摘要/Abstract
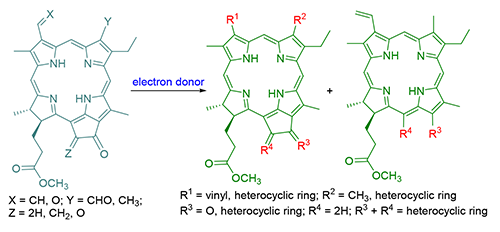
为了拓展叶绿素类二氢卟吩的研究和应用,以焦脱镁叶绿酸-a甲酯为起始原料,通过氧化和缩合反应,对其N21-N23轴向两端的取代基团进行化学修饰和结构转换,在四吡咯大环分子的周环上构建了醛、邻位二酮、烯腈和烯酮等活性受电子官能结构,再与不同的富电子体系实施关环,合成了一系列未见报道的含有多种杂环结构的焦脱镁叶绿酸衍生物,其化学结构均经UV-Vis,IR,1H NMR,MS及元素分析予以证实.同时,对相应杂环的形成过程、立体化学选择性以及电子光谱变化进行了讨论.
关键词: 叶绿素-a, 二氢卟吩, 化学修饰, 杂环化反应, 合成
In order to expand study and application for the chlorophllous chlorins, pyropheophorbide-a methyl ester was used as a starting material. The chemical modifications and structural transformations along the terminals of N21-N23 axis were carried out to build active electron-accepting functional structures such as aldehyde, α-diketone, enenitrile and ketene moieties. The cyclizations with different electron-sufficient systems were accomplished to synthesize a series of unreported chlorins related to chlorophyll with multiple heterocyclic structures. The chemical structures of new compounds were characterized by elemental analysis, MS, UV-Vis, IR and 1H NMR spectra. Meanwhile the relevant formation process of the heterocyclic ring, the stereochemistry selectivity and the change of the electronic spectrum were discussed.
Key words: chlorophyll-a, chlorin, chemical modification, heterocyclization reaction, synthesis
PDF全文下载地址:
点我下载PDF
