摘要/Abstract
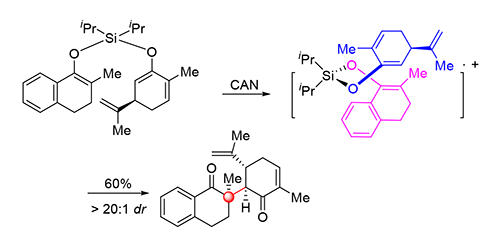
立体位阻拥挤C—C键,尤其是包含全碳季碳中心C—C键的构建一直是现代有机合成化学的挑战.利用硝酸铈铵(CAN)诱导的双烯醇硅醚化合物分子内交叉氧化偶联反应来非对映选择性地构建连续全碳季碳中心以及叔碳中心.该方法在相对温和的反应条件下,以CAN作为单电子氧化剂,高产率、高非对映选择性地构建立体位阻拥挤的C—C键.本研究提供了一种通过分子内交叉氧化偶联反应来实现两个不同片段高效连接的有效手段,可以应用到复杂天然产物的全合成研究中去.
关键词: 氧化偶联反应, 季碳中心, 叔碳中心, 方法学
The formation of sterically hindered C—C bond represents a great challenge in modern synthetic organic chemistry. A particularly challenging issue is the construction of all-carbon quaternary stereocenters. Herein, a ceric ammonium nitrate (CAN)-mediated intramolecular oxidative cross-coupling of silyl ethers for direct construction of valuable polycyclic scaffolds is described. The reaction enables sterically congested vicinal all-carbon quaternary and tertiary stereocenters to be installed diastereoselectively. The developed method provides a concise and efficient approach for ligation of two different segments through a compact C—C bond formation, which has potential applications in the synthesis of complex molecules as well as sterically congested natural products.
Key words: oxidative coupling reaction, quaternary stereocenter, tertiary stereocenter, methodology
PDF全文下载地址:
点我下载PDF
