类基因, IC与EA间得到了49类基因, OC与EA间得到了46类基因。其中BnaA02g11570D、BnaA03g23490D、BnaA03g13590D和BnaC03g27860D为3种组合对比中共有的差异基因。而IC与EA、OC与EA两种对比组合间共有匹配到通路上的和油脂合成相关的差异表达基因有38个, IC与EA间特有7个, OC与EA间特有4个。我们将得到的53个基因根据不同对比组合进行了注释和分析(图4和表1)。油脂合成途径中IC和OC中的基因表达量都普遍高于EA, 而IC和OC之间的基因表达水平差别不显著(图4)。Table 1
表1
表1甘蓝型油菜皖油20种子不同部位油脂合成差异表达基因
Table 1
| 基因名 Gene ID | 注释描述 Annotation description | 蛋白家族缩写 Protein family abbreviations | 分组 Group | |
|---|---|---|---|---|
| BnaA03g13590D | Fatty acid desaturase 3 | FAD3 | EA-IC-OC | |
| BnaC03g16520D | Fatty acid desaturase 3 | FAD3 | EA-IC/EA-OC | |
| BnaC04g14820D | Fatty acid desaturase 3 | FAD3 | EA-IC/EA-OC | |
| BnaC04g40760D | Fatty acid desaturase 3 | FAD3 | EA-IC/EA-OC | |
| BnaA04g17150D | Fatty acid desaturase 3 | FAD3 | EA-IC/EA-OC | |
| BnaAnng09250D | Fatty acid desaturase 2 | FAD2 | EA-IC/EA-OC | |
| BnaA02g11570D | Hydroxysteroid dehydrogenase 1 | OBO | EA-IC-OC | |
| BnaA03g23490D | Hydroxysteroid dehydrogenase 1 | OBO | EA-IC-OC | |
| BnaC03g27860D | Hydroxysteroid dehydrogenase 1 | OBO | EA-IC-OC | |
| BnaCnng57830D | Hydroxysteroid dehydrogenase 1 | OBO | EA-IC | |
| BnaA09g02110D | Oleosin | OBO | EA-OC | |
| BnaA03g20420D | Stearoyl-acyl-carrier-protein desaturase protein | SAD | EA-IC/EA-OC | |
| BnaA01g32860D | Stearoyl-acyl-carrier-protein desaturase protein | SAD | EA-IC/EA-OC | |
| BnaC03g24420D | Stearoyl-acyl-carrier-protein desaturase protein | SAD | EA-IC/EA-OC | |
| BnaC09g41580D | Stearoyl-acyl-carrier-protein desaturase protein | SAD | EA-IC/EA-OC | |
| BnaA10g18080D | Stearoyl-acyl-carrier-protein desaturase protein | SAD | EA-IC/EA-OC | |
| BnaA05g03490D | Stearoyl-acyl-carrier-protein desaturase | SAD | EA-OC | |
| BnaC04g03030D | Stearoyl-acyl-carrier-protein desaturase | SAD | EA-OC | |
| BnaC09g19280D | 3-ketoacyl-acyl carrier protein synthase I | KASI | EA-IC/EA-OC | |
| BnaA02g24400D | 3-ketoacyl-acyl carrier protein synthase I | KASI | EA-IC/EA-OC | |
| BnaA06g36060D | 3-ketoacyl-acyl carrier protein synthase I | KASI | EA-IC/EA-OC | |
| BnaC06g35760D | 3-ketoacyl-acyl carrier protein synthase II | KAS II | EA-IC/EA-OC | |
| BnaA07g31890D | 3-ketoacyl-acyl carrier protein synthase II | KAS II | EA-IC/EA-OC | |
| BnaA07g21940D | 3-ketoacyl-acyl carrier protein synthase II | KAS II | EA-IC/EA-OC | |
| BnaC06g22680D | 3-ketoacyl-acyl carrier protein synthase II | KAS II | EA-IC | |
| BnaA04g07120D | Acyl-ACP thioesterase | FATA | EA-IC/EA-OC | |
| BnaCnng41490D | Acyl-ACP thioesterase | FATA | EA-IC/EA-OC | |
| BnaCnng00070D | FATA acyl-ACP thioesterase FATA | FATA | EA-IC/EA-OC | |
| BnaA07g05070D | FATA acyl-ACP thioesterase FATA | FATA | EA-IC/EA-OC | |
| BnaC03g75820D | Ketoacyl-ACP Reductase | KAR | EA-IC/EA-OC | |
| BnaA02g13310D | Beta-ketoacyl reductase | KAR | EA-IC/EA-OC | |
| BnaA07g26670D | Beta-ketoacyl reductase | KAR | EA-IC/EA-OC | |
| BnaC06g28830D | Beta-ketoacyl reductase | KAR | EA-IC/EA-OC | |
| BnaC09g16320D | Acyl carrier protein | ACP | EA-IC/EA-OC | |
| BnaC09g03000D | Acyl carrier protein | ACP | EA-IC/EA-OC | |
| BnaA09g03610D | Acyl carrier protein | ACP | EA-IC/EA-OC | |
| BnaAnng23710D | Acyl carrier protein | ACP | EA-OC | |
| BnaC03g45040D | Enoyl-ACP Reductase | ENR | EA-IC/EA-OC | |
| BnaC07g04330D | Enoyl-ACP Reductase | ENR | EA-IC/EA-OC | |
| BnaA03g38220D | Enoyl-ACP Reductase | ENR | EA-IC/EA-OC | |
| BnaAnng02240D | E2 component of pyruvate dehydrogenase complex | DHLAT | EA-IC/EA-OC | |
| BnaC06g08280D | E2 component of pyruvate dehydrogenase complex | DHLAT | EA-IC/EA-OC | |
| BnaC07g23030D | E2 component of pyruvate dehydrogenase complex | DHLAT | EA-IC | |
| BnaA06g33300D | E2 component of pyruvate dehydrogenase complex | DHLAT | EA-IC | |
| BnaAnng22560D | Chloroplasticacetyl coenzyme A carboxylase | BCCP | EA-IC/EA-OC | |
| BnaC09g42420D | Biotin carboxyl carrier protein 2 | BCCP | EA-IC/EA-OC | |
| BnaA03g02830D | Thioesterase superfamily protein | HAD | EA-IC/EA-OC | |
| BnaA02g00390D | Thioesterase superfamily protein | HAD | EA-IC | |
| BnaA07g20920D | Long chain acyl-CoA synthetase 9 | LACS9 | EA-IC/EA-OC | |
| BnaC06g20910D | Long chain acyl-CoA synthetase 9 | LACS9 | EA-IC | |
| BnaA01g17630D | E3 component of pyruvate dehydrogenase complex | LPD | EA-IC/EA-OC | |
| BnaCnng75250D | Acetyl-CoA carboxylase | ACCase | EA-IC | |
| BnaA05g12180D | Acyl-carrier-protein | MCMT | EA-IC/EA-OC | |
新窗口打开|下载CSV
图4
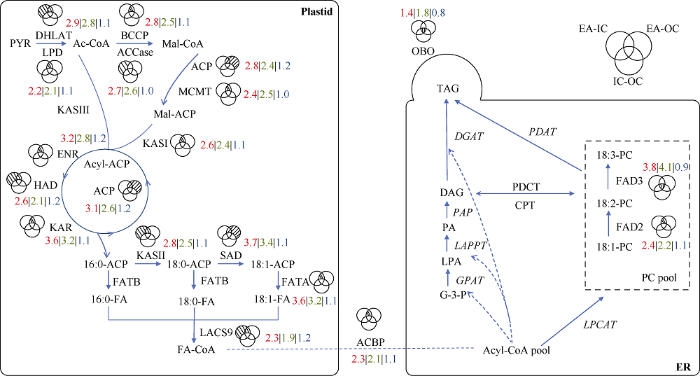 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4油脂合成相关差异表达基因在油脂代谢通路中的比较分析
维恩图中阴影代表差异表达基因的分布。红色数字表示蛋白家族对应基因TPM值的平均值在IC和EA间的比值, 绿色数字表示蛋白家族对应基因TPM值的平均值在OC和EA间的比值, 蓝色数字表示蛋白家族对应基因TPM值的平均值在IC和OC间的比值。编码蛋白质的基因: DHLAT: 二氢硫辛酰胺乙酰转移酶; LPD: 二氢硫辛酰胺脱氢酶; BCCP: 生物素羧基载体蛋白; ACCase: 乙酰辅酶a羧化酶; MCMT: 酰基载体蛋白; KASI: 3-酮酰基-酰基载体蛋白合酶I; KASII: 3-酮酰基-酰基载体蛋白合酶II; KASIII: 3-酮酰基-酰基载体蛋白合酶III; KAR: 酮脂酰还原酶; HAD: 硫酯酶蛋白; ENR: 烯酰ACP还原酶; ACP: 酰基载体蛋白; SAD: 硬脂酰-酰基载体蛋白脱饱和酶蛋白; FATA: FATA硫酯酶; FATB: FATB硫酯酶; LACS9: 长链酰基辅酶9; FAD2: 脂肪酸去饱和酶2; FAD3: 脂肪酸去饱和酶3; CPT: CDP胆碱-甘油二酯胆碱酯酶; PDCT: 磷脂酰胆碱-甘油二酯胆碱酯酶; GPAT: 磷酸甘油脂酰转移酶; PDAT: 磷脂-二酰甘油酰基转移酶; LPAAT: 溶血磷脂酸酰基转移酶; PAP: 磷脂酸磷酸酶; DGAT: 二酰甘油酰基转移酶; OBO: 油体蛋白; LTP: 脂质转运蛋白; ER: 内质网。
Fig. 4Analysis of differentially expressed oil biosynthesis-related genes in lipid metabolism pathway
The shadow in venn diagram represents where the differentially expressed genes are located. Number in red indicates the ratio of genes’ average TPM of between IC and EA, number in green indicates the ratio of genes’ average TPM of between OC and EA, number in blue indicates the ratio of genes’ average TPM of between IC and OC.Abbreviation of genes that encode proteins: DHLAT: dihydrolipoamide acetyltransferase; LPD: dihydrolipoamide dehydrogenase; BCCP: biotin carboxyl carrier protein; ACCase: acetyl-CoA carboxylase; MCMT: malonyl-CoA: ACP malonyltransferase; KASI: 3-ketoacyl-acyl carrier protein synthase I; KASII: 3-ketoacyl-acyl carrier protein synthase II; KASIII: 3-ketoacyl-acyl carrier protein synthase III; KAR: ketoacyl-ACP reductase; HAD: hydroxyacyl-ACP dehydrase; ENR: enoyl-ACP reductase; ACP: acyl carrier protein; SAD: stearoyl-acyl carrier protein desaturase; FATA: acyl-ACP thioesterase A; FATB: acyl-ACP thioesterase B; LACS: long-chain acyl-CoA synthetase; FAD2: FA desaturase 2; FAD3: FA desaturase 3; CPT: CDP-choline: diacylglycerol cholinephosphotransferase; PDCT: phosphatidylcholine:diacylglycerol cholinephosphotransferase; GPAT: glycerol-3-phosphate acyltransferase; PDAT: phospholipid:diacylglycerol acyltransferase; LPAAT: lysophosphatidic acid acyltransferase; PAP: phosphatidic acid phosphatase; DGAT: diacylglycerol acyltransferase; OBO: oil body oleosin; LTP: lipid transfer protein; ER: endoplasmic reticulum.
3 讨论
植物种子中的油脂合成过程是一个复杂的网络, 发生在细胞的多个细胞器中, 受多种酶、代谢物转运、转录因子和能量代谢等影响[31]。近年来研究表明, 脂质代谢途径在油料植物种子胚的不同部位存在着差异[14,15,16,17]。本研究对低芥酸油菜WY20种子不同部位的分析结果表明, 含油量在IC、OC和EA中存在着显著差异, OC含油量最高, IC含油量次之, EA含油量最低。C16:0和C18:2在EA中的比例显著高于IC和OC, 而C18:3在EA中比例最低(图1-B, C)。本研究结果与前人利用不同的油菜材料研究结果基本一致[26], 表明油菜种子不同部位中油脂合成和脂肪酸脱饱和过程受到不同的调控。Borisjuk等[32]研究表明油菜种子不同部位能量代谢有明显差异, 这可能与油菜种子结构有关, 不同种子部位获得的空间和光不同, 导致光合作用等存在着差别。Lu等[26]对ZS11和WH5557两个油菜材料的种子不同部位进行分析表明, IC、OC和EA中脂质代谢物含量存在显著差异, IC、OC和EA中糖酵解途径和油脂合成途径的基因表达水平普遍存在着显著差异。并进一步鉴定了编码LPAAT、PAP、DGAT、Oleosin等调控种子不同部位脂质含量差异的关键基因。本研究在前期工作基础上, 对低芥酸油菜WY20种子不同部位进行转录组分析, 进一步从转录水平解析油菜种子不同部位含油量和脂肪酸差异的调控机制。WY20种子3个部位转录组分析结果显示, EA与IC和OC之间的差异表达基因主要富集在光合作用、一元羧酸合成和脂肪酸合成与代谢过程, 这些差异表达基因可能是造成不同部位含油量和脂肪酸差异的主要因素(图3-B, C)。IC和OC之间差异表达基因仅有18个基因与脂质代谢相关, 而EA与IC和OC之间与脂质代谢相关的差异表达基因分别为268个和286个, 与EA含油量和脂肪酸组成与IC和OC差异比较一致(图1-B, D)。对油脂合成途径进行深入分析显示, IC和OC中与油脂合成相关的基因的表达量普遍是EA的2~3倍, 例如ACCase在IC和OC中的表达量分别是EA中的2.8倍和2.5倍(图4)。这些差异表达基因主要集中在质体中脂肪酸合成途径, 因此我们推断这些关键基因在转录水平的调控是造成EA含油量比IC和OC低的主要原因(图4)。
脂肪酸组成主要由脂肪酸脱饱和酶和脂肪酸延长酶调控[33]。植物质体中由SAD催化合成18:1-ACP, 而其余不饱和脂肪酸合成的反应主要发生在内质网中。从质体转运出来的18:1-CoA被合成PC, 接着在油酸去饱和酶(FAD2)和亚油酸去饱和酶(FAD3)作用下分别生成18:2-PC和18:3-PC。本研究结果表明FAD2在IC和OC中表达量分别为EA的2.4倍和2.2倍, 与EA中C18:1比例最低而C18:2比例最高结果一致。FAD3在IC和OC中表达量分别为EA的3.8倍和4.1倍, 与EA中C18:3比例最低结果一致。而EA中C16:0的含量约为IC和OC的2倍, 从代谢途径看, KASII、SAD和FATA在IC和OC中的表达量均显著高于EA (2.5~3.7倍), 因此C16:0在IC和OC中被更高效地转化为C18:1, 这与IC和OC中C16:0比例比EA低一致(图1-D和图4)。说明FAD2、FAD3、KASII、SAD和FATA等关键基因在WY20种子的表达有部位特异性, 这些基因的表达量决定了种子不同部位中脂肪酸的组成。
4 结论
WY20油菜种胚不同部位的含油量和脂肪酸组成存在着差异, 尤其是EA的含油量最低并且脂肪酸组成与子叶存在较大差异。EA与子叶之间参与油脂合成的差异表达基因较多。EA中参与质体中脂肪酸合成的基因表达量普遍低于子叶, 造成EA含油量比子叶低。EA中FAD2、FAD3、KASII、SAD和FATA等关键基因表达量显著低于子叶, 造成EA中C16:0和C18:2高于子叶。转录调控是WY20油菜种子不同部位油脂合成差异的主要机制, 该研究对于理解油菜种子不同部位含油量和脂肪酸组成差异具有一定的科学意义, 对油菜和其他油料作物高含油量育种和品质改良具有重要的指导意义。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.3969/j.issn.1008-0864.2011.01.01URL [本文引用: 1]

通过比较大宗食用植物油的品质特性,以及分析我国食用植物油的消费与供给、油菜的生产现状及存在问题与发展潜力,明确了我国油菜生产在我国植物油供给中的重要地位,发展油菜生产是保障我国食用油供给的根本途径。并由此提出了发展油菜生产的主要措施:加强油菜相关研究,着实解决我国油菜生产上存在的问题;规范种业市场,促进我国油菜种业健康发展;加强油菜籽的加工研究与利用,提高附加值。
DOI:10.3969/j.issn.1008-0864.2011.01.01URL [本文引用: 1]

通过比较大宗食用植物油的品质特性,以及分析我国食用植物油的消费与供给、油菜的生产现状及存在问题与发展潜力,明确了我国油菜生产在我国植物油供给中的重要地位,发展油菜生产是保障我国食用油供给的根本途径。并由此提出了发展油菜生产的主要措施:加强油菜相关研究,着实解决我国油菜生产上存在的问题;规范种业市场,促进我国油菜种业健康发展;加强油菜籽的加工研究与利用,提高附加值。
DOI:10.3969/j.issn.1671-9646-B.2008.08.016URL [本文引用: 1]

在综述前人对中国油菜产业国际竞争力研究成果的基础上,结合人世 以来国内外油菜产业的发展情况,具体分析了中国油菜产业的竞争优势与劣势.研究表明,中同油菜产业具有气候、品种,劳动力资源丰富,以及国内市场需求空间 大的优势,然而在产品质量与流通加工能力上,其竞争劣势显著.
DOI:10.3969/j.issn.1671-9646-B.2008.08.016URL [本文引用: 1]

在综述前人对中国油菜产业国际竞争力研究成果的基础上,结合人世 以来国内外油菜产业的发展情况,具体分析了中国油菜产业的竞争优势与劣势.研究表明,中同油菜产业具有气候、品种,劳动力资源丰富,以及国内市场需求空间 大的优势,然而在产品质量与流通加工能力上,其竞争劣势显著.
DOI:10.7606/j.issn.1004-1389.2011.12.016URL [本文引用: 1]

This article summarized the development of high oil content breeding of rapeseed in the world. It mainly introduced the progress of high oil content breeding on using the different breeding technology, such as single cross, multiple cross, ecological breeding, yellow seeded breeding, DH breeding and heterosis breeding with high oil content germplasm, and so on. The achievements in the past years showed that the oil content of rapeseed germplasm have been improved from 40% to 60%, even to 61.7%, that of the hybrids have been raised to 50% from 40%. At the same time, the authors put forward the new hybrid breeding mode igh oil germplasm + Chemical induced male sterility on the heterosis utilization for high oil content. Compared with CMS and GMS, the new technology has many advantages, such as, more efficiency and safety, more easily to get high oil content cross combinations using high oil content gemplasms and significantly improve the oil production of unit area, and so on. Therefore, this breeding mode promoted the innovation of rapeseed breeding technology. For the potency of oil content in rapeseed, authors also discussed. Based on the analysis of cell physiology and component as well as sunflower breeding experience, the author put forward 70% as the maximal oil content, and this index would possibly become the new target in the future rapeseed breeding.
DOI:10.7606/j.issn.1004-1389.2011.12.016URL [本文引用: 1]

This article summarized the development of high oil content breeding of rapeseed in the world. It mainly introduced the progress of high oil content breeding on using the different breeding technology, such as single cross, multiple cross, ecological breeding, yellow seeded breeding, DH breeding and heterosis breeding with high oil content germplasm, and so on. The achievements in the past years showed that the oil content of rapeseed germplasm have been improved from 40% to 60%, even to 61.7%, that of the hybrids have been raised to 50% from 40%. At the same time, the authors put forward the new hybrid breeding mode igh oil germplasm + Chemical induced male sterility on the heterosis utilization for high oil content. Compared with CMS and GMS, the new technology has many advantages, such as, more efficiency and safety, more easily to get high oil content cross combinations using high oil content gemplasms and significantly improve the oil production of unit area, and so on. Therefore, this breeding mode promoted the innovation of rapeseed breeding technology. For the potency of oil content in rapeseed, authors also discussed. Based on the analysis of cell physiology and component as well as sunflower breeding experience, the author put forward 70% as the maximal oil content, and this index would possibly become the new target in the future rapeseed breeding.
DOI:10.13856/j.cn11-1097/s.2015.04.022URL [本文引用: 1]

油菜是中国大宗油料作物,对于中国食用油供给安全具有重要意义。近年来,中国食用油市场份额被进口油籽及植物油大量挤占,菜油消费不旺,产业发展严重受阻。而低价油菜籽及菜油进口的快速增加,对中国油菜产业冲击进一步加大,临时收储政策也陷入政策失灵和财政难以负担的窘境。中国油菜产业本身竞争力较弱,在进口冲击下,可持续发展面临严峻挑战,必须采取有力措施维护其产业安全。
DOI:10.13856/j.cn11-1097/s.2015.04.022URL [本文引用: 1]

油菜是中国大宗油料作物,对于中国食用油供给安全具有重要意义。近年来,中国食用油市场份额被进口油籽及植物油大量挤占,菜油消费不旺,产业发展严重受阻。而低价油菜籽及菜油进口的快速增加,对中国油菜产业冲击进一步加大,临时收储政策也陷入政策失灵和财政难以负担的窘境。中国油菜产业本身竞争力较弱,在进口冲击下,可持续发展面临严峻挑战,必须采取有力措施维护其产业安全。
DOI:10.7505/j.issn.1007-9084.2014.03.020URL [本文引用: 1]

在当前我国食用植物油对外依存度居高不下的严峻形势下,本文通过对世界及我国油料产需及贸易形势进行分析,明确了当前我国油料产业发展存在的主要问题,针对存在的问题从政策、技术等方面提出了相应的对策建议。
DOI:10.7505/j.issn.1007-9084.2014.03.020URL [本文引用: 1]

在当前我国食用植物油对外依存度居高不下的严峻形势下,本文通过对世界及我国油料产需及贸易形势进行分析,明确了当前我国油料产业发展存在的主要问题,针对存在的问题从政策、技术等方面提出了相应的对策建议。
DOI:10.3969/j.issn.1003-0174.2014.06.023URL [本文引用: 1]

从脂肪酸组成、植物甾醇、微量元素与维生素E等几个植物油营养品质方面比较了菜籽油与其他食用植物油的营养价值,结果显示,菜籽油中的饱和脂肪酸含量最低,且油酸含量较高,亚油酸、亚麻酸含量合理;胆固醇含量较低,对身体有益的菜籽甾醇、菜油甾醇和豆甾醇含量比其他食用植物油高;主要微量元素如铁、铜、锌等和维生素E含量较高且维生素E功能性成分比例适当。另外,菜籽油含有特有的天然抗氧化剂菜籽多酚;具有降低血液胆固醇及其有害组分且增加有益组分含量、降低血液纤维蛋白原的作用。分析了菜籽油存在的不足,提出了"四高三低二适中"的油菜品质改良目标及实现方法。
DOI:10.3969/j.issn.1003-0174.2014.06.023URL [本文引用: 1]

从脂肪酸组成、植物甾醇、微量元素与维生素E等几个植物油营养品质方面比较了菜籽油与其他食用植物油的营养价值,结果显示,菜籽油中的饱和脂肪酸含量最低,且油酸含量较高,亚油酸、亚麻酸含量合理;胆固醇含量较低,对身体有益的菜籽甾醇、菜油甾醇和豆甾醇含量比其他食用植物油高;主要微量元素如铁、铜、锌等和维生素E含量较高且维生素E功能性成分比例适当。另外,菜籽油含有特有的天然抗氧化剂菜籽多酚;具有降低血液胆固醇及其有害组分且增加有益组分含量、降低血液纤维蛋白原的作用。分析了菜籽油存在的不足,提出了"四高三低二适中"的油菜品质改良目标及实现方法。
DOI:10.3969/j.issn.1007-4821.2014.06.023URL [本文引用: 1]

正目前主导食油市场的是植物 油。不同的食用植物油,对人的健康影响也不同。这里介绍几种常见的食用植物油对人的健康影响。1.芝麻油:芝麻油的消化吸收率达98%,是食用品质好、营 养价值高的优良食油。芝麻油中含有特别丰富的维生素E和比较丰富的亚油酸,可调节毛细血管的渗透作用,加强人体组织对氧的吸收能力,改善血液循环,促进性 腺发育,延缓衰老。2.茶油:茶油中丰富的不饱和脂肪酸和抗氧化
DOI:10.3969/j.issn.1007-4821.2014.06.023URL [本文引用: 1]

正目前主导食油市场的是植物 油。不同的食用植物油,对人的健康影响也不同。这里介绍几种常见的食用植物油对人的健康影响。1.芝麻油:芝麻油的消化吸收率达98%,是食用品质好、营 养价值高的优良食油。芝麻油中含有特别丰富的维生素E和比较丰富的亚油酸,可调节毛细血管的渗透作用,加强人体组织对氧的吸收能力,改善血液循环,促进性 腺发育,延缓衰老。2.茶油:茶油中丰富的不饱和脂肪酸和抗氧化
DOI:10.1104/pp.106.084079URLPMID:1533943 [本文引用: 1]

Triacylglycerols (TAGs) are the most important storage form of energy for eukaryotic cells. TAG biosynthetic activity was identified in the cytosolic fraction of developing peanut (Arachis hypogaea) cotyledons. This activity was NaF insensitive and acyl-coenzyme A (CoA) dependent. Acyl-CoA:diacylglycerol acyltransferase (DGAT) catalyzes the final step in TAG biosynthesis that acylates diacylglycerol to TAG. Soluble DGAT was identified from immature peanuts and purified by conventional column chromatographic procedures. The enzyme has a molecular mass of 41 1.0 kD. Based on the partial peptide sequence, a degenerate probe was used to obtain the full-length cDNA. The isolated gene shared less than 10% identity with the previously identified DGAT1 and 2 families, but has 13% identity with the bacterial bifunctional wax ester/DGAT. To differentiate the unrelated families, we designate the peanut gene as AhDGAT. Expression of peanut cDNA in Escherichia coli resulted in the formation of labeled TAG and wax ester from [ ]acetate. The recombinant E. coli showed high levels of DGAT activity but no wax ester synthase activity. TAGs were localized in transformed cells with Nile blue A and oil red O staining. The recombinant and native DGAT was specific for 1,2-diacylglycerol and did not utilize hexadecanol, glycerol-3-phosphate, monoacylglycerol, lysophosphatidic acid, and lysophosphatidylcholine. Oleoyl-CoA was the preferred acyl donor as compared to palmitoyl- and stearoyl-CoAs. These data suggest that the cytosol is one of the sites for TAG biosynthesis in oilseeds. The identified pathway may present opportunities of bioengineering oil-yielding plants for increased oil production.
DOI:10.1006/mben.2001.0204URLPMID:11800570 [本文引用: 1]

Fatty acids are the most abundant form of reduced carbon chains available from nature and have diverse uses ranging from food to industrial feedstocks. Plants represent a significant renewable source of fatty acids because many species accumulate them in the form of triacylglycerol as major storage components in seeds. With the advent of plant transformation technology, metabolic engineering of oilseed fatty acids has become possible and transgenic plant oils represent some of the first successes in design of modified plant products. Directed gene down-regulation strategies have enabled the specific tailoring of common fatty acids in several oilseed crops. In addition, transfer of novel fatty acid biosynthetic genes from noncommercial plants has allowed the production of novel oil compositions in oilseed crops. These and future endeavors aim to produce seeds higher in oil content as well as new oils that are more stable, are healthier for humans, and can serve as a renewable source of industrial commodities. Large-scale new industrial uses of engineered plant oils are on the horizon but will require a better understanding of factors that limit the accumulation of unusual fatty acid structures in seeds.
DOI:10.1073/pnas.120067297URL [本文引用: 1]

Triacylglycerol (TAG) is known to be synthesized in a reaction that uses acyl-CoA as acyl donor and diacylglycerol (DAG) as acceptor, and which is catalyzed by the enzyme acyl-CoA:diacylglycerol acyltransferase. We have found that some plants and yeast also have an acyl-CoA-independent mechanism for TAG synthesis, which uses phospholipids as acyl donors and DAG as acceptor. This reaction is catalyzed by an enzyme that we call phospholipid:diacylglycerol acyltransferase, or PDAT. PDAT was characterized in microsomal preparations from three different oil seeds: sunflower, castor bean, and Crepis palaestina. We found that the specificity of the enzyme for the acyl group in the phospholipid varies between these species. Thus, C. palaestina PDAT preferentially incorporates vernoloyl groups into TAG, whereas PDAT from castor bean incorporates both ricinoleoyl and vernoloyl groups. We further found that PDAT activity also is present in yeast microsomes. The substrate specificity of this PDAT depends on the head group of the acyl donor, the acyl group transferred, and the acyl chains of the acceptor DAG. The gene encoding the enzyme was identified. The encoded PDAT protein is related to lecithin:cholesterol acyltransferase, which catalyzes the acyl-CoA-independent synthesis of cholesterol esters. However, budding yeast PDAT and its relatives in fission yeast and Arabidopsis form a distinct branch within this protein superfamily, indicating that a separate PDAT enzyme arose at an early point in evolution.
URL [本文引用: 1]

In this review, the basic pathway of fatty acid metabolism in plant seeds was introduced, and the recent developments in genetic manipulation and gene engineering of plant fatty acid metabolism were also summarized in detail. The composition of fatty acid in plant seeds can be changed artificially through genetic manipulation. Meanwhile, this review clarified that making use of gene engineering to manipulate fatty acid metabolism in plant seeds has already created a new research area in resent years, which possesses great potentialities, and would play immense effects in people's life.
URL [本文引用: 1]

In this review, the basic pathway of fatty acid metabolism in plant seeds was introduced, and the recent developments in genetic manipulation and gene engineering of plant fatty acid metabolism were also summarized in detail. The composition of fatty acid in plant seeds can be changed artificially through genetic manipulation. Meanwhile, this review clarified that making use of gene engineering to manipulate fatty acid metabolism in plant seeds has already created a new research area in resent years, which possesses great potentialities, and would play immense effects in people's life.
DOI:10.7685/j.issn.1000-2030.2012.05.009URL [本文引用: 1]

植物油脂是人类营养的重要组分之一,其主要化学成分是三酰甘油,由甘油上的3个羟基与脂肪酸分别通过酯化作用连接而成。目前研究的关注点主要是如何提高种子油脂含量、改善脂肪酸组分。本文综述植物三酰甘油合成、代谢过程中的关键酶及其基因,并介绍三酰甘油含量及其组分调控基因工程的研究进展。
DOI:10.7685/j.issn.1000-2030.2012.05.009URL [本文引用: 1]

植物油脂是人类营养的重要组分之一,其主要化学成分是三酰甘油,由甘油上的3个羟基与脂肪酸分别通过酯化作用连接而成。目前研究的关注点主要是如何提高种子油脂含量、改善脂肪酸组分。本文综述植物三酰甘油合成、代谢过程中的关键酶及其基因,并介绍三酰甘油含量及其组分调控基因工程的研究进展。
DOI:10.1016/j.pbi.2013.02.015URLPMID:23529069 [本文引用: 1]

Oil produced in plant seeds is utilized as a major source of calories for human nutrition, as feedstocks for non-food uses such as soaps and polymers, and can serve as a high-energy biofuel. The biochemical pathways leading to oil (triacylglycerol) synthesis in seeds involve multiple subcellular organelles, requiring extensive lipid trafficking. Phosphatidylcholine plays a central role in these pathways as a substrate for acyl modifications and likely as a carrier for the trafficking of acyl groups between organelles and membrane subdomains. Although much has been clarified regarding the enzymes and pathways responsible for acyl-group flux, there are still major gaps in our understanding. These include the identity of several key enzymes, how flux between alternative pathways is controlled and the specialized cell biology leading to biogenesis of oil bodies that store up to 80% of carbon in seeds.
DOI:10.1105/tpc.111.094581URLPMID:22337917 [本文引用: 2]

Advances in mass spectrometry (MS) have made comprehensive lipidomics analysis of complex tissues relatively commonplace. These compositional analyses, although able to resolve hundreds of molecular species of lipids in single extracts, lose the original cellular context from which these lipids are derived. Recently, high-resolution MS of individual lipid droplets from seed tissues indicated organelle-to-organelle variation in lipid composition, suggesting that heterogeneity of lipid distributions at the cellular level may be prevalent. Here, we employed matrix-assisted laser desorption/ionization-MS imaging (MALDI-MSI) approaches to visualize lipid species directly in seed tissues of upland cotton (Gossypium hirsutum). MS imaging of cryosections of mature cotton embryos revealed a
DOI:10.1111/tpj.12278URLPMID:23808562 [本文引用: 2]

Engineering compositional changes in oilseeds is typically accomplished by introducing new enzymatic step(s) and/or by blocking or enhancing an existing enzymatic step(s) in a seed-specific manner. However, in practice, the amounts of lipid species that accumulate in seeds are often different from what one would predict from enzyme expression levels, and these incongruences may be rooted in an incomplete understanding of the regulation of seed lipid metabolism at the cellular/tissue level. Here we show by mass spectrometry imaging approaches that triacylglycerols and their phospholipid precursors are distributed differently within cotyledons and the hypocotyl/radicle axis in embryos of the oilseed crop Camelina sativa, indicating tissue-specific heterogeneity in triacylglycerol metabolism. Phosphatidylcholines and triacylglycerols enriched in linoleic acid (C18:2) were preferentially localized to the axis tissues, whereas lipid classes enriched in gadoleic acid (C20:1) were preferentially localized to the cotyledons. Manipulation of seed lipid compositions by heterologous over-expression of an acyl cyl carrier protein thioesterase, or by suppression of fatty acid desaturases and elongases, resulted in new overall seed storage lipid compositions with altered patterns of distribution of phospholipid and triacylglycerol in transgenic embryos. Our results reveal previously unknown differences in acyl lipid distribution in Camelina embryos, and suggest that this spatial heterogeneity may or may not be able to be changed effectively in transgenic seeds depending upon the targeted enzyme(s)/pathway(s). Further, these studies point to the importance of resolving the location of metabolites in addition to their quantities within plant tissues.
DOI:10.1016/j.bbalip.2016.11.012URLPMID:27919665 [本文引用: 2]

Arabidopsis thaliana has been widely used as a model plant to study acyl lipid metabolism. Seeds of A. thaliana are quite small (approximately 50002×0230002μm and weigh ~022002μg), making lipid compositional analyses of single seeds difficult to achieve. Here we have used matrix assisted laser desorption/ionization-mass spectrometry imaging (MALDI-MSI) to map and visualize the three-dimensional spatial distributions of two common membrane phospholipid classes, phosphatidylcholine (PC) and phosphatidylinositol (PI), in single A. thaliana seeds. The 3D images revealed distinct differences in distribution of several molecular species of both phospholipids among different seed tissues. Using data from these 3D reconstructions, the PC and PI mol% lipid profiles were calculated for the embryonic axis, cotyledons, and peripheral endosperm, and these data agreed well with overall quantification of these lipids in bulk seed extracts analyzed by conventional electrospray ionization-mass spectrometry (ESI-MS). In addition, MALDI-MSI was used to profile PC and PI molecular species in seeds of wild type, fad2–1 , fad3–2 , fad6–1 , and fae1–1 acyl lipid mutants. The resulting distributions revealed previously unobserved changes in spatial distribution of several lipid molecular species, and were used to suggest new insights into biochemical heterogeneity of seed lipid metabolism. These studies highlight the value of mass spectrometry imaging to provide unprecedented spatial and chemical resolution of metabolites directly in samples even as small as a single A. thaliana seeds, and allow for expanded imaging of plant metabolites to improve our understanding of plant lipid metabolism from a spatial perspective.
DOI:10.1104/pp.16.01705URLPMID:28188274 [本文引用: 2]

The regulation of lipid synthesis in oil seeds is still not fully understood. Oilseed rape is the third most productive vegetable oil crop on the global market. Therefore, increasing our understanding of lipid accumulation in oilseed rape seeds is of great economic, as well as intellectual, importance. Matrix-assisted laser/desorption ionisation - mass spectrometry imaging (MALDI-MSI) is a technique that allows the mapping of metabolites directly onto intact biological tissues, giving a spatial context to metabolism. We have used MADLI-MSI to study the spatial distribution of two major lipid species, triacylglycerols (TAGs) and phosphatidylcholines (PCs). A dramatic, heterogeneous landscape of molecular species was revealed, demonstrating significantly different lipid compositions between the various seed tissues. The embryonic axis was particularly enriched in lipid species containing palmitate, while the seed coat/aleurone layer accumulated vaccenic, linoleic and -linoleic acids. Furthermore, the lipid composition of the inner and outer cotyledons differed to each other, a remarkable discovery given the supposed identical functionality of these two tissues. TAG and PC molecular species distribution was analysed through a developmental time series covering early seed lipid accumulation to the end of lipid accumulation. The spatial patterning of lipid molecular species did not vary much during seed development, although there were exceptions. Data gathered using MALDI-MSI was verified through gas chromatography analysis of dissected seeds. The distinct lipid distribution profiles observed implies differential regulation of lipid metabolism between the different seed tissues. Further understanding of this differential regulation will enhance efforts to improve oilseed rape productivity and quality.
DOI:10.1016/j.copbio.2015.10.004URLPMID:26613199 [本文引用: 1]

Direct visualization of plant tissues by matrix assisted laser desorption ionization-mass spectrometry imaging (MALDI-MSI) has revealed key insights into the localization of metabolitesin situ. Recent efforts have determined the spatial distribution of primary and secondary metabolites in plant tissues and cells. Strategies have been applied in many areas of metabolism including isotope flux analyses, plant interactions, and transcriptional regulation of metabolite accumulation. Technological advances have pushed achievable spatial resolution to subcellular levels and increased instrument sensitivity by several orders of magnitude. It is anticipated that MALDI-MSI and other MSI approaches will bring a new level of understanding to metabolomics as scientists will be encouraged to consider spatial heterogeneity of metabolites in descriptions of metabolic pathway regulation.
DOI:10.1104/pp.113.214874URLPMID:23478895 [本文引用: 1]

Abstract Transcriptome analysis of early-developing maize (Zea mays) seed was conducted using Illumina sequencing. We mapped 11,074,508 and 11,495,788 paired-end reads from endosperm and embryo, respectively, at 9 d after pollination to define gene structure and alternative splicing events as well as transcriptional regulators of gene expression to quantify transcript abundance in both embryo and endosperm. We identified a large number of novel transcribed regions that did not fall within maize annotated regions, and many of the novel transcribed regions were tissue-specifically expressed. We found that 50.7% (8,556 of 16,878) of multiexonic genes were alternatively spliced, and some transcript isoforms were specifically expressed either in endosperm or in embryo. In addition, a total of 46 trans-splicing events, with nine intrachromosomal events and 37 interchromosomal events, were found in our data set. Many metabolic activities were specifically assigned to endosperm and embryo, such as starch biosynthesis in endosperm and lipid biosynthesis in embryo. Finally, a number of transcription factors and imprinting genes were found to be specifically expressed in embryo or endosperm. This data set will aid in understanding how embryo/endosperm development in maize is differentially regulated.
[本文引用: 1]
DOI:10.1038/nmeth1156URLPMID:18165802 [本文引用: 1]

http://www.nature.com/doifinder/10.1038/nmeth1156
DOI:10.1038/nrg2626URLPMID:19997069 [本文引用: 1]

Demand has never been greater for revolutionary technologies that deliver fast, inexpensive and accurate genome information. This challenge has catalysed the development of next-generation sequencing (NGS) technologies. The inexpensive production of large volumes of sequence data is the primary advantage over conventional methods. Here, I present a technical review of template preparation, sequencing and imaging, genome alignment and assembly approaches, and recent advances in current and near-term commercially available NGS instruments. I also outline the broad range of applications for NGS technologies, in addition to providing guidelines for platform selection to address biological questions of interest.
DOI:10.1038/nrg2423URL [本文引用: 1]
DOI:10.1111/j.1365-313X.2011.04751.xURLPMID:21851431 [本文引用: 1]

Transcriptome analysis based on deep expressed sequence tag (EST) sequencing allows quantitative comparisons of gene expression across multiple species. Using pyrosequencing, we generated over 7 million ESTs from four stages of developing seeds ofRicinus communis,Brassica napus,Euonymus alatusandTropaeolum majus, which differ in their storage tissue for oil, their ability to photosynthesize and in the structure and content of their triacylglycerols (TAG). The larger number of ESTs in these 16 datasets provided reliable estimates of the expression of acyltransferases and other enzymes expressed at low levels. Analysis of EST levels from these oilseeds revealed both conserved and distinct species-specific expression patterns for genes involved in the synthesis of glycerolipids and their precursors. Independent of the species and tissue type, ESTs for core fatty acid synthesis enzymes maintained a conserved stoichiometry and a strong correlation in temporal profiles throughout seed development. However, ESTs associated with non-plastid enzymes of oil biosynthesis displayed dissimilar temporal patterns indicative of different regulation. The EST levels for several genes potentially involved in accumulation of unusual TAG structures were distinct. Comparison of expression of members from multi-gene families allowed the identification of specific isoforms with conserved function in oil biosynthesis. In all four oilseeds, ESTs for Rubisco were present, suggesting its possible role in carbon metabolism, irrespective of light availability. Together, these data provide a resource for use in comparative and functional genomics of diverse oilseeds. Expression data for more than 350 genes encoding enzymes and proteins involved in lipid metabolism are available at the RALIP website (http://aralip.plantbiology.msu.edu/).
DOI:10.1104/pp.113.220525URL [本文引用: 1]

Oil palm (Elaeis guineensis) produces two oils of major economic importance, commonly referred to as palm oil and palm kernel oil, extracted from the mesocarp and the endosperm, respectively. While lauric acid predominates in endosperm oil, the major fatty acids (FAs) of mesocarp oil are palmitic and oleic acids. The oil palm embryo also stores oil, which contains a significant proportion of linoleic acid. In addition, the three tissues display high variation for oil content at maturity. To gain insight into the mechanisms that govern such differences in oil content and FA composition, tissue transcriptome and lipid composition were compared during development. The contribution of the cytosolic and plastidial glycolytic routes differed markedly between the mesocarp and seed tissues, but transcriptional patterns of genes involved in the conversion of sucrose to pyruvate were not related to variations for oil content. Accumulation of lauric acid relied on the dramatic up-regulation of a specialized acyl-acyl carrier protein thioesterase paralog and the concerted recruitment of specific isoforms of triacylglycerol assembly enzymes. Three paralogs of the WRINKLED1 (WRI1) transcription factor were identified, of which EgWRI1-1 and EgWRI1-2 were massively transcribed during oil deposition in the mesocarp and the endosperm, respectively. None of the three WRI1 paralogs were detected in the embryo. The transcription level of FA synthesis genes correlated with the amount of WRI1 transcripts and oil content. Changes in triacylglycerol content and FA composition of Nicotiana benthamiana leaves infiltrated with various combinations of WRI1 and FatB paralogs from oil palm validated functions inferred from transcriptome analysis.
DOI:10.1111/tpj.13959URLPMID:29752761 [本文引用: 4]

Abstract Despite the importance of oilseeds to worldwide human nutrition, and more recently to the production of bio-based diesel fuels, the detailed mechanisms regulating seed oil biosynthesis remain partly understood, especially from a tissue-specific perspective. Here, we investigated the spatial distributions of lipid metabolites and transcripts involved in oil biosynthesis from seeds of two low-erucic acid genotypes of Brassica napus with high and low seed oil content. Integrated results from matrix-assisted laser desorption/ionization-mass spectrometry imaging (MALDI-MSI) of lipids in situ, lipidome profiling of extracts from seed tissues, and tissue-specific transcriptome analysis revealed complex spatial distribution patterns of lipids and transcripts. In general, it appeared that many triacylglycerol and phosphatidylcholine species distributed heterogeneously throughout the embryos. Tissue-specific transcriptome analysis identified key genes involved in de novo fatty acid biosynthesis in plastid, TAG assembly and lipid droplet packaging in ER that may contribute to high and low oil phenotype and heterogeneity of lipids distribution. Our results imply that transcriptional regulation represents an important means of impacting lipid compartmentalization in oilseeds. While much remains to be learned about the intricacies of seed oil accumulation and distribution, these studies highlight the advances that come from evaluating lipid metabolism within a spatial context and with multiple-omics-level datasets. This article is protected by copyright. All rights reserved.
DOI:10.1038/nprot.2012.016URLPMID:3334321 [本文引用: 1]

INTRODUCTIONHigh-throughput mRNA sequencing (RNA-seq) offers the abil- ity to discover new genes and transcripts and measure transcript expression in a single assay1-3. However, even small RNA-seq experi- ments involving only a single sample produce enormous volumes of raw sequencing reads--current instruments generate more than 500 gigabases in a single run. Moreover, sequencing costs are reducing exponentially, opening the door to affordable personal- ized sequencing and inviting comparisons with commodity com- puting and its impact on society4. Although the volume of data from RNA-seq experiments is often burdensome, it can provide enormous insight. Just as cDNA sequencing with Sanger sequencers drastically expanded our catalog of known human genes5, RNA- seq reveals the full repertoire of alternative splice isoforms in our transcriptome and sheds light on the rarest and most cell- and context-specific transcripts6. Furthermore, because the number of reads produced from an RNA transcript is a function of that tran- script's abundance, read density can be used to measure transcript7,8 and gene2,3,9,10 expression with comparable or superior accuracy to expression microarrays1,11.RNA-seq experiments must be analyzed with robust, efficient and statistically principled algorithms. Fortunately, the bioinformat- ics community has been hard at work developing mathematics, statistics and computer science for RNA-seq and building these ideas into software tools (for a recent review of analysis concepts and software packages see Garber et al.12). RNA-seq analysis tools generally fall into three categories: (i) those for read alignment; (ii) those for transcript assembly or genome annotation; and (iii) those for transcript and gene quantification. We have developed
DOI:10.1093/bioinformatics/btu638URLPMID:25260700 [本文引用: 1]

Motivation: A large choice of tools exists for many standard tasks in the analysis of high-throughput sequencing (HTS) data. However, once a project deviates from standard workflows, custom scripts are needed. Results: We present HTSeq, a Python library to facilitate the rapid development of such scripts. HTSeq offers parsers for many common data formats in HTS projects, as well as classes to represent data, such as genomic coordinates, sequences, sequencing reads, alignments, gene model information and variant calls, and provides data structures that allow for querying via genomic coordinates. We also present htseq-count, a tool developed with HTSeq that preprocesses RNA-Seq data for differential expression analysis by counting the overlap of reads with genes.
DOI:10.1093/bioinformatics/btp612URL [本文引用: 1]
DOI:10.1186/s13059-014-0550-8URLPMID:25516281 [本文引用: 1]

Abstract In comparative high-throughput sequencing assays, a fundamental task is the analysis of count data, such as read counts per gene in RNA-seq, for evidence of systematic changes across experimental conditions. Small replicate numbers, discreteness, large dynamic range and the presence of outliers require a suitable statistical approach. We present DESeq2, a method for differential analysis of count data, using shrinkage estimation for dispersions and fold changes to improve stability and interpretability of estimates. This enables a more quantitative analysis focused on the strength rather than the mere presence of differential expression. The DESeq2 package is available at http://www.bioconductor.org/packages/release/bioc/html/DESeq2.html webcite.
DOI:10.1016/j.plipres.2010.01.001URLPMID:20102727 [本文引用: 1]

Triacylglycerols (TAGs) constitute a highly efficient form of energy storage. In seeds of angiosperms, they can act as a reserve of carbon and energy allowing to fuel post-germinative seedling growth until photosynthesis becomes effective. They also constitute the economic value of seeds in many crops. In the past years, extensive tools allowing the molecular dissection of plant metabolism have been developed together with analytical and cytological procedures adapted for seed material. These tools have allowed gaining a comprehensive overview of the metabolic pathways leading to TAG synthesis. They have also unravelled factors limiting oil production such as metabolic bottlenecks and light or oxygen availability in seed tissues. Beyond these physiological aspects, accumulation of TAGs is developmentally regulated in seeds. The oil biosynthetic process is initiated at the onset of the maturation phase, once embryo morphogenesis is achieved. A wealth of recent studies has shed new lights on the intricate regulatory network controlling the seed maturation phase, including reserve deposition. This network involves a set of regulated transcription factors that crosstalk with physiological signaling. The knowledge thus acquired paves the way for the genetic engineering of oilseed crops dedicated to food applications or green chemistry.
DOI:10.1105/tpc.113.111740URLPMID:23709628 [本文引用: 1]

Constrained to develop within the seed, the plant embryo must adapt its shape and size to fit the space available. Here, we demonstrate how this adjustment shapes metabolism of photosynthetic embryo. Noninvasive NMR-based imaging of the developing oilseed rape (Brassica napus) seed illustrates that, following embryo bending, gradients in lipid concentration became established. These were correlated with the local photosynthetic electron transport rate and the accumulation of storage products. Experimentally induced changes in embryo morphology and/or light supply altered these gradients and were accompanied by alterations in both proteome and metabolome. Tissue-specific metabolic models predicted that the outer cotyledon and hypocotyl/radicle generate the bulk of plastidic reductant/ATP via photosynthesis, while the inner cotyledon, being enclosed by the outer cotyledon, is forced to grow essentially heterotrophically. Under field-relevant highlight conditions, major contribution of the ribulose-1,5-bisphosphate carboxylase/oxygenase-bypass to seed storage metabolism is predicted for the outer cotyledon and the hypocotyl/radicle only. Differences between in vitro-versus in planta-grown embryos suggest that metabolic heterogeneity of embryo is not observable by in vitro approaches. We conclude that in vivo metabolic fluxes are locally regulated and connected to seed architecture, driving the embryo toward an efficient use of available light and space.
DOI:10.1016/j.pbi.2014.04.001URLPMID:4070482 [本文引用: 1]

The manipulation of plant seed oil composition so as to deliver enhanced fatty acid compositions suitable for feed or fuel has long been a goal of metabolic engineers. Recent advances in our understanding of the flux of acyl-changes through different key metabolic pools such as phosphatidylcholine and diacylglycerol have allowed for more targeted interventions. When combined in iterative fashion with further lipidomic analyses, significant breakthroughs in our capacity to generate plants with novel oils have been achieved. Collectively these studies, working at the interface between metabolic engineering and synthetic biology, demonstrate the positive fundamental and applied outcomes derived from such research.
