摘要/Abstract
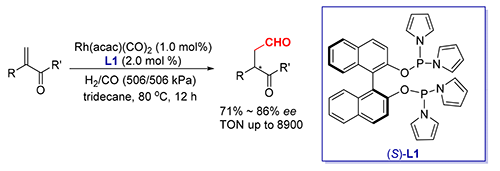
发展了一类吡咯取代的手性亚磷酰胺配体,并将其成功地应用于铑催化的1,1-双取代烯烃的不对称氢甲酰化反应,以优秀的区域选择性、良好的化学选择性和对映选择性(71%~86%ee)得到相应的手性直链醛,反应的转化数(Turnover Number,TON)值最高达到8900.该类催化剂容易制备且具有广泛的官能团兼容性,通过不对称氢甲酰化反应为手性α-烷基-β-甲酰基丙酸酯类化合物的合成提供了一类新的方法.
关键词: 氢甲酰化, 烯烃, 铑, 不对称催化, 亚磷酰胺
A readily prepared chiral pyrrolylphosphinite has been found highly efficient for Rh(I) catalyzed asymmetric hydroformylation of 1,1-disubstituted olefins. Chiral linear aldehydes have been synthesized with high productivity (turnover number (TON) up to 8900), excellent regioselectivity, and good to high chemo- and enantio-selectivites (71%~86% ee). The reaction features ready catalyst preparation and wide functional group tolerance, thus will be of practical value in the use of asymmetric hydroformylation (AHF) for the synthesis of chiral α-alkyl-β-formylpropanoate analogues.
Key words: hydroformylation, olefin, Rhodium, asymmetric catalysis, phosphoramidite
PDF全文下载地址:
点我下载PDF
