摘要/Abstract
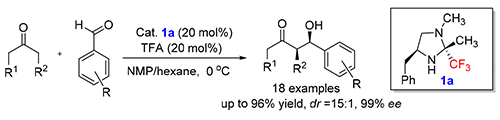
手性1,2-二醇骨架是天然产物或生物活性分子构建过程中的重要骨架,而α-羟基酮参与的不对称Aldol缩合反应是实现手性1,2-二醇骨架的重要手段.设计并合成了含三氟甲基的咪唑啉型化合物,并将其应用于羟基丙酮和醛的不对称Aodol缩合反应.研究结果表明,当采用含氟咪唑啉(2R,4S)-4-苄基-1,2-二甲基-2-三氟甲基咪唑啉(1a)作为不对称Aldol反应的催化剂时,能够以产率高达96%、最高ee值达到99%及dr值达到15∶1的效率高效构建一系列顺式1,2-二醇产物.同时,我们也初步探讨了氟-氢键在不对称催化反应中的作用.
关键词: 不对称Aldol反应, 有机催化, 咪唑啉, 三氟甲基, 合成
Aldol reaction of hydroxyacetone is an all-purpose route to construct the 1,2-diol building blocks for the synthesis of multifarious natural products and biological active molecules. In this work, a new series of trifluoromethylated-imidazoline organocatalysts have been designed and synthesized. It is found that the trifluoromethylated chiral organocatalyst (2R,4S)-4-benzyl-1,2-dimethyl-2-(trifluoromethyl) imidazolidine (1a) has proved to be very efficient for the direct asymmetric aldol reaction of α-hydroxyketones with aldehydes to build the syn-1,2-diol building blocks. Among the synthesized syn-aldol products, a good yield (up to 96%) and high stereoselectivity (up to dr=15:1, 99% ee) could be obtained. The F—H bonding derived from trifluoromethyl group was proposed to play an important role in the stabilization of the transition state.
Key words: asymmetric aldol reaction, organocatalysis, imidazoline, trifluoromethyl, synthesis
PDF全文下载地址:
点我下载PDF
