摘要/Abstract
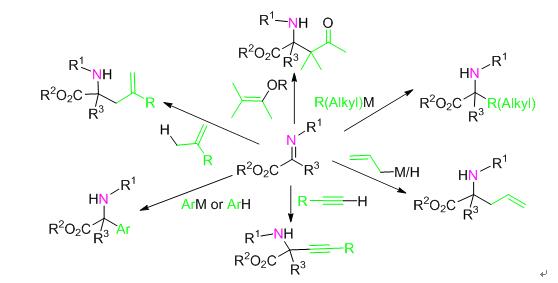
手性α-氨基酸衍生物在生命医药、精细化工等领域的广泛应用极大地促进了其合成方法的发展.目前在众多合成手性α-氨基酸衍生物的方法中,α-亚胺酯的不对称亲核加成反应是合成手性α-氨基酸衍生物的有效方法之一,成为不对称催化研究的热点.从反应类型和亲核试剂类型的角度出发,总结了α-亚胺酯不对称亲核加成反应合成手性α-氨基酸衍生物的研究进展.具体介绍了α-亚胺酯的烯丙基化反应、芳基化反应、Mannich反应、烯基化反应、炔基化反应及烷基化反应等六种合成手性α-氨基酸衍生物的主要方法以及相应反应机理及发展现状,并对合成手性α-氨基酸衍生物的发展方向进行了展望.
关键词: α-亚胺酯, 手性α-氨基酸衍生物, 不对称, 加成反应
The wide applications of chiral α-amino acid derivatives in the pharmaceutical and fine chemical industry field has greatly arisen the development of its synthetic methods. So far, asymmetric nucleophilic addition reaction of α-imino ester has been proven to be one of the most effective methods to synthesize chiral α-amino acid derivatives and has been focused by chemists in the field of asymmetric catalysis. The development of such method on the view of reaction types and different kinds of nucleophiles is described. Specifically, allylation reaction, arylation reactions, Mannich reactions, alkenylation reactions, alkynylation reactions and alkylation reactions are introduced, together with the associated reaction mechanisms and recent developments. Additionally, a prospect on this research field is given.
Key words: α-imino ester, chiral α-amino acid derivative, asymmetric, addition reaction References
PDF全文下载地址:
点我下载PDF
