 ,, 郑博元, 李蕾蕾, 贾双杰, 韩心培, 郭家萌, 王泳超, 邵瑞鑫
,, 郑博元, 李蕾蕾, 贾双杰, 韩心培, 郭家萌, 王泳超, 邵瑞鑫 ,*河南农业大学农学院, 河南郑州450046
,*河南农业大学农学院, 河南郑州450046Effect of Exogenous Nitric Oxide Donor on Carbon Assimilation and Antioxidant System in Leaves of Maize Seedlings under PEG-induced Water Deficit Stress
YANG Qing-Hua ,, ZHENG Bo-Yuan, LI Lei-Lei, JIA Shuang-Jie, HAN Xin-Pei, GUO Jia-Meng, WANG Yong-Chao, SHAO Rui-Xin
,, ZHENG Bo-Yuan, LI Lei-Lei, JIA Shuang-Jie, HAN Xin-Pei, GUO Jia-Meng, WANG Yong-Chao, SHAO Rui-Xin ,*College of Agronomy, Henan Agricultural University, Zhengzhou 450046, Henan, China
,*College of Agronomy, Henan Agricultural University, Zhengzhou 450046, Henan, China通讯作者:
第一联系人:
收稿日期:2017-12-22接受日期:2018-06-12网络出版日期:2018-07-02
| 基金资助: |
Received:2017-12-22Accepted:2018-06-12Online:2018-07-02
| Fund supported: |

摘要
关键词:
Abstract
Keywords:
PDF (1883KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
杨青华, 郑博元, 李蕾蕾, 贾双杰, 韩心培, 郭家萌, 王泳超, 邵瑞鑫. 外源NO供体对水分亏缺下玉米叶片碳同化关键酶及抗氧化系统的影响[J]. 作物学报, 2018, 44(9): 1393-1399. doi:10.3724/SP.J.1006.2018.01393
YANG Qing-Hua, ZHENG Bo-Yuan, LI Lei-Lei, JIA Shuang-Jie, HAN Xin-Pei, GUO Jia-Meng, WANG Yong-Chao, SHAO Rui-Xin.
水资源短缺是制约全球农业生产发展的一个严峻生态问题[1], 目前我国水资源已成为限制粮食持续增产的主要瓶颈。玉米作为我国第一大粮食作物, 在国家粮食安全中起着重要作用。近年来, 由于气候的变化, 我国约有60%的玉米面积受到干旱影响, 每年因旱灾减产15%~20%, 干旱灾害已成为制约玉米持续增产的关键生态因素[2]。因此, 提高玉米的抗旱性, 对实现玉米可持续生产及保障国家粮食安全具有十分重要的现实意义。
光合作用作为植物生长发育关键的代谢过程, 对干旱胁迫反应非常敏感[3,4]。在水分亏缺下, 光合作用的下调与叶绿体内的核酮糖-1,5-二磷酸羧化酶/加氧酶(Rubisco)、Rubisco活化酶(RCA)、磷酸烯醇丙酮酸羧化酶、丙酮酸磷酸激酶、NADP-苹果酸脱氢酶等光合酶活性的变化有关[5]。其中, Rubisco和RCA是在光合作用中固定CO2的关键酶, 研究表明耐旱型植株在干旱胁迫后Rubisco和RCA具有相对较高的酶活性和转录水平[6,7]。光合碳同化对干旱胁迫的适应性主要通过调节其基因表达来实现, 已表明Rubisco大亚基rbc L由叶绿体基因编码, 小亚基rbc S由核基因编码, 二者在受到干旱胁迫时的表达量明显影响着植物对胁迫的适应性[3]。此外, 植物抵御不利环境的能力还与其细胞具有较强的抗氧化能力密切相关, 因为生物体处于逆境下时, 体内的抗氧化酶同工酶表达会发生显著的变化, 若维持较高的超氧化物歧化酶(SOD)、过氧化物酶(POD)、过氧化氢酶(CAT)活性及类胡萝卜素(Car)、抗坏血酸(AsA)含量, 以降低膜质过氧化程度, 可提高植物的抗逆性[8]。
一氧化氮(NO)调节植物的生长和发育, 是逆境胁迫下植物防御响应的信号分子, 被确认为是一种新的植物生长调节物质[9,10]。近年来, 较多研究报道了外源NO供体(硝普钠, SNP)提高植物的抗旱性。SNP可诱导小麦D1蛋白快速周转以激活失去活性的PSII反应中心, 维持较高的PSII最大光化学效率, 保护叶绿体结构不受干旱胁迫下ROS积累的破坏[11,12]; 能促进红芸豆叶片SOD、CAT、POD同工酶的表达, 提高干旱胁迫下小麦、水稻叶片保护酶的活性, 从而增强细胞膜的稳定性[13,14,15]。但这些研究主要集中在小麦、水稻、拟南芥等作物, 而关于SNP对水分亏缺下玉米叶片碳同化关键酶及抗氧化系统的影响及其调控机制研究还未见报道。本文在前期试验基础上, 深入探讨了SNP在PEG模拟水分亏缺胁迫下对玉米叶片碳同化相关酶Rubisco、RCA及其基因表达和抗氧化酶系统活性及其同工酶谱的影响, 旨在明确SNP的调控机制, 并为生产上利用SNP提高玉米抗旱性提供理论依据。
1 材料与方法
1.1 试验材料
精选水分敏感玉米品种驻玉309 [16]的种子, 用1.8% (v/v)次氯酸钠表面消毒5 min, 蒸馏水反复冲洗后, 浸种10 h, 在25°C恒温培养箱内暗催芽4 d, 转入1/2 Hoagland 营养液中培养至三叶一心, 改用全营养液培养, 同时用100 μmol L-1的SNP预处理3 d (四叶一心), 20% PEG-6000 (-0.8 MPa)模拟中度水分亏缺胁迫处理3 d, 处理期间每天更换营养液。幼苗生长的昼/夜温度为(27 ± 1) °C /(22 ± 1) °C, 光强为250 μmol m-2 s-1, 湿度为(60 ± 5)%。在前期试验的基础上设4个处理, 即CK (0% PEG, 对照)、SNP (100 μmol L-1 SNP)、PEG (20% PEG)、SNP + PEG (100 μmol L-1 SNP + 20% PEG), 每个处理重复3次, 每盆定苗12株[盆大小为21 cm ×16 cm × 13 cm (长×宽×高)]。在胁迫后第3天选取完全展开的倒二叶于液氮中保存, 选取叶片中部, 除去叶脉, 进行生理生化指标和Real time PCR检测。1.2 测定项目与方法
1.2.1 活性氧含量、抗氧化系统、Rubisco和RCA活性的测定 根据李忠光和龚明方法测定O2-产生速率[17]。采用硫酸钛比色法测定H2O2含量[18]。参照邹琦[19]的方法略加改进测定SOD、POD、CAT的活性。采用植物酶联免疫分析试剂盒(苏州科铭生物技术有限公司生产)测定Rubisco、RCA活性。取叶片中部0.1 g, 置研钵中加液氮及1%不溶性PVP研磨, 加入1.9 mL预冷提取液(50 mmol L-1 HEPES- KOH pH 7.0, 1 mmol L-1 EDTA, 5 mmol L-1 MgCl2, 0.4 mmol L-1 ATP, 15 mmol L-1 DTT, 1 mmol L-1 PMSF, 2 mmol L-1 Benzamidine, 0.01 mmol L-1 Leupeptin), 磨成均浆, 15 000 × g离心10 min, 上清液用于测定酶活性。
1.2.2 Rubisco和RCA基因的Real-time PCR分析
根据GenBank已上传编码D1蛋白的编码Rubisco的小亚基蛋白rbc S基因序列、编码Rubisco大亚基蛋白rbc L基因序列、编码RCA蛋白的rca β基因序列同源性设计Real-time PCR的引物, 选其内参基因为actin, 利用DNAMAN和Premier 5.0软件依据GenBank 已上传的序列(GI: 1498383; GI: 1498383)的同源性设计引物序列(表1)。
Table 1
表1
表1本实验引物序列
Table 1
| 引物名称 Primer name | 序列 Sequence (5'-3') | Tm (°C) |
|---|---|---|
| actin-F | CTGAACCCCAAGGCAAACA | 59.0 |
| actin-R | ACTGGCGTACAGGGAAAGAA | 57.3 |
| rca β-F | TCCTTGAGACCTTCTTGACGG | 59.8 |
| rca β-R | ATCGCCTTGAACCTGCTGT | 57.8 |
| rbc L-F | CCGTTTCGTCTTTTGTGCC | 58.9 |
| rbc L-R | TGCGGTGAATCCTCCTGTT | 58.3 |
| rbc S-F | CGCTACTGGACCATGTGGAA | 59.1 |
| rbc S-R | ACTGCGTCTGCTTGATGTTGT | 58.1 |
新窗口打开|下载CSV
在20 μL反应体系中包含10 μL SYBR Green QPK-201、0.8 μL正义及反义端的引物、1 μL cDNA模板以及7.4 μL ddH2O。PCR条件为95°C预变性3min; 95°C变性7 s, 57°C退火10 s, 72°C延伸10 s, 40个循环; 72°C延伸10 min, 65~95°C溶解曲线。
以actin基因作为内参, 采用相对定量方法, 通过比较CT值法(2-ΔΔCT法)进行荧光定量数据分析。
改变的倍数=2-ΔΔCT, ΔΔCT=(CT靶基因-CT内参)处理组-(CT靶基因-CT内参)未处理组。
1.2.3 抗氧化酶同工酶的测定 准确称取1 g叶片, 加入少量提样缓冲液, 置冰浴研磨匀浆后定容至5 mL, 10 000×g离心15 min, 上清液为可溶性蛋白的粗提液, 用考马斯亮蓝染色法测定蛋白质含量, 粗提液贮于冰箱备用。参照李文鹤[20]的方法对SOD、POD、CAT的同工酶染色测定。
1.3 数据分析
采用SPSS 19.0软件对数据进行统计分析, 并用SigmaPlot 10.0软件作图。2 结果与分析
2.1 NO对干旱胁迫下玉米叶片Rubisco活性以及RCA活性的影响
干旱条件下, 玉米叶片Rubisco及RCA活性与CK相比分别降低了42.3%和33.3% (图1)。SNP + PEG处理的幼苗, 其叶片Rubisco及RCA的活性分别较PEG胁迫处理上升32.70%和14.67%, 而单独的SNP处理之后, 其玉米幼苗叶片的RCA活性与CK无显著差异, 叶片Rubisco活性则较CK有所降低。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1外源NO对干旱胁迫下玉米幼苗Rubisco活性(A)及活化酶RCA活性(B)的影响
处理CK、SNP、PEG、SNP + PEG分别为0% PEG、100 μmol L-1 SNP、20% PEG和100 μmol L-1 SNP + 20% PEG。
Fig. 1Effects of exogenous NO on the Rubisco activity (A) and RCA activity (B) in leaves of maize seedlings under drought stress
Treatments including CK, SNP, PEG, and SNP + PEG indicate 0% PEG, 100 μmol L-1 SNP, 20% PEG, and 100 μmol L-1 SNP + 20% PEG, respectively.
2.2 NO对干旱胁迫下玉米叶片rbc L、rbc S、rca β基因表达水平的影响
干旱胁迫后, 玉米叶片rbc L基因的相对转录水平下降, 而rbc S基因的相对转录水平略有上升(图2), rcaβ基因的相对表达量较CK降低了77.1%; SNP + PEG处理的幼苗, 其叶片rbc L、rbc S和rca β基因的转录水平较PEG处理分别提高73.4%、78.1%和109.1%; 单独SNP处理之后的幼苗, 其叶片rbc L、rbc S和rca β基因的相对表达量与CK无显著差异。图2
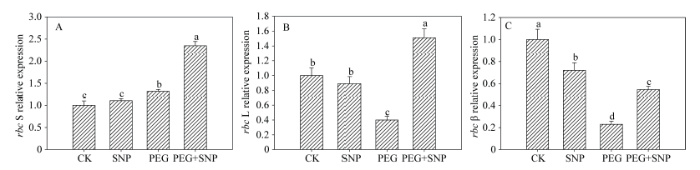 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2外源NO对干旱胁迫下玉米rbc S (A)、rbc L (B)和rca β (C)的影响
处理CK、SNP、PEG、SNP + PEG分别为0% PEG、100 μmol L-1 SNP、20% PEG和100 μmol L-1 SNP + 20% PEG。
Fig. 2Effects of exogenous NO on the expression of rbc S (A), rbc L (B), and rca β (C) in leaves of maize seedlings under drought stress
Treatments including CK, SNP, PEG, and SNP + PEG indicate 0% PEG, 100 μmol L-1 SNP, 20% PEG, and 100 μmol L-1 SNP + 20% PEG, respectively.
2.3 NO对干旱胁迫下玉米叶片ROS积累的影响
干旱条件下, 玉米幼苗叶片的O2-和H2O2含量均显著升高(图3), 与CK相比分别上升284.3%和21.0%。SNP + PEG处理的幼苗, 其叶片O2-、H2O2含量比干旱处理分别降低了27.4%和17.9%, 而单独的SNP处理之后, 其叶片O2-、H2O2含量与CK无显著差异。这表明NO预处理可明显改善干旱胁迫条件下玉米幼苗叶片活性氧的积累。图3
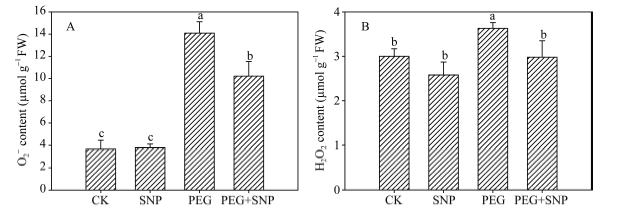 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3外源NO处理对干旱胁迫下玉米幼苗O2-(A)和H2O2(B)含量的影响
处理CK、SNP、PEG、SNP + PEG分别为0% PEG、100 μmol L-1 SNP、20% PEG和100 μmol L-1 SNP + 20% PEG。
Fig. 3Effects of exogenous NO on O2-(A) and H2O2(B) contents in leaves of maize seedlings under drought stress
Treatments including CK, SNP, PEG, and SNP + PEG indicate 0% PEG, 100 μmol L-1 SNP, 20% PEG, and 100 μmol L-1 SNP + 20% PEG, respectively.
2.4 NO对干旱胁迫下玉米叶片抗氧化酶活性及其同工酶的影响
干旱胁迫和单独SNP处理与CK相比, 玉米幼苗叶片SOD活性分别提高66.5%和108.1%。SNP预处理之后进行干旱胁迫, 其活性进一步增加, 较PEG处理提升37.7% (图4-A)。SNP 处理和PEG胁迫后SOD同工酶谱带(图4-B)的宽度和亮度都高于CK, 尤其是SNP处理。图4
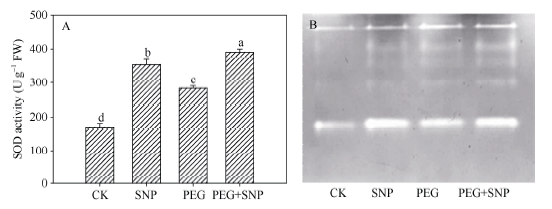 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4外源NO处理对干旱胁迫下玉米幼苗SOD活性(A)及SOD同工酶(B)的影响
处理CK、SNP、PEG、SNP + PEG分别为0% PEG、100 μmol L-1 SNP、20% PEG和100 μmol L-1 SNP + 20% PEG。
Fig. 4Effects of exogenous NO on SOD activity (A) and SOD isoenzyme (B) in leaves of maize seedlings under drought stress
Treatments including CK, SNP, PEG, and SNP + PEG indicate 0% PEG, 100 μmol L-1 SNP, 20% PEG, and 100 μmol L-1 SNP + 20% PEG, respectively.
玉米幼苗叶片的POD和CAT活性在PEG胁迫后分别较CK降低33.3%和56.0% (图5-A, 6-A)。单独SNP处理之后, 其叶片的POD活性较CK提高10.8%, 而叶片CAT酶活性与CK差异不显著。SNP + PEG处理的幼苗, 其叶片的POD、CAT活性与PEG处理相比分别提高25.0%和67.9%。图5-B和图6-B结果显示, PEG胁迫处理叶片的POD、CAT同工酶谱带在宽度和亮度上弱于CK, 单独SNP预处理之后, 增强不显著, 而SNP + PEG处理的幼苗叶片POD、CAT同工酶谱带, 较PEG胁迫处理的宽度和亮度明显增强。
图5
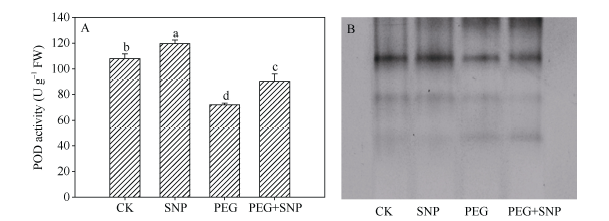 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5外源NO处理对干旱胁迫下玉米幼苗POD活性(A)及POD同工酶(B)的影响
处理CK、SNP、PEG、SNP + PEG分别为0% PEG、100 μmol L-1 SNP、20% PEG和100 μmol L-1 SNP + 20% PEG。
Fig. 5Effects of exogenous NO on POD activity (A) and POD isoenzyme (B) in leaves of maize seedlings under drought stress
Treatments including CK, SNP, PEG, and SNP + PEG indicate 0% PEG, 100 μmol L-1 SNP, 20% PEG, and 100 μmol L-1 SNP + 20% PEG, respectively.
图6
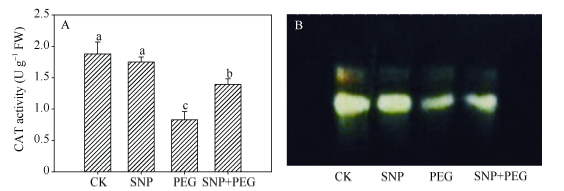 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6外源NO处理对干旱胁迫下玉米幼苗CAT活性(A)及CAT同工酶(B)的影响
处理CK、SNP、PEG、SNP + PEG分别为0% PEG、100 μmol L-1 SNP、20% PEG和100 μmol L-1 SNP + 20% PEG。
Fig. 6Effects of exogenous NO on CAT activity (A) and CAT isoenzyme (B) in leaves of maize seedlings under drought stress
Treatments including CK, SNP, PEG, and SNP + PEG indicate 0% PEG, 100 μmol L-1 SNP, 20% PEG, and 100 μmol L-1 SNP + 20% PEG, respectively.
3 讨论
光合作用是植物生命活动过程中的重要组成部分, 已有的研究结果表明, 水分亏缺胁迫引起光合作用能力下降主要是因为非气孔因素限制[16], 与Rubisco活性和激活状态有关, 而Rubisco在植物体内的活性取决于RCA对它的活化[5]。在水分亏缺胁迫下, Rubisco和RCA的活性下降会影响叶片的气体交换和光合作用的正常进行[21]。Rubisco由8个大亚基和8个小亚基组成, 大亚基由叶绿体基因rbc L编码, 小亚基由细胞核中的多基因家族rbc S编码[22]。而且不同植物RCA亚基的数量和种类也不相同, 但玉米中只发现了β亚基[23]。本试验结果表明, 玉米叶片Rubisco和RCA的活性及rbc L、rca β基因表达量在水分亏缺时均显著降低, 而rbc S基因的表达量略有上升, 这与前人对水分亏缺胁迫下番茄、水稻、拟南芥等植物叶片rbc S基因表达量急剧下降的研究结果不一致, 说明rbc S基因对环境的敏感程度可能因植物种类不同而异。NO作为新型的植物生长调节物质, 研究表明外源NO可通过上调光合碳同化过程中相关酶基因的mRNA表达水平, 提高NaCl胁迫下番茄Rubisco和RCA活性[24]。本试验结果也证明了外源NO能上调Rubisco和 RCA的活性及rbc L、rbc S、rca β基因表达, 有利于增强CO2的同化效率和光合电子传递效率, 并促进光反应同化力(NADPH和ATP)的积累, 增加 RuBP固定CO2的量, 从而提高 Rubisco的羧化效率。ROS产生的主要部位是在叶绿体和线粒体, 研究表明干旱条件下光合作用的下调与抗氧化酶的活性有关[25]。SOD、POD和CAT是植物组织内重要的抗氧化酶, 它们通过清除O2-、·OH和H2O2来减少ROS对叶绿体细胞膜的伤害、减轻膜质过氧化和稳定膜的透性[26]。当植物处于逆境时, 体内的抗氧化酶活性及同工酶表达升高, 是保障植物光合作用正常进行的重要酶系统[27]。而在本试验中, 水分亏缺胁迫后, 玉米幼苗叶片仅SOD活性上升及同工酶谱带增宽, CAT、POD活性的下降引起了ROS类物质O2-和H2O2大量积累, 抗氧化防御系统作用减弱, 体内自由基不能被完全清除而造成玉米叶片膜脂过氧化损伤, 从而导致了玉米光合碳同化能力下降。NO本身是一个活性氮中间体(RNS), 低浓度NO可通过各种方式与ROS作用并发挥抗氧化胁迫功能[3,10,28]。外源NO处理后抗氧化酶SOD、CAT、POD活性的提高, 及其同工酶带宽度和亮度增强, 表明了NO对细胞内ROS的动态平衡和细胞膜稳定性的调控作用, 预示着NO对植物光合作用的调节作用与其对抗ROS代谢水平的调节也密切相关。
4 结论
水分亏缺诱导的NO调节物质在玉米抗旱机制中扮演着非常重要的角色, 外源NO预处理可以提高干旱胁迫条件下玉米幼苗叶片的光合碳同化能力和抗氧化酶活性, 缓解干旱胁迫对叶片光合作用的抑制。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1093/jxb/erq340URLPMID:21172816 [本文引用: 3]

Abstract Photosynthesis is one of the key processes to be affected by water deficits, via decreased CO2 diffusion to the chloroplast and metabolic constraints. The relative impact of those limitations varies with the intensity of the stress, the occurrence (or not) of superimposed stresses, and the species we are dealing with. Total plant carbon uptake is further reduced due to the concomitant or even earlier inhibition of growth. Leaf carbohydrate status, altered directly by water deficits or indirectly (via decreased growth), acts as a metabolic signal although its role is not totally clear. Other relevant signals acting under water deficits comprise: abscisic acid (ABA), with an impact on stomatal aperture and the regulation at the transcription level of a large number of genes related to plant stress response; other hormones that act either concurrently (brassinosteroids, jasmonates, and salycilic acid) or antagonistically (auxin, cytokinin, or ethylene) with ABA; and redox control of the energy balance of photosynthetic cells deprived of CO2 by stomatal closure. In an attempt to systematize current knowledge on the complex network of interactions and regulation of photosynthesis in plants subjected to water deficits, a meta-analysis has been performed covering >450 papers published in the last 15 years. This analysis shows the interplay of sugars, reactive oxygen species (ROS), and hormones with photosynthetic responses to drought, involving many metabolic events. However, more significantly it highlights (i) how fragmented and often non-comparable the results are and (ii) how hard it is to relate molecular events to plant physiological status, namely photosynthetic activity, and to stress intensity. Indeed, the same data set usually does not integrate these different levels of analysis. Considering these limitations, it was hard to find a general trend, particularly concerning molecular responses to drought, with the exception of the genes ABI1 and ABI3. These genes, irrespective of the stress type (acute versus chronic) and intensity, show a similar response to water shortage in the two plant systems analysed (Arabidopsis and barley). Both are associated with ABA-mediated metabolic responses to stress and the regulation of stomatal aperture. Under drought, ABI1 transcription is up-regulated while ABI3 is usually down-regulated. Recently ABI3 has been hypothesized to be essential for successful drought recovery.
[本文引用: 1]
[本文引用: 1]
DOI:10.1093/jxb/eru406URLPMID:25324402 [本文引用: 2]

Abstract Incident irradiance on plant leaves often fluctuates, causing dynamic photosynthesis. Whereas steady-state photosynthetic responses to environmental factors have been extensively studied, knowledge of dynamic modulation of photosynthesis remains scarce and scattered. This review addresses this discrepancy by summarizing available data and identifying the research questions necessary to advance our understanding of interactions between environmental factors and dynamic behaviour of photosynthesis using a mechanistic framework. Firstly, dynamic photosynthesis is separated into sub-processes related to proton and electron transport, non-photochemical quenching, control of metabolite flux through the Calvin cycle (activation states of Rubisco and RuBP regeneration, and post-illumination metabolite turnover), and control of CO090202 supply to Rubisco (stomatal and mesophyll conductance changes). Secondly, the modulation of dynamic photosynthesis and its sub-processes by environmental factors is described. Increases in ambient CO090202 concentration and temperature (up to ~3500°C) enhance rates of photosynthetic induction and decrease its loss, facilitating more efficient dynamic photosynthesis. Depending on the sensitivity of stomatal conductance, dynamic photosynthesis may additionally be modulated by air humidity. Major knowledge gaps exist regarding environmental modulation of loss of photosynthetic induction, dynamic changes in mesophyll conductance, and the extent of limitations imposed by stomatal conductance for different species and environmental conditions. The study of mutants or genetic transformants for specific processes under various environmental conditions could provide significant progress in understanding the control of dynamic photosynthesis. 0008 The Author 2014. Published by Oxford University Press on behalf of the Society for Experimental Biology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1111/tpj.13299URLPMID:27801967 [本文引用: 1]

Reactive oxygen species (ROS) play a key role in the acclimation process of plants to abiotic stress. They primarily function as signal transduction molecules that regulate different pathways during plant acclimation to stress, but are also toxic byproducts of stress metabolism. Because each subcellular compartment in plants contains its own set of ROS‐producing and ROS‐scavenging pathways, the steady‐state level of ROS, as well as the redox state of each compartment, is different at any given time giving rise to a distinct signature of ROS levels at the different compartments of the cell. Here we review recent studies on the role of ROS in abiotic stress in plants, and propose that different abiotic stresses, such as drought, heat, salinity and high light, result in different ROS signatures that determine the specificity of the acclimation response and help tailor it to the exact stress the plant encounters. We further address the role of ROS in the acclimation of plants to stress combination as well as the role of ROS in mediating rapid systemic signaling during abiotic stress. We conclude that as long as cells maintain high enough energy reserves to detoxify ROS, ROS is beneficial to plants during abiotic stress enabling them to adjust their metabolism and mount a proper acclimation response.
