摘要/Abstract
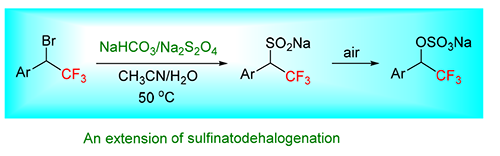
脱卤亚磺化反应是引入氟烷基基团的一种常用方法.探索了α-三氟甲基苄溴在脱卤亚磺化条件下的反应,发现产物并不是亚磺酸盐[ArCH(CF3)SO2Na],而是烷基硫酸盐[ArCH(CF3)OSO3Na].即使在烯烃的存在的条件下,α-三氟甲基苄溴在脱卤亚磺化条件下产生了自由基,也不与烯烃发生加成反应,而是直接生成亚磺酸盐,亚磺酸盐被空气氧化成烷基硫酸盐.
关键词: 三氟甲基, 自由基, 脱卤亚磺化, 烷基硫酸盐
The sulfinatodehalogenation reaction is a common method of introducing a fluoroalkyl group. In this paper, the reaction of α-trifluoromethylbenzyl bromide under sulfinatodehalogenation conditions was investigated. It was found that the product was an sodium alkyl sulfate (ArCH(CF3)OSO3Na) instead of an sodium alkyl sulfinate (ArCH(CF3)SO2Na) which was normal produced. α-Trifluoromethylbenzyl bromide did not react with the olefin after its generation of a radical intermediate under sulfinatodehalogenation conditions even though an olefin was presented. Instead, the reaction directly gave an alkyl sulfinate, and then oxidized by air to provide a product as an alkyl sulfate.
Key words: Trifiluoromethyl, free radical, sulfinatodehalogenation, alkyl sulfate
PDF全文下载地址:
点我下载PDF
